Abstract
Context:
Anti-Müllerian hormone (AMH) is an accurate marker of ovarian reserve. However, sufficiently large sets of normative data from infancy to the end of reproductive life are scarce.
Objective:
This study was an assessment of serum AMH levels in healthy females.
Subjects:
In 804 healthy females ranging from infancy until the end of the reproductive period, serum AMH levels were measured with an enzyme-linked immunometric assay. All adults had regular menstrual cycles. The majority was proven fertile and none of them had used oral contraceptive pills prior to study inclusion.
Results:
In the total cohort, AMH was inversely correlated with age (r = −0.24; P < 0.001). The age at which the maximum AMH value was attained was at 15.8 yr. In girls younger than 15.8 yr, serum AMH and age were positively correlated (r = +0.18; P = 0.007). Thereafter AMH levels remained stable (r = −0.33; P = 0.66), whereas from the age of 25.0 yr onward, an inverse correlation between AMH and age (r = −0.47; P < 0.001) was observed. At any given age, considerable interindividual differences in serum AMH levels were observed.
Conclusion:
During infancy AMH levels increase, whereas during adolescence, a plateau until the age of 25 yr was observed. From the age of 25 yr onward, serum AMH levels correlate inversely with age, implying that AMH is applicable as a marker of ovarian reserve only in women of 25 yr old and older. Our nomogram may facilitate counseling women on their reproductive potential.
Serum anti-Müllerian hormone (AMH) is solely produced in the human ovary by granulosa cells (1). AMH expression starts in primary follicles as soon as recruitment from the primordial follicle pool is initiated, until the early antral stage during which expression is strongest (1–3). Expression ceases in follicles with a diameter between 8 and 10 mm (1). Based on this expression pattern, serum AMH was suggested to reflect the number of early growing follicles (1). Indeed, in mice, serum AMH levels correlated strongly with the number of small growing follicles and, more importantly, with the number of primordial follicles (4). Although direct assessment of the primordial follicle pool in women is difficult, one study using oophorectomy for benign gynecological reasons confirmed the correlation between serum AMH and primordial follicle number in women (5). In addition, several clinical studies have confirmed the strong correlation between serum AMH and antral follicle count (AFC) (6–8). Serum AMH reflects the cohort of small growing follicles and therefore constitutes a proxy for the size of the primordial follicle pool (6). Furthermore, it was shown that AMH can be used as a marker of diminished ovarian reserve (6, 8, 9). Similar to AFC, AMH has been shown to be a better predictor of poor response to controlled ovarian hyperstimulation than age, serum FSH, or serum inhibin B concentrations (10–12).
In addition to being a marker of ovarian reserve, AMH has also been described as marker for the excess of growing follicles in normogonadotropic normoestrogenic anovulatory infertility and polycystic ovary syndrome (PCOS). As compared with women with regular menstrual cycles, 2- to 3-fold higher AMH levels have been observed in women with PCOS (13, 14). Although serum AMH is used as a marker for ovarian function in clinical practice, data on the range of AMH levels in the normal population are scarce, and thus, information on cutoff levels of the normal range is still lacking. Recently several studies have aimed to fill this gap in knowledge (5, 12, 15–20). Although large cohorts were studied, the vast majority of women included in these studies were recruited at fertility clinics (12, 15, 17, 18, 20) and may therefore not represent normoovulatory women. The present study is unique, by measuring serum AMH levels in a large cohort of healthy females, ranging from infancy to the end of reproductive lifespan, in a single laboratory. In addition, most of the described women of reproductive age were proven fertile.
Materials and Methods
Subjects
Healthy children, adolescents, and adult women were included. Most subjects had participated in earlier studies as healthy control subjects. For detailed information on recruitment strategy and study populations, we refer to the original papers (6, 8, 21–25). All studies had been previously approved by the different local ethical review boards. All subjects had given written informed consent. Healthy girls aged up to 18 yr were recruited at local primary and secondary schools or at the Sophia Children's Hospital before minor surgery. Adults (aged 18 yr and older) had regular menstrual cycles between 25 and 35 d or were proven fertile. Adults did not use any hormones or oral contraceptive pills during the last 3 months before participation. Proven fertile women had given birth at least 6 months earlier (24). Serum was drawn randomly during the menstrual cycle. In those adult women screened during the early follicular phase, serum FSH was also measured. In adults, transvaginal ultrasound was performed on cycle d 3, 4, or 5 to assess AFC.
Hormone assays
All serum measurements were performed at the diagnostic endocrine laboratory at the Erasmus Medical Center (Rotterdam, The Netherlands) (by F.H.d.J., Y.B.d.R.). All samples had been stored at −20 C until assayed. AMH immunoreactivity in serum samples was stable after repeated freeze-thaw cycles (4). In the older cohorts (6, 8, 25), AMH was measured with an ELISA (Immunotech-Coulter, Marseilles, France) (6) and an in-house ELISA (commercially available as the GenII Beckman Coulter, Beckman Coulter, Inc., Webster, TX) was used in study subjects, in whom serum was drawn more recently (4). The standard curve of the Immunotech-Coulter assay ranged from 0.7 to 175 pmol/liter (0.1–24.5 ng/ml). The range of the AMH standards used in the in-house assay was from 0.037 to 5 ng/ml. To construct a nomogram of normal AMH levels, the values obtained from both assays need to be comparable. Consequently, values obtained with the Immunotech assay were adjusted with a factor 1.564 to be comparable with values measured with the currently used ELISA. Intra- and interassay coefficients of variation in the Immunotech-Coulter assay were less than 5% and 8% and less than 5% and less than 10% in the in-house ELISA. The detection limit of the currently used ELISA, defined as the mean of the absorbance of the blank replicates +2 sd, was 6.3 pg/ml. For analysis, serum AMH levels below 0.1 μg/liter were valued at 0.0 μg/liter. FSH was measured with a chemoluminescence-based immunometric assay (Immulite 2000; Siemens, Los Angeles, CA), with intra- and interassay coefficients of variance less than 3% and 8%.
Data analysis
Because samples were obtained from subjects who had participated in different studies, AMH levels were compared between the included study cohorts with a univariate analysis of covariance with adjustment for age. Because serum was drawn across the follicular and luteal phase of the menstrual cycle, a similar test was used to compare AMH levels drawn during the follicular phase with those drawn during the luteal phase.
Reference curves for serum AMH levels as function of age were calculated from a linear regression model using a natural cubic spline fitted on log-transformed AMH values. From the fitted curve, a piecewise linear function was calculated to describe variation in AMH levels with increasing age. In addition, we determined the age at which AMH values attained their maximum value, and the 95% confidence interval of this age was determined by bootstrapping 5000 times. Consequently, two subgroups could be distinguished: one with increasing AMH levels and a subgroup with decreasing AMH levels. In the total cohort as well as in subgroups, bivariate correlations were performed between serum AMH and age. In addition, a bivariate correlation was performed between serum AMH levels and the number of follicles. This correlation was also performed between serum AMH levels and FSH levels in samples drawn during the early follicular phase.
Results
A total of 804 healthy infants, children, adolescents, and adult women were included. The median age was 24.6 yr. The youngest subject was 3.1 months old and the oldest was 46.8 yr old. Measurement of AMH concentrations using the two AMH ELISAs did not result in significant differences [median 1.6 (range 0.0–15.5) vs. 3.3 (range 0.0–18.3) μg/liter; P = 0.11] (6). There were no differences in AMH levels drawn during the follicular phase and those measured during the luteal phase after adjustment for age differences (median 1.9 vs. 3.3 μg/liter, respectively; P = 0.08).
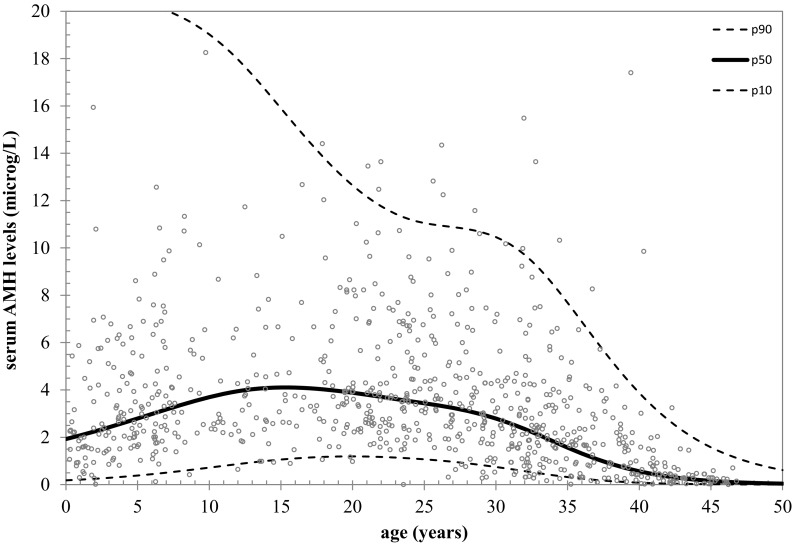
In the total cohort, serum AMH levels were inversely correlated with age (r = −0.24; P < 0.001). The natural cubic spline function explained 41% of the variance in log(AMH), indicating that variance in AMH was 41% determined by age. The fitted curve showed that, from birth onward, serum AMH levels increased slightly until the end of puberty and seemed to remain rather constant until levels decreased from the age of 25.0 yr onward (Figs. 1 and 2). Using a piecewise linear function, the rise in the AMH levels during childhood and the attenuation in AMH during adolescence did not reach statistical significance (data not shown). The age at which the maximum value of AMH was attained was estimated as 15.8 yr (95% confidence interval 13.5–20.3). Before the age of 15.8 yr, serum AMH and age were positively correlated (r = +0.18; P = 0.007). Thereafter AMH levels remained stable, and consequently, the correlation between AMH and age did not reach statistical significance (r = −0.33; P = 0.66). From the age of 25.0 yr, there was an inverse correlation between AMH and age (r = −0.47; P < 0.001) (Fig. 2).
Fig. 1.
AMH nomogram based on natural linear spline interpolation. Reference lines of serum AMH for the 10th, 50th, and 90th percentiles of predicted AMH values vs. age.
Fig. 2.
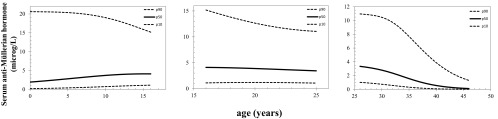
Serum AMH levels (micrograms per liter) per subgroup based on age. Reference lines of serum AMH for the 10th, 50th, and 90th percentiles of predicted AMH values vs. age in subgroup 0–15.8 yr (left panel), 16–25 yr (middle panel), and in women older than 25 yr (right panel).
Antral follicle counts were not available in girls younger than 18 yr. In 465 women, aged 18 yr and older, antral follicle counts were available. The median antral follicle count was 13 (range 0–48). Serum AMH and antral follicle count were positively correlated (r = +0.67; P < 0.001). In 253 adults, serum was drawn during the early follicular phase and the median FSH was 6.4 IU/liter (range 2.0–35.9 IU/liter). Serum AMH and FSH levels were negatively correlated (r = −0.29; P < 0.001).
Discussion
In this study, serum AMH levels were assessed in healthy females from birth to the end of reproductive life in one laboratory. Our data clearly indicate that the correlation between serum AMH levels and age throughout childhood is different from that during the reproductive life span. Serum AMH levels slightly increased during childhood until the midteens and reached a maximum at the age of 15.8 yr. Thereafter AMH levels remained stable until the early 20s, and, from the age of 25 yr, AMH levels decreased with increasing age. Recently a study in healthy children has described increasing AMH levels during early childhood and thereafter stable AMH concentrations until early adulthood (16). These findings were confirmed in a nomogram based on previously described AMH levels in children and adults (19). In addition, the authors concluded that the variation in AMH concentrations was mainly determined by factors other than age (19). Indeed, in our data the variance in AMH was for 41% determined by age, and hence, variation in AMH concentrations may also be determined by the variation in the number of AMH-producing follicles or by genetic predisposition.
Our data support the earlier described correlation between serum AMH levels and the decreasing number of antral follicles in women and rodents (4, 6). In an earlier study, it was shown that a quadratic equation was the best model to describe declining serum AMH values with increasing age (17). In our data, a quadratic model could not fit the results of our total cohort. However, when the quadratic equation was applied to AMH levels in women aged 25 yr and older, a similar curve was obtained as obtained with the natural linear spline interpolation model (data not shown). Hence, our results suggest that AMH is applicable only as a marker of the decline in ovarian reserve in women from 25 yr onward.
As described in the previously mentioned studies (16, 19), we observed a large interindividual variation in AMH levels. In the general population, up to 10% of women of reproductive age are affected by PCOS (26, 27), which is characterized by increased AMH levels (13, 14). In the current study, only women with regular cycles were included, thus excluding women with oligoanovulation, one of the major PCOS characteristics (27). In addition, most women had conceived spontaneously. Nevertheless, women with hyperandrogenism and polycystic ovarian morphology, and thus with PCOS according to the Rotterdam consensus criteria, might also have been included and might cause some of the variation in AMH levels at the upper limit of normal values. However, the incidence of PCOS women with hyperandrogenism and polycystic ovarian morphology in our study cohort was estimated to be less than 3% (27–29) and was therefore considered too low to explain the observed range in AMH levels. Furthermore, similar ranges in AMH levels have been described in a large population of infertile women, with normal ovarian morphology and proven ovulation (30, 31), and in healthy volunteers with regular menstrual cycles and no history of either infertility or hyperandrogenism (5).
Most likely, the variation in AMH levels may reflect the range in reproductive capacity and age of menopause, which shows a similar large variation in normal women, ranging from 40 to 60 yr (32). Indeed, it has been shown that the age of menopause was more accurately predicted by serum AMH concentrations than by chronological age (33). Recent longitudinal cohort studies showed that serum AMH levels drawn approximately 10 yr earlier were highly predictive of the age of menopausal transition (34, 35), suggesting that AMH is capable of predicting age at menopause for a given woman. Hence, it may be proposed that at any age, women with AMH at the upper limit of the normal range will enter menopause at a later age compared with women with AMH levels at the lower limit of the normal range. However, the mean age of women included in those cohort studies was 35 and 41 yr, at which the interindividual variation in AMH is smaller than at younger ages, as shown in our data. Consequently, a prediction of age of menopause based on AMH levels measured at a young age has to be interpreted with caution because such a prediction may be less accurate. Furthermore, it remains unknown whether follicle loss follows a similar rate in all women and whether the initial number of primordial follicles, endowed during early fetal life, is similar in all women. Additional studies with long-term follow-up of frequently measured AMH levels in young women are needed to confirm whether AMH indeed can predict age of menopausal transition.
Recent studies in regularly cycling women have remained inconclusive on the relation between serum AMH levels and FSH levels (6, 36–38). In our results, serum AMH and FSH levels in women older than 25 yr were negatively correlated, despite a large interindividual variation of both hormones. In addition, the inverse correlation between AMH and age was stronger than that between FSH and age, suggesting that AMH is a more accurate marker of ovarian ageing than FSH (6).
In conclusion, our results confirm and expand earlier findings of AMH levels across the reproductive life span. We demonstrated increasing AMH levels during childhood until a maximum is reached at the age of 15.8 yr. Thereafter a plateau until the age of 25 yr was observed. From the age of 25 yr onward, serum AMH levels correlate inversely with age, implying that AMH is applicable as marker of ovarian reserve only in women aged 25 yr and older. Therefore, our data suggest that follicular dynamics during childhood might be different from that at adult age. The nomogram based on our data may facilitate counseling of women on their reproductive potential.
Acknowledgments
The authors had the following roles in the writing of this report: S.L.F. is responsible for the study design, analysis, and manuscript drafting; J.A.V., I.S., and J.S.E.L. are responsible for the study design, data collection, manuscript drafting, critical discussion, and review; C.K.W., A.C.S.H.-K., E.M.R., W.H.M.P., F.J.B., and B.C.J.M.F. are responsible for the data collection, critical discussion, and review; A.P.N.T., Y.B.d.R., and F.H.d.J. are responsible for the hormone measurements, technical support, and critical review; and M.J.C.E. is responsible for the statistical analysis.
Disclosure Summary: A.C.S.H.-K. receives financial support from Pfizer and Novo Nordisk to carry out investigator-initiated clinical trial; B.C.J.M.F. has received fees and grant support from the following companies: Andromed, Ardana, Ferring, Merck Serono, Organon, Pantharei Bioscience, PregLem, Schering Plough, Schering, Serono, and Wyeth; A.P.N.T. consults for ANSHlabs; and J.S.E.L. has received fees and grant support from the following companies: Ferring, Genovum, Merck-Serono, MSD, Organon, Schering-Plough, and Serono.
Footnotes
- AFC
- Antral follicle count
- AMH
- anti-Müllerian hormone
- PCOS
- polycystic ovary syndrome.
References
- 1. Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. 2004. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 10:77–83 [DOI] [PubMed] [Google Scholar]
- 2. Andersen CY, Byskov AG. 2006. Estradiol and regulation of anti-Mullerian hormone, inhibin-A, and inhibin-B secretion: analysis of small antral and preovulatory human follicles' fluid. J Clin Endocrinol Metab 91:4064–4069 [DOI] [PubMed] [Google Scholar]
- 3. Durlinger AL, Visser JA, Themmen AP. 2002. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction 124:601–609 [DOI] [PubMed] [Google Scholar]
- 4. Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP, Themmen AP, Visser JA. 2006. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology 147:3228–3234 [DOI] [PubMed] [Google Scholar]
- 5. La Marca A, Spada E, Grisendi V, Argento C, Papaleo E, Milani S, Volpe A. 2012. Normal serum anti-Mullerian hormone levels in the general female population and the relationship with reproductive history. Eur J Obstet Gynecol Reprod Biol 163:180–184 [DOI] [PubMed] [Google Scholar]
- 6. de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. 2002. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril 77:357–362 [DOI] [PubMed] [Google Scholar]
- 7. Pigny P, Jonard S, Robert Y, Dewailly D. 2006. Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab 91:941–945 [DOI] [PubMed] [Google Scholar]
- 8. van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, Themmen AP. 2002. Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod 17:3065–3071 [DOI] [PubMed] [Google Scholar]
- 9. Lie Fong S, Baart EB, Martini E, Schipper I, Visser JA, Themmen AP, de Jong FH, Fauser BJ, Laven JS. 2008. Anti-Mullerian hormone: a marker for oocyte quantity, oocyte quality and embryo quality? Reprod Biomed Online 16:664–670 [DOI] [PubMed] [Google Scholar]
- 10. Fanchin R, Schonäuer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. 2003. Serum anti-Mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod 18:323–327 [DOI] [PubMed] [Google Scholar]
- 11. van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, Fauser BJ, Themmen AP, te Velde ER. 2005. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril 83:979–987 [DOI] [PubMed] [Google Scholar]
- 12. Broer SL, Dólleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJ. 2011. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update 17:46–54 [DOI] [PubMed] [Google Scholar]
- 13. Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC. 2004. Anti-Mullerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab 89:318–323 [DOI] [PubMed] [Google Scholar]
- 14. Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, Dewailly D. 2003. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab 88:5957–5962 [DOI] [PubMed] [Google Scholar]
- 15. Almog B, Shehata F, Suissa S, Holzer H, Shalom-Paz E, La Marca A, Muttukrishna S, Blazar A, Hackett R, Nelson SM, Cunha-Filho JS, Eldar-Geva T, Margalioth EJ, Raine-Fenning N, Jayaprakasan K, McIlveen M, Wunder D, Freour T, Nardo LG, Balasch J, Peñarrubia J, Smeenk J, Gnoth C, Godehardt E, Lee TH, Lee MS, Levin I, Gamzu R, Tulandi T. 2011. Age-related normograms of serum antimullerian hormone levels in a population of infertile women: a multicenter study. Fertil Steril 95:2359–2363, 2363.e2351 [DOI] [PubMed] [Google Scholar]
- 16. Hagen CP, Aksglaede L, Sørensen K, Main KM, Boas M, Cleemann L, Holm K, Gravholt CH, Andersson AM, Pedersen AT, Petersen JH, Linneberg A, Kjaergaard S, Juul A. 2010. Serum levels of anti-Mullerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab 95:5003–5010 [DOI] [PubMed] [Google Scholar]
- 17. Broekmans FJ, Visser JA, Laven JS, Broer SL, Themmen AP, Fauser BC. 2008. Anti-Mullerian hormone and ovarian dysfunction. Trends Endocrinol Metab 19:340–347 [DOI] [PubMed] [Google Scholar]
- 18. Broer SL, Mol BW, Hendriks D, Broekmans FJ. 2009. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril 91:705–714 [DOI] [PubMed] [Google Scholar]
- 19. Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. 2011. A validated model of serum anti-mullerian hormone from conception to menopause. PLoS One 6:e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee JY, Jee BC, Lee JR, Kim CH, Park T, Yeon BR, Seo SY, Lee WD, Suh CS, Kim SH. 2012. Age-related distributions of anti-Mullerian hormone level and anti-Mullerian hormone models. Acta Obstet Gynecol Scand 91:970–975 [DOI] [PubMed] [Google Scholar]
- 21. Adams JM, Taylor AE, Crowley WF, Jr, Hall JE. 2004. Polycystic ovarian morphology with regular ovulatory cycles: insights into the pathophysiology of polycystic ovarian syndrome. J Clin Endocrinol Metab 89:4343–4350 [DOI] [PubMed] [Google Scholar]
- 22. Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega AC. 2008. Fat mass and apolipoprotein E genotype influence serum lipoprotein levels in early adulthood, whereas birth size does not. J Clin Endocrinol Metab 93:4307–4314 [DOI] [PubMed] [Google Scholar]
- 23. Murphy MK, Hall JE, Adams JM, Lee H, Welt CK. 2006. Polycystic ovarian morphology in normal women does not predict the development of polycystic ovary syndrome. J Clin Endocrinol Metab 91:3878–3884 [DOI] [PubMed] [Google Scholar]
- 24. Roes EM, Sieben R, Raijmakers MT, Peters WH, Steegers EA. 2005. Severe preeclampsia is associated with a positive family history of hypertension and hypercholesterolemia. Hypertens Pregnancy 24:259–271 [DOI] [PubMed] [Google Scholar]
- 25. Scheffer GJ, Broekmans FJ, Looman CW, Blankenstein M, Fauser BC, teJong FH, teVelde ER. 2003. The number of antral follicles in normal women with proven fertility is the best reflection of reproductive age. Hum Reprod 18:700–706 [DOI] [PubMed] [Google Scholar]
- 26. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF. 2009. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91:456–488 [DOI] [PubMed] [Google Scholar]
- 27. Broekmans FJ, Knauff EA, Valkenburg O, Laven JS, Eijkemans MJ, Fauser BC. 2006. PCOS according to the Rotterdam consensus criteria: change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG 113:1210–1217 [DOI] [PubMed] [Google Scholar]
- 28. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. 2010. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 25:544–551 [DOI] [PubMed] [Google Scholar]
- 29. Nidhi R, Padmalatha V, Nagarathna R, Amritanshu R. 2011. Prevalence of polycystic ovarian syndrome in Indian adolescents. J Pediatr Adolesc Gynecol 24:223–227 [DOI] [PubMed] [Google Scholar]
- 30. Nelson SM, Messow MC, Wallace AM, Fleming R, McConnachie A. 2011. Nomogram for the decline in serum antimullerian hormone: a population study of 9,601 infertility patients. Fertil Steril 95:736–741.e1–3 [DOI] [PubMed] [Google Scholar]
- 31. Shebl O, Ebner T, Sir A, Schreier-Lechner E, Mayer RB, Tews G, Sommergruber M. 2011. Age-related distribution of basal serum AMH level in women of reproductive age and a presumably healthy cohort. Fertil Steril 95:832–834 [DOI] [PubMed] [Google Scholar]
- 32. te Velde ER, Pearson PL. 2002. The variability of female reproductive ageing. Hum Reprod Update 8:141–154 [DOI] [PubMed] [Google Scholar]
- 33. van Disseldorp J, Faddy MJ, Themmen AP, de Jong FH, Peeters PH, van der Schouw YT, Broekmans FJ. 2008. Relationship of serum antimullerian hormone concentration to age at menopause. J Clin Endocrinol Metab 93:2129–2134 [DOI] [PubMed] [Google Scholar]
- 34. Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP, Laven JS, de Jong FH, Te Velde ER, Fauser BC, Broekmans FJ. 2011. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab 96:2532–2539 [DOI] [PubMed] [Google Scholar]
- 35. Freeman EW, Sammel MD, Lin H, Gracia CR. 2012. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab 97:1673–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rashidi BH, Abediasl Z, Tehraninejad ES, Shariat M, Mahdavi A. 2009. Menstrual cycle length in relation to antimullerian hormone and follicle-stimulating hormone. J Reprod Med 54:315–318 [PubMed] [Google Scholar]
- 37. Singer T, Barad DH, Weghofer A, Gleicher N. 2009. Correlation of antimullerian hormone and baseline follicle-stimulating hormone levels. Fertil Steril 91:2616–2619 [DOI] [PubMed] [Google Scholar]
- 38. Wachs DS, Coffler MS, Malcom PJ, Chang RJ. 2007. Serum anti-mullerian hormone concentrations are not altered by acute administration of follicle stimulating hormone in polycystic ovary syndrome and normal women. J Clin Endocrinol Metab 92:1871–1874 [DOI] [PubMed] [Google Scholar]