Abstract
Cardiac hypertrophy is a strong predictor of morbidity and mortality in patients with heart failure. Small molecule histone deacetylase (HDAC) inhibitors have been shown to suppress cardiac hypertrophy through mechanisms that remain poorly understood. We report that class I HDACs function as signal-dependent repressors of cardiac hypertrophy via inhibition of the gene encoding dual-specificity phosphatase 5 (DUSP5) DUSP5, a nuclear phosphatase that negatively regulates prohypertrophic signaling by ERK1/2. Inhibition of DUSP5 by class I HDACs requires activity of the ERK kinase, mitogen-activated protein kinase kinase (MEK), revealing a self-reinforcing mechanism for promotion of cardiac ERK signaling. In cardiac myocytes treated with highly selective class I HDAC inhibitors, nuclear ERK1/2 signaling is suppressed in a manner that is absolutely dependent on DUSP5. In contrast, cytosolic ERK1/2 activation is maintained under these same conditions. Ectopic expression of DUSP5 in cardiomyocytes results in potent inhibition of agonist-dependent hypertrophy through a mechanism involving suppression of the gene program for hypertrophic growth. These findings define unique roles for class I HDACs and DUSP5 as integral components of a regulatory signaling circuit that controls cardiac hypertrophy.
Keywords: cardiac remodeling, gene transcription, lysine acetylation
Cardiovascular disease remains the leading cause of death in the United States. In 2008, more than 2,200 Americans died of cardiovascular disease each day on average, with heart failure as a major cause of these deaths. Approximately 6 million Americans suffer from heart failure, placing an economic burden on the United States that is projected to rise to nearly $100 billion annually by 2030 (1). The 5-y mortality rate following first admission for heart failure is 42.3%, further highlighting an urgent need for novel therapeutic approaches (2).
A common outcome of stress in the heart is cardiomyocyte hypertrophy, a growth response during which individual myocytes increase in size without dividing, assemble additional contractile units (sarcomeres) to maximize force generation, and reactivate a fetal program of gene expression. Cardiac hypertrophy has long been viewed as a compensatory mechanism that normalizes wall stress and enhances cardiac performance. However, long-term suppression of left ventricular hypertrophy (LVH) is associated with reduced morbidity and mortality in patients with hypertension (3, 4), and studies in animal models have demonstrated that blocking “compensatory” hypertrophy can result in improved cardiac function and provide long-term survival benefit (5). As such, chronic cardiac hypertrophy is now considered maladaptive and, given that LVH is an independent predictor of adverse outcomes in patients with cardiovascular disease (6–9), represents an attractive target for novel therapeutic intervention (10).
A variety of biochemical pathways have been shown to regulate hypertrophic growth of cardiac myocytes (11). Biomechanical stress triggers cardiac hypertrophy, in part, through activation of autocrine/paracrine signaling pathways that stimulate Gαq/Gα11 protein-coupled receptors (GPCRs), including the angiotensin, endothelin, and α1-adrenergic receptors. Indeed, mice with compound knockout of the genes encoding Gαq and Gα11, which are functionally redundant, are resistant to pressure overload-induced cardiac hypertrophy (12). Downstream mediators of hypertrophy include the Ca2+/calmodulin-dependent protein phosphatase, calcineurin (13), Ca2+/calmodulin-dependent kinase (CaM kinase) (14), protein kinases C and D (15–17), as well as members of the mitogen-activated protein kinase (MAPK) family (18, 19). Given the plethora of redundant signaling pathways capable of triggering pathological cardiac hypertrophy, many in the field have speculated that the most promising approach for therapeutic intervention would involve targeting distal signaling mediators that function as nodal integrators of upstream prohypertrophic signaling cascades. Recent studies have suggested that epigenetic regulators, such as histone deacetylases (HDACs), may represent such targets.
HDACs catalyze removal of acetyl groups from lysine residues in a variety of proteins. Historically, HDACs have been studied in the context of chromatin, where they deacetylate nucleosomal histones and alter the electrostatic properties of chromatin in a manner that favors gene repression. However, it is now clear that HDACs regulate the acetylation state of thousands of distinct proteins in the nuclear and cytoplasmic compartments of diverse cell types (20, 21). The 18 HDACs are encoded by distinct genes and are grouped into four classes. Class I, II, and IV HDACs are zinc-dependent enzymes, whereas class III HDACs, which are also known as sirtuins, require nicotinamide adenine dinucleotide (NAD+) for catalytic activity (22).
Multiple small molecule inhibitors of zinc-dependent HDACs have been shown to be efficacious in rodent models of heart failure, blocking pathological cardiac hypertrophy and improving cardiac function, suggesting an unexpected application for HDAC inhibitors for the treatment of human heart failure (23). However, the molecular mechanisms by which specific HDAC isoforms promote cardiomyocyte hypertrophy remain incompletely defined. Here, we describe a unique pathway for regulation of cardiac hypertrophy by class I HDACs. We show that selective small molecule inhibitors of class I HDACs block agonist-dependent activation of ERK1/2 in cardiomyocytes via induction of an ERK-specific phosphatase, dual-specificity phosphatase 5 (DUSP5). Hypertrophic stimuli concomitantly stimulate ERK1/2 phosphorylation and repress expression of DUSP5 through a mechanism that is dependent on class I HDACs and the ERK kinase, mitogen-activated protein kinase kinase (MEK). Class I HDAC inhibitors depress expression of DUSP5, which is a nuclear phosphatase, resulting in suppression of nuclear ERK1/2 signaling and inhibition of downstream target genes that drive cardiac hypertrophy. Consistent with this, ectopically expressed DUSP5 potently inhibits cardiomyocyte hypertrophy. Our findings demonstrate a unique role for class I HDACs in the control of cardiac MAPK signaling and reveal an unforeseen function for DUSP5 in the regulation of myocyte growth.
Results
Class I HDACs Regulate Stress-Induced Activation of ERK1/2 Signaling in Cardiac Myocytes.
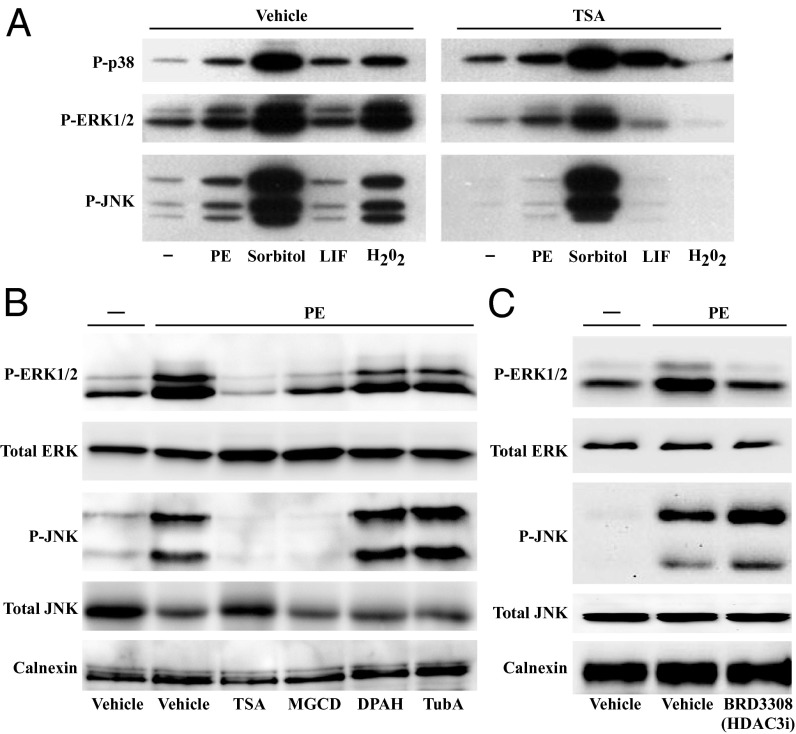
Data from nonmyocytes suggested roles for HDACs in mediating intracellular signaling events involved in growth, differentiation, inflammation, and oxidative stress (24). To begin to address whether HDACs regulate stress signaling in the heart, neonatal rat ventricular myocytes (NRVMs) were treated for 48 h with a panel of stress-inducing agents in the absence or presence of the pan-HDAC inhibitor, trichostatin A (TSA). Immunoblotting was performed with NRVM lysates and phospho-specific antibodies to detect p38, JNK, and ERK1/2 only when dually phosphorylated on specific threonine and tyrosine residues, a condition that is necessary and sufficient for stress-induced MAPK activation. As shown in Fig. 1A, treatment of NRVMs with TSA dramatically reduced ERK1/2 and JNK phosphorylation in response to phenylephrine (PE; α1-adrenergic receptor agonist), sorbitol (osmotic stress), leukemia inhibitory factory (LIF; gp130 receptor agonist), or hydrogen peroxide (H2O2; oxidative stress); signal-dependent phosphorylation of p38 was not suppressed by TSA.
Fig. 1.
Class I HDAC inhibition attenuates signal-dependent phosphorylation of ERK1/2 and JNK in cardiomyocytes. (A) Neonatal rat ventricular myocytes (NRVMs) were stimulated with the indicated agents for 48 h in the absence or presence of the pan-HDAC inhibitor trichostatin A (TSA). Phospho-p38, JNK, and ERK were examined by immunoblotting. PE, phenylephrine; LIF, leukemia inhibitory factor. (B) NRVMs were stimulated for 48 h with PE in the absence or presence of the following HDAC inhibitors: TSA, MGCD0103 (class I HDAC inhibitor), DPAH (class IIa HDAC inhibitor), and tubastatin A (HDAC6 inhibitor); DMSO (0.1% final concentration) was used as the vehicle for each HDAC inhibitor. Immunoblotting was performed to detect phospho and total forms of ERK1/2 and JNK. Calnexin served as a loading control. (C) NRVMs were stimulated for 48 h with PE in the absence or presence of BRD3308 (HDAC3 inhibitor) before immunoblotting.
To address which HDACs control ERK1/2 and JNK phosphorylation in cardiac myocytes, subsequent studies were performed with isoform-selective HDAC inhibitors. Agonist-dependent phosphorylation of ERK1/2 and JNK was found to be significantly attenuated by MGCD0103, which is a highly selective inhibitor of class I HDACs -1, -2, and -3 (25) (Fig. 1B). MS-275, an independent class I HDAC inhibitor, also blocked ERK1/2 and JNK phosphorylation in cardiomyocytes (Fig. S1A). In contrast, diphenylacetohydroxamic acid (DPAH), which blocks class IIa HDACs (26), and tubastatin A, which selectively inhibits class IIb HDAC6 (27), had no effect on ERK1/2 or JNK phosphorylation in NRVMs (Fig. 1B). Pan- and class I HDAC-selective inhibition blocked both basal and agonist-dependent ERK and JNK phosphorylation (Fig. S1B). These data suggest a unique role for class I HDACs in the control of cardiac MAPK signaling.
MGCD0103 and MS-275 inhibit class I HDACs -1, -2, and -3. Additional small molecule inhibitors were used to determine which class I HDAC(s) controls ERK1/2 and JNK phosphorylation in cardiac myocytes. BRD3308, which selectively inhibits HDAC3, attenuated PE-mediated phosphorylation of ERK but not JNK (Fig. 1C); small interfering RNA targeting HDAC3 also preferentially blunted ERK phosphorylation in cardiac myocytes (Fig. S1C). In contrast, biaryl-60 (BA-60), which is highly selective for HDACs -1 and -2 (28, 29), suppressed agonist-dependent phosphorylation of JNK but not ERK1/2 (Fig. S1D). Together, these data suggest that phosphorylation of ERK1/2 and JNK in cardiac myocytes is controlled by HDAC3 and HDAC1/2, respectively.
De Novo Phosphatase Expression Is Required for Class I HDAC Inhibitor-Mediated Suppression of Cardiac ERK1/2 Phosphorylation.
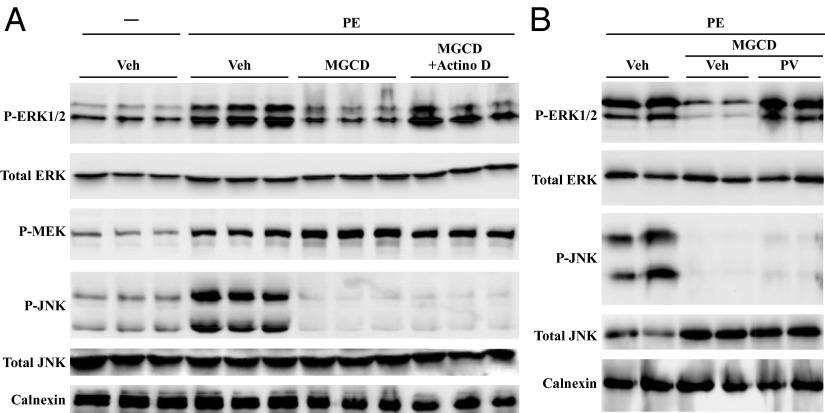
Given the ability of HDAC inhibitors to alter gene expression, an experiment was performed to determine whether de novo gene transcription is required for class I HDAC inhibitor-mediated suppression of cardiac MAPK phosphorylation. NRVMs were treated for 48 h with PE in the absence or presence of MGCD0103. For the final 6 h of the study a subset of cells was exposed to actinomycin D, which prevents RNA polymerase elongation. As shown in Fig. 2A, actinomycin D rescued ERK1/2 phosphorylation in MGCD0103-treated NRVMs. In contrast, actinomycin D failed to rescue JNK phosphorylation. These data suggest that HDAC inhibitors block cardiac ERK1/2 and JNK phosphorylation through distinct mechanisms, which are dependent and independent on new gene expression, respectively.
Fig. 2.
Class I HDAC inhibitor-mediated suppression of ERK1/2 phosphorylation requires gene transcription and phosphatase activity. (A) NRVMs were stimulated with PE in the absence or presence of the class I HDAC inhibitor MGCD0103 for 42 h before treatment with DMSO vehicle or actinomycin D (Actino D) for 6 h. (B) NRVMs were treated with PE in the absence or presence of MGCD0103 for 46 h. Cells were further treated with the phosphatase inhibitor, pervanadate (PV; 75 μM), for 2 h before harvesting for immunoblotting with the indicated antibodies.
Phosphorylation of the ERK1/2 kinase, MEK1/2, was not attenuated in the presence of MGCD0103 (Fig. 2A), supporting the notion that HDAC inhibitors block cardiac ERK1/2 phosphorylation by stimulating expression of a negative regulator rather than through inhibition of an upstream kinase. In further support of this mechanism, HDAC inhibitor-mediated suppression of ERK1/2 phosphorylation in NRVMs was completely rescued by pervanadate (PV), a potent inhibitor of protein tyrosine phosphatases; JNK phosphorylation was not rescued under the same conditions (Fig. 2B). These data suggest that class I HDAC inhibitors block ERK1/2 phosphorylation by stimulating expression of a phosphatase, whereas inhibition of JNK phosphorylation likely involves suppression of an upstream kinase. The remainder of the studies presented here focus on elucidating the mechanism of cardiac ERK1/2 regulation by class I HDACs.
Class I HDAC Inhibition Derepresses Hypertrophic Agonist-Dependent Suppression of DUSP5 Expression.
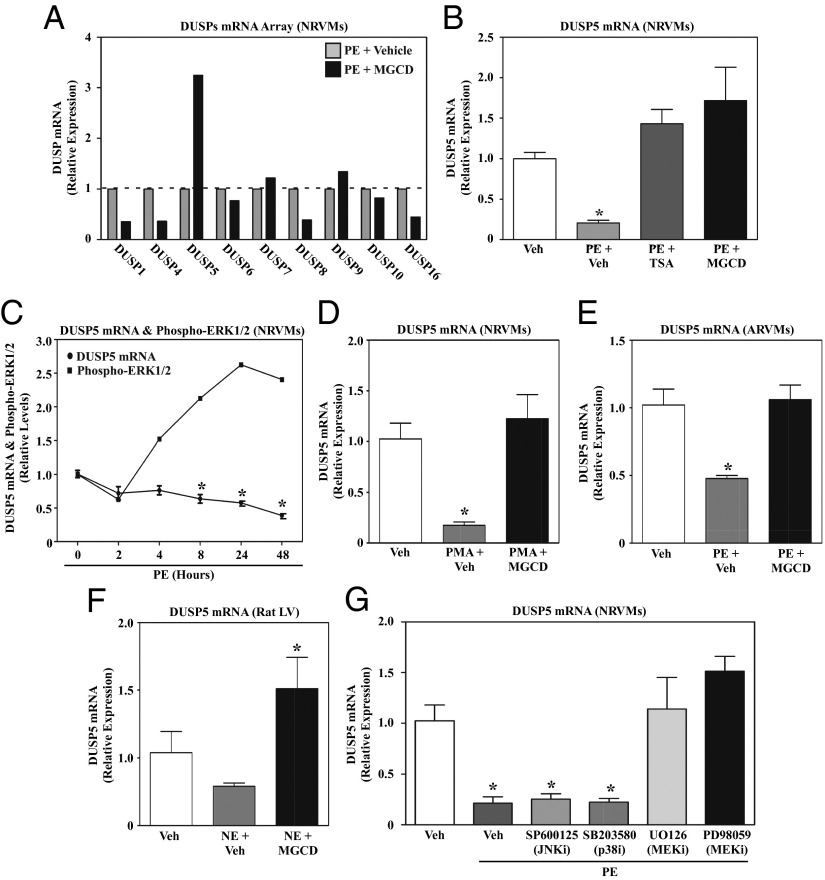
To begin to address whether HDAC inhibitors stimulate MAPK phosphatase expression in cardiac myocytes, RNA from NRVMs treated with PE in the absence or presence of MGCD0103 was analyzed using a protein phosphatase-focused PCR array. Of all of the MAPK-specific phosphatases that were analyzed, only DUSP5 was significantly induced by MGCD0103 (Fig. 3A). Follow-up quantitative PCR (qPCR) with independent samples confirmed the effect of MGCD0103 on DUSP5 expression and also demonstrated an equivalent stimulatory effect of TSA (Fig. 3B). Consistent with a role for HDAC3 in the control of ERK1/2 in cardiac myocytes, DUSP5 expression was also induced by BRD3308 and small interfering RNA targeting HDAC3 (Fig. S2 A and B). Interestingly, these qPCR studies also revealed that PE treatment dramatically reduced DUSP5 mRNA expression in cardiomyocytes, suggesting that HDAC inhibitors derepress the dusp5 gene. Kinetic studies demonstrated that down-regulation of DUSP5 mRNA transcripts in PE-treated NRVMs correlated with maximal ERK1/2 phosphorylation (Fig. 3C). As DUSP5 has been previously shown to selectively target ERK1/2 for dephosphorylation (30), these data implicate DUSP5 as a prime candidate for mediating ERK1/2 suppression by HDAC inhibitors in cardiac myocytes.
Fig. 3.
Signal-dependent repression of DUSP5 expression in cardiomyocytes requires class I HDAC and MEK catalytic activity. (A) NRVMs were stimulated with PE for 48 h in the absence or presence of MGCD0103 or DMSO vehicle control. RNA was harvested and expression of the indicated MAPK-specific dual-specificity phosphatases (DUSPs) was quantified using a PCR array. (B) NRVMs were left untreated or treated with PE for 48 h in the absence or presence of TSA or MGCD0103. DUSP5 mRNA expression was analyzed by qPCR. (C) NRVMs were stimulated with PE and harvested over time for analysis of DUSP5 mRNA expression and phospho-ERK1/2 levels. Densitometry was used to quantify phospho-ERK normalized to total ERK. (D) NRVMs were left untreated or stimulated with phorbol myristate acetate (PMA) for 48 h in the absence or presence of MGCD0103, and DUSP5 mRNA expression was analyzed by qPCR. (E) Adult rat ventricular myocytes (ARVMs) were treated for 48 h with PE in the absence or presence of MGCD0103, and DUSP5 mRNA levels were measured by qPCR. (F) Adult rats were administered MGCD0103 (10 mg/kg) or vehicle control by i.p. injection for 3 d and were subsequently given a single i.p. injection of norepinephrine (NE). Animals were killed 2 h post-NE injection and DUSP5 mRNA expression in the LV was analyzed by qPCR. n = 3 animals per condition; *P < 0.05 vs. unstimulated, vehicle-treated rats. (G) NRVMs were left untreated or stimulated with PE for 48 h in the absence or presence of a JNK inhibitor (SP600125), a p38 inhibitor (SB203850), or two different MEK inhibitors (U0126 and PD98059). DUSP5 mRNA expression was assessed by qPCR. For qPCR analysis in B–E and G, three plates of cells were used per condition; *P < 0.05 vs. unstimulated, vehicle-treated cells.
Additional experiments were performed to address whether agonist-dependent inhibition of DUSP5 expression, and derepression by HDAC inhibitors, is specific for PE and NRVMs, or is more generalizable. Other hypertrophic agonists caused class I HDAC-dependent repression of DUSP5 expression in NRVMs, including phorbol myristate acetate (Fig. 3D), which functions intracellularly to trigger hypertrophy via PKC activation. Furthermore, HDAC inhibitor-sensitive, agonist-dependent repression of DUSP5 was noted in cultured adult rat ventricular myocytes (Fig. 3E) and in vivo in LVs of adult rats treated with norepinephrine (Fig. 3F). Currently available antibodies fail to recognize rat DUSP5 protein, necessitating the reliance on mRNA analysis for the current studies. These data suggest that DUSP5 down-regulation is a common response to stress signaling in the heart.
To begin to address the mechanism for stimulus-dependent down-regulation of DUSP5 expression, NRVMs were treated with PE in the absence or presence of various pharmacological inhibitors. As shown in Fig. 3G, inhibitors of MEK potently rescued DUSP5 mRNA expression in PE-treated cells, whereas inhibitors of JNK and p38 were without effect. These data suggest that MEK/ERK signaling promotes class I HDAC-mediated suppression of DUSP5 expression, thereby providing a self-reinforcing mechanism to sustain prohypertrophic ERK1/2 signaling in response to stress stimuli.
DUSP5 Governs Class I HDAC Inhibitor-Mediated Suppression of Nuclear ERK1/2 Signaling in Cardiac Myocytes.
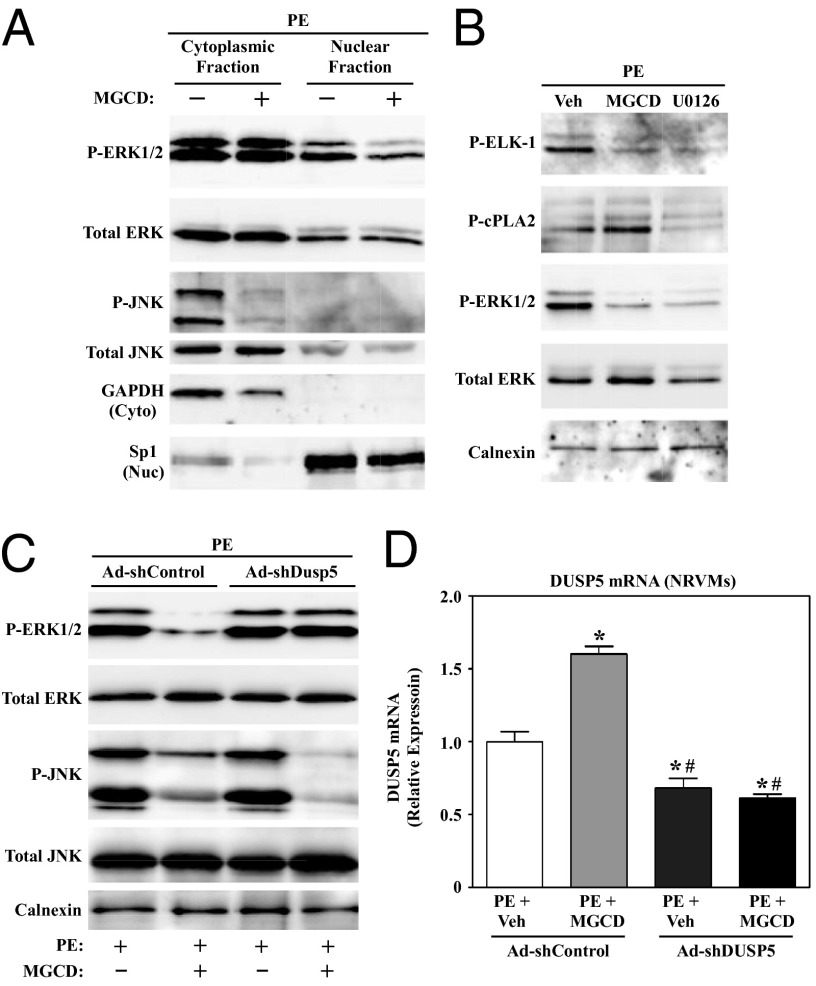
Because DUSP5 is a nuclear phosphatase, we hypothesized that class I HDAC inhibition selectively inhibits ERK1/2 signaling in cardiomyocyte nuclei. To address this possibility, fractionation studies were performed with NRVMs treated with MGCD0103. As shown in Fig. 4A, phosphorylation of the cytoplasmic pool of ERK1/2 was unaffected by the class I HDAC inhibitor. In contrast, nuclear ERK1/2 phosphorylation was attenuated by MGCD0103 treatment. MGCD0103 dramatically reduced the level of phospho-JNK, which was predominantly cytoplasmic, further establishing that class I HDACs control cardiac ERK1/2 and JNK through distinct mechanisms. Additionally, these results strongly suggest that class I HDACs specifically control nuclear ERK1/2 signaling. Consistent with this, subsequent analyses demonstrated that MGCD0103 blocked agonist-dependent phosphorylation of the nuclear ERK1/2 substrate, ETS domain-containing protein (ELK-1), but had no effect on ERK-mediated phosphorylation of cytosolic phospholipase A2 (cPLA2) (Fig. 4B).
Fig. 4.
Class I HDAC inhibition selectively blocks nuclear ERK1/2 signaling in cardiomyocytes via induction of DUSP5. (A) NRVMs were treated with PE for 48 h in the absence or presence of MGCD0103. Nuclear and cytoplasmic protein fractions were prepared and immunoblotted with the indicated antibodies. GAPDH and Sp1 served as controls to establish the purity of cytoplasmic and nuclear fractions, respectively. (B) NRVMs were left untreated or treated for 48 h with PE in the absence or presence of MGCD0103 or the MEK inhibitor, U0126. Immunoblot analysis was performed with whole-cell lysates to assess the degree of phosphorylation of a cytoplasmic ERK1/2 substrate (cPLA2) and a nuclear ERK substrate (ELK-1). (C) NRVMs were infected with adenoviruses encoding shRNA to knockdown expression of endogenous DUSP5 (Ad-shDUSP5) or scrambled negative control (Ad-shControl); multiplicity of infection (MOI) = 50 for each virus. After 24 h of infection, cells were treated with PE in the absence or presence of MGCD0103 for an additional 48 h and whole-cell lysates were analyzed by immunoblotting. (D) RNA was harvested from parallel plates of NRVMs to assess DUSP5 mRNA expression by qPCR. n = 3 plates of cells per condition. *P < 0.05 vs. PE plus vehicle in cells infected with Ad-shControl; #P < 0.05 vs. PE plus MGCD0103-treated cells infected with Ad-shControl.
These data implicate DUSP5 as a negative regulator of nuclear ERK1/2 signaling in HDAC inhibitor-treated cardiac myocytes. To further address this possibility, an experiment was performed with adenovirus encoding short hairpin RNA designed to knockdown expression of endogenous DUSP5 in NRVMs (Ad-shDUSP5). As shown in Fig. 4C, ERK1/2 phosphorylation in class I HDAC inhibitor-treated NRVMs was completely rescued by Ad-shDUSP5; DUSP5 knockdown failed to increase JNK phosphorylation in the face of HDAC inhibition. The degree of rescue of ERK1/2 phosphorylation correlated with the extent of DUSP5 knockdown by the shRNA, as determined by qPCR (Fig. 4D). Ad-shDUSP5 also modestly increased basal ERK1/2 phosphorylation in cardiac myocytes (Fig. S3). These data demonstrate that DUSP5 is responsible for HDAC inhibitor-mediated suppression of nuclear ERK1/2 signaling in cardiac myocytes.
DUSP5 Inhibits Cardiomyocyte Hypertrophy.
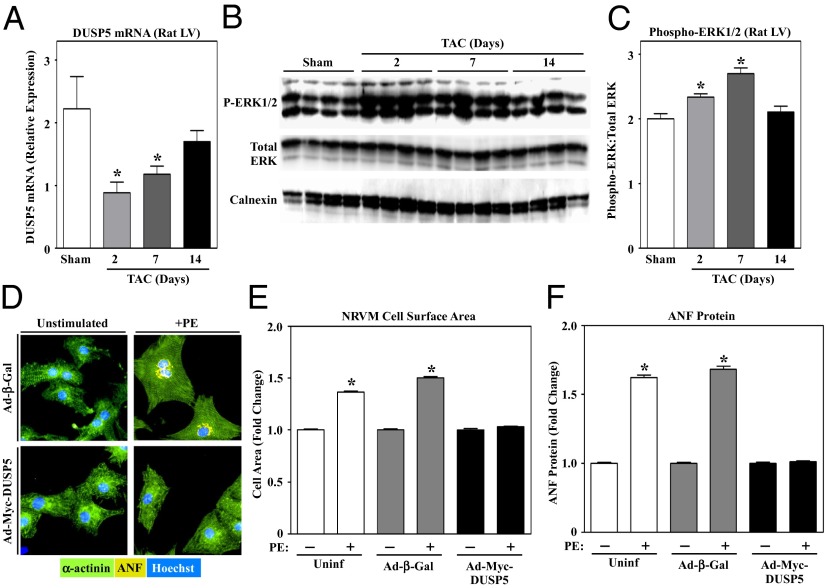
Given that ERK1/2 signaling can impact hypertrophic growth of the heart, we next assessed the potential involvement of DUSP5 in the control of cardiomyocyte hypertrophy. Consistent with data obtained using cultured cardiac myocytes (Fig. 3), DUSP5 mRNA levels were significantly decreased in rat LVs undergoing hypertrophy in response to transverse aortic constriction (TAC) (Fig. 5A). The kinetics of DUSP5 down-regulation in response to TAC correlated with the degree of ERK1/2 phosphorylation in the myocardium (Fig. 5 B and C). Next, we tested whether ectopically expressed DUSP5 is capable of altering agonist-dependent NRVM growth. Adenovirus-mediated expression of Myc-tagged DUSP5 led to dose-dependent inhibition of PE-mediated ERK1/2 phosphorylation in NRVMs (Fig. S4A). Strikingly, ectopic DUSP5 also blocked PE-mediated increases in sarcomere organization, cell area, and expression of a hypertrophic marker protein, atrial natriuretic factor (ANF) (Fig. 5 D–F). Induction of brain natriuretic peptide (BNP) and α-skeletal actin mRNA expression, as well as suppression of sarcoendoplasmic reticulum calcium ATPase (SERCA2a), are hallmark features of pathological cardiac hypertrophy. Ectopic DUSP5 completely reversed these changes in gene expression in PE-treated NRVMs (Fig. S4 B–D); DUSP5 also blocked PE-induced β- and α-myosin heavy chain isoform switching in NRVMs (Fig. S4 E and F). Consistent with these findings, Ad-shDUSP5 recused PE-mediated increases in cell size in HDAC inhibitor-treated NRVMs (Fig. S4G). Together, these data support a role for DUSP5 in the control of cardiac hypertrophy.
Fig. 5.
DUSP5 blocks cardiomyocyte hypertrophy. Adult rats were subjected to transverse aortic constriction (TAC) and killed at the indicated times postsurgery. DUSP5 mRNA expression in the LV was assessed by qPCR (A), and the ratio of phospho- to total ERK1/2 in the LV was analyzed by immunoblotting (B); ERK levels were quantified by densitometry (C). For A and C, n = 4 animals per condition; *P < 0.05 vs. rats subjected to sham surgery. (D) NRVMs were infected with adenovirus encoding β-galactosidase control (Ad-β-Gal) or Myc-tagged DUSP5 (Ad-Myc-DUSP5), each at an MOI of 1.0, and stimulated with PE for 48 h. Cells were fixed and subjected to indirect immunofluorescence to detect α-actinin (green) or ANF (yellow); nuclei were stained with Hoechst dye (blue). Cell area (E) and ANF protein expression (F) were quantified by high content imaging of NRVMs cultured on 96-well plates. n = 16 wells and >500 cells per condition; *P < 0.05 vs. unstimulated, uninfected cells.
Discussion
The findings of this study define DUSP5 as a negative regulator of cardiac hypertrophy that functions by blocking signaling of a nuclear pool of ERK1/2 in cardiomyocytes. The ability of HDAC inhibitors to stimulate DUSP5 expression and block ERK1/2 activation further explains the molecular basis of the antihypertrophic action of this compound class. We propose a model in which hypertrophic stimuli promote sustained nuclear ERK1/2 signaling by suppressing expression of DUSP5 through a class I HDAC-dependent mechanism (Fig. 6). Agonist-mediated repression of DUSP5 is also dependent on the ERK kinase, MEK, revealing a self-reinforcing mechanism for enhancing nuclear ERK1/2 signaling in response to stress stimuli. These findings establish a previously unappreciated role for HDACs in the control of cardiac MAPK signaling and a unique function for DUSP5 as a regulator of cardiac growth.
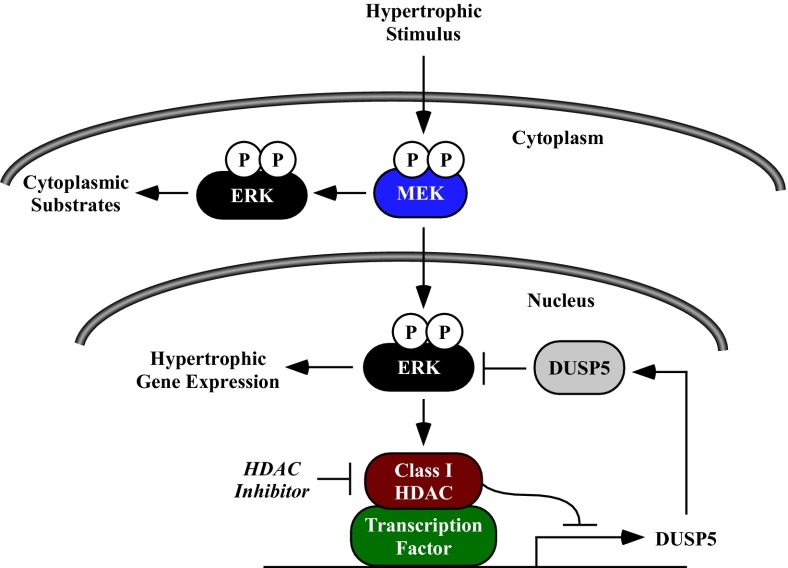
Fig. 6.
A model for the regulation of nuclear ERK1/2 signaling and cardiomyocyte hypertrophy by class I HDACs and DUSP5. Stress stimuli trigger MEK-dependent recruitment of a class I HDAC(s) to dusp5 gene regulatory elements, leading to repression of DUSP5 expression and enhanced ERK1/2 phosphorylation. Derepression of the dusp5 gene by a class I HDAC inhibitor stimulates DUSP5 expression, leading to dephosphorylation of nuclear ERK1/2 and inhibition of prohypertrophic gene expression. Because DUSP5 localizes to the nucleus, phosphorylation of cytosolic ERK1/2 substrates is preserved in HDAC inhibitor-treated cells.
Many studies have demonstrated roles for ERK1/2 in the control of cardiac hypertrophy, although the extent to which ERK-mediated hypertrophy is compensatory versus pathological remains in question (18, 19). Work by the Molkentin group has clearly shown that cardiac ERK1/2 activation through transgenic expression of activated MEK1 results in concentric cardiac hypertrophy (31). However, genetic deletion of ERK1/2 failed to block hypertrophy in response to pressure overload and instead promoted eccentric, pathological cardiac hypertrophy and myocyte apoptosis, suggesting that ERK is cardioprotective (32, 33). In contrast, studies in humans have suggested a direct correlation between ERK activation and pathological cardiac remodeling. For example, cardiac dysfunction in patients with Noonan or LEOPARD syndromes was found to be associated with augmented MEK/ERK activity (34); pharmacological inhibition of MEK in a mouse model of Noonan syndrome led to inhibition of cardiac hypertrophy and improved ventricular function (35). Additionally, dramatic decreases in LV ERK1/2 phosphorylation were observed during reverse remodeling of the human heart upon mechanical unloading with a left ventricular assist device (36).
Complexity regarding ERK1/2 in the heart is likely related to the fact that changes in the intensity, duration, and subcellular localization of ERK1/2 signaling can specify distinct biological outcomes (37). This is exemplified by work done by Lohse and colleagues, who showed that binding of G protein βγ subunits to ERK2 upon stimulation of GPCRs that couple to Gαq results in autophosphorylation and nuclear accumulation of ERK2 in cardiomyocytes (38, 39). Unlike MEK1 overexpression, which results in compensatory hypertrophy (31), transgenic expression of constitutively nuclear ERK2 leads to exaggerated pathological cardiac remodeling and contractile dysfunction in response to pressure overload (38). Additionally, expression of an autophosphorylation-resistant form of ERK2 in the heart was shown to block cardiac hypertrophy in response to β-adrenergic receptor signaling (39). These results suggest that nuclear ERK signaling leads to pathological cardiac hypertrophy, whereas phosphorylation of cytoplasmic substrates by ERK1/2 is cardioprotective. As such, strategies designed to selectively suppress nuclear ERK1/2 activity could be beneficial in the setting of heart failure. Based on our data, one approach to achieve this goal is through class I HDAC inhibitor-mediated induction of DUSP5, which possesses a strong nuclear localization signal and is highly selective for dephosphorylation of ERK versus p38 and JNK (30, 40). Validation of this approach was provided by the demonstration that class I HDAC inhibition selectively blocks nuclear ERK1/2 activation (Fig. 4 A and B), and ectopic DUSP5 potently inhibits cardiomyocyte hypertrophy (Fig. 5).
DUSPs are the largest group of MAPK phosphatases and constitute a structurally distinct family of 10 proteins characterized by a carboxyl-terminal dual-specificity phosphatase domain and an amino-terminal MAPK binding domain (41). DUSPs fall into three groups: (i) nuclear DUSPs, (ii) cytosolic ERK-specific DUSPs, and (iii) DUSPs that selectively inactivate stress-activated MAPKs (i.e., JNK and p38). Relatively little is known about the functions of DUSPs in the heart. Cardiac-specific overexpression of DUSP1 results in suppression of ERK, JNK, and p38 signaling and blunting of cardiac hypertrophy in response to pressure overload (42). Dual knockout of DUSP1 and -4 was recently shown to stimulate p38 signaling and cause cardiomyopathy (43). DUSP6 is a cytoplasmic phosphatase that is thought to specifically regulate ERK1/2. Cardiac-specific overexpression of DUSP6 was shown to increase cardiac fibrosis and apoptosis in response to pressure overload; ventricular hypertrophy was unaffected by DUSP6 overexpression (33). In contrast, dusp6 null mice were found to exhibit hypercellularity in the heart and attenuated cardiac remodeling in response to stress signaling, although dusp6 gene deletion had minimal effects on MAPK phosphorylation in the heart (33, 44). Our findings reveal a unique function for DUSP5 as an endogenous inhibitor of cardiac growth.
Class I HDAC inhibition potently suppresses agonist-dependent phosphorylation of JNK in cardiomyocytes. Several lines of evidence suggest that the mechanisms governing inhibition of ERK1/2 and JNK phosphorylation by HDAC inhibitors are distinct. For example, suppression of gene transcription (Fig. 2A) or tyrosine phosphatase activity (Fig. 2B) was sufficient to rescue phosphorylation of ERK1/2 but not JNK in HDAC inhibitor-treated cardiomyocytes. Furthermore, knockdown of endogenous DUSP5 expression in cardiomyocytes exposed to HDAC inhibitors led to complete normalization of ERK1/2 phosphorylation, while having no effect on the phosphorylation status of JNK (Fig. 4C). Based on these findings, we hypothesize that HDAC inhibitors block cardiac JNK phosphorylation by suppressing the activity of an upstream kinase.
Our data suggest that HDAC3 regulates ERK1/2 in cardiac myocytes, whereas HDAC1 and/or HDAC2 controls JNK in these cells (Fig. 1 and Fig. S1). Consistent with our findings, a prior report revealed that HDAC3 controls TGF-β–mediated phosphorylation of ERK in C3H10T1/2 cells, although the mechanism for HDAC3-mediated control of ERK in these cells was not defined (45). It is possible that HDAC3-dependent control of DUSP5 provides a generalizable mechanism for signal-dependent control of ERK1/2 in myocytes and nonmyocytes. It should be noted, however, that the degree of ERK1/2 inhibition obtained with the selective HDAC3 inhibitor, BRD3308 (Fig. 1C), and siRNA against HDAC3 (Fig. S1C), was less than that seen with MGCD0103 (Fig. 1B), suggesting the possibility of cooperative control of cardiac ERK1/2 by multiple class I HDAC isoforms.
The mechanisms by which HDACs regulate the hypertrophic response have not been fully elucidated. It has been proposed that HDAC1 and HDAC2 stimulate hypertrophy by enhancing autophagy in the heart through an undefined mechanism (46). Additionally, association of HDAC2 with the Ying Yang 1 (YY1) transcription factor has been shown to promote expression of BNP in response to a hypertrophic stimulus (47), and HDAC1 activity stimulates sodium calcium exchanger (NCX1) gene expression during cardiac hypertrophy (48). Our data demonstrate that class I HDACs prevent induction of a negative feedback loop that serves to inhibit a central prohypertrophic signaling network governed by nuclear ERK1/2. By derepressing the gene encoding DUSP5, class I HDAC inhibitors restore this feedback loop and thereby suppress cardiac hypertrophy. Future investigation to define the signaling mechanisms and epigenetic events that control class I HDACs and DUSP5 during cardiac hypertrophy will likely uncover novel pathways for the regulation of pathological cardiac remodeling, and may reveal innovative therapeutic strategies for the treatment of heart failure.
Materials and Methods
In vitro, cellular and in vivo assays are described in detail in SI Materials and Methods. Oligonucleotide sequences for DUSP5 knockdown and quantitative PCR are provided in Tables S1 and S2, respectively. HDAC inhibitors were purchased or synthesized in-house, as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank M. Cavasin, K. Demos-Davies, and A. Perry for assistance with in vivo studies; J. Mahaffey for ARVM preparation; and S. M. Keyse (Medical Research Institute, Dundee, United Kingdom) for the Myc-DUSP5 cDNA construct. B.S.F. was supported by a postdoctoral fellowship from the American Heart Association (12POST10680000) and a T32 training grant from the National Institutes of Health (5T32HL007822-12).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301509110/-/DCSupplemental.
References
- 1.Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, et al. WRITING GROUP MEMBERS American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Devereux RB, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292(19):2350–2356. doi: 10.1001/jama.292.19.2350. [DOI] [PubMed] [Google Scholar]
- 4.Gardin JM, Lauer MS. Left ventricular hypertrophy: The next treatable, silent killer? JAMA. 2004;292(19):2396–2398. doi: 10.1001/jama.292.19.2396. [DOI] [PubMed] [Google Scholar]
- 5.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: A new therapeutic target? Circulation. 2004;109(13):1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 6.Casale PN, et al. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med. 1986;105(2):173–178. doi: 10.7326/0003-4819-105-2-173. [DOI] [PubMed] [Google Scholar]
- 7.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32(5):1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 8.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114(5):345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 9.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 10.McKinsey TA, Kass DA. Small-molecule therapies for cardiac hypertrophy: Moving beneath the cell surface. Nat Rev Drug Discov. 2007;6(8):617–635. doi: 10.1038/nrd2193. [DOI] [PubMed] [Google Scholar]
- 11.Sciarretta S, Sadoshima J. New insights into the molecular phenotype of eccentric hypertrophy. J Mol Cell Cardiol. 2010;49(2):153–156. doi: 10.1016/j.yjmcc.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wettschureck N, et al. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Galphaq/Galpha11 in cardiomyocytes. Nat Med. 2001;7(11):1236–1240. doi: 10.1038/nm1101-1236. [DOI] [PubMed] [Google Scholar]
- 13.Molkentin JD, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93(2):215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra S, et al. Cardiac hypertrophy and heart failure development through Gq and CaM kinase II signaling. J Cardiovasc Pharmacol. 2010;56(6):598–603. doi: 10.1097/FJC.0b013e3181e1d263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison BC, et al. Regulation of cardiac stress signaling by protein kinase d1. Mol Cell Biol. 2006;26(10):3875–3888. doi: 10.1128/MCB.26.10.3875-3888.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q, Molkentin JD. Protein kinase Cα as a heart failure therapeutic target. J Mol Cell Cardiol. 2011;51(4):474–478. doi: 10.1016/j.yjmcc.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vega RB, et al. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24(19):8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kehat I, Molkentin JD. Extracellular signal-regulated kinase 1/2 (ERK1/2) signaling in cardiac hypertrophy. Ann N Y Acad Sci. 2010;1188:96–102. doi: 10.1111/j.1749-6632.2009.05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenz K, Schmitt JP, Vidal M, Lohse MJ. Cardiac hypertrophy: Targeting Raf/MEK/ERK1/2-signaling. Int J Biochem Cell Biol. 2009;41(12):2351–2355. doi: 10.1016/j.biocel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Choudhary C, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 21.Lundby A, et al. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep. 2012;2(2):419–431. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: Functional implications of phylogenetic analysis. J Mol Biol. 2004;338(1):17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Bush EW, McKinsey TA. Protein acetylation in the cardiorenal axis: The promise of histone deacetylase inhibitors. Circ Res. 2010;106(2):272–284. doi: 10.1161/CIRCRESAHA.109.209338. [DOI] [PubMed] [Google Scholar]
- 24.McKinsey TA. Isoform-selective HDAC inhibitors: Closing in on translational medicine for the heart. J Mol Cell Cardiol. 2011;51(4):491–496. doi: 10.1016/j.yjmcc.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Fournel M, et al. MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther. 2008;7(4):759–768. doi: 10.1158/1535-7163.MCT-07-2026. [DOI] [PubMed] [Google Scholar]
- 26.Tessier P, et al. Diphenylmethylene hydroxamic acids as selective class IIa histone deacetylase inhibitors. Bioorg Med Chem Lett. 2009;19(19):5684–5688. doi: 10.1016/j.bmcl.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Butler KV, et al. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J Am Chem Soc. 2010;132(31):10842–10846. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Methot JL, et al. Exploration of the internal cavity of histone deacetylase (HDAC) with selective HDAC1/HDAC2 inhibitors (SHI-1:2) Bioorg Med Chem Lett. 2008;18(3):973–978. doi: 10.1016/j.bmcl.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 29.Moradei OM, et al. Novel aminophenyl benzamide-type histone deacetylase inhibitors with enhanced potency and selectivity. J Med Chem. 2007;50(23):5543–5546. doi: 10.1021/jm701079h. [DOI] [PubMed] [Google Scholar]
- 30.Mandl M, Slack DN, Keyse SM. Specific inactivation and nuclear anchoring of extracellular signal-regulated kinase 2 by the inducible dual-specificity protein phosphatase DUSP5. Mol Cell Biol. 2005;25(5):1830–1845. doi: 10.1128/MCB.25.5.1830-1845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bueno OF, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19(23):6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kehat I, et al. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108(2):176–183. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purcell NH, et al. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci USA. 2007;104(35):14074–14079. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandit B, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39(8):1007–1012. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- 35.Wu X, et al. MEK-ERK pathway modulation ameliorates disease phenotypes in a mouse model of Noonan syndrome associated with the Raf1(L613V) mutation. J Clin Invest. 2011;121(3):1009–1025. doi: 10.1172/JCI44929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baba HA, et al. Dynamic regulation of MEK/Erks and Akt/GSK-3beta in human end-stage heart failure after left ventricular mechanical support: Myocardial mechanotransduction-sensitivity as a possible molecular mechanism. Cardiovasc Res. 2003;59(2):390–399. doi: 10.1016/s0008-6363(03)00393-6. [DOI] [PubMed] [Google Scholar]
- 37.Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: Mechanisms for providing signaling specificity. J Cell Sci. 2005;118(Pt 14):2997–3002. doi: 10.1242/jcs.02505. [DOI] [PubMed] [Google Scholar]
- 38.Lorenz K, Schmitt JP, Schmitteckert EM, Lohse MJ. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med. 2009;15(1):75–83. doi: 10.1038/nm.1893. [DOI] [PubMed] [Google Scholar]
- 39.Vidal M, Wieland T, Lohse MJ, Lorenz K. β-Adrenergic receptor stimulation causes cardiac hypertrophy via a Gβγ/Erk-dependent pathway. Cardiovasc Res. 2012;96(2):255–264. doi: 10.1093/cvr/cvs249. [DOI] [PubMed] [Google Scholar]
- 40.Caunt CJ, Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs): Shaping the outcome of MAP kinase signalling. FEBS J. 2013;280(2):489–504. doi: 10.1111/j.1742-4658.2012.08716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci. 2006;119(Pt 22):4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- 42.Bueno OF, et al. The dual-specificity phosphatase MKP-1 limits the cardiac hypertrophic response in vitro and in vivo. Circ Res. 2001;88(1):88–96. doi: 10.1161/01.res.88.1.88. [DOI] [PubMed] [Google Scholar]
- 43. Auger-Messier M et al. (2012) Unrestrained p38 MAPK activation in Dusp1/4 double null mice induces cardiomyopathy. Circ Res 112(1):48–56. [DOI] [PMC free article] [PubMed]
- 44.Maillet M, et al. DUSP6 (MKP3) null mice show enhanced ERK1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility. J Biol Chem. 2008;283(45):31246–31255. doi: 10.1074/jbc.M806085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barter MJ, et al. HDAC-mediated control of ERK- and PI3K-dependent TGF-β-induced extracellular matrix-regulating genes. Matrix Biol. 2010;29(7):602–612. doi: 10.1016/j.matbio.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Cao DJ, et al. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci USA. 2011;108(10):4123–4128. doi: 10.1073/pnas.1015081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glenn DJ, Wang F, Chen S, Nishimoto M, Gardner DG. Endothelin-stimulated human B-type natriuretic peptide gene expression is mediated by Yin Yang 1 in association with histone deacetylase 2. Hypertension. 2009;53(3):549–555. doi: 10.1161/HYPERTENSIONAHA.108.125088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chandrasekaran S, et al. Histone deacetylases facilitate sodium/calcium exchanger up-regulation in adult cardiomyocytes. FASEB J. 2009;23(11):3851–3864. doi: 10.1096/fj.09-132415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.