Abstract
The metal-reducing bacterium Shewanella oneidensis MR-1 displays remarkable anaerobic respiratory plasticity, which is reflected in the extensive number of electron transport components encoded in its genome. In these studies, several cell components required for the reduction of vanadium(V) were determined. V(V) reduction is mediated by an electron transport chain which includes cytoplasmic membrane components (menaquinone and the tetraheme cytochrome CymA) and the outer membrane (OM) cytochrome OmcB. A partial role for the OM cytochrome OmcA was evident. Electron spin resonance spectroscopy demonstrated that V(V) was reduced to V(IV). V(V) reduction did not support anaerobic growth. This is the first report delineating specific electron transport components that are required for V(V) reduction and of a role for OM cytochromes in the reduction of a soluble metal species.
From the perspective of anaerobic respiratory versatility, the metal-reducing bacterium Shewanella oneidensis (formerly putrefaciens) MR-1 is arguably the most versatile bacterium studied to date. It utilizes a variety of anaerobic electron acceptors, including insoluble iron (Fe) and manganese (Mn) oxides and a variety of soluble compounds, including fumarate, nitrate, nitrite, dimethyl sulfoxide (DMSO), trimethylamine N-oxide (TMAO), thiosulfate, and others (21, 27, 28, 31). This respiratory versatility is reflected in the large array of electron transport components encoded in its genome (7). Its use of soluble electron acceptors requires both cytoplasmic membrane (CM) and periplasmic components, whereas the use of insoluble metal oxides also relies on outer membrane (OM) components.
Vanadium is distributed worldwide and ranks 14th in overall geochemical abundance (9). Approximately 46,000 tons are mined annually (L. Perron, Abstr. Int. Symp. Vanadium, abstr. 9.2, 2002). While the vast majority of vanadium is used in the steel industry, it is also used in ceramics and glass pigments and as a catalyst for gas-phase oxidations (3). Greater than 57,000 tons vanadium are released into the atmosphere annually as a result of oil and coal combustion (3, 9). Atmospheric deposition rates near industrial sources of up to 10 kg per hectare have been reported (3).
The most common oxidation state of vanadium for industrial uses is V(V) (e.g., vanadate) (3). V(V) is toxic and is known to cause oxidative cell damage (4, 5, 35, 37). The reduction of V(V) to V(IV) represents a major way to reduce the impact of vanadium on living systems (9). A recent report noted that MR-1 can reduce vanadate (2), although the cell components responsible for vanadate reduction were not identified. At neutral pH, vanadium(V) exists as vanadate, which is very water soluble (40). The reduced form, V(IV) or vanadyl, is much less water soluble at neutral pH. The reduction of V(V) to V(IV) could therefore be used to facilitate removal of vanadium from contaminated effluents. Understanding the bacterial mechanisms of V(V) reduction is, therefore, an important step to exploiting their potential use for bioremediation. In this study, a variety of mutants of MR-1 were used to elucidate cell components required for V(V) reduction. These include electron transport components required for the use of other electron acceptors and are located in both the CM and the OM.
MATERIALS AND METHODS
Sodium metavanadate (NaVO3), vanadium(V) oxide (V2O5), and vanadyl sulfate were obtained from Aldrich Chemical (Milwaukee, Wis.). Stock solutions (0.1 M) of NaVO3 and V2O5 were prepared in warm deionized water and 0.48 M NaOH, respectively. All other chemicals and reagents were obtained from sources described previously (22, 30).
Bacterial strains, plasmids, media, and vanadium reduction.
A list of the bacteria and plasmids used in this study is presented in Table 1. Cells for vanadium reduction experiments were pregrown under anaerobic conditions for 22 to 24 h at room temperature (23 to 25°C) in M1 defined medium (28) supplemented with 15 mM lactate, 20 mM TMAO, 30 mM HEPES (pH 7.4), and Bacto-Casamino Acids (0.1 g liter−1). For the appropriate strains, the medium was supplemented with kanamycin (50 μg ml−1) or gentamicin (10 μg ml−1). The cells were harvested by centrifugation for 15 min at 2,800 × g at 4°C, washed once, and resuspended in sterile distilled water. The inoculum densities were adjusted to equalize turbidity (optical density at 500 nm [OD500; Beckman DU-64 spectrophotometer).
TABLE 1.
Bacteria and plasmids used in this study
| Bacterial strain or plasmid | Description | Reference | Source |
|---|---|---|---|
| Shewanella strains | |||
| MR-1 | Manganese-reducing strain from Lake Oneida, N.Y., sediments | 27 | Previous study |
| MR-1A | Spontaneous Rfr mutant of MR-1 | 20 | Previous study |
| OMCA1 | omcA mutant derived from MR-1; omcA::Kmr | 31 | Previous study |
| OMCB1 | omcB mutant derived from MR-1; omcB::Kmr | 31 | Previous study |
| MTRB1 | mtrB mutant derived from MR-1; mtrB::Kmr | 25 | Previous study |
| MTRF1 | mtrF mutant derived from MR-1; mtrF::Kmr | 25 | Previous study |
| MR1-CYMA | cymA mutant derived from MR-1; cymA::Kmr | 30 | Previous study |
| ETRA-153 | etrA mutant derived from MR-1; etrA::Kmr | 13 | Previous study |
| CMA-1 | Acridine orange-generated mutant of MR-1 that lacks menaquinone | 20 | Previous study |
| CMTn-3 | TnphoA-generated mutant of MR-1A unable to use fumarate as an electron acceptor and lacking the sole physiological fumarate reductase | 14, 23 | Previous study |
| MR-4 | Manganese-reducing strain from the Black Sea water column | 34 | Previous study |
| MR-8 | Manganese-reducing strain from the Black Sea water column | 34 | Previous study |
| MR-30 | Manganese-reducing strain from Lake Michigan sediments | —a | Previous study |
| MR-42 | Manganese-reducing strain from Lake Michigan sediments | —a | Previous study |
| Plasmids | |||
| pVK100 | 23-kb broad-host-range cosmid; Tcr Kmr Tra+ | 10 | ATCC 37156 |
| pVK21 | 21-kb MR-1 genomic DNA fragment containing omcB and 19 kb of downstream DNA cloned into the HindIII site of pVK100; complements OMCB1 and MTRB1 | 32 | Previous study |
| pCMTN1-VK | pVK100 with MR-1 cymA open reading frame plus associated 5′ and 3′ regions; complements MR1-CYMA | 22 | Previous study |
| pJQ200KS | Mobilizable vector; P15A origin; Gmr; replicates in MR-1 | 39 | G. Reid |
| pJQ60.4F | MR-1 genomic DNA fragment including the fumarate reductase gene and 0.5 kb of upstream DNA cloned into pJQ200KS in the forward direction; restores fumarate reduction and fumarate reductase to CMTn-3 | 14 | Previous study |
C. R. Myers, B. B. Wimpee, and K. H. Nealson, Abstr. 32nd Conf. Great Lakes Res., p. 87, 1989.
To test anaerobic vanadium reduction, M1 defined medium was similarly supplemented with lactate and Casamino Acids plus 2 mM NaVO3. Inoculum was added to establish an initial calculated turbidity (OD500) of 0.019 (ca. 5 × 107 CFU ml−1). Samples taken at periodic intervals were assayed for V(V) using a diphenylcarbazide assay (2, 42), and a standard curve was established with varied levels of NaVO3 as the source of V(V). Experiments were conducted at room temperature under anaerobic conditions in a Coy anaerobic chamber (4 to 5% H2, balance N2). All media were preequilibrated under anaerobic conditions before use.
Growth in the presence of vanadium.
To test the ability of MR-1 to grow in the presence of V(V), inocula were pregrown overnight at room temperature in M1 defined medium (supplemented with Casamino Acids and lactate as described above) under either aerobic conditions (200 rpm on a gyrotary shaker) or anaerobic conditions with 30 mM fumarate. The cells were harvested by centrifugation for 15 min at 2,800 × g at 4°C, washed once, and resuspended in sterile distilled water. Washed cells (0.1 ml) were inoculated into 10 ml of defined medium containing various concentrations of NaVO3 or V2O5. In order to prevent alkalinization by the V2O5 stock, the medium was supplemented using a stock of 1 M HEPES, pH 7.4 (equivalent to the volume of V2O5). Aerobic inocula were used to test growth under aerobic conditions (room air on a gyrotary shaker), and anaerobic inocula were used to test growth anaerobically either with V(V) as the sole electron acceptor or with V(V) plus fumarate. Growth was assessed by changes over time in culture turbidity at 500 nm (using a Beckman DU-64 spectrophotometer), by colony counts of serial dilutions of cultures, or by direct cell counts of acridine orange-stained cells examined by epifluorescence microscopy (Eclipse E600; Nikon Instruments, Melville, N.Y.) (12).
Antibodies and Western blotting.
Polyclonal antibodies specific for OmcB and MtrB were prepared in previous studies (25, 32). Recombinant technology was used to generate a protein fusion of thioredoxin (TR) to an internal 160-residue fragment of CymA. Specifically, a 480-bp fragment of cymA was generated by PCR of MR-1 genomic DNA using the oligonucleotides 5′-ATCGTGATTGGTGTTGTGGGCTATT-3′ and 5′-GTGAGCGACACCTTTGTGGCAATCC-3′. This PCR product was cloned into pBAD/Thio-TOPO (Invitrogen, Carlsbad, Calif.) and transformed into Escherichia coli TOP10 cells. After identifying a clone in which the cymA insert was in the proper orientation, expression of the fusion protein was induced with 0.02% arabinose for 2 h at 37°C. The cells were harvested by centrifugation and lysed using Bugbuster protein extraction reagent (Novagen, Madison, Wis.). The resulting fusion protein, which contained the 160-residue fragment of CymA sandwiched between TR at the N terminus and a six-histidine tag at the C terminus, was localized primarily in inclusion bodies. After solubilization with 6 M urea, the TR-CymA fusion was purified using His-Bind Quick resin (Novagen) according to the manufacturer's instructions. The purified fusion protein was dialyzed at 4°C against 20 mM Tris-HCl (pH 7.4)-50 mM NaCl-4 M urea and then concentrated by ultrafiltration. This concentrated TR-CymA fusion protein was used as an antigen to generate polyclonal antisera in New Zealand White rabbits as previously described (24), except that Titermax (CytRx Corp., Norcross, Ga.) was used as an adjuvant.
Purification of immunoglobulin G from rabbit sera and preabsorption of anti-CymA with E. coli cells were performed as previously described (24, 25). Whole cells of Shewanella were lysed in hot sodium dodecyl sulfate (SDS) and urea (26) prior to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) separation and Western blot analysis (24, 25).
ESR spectroscopy.
V(IV) has a distinct eight-line electron spin resonance (ESR) spectrum at conventional X-band frequency (45, 46). V(V) reduction experiments were performed as described above, and aliquots were placed in a flat cell for ESR analysis at room temperature. The spectra were recorded using a Varian Century Series spectrometer, which had a Gauss meter for magnetic field calibration and a frequency counter. Instrument settings were as follows: 10-G modulation amplitude, 2 × 103 receiver gain, 0.128-s time constant, 9.5026 to 9.5036 GHz microwave frequency, sweep width of 2,000 G, field set of 3,300 G, modulation frequency of 100 kHz, scan time of 4 min, and 10-dB microwave power.
RESULTS
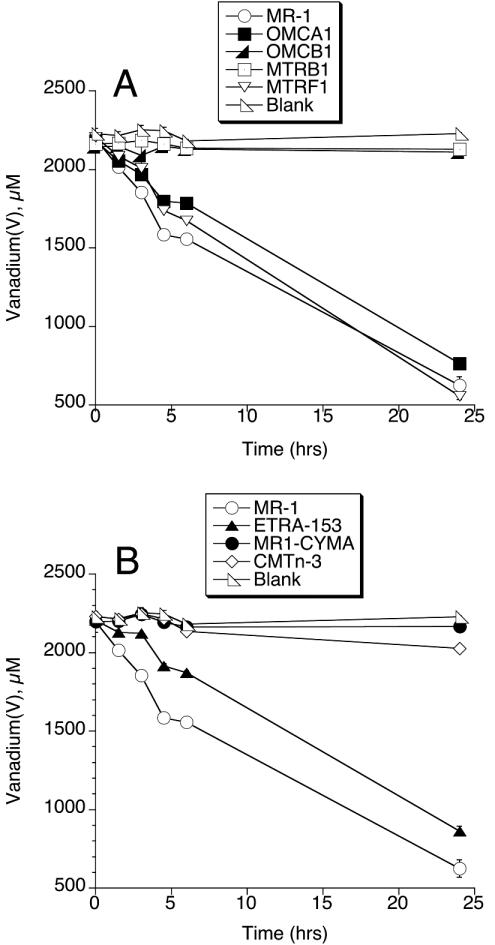
Reduction of metavanadate by MR-1 under anaerobic conditions was compared to that of several mutants of MR-1 that are deficient in specific electron transport components. Since all strains could grow anaerobically on TMAO, all inocula were grown under these conditions to provide for direct comparison of all strains. MR-1 reduced most of the V(V) over 24 h (Fig. 1). Strains that cannot synthesize the OM cytochrome OmcB (OMCB1) or the OM protein MtrB (MTRB1) showed little to no reduction of V(V) (Fig. 1A). Strains lacking the OM cytochrome OmcA (OMCA1) and the putative OM cytochrome MtrF (MTRF1) reduced a similar amount of V(V), as did MR-1 over 24 h (Fig. 1A). However, the results for OMCA1 and MTRF1 were significantly different from those of MR-1 at 3 h (P < 0.05) and 6 h (P < 0.001), but the scale of the line graph (Fig. 1A) does not provide for this visual distinction. Over the first 6 h, OMCA1 and MTRF1 had rates that were 62 and 83% of those of MR-1, respectively. MR1-CYMA (which lacks the CM cytochrome CymA) showed essentially no V(V) reduction (Fig. 1B), and CMTn-3 (which lacks the periplasmic fumarate reductase) exhibited a very slow rate of V(V) reduction (Fig. 1B). ETRA-153, which lacks the transcriptional regulator EtrA, was 48% slower than MR-1 over the first 6 h (P < 0.001) but exhibited near-wild-type rates over 24 h (Fig. 1B).
FIG. 1.
Reduction of NaVO3 by MR-1 and various derived mutants. The decline in vanadium(V) concentration was followed over time. All values represent the mean ± standard deviation (n = 3 cultures of each strain). For points lacking apparent error bars, the bars were smaller than the points.
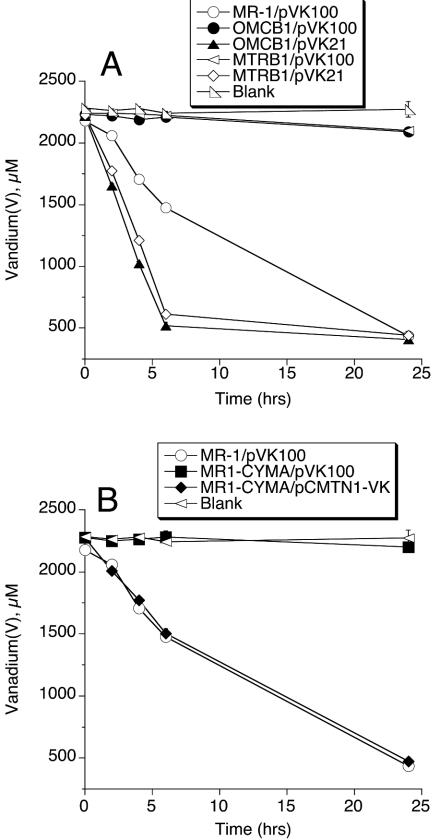
To better assess the role of components whose absence caused near-complete loss of V(V) reduction, experiments were conducted that compared these mutants with those mutants for which the deficiency was restored. The plasmid pVK21, which contains an MR-1 genomic fragment that restores both OmcB and MtrB (25, 32), restored V(V) reduction to OMCB1 and MTRB1 to rates that were even greater than those of wild type (Fig. 2A). Complementation with pVK21 can similarly result in greater-than-wild-type levels of Mn(IV) reduction (33), which corresponds with overexpression of OmcB in these strains (32, 33). The plasmid pCMTN-1, which contains wild-type cymA (30), corrected the V(V) reduction deficiency of MR1-CYMA (Fig. 2B).
FIG. 2.
Reduction of NaVO3 by various mutants and complemented mutants. The decline in vanadium(V) concentration was followed over time. All values represent the mean ± standard deviation (n = 3 cultures of each strain). For points lacking apparent error bars, the bars were smaller than the points.
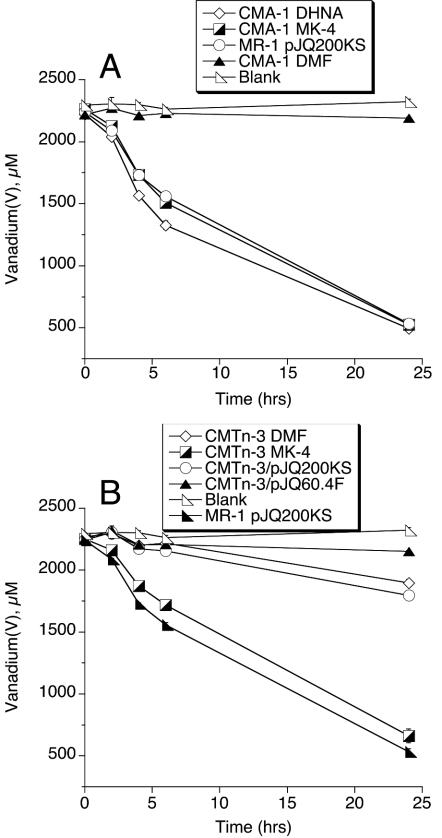
Since the absence of CymA results in an electron acceptor phenotype similar to that of menaquinone deficiency (20, 22), the potential role of menaquinone in V(V) reduction was also explored. The menaquinone-minus mutant CMA-1 is unable to reduce V(V) (Fig. 3A). Relative to the dimethylformamide (DMF) vehicle control, menaquinone-4 (MK-4) and 1,4-dihydroxy-2-naphthoic acid (DHNA) restored V(V) reduction to CMA-1 (Fig. 3A). Both MK-4 and DHNA have previously been shown to restore menaquinone in CMA-1 (20).
FIG. 3.
Reduction of NaVO3 by strains with or without menaquinone. The decline in vanadium(V) concentration was followed over time. All values represent the mean ± standard deviation (n = 3 cultures of each strain). For points lacking apparent error bars, the bars were smaller than the points.
The V(V) reduction phenotype of the fumarate reductase-minus mutant CMTn-3 was also explored further. Plasmid pJQ60.4F has previously been shown to restore fumarate reductase protein and activity to CMTn-3 as well as its ability to use fumarate as an electron acceptor (14). However, pJQ60.4F did not restore V(V) reduction to CMTn-3. CMTn-3 was derived from MR-1A, a spontaneous rifampin (Rf)-resistant mutant of MR-1. This Rfr phenotype in MR-1A and its derived strains is associated with markedly depressed menaquinone levels, and supplementation with MK-4 results in increased menaquinone content and more-wild-type rates of electron acceptor use that are dependent on MK (30). Growth of the CMTn-3 inoculum with MK-4 restored V(V) reduction to rates that were very similar to those of the wild type (Fig. 3B). The deficiency in V(V) reduction in CMTn-3 was therefore the result of MK deficiency due to the Rfr phenotype and was not linked to the absence of fumarate reductase.
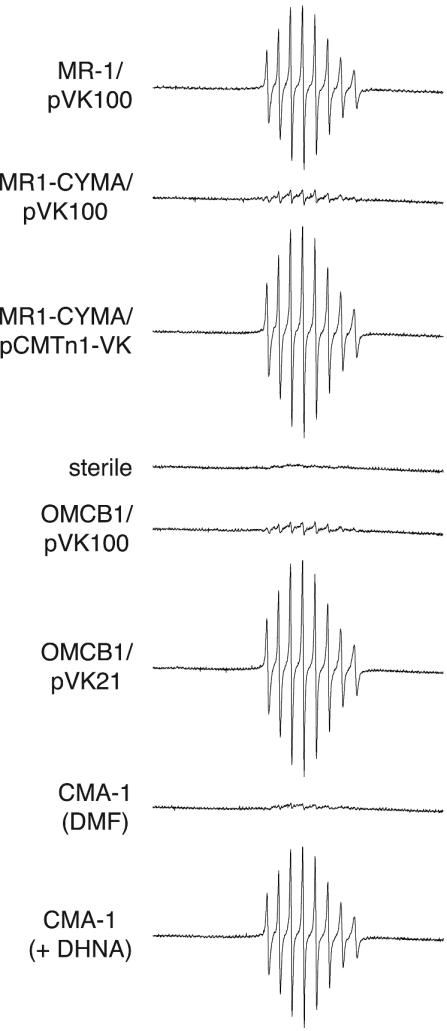
Throughout these studies, a blue color developed over time and remained in cultures that showed prominent V(V) reduction. This color did not appear in those that reduced little or no V(V). This blue is characteristic of V(IV) (11). The blue color remained in suspension throughout the experiments, and there was no apparent aggregation or precipitation of V(IV). ESR spectra were done to verify reduction of V(V) to V(IV). For those strains that showed prominent reduction of V(V) by the diphenylcarbazide assay (MR-1/pVK100, MR1-CYMA/pCMTn1-VK, OMCB1/pVK21, and CMA-1 plus DHNA) (Fig. 2 and 3), the ESR spectra showed a prominent eight-line spectrum characteristic for V(IV) (Fig. 4). V(IV) contains a 3d1 electron coupled to its nuclear spin [I = 7/2 for 51V(IV)] (46). This spectrum closely resembles that of the V(IV) compound vanadyl sulfate (VOSO4) (45, 46). The V(IV) spectrum was absent in the sterile control and was of very small intensity for strains that did not significantly reduce V(V), e.g., OMCB1/pVK100 and CMA-1(DMF) (Fig. 4).
FIG. 4.
ESR spectra for the presence of V(IV) after 18 h of anaerobic incubation of the indicated strains with NaVO3 at room temperature. The ESR instrument settings are defined in Materials and Methods. Each scan spanned 2,000 G (from 2,300 to 4,300 G).
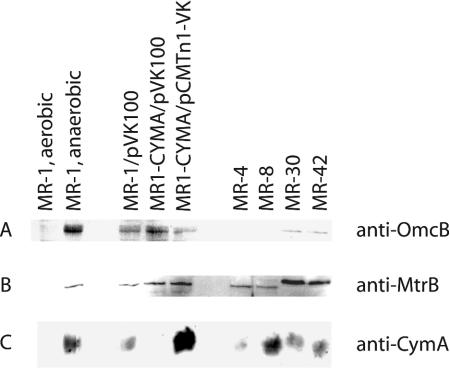
To determine if other Shewanella isolates can reduce V(V), V(V) reduction was examined with four other strains isolated from the Black Sea (MR-4 and MR-8) and Lake Michigan (MR-30 and MR-42). All four strongly resembled MR-1, with 1.85 to 1.89 mM V(V) reduced at 24 h versus 1.78 mM for MR-1. This implies that these strains may have analogs of the MR-1 components that are required for V(V) reduction. With respect to OM cytochromes, all four of these strains have at least two high-molecular-weight OM cytochromes, with OmcA homologs detected in MR-4 and MR-8 (29). In these studies, Western blotting detected the presence of OmcB homologs in MR-30 and MR-42, but not in MR-4 or MR-8 (Fig. 5). MtrB homologs were detected in all of the strains and were of slightly larger mass in MR-30 and MR-42 than in MR-4 and MR-8 (Fig. 5). CymA homologs were detected in all four strains as well, with slight differences in protein migration (Fig. 5). The blots also showed that all three of these proteins were significantly upregulated during anaerobic growth (Fig. 5). While previous studies had noted that pCMTn1-VK provided functional restoration of CymA-dependent processes to cymA mutants (22, 30), the development of the antibody for these studies provided direct evidence that pCMTN1-VK restores CymA protein to MR1-CYMA (Fig. 5).
FIG. 5.
Western blots of whole cells from various strains of Shewanella using immunoglobulin G specific for the OM cytochrome OmcB (A), the OM protein MtrB (B), and the CM cytochrome CymA (C). All cells were grown anaerobically with TMAO, except for the following: MR-1 aerobic was grown aerobically overnight on an orbital shaker (200 rpm) in 30 ml of defined medium in a 125-ml Erlenmeyer flask, and MR-1 anaerobic was grown anaerobically with fumarate as the electron acceptor. The bands for OmcB, MtrB, and CymA in MR-1 migrated with apparent masses of 75, 76.5, and 21.3 kDa, respectively, all very near their predicted masses.
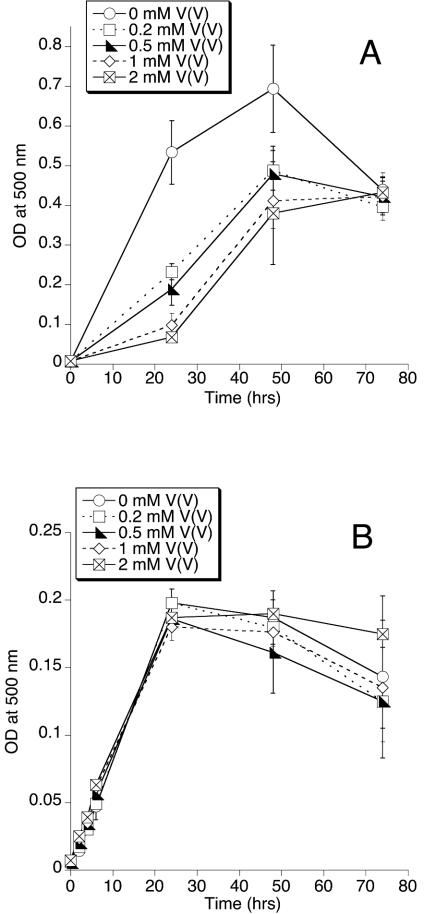
The MR-1 components required for V(V) reduction are also required for its use of other anaerobic electron acceptors (20, 22, 30, 31, 33). Experiments were therefore done to determine if V(V) could serve as an electron acceptor to support anaerobic growth of MR-1. Since V(V) is potentially toxic to cells, it was also necessary to determine if various concentrations of V(V) could inhibit growth. For aerobically grown cells, NaVO3 (0.2 to 2 mM) clearly inhibited growth during the first 24 h (Fig. 6A). While cells recovered somewhat after 48 h, they never attained the same final density as cells without V(V) (Fig. 6A). Under anaerobic conditions in the presence of fumarate, NaVO3 (0.2 to 2 mM) did not inhibit the growth of MR-1 (Fig. 6B). The cells in Fig. 6B generated the blue color characteristic of V(IV) within the first few hours; this ability to rapidly reduce V(V) corresponded with the marked upregulation of the components required for V(V) reduction under anaerobic conditions (Fig. 5). V(V) reduction therefore likely protects the cells from V(V) toxicity. This is consistent with the toxicity of V(V) to aerobic cells (Fig. 6A) and the minimal expression of key components required for V(V) reduction in aerobic cells (Fig. 5).
FIG. 6.
Growth of MR-1 in the presence of various concentrations of NaVO3 under room air (A) or under anaerobic conditions with fumarate as an electron acceptor (B). Growth was assessed by increases in culture turbidity at 500 nm over time. All values represent the mean ± standard deviation (n = 3 cultures for each vanadium concentration). For points lacking apparent error bars, the bars were smaller than the points.
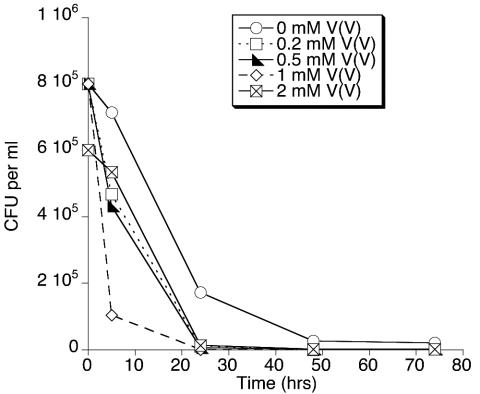
The ability of MR-1 to grow anaerobically with NaVO3 as the sole electron acceptor was examined. Cells were pregrown on fumarate, because such cells exhibit even higher levels of OM cytochromes and menaquinone than do TMAO-grown cells (18, 22, 24, 30). In studies which examined culture turbidity, small increases (<0.02) in the OD500 were observed over time in the presence of NaVO3, but these differences were not significant and were likely due to absorbance by V(IV) rather than to cell growth. Despite the rapid formation of the blue color characteristic of V(IV), colony counts declined over time (Fig. 7), suggesting that MR-1 cannot obtain sufficient energy from NaVO3 reduction to support anaerobic growth. Such declines in colony counts in the absence of an electron acceptor have been previously reported (27) and are consistent with the obligate respiratory nature of MR-1. The declines in Fig. 7 are not the result of cells aggregated to V(IV), because aggregates or precipitates were not observed in the medium and cell aggregates were not seen in epifluorescence cell counts of metavanadate cultures. Direct cell counts also demonstrated that 2 and 4 mM metavanadate concentrations did not support anaerobic cell growth (data not shown). The inability of V(V) to significantly stimulate anaerobic growth on fumarate (Fig. 6B) is also consistent with the inability of V(V) reduction to supply significant respiratory energy.
FIG. 7.
Colony counts of MR-1 under anaerobic conditions with various concentrations of NaVO3 as the sole electron acceptor. The inoculum was pregrown under anaerobic conditions on fumarate to ensure the presence of the components necessary for V(V) reduction. The points which appear to lie on the x axis represent ≤104 CFU ml−1. The data are from a representative experiment.
V2O5 similarly failed to support anaerobic growth. No significant increase in direct cell counts was observed over 48 h with 2 mM V2O5 as the sole electron acceptor, and colony counts declined significantly (data not shown). Higher concentrations of V2O5 proved toxic; 5 mM V2O5 decreased the anaerobic growth yields on fumarate, and 10 mM V2O5 largely prevented growth on fumarate (data not shown). Various concentrations of V2O5 and NaVO3 (2, 5, and 10 mM) also failed to support growth (colony formation) on solid medium under anaerobic conditions. Altogether, the data indicated that reduction of NaVO3 or V2O5 by MR-1 does not supply significant respiratory energy to support anaerobic growth.
DISCUSSION
The sum total of evidence indicates that a multicomponent electron transport chain is involved in the reduction of V(V) by MR-1. This chain involves cytochromes and a quinone and includes components of both the CM (CymA and MK) and the OM (OmcB, and a partial role for OmcA). These same components are involved in the respiratory reduction of Mn(IV) by MR-1 (20, 22, 31, 32), and the observed effects are quantitatively similar for Mn(IV) and V(V), i.e., the absence of CymA, MK, or OmcB results in loss of the overwhelming majority of activity, whereas the absence of OmcA results in rates that are 55 and 62% of those of MR-1 for Mn(IV) (31) and V(V), respectively. The OM protein MtrB does not likely have an electron transport role in V(V) reduction. Rather, its absence results in the mislocalization and improper incorporation of the OM cytochromes (25), with the effects on OmcB being most critical for V(V) reduction. Even though metavanadate is readily soluble, the results with MTRB1 suggest that proper OM localization is necessary for the role of OM cytochromes in V(V) reduction. Microscopic analysis of MR-1 incubated with V2O5 for 5 days noted the presence of extensive extracellular V(IV) granules (2). This extracellular formation of V(IV) is consistent with a role for OM cytochromes reported here.
The need for cell-surface-exposed OM cytochromes (26) to mediate the reduction of insoluble Mn(IV) oxides is apparent, whereas the advantage for this mechanism in the reduction of a soluble species such as V(V) is not clear. One possibility is that reduction at the cell surface might prevent potential toxic effects from the intracellular accumulation of V(V). This is supported by the observations in Fig. 6, in which anaerobic cells which expressed V(V)-reducing components were not inhibited by vanadate (Fig. 6B), whereas vanadate did partially inhibit the growth of aerobic cells (Fig. 6A) which only poorly express essential V(V)-reducing components. In contrast, anaerobic E. coli cells are more susceptible to inhibition by vanadate compared to aerobic cells (15), but significant vanadate reduction by E. coli has not been reported. Through its ability to mimic phosphate, vanadate can inhibit P-type ATPases, kinases, and ATP-binding cassette transporters (8). Vanadate can also partially inhibit nitrate reduction by E. coli, fumarate reduction by Campylobacter jejuni, the nitrate reductase of Aspergillus, and the formate dehydrogenase of Pseudomonas oxalaticus (15).
In addition to their role in V(V) reduction, the CM components CymA and MK are required for the use of other anaerobic electron acceptors, including Fe(III), nitrate, and fumarate (20, 22, 30). MK is also required for the reduction of thiosulfate, nitrite, DMSO, and anthraquinone-2,6-disulfonate (AQDS) (36, 41), and CymA is required for the reduction of DMSO and nitrite (44) and AQDS (unpublished data). Both CymA and MK are therefore common central components in electron transport chains that branch to several terminal reductases downstream. These downstream reductases are differentially localized in MR-1. The fumarate reductase is periplasmic (16, 17, 23, 38), and the genomic sequence predicts that the MR-1 reductases for DMSO, nitrate, and nitrite are also periplasmic. OM components are required for the reduction of Mn(IV) and Fe(III) (19, 25, 29, 31, 32). It was originally proposed that CymA, a member of the NapC/NirT family of cytochromes, is anchored to the periplasmic face of the CM and functions as an electron carrier between MK and downstream electron transport components (22). These properties of CymA have been supported by recent studies (43, 44). Electron transfer from MK to CymA is apparently true for most electron acceptors, but not for thiosulfate. While MK is required for thiosulfate use by MR-1, CymA is not (unpublished data). This further indicates that CymA is downstream of MK, because if it were upstream of MK then both CymA and MK would be required for thiosulfate reduction.
Since the other Shewanella strains examined also reduced V(V) and contained homologs of the components required in MR-1, it is likely that they reduce V(V) using a similar electron transport system. MtrB and CymA homologs were detected in all four of these strains, whereas OmcB homologs were detected in two. It is possible that the other two have an OmcB homolog, because they contain high-molecular-weight OM cytochromes of a similar mass (29). The anti-OmcB was generated against an internal 195-residue fragment of the MR-1 OmcB (32), so it is possible that this region of OmcB was sufficiently divergent in MR-4 and MR-8 that it was not recognized by anti-OmcB.
The anaerobic upregulation of the components required for V(V) reduction (Fig. 5) makes sense for their role in the use of various anaerobic electron acceptors. Consistent with this, previous studies demonstrated that OmcA is upregulated sevenfold under anaerobic conditions (24), and MK content is threefold higher in TMAO-grown cells than in aerobic cells (20).
The finding that ETRA-153 lags 48% behind wild type in V(V) reduction over the first 6 h is interesting. EtrA is a putative analog of Fnr, which participates in the transcriptional activation of genes required for anaerobic growth in E. coli (6). This initial V(V) lag with ETRA-153 resembles that previously reported for fumarate and nitrate reduction (13). For fumarate reduction, this corresponded with initially lower levels of the transcript and protein for the flavocytochrome fumarate reductase (13). However, several components required for V(V) reduction are unaffected in ETRA-153, including subcellular cytochrome distribution, OM cytochrome content, menaquinone levels, and SDS-PAGE profiles of subcellular fractions (13). While microarray transcription profiling of MR-1 indicated that the expression of 69 genes can be positively or negatively affected by the absence of EtrA, none of the components implicated in V(V) reduction in this report were included in these 69 (1). Several putative electron transport components were influenced by the loss of EtrA (nitrate reductase, fumarate reductase, DMSO reductase, formate dehydrogenase, hydrogenase, and various cytochromes and oxidases) (1), but additional studies will be required to determine which, if any, of these may impact V(V) reduction. Some putative transcriptional regulators were also impacted by the loss of EtrA (1), one or more of which may indirectly affect unidentified components involved in V(V) reduction.
The reduction of V(V) by MR-1 does not appear to supply sufficient energy to support anaerobic growth. This is interesting because the components required for V(V) reduction (MK, CymA, and OmcB) are required for the use of other electron acceptors, including Mn(IV) (20, 22, 30-32). The standard redox potential for the reduction of V(V) to V(IV) (VO+2 → VO2+) at pH 7.4 is +0.127 V. This is more positive than that for fumarate reduction (+0.030 V), which is mediated by a periplasmic enzyme, but significantly less positive than that for MnO2 reduction (+0.42 V), which requires the OM cytochrome OmcB. It is therefore possible that the reduction potential of vanadate is insufficient to support anaerobic growth when the reduction is mediated by OM components. Alternatively, it is possible that higher concentrations of V(V) are required to support measurable growth, but toxicity at higher concentrations precluded such studies. However, 2 mM levels of several other electron acceptors are sufficient to support anaerobic growth by MR-1 (27). Such high levels would be highly unlikely in most environments in which vanadium levels are typically micromolar (3). As shown in these studies, MR-1 does not need to respire V(V) to facilitate its reduction and can survive on other electron acceptors, such as Fe(III), which is typically far more abundant in soils, sediments, and aquatic systems than is vanadium.
Prior to the recent report of V(V) reduction by MR-1 (2), there were other reports of bacterial vanadium reduction, including qualitative observations with Pseudomonas isachenkovii and Pseudomonas vanadium reductans (47). However, this is the first report delineating specific components in an electron transport chain that are required for V(V) reduction. The requirement for cytochromes and MK contrasts with that of eukaryotic enzymes with V(V) reductase activity which are NADPH-dependent flavoenzymes, e.g., glutathione reductase, lipoyl dehydrogenase, and ferredoxin-NADP oxidoreductase (45). In addition, this is the first report of a requirement for OM cytochromes in the reduction of a soluble metal species.
Acknowledgments
This work was supported by National Science Foundation grant MCB-0315875 to C.R.M. We are grateful for the support of the STRATTEC Foundation.
We thank Paulo Ferreira for the use of the epifluorescence microscope.
REFERENCES
- 1.Beliaev, A. S., D. K. Thompson, M. W. Fields, L. Wu, D. P. Lies, K. H. Nealson, and J. Zhou. 2002. Microarray transcription profiling of a Shewanella oneidensis etrA mutant. J. Bacteriol. 184:4612-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpentier, W., K. Sandra, I. De Smet, A. Brigé, L. De Smet, and J. Van Beeumen. 2003. Microbial reduction and precipitation of vanadium by Shewanella oneidensis. Appl. Environ. Microbiol. 69:3636-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costigan, M., R. Cary, and S. Dobson. 2001. Vanadium pentoxide and other inorganic vanadium compounds. World Health Organization, Geneva, Switzerland.
- 4.Domingo, J. L., J. M. Llobet, and J. Corbella. 1985. Protection of mice against the lethal effects of sodium metavanadate: a quantitative comparison of a number of chelating agents. Toxicol. Lett. 26:95-99. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson, J., R. Hemming, and F. LaBella. 1985. Vanadium exposure enhances lipid peroxidation in the kidney of rats and mice. Can. J. Physiol. Pharmacol. 63:196-199. [DOI] [PubMed] [Google Scholar]
- 6.Gunsalus, R. P. 1992. Control of electron flow in Escherichia coli: coordinated transcription of respiratory pathway genes. J. Bacteriol. 174:7069-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heidelberg, J. F., I. T. Paulsen, K. E. Nelson, E. J. Gaidos, W. C. Nelson, T. D. Read, J. A. Eisen, R. Seshadri, N. Ward, B. Methe, R. A. Clayton, T. Meyer, A. Tsapin, J. Scott, M. Beanan, L. Brinkac, S. Daugherty, R. T. DeBoy, R. J. Dodson, A. S. Durkin, D. H. Haft, J. F. Kolonay, R. Madupu, J. D. Peterson, L. A. Umayam, O. White, A. M. Wolf, J. Vamathevan, J. Weidman, M. Impraim, K. Lee, K. Berry, C. Lee, J. Mueller, H. Khouri, J. Gill, T. R. Utterback, L. A. McDonald, T. V. Feldblyum, H. O. Smith, J. C. Venter, K. H. Nealson, and C. M. Fraser. 2002. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat. Biotechnol. 20:1118-1123. [DOI] [PubMed] [Google Scholar]
- 8.Hunke, S., S. Dose, and E. Schneider. 1995. Vanadate and bafilomycin A1 are potent inhibitors of the ATPase activity of the reconstituted bacterial ATP-binding cassette transporter for maltose (MalFGK2). Biochem. Biophys. Res. Commun. 216:589-594. [DOI] [PubMed] [Google Scholar]
- 9.Irwin, R. J., M. VanMouwerick, L. Stevens, M. D. Sesse, and W. Basham. 1997. Vanadium. In Environmental contaminants encyclopedia. National Park Service, Water Resources Division, Fort Collins, Colo. [Online.]
- 10.Knauf, V. C., and E. W. Nester. 1982. Wide host range cloning vectors: cosmid clone bank of Agrobacterium Ti plasmids. Plasmid 8:45-54. [DOI] [PubMed] [Google Scholar]
- 11.Lovley, D. R. 1993. Dissimilatory metal reduction. Annu. Rev. Microbiol. 47:263-290. [DOI] [PubMed] [Google Scholar]
- 12.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maier, T. M., and C. R. Myers. 2001. Isolation and characterization of a Shewanella putrefaciens MR-1 electron transport regulator etrA mutant: reassessment of the role of EtrA. J. Bacteriol. 183:4918-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maier, T. M., J. M. Myers, and C. R. Myers. 2003. Identification of the gene encoding the sole physiological fumarate reductase of Shewanella putrefaciens MR-1. J. Basic Microbiol. 43:312-327. [DOI] [PubMed] [Google Scholar]
- 15.Mendz, G. L. 1999. Redox and specific effects of vanadium ions on respiratory enzymes. Biometals 12:35-45. [DOI] [PubMed] [Google Scholar]
- 16.Morris, C. J., A. C. Black, S. L. Pealing, F. D. C. Manson, S. K. Chapman, G. A. Reid, D. M. Gibson, and B. F. Ward. 1994. Purification and properties of a novel cytochrome: flavocytochrome c from Shewanella putrefaciens. Biochem. J. 302:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers, C. R., and J. M. Myers. 1992. Fumarate reductase is a soluble enzyme in anaerobically grown Shewanella putrefaciens MR-1. FEMS Microbiol. Lett. 98:13-20. [Google Scholar]
- 18.Myers, C. R., and J. M. Myers. 1992. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J. Bacteriol. 174:3429-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers, C. R., and J. M. Myers. 1993. Ferric reductase is associated with the membranes of anaerobically grown Shewanella putrefaciens MR-1. FEMS Microbiol. Lett. 108:15-22. [Google Scholar]
- 20.Myers, C. R., and J. M. Myers. 1993. Role of menaquinone in the reduction of fumarate, nitrate, iron(III) and manganese(IV) by Shewanella putrefaciens MR-1. FEMS Microbiol. Lett. 114:215-222. [Google Scholar]
- 21.Myers, C. R., and J. M. Myers. 1994. Ferric iron reduction-linked growth yields of Shewanella putrefaciens MR-1. J. Appl. Bacteriol. 76:253-258. [DOI] [PubMed] [Google Scholar]
- 22.Myers, C. R., and J. M. Myers. 1997. Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciens MR-1. J. Bacteriol. 179:1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers, C. R., and J. M. Myers. 1997. Isolation and characterization of a transposon mutant of Shewanella putrefaciens MR-1 deficient in fumarate reductase. Lett. Appl. Microbiol. 25:162-168. [DOI] [PubMed] [Google Scholar]
- 24.Myers, C. R., and J. M. Myers. 1997. Outer membrane cytochromes of Shewanella putrefaciens MR-1: spectral analysis, and purification of the 83-kDa c-type cytochrome. Biochim. Biophys. Acta 1326:307-318. [DOI] [PubMed] [Google Scholar]
- 25.Myers, C. R., and J. M. Myers. 2002. MtrB is required for proper incorporation of the cytochromes OmcA and OmcB into the outer membrane of Shewanella putrefaciens MR-1. Appl. Environ. Microbiol. 68:5585-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers, C. R., and J. M. Myers. 2003. Cell surface exposure of the outer membrane cytochromes of Shewanella oneidensis MR-1. Lett. Appl. Microbiol. 37:254-258. [DOI] [PubMed] [Google Scholar]
- 27.Myers, C. R., and K. H. Nealson. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319-1321. [DOI] [PubMed] [Google Scholar]
- 28.Myers, C. R., and K. H. Nealson. 1990. Respiration-linked proton translocation coupled to anaerobic reduction of manganese(IV) and iron(III) in Shewanella putrefaciens MR-1. J. Bacteriol. 172:6232-6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers, J. M., and C. R. Myers. 1998. Isolation and sequence of omcA, a gene encoding a decaheme outer membrane cytochrome c of Shewanella putrefaciens MR-1, and detection of omcA homologs in other strains of S. putrefaciens. Biochim. Biophys. Acta 1373:237-251. [DOI] [PubMed] [Google Scholar]
- 30.Myers, J. M., and C. R. Myers. 2000. Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. J. Bacteriol. 182:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers, J. M., and C. R. Myers. 2001. Role for outer membrane cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in reduction of manganese dioxide. Appl. Environ. Microbiol. 67:260-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers, J. M., and C. R. Myers. 2002. Genetic complementation of an outer membrane cytochrome omcB mutant of Shewanella putrefaciens MR-1 requires omcB plus downstream DNA. Appl. Environ. Microbiol. 68:2781-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers, J. M., and C. R. Myers. 2003. Overlapping role of the outer membrane cytochromes of Shewanella oneidensis MR-1 in the reduction of manganese(IV) oxide. Lett. Appl. Microbiol. 36:21-25. [DOI] [PubMed] [Google Scholar]
- 34.Nealson, K. H., C. R. Myers, and B. B. Wimpee. 1991. Isolation and identification of manganese-reducing bacteria and estimates of microbial Mn(IV)-reducing potential in the Black Sea. Deep-Sea Res. 38(Suppl. 2):S907-S920. [Google Scholar]
- 35.Nechay, B. R. 1984. Mechanisms of action of vanadium. Annu. Rev. Pharmacol. Toxicol. 24:501-524. [DOI] [PubMed] [Google Scholar]
- 36.Newman, D. K., and R. Kolter. 2000. A role for excreted quinones in extracellular electron transfer. Nature 405:94-97. [DOI] [PubMed] [Google Scholar]
- 37.Patole, M. S., and T. Ramasarma. 1988. Occurrence of lipid peroxidation in brain microsomes in the presence of NADH and vanadate. J. Neurochem. 51:491-496. [DOI] [PubMed] [Google Scholar]
- 38.Pealing, S. L., A. C. Black, F. D. C. Manson, F. B. Ward, S. K. Chapman, and G. A. Reid. 1992. Sequence of the gene encoding flavocytochrome c from Shewanella putrefaciens: a tetraheme flavoenzyme that is a soluble fumarate reductase related to the membrane-bound enzymes from other bacteria. Biochemistry 31:12132-12140. [DOI] [PubMed] [Google Scholar]
- 39.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 40.Rehder, D. 1992. Structure and function of vanadium compounds in living organisms. Biometals 5:3-12. [DOI] [PubMed] [Google Scholar]
- 41.Saffarini, D. A., S. L. Blumerman, and K. J. Mansoorabadi. 2002. Role of menaquinones in Fe(III) reduction by membrane fractions of Shewanella putrefaciens. J. Bacteriol. 184:846-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandell, E. B. 1959. Colorimetric determinations of traces of metals. Interscience, New York, N.Y.
- 43.Schwalb, C., S. K. Chapman, and G. A. Reid. 2002. The membrane-bound tetrahaem c-type cytochrome CymA interacts directly with the soluble fumarate reductase in Shewanella. Biochem. Soc. Trans. 30:658-662. [DOI] [PubMed] [Google Scholar]
- 44.Schwalb, C., S. K. Chapman, and G. A. Reid. 2003. The tetraheme cytochrome CymA is required for anaerobic respiration with dimethyl sulfoxide and nitrite in Shewanella oneidensis. Biochemistry 42:9491-9497. [DOI] [PubMed] [Google Scholar]
- 45.Shi, X., and N. S. Dalal. 1991. Flavoenzymes reduce vanadium(V) and molecular oxygen and generate hydroxyl radical. Arch. Biochem. Biophys. 289:355-361. [DOI] [PubMed] [Google Scholar]
- 46.Shi, X., and N. S. Dalal. 1993. One-electron reduction of vanadium(V) by flavoenzymes/NADPH. Arch. Biochem. Biophys. 302:300-303. [DOI] [PubMed] [Google Scholar]
- 47.Yurkova, N. A., and N. N. Lyalikova. 1991. New vanadate-reducing facultative chemolithotrophic bacteria. Microbiology 59:672-677. [Google Scholar]