Abstract
The bone-resorbing osteoclast is essential for skeletal remodeling, yet its deregulation contributes to diseases such as osteoporosis and cancer bone metastasis. Here we identify histone deacetylase 7 (HDAC7) as a key negative regulator of osteoclastogenesis and bone resorption using both in vitro cellular and molecular analyses and in vivo characterization of conditional HDAC7-knockout mice. Bone marrow osteoclast differentiation assays reveal that HDAC7 overexpression suppresses, whereas HDAC7 deletion enhances, osteoclastogenesis. Mechanistically, in the absence of receptor activator of nuclear factor κ-B ligand (RANKL), HDAC7 attenuates β-catenin function and cyclin D1 expression, thereby reducing precursor proliferation; upon RANKL activation, HDAC7 suppresses NFATc1 and prevents β-catenin down-regulation, thereby blocking osteoclast differentiation. Consequently, HDAC7 deletion in the osteoclast lineage results in a 26% reduction in bone mass (P = 0.003) owing to 102% elevated bone resorption (P = 0.01). These findings are clinically significant in light of the remarkable therapeutic potentials of HDAC inhibitors for several diseases such as cancer, diabetes, and neurodegeneration.
Skeletal homeostasis is controlled by the delicate balance between osteoclast-mediated bone resorption and osteoblast-mediated bone formation. Osteoclasts are multinucleated cells differentiated from myeloid progenitors by mainly two cytokines, macrophage colony-stimulating factor (MCSF) and receptor activator of nuclear factor κ-B ligand (RANKL) (1–3). RANKL induces the expression of key transcription factors such as NFATc1 and c-fos that are essential for osteoclast differentiation and maturation (4, 5). Osteoclasts are required for normal functions such as bone remodeling and fracture repair. However, excessive osteoclast activity can lead to several disorders such as osteoporosis, arthritis, and cancer bone metastasis. Therefore, discovery of novel mechanisms underlying the regulation of osteoclastogenesis is pivotal to enhance our understanding of bone physiology, and the identification of key modulators of bone resorption may reveal new therapeutic targets for the prevention and treatment of bone diseases.
Wnt/β-catenin signaling is a key regulator of skeletal physiology. This pathway is clinically important because neutralizing antibodies against Wnt antagonists are promising new drugs for bone diseases (6–11). Previous studies have shown that activation of Wnt/β-catenin signaling can increase osteoblast-mediated bone formation and modulate osteoblast functions (12–15). Our recent study has identified β-catenin as a novel and critical regulator of osteoclast differentiation and bone resorption (16). β-Catenin protein and its target gene cyclin D1 are induced during the MCSF-mediated precursor proliferation but must be down-regulated during RANKL-mediated osteoclast differentiation; β-catenin constitutive activation blocks, whereas β-catenin dosage reduction enhances, osteoclast differentiation (16, 17). However, the upstream factors that govern β-catenin regulation in this process are unknown.
Histone deacetylases (HDACs) are important regulators of a wide variety of biological processes, such as muscle differentiation and neuronal survival, by deacetylating both histone and nonhistone proteins (18). Based on structural and functional similarities, the 18 HDACs in the human genome are classified into four groups. Class I HDACs (HDACs 1, 2, 3, and 8) are broadly expressed and represent the major enzymes for histone deacetylation. In contrast, class II HDACs (HDACs 4–7, 9, and 10) are expressed in a more tissue-restricted manner and appear to have less contribution to histone deacetylation. Class III HDACs (Sirtuins 1–7) require reduced nicotinamide adenine dinucleotide for enzymatic activity. Class IV HDAC (HDAC11) is still poorly understood (19).
HDACs are also emerging as key regulators of skeletal homeostasis (19). Several in vitro studies report that osteoclast differentiation can be suppressed by HDAC inhibitors (20–24), which are broad-spectrum compounds that target several HDAC members (18). Nonetheless, the specific physiological roles of individual HDAC in bone, particularly in osteoclasts, are largely unknown (19). This is an important question because HDAC inhibitors, many of which are currently in clinical trials, hold tremendous therapeutic potential for numerous diseases such as cancer, diabetes, and neurodegeneration (25, 26).
HDAC7 is a member of the class IIa HDACs. In vitro studies suggest that HDAC7 regulates the maturation of both osteoclast and osteoblast (27, 28). However, whether HDAC7 is a physiologically relevant regulator of osteoclastogenesis and bone resorption in vivo is still an open question, and the mechanisms underlying potential HDAC7 regulation of osteoclast is unclear. In this study, by generating two new mouse genetic models that target HDAC7 in the osteoclast lineage, we reveal a critical role of HDAC7 in suppressing osteoclastogenesis and bone resorption via a novel molecular mechanism that involves the dual regulation of β-catenin and NFATc1.
Materials and Methods
Mice
HDAC7 flox mice (29) and peroxisome proliferator-activated receptor γ (PPARγ)-tTA;TRE-cre mice (16, 30, 31) were previously described. Lysozyme-cre mice (32) were purchased from The Jackson Laboratory (Bar Harbor, ME). All experiments were performed with littermates. All protocols for mouse experiments were approved by the Institutional Animal Care and Use Committee of University of Texas Southwestern Medical Center.
Bone analyses
Micro-computed tomography (μCT) was performed on tibias to evaluate bone volume and architecture using a Scanco μCT-35 instrument (SCANCO Medical, Wayne, PA) as previously described (33, 34). Histomorphometry of femoral sections was conducted using the BIOQUANT Image Analysis software (Bioquant, Nashville, TN). Tartrate-resistant acid phosphatase (TRAP) staining of osteoclasts was performed using a Leukocyte Acid Phosphatase staining kit (Sigma, St. Louis, MO). As a bone resorption marker, serum CTX-1 was measured with the RatLaps enzyme immunoassay kit (Immunodiagnostic Systems, Fountain Hills, AZ) (33). As a bone formation marker, serum amino-terminal propeptide of type I collagen (P1NP) was measured with the Rat/Mouse P1NP enzyme immunoassay kit (Immunodiagnostic Systems) (33).
Ex vivo bone marrow osteoclast differentiation
Osteoclasts were differentiated from mouse bone marrow cells as described previously (16, 35). Briefly, hematopoietic bone marrow cells were purified with a 40-μm cell strainer to remove mesenchymal cells, and differentiated with 40 ng/ml of MCSF (R&D systems, Minneapolis, MN) in α-MEM containing 10% FBS for 3 d and then with 40 ng/ml of MCSF and 100ng/ml of RANKL (R&D systems) for 3 d (for mRNA analysis) or 9–12 d (for TRAP staining), in the presence or absence of 1 μm rosiglitazone (Cayman Chemical, Ann Arbor, MI). Mature osteoclasts were identified as multinucleated (>3 nuclei) TRAP+ cells. Osteoclast differentiation was quantified by the RNA expression of RANKL-induced transcription factors and osteoclast function genes using quantitative RT-PCR analysis. For HDAC7 transfection, bone marrow cells were cultured with MCSF for 2 d, and the attached cells were transfected with HDAC7 expression plasmid or vector control with FuGENE HD (Roche, Indianapolis, IN) for 24 h before changing to medium containing MCSF and RANKL for osteoclast differentiation.
Osteoclast precursor proliferation assay
Osteoclast precursor proliferation was quantified using a bromodeoxyuridine (BrdU) Cell Proliferation Assay Kit (GE Healthcare Life Sciences, Piscataway, NJ) (16, 36). Mouse bone marrow cells were treated with MCSF (40 ng/ml) for 1 or 3 d. The cells were MCSF-starved for 6 h, and then restimulated with MCSF for 4 h to induce S phase, during which BrdU was provided in the culture medium. Cell proliferation was quantified as BrdU incorporation using the BrdU ELISA assay in the kit.
Gene expression analyses
RNA expression was analyzed by quantitative RT-PCR. RNA was reverse transcribed into cDNA using an ABI High Capacity cDNA RT Kit, and analyzed using real-time quantitative PCR (SYBR Green) in triplicate. All RNA expression was normalized by L19. Genes analyzed and PCR primer sequences are listed below. Protein expression was analyzed by Western blot using whole-cell extract or nuclear extract. The following antibodies were used: anti-β-catenin and anticyclin D1 (BD Biosciences, Palo Alto, CA); anti-HDAC7 (Abcam, Inc., Cambridge, MA) and anti-β-actin (Sigma).
HDAC7a-forward (F) CTCGGCTGAGGACCTAGAGA
HDAC7a-reverse (R) CAGAGAAATGGAGCCTCTGC
NFATc1-F AGTCTCTTTCCCCGACATCA
NFATc1-R GATCCGAAGCTCGTATGGAC
c-fos-F AAGTATGCCCACACCAACTGATC
c-fos-R GAAAGCCCGTTCCCAAGAAA
TRAP-F AAGTATGCCCACACCAACTGATC
TRAP-R GAAAGCCCGTTCCCAAGAAA
β-catenin-F AGCTGATATTGACGGGCAGTATG
β-catenin-R GCCAAGCGCTGGACATTAGT
CyclinD1-F TCCCTAGCAAGCTGCCAAAC
CyclinD1-R TGGACCCACCACCAGTCTATG
CTSK-F AGCAGGCTGGAGGACTAAGGT
CTSK-R GATTTGTGCATCTCAGTGGAAGAC
CAR2-F TGGTCAACTTAGGGCATCTTTTC
CAR2-R TCCTATGGCTGTGAAGAGAAGCA
MMP-9-F CCAAGGGTACAGCCTGTTCCT
MMP-9-R GCACGCTGGAATGATCTAAGC
Atp6v0d2-F GACCAACACACCTTCTCAACCA
Atp6v0d2-R GCCACAAAATACCTGAAACAACAG
Clcn7-F TCTGTCAGGGAACCCACATGT
Clcn7-R GGGCAGATGTTGGTGAATTCA
Transient transfection and reporter analysis
To quantify β-catenin activity, human embryonic kidney (HEK)293 cells were transfected with a TOP-flash luciferase reporter, or FOP-flash negative control, together with a cytomegalovirus-β-gal reporter (as internal control for transfection efficiency), as well as expression plasmids for β-catenin-Δex3, HDAC7, NFATc1, or vector control. To quantify NFATc1 activity, HEK293 cells were transfected with an NFATc1-luciferase reporter (Stratagene, La Jolla, CA), together with a cytomegalovirus-β-gal reporter, as well as expression plasmids for NFATc1, HDAC7, or vector control. HDAC7 plasmid was generously provided by Dr. Eric Olson (University of Texas Southwestern). β-Catenin-Δex3 plasmid was generously provided by Dr. Chi Zhang (Texas Scottish Rite Hospital for Children). NFATc1 plasmid was purchased from Open Biosystems (Huntsville, AL). All transfection was performed using FuGENE HD (Roche) (n = 6) and repeated for at least three times. Reporter assays were conducted 48 h after transfection, and luciferase activity was normalized by β-gal activity.
Statistical analyses
Statistical analyses were performed with Student's t test and represented as mean ± sd; *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001; n.s., nonsignificant, P > 0.05.
Results
HDAC7 expression during osteoclast differentiation
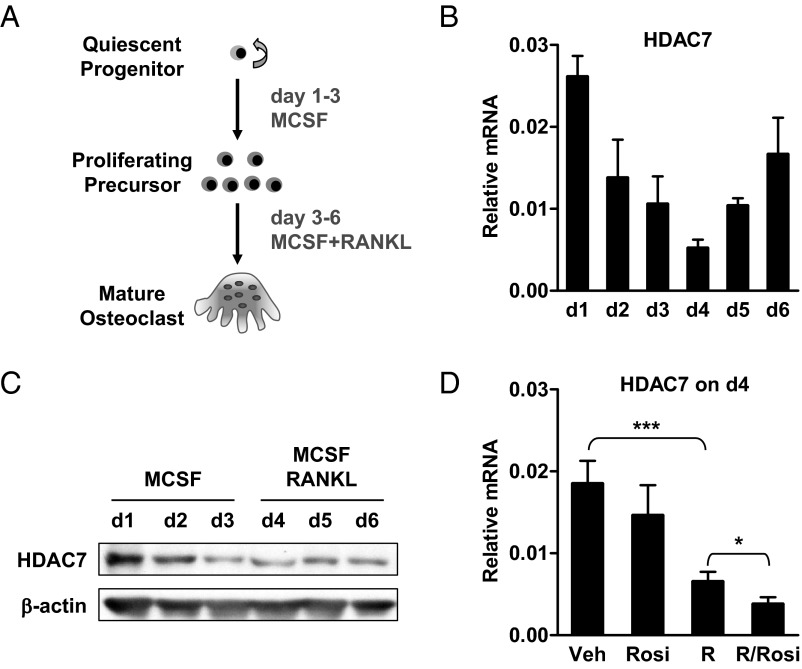
We first examined HDAC7 expression during a time course of osteoclast differentiation. Bone marrow cells were cultured with MCSF for 3 d to promote osteoclast precursor proliferation (d 1—d 3), and then treated with RANKL and MCSF for 3 d to induce osteoclast differentiation (d 4—d 6) (Fig. 1A). HDAC7 mRNA was decreased during MCSF-mediated proliferation (d 1—d 3), and further reduced by RANKL treatment (d 4) but then gradually returned (Fig. 1B). HDAC7 protein showed a similar expression pattern (Fig. 1C). Furthermore, HDAC7 mRNA was significantly down-regulated after 24 h of RANKL treatment, and further suppressed by the cotreatment with rosiglitazone, an agonist of the nuclear receptor PPARγ that has been shown to accelerate osteoclastogenesis (16, 35) (Fig. 1D). These results suggest that HDAC7 may be a negative regulator of osteoclast differentiation.
Fig. 1.
HDAC7 expression during osteoclast differentiation. A, A schematic diagram of the ex vivo bone marrow osteoclast differentiation assay. B, HDAC7 mRNA expression on each day of osteoclast differentiation (n = 3). C, HDAC7 protein expression on each day of osteoclast differentiation (n = 3). D, HDAC7 mRNA expression 24 h after RANKL treatment in the absence or presence of rosiglitazone (Rosi). R, RANKL; V, vehicle. Statistical analyses were performed with Student's t test and are shown as mean ± sd; *, P < 0.05; ***, P < 0.005.
HDAC7 overexpression inhibits osteoclast differentiation
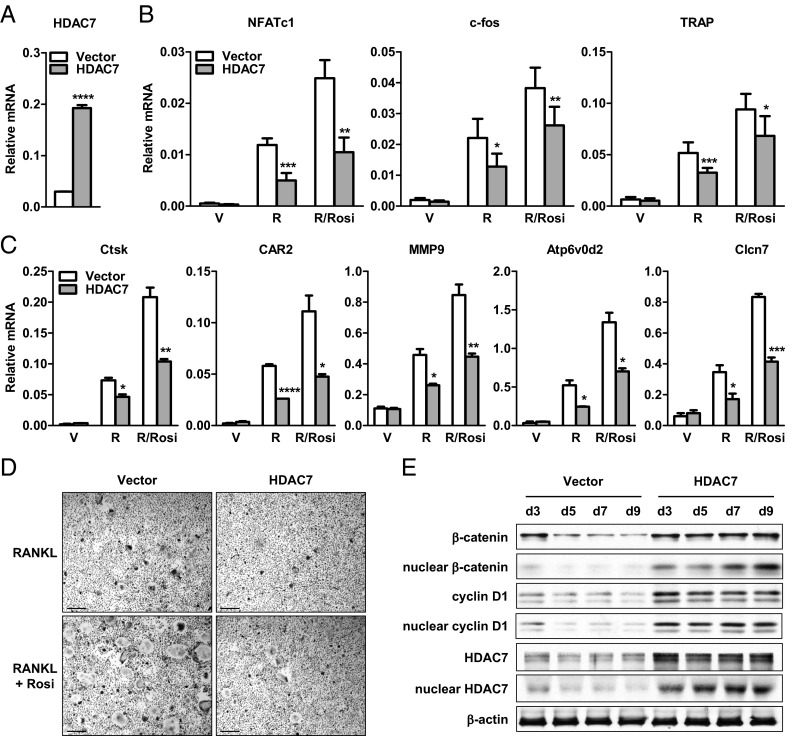
To investigate the roles of HDAC7 in osteoclastogenesis, we first examined the effects of HDAC7 gain of function. Bone marrow cells from wild-type mice were transfected with a HDAC7 expression plasmid or a vector control before osteoclast differentiation. HDAC7 expression was effectively elevated at both mRNA and protein levels (Fig. 2, A and E). As a result, osteoclast differentiation was suppressed, illustrated by the reduced expression of RANKL-induced and rosiglitazone-stimulated differentiation marker genes such as NFATc1, c-fos, and TRAP (Fig. 2B), as well as resorptive activity marker genes such as Ctsk, CAR2, MMP9, Atp6v0d2, and Clcn7 (Fig. 2C), leading to decreased number and size of TRAP+ multinucleated mature osteoclasts (Fig. 2D).
Fig. 2.
HDAC7 overexpression inhibits osteoclast differentiation. Bone marrow cells from wild-type (WT) mice were transfected with HDAC7 or vector control before osteoclast differentiation with RANKL (R), with or without rosiglitazone (Rosi). A, HDAC7 mRNA expression was increased by HDAC7 transfection (n = 3). B, RANKL-induced and Rosi-stimulated expression of osteoclast transcription factors (NFATc1 and c-fos) and differentiation marker (TRAP) were attenuated by HDAC7 overexpression (n = 3). C, RANKL-induced and Rosi-stimulated expression of osteoclast resorptive activity markers were decreased by HDAC7 overexpression (n = 3). D, Osteoclast formation was suppressed by HDAC7 overexpression. Mature osteoclasts were identified as multinucleated TRAP+ (purple) cells (n = 3). Scale bar, 25μm. E, RANKL-mediated down-regulation of β-catenin and cyclin D1 proteins was abolished by HDAC7 overexpression. Whole-cell extract or nuclear extract were isolated from the differentiation cultures 3, 5, 7, or 9 d after RANKL stimulation, and immunoblotted with antibodies for β-catenin, cyclin D1, HDAC7, or β-actin. Statistical analyses were performed with Student's t test and are shown as mean ± sd; *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001. V, Vehicle.
To explore the mechanisms for the antiosteoclastogenic effects of HDAC7 overexpression, we examined the levels of β-catenin and cyclin D1, the down-regulation of which upon RANKL treatment is required for osteoclast differentiation (16). HDAC7 overexpression prevented the down-regulation of β-catenin and cyclin D1 proteins in whole cells, and more dramatically in the nucleus, leading to their constitutive expression (Fig. 2E). This indicates that HDAC7 gain of function inhibits osteoclast differentiation by sustaining β-catenin and cyclin D1 levels.
HDAC7 deletion enhances osteoclast differentiation
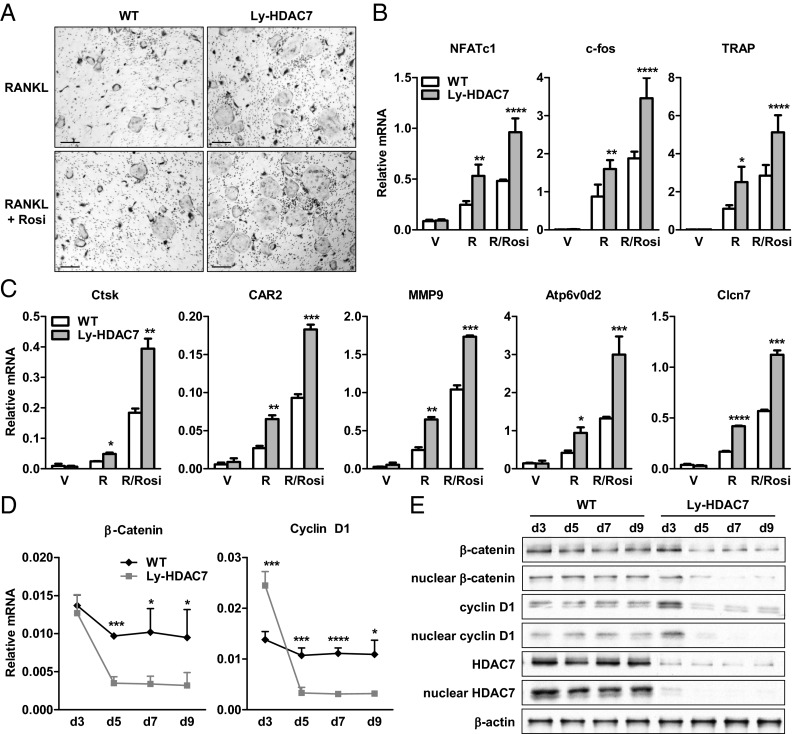
We next determined the effects of HDAC7 loss of function on osteoclastogenesis. To delete HDAC7 in the macrophage/osteoclast lineage, we crossed HDAC7 flox mice (29) with lysozyme-cre (Ly-cre) mice (32) to generate Ly-HDAC7 (HDAC7flox/flox; Ly-cre+) conditional knockout mice. Osteoclast differentiation from the bone marrow of Ly-HDAC7 mice was enhanced compared with the littermate HDAC7flox/flox controls, illustrated by more and larger TRAP+ multinucleated mature osteoclasts in culture (Fig. 3A) and higher expression of osteoclast differentiation and activity marker genes (Fig. 3, B and C).
Fig. 3.
HDAC7 deletion enhances osteoclast differentiation. Bone marrow cells from Ly-HDAC7 knockout mice or littermate controls were differentiated into osteoclasts with MCSF and RANKL in the absence or presence of rosiglitazone (Rosi). A, RANKL-induced and Rosi-stimulated osteoclast formation was enhanced by HDAC7 deletion. Mature osteoclasts were multinucleated TRAP+ (purple) cells (n = 3). Scale bar, 25 μm. B, RANKL-induced and Rosi-stimulated expression of osteoclast transcription factors (NFATc1 and c-fos) and differentiation marker (TRAP) were increased by HDAC7 deletion (n = 3). C, RANKL-induced and Rosi-stimulated expression of osteoclast resorptive activity markers were increased by HDAC7 deletion (n = 3). D and E, RANKL-mediated down-regulation of β-catenin and cyclin D1 mRNA (D) and protein (E) were accelerated by HDAC7 deletion (n = 3). RNA, whole-cell extract, or nuclear extract were isolated from the differentiation cultures 3, 5, 7, or 9 d after RANKL stimulation and immunoblotted with antibodies for β-catenin, cyclin D1, HDAC7, or β-actin. Statistical analyses were performed with Student's t test and are shown as mean ± sd; *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001. R, RANKL, V, vehicle; WT, wild type.
Upon RANKL treatment, HDAC7 deletion resulted in a more rapid down-regulation of β-catenin and cyclin D1 mRNAs (Fig. 3D), along with an accelerated reduction in β-catenin and cyclin D1 proteins in whole cells, and more profoundly in the nucleus (Fig. 3E). Western blot analysis showed that HDAC7 protein expression was severely blunted in the Ly-HDAC7 differentiation cultures, confirming efficient HDAC7 gene deletion by Ly-cre (Fig. 3E). Together, these results show that HDAC7 loss of function stimulates osteoclast differentiation by accelerating RANKL-mediated β-catenin and cyclin D1 down-regulation.
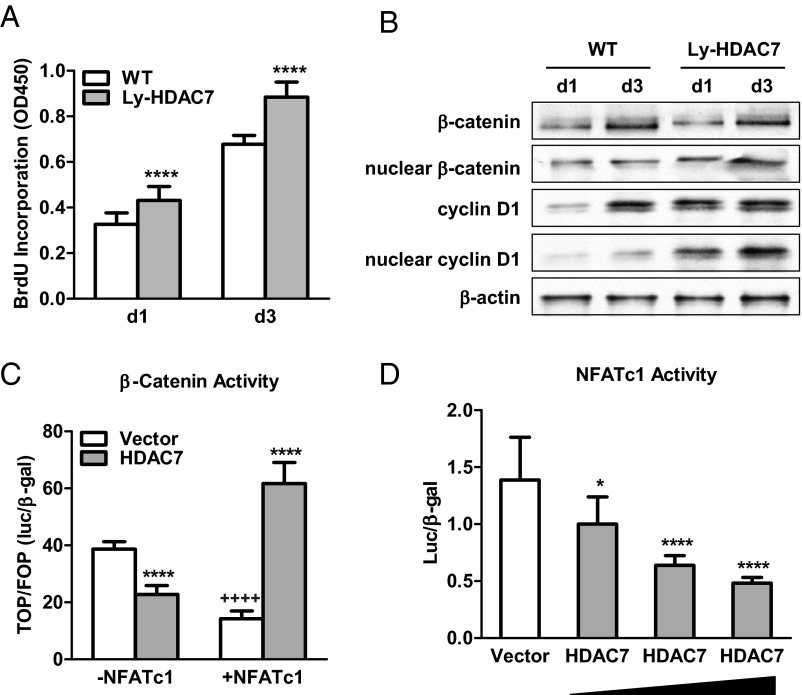
HDAC7 deletion promotes osteoclast precursor proliferation
We next examined the effect of HDAC7 deletion on MCSF-mediated osteoclast precursor proliferation, which requires the up-regulation of β-catenin and cyclin D1 (16). BrdU incorporation was significantly increased in the bone marrow cultures from Ly-HDAC7 mice compared with littermate control mice after 1 d or 3 d of MCSF treatment (Fig. 4A). Consistent with this observation, nuclear β-catenin and cyclin D1 protein levels were elevated in the Ly-HDAC7 proliferation cultures (Fig. 4B). This was opposite from the decreased β-catenin and cyclin D1 protein levels in the Ly-HDAC7 differentiation cultures (Fig. 3E). Our previous study showed that RANKL functions as a β-catenin switch so that β-catenin is increased before RANKL treatment but decreased upon RANKL stimulation (16). Our current results suggest that HDAC7 also differentially regulates β-catenin function so that it inhibits β-catenin before RANKL treatment but promotes β-catenin upon RANKL stimulation.
Fig. 4.
HDAC7 Reverses RANKL-mediated β-catenin switch by suppressing NFATc1. A, Osteoclast precursor proliferation was increased by HDAC7 deletion (n = 10). BrdU incorporation was compared between bone marrow cultures from Ly-HDAC7 knockout mice or littermate controls 1 or 3 d after MCSF treatment. B, β-Catenin and cyclin D1 protein levels during precursor proliferation were elevated by HDAC7 deletion. Whole-cell extract or nuclear extract was isolated from the bone marrow cultures 1 or 3 d after MCSF treatment, and immunoblotted with antibodies for β-catenin, cyclin D1, or β-actin. C, β-Catenin activity was inhibited by HDAC7 in the absence of NFATc1, but stimulated by HDAC7 in the presence of NFATc1 in a transient transfection assay. TOP-flash activity was normalized by FOP-flash activity control (n = 6). *, HDAC7 compared with vector control; +, −NFATc1 compared with +NFATc1. D, NFATc1 activity was suppressed in a dose-dependent manner by HDAC7 in a transient transfection assay (n = 6). Statistical analyses were performed with Student's t test and are shown as mean ± sd; *, P < 0.05; **** or ++++, P < 0.001. WT, Wild type.
HDAC7 reverses RANKL-mediated β-catenin switch by suppressing NFATc1
To further investigate the mechanisms for RANKL-dependent regulation of β-catenin by HDAC7, we next examined how HDAC7 and RANKL-induced transcription factors regulate β-catenin activity using a transient transfection assay of the TOP-flash luciferase reporter in a heterologous cell line. Although HEK293 cells represent a different cellular context from osteoclasts, this cell line is widely used for transient transfection and reporter analysis to dissect the functions of individual transcription factor. First, we identified NFATc1 as the transcription factor that mediates the RANKL switch, because β-catenin activity was specifically suppressed by NFATc1 (Fig. 4C), but not other RANKL-induced transcription factors such as c-fos (data not shown). Second, the effects of HDAC7 on β-catenin activity was NFATc1 dependent because β-catenin activity was inhibited by HDAC7 in the absence of NFATc1, but stimulated by HDAC7 in the presence of NFATc1 (Fig. 4C). Third, a transient transfection assay of the NFATc1-luciferase reporter revealed that HDAC7 dose-dependently inhibited NFATc1 activity (Fig. 4D). Together with the HDAC7 suppression of NFATc1 expression during osteoclast differentiation (Figs. 2B and 3B), these results show that HDAC7 attenuates NFATc1 function and abolishes NFATc1 suppression of β-catenin in the presence of RANKL, consequently blocking osteoclast differentiation. On the other hand, in the absence of RANKL, HDAC7 inhibits β-catenin expression and activity (Fig. 4, B and C), thereby decreasing osteoclast precursor proliferation. Therefore, the combined suppression of precursor proliferation and osteoclast differentiation by HDAC7 would predict that HDAC7 deletion in the osteoclast lineage increases bone resorption and decreases bone mass in vivo.
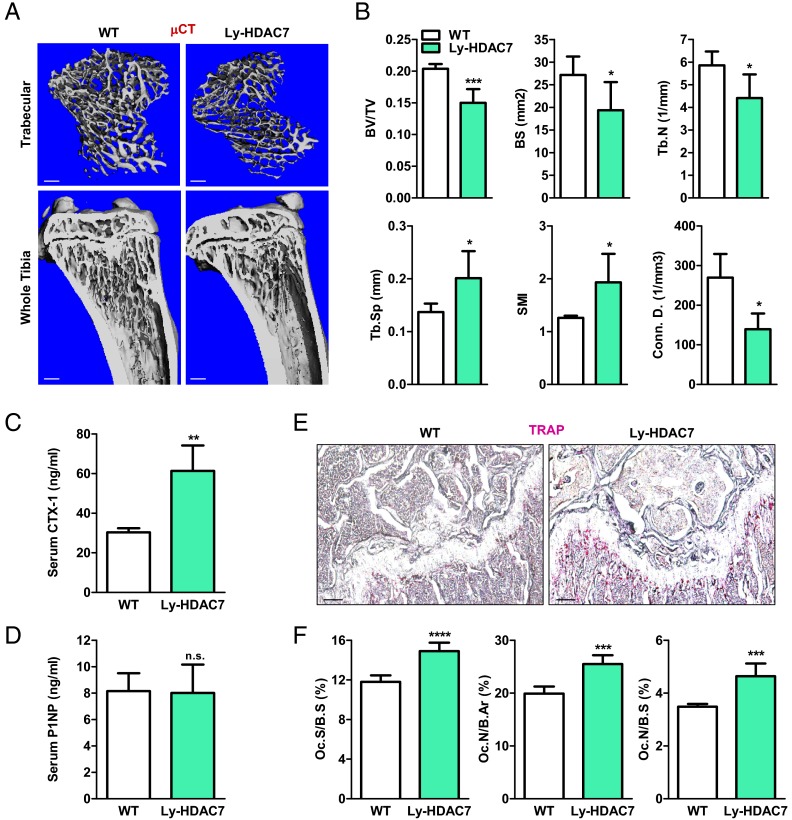
Osteoclastic HDAC7 deletion in mice decreases bone mass by increasing bone resorption
To determine the in vivo consequences of osteoclastic HDAC7 deletion, we next analyzed the bone phenotype in Ly-HDAC7 mice. Micro-computed tomography (μCT) analysis revealed that Ly-HDAC7 mice exhibited a lower bone mass manifested as a significantly decreased bone volume/tissue volume ratio (BV/TV, −26%), bone surface (BS, −29%), and trabecular number (−25%), along with a significantly increased trabecular separation (+47%), leading to weaker bones with a higher structure model index (+53%) and a lower connectivity density (−48%) (Fig. 5A-B). ELISA analyses of serum markers showed that this low-bone mass phenotype was specifically caused by an increased bone resorption because the bone resorption marker CTX-1 (C-terminal telopeptides of Type I collagen) was 102% higher (Fig. 5C), whereas the bone formation marker P1NP was unchanged (Fig. 5D). Consistent with these observations, histomorphometry analysis showed that osteoclast number and surface were significantly increased in the Ly-HDAC7 knockout mice compared with littermate control mice (Fig. 5, E and F). These findings indicate that HDAC7 deletion in the macrophage/osteoclast population results in osteopenia due to elevated bone resorption.
Fig. 5.
Ly-HDAC7 knockout mice have low bone mass due to high bone resorption. A and B, Ly-HDAC7 mice displayed a low-bone mass phenotype. Tibias from Ly-HDAC7 mice or wild-type (WT) littermate controls (3-month-old males, n = 4) were analyzed by μCT. A, Representative images of the trabecular bone of the tibial metaphysis (top) (scale bar, 10 μm) and the entire proximal tibia (bottom) (scale bar, 1mm). B, Quantification of trabecular bone volume and architecture. Tb.N, Trabecular number; Tb.Sp, trabecular separation; SMI, structure model index; Conn.D., connectivity density. C, Serum CTX-1 was increased (3-month-old males, n = 6). D, Serum P1NP was unaltered (3-month-old males, n = 6). E and F, Bone histomorphometry analysis shows increased osteoclasts in the Ly-HDAC7 mice compared with littermate controls (3-month-old males, n =4). E, Representative images of TRAP-stained femoral sections. Osteoclasts were identified as multinucleated TRAP+ (purple) cells. Scale bar, 100 μm. F, Quantification of osteoclast surface and osteoclast number. Oc.S, osteoclast surface; B.Ar, bone area. Statistical analyses were performed with Student's t test and are shown as mean ± sd; *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001; n.s., nonsignificant., P > 0.05.
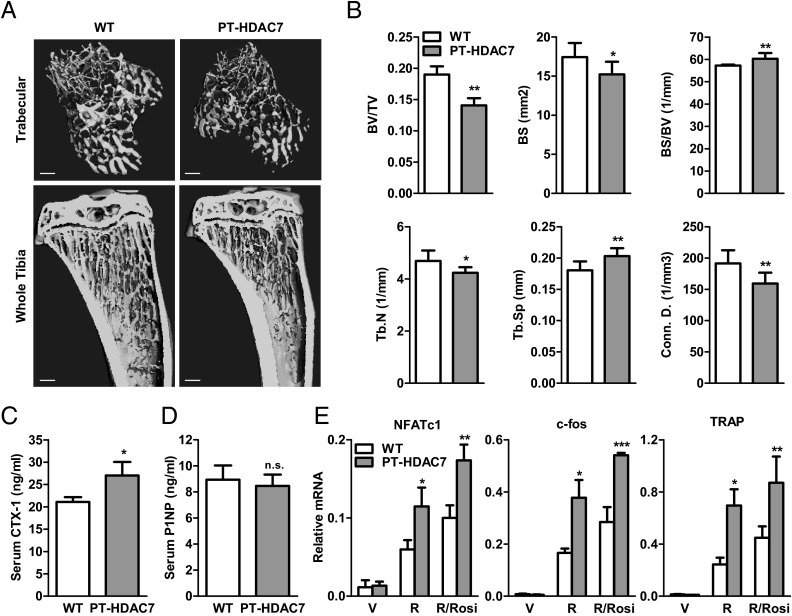
To further investigate the in vivo effects of HDAC7 deletion in the entire osteoclast lineage, we decided to examine another cre driver that can target early osteoclast progenitor cells. Our previous study used Tie2cre for this purpose (35); however, Tie2cre-mediated HDAC7 deletion results in early embryonic lethality (29). We have recently shown that osteoclast progenitors reside in PPARγ+ hematopoietic bone marrow cells; and PPARγ-tTA;TRE-cre (PT-cre) driver can efficiently target the entire osteoclast lineage (16, 31). Thus, we bred HDAC7flox/flox mice with PT-cre mice to generate PT-HDAC7 (HDAC7flox/flox; PT-cre+) conditional knockout mice. μCT analysis showed that the PT-HDAC7 mice had a similar low-bone-mass phenotype as the Ly-HDAC7 mice with reduced BV/TV, bone surface, trabecular number, and connectivity density, along with increased bone surface/bone volume (BS/BV) ratio and trabecular separation (Fig. 6, A and B). ELISA analyses of serum markers showed that the PT-HDAC7 mice had increased bone resorption (Fig. 6C) but unaltered bone formation (Fig. 6D). Consistently, osteoclast differentiation from the bone marrow of PT-HDAC7 mice was enhanced compared with the littermate HDAC7flox/flox controls (Fig. 6E). These data further confirm the in vivo antiosteoclastogenic role of HDAC7. Together, the findings from two osteoclastic HDAC7 knockout mouse models convincingly demonstrated that HDAC7 is a physiologically relevant negative regulator of bone resorption that exerts profound consequences on skeletal mass.
Fig. 6.
PT-HDAC7 knockout mice have low bone mass due to high bone resorption. A and B, PT-HDAC7 mice displayed a low-bone mass phenotype. Tibias from PT-HDAC7 mice or littermate controls (3-month-old males, n = 4) were analyzed by μCT. A, Representative images of the trabecular bone of the tibial metaphysis (top) (scale bar, 10 μm) and the entire proximal tibia (bottom) (scale bar, 1 mm). B, Quantification of trabecular bone volume and architecture. Tb.N, Trabecular number; Tb.Sp, trabecular separation; Conn.D., connectivity density. C, Serum CTX-1 was increased (3-month-old males, n = 4). D, Serum P1NP was unaltered (3-month-old males, n = 4). E, RANKL-induced and rosi-stimulated expression of osteoclast transcription factors (NFATc1 and c-fos) and functional gene (TRAP) were increased in PT-HDAC7 bone marrow differentiation culture (n = 3). R, RANKL; Rosi, rosiglitazone; V, vehicle. Statistical analyses were performed with Student's t test and are shown as mean ± sd; *, P < 0.05; **, P < 0.01; ***, P < 0.005; n.s., nonsignificant., P > 0.05.
Discussion
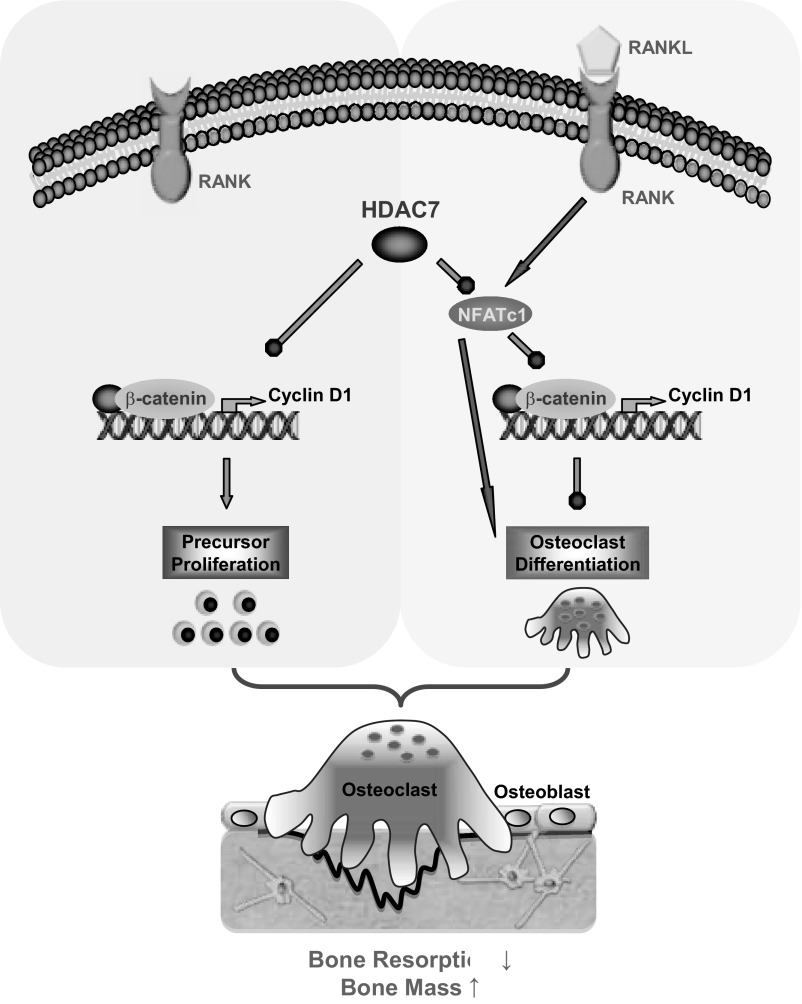
In this study, using both in vitro and in vivo strategies, we have identified HDAC7 as an important and physiologically relevant negative regulator of osteoclastogenesis and bone resorption. Mechanistic studies reveal that HDAC7 suppresses both osteoclast precursor proliferation and osteoclast differentiation via a dual regulation of β-catenin and NFATc1. In the absence of RANKL, HDAC7 inhibits precursor proliferation by attenuating β-catenin and cyclin D1 expression as well as β-catenin activity. Upon RANKL stimulation, HDAC7 inhibits osteoclast differentiation by suppressing NFATc1 expression and activity and preventing the down-regulation of β-catenin and cyclin D1. Therefore, HDAC7 impedes osteoclastogenesis by a novel mechanism of reversing the RANKL/NFATc1-mediated β-catenin switch (Fig. 7). Consequently, HDAC7 deletion in the osteoclast lineage results in higher bone resorption and lower bone mass in two mouse genetic models. This is the first in vivo study that reports a critical physiological function of an individual HDAC in osteoclastogenesis and bone resorption.
Fig. 7.
A simplified model for how HDAC7 suppresses osteoclastogenesis. In the absence of RANKL, HDAC7 inhibits osteoclast precursor proliferation by attenuating β-catenin function and cyclin D1 expression. Upon RANKL stimulation, HDAC7 inhibits osteoclast differentiation by suppressing NFATc1 and prevents RANKL/NFATc1-mediated down-regulation of β-catenin and cyclin D1. Consequently, HDAC7 decreases osteoclastogenesis and bone resorption, thereby increasing bone mass.
Future studies are required to elucidate the detailed molecular mechanisms for how HDAC7 regulates the expression and activity of β-catenin, NFATc1, and cyclin D1. Because both histone and nonhistone proteins can be modified by acetylation, HDAC7 may function via multiple targets to alter chromatin structure as well as to modulate the activity, stability, recruitment, and/or subcellular localization of these transcription factors. Another important question is which histone acetyltransferases oppose the function of HDAC7 during osteoclastogenesis to balance the acetylation switches.
Numerous HDAC inhibitors are currently in clinical trials for the treatment of a bevy of diseases including cancer and neurodegeneration (25, 26). Interestingly, inhibition of class IIa HDACs (HDAC 4, 5, and 7) has also been recently implicated as a potential therapeutic strategy for ameliorating type 2 diabetes (37, 38). In light of the bone loss side effects of several current diabetes and cancer drugs such as rosiglitazone and exemestane (39–41), it is important to understand the functional roles of each HDAC in bone physiology and the effects of HDAC inhibition on skeletal health. On the one hand, some HDAC inhibitors may be beneficial to the skeleton and represent potential therapy for bone diseases. On the other hand, some HDAC inhibitors may cause bone fragility, and combined treatment with bone-boosting drugs such as bisphosphonates or denosumab may be beneficial (42).
Although in vitro studies have reported that broad-spectrum HDAC inhibitors suppress osteoclast differentiation (20–24), our in vivo study has unexpectedly revealed that the specific deletion of HDAC7 in mice promotes osteoclastogenesis and bone resorption. The physiological roles of other HDACs, including other class IIa HDAC members, in osteoclasts are still unknown. Hence, the dissection of the specific regulation by each individual HDAC using mouse genetics will be the key to distinguish the friends and foes of bone and to facilitate the development of new HDAC inhibitors with maximum therapeutic benefits but less bone loss side effects. To this end, in vitro studies suggest that HDAC7 (a class II HDAC) and HDAC3 (a class I HDAC) may exert opposite effects on osteoclast differentiation (28). Initiated by our identification of HDAC7 as the first antiosteoclastogenic HDAC in vivo, future studies will promise to elucidate the many more versatile functions of the HDAC family in skeletal physiology.
Acknowledgments
We thank Drs. Eric Olson and Rhonda Bassel-Duby at University of Texas Southwestern for providing HDAC7 flox mice and HDAC7 expression plasmid.
This work was supported by grants from National Institutes of Health (R01 DK089113, to Y.W.), Cancer Prevention Research Institute of Texas (CPRIT) (RP100841, to Y.W.), The Welch Foundation (I-1751, to Y.W.), and University of Texas Southwestern Endowed Scholar Startup Fund (to Y.W.). Y.W. is a Virginia Murchison Linthicum Scholar in Medical Research and a recipient of the Basil O'Connor Scholar Award.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- Bromodeoxyuridine
- BS
- bone surface
- BV
- bone volume
- μCT
- micro-computed tomography
- HDAC
- histone deacetylase
- HEK
- human embryonic kidney
- MCSF
- macrophage colony-stimulating factor
- P1NP
- serum amino-terminal propeptide of type I collagen
- PPARγ
- peroxisome proliferator-activated receptor γ
- RANKL
- receptor activator of nuclear factor κ-B ligand
- TRAP
- tartrate-resistant acid phosphatase
- TV
- tissue volume.
References
- 1. Bruzzaniti A, Baron R. 2006. Molecular regulation of osteoclast activity. Rev Endocr Metab Disord 7:123–139 [DOI] [PubMed] [Google Scholar]
- 2. Novack DV, Teitelbaum SL. 2008. The osteoclast: friend or foe? Annu Rev Pathol 3:457–484 [DOI] [PubMed] [Google Scholar]
- 3. Wan Y. 2010. PPARγ in bone homeostasis. Trends Endocrinol Metab 21:722–728 [DOI] [PubMed] [Google Scholar]
- 4. Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. 1994. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266:443–448 [DOI] [PubMed] [Google Scholar]
- 5. Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. 2002. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901 [DOI] [PubMed] [Google Scholar]
- 6. Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G. 2007. Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13:156–163 [DOI] [PubMed] [Google Scholar]
- 7. Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai YT, Chauhan D, Mitsiades C, Prabhala R, Raje N, Anderson KC, Stover DR, Munshi NC. 2009. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 114:371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C. 2009. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24:578–588 [DOI] [PubMed] [Google Scholar]
- 9. Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, Gong J, Gao Y, Cao J, Graham K, Tipton B, Cai J, Deshpande R, Zhou L, Hale MD, Lightwood DJ, Henry AJ, Popplewell AG, Moore AR, Robinson MK, Lacey DL, Simonet WS, Paszty C. 2010. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25:948–959 [DOI] [PubMed] [Google Scholar]
- 10. Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Löwik CW, Reeve J. 2005. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19:1842–1844 [DOI] [PubMed] [Google Scholar]
- 11. Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr 2003. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349:2483–2494 [DOI] [PubMed] [Google Scholar]
- 12. Day TF, Guo X, Garrett-Beal L, Yang Y. 2005. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8:739–750 [DOI] [PubMed] [Google Scholar]
- 13. Glass DA, II, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. 2005. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8:751–764 [DOI] [PubMed] [Google Scholar]
- 14. Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. 2005. Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8:727–738 [DOI] [PubMed] [Google Scholar]
- 15. Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL, Williams BO. 2005. Essential role of β-catenin in postnatal bone acquisition. J Biol Chem 280:21162–21168 [DOI] [PubMed] [Google Scholar]
- 16. Wei W, Zeve D, Suh JM, Wang X, Du Y, Zerwekh JE, Dechow PC, Graff JM, Wan Y. 2011. Biphasic and dosage-dependent regulation of osteoclastogenesis by β-catenin. Mol Cell Biol 31:4706–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Otero K, Shinohara M, Zhao H, Cella M, Gilfillan S, Colucci A, Faccio R, Ross FP, Teitelbaum SL, Takayanagi H, Colonna M. 2012. TREM2 and β-catenin regulate bone homeostasis by controlling the rate of osteoclastogenesis. J Immunol 188:2612–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haberland M, Montgomery RL, Olson EN. 2009. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10:32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGee-Lawrence ME, Westendorf JJ. 2011. Histone deacetylases in skeletal development and bone mass maintenance. Gene 474:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cantley MD, Fairlie DP, Bartold PM, Rainsford KD, Le GT, Lucke AJ, Holding CA, Haynes DR. 2011. Inhibitors of histone deacetylases in class I and class II suppress human osteoclasts in vitro. J Cell Physiol 226:3233–3241 [DOI] [PubMed] [Google Scholar]
- 21. Kim HN, Lee JH, Jin WJ, Ko S, Jung K, Ha H, Lee ZH. 2012. MS-275, a benzamide histone deacetylase inhibitor, prevents osteoclastogenesis by down-regulating c-Fos expression and suppresses bone loss in mice. Eur J Pharmacol 691:69–76 [DOI] [PubMed] [Google Scholar]
- 22. Nakamura T, Kukita T, Shobuike T, Nagata K, Wu Z, Ogawa K, Hotokebuchi T, Kohashi O, Kukita A. 2005. Inhibition of histone deacetylase suppresses osteoclastogenesis and bone destruction by inducing IFN-β production. J Immunol 175:5809–5816 [DOI] [PubMed] [Google Scholar]
- 23. Rahman MM, Kukita A, Kukita T, Shobuike T, Nakamura T, Kohashi O. 2003. Two histone deacetylase inhibitors, trichostatin A and sodium butyrate, suppress differentiation into osteoclasts but not into macrophages. Blood 101:3451–3459 [DOI] [PubMed] [Google Scholar]
- 24. Williams PJ, Nishu K, Rahman MM. 2011. HDAC inhibitor trichostatin A suppresses osteoclastogenesis by upregulating the expression of C/EBP-β and MKP-1. Ann NY Acad Sci 1240:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnstone RW. 2002. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov 1:287–299 [DOI] [PubMed] [Google Scholar]
- 26. Kazantsev AG, Thompson LM. 2008. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov 7:854–868 [DOI] [PubMed] [Google Scholar]
- 27. Jensen ED, Schroeder TM, Bailey J, Gopalakrishnan R, Westendorf JJ. 2008. Histone deacetylase 7 associates with Runx2 and represses its activity during osteoblast maturation in a deacetylation-independent manner. J Bone Miner Res 23:361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pham L, Kaiser B, Romsa A, Schwarz T, Gopalakrishnan R, Jensen ED, Mansky KC. 2011. HDAC3 and HDAC7 have opposite effects on osteoclast differentiation. J Biol Chem 286:12056–12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang S, Young BD, Li S, Qi X, Richardson JA, Olson EN. 2006. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell 126:321–334 [DOI] [PubMed] [Google Scholar]
- 30. Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. 2008. White fat progenitor cells reside in the adipose vasculature. Science 322:583–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wei W, Zeve D, Wang X, Du Y, Tang W, Dechow PC, Graff JM, Wan Y. 2011. Osteoclast progenitors reside in the peroxisome proliferator-activated receptor γ-expressing bone marrow cell population. Mol Cell Biol 31:4692–4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8:265–277 [DOI] [PubMed] [Google Scholar]
- 33. Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC, Mangelsdorf DJ, Kliewer SA, Wan Y. 2012. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc Natl Acad Sci USA 109:3143–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wei W, Wang X, Yang M, Smith LC, Dechow PC, Sonoda J, Evans RM, Wan Y. 2010. PGC1β mediates PPARγ activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab 11:503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wan Y, Chong LW, Evans RM. 2007. PPAR-γ regulates osteoclastogenesis in mice. Nat Med 13:1496–1503 [DOI] [PubMed] [Google Scholar]
- 36. Bai S, Kopan R, Zou W, Hilton MJ, Ong CT, Long F, Ross FP, Teitelbaum SL. 2008. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem 283:6509–6518 [DOI] [PubMed] [Google Scholar]
- 37. Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ. 2011. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell 145:607–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang B, Moya N, Niessen S, Hoover H, Mihaylova MM, Shaw RJ, Yates JR, III, Fischer WH, Thomas JB, Montminy M. 2011. A hormone-dependent module regulating energy balance. Cell 145:596–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheung AM, Tile L, Cardew S, Pruthi S, Robbins J, Tomlinson G, Kapral MK, Khosla S, Majumdar S, Erlandson M, Scher J, Hu H, Demaras A, Lickley L, Bordeleau L, Elser C, Ingle J, Richardson H, Goss PE. 2012. Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP. 3 randomised controlled trial. Lancet Oncol 13:275–284 [DOI] [PubMed] [Google Scholar]
- 40. Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ. 2009. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373:2125–2135 [DOI] [PubMed] [Google Scholar]
- 41. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. 2006. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 42. Baron R, Ferrari S, Russell RG. 2011. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 48:677–692 [DOI] [PubMed] [Google Scholar]