Significance
Macrophages—cells crucially involved in defense against infections—exhibit, depending on their anatomical location, distinct biological properties. Studies of the underlying mechanisms are of scientific and clinical interest, but are hampered by the difficulty of obtaining primary tissue macrophages in sufficient numbers and purity. Here, we report the generation of nontransformed murine macrophages, which are similar to alveolar macrophages and can be grown continuously without change of phenotype and in unlimited amounts. Such macrophages helped us to recognize several innate immune properties of alveolar macrophages that are involved in the pathogenesis of infectious lung inflammation.
Keywords: LPS recognition, innate immunity
Abstract
Macrophages are diverse cell types in the first line of antimicrobial defense. Only a limited number of primary mouse models exist to study their function. Bone marrow-derived, macrophage-CSF–induced cells with a limited life span are the most common source. We report here a simple method yielding self-renewing, nontransformed, GM-CSF/signal transducer and activator of transcription 5-dependent macrophages (Max Planck Institute cells) from mouse fetal liver, which reflect the innate immune characteristics of alveolar macrophages. Max Planck Institute cells are exquisitely sensitive to selected microbial agents, including bacterial LPS, lipopeptide, Mycobacterium tuberculosis, cord factor, and adenovirus and mount highly proinflammatory but no anti-inflammatory IL-10 responses. They show a unique pattern of innate responses not yet observed in other mononuclear phagocytes. This includes differential LPS sensing and an unprecedented regulation of IL-1α production upon LPS exposure, which likely plays a key role in lung inflammation in vivo. In conclusion, Max Planck Institute cells offer an useful tool to study macrophage biology and for biomedical science.
Macrophages comprise a group of tissue-resident mononuclear phagocytes, crucially involved in antimicrobial defense and tissue homeostasis and diverse in their origin, development, life span, and function. The major part of tissue macrophages develops from bone-marrow (BM) hematopoietic stem cells (HSCs) under the influence of macrophage-CSF (M-CSF) signaling through blood monocytes that colonize various organs and become specialized cells with a limited life span (1). However, some macrophage subsets derive from the yolk sack; and microglia, Langerhans cells, and alveolar and pleural macrophages can proliferate in situ (2–4). In addition to M-CSF, GM-CSF can also support macrophage growth and is critical in steady-state lung alveolar macrophage (AM) homeostasis (5, 6).
Macrophages sense pathogens via pattern recognition receptors, including toll-like receptors (TLRs), and the subsequent production of pro- and anti-inflammatory mediators such as TNF-α, IL-6, IL-1α, IL-1β, and IL-10 is crucial to combat infection (7). Stimulation of macrophages by TLR ligands such as LPS leads to the production of immature pro-IL1α and calpain-mediated cleavage and secretion of mature IL-1α (mIL-1α) are induced via separate signal transduction pathways (8).
Both quantitative and qualitative differences in receptor distribution and cytokine production exist among distinct macrophage types, and the role of various receptors and signaling pathways has been intensively studied in primary macrophages that are isolated from tissues, ex vivo-differentiated cells, and immortalized macrophage lines. The use of freshly isolated macrophages is hampered by the elaborate isolation procedures, insufficient purity, limited quantities, and the large number of human or animal donors required. Transformed macrophage lines are frequently used; however, such cells often loose important macrophage functions, and their genetic background is often not well defined. Therefore, primary macrophages generated from BM precursors with M-CSF in vitro (BMMs) are used preferentially for functional studies and high-throughput screening. BMMs can be obtained in large numbers; however, they have a limited life span and represent only a particular subset of macrophage populations (1, 9).
Here we present a simple method to generate self-renewing, nontransformed, GM-CSF-dependent, differentiated macrophages from different wild-type and mutant mice [Max Planck Institute (MPI) cells]. Functionally, these cells closely resemble AMs. Phenotypically stable MPI cells can be grown for a long period (at least 2 y) in almost unlimited numbers. The unique innate reactivity pattern of MPI cells and AMs is clearly different from that of BMMs and characterized by differences in LPS sensing, strikingly increased sensitivity to several microbial agents, the lack of IL-10 production, and a strongly proinflammatory cytokine response including unconventional IL-1α secretion. Thus, we delineate an unusual innate response type and present a powerful tool for macrophage studies.
Results
MPI Cells Are Self-Renewing, Nontransformed, GM-CSF–Dependent Phagocytes.
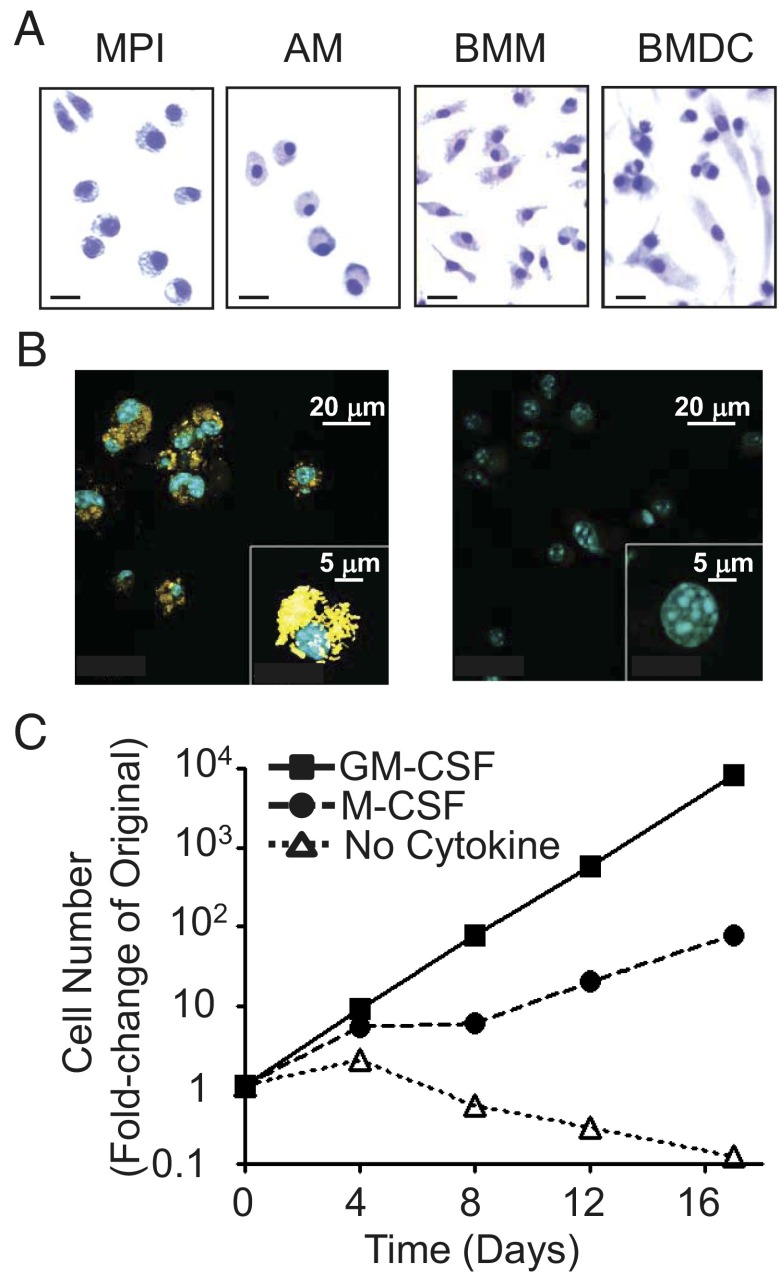
Culturing of unseparated fetal liver cells for ∼8 wk in the presence of GM-CSF resulted in predominantly adherent cells with a characteristic round shape, similar to that of AMs and different from the multiangular shape of BMMs or GM-CSF–dependent BM-derived dendritic cells (BMDCs) (Fig. 1A). These cells were designated MPI cells. About 0.1% of the cells are multinuclear giant cells that may occur in macrophage cultures (10). To test the phagocytic activity of the MPI cells, we incubated them with heat-killed Propionibacterium acnes, which resulted in the efficient uptake of the bacteria (Fig. 1B).
Fig. 1.
MPI cells are factor-dependent, self-renewing phagocytes. (A) Giemsa staining of MPI cells, AMs, BMMs, and BMDCs. (Scale bars, 20 μm.) (B) Phagocytosis of Alexa 647-stained P. acnes (Left, yellow) in MPI cells and in mock-treated cells (Right) using confocal microscopy. Blue, DAPI-stained nuclei. (Insets) Single P. acnes- and mock-treated cells at high power. (C) Growth curve of MPI cells with GM-CSF (30 ng/mL), M-CSF (30 ng/mL), or without any growth factor.
In the presence of GM-CSF, MPI cells grow exponentially (Fig. 1C) without changes in morphology for at least 100 weekly passages. The replacement of GM-CSF by M-CSF slowed down their growth, but no significant change in morphology was observed. The complete removal of GM-CSF resulted in G1 arrest and a slow reduction in the number of living cells (Fig. 1C and Fig. S1A).
To test the tumorigenic potential of the MPI cells, we injected them to recombination-activating gene-2 (RAG2)−/− mice. In line with their nontransformed character, no visible signs of illness, ascites, or tumors were found in the skin or inner organs. Control carcinoma cell-injected mice developed ascites (Fig. S1B).
Surface Markers and Global Gene Expression Profiling Indicates That MPI Cells Represent a Subtype of Differentiated Macrophages.
We examined the expression of myeloid cell-specific surface antigens on the MPI cells (Fig. S2A), which were, like BMMs and BMDCs, positive for cluster of differentiation molecule (CD)11b, Cell surface glycoprotein F4/80 (EGF-like module-containing mucin-like hormone receptor-like 1; Emr1), and CD32 and negative for granulocyte differentiation antigen-1. Being weakly CD11c positive, MPI cells differed from both the CD11c-negative BMMs and the strongly positive BMDCs. Similar to BMMs and unlike BMDCs, they did not express MHC class II proteins.
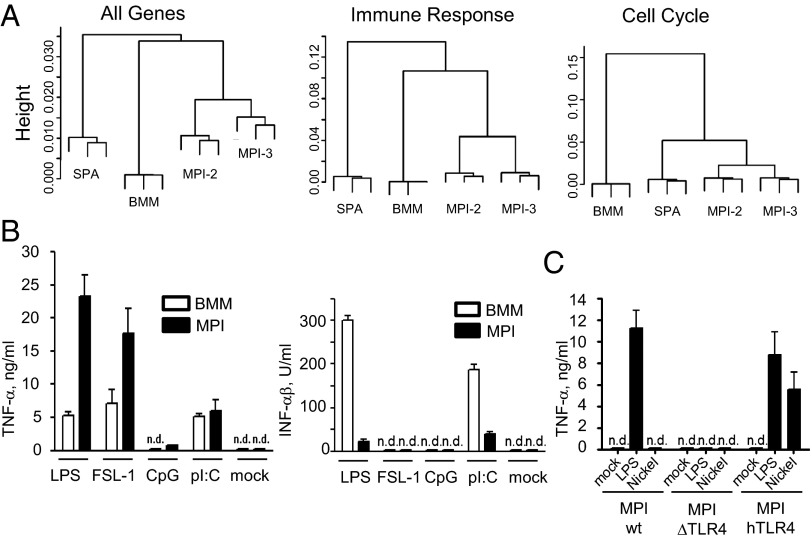
To gain a comprehensive view of gene expression relative to other mononuclear phagocytes, we analyzed mRNA levels in MPI cells, in BMMs, and in the GM-CSF–dependent immature myeloid dendritic cell (DC) line SP37A3 using microarrays. Cluster analysis showed that two independently made MPI lines are very close to each other and significantly closer to BMMs than to SP37A3 cells when all of the genes or immune response genes were analyzed (Fig. 2A). However, when the analysis was done on cell cycle genes, MPI cells were more similar to SP37A3 cells than to BMMs, reflecting their self-renewing capacity (Fig. 2A). For example, the G2 phase-specific genes budding uninhibited by benzimidazoles 1 homolog, beta (Bub1b) and myelocytomatosis oncogene (Myc) are expressed in cycling SP37A3 and MPI cells, but not in G1-arrested BMMs. MPI cells, unlike BMMs and SP737A3, exhibit high levels of Kruppel-like factor 4 (KLF4) and lack v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B (MafB) mRNA. Furthermore, unlike BMMs, they expressed very low Maf mRNA levels (Fig. S2B). In line with these results, overlap and gene set analysis for enriched pathways revealed that the main differences between MPI cells and BMMs are mainly in mitotic process genes (e.g., cell cycle, DNA binding, chromatin assembly, DNA replication, etc.) and in immune response categories (e.g., immune response, TLR pathway, JNK pathway) (Datasets S1 and S2; Fig. S2C). The main differences between MPI and SP37A3 cells are in broad and specific categories of immune function (e.g., immune system process, immune response, chemokine receptor binding) and of immune development (e.g., immune system development, myeloid differentiation) (Dataset S3). Collectively, the morphology and phagocytic properties, the expression of surface antigens, and the global gene expression profiles indicate that MPI cells are macrophages.
Fig. 2.
Gene expression and reactivity to TLR ligands in MPI cells, BMMs, and BMDCs. Global gene expression using total cellular RNA of the DC line SP37A3 (SPA), BMMs, and two independently made MPI cell lines (MPI-2 and MPI-3) with microarrays. (A) Cluster analysis for all, immune response, and cell-cycle genes. (B) TNF-α and IFN-αβ levels in supernatants of MPI cells and BMMs stimulated with 0.1 μg/mL S-LPS, 0.1 μg/mL FSL-1, 8 nM CpG ODN 1668, and 25 μg/mL poly I:C. (C) TNF-α levels of WT (MPI-wt), TLR4-deficient (MPI-ΔTLR4), and TLR4-deficient human TLR4-expressing (MPI-hTLR4) MPI cells in response to LPS (0.1 μg/mL) and nickel chloride (0.5 mM).
The enrichment analysis of rather broad functional annotations does not allow the comparison of MPI cells with specific macrophage subsets. To this end, we searched specific markers, differentially expressed in BMMs and MPI cells (Fig. S2B). We found significant levels of Emr1 (F4/80) mRNA in both MPI cells and BMMs. On the contrary, high mRNA levels of chitinase 3-like 3 (Chl3l), a marker of alternatively activated macrophages (11) and of the scavenger receptor macrophage receptor with collagenous structure (MARCO), were expressed only in MPI cells. Accordingly, the F4/80 protein was detectable on both macrophage types (Fig. S2A), whereas MARCO was detectable only on MPI cells (Fig. S2D). The expression of the LPS coreceptor CD14 was significantly higher in BMMs on both mRNA (Fig. S2B) and protein (Fig. S2D) levels. Notably, the presence of MARCO, Chl3l, CD11c, and the low levels of CD14 observed here for MPI cells is characteristic for lung AMs as well (12–14).
MPI Cells and BMMs Exhibit Large Differences in the Extent of TNF-α, IL-6, and IFN-IFN-αβ Responses to TLR2 and TLR4 Ligands.
Recognition of microbial agents via TLRs and the subsequent production of cytokines is a hallmark of macrophage function. Compared with BMMs, MPI cells produced three to five times higher amounts of TNF-α and IL-6 upon stimulation with the TLR4-dependent LPS or the TLR2-dependent fibroblast stimulated lipopeptide-1 (FSL-1) but comparable levels after stimulation with the TLR3 ligand polyriboinosinic polycytidylic acid (poly I:C). Furthermore, MPI cells produced ∼10 times less IFN-αβ in response to LPS and poly I:C, and neither cell types produced IFN-αβ upon stimulation with FSL-1. The cytokine responses to the TLR9 ligand cytosine triphosphate deoxynucleotide phosphodiester quanine triphosphate deoxynucleotide (CpG) DNA were marginal or absent in both cell types (Fig. 2B and Fig. S2E). Thus, the height of the responses to LPS and FSL-1 is different in MPI cells and BMMs.
In addition to cells from wild type (WT) mice, we generated MPI cells, also from TLR4-deficient and human TLR4-expressing mice. As expected, the absence of TLR4 in MPI cells resulted in the loss of LPS responsiveness (Fig. 2C and Fig. S2F), whereas the presence of human TLR4 not only restored the LPS response but also allowed a response to nickel (Fig. 2C), confirming that nickel stimulates human but not murine TLR4 (15). In a control experiment, WT and TLR4-deficient cells responded comparably to FSL-1 (Fig. S2F).
Several MPI cell lines were kept in culture continuously, the oldest for more than 2 y. Their reactivity to LPS and FSL-1 was regularly checked. Similarly, the reactivity and proliferative capacity were also tested after freezing and thawing of MPI cells. In all cases, no changes in growth properties or the cytokine response were observed, confirming the stable character of the MPI cells.
LPS Induces Differential Changes in the Gene Expression Pattern of MPI Cells and BMMs.
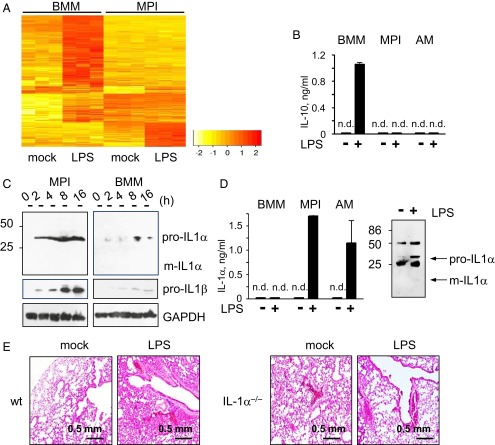
LPS is probably the most potent macrophage activator. To compare its primary effects on global gene expression in MPI cells and BMMs, we analyzed the newly synthesized (30–90 min after stimulation) mRNA levels after stimulation using microarrays. Gene set analysis revealed the enrichment of common activated pathways characteristic of innate responses (e.g., inflammatory response, cytokine production, defense response) in both MPI cells and BMMs (Datasets S4 and S5). At the level of individual genes, cytokines such as IL-6 were strongly induced in both cell types (Fig. S3A). However, there were also clear differences between the induced gene expression patterns. Many genes were induced exclusively in either MPI cells or BMMs (Fig. 3A). For example, colony stimulating factor 3 (granulocyte), epiregulin, and chemokine (C-C motif) ligand 20 were activated exclusively in MPI cells (Fig. S3A and Dataset S6), whereas IL-10, chemokine (C-X-C motif) ligand 11, myxovirus (influenza virus) resistance 1, and IFN-β were induced only in BMMs (Fig. S3A and Dataset S7). In line with these results, LPS up-regulated the expression of soluble and membrane-bound CD14 protein only in MPI cells (Fig. S3B) and the secretion of IL-10 only in BMMs (Fig. 3B). Also, LPS-stimulated BMMs produced much higher levels of IFN-αβ (Fig. S3C) than MPI cells. Interestingly, AMs, like MPI cells, also secreted high levels of proinflammatory TNF-α, little IFN-αβ, and no anti-inflammatory IL-10 in response to LPS (Fig. 3B and Fig. S3C).
Fig. 3.
Differences between LPS-stimulated responses of MPI cells, AMs, and BMMs. (A) Heatmap of genes differentially induced by 0.1 μg/mL LPS in BMMs and MPI cells. (B) IL-10 levels in supernatants of BMMs, MPI cells, and AMs stimulated with 0.1 μg/mL LPS. (C) Pro- and mIL-1α and IL-1β levels in lysates of MPI cells and BMMs at various time points after stimulation with 0.1 μg/mL LPS. (D) IL-1α levels in supernatants of 0.1 μg/mL LPS-stimulated BMMs, MPI cells, and AMs (Left). Western blot analysis of IL-1α isoforms immunoprecipitated from MPI cell supernatants 12 h after stimulation with 0.1 μg/mL (Right). (E) Role of IL-1α in LPS-induced lung pathology. WT and IL-1α−/− mice were treated with 10 μg LPS intranasally, and lungs were examined histologically 2 d later (H&E staining).
GM-CSF treatment of BMMs was reported to result in a partial phenotype change (increased IL-6 and decreased but not absent IL-10 induction) in response to LPS (16). We found, however, that the replacement of GM-CSF by M-CSF in MPI cell cultures just before LPS activation had neither an influence on the strength of the IL-6 response nor led to IL-10 production (Fig. S3D). The substitution of GM-CSF for M-CSF for 3 wk in MPI cells lowered the IL-6 response to LPS and FSL-1 but did not lead to IL-10 induction (Fig. S3E).
LPS Induces the Secretion of IL-1α in MPI Cells and AMs and Triggers IL-1α–Dependent Inflammation in the Lung.
The pattern of the LPS-stimulated cytokine response in MPI cells and freshly isolated AMs is remarkably similar (Fig. 3B and Fig. S3C) and highly proinflammatory. We also analyzed the induction of the proinflammatory IL-1α and IL-1β in MPI cells, BMMs, and AMs. According to the current consensus, LPS induces both proteins as procytokines, and an independent, second signal is required to obtain the protease-cleaved mature, secreted form of the proteins. In agreement with this consensus (8), LPS induced the immature cytokines in both MPI cells and BMMs, whereby much higher levels were found in MPI cells (Fig. 3C). mIL-1α and mIL-1β were not found in lysates of stimulated cells, but LPS-stimulated MPI cells and AMs excreted very low levels of IL-1β (Fig. 3 C and D and Fig. S3C). Most surprisingly, however, LPS-stimulated MPI cells and AMs secreted substantial amounts of IL-1α (Fig. 3D, Left). Western blot analysis revealed that this secreted IL-1α represents the immature proprotein (Fig. 3D, Right). Because there was no difference between the viability of LPS and mock-stimulated MPI cells, it can be concluded that pro–IL-1α did not stem from LPS-damaged dying cells (Fig. S3F).
To test the potential contribution of IL-1α to LPS-induced pathology in vivo, we challenged WT and IL-1α–deficient mice with ultrapure LPS intranasally. WT mice developed lung edema and inflammation corresponding to earlier findings (17); however, much less damage was found in the lungs of IL-1α−/− mice (Fig. 3E).
MPI Cells and AMs Require LPS-Binding Protein and CD14 to Sense Rough Form LPS.
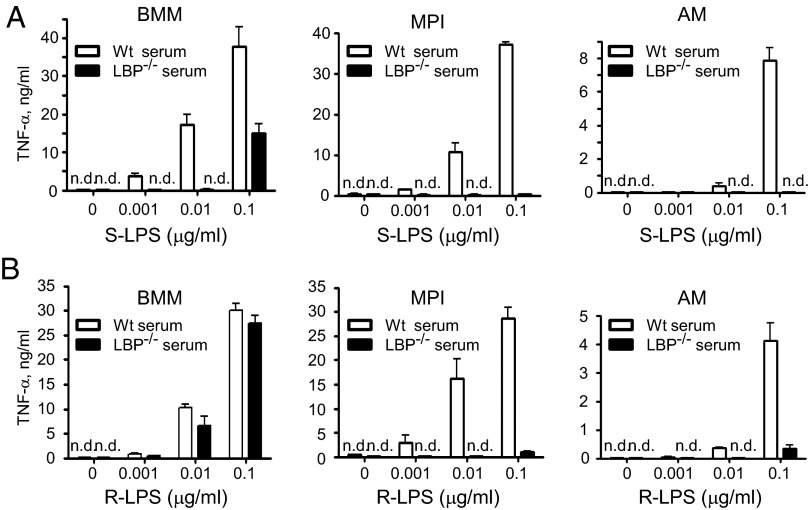
Two types of biologically active LPS are synthetized by Gram-negative bacteria, smooth (S) and rough (R) form LPS (S-LPS and R-LPS). LPS-binding proteins (LBP) and CD14 are required for the activation of cells by S-LPS via the TLR4/myeloid differentiation factor-2 receptor complex, whereas R-LPS activates various target cells including BMMs in the absence of these proteins (18, 19). In agreement, we found that the TNF-α response to S-LPS in MPI cells, AMs, and BMMs is highly dependent on LBP and CD14 (Fig. 4A and Fig. S4A). Surprisingly, the responses to R-LPS in both MPI cells and AMs, but not in BMMs, were also highly dependent on LBP and CD14 (Fig. 4B and Fig. S4 A–C). Thus, whereas similar R-LPS amounts were needed to induce comparable TNF-α levels in the presence or absence of LBP in BMMs, more than 100 times higher amounts (0.1 µg/mL vs. 0.001 µg/mL) were needed in MPI cells when LBP was absent (Fig. 4B). Furthermore, recombinant LBP enhanced the TNF-α response to R-LPS only minimally in BMM cultures, but strongly in MPI cells and AMs (Fig. S4 A and B). Finally, CD14 deficiency did not influence the TNF-α response of BMMs to R-LPS but abolished the response of MPI cells and AMs, and addition of recombinant LBP did not substitute for CD14 deficiency (Fig. S4 B and C). Thus, the activation of MPI cells and AMs by R-LPS, unlike that of BMMs, requires LBP and CD14.
Fig. 4.
Unlike BMMs, MPI cells and AMs require LBP to sense R-LPS. TNF-α response of BMMs, MPI cells, and AMs to a range of S-LPS (A) and R-LPS (B) induced in the presence of 5% serum from WT or LBP−/− mice (A and B).
MPI Cells and AMs, Unlike BMMs, Produce High Levels of Proinflammatory Cytokines, but No Anti-Inflammatory IL-10 in Response to Mycobacterium tuberculosis, Trehalose Dimycolate, and Adenovirus.
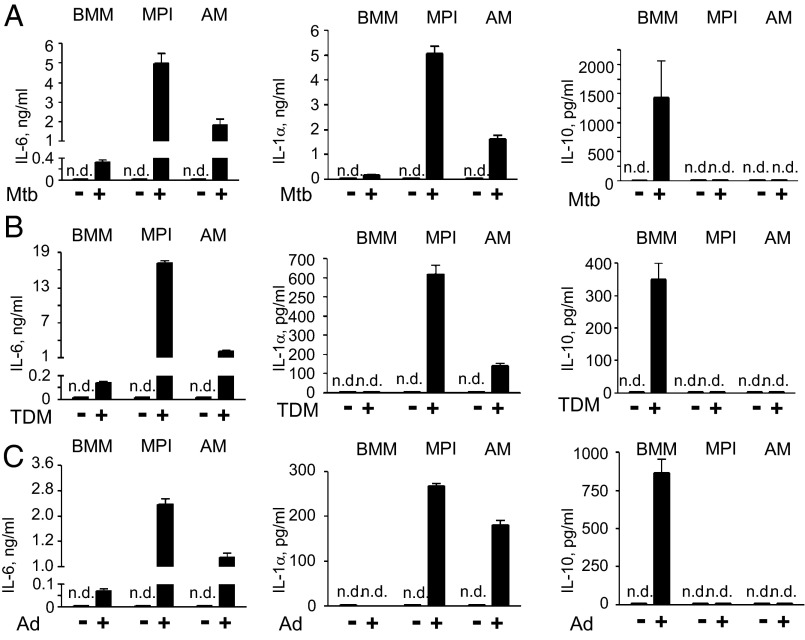
The striking similarities between MPI cells and AMs shown above raised the question if these cells react similarly to air-born pathogens as well. In response to the heat-killed lung pathogen Mycobacterium tuberculosis and its component, trehalose dimycolate (cord factor, TDM), as well as to adenovirus (Ad), MPI cells and AMs secreted much higher amounts of IL-6 (Fig. 5 A and B) than BMMs. However, in sharp contrast to BMMs, they secreted IL-1α but not IL-10 (Fig. 5 A and B). The finding of a poor proinflammatory and a strong IL-10 response of BMMs to M. tuberculosis and TDM is in agreement with previous findings (20). Overall, in contrast to BMMs, MPI cells and AMs exhibit a similar highly proinflammatory phenotype to the air-born microbes used.
Fig. 5.
Cytokine responses to heat-killed M. tuberculosis, TDM, and Ad in MPI cells, AMs, and BMMs. IL-6, IL-1α, and IL-10 levels in supernatants of BMMs, MPI cells, and AMs stimulated with M. tuberculosis at 20 bacterial particles per cell (A), with 25 μg/mL TDM (B), or with Ad5GFP at 100 pfu/cell (C).
GM-CSF–Induced STAT5 Confers Self-Renewing Capacity to MPI Cells and Maintains Their Innate Reactivity.
The presence of GM-CSF is required for the proliferation of MPI cells (Fig. 1C and Fig. S1A). GM-CSF signaling leads to the induction of several pathways including the activation of STAT5 (6, 21). We found that proliferating MPI cells express activated STAT5, as evidenced by its nuclear localization (Fig. 6A). Removal of GM-CSF resulted in the disappearance of the nuclear STAT5 levels (Fig. 6A), proliferation arrest (Fig. 1C and Fig. S1A), and a strong reduction of the IL-6 response to microbial stimulation (Fig. 6D). To evaluate the functional role of STAT5, we transduced MPI cells with retroviruses expressing CD4 and a constitutively active form (caSTAT5) (22). The transduced cells expressed STAT5 in the nucleus and proliferated without GM-CSF at the same rate as cells transduced with a control CD4-expressing retrovirus in its presence (Fig. 6 A and B). In addition, the response of transduced cells to R-LPS, like that of GM-CSF–dependent WT cells, was entirely LBP dependent (Fig. 6C). Furthermore, the cytokine responses of caSTAT5-expressing cells to various microbial agents were comparable to those of WT cells grown in the presence of GM-CSF (Fig. 6D).
Fig. 6.
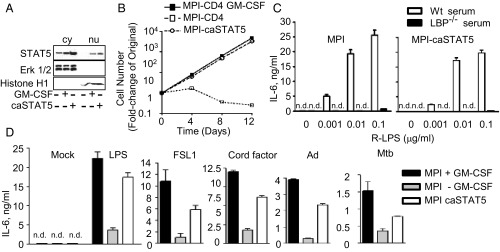
STAT5 is important for the self-renewing capacity and innate reactivity of MPI cells. (A) Western blot analysis of STAT5, Erk1/2, and histone H1 in cytoplasmic (cy) and nuclear (nu) extracts of GM-CSF–starved and –incubated MPI cells and in GM-CSF–starved cells expressing caSTAT5. (B) Growth curves of GM-CSF–incubated and –starved MPI cells expressing CD4 (MPI-CD4; controls) or of GM-CSF–starved cells expressing caSTAT5 and CD4 (MPI-caSTAT5). (C) IL-6 levels of MPI cells and caSTAT5 MPI cells stimulated with a range of LPS in the presence of 5% serum from WT or LBP−/− mice. (D) IL-6 responses of GM-CSF–incubated or –starved MPI cells and GM-CSF–starved caSTAT5-expressing MPI cells to 0.1 μg/mL LPS, 0.1 μg/mL FSL-1, 25 μg/mL cord factor (TDM), 100 pfu/cell Ad5 GFP (Ad), and 10 bacterial particles per cell of heat-killed M. tuberculosis.
Discussion
In the present study we report the establishment and properties of self-renewing, primary, GM-CSF–dependent macrophages (MPI cells). MPI cells are model cells of differentiated tissue macrophages prepared without genetic manipulation or oncogenic transformation and share a number of properties with alveolar macrophages. Using GM-CSF and M-CSF, DC lines have been established previously from mouse spleen (23, 24). We compared one of them, the SP37A3 cells (24), to the MPI cells. Our analysis of the gene expression profiles, surface markers, and responses to innate stimuli showed that SP37A3 cells are quite different from MPI cells.
Depending on its concentration, GM-CSF induces the generation of macrophages, DCs, and neutrophil or eosinophil granulocytes from HSCs in vitro (25). The concentration of GM-CSF that we used to establish MPI cells from fetal liver (20–50 ng/mL) leads to the development of DCs with a limited life span from BM (26) or from isolated fetal liver HSCs (27). This suggests also that the original GM-CSF–responding cell determines which cell type is generated and that the precursor of MPI cells is probably not the HSC.
GM-CSF triggers several signaling pathways and transcription factors such as c-Myc, pim-1, CCAAT/enhancer-binding protein alpha, PU.1, and STAT5 (6, 21). Our finding that the expression of caSTAT5 abolished the need for GM-CSF indicates a pivotal role for STAT5 in the proliferation and maintenance of the characteristic proinflammatory phenotype of MPI cells. A role for STAT5 in the proliferation of B and T cells, DC, and mast cell progenitors, but not in macrophage self-renewal, has been demonstrated (28, 29).
Concomitant up-regulation of two stem cell-inducing factors, KLF4 and Myc, enables the continuous proliferation of MafB/c-Maf−/−–deficient mature monocytes and macrophages to high concentration of M-CSF in vitro (30). Similarly, MPI cells do not express MafB and c-Maf and, compared with BMMs, exhibit elevated levels of KLF4 and c-Myc. Further studies should clarify the potential role of these factors in the self-renewing capacity of the MPI cells.
BMMs generated in vitro are widely used in macrophage studies. We show that MPI cells and BMMs represent two functionally very distinct macrophage types. In response to LPS, MPI cells and BMMs exhibit several exclusively activated genes. MPI cells show stronger proinflammatory cytokine responses, reduced IFN αβ, and no anti-inflammatory IL-10 response to several microbial agents.
The finding that the cytokine response of MPI cells to R-LPS strictly depends on LBP and CD14 is surprising. According to the current consensus, LBP/CD14 help is required for S-LPS– but not R-LPS–triggered cell activation (18, 19, 31). Therefore, our present finding suggests the existence of an alternative, cell type-specific activation mechanism in MPI cells and AMs.
Another remarkable property of the MPI cells is their high sensitivity to the air-born pathogens M. tuberculosis and adenovirus and to mycobacterial TDM. All these agents, like the TLR ligands LPS and FSL-1, induce a strong proinflammatory but no IL-10 response. Clearly, GM-CSF–induced cell differentiation is an important factor in the high sensitivity of MPI cells and AMs to M. tuberculosis and TDM. In agreement, human monocyte-derived macrophages differentiated under GM-CSF could survive an otherwise lethal M. tuberculosis infection and could severely limit M. tuberculosis replication (32). The expression of the scavenger receptor MARCO probably explains the high sensitivity of MPI cells and AMs to M. tuberculosis and TDM (33). MARCO, however, is not essential for the M. tuberculosis and TDM-induced IL-10 response because MARCO-negative BMMs produced substantial amounts of this cytokine upon activation. Notably, the absence of IL-10 production to all microbial agents tested suggests a general lack of the IL-10 response in MPI cells and is likely to amplify the proinflammatory cytokine response of these cells to microbial stimuli.
Cell morphology, expression of selected surface markers, high sensitivity, and the unique proinflammatory cytokine responses to microbial agents, including LPS, M. tuberculosis, TDM, and Ad, as well as the need for CD14 and LBP to sense R-LPS indicated a strong functional relationship between MPI cells and AMs. Importantly, AMs can self-renew in vivo (2, 13), proliferate, and differentiate into multinucleated giant cells in response to GM-CSF in vitro (34) similarly to findings on the MPI cells. In naive animals, GM-CSF is required for the terminal differentiation of AMs (6). Impaired GM-CSF signaling leads to defective innate activity in AMs and to high susceptibility to lung infections (35). In line with these data, GM-CSF–deprived or M-CSF–grown MPI cells show reduced cytokine production to various microbial agents, which further supports their similarity to AMs.
The finding that MPI cells and AMs secrete high levels of pro–IL-1α in response to LPS was unexpected. As is so far known, LPS induces only pro–IL-1α in BMMs and DCs and a second stimulus is needed for the cleavage and secretion of mIL-1α; the involvement of calpain enzymatic activity and caspase 1 have been shown (8, 36, 37). Thus, the secretion of large amounts of pro-ILα by LPS-stimulated MPI cells points to a not-yet-characterized IL-1α secretory pathway. Because both pro- and mIL-1α are functionally active (38), this mechanism is of particular interest.
The microbial agents used in this study elicit potent inflammatory responses in the lung (39–42). We show that IL-1α is decisively involved in LPS-induced lung inflammation. Because in humans LPS contributes to acute lung injury (17), this finding may have therapeutic consequences. We expect that AMs, cells in the first line of defense against air-born pathogens, contribute to this pathology by their IL-1α response. As AMs and MPI cells respond similarly to microbial stimuli, MPI cells represent a valuable model to study the not completely understood molecular mechanisms of IL-1α production.
Unlike other types of primary macrophages, MPI cells from various WT, gene-deficient, or transgenic mice strains can be propagated indefinitely in unlimited quantities and can be easily manipulated genetically. Their use therefore can reduce the need for living animals in macrophage studies. Due to their high sensitivity to microbial ligands, selected MPI lines can be used for biological testing of compounds of interest. As an example, TLR4-deficient MPI cells represent a sensitive tool to identify contaminants in LPS preparations (detection limit: 10–50 pg/mL).
In summary, we established a type of GM-CSF/STAT5–dependent macrophage model cell that reproduces the innate immune characteristics of AMs. We report a unique pattern of innate responses in this system, not yet observed in other mononuclear phagocytes, and an unprecedented regulation of IL-1α production that likely plays a central role in lung inflammation in vivo. Our studies therefore reveal as yet unknown aspects of macrophage biology, and MPI cells may prove useful in future biomedical research.
Materials and Methods
Mouse Strains and Lung Disease.
C57BL/6 (BL6) and C57BL/10 (BL10) mice, as well as TLR4-deficient BL10 (ScN), human TLR4 transgenic ScN (15), CD14−/− BL6 (43), Rag2−/− BALB/c (44), and IL-1Receptor−/− BL6 (45) mice were bred under specific pathogen-free conditions at the Max Planck Institute and IL1α−/− BL6 (46) mice at Ben-Gurion University. LPS-induced lung injury was elicited as described (17). The animal experiments were approved by the animal welfare committee at the Regierungspräsidium (regional board) Freiburg and by the Animal Committee, Ben Gurion University of the Negev.
Generation of MPI Cells.
As a matter of routine, MPI cells were prepared from fetal livers of 15- to 19-d-old mouse embryos. Moreover, we succeeded in preparing the cells also from livers of mice up to 2 wk after birth. Liver single-cell suspensions (0.5 × 106 cell/mL) were washed in PBS and resuspended in MPI cell medium consisting of RPMI 1640 containing 10% (vol/vol) FCS and supplemented with 20–50 ng/mL murine GM-CSF (usually 30 ng/mL). Both recombinant GM-CSF or supernatants from the GM-CSF–producing line ×63-Ag8 (47) can be used. After 4–6 d, rapidly proliferating cells were subcultured by splitting them 1:5. For this purpose, floating cells and adherent cells, detached with PBS/1.5 mM EDTA, were combined, centrifuged, and resuspended in fresh MPI medium. Cells proliferated more slowly after 2–3 wk of culture. GM-CSF was replenished weekly, and subcultures in MPI medium were made when total (attached and floating) cell density reached ∼0.5 × 106 cell/mL. After 6–8 wk in culture, cells proliferated at a stable rate, and stocks (MPI cells) were cryopreserved. Further subcultures could be done weekly for at least 90 passages without morphological or functional changes.
Cells, Stimulations, and Detection of Intracellular, Surface, and Secreted Proteins
If not stated otherwise, MPI cells refer to the WT MPI-2 line. MPI cells were transduced with pMys retroviruses (Cell Biolabs) expressing the CD4 antigen or CD4 and the Stat5b-CA gene or the mKO2-hCdt1 reporter of the Fucci technology (48). CD4-expressing MPI cells were purified by FACS before further culture. BMMs and BMDCs were generated as described (26, 49). SP37A3 cells were grown as described (24) but without M-CSF. For induction of cytokines and sCD14, the different cell types were stimulated in 96-well plates (105 cells/0.2 mL per well) and for Western blot or microarray analysis in six-well plates (3 × 106 cells/3 mL per well). The levels of secreted TNF-α and IFN-αβ were determined after 6 h of stimulation; of IL-1α, I-1β, IL-6, IL-10, and sCD14 after 24 h; or as indicated.
Mouse AMs were obtained by bronchoalveolar lavage as described (50). Briefly, lungs were washed with Ca- and Mg-free PBS five times through a catheter inserted into the trachea. The cells obtained from several mice (6–10) were pooled, washed, resuspended in RPMI 1640 containing 10% FCS and 30 ng/mL GM-CSF (0.5 × 106 cells/mL) and plated in 96-well plates (105 cells/0.2 mL per well). After 2 h of culture, the nonadherent cells were removed, and the adherent cells were stimulated in fresh culture medium as described above.
Lewis lung carcinoma cells were grown and injected as described (51). P. acnes, adenovirus, and ultra pure LPS preparations were prepared as described (49, 52, 53). Unless otherwise indicated, R-LPS was used. P. acnes was stained with an Alexa 647 labeling kit from Invitrogen. TDM, CpG ODN 1668, and poly I:C were from Enzo Life Sciences. FSL-1 and early log phase H37Rv M. tuberculosis were kindly provided by K. Wiesmüller (EMC Microcollections, Tübingen, Germany) and N. Reiling and C. Hölscher (Forschungsinstitut Borstel, Borstel, Germany), respectively. All nonendotoxin activators were LPS-free (less than 1 pg LPS/50 µg agent or 1 pg LPS/1011 viral particles). Murine LBP was from Biometec. Secreted cytokines and intracellular proteins were detected by commercial antibodies using ELISA or immunoblotting. Cell-surface antigens were detected by commercial antibodies using FACS.
Global Gene Expression Profiling.
Total cellular RNA was prepared with TRIzol (Invitrogen). Newly synthesized RNA obtained with 4-thiouracil labeling of cells at 250 μM in culture medium for 60 min was affinity-purified as described (54). RNA samples were amplified and labeled using the Affymetrix One-Cycle Target Labeling Kit and were hybridized to Affymetrix MG 430 2.0 arrays.
Data Analysis and Statistics.
Data were analyzed using Prism GraphPad software. Data in all figures are presented as mean, and error bars show SEM from at least three independent experiments.
Supplementary Material
Acknowledgments
We thank A. Sutter for the SP37A3 cells; N. Reiling and C. Hölscher for M. tuberculosis; K. Wiesmüller for FSL-1; J. Ippisch, P. Lüderitz, and H. Garbers for technical assistance; and P. Nielsen and T. Boehm for discussions. G.F. was supported partially with funds from the European Regional Development Fund to the University of Exeter's European Centre for Environment and Human Health, I.C. was supported by an Alexander von Humboldt fellowship, and O.P.d.C. was supported by the Deutsche Forschungsgemeinschaft (SFB-TR22).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302877110/-/DCSupplemental.
References
- 1.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10(6):453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hume DA, et al. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72(4):621–627. [PubMed] [Google Scholar]
- 3.Jenkins SJ, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332(6035):1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 5.Dranoff G, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264(5159):713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 6.Shibata Y, et al. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15(4):557–567. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 9.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol. 2011;11(11):788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 10.Helming L, Gordon S. The molecular basis of macrophage fusion. Immunobiology. 2007;212(9–10):785–793. doi: 10.1016/j.imbio.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palecanda A, et al. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J Exp Med. 1999;189(9):1497–1506. doi: 10.1084/jem.189.9.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol. 2007;179(6):3488–3494. doi: 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- 14.Nio J, et al. Cellular expression of murine Ym1 and Ym2, chitinase family proteins, as revealed by in situ hybridization and immunohistochemistry. Histochem Cell Biol. 2004;121(6):473–482. doi: 10.1007/s00418-004-0654-4. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M, et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol. 2010;11(9):814–819. doi: 10.1038/ni.1919. [DOI] [PubMed] [Google Scholar]
- 16.Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: Implications for CSF blockade in inflammation. J Immunol. 2007;178(8):5245–5252. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- 17.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Z, et al. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6(6):565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 19.Huber M, et al. R-form LPS, the master key to the activation ofTLR4/MD-2-positive cells. Eur J Immunol. 2006;36(3):701–711. doi: 10.1002/eji.200535593. [DOI] [PubMed] [Google Scholar]
- 20.Klug K, Ehlers S, Uhlig S, Reiling N. Mitogen-activated protein kinases p38 and ERK1/2 regulated control of Mycobacterium avium replication in primary murine macrophages is independent of tumor necrosis factor-α and interleukin-10. Innate Immun. 2011;17(5):470–485. doi: 10.1177/1753425910377799. [DOI] [PubMed] [Google Scholar]
- 21.Hercus TR, et al. The granulocyte-macrophage colony-stimulating factor receptor: Linking its structure to cell signaling and its role in disease. Blood. 2009;114(7):1289–1298. doi: 10.1182/blood-2008-12-164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burchill MA, et al. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: Development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J Immunol. 2003;171(11):5853–5864. doi: 10.4049/jimmunol.171.11.5853. [DOI] [PubMed] [Google Scholar]
- 23.Winzler C, et al. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185(2):317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bros M, et al. A newly established murine immature dendritic cell line can be differentiated into a mature state, but exerts tolerogenic function upon maturation in the presence of glucocorticoid. Blood. 2007;109(9):3820–3829. doi: 10.1182/blood-2006-07-035576. [DOI] [PubMed] [Google Scholar]
- 25.Inaba K, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, et al. Development of dendritic cells in vitro from murine fetal liver-derived lineage phenotype-negative c-kit(+) hematopoietic progenitor cells. Blood. 2000;95(1):138–146. [PubMed] [Google Scholar]
- 28.Yao Z, et al. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci USA. 2006;103(4):1000–1005. doi: 10.1073/pnas.0507350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miah MA, et al. CISH is induced during DC development and regulates DC-mediated CTL activation. Eur J Immunol. 2012;42(1):58–68. doi: 10.1002/eji.201141846. [DOI] [PubMed] [Google Scholar]
- 30.Aziz A, Soucie E, Sarrazin S, Sieweke MH. MafB/c-Maf deficiency enables self-renewal of differentiated functional macrophages. Science. 2009;326(5954):867–871. doi: 10.1126/science.1176056. [DOI] [PubMed] [Google Scholar]
- 31.Minguet S, et al. Enhanced B-cell activation mediated by TLR4 and BCR crosstalk. Eur J Immunol. 2008;38(9):2475–2487. doi: 10.1002/eji.200738094. [DOI] [PubMed] [Google Scholar]
- 32.Vogt G, Nathan C. In vitro differentiation of human macrophages with enhanced antimycobacterial activity. J Clin Invest. 2011;121(10):3889–3901. doi: 10.1172/JCI57235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowdish DM, et al. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009;5(6):e1000474. doi: 10.1371/journal.ppat.1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemaire I, Yang H, Lauzon W, Gendron N. M-CSF and GM-CSF promote alveolar macrophage differentiation into multinucleated giant cells with distinct phenotypes. J Leukoc Biol. 1996;60(4):509–518. doi: 10.1002/jlb.60.4.509. [DOI] [PubMed] [Google Scholar]
- 35.Greenhill SR, Kotton DN. Pulmonary alveolar proteinosis: A bench-to-bedside story of granulocyte-macrophage colony-stimulating factor dysfunction. Chest. 2009;136(2):571–577. doi: 10.1378/chest.08-2943. [DOI] [PubMed] [Google Scholar]
- 36.Fettelschoss A, et al. Inflammasome activation and IL-1β target IL-1α for secretion as opposed to surface expression. Proc Natl Acad Sci USA. 2011;108(44):18055–18060. doi: 10.1073/pnas.1109176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gross O, et al. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36(3):388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Keller M, Rüegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132(5):818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 39.Mayer-Barber KD, et al. Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35(6):1023–1034. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Bai C, Wang X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev Respir Med. 2010;4(6):773–783. doi: 10.1586/ers.10.71. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa E, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206(13):2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ginsberg HS, et al. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci USA. 1991;88(5):1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore KJ, et al. Divergent response to LPS and bacteria in CD14-deficient murine macrophages. J Immunol. 2000;165(8):4272–4280. doi: 10.4049/jimmunol.165.8.4272. [DOI] [PubMed] [Google Scholar]
- 44.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 45.Labow M, et al. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol. 1997;159(5):2452–2461. [PubMed] [Google Scholar]
- 46.Horai R, et al. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187(9):1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zal T, Volkmann A, Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J Exp Med. 1994;180(6):2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakaue-Sawano A, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132(3):487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 49.Fejer G, et al. Key role of splenic myeloid DCs in the IFN-alphabeta response to adenoviruses in vivo. PLoS Pathog. 2008;4(11):e1000208. doi: 10.1371/journal.ppat.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Goncalves R, Mosser DM. 2008. The isolation and characterization of murine macrophages. Curr Protoc Immunol Chapter 14:Unit 14 11.
- 51.Bartholeyns J, Freudenberg M, Galanos C. Growing tumors induce hypersensitivity to endotoxin and tumor necrosis factor. Infect Immun. 1987;55(9):2230–2233. doi: 10.1128/iai.55.9.2230-2233.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalis C, et al. Requirement for TLR9 in the immunomodulatory activity of Propionibacterium acnes. J Immunol. 2005;174(7):4295–4300. doi: 10.4049/jimmunol.174.7.4295. [DOI] [PubMed] [Google Scholar]
- 53.Galanos C, Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- 54.Dölken L, et al. High-resolution gene expression profiling for simultaneous kinetic parameter analysis of RNA synthesis and decay. RNA. 2008;14(9):1959–1972. doi: 10.1261/rna.1136108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.