Abstract
GH and prolactin (PRL) are structurally related hormones that exert important effects in disparate target tissues. Their receptors (GHR and PRLR) reside in the cytokine receptor superfamily and share signaling pathways. In humans, GH binds both GHR and PRLR, whereas PRL binds only PRLR. Both hormones and their receptors may be relevant in certain human and rodent cancers, including breast cancer. GH and PRL promote signaling in human T47D breast cancer cells that express both GHR and PRLR. Furthermore, GHR and PRLR associate in a fashion augmented acutely by GH, even though GH primarily activates PRLR, rather than GHR, in these cells. To better understand PRLR's impact, we examined the effects of PRLR knockdown on GHR availability and GH sensitivity in T47D cells. T47D-ShPRLR cells, in which PRLR expression was reduced by stable short hairpin RNA (shRNA) expression, were compared with T47D-SCR control cells. PRLR knockdown decreased the rate of GHR proteolytic turnover, yielding GHR protein increase and ensuing sensitization of these cells to GHR signaling events including phosphorylation of GHR, Janus kinase 2, and signal transducer and activator of transcription 5 (STAT5). Unlike in T47D-SCR cells, acute GH signaling in T47D-ShPRLR cells was not blocked by the PRLR antagonist G129R but was inhibited by the GHR-specific antagonist, anti-GHRext-mAb. Thus, GH's use of GHR rather than PRLR was manifested when PRLR was reduced. In contrast to acute effects, GH incubation for 2 h or longer yielded diminished STAT5 phosphorylation in T47D-ShPRLR cells compared with T47D-SCR, a finding perhaps explained by markedly greater GH-induced GHR down-regulation in cells with diminished PRLR. However, when stimulated with repeated 1-h pulses of GH separated by 3-h washout periods to more faithfully mimic physiological GH pulsatility, T47D-ShPRLR cells exhibited greater transactivation of a STAT5-responsive luciferase reporter than did T47D-SCR cells. Our data suggest that PRLR's presence meaningfully affects GHR use in breast cancer cells.
GH and prolactin (PRL) share important structural and functional features. Both are peptide hormones of slightly greater than 20 kDa molecular mass that emanate largely from the anterior pituitary gland in humans and other vertebrates. Human (h) GH and hPRL share 16% sequence identity and they are very similar topologically, being members of the class I cytokine family (1, 2). Both hormones elicit multiple effects. Although GH is most known for its anabolic and metabolic properties (3–6) and PRL has important impact in breast development and lactation (7, 8), both GH (9–14) and PRL (15–17) have been implicated in breast cancer pathogenesis and behavior.
GH and PRL also activate similar intracellular signaling cascades; both use the Janus kinase 2 (JAK2)-signal transducer and activator of transcription 5 (STAT5) pathway, although each elicits other biochemical signals as well (18–21). GH receptor (GHR) and PRL receptor (PRLR) also share significant similarities, both being type 1 transmembrane glycoprotein cytokine receptor superfamily members with substantial homology, especially in their extracellular domains (22) and interaction with the JAK2 kinase via their proximal intracellular domains (23–26). In humans, hGH can interact with both the GHR and the PRLR, whereas hPRL interacts with PRLR but not GHR. The ability of hGH to productively interact with both receptors suggests potential physiologically relevant diversification of GH actions (27–30). Rational exploitation or inhibition of those actions requires intimate knowledge how GHR and PRLR may influence each other.
In response to their ligands, GHR and PRLR are believed to signal as dimers (31–38). Each receptor is typically envisioned to exist as a homodimer. However, several recent findings suggest the possibility that GHR and PRLR can engage each other, forming either heterodimers or at least existing together in a complex in cells in which they are coexpressed (39–42). We recently studied GH and PRL signaling in the estrogen receptor- and progesterone receptor-positive human T47D breast cancer cell, which endogenously expresses ample GHR and PRLR (42), both of which are detected by immunoprecipitation and immunoblotting. T47D responded well to both human GH and PRL in terms of activation of the JAK2/STAT5 pathway. Although GH engaged GHR, little acute GH-induced GHR tyrosine phosphorylation was detected; rather, GH-induced PRLR tyrosine phosphorylation was more pronounced. Furthermore, GH-induced STAT5 phosphorylation in T47D cells was reduced by cotreatment with the non-GHR-specific GH antagonist, G120R, or the PRL antagonist, G129R, but not affected by cotreatment with either a GHR-specific antagonists such as a mutant ligand (B2036) or an antibody (anti-GHRext-mAb). These data suggested that when both receptors were expressed, GH strongly used PRLR and that the presence of PRLR might influence aspects of GHR availability or action in human cancer cells. To better understand PRLR's impact, we now wanted to examine the effects of PRLR knockdown on GHR availability and GH sensitivity in T47D cells.
Materials and Methods
Materials
Routine reagents were purchased from Sigma Aldrich Corp. (St. Louis, MO) unless otherwise noted. Fetal bovine serum was purchased from Atlanta Biologicals (Lawrenceville, GA). Gentamicin sulfate, penicillin, and streptomycin were purchased from Mediatech (Manassas, VA). Puromycin was purchased from Invitrogen (Grand Island, NY). Recombinant hGH was kindly provided by Eli Lilly & Co. (Indianapolis, IN). Recombinant hPRL was obtained from the National Hormone and Pituitary Program (Torrance, CA). Recombinant hGH-G120R and recombinant hPLR-G129R were produced and prepared as previously described (41, 43).
Antibodies
Polyclonal antiphospho-STAT5 was purchased from Zymed Laboratories (San Francisco, CA). Anti-STAT5 and anti-PRLR (polyclonal against human PRLR amino acids 322–622) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antiphosphotyrosine (4G10) was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). Polyclonal anti-GHRcytAL-47 against the intracellular domain of GHR (44), polyclonal anti-JAK2AL33 against the C-terminal one third of JAK2 (45), and monoclonal anti-GHRext-mAb against the extracellular domain of GHR (46) were described previously.
Cells and cell culture
T47D-SCR and T47D-ShPRLR cells and their derivation and preparation have been previously described (47). The cells were cultured in DMEM/F12 medium, supplemented with 10% fetal bovine serum, 50 μg/ml gentamicin sulfate, 100 U/ml penicillin, and 100 μg/ml streptomycin and puromycin, as described (47).
Cell starvation, cell stimulation, and protein extraction
Serum starvation of cells was accomplished by substitution of 0.5% (wt/vol) BSA (fraction V; Roche Molecular Biochemicals, Indianapolis, IN) for fetal bovine serum in the culture medium for 16–20 h before experiments. Pretreatments and stimulations were carried out at 37 C in serum-free medium. For experiments examining receptor stability, serum-starved cells were incubated with cycloheximide (CHX; 20 μg/ml) for 0–5 h before extraction and analysis. Stimulations were terminated by washing the cells once with ice-cold PBS supplemented with 0.4 mm sodium orthovanadate and then harvested in lysis buffer (1% Triton X-100, 150 mm NaCl, 10% glycerol, 50 mm Tris-HCL, 100 mm NaF, 2 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1 mm sodium orthovanadate, 10 mm benzamidine, 5 μg/ml aprotinin, and 5 μg/ml leupeptin). Cells were lysed for 30 min at 4C in lysis buffer before centrifugation at 15,000 × g for 10 min at 4 C. The protein concentration was determined and equal aliquots of protein extracts (supernatant) were subjected to immunoprecipitation or were directly electrophoresed and immunoblotted as indicated below.
Immunoprecipitation, enzymatic deglycosylation, and Western immunoblot analysis
For immunoprecipitation, 0.5–1 mg protein was incubated with an antibody against JAK2 or GHR overnight at 4 C. Protein A-agarose (fast flow; Pharmacia Biotech, Providence, RI) was then added, and the incubations continued for 4 h at 4 C. The immunoprecipitated proteins were resolved by sodium dodecyl sulfate and 8% PAGE and then transferred to nitrocellulose paper. Western transfers were immunoblotted with antiphosphotyrosine (anti-pY; 4G10), anti-JAK2AL33, anti-GHRcytAL-47, or anti-PRLR antibodies. The enzymatic deglycosylation of the immunoprecipitated GHR with endoglycosidase H was accomplished as previously described (48).
For Western blotting, 50–150 μg protein per lane was resolved by 7.5% SDS-PAGE and transferred to nitrocellulose membrane (Amersham Biosciences, Pittsburgh, PA). Western transfers were immunoblotted with primary antibodies, after which horseradish peroxidase-conjugated secondary antibody (Pierce Chemical Co., Rockford, IL) was added for detection of bound antibody by Supersignal Femto maximum sensitivity substrate reagent (Pierce Biotechnology, Rockford, IL). Membrane stripping was performed according to the manufacturer's instructions (Amersham Biosciences).
RNA preparation and real-time PCR analysis
Total RNA was isolated from cells using the RNeasy RNA isolation kit as recommended by the manufacturer (QIAGEN, Valencia, CA). cDNA was synthesized using the SuperScript first-strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA). The cDNA was amplified in the Bio-Rad iQ5 real-time PCR system (Bio-Rad Laboratories, Hercules, CA) using SYBR Green reagent (Bio-Rad Laboratories) and sequence-specific primers. PCR was performed in triplicate for each cDNA, averaged, and normalized to endogenous 18S rRNA reference transcripts. Primer sequences used were: GHR, 5′-GAGGCCACAGCAGCTATCCT-3′ and 5′-ATTTGTCTTTAGGCCTGGATTAACA-3′ (49); 18S rRNA, 5′-CCCCATGAACGAGGGAATT-3′ and 5′-GGGACTTAATCAACGCAAGCTT-3′ (50).
GH-induced STAT5-dependent luciferase activity
As detailed in the text, serum-starved T47D-ShPRLR vs. T47D-SCR cells were cotransfected as previously described (21) with a plasmid encoding the luciferase gene under the control of the STAT5-responsive element of the CISH gene (51) (a gift of Dr. Charles Clevenger, Northwestern University, Chicago, IL) and a constitutively (Simian virus 40) driven renilla luciferase plasmid. Each transfected cell type was then treated in replicate with GH (0–500 ng/ml), using the protocols described in the text. Luciferase activity was assayed as previously described (21) and is expressed as the GH-induced fold luciferase (firefly normalized for renilla) activity.
Densitometric and statistical analysis
Immunoblots were scanned using a high-resolution scanner (Hewlett-Packard Co., Palo Alto, CA). Densitometric quantification of images was performed using an image analysis program, ImageJ (developed by W. S. Rasband Research Services Branch, National Institute of Mental Health, Bethesda, MD). Pooled data of densitometry assays from several experiments are displayed as mean ±se. The significance (P value) of the differences of pooled results was estimated using t tests.
Results
Knockdown of PRLR in T47D cells results in enhancement of GHR protein abundance without increased GHR mRNA
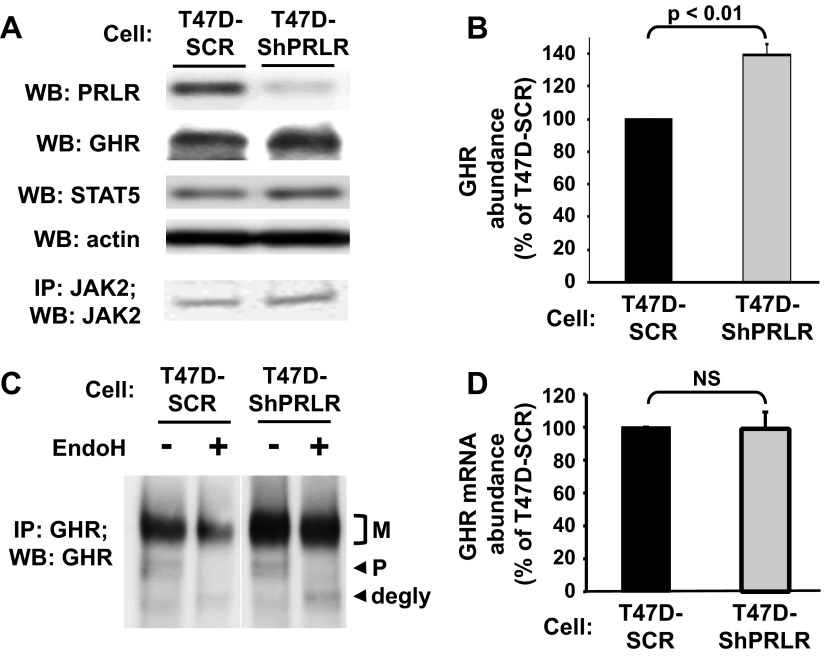
To assess the influence of PRLR on GH signaling in T47D cells, we used our previously described T47D-ShPRLR cells (47). In these cells, stable expression of a short hairpin RNA (shRNA) that targets human PRLR yielded markedly reduced PRLR abundance, as verified by anti-PRLR immunoblotting of serum-starved T47D-ShPRLR compared with T47D-SCR control cells in which a scrambled short hairpin RNA sequence was expressed (Fig. 1A). Immunoblotting confirmed that both cells expressed similar levels of both JAK2 and STAT5. Notably, in contrast to the dramatic knockdown of PRLR protein in T47D-ShPRLR cells, these same cells exhibited markedly increased abundance of immunoblottable GHR. Densitometric analysis of several similar experiments revealed that the GHR level was nearly 40% greater in T47D-ShPRLR cells compared with T47D-SCR cells (Fig. 1B).
Fig. 1.
Knockdown of PRLR in T47D cells results in augmented mature GHR abundance. A, PRLR, GHR, STAT5, and JAK2 levels. Serum-starved T47D-SCR and T47D-ShPRLR cells were extracted in detergent-containing lysis buffer. Equal amounts of protein from each cell extract were evaluated without immunopreciptation or, in a separate experiment, immunoprecipitated with anti-JAK2. Precipitated eluates or extracts were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. Actin blotting served as a loading control for cell extracts. B, Densitometric analysis of data from three separate experiments, performed such as in A, was used to estimate GHR abundance. The T47D-SCR GHR level in each experiment considered 100%. Data are expressed as mean ± se. C, EndoH treatment. Serum-starved T47D-SCR and T47D-ShPRLR cells were extracted in detergent-containing lysis buffer. Equal amounts of protein from each cell extract were immunoprecipitated with anti-GHR, and precipitates were treated with EndoH or vehicle, as indicated. Eluates or extracts were resolved by SDS-PAGE and immunoblotted with anti-GHR. The positions of the mature (M; endoH resistant) and precursor (P; EndoH sensitive) forms of GHR are indicated. D, GHR mRNA levels. Total RNA was extracted from serum-starved T47D-SCR and T47D-ShPRLR cells, and quantitative real-time PCR for GHR (normalized for 18S rRNA) was performed. Pooled data are represented as mean ± se. The GHR mRNA level in the T47D-SCR cells were arbitrarily set to 100%. NS, No significant difference; WB, Western blot; IP, immunoprecipitated.
To further evaluate GHRs in the two stable cell lines, we compared the levels of both the mature and precursor forms of the receptor, as identified by sensitivity to endoglycosidase H (endoH) digestion. GHR is heavily N-glycosylated in its extracellular domain. The precursor GHR form is seen early in the biogenesis process and contains high-mannose sugars that are susceptible to removal by endoH, which causes the deglycosylated receptor to migrate faster in SDS-PAGE. As the precursor form traverses the trans-Golgi, high-mannose sugars are trimmed and the GHR acquires resistance to endoH digestion. This endoH-resistant mature GHR exhibits retarded migration compared with the precursor and is the form believed to populate the cell surface and respond to GH binding with initiation of signaling (48, 52–54). In the experiment shown in Fig. 1C, GHR was immunoprecipitated from T47D-SCR and T47D-ShPRLR cells with an antiserum directed at the intracellular domain and precipitates were incubated either in control buffer (−) or endoH (+), and eluted proteins were resolved by SDS-PAGE. In both cells, GHR migrated as two forms: a more intense broad band (mature) and a faster-migrating closely spaced doublet (precursor). When subjected to endoH treatment, the precursor forms displayed enhanced migration and are referred to as the deglycosylated precursor form. As expected, the mature form did not change its migration, confirming its designation as the endoH-resistant mature GHR. Importantly, comparison of the two cells indicated that the increase in GHR abundance in the PRLR knockdown cells was mainly contributed to by the mature GHR rather than the precursor form. These data suggest that PRLR knockdown enhanced either the stability of the mature GHR or the conversion of the precursor to mature GHR during biogenesis but that the increased GHR translation per se was unlikely to account for the increase in immunoblotted GHR in T47D-ShPRLR cells. To further address this issue, we measured the GHR mRNA abundance by real-time RT-PCR (Fig. 1D). Consistent with the notion that the GHR level was increased at a posttranscriptional level in T47D-ShPRLR cells, we found no difference in the levels of GHR message between T47D-SCR and T47D-ShPRLR cells.
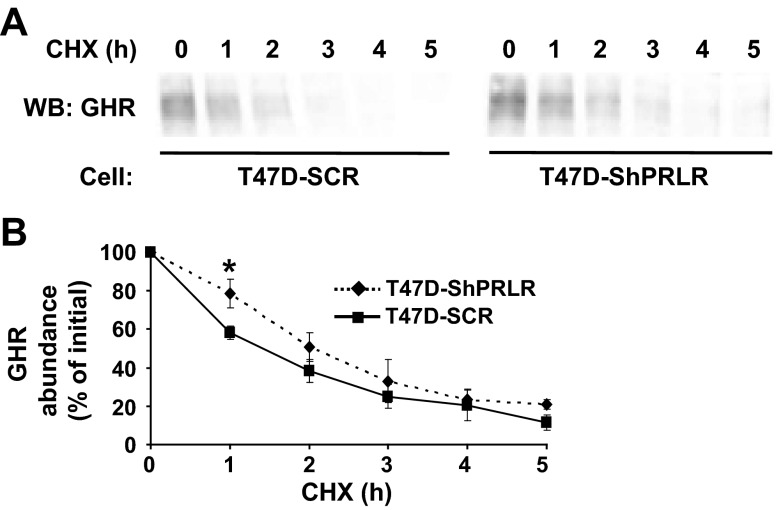
Because we found that mature (already synthesized) GHR was disproportionately more abundant relative to precursor in T47D-ShPRLR cells than in T47D-SCR cells, we hypothesized that stability of the mature GHR might be enhanced in the PRLR knockdown cells. To assess this, we performed CHX chase experiments (Fig. 2). We previously used this technique to compare the stability of various GHR mutants and the effects of JAK2 on GHR stability by immunoblotting under conditions when new protein synthesis is inhibited; thus, the fate of previously synthesized mature GHR is tracked and its rate of disappearance is inversely related to its stability (48, 54, 55). T47D-SCR and T47D-ShPRLR cells were treated with CHX for 0–5 h, and detergent extracts were immunoblotted with anti-GHR (Fig. 2A). As expected, basal (no CHX treatment) GHR abundance was greater in T47D-ShPRLR cells and the receptor level progressively declined with CHX treatment in both cells. However, when measured densitometrically in several such experiments and normalized for basal levels within each cell, the loss of GHR was significantly retarded in T47D-ShPRLR cells compared with T47D-SCR cells (Fig. 2B). By this method, we estimated that the half-life of GHR was extended by roughly 50% in the cells in which PRLR was knocked down. Because these experiments were carried out in serum-free conditions and without the addition of GH, we concluded that the presence of PRLR in breast cancer cells that also express GHR contributes to a more rapid ligand-independent turnover of GHR and may therefore diminish cell surface GHR availability in comparison with breast cancer cells in which PRLR is depleted.
Fig. 2.
Enhanced GHR stability in T47D cells with reduced PRLR. A, Cycloheximide treatment. Serum-starved T47D-SCR and T47D-ShPRLR cells were treated for 0–5 h with CHX, as indicated. Detergent extracts were resolved by SDS-PAGE and immunoblotted. Note that all samples were resolved on the same gels but are separated in the figure according to cell type for ease of visualization. B, Densitometric analysis of data from three separate experiments, performed as in A, was used to estimate GHR abundance. For each cell type, the GHR level present in untreated cells was considered as 100%. Data are expressed as mean ± se for each CHX treatment duration relative to untreated cells for each cell type. *, P < 0.05 when comparing T47D-SCR vs. T47D-ShPRLR cells incubated for the same duration in CHX. WB, Western blot.
Acute GH sensitivity is greatly augmented in T47D cells with reduced PRLR
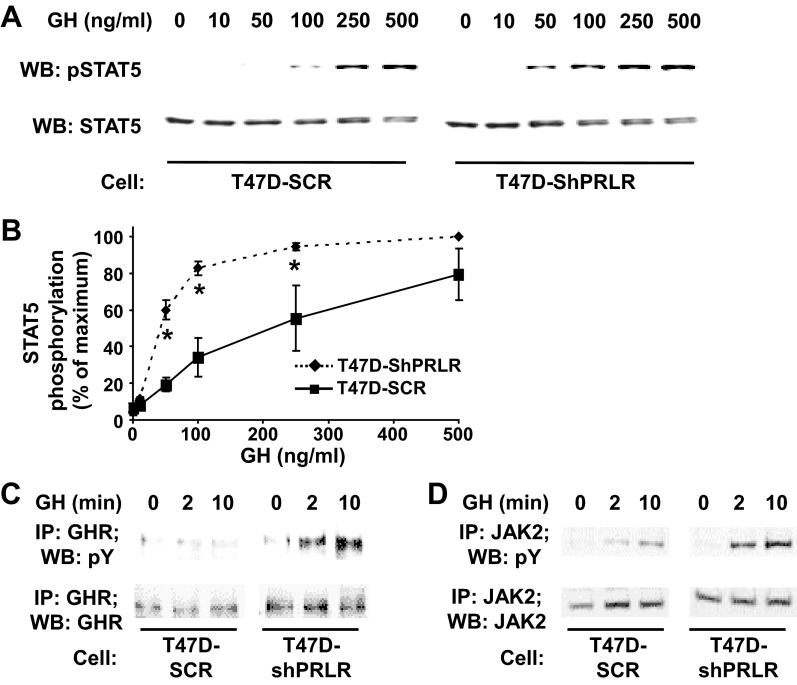
Because PRLR knockdown affected GHR stability and steady state abundance in T47D cells, we next asked whether GH responsiveness was also affected (Fig. 3). Serum-starved T47D-SCR and T47D-ShPRLR cells were treated for 10 min with varying concentrations (0–500 ng/ml) of GH and detergent extracts were assayed for phosphorylated STAT5 by immunoblotting. As in the representative experiment shown in Fig. 3A, GH induced concentration-dependent acute STAT5 phosphorylation in T47D-SCR cells. When the results of multiple experiments were analyzed densitometrically, the peak response to GH required 250–500 ng/ml and appreciable signal was difficult to detect when the stimulatory concentration was less than 100 ng/ml (Fig. 3B). In contrast, acute GH treatment of T47D-ShPRLR cells elicited STAT5 phosphorylation at far lower GH concentrations than in the T47D-SCR cells (Figs. 3, A and B). The EC50 for GH-induced STAT5 phosphorylation was greater than 4.5-fold in T47D-SCR cells than in T47D-ShPRLR cells, indicating a marked enhancement in acute GH sensitivity in cells in which PRLR was knocked down.
Fig. 3.
Enhanced acute GH-induced signaling in T47D cells with reduced PRLR. A and B, STAT5 phosphorylation concentration-dependence. A, Serum-starved T47D-SCR vs. T47D-ShPRLR cells were treated without or with the indicated concentrations of GH for 10 min. Detergent cell extracts were resolved by SDS-PAGE and immunoblotted with an antibody that recognizes tyrosine phosphorylated STAT5 (pSTAT5). The blot was stripped and reprobed with antibody for total STAT5 (STAT5) as a loading control. B, Densitometric analysis of data from three separate experiments, performed as in A, was used to estimate pSTAT5 levels. For each experiment, the pSTAT5 level achieved in response to 500 ng/ml GH in T47D-ShPRLR cells was considered 100%. Data are expressed as mean ± se for each GH concentration relative to 100%. *, P < 0.05 when comparing T47D-SCR vs. T47D-ShPRLR cells incubated with the same GH concentration. Note that all samples in A were resolved on the same gels but are separated in the figure according to cell type for ease of visualization. C and D, GHR and JAK2 tyrosine phosphorylation. Serum-starved T47D-SCR vs. T47D-ShRPRL cells were treated with GH (100 ng/ml) for 0, 2, or 10 min. Detergent extracts were immunoprecipitated with either anti-GHR (C) or anti-JAK2 (D), and eluates were resolved by SDS-PAGE and immunoblotted with sequentially with anti-pY and anti-GHR (C) or anti-pY and anti-JAK2 (D). The data shown are representative of three independent experiments. Note that all samples in C and D were resolved on the same gels but are separated in the figure according to cell type for ease of visualization. WB, Western blot; IP, immunoprecipitated.
We similarly examined the ability of GH to cause acute tyrosine phosphorylation of the GHR and JAK2 in T47D-SCR vs. T47D-ShPRLR cells. In the experiments shown in Fig. 3C, serum-starved T47D-SCR and T47D-ShPRLR cells were stimulated with 100 ng/ml GH for 2 or 10 min and compared with cells not exposed to GH. Detergent extracts were immunoprecipitated with antiserum against the intracellular domain of GHR and eluates were resolved by SDS-PAGE and sequentially immunoblotted with antiphosphotyrosine (anti-pY) and anti-GHR antibodies. In concert with prior findings in parental T47D cells, their derivative harboring control shRNA exhibited little (if any) tyrosine phosphorylation of GHR in response to GH. However, GH promoted easily detectable acute GHR tyrosine phosphorylation in T47D-ShPRLR cells. Concurrent analysis of GHR levels verified the augmented GHR level in T47D-ShPRLR cells. Although JAK2 levels were similar in both cell lines, GH-induced acute JAK2 tyrosine phosphorylation was also augmented in T47D-ShPRLR cells, as verified by anti-JAK2 immunoprecipitation and sequential anti-pY and anti-JAK2 blotting (Fig. 3D) of the same extracts examined in Fig. 3C. These data strongly suggest that the enhanced sensitivity to GH with regard to acute STAT5 tyrosine phosphorylation in T47D-ShPRLR cells is related to the greater abundance of GHR and/or more efficient coupling of GH stimulation to GHR/JAK2 tyrosine phosphorylation in the cells in which PRLR abundance is reduced.
GH use of GHR is enhanced upon PRLR knockdown
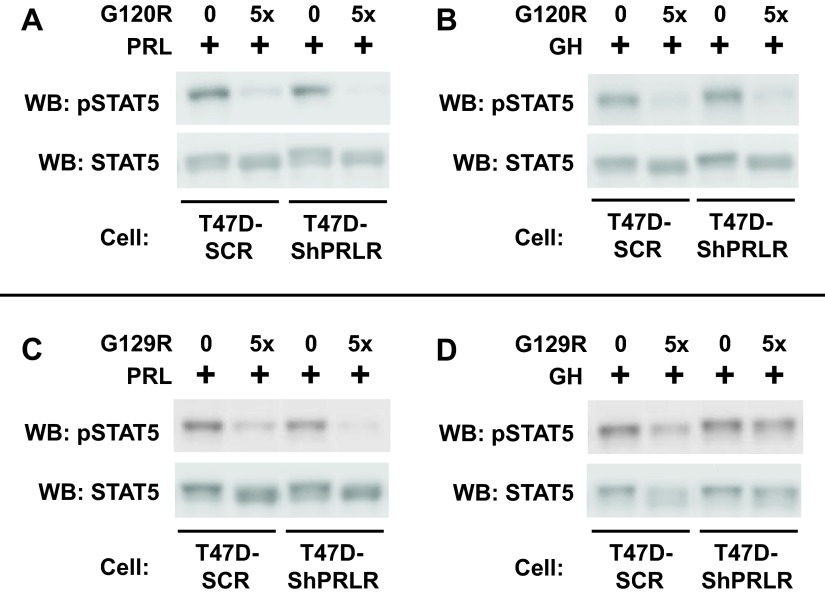
To better understand the effects of PRLR knockdown on GH signaling in T47D cells, we tested the effects of GHR and PRLR antagonists on acute GH- or PRL-induced STAT5 activation. hGH-G120R (G120R) is a mutated human GH molecule in which the natural glycine at residue 120 is changed to arginine; the result is a GH antagonist that binds to both GHR and PRLR and can antagonize signaling from both receptors (56). As anticipated based on our previous findings in T47D cells (42), G120R pretreatment blunted both PRL- and GH-induced acute STAT5 phosphorylation in T47D-SCR cells (Fig. 4, A and B, respectively). Furthermore, both the diminished PRL-induced STAT5 activation (Fig. 4A) and the enhanced GH-induced STAT5 activation (Fig. 4B) in T47D-ShPRLR cells were inhibited by G120R, consistent with this antagonist's ability to block signaling via either PRLR or GHR.
Fig. 4.
The PRLR antagonist, G129R, fails to block GH-induced STAT5 tyrosine phosphorylation in T47D-ShPRLR cells. Serum-starved T47D or T47D-ShPRLR cells were treated with vehicle, PRL (A and C), or GH (B and D) (500 ng/ml each; 15 min) in the presence or absence of G120R (A and B) or G129R (C and D) (5-fold molar excess of antagonist compared with PRL or GH). The detergent cell extracts were resolved by SDS-PAGE and serially immunoblotted with anti-pSTAT5 and anti-STAT5. The data shown are representative of three independent experiments. WB, Western blot; pSTAT5, phosphorylated STAT5.
In contrast to G120R, hPRL-G129R (G129R), which is a mutated human PRL molecule with glycine-129 changed to arginine, specifically antagonizes signaling via PRLR, not GHR (57). Thus, the effects of G129R on GH signaling could be helpful in interpreting whether GH is utilizing GHR vs. PRLR, both of which can be productively engaged by human GH. As anticipated, G129R pretreatment substantially reduced PRL-induced STAT5 phosphorylation in T47D-SCR cells and similarly antagonized the residual PRL-induced STAT5 phosphorylation in T47D-ShPRLR cells (Fig. 4C), consistent with the conclusion that PRL-induced signaling in both cells is via PRLR, albeit to a reduced level in the PRLR knockdown cells. In contrast, G129R's effects on GH signaling differed between the two cell lines (Fig. 4D). The PRLR-specific antagonist was much more effective in reducing GH-induced STAT5 phosphorylation in T47D-SCR cells than in T47D-ShPRLR cells, although a small degree of inhibition of GH signaling by G129R (perhaps via residual PRLR) was evident in T47D-ShPRLR cells. Thus, the knockdown of PRLR allowed greater GH-induced STAT5 phosphorylation that was less sensitive to inhibition by G129R. This is consistent with the findings that GH-induced GHR tyrosine phosphorylation was enhanced in T47D-ShPRLR cells and suggests a difference in receptor use by GH, depending on the relative availability of GHR vs. PRLR and/or the effects of each receptor on the other in dictating receptor availability.
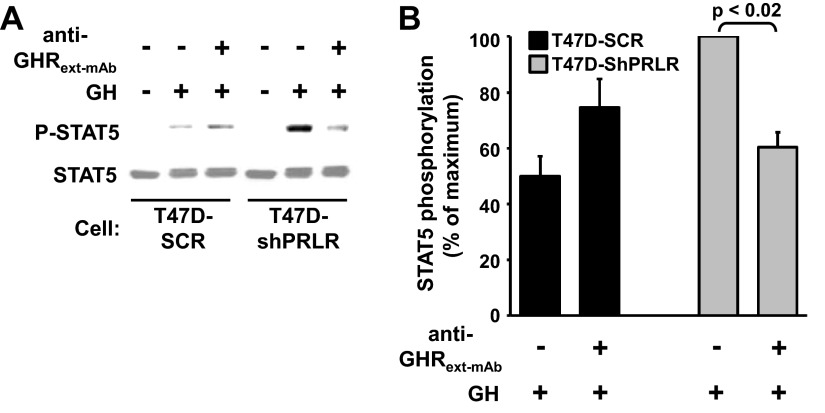
To further pursue this notion, we also used our GHR-specific antagonist monoclonal antibody, anti-GHRext-mAb. This antibody was raised against the recombinant extracellular domain of the rabbit GHR and cross-reacts with human GHR; its conformation-sensitive epitope resides in extracellular subdomain 2 and pretreatment of cells that bear GHR with anti-GHRext-mAb can effectively inhibit GH-induced GHR-mediated signaling without significantly impairing GH binding (46, 58). T47D-SCR and T47D-ShPRLR cells were treated with GH or vehicle for 10 min in the absence or presence of anti-GHRext-mAb, which was added 10 min before GH (Fig. 5A). The degree of GH-induced STAT5 tyrosine phosphorylation was assessed by immunoblotting of the cell extracts. As anticipated, acute GH-induced STAT5 tyrosine phosphorylation was markedly augmented in T47D-ShPRLR cells compared with T47D-SCR cells. As we previously reported for the parental T47D cells (42), anti-GHRext-mAb failed to inhibit GH-induced STAT5 activation in the T47D-SCR control cells. Notably, however, PRLR knockdown rendered GH-induced STAT5 activation in T47D-ShPRLR cells quite sensitive to inhibition by anti-GHRext-mAb, with approximately 40% reduction of GH signaling in the presence of the antibody in these cells (Fig. 5B). Thus, this conformation-specific GHR antagonist antibody is dramatically more able to exert its inhibitory effect in cells depleted of PRLR. Whether this is due to the augmented GHR level present when PRLR levels are reduced or an inhibitory effect of PRLR on the antibody's ability to interact with GHR present in GHR-PRLR complexes is as yet unknown.
Fig. 5.
The GHR antagonist monoclonal antibody, anti-GHRext-mAb, inhibits GH-induced signaling in T47D-ShPRLR, but not T47D-SCR, cells. A and B, Serum-starved T47D-SCR vs. T47D-ShPRLR cells were pretreated with or without anti-GHRext-mAb for 15 min before treatment with GH (100 ng/ml) or vehicle for 10 min. Detergent cell extracts were resolved by SDS-PAGE and sequentially immunoblotted with anti-pSTAT5 and anti-STAT5 (A). B, Densitometric analysis of data from three separate experiments, performed as in A, was used to estimate pSTAT5 levels. For each experiment, the pSTAT5 level achieved in response to 100 ng/ml GH in T47D-ShPRLR cells in the absence of antibody pretreatment was considered 100%. Data are expressed as mean ± S.E. for each GH concentration relative to 100%. pSTAT5, Phosphorylated STAT5.
Impact of PRLR on GH signaling time course, GH-induced GHR down-regulation, and the response to pulsatile GH delivery
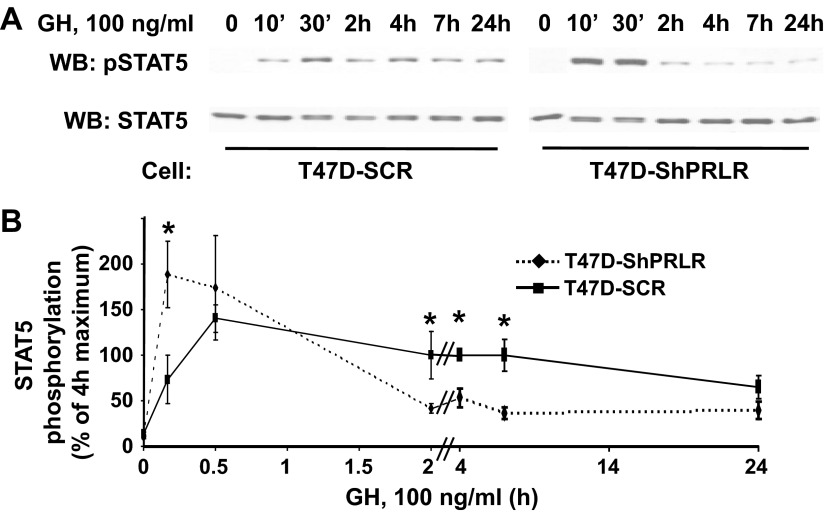
The findings in Figs. 1–5 suggest that PRLR, perhaps by virtue of association with GHR, affects GHR abundance at a posttranscriptional level and also impacts acute GH sensitivity. That is, depletion of PRLR significantly sensitizes cells to lower concentrations of GH. We next examined the effect of PRLR depletion on the time course of GH-induced STAT5 phosphorylation, focusing on both the acute (0–30 min) response and the response to GH exposure for prolonged periods (2–24 h). Serum-deprived T47D-SCR and T47D-ShPRLR cells were treated with 100 ng/ml GH for the indicated durations and detergent cell extracts were resolved by SDS-PAGE and sequentially immunoblotted to detect tyrosine phosphorylated and total STAT5 levels (Fig. 6A). As expected, STAT5 levels were similar between the two cell lines, and GH-induced STAT5 tyrosine phosphorylation after 10 min was markedly greater in T47D-ShPRLR cells than in T47D-SCR cells. GH-induced STAT5 tyrosine phosphorylation in T47D-ShPRLR cells remained high after 30 min but then precipitously declined. In contrast, STAT5 tyrosine phosphorylation in response to GH was relatively delayed and markedly prolonged in T47D-SCR cells. Densitometric analysis of multiple such experiments is shown in Fig. 6B.
Fig. 6.
Continuous GH treatment causes enhanced, but more transient, STAT5 activation in T47D-ShPRLR vs. T47D-SCR cells. A and B, Serum-starved T47D-SCR vs. T47D-ShPRLR cells were treated with GH (100 ng/ml) for the indicated durations. Detergent cell extracts were resolved by SDS-PAGE and sequentially immunoblotted with anti-pSTAT5 and anti-STAT5 (A). B, Densitometric analysis of data from three separate experiments, performed as in A, was used to estimate pSTAT5 levels. For each experiment, the pSTAT5 level achieved in response to GH for 4 h in T47D-SCR cells was considered 100%. Data are expressed as mean ± se for each GH treatment duration relative to 100%. *, P < 0.05 when comparing T47D-SCR vs. T47D-ShPRLR cells incubated with GH for the same duration. Note that all samples in A were resolved on the same gels but are separated in the figure according to cell type for ease of visualization. WB, Western blot; pSTAT5, phosphorylated STAT5.
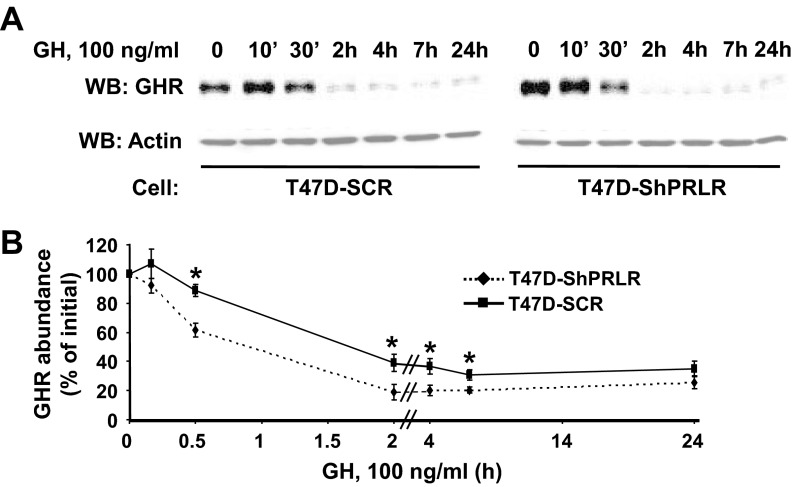
To further explore how the presence of PRLR might influence the dynamics of GH's effects, we examined the time course of GH-induced GHR down-regulation in T47D-SCR vs. T47D-ShPRLR cells (Fig. 7). In prior studies, we have shown that GH treatment relatively rapidly down-regulates GHR, as monitored by immunoblotting, and that the pace and degree of GH-induced GHR down-regulation are affected by whether the receptor can activate JAK2 and undergo GH-induced tyrosine phosphorylation (55, 59). Serum-deprived cells were treated with GH continuously for either a relatively short exposure (30 min or less) or a longer duration (2–24 h) and GHR levels remaining after GH exposure were assessed by immunoblotting (Fig. 7A). Acute GH treatment of T47D-SCR cells (in which acute GHR tyrosine phosphorylation is minimal (see Fig. 3C and Ref. 42) produced little loss of GHR over the first 30 min (see Fig. 7B for densitometric quantification). In contrast, nearly half of the GHR present in T47D-ShPRLR cells was lost after 30 min incubation with GH. With continuous GH incubation for 2 h or more, GHR loss was progressive in both cells, but significantly more diminished in T47D-ShPRLR cells over the 2- to 7-h incubation duration. This difference in GH-induced down-regulation appeared to be driven largely by the more robust acute down-regulation caused by GH in the PRLR-deficient cells.
Fig. 7.
Continuous GH treatment causes enhanced and sustained GHR down-regulation in T47D-ShPRLR vs. T47D-SCR cells. A and B, Serum-starved T47D-SCR vs. T47D-ShPRLR cells were treated with GH (100 ng/ml) for the indicated durations. Detergent cell extracts were resolved by SDS-PAGE and sequentially immunoblotted with anti-GHR and antiactin (A). B, Densitometric analysis of data from three separate experiments, performed as in A, was used to estimate GHR abundance. For each cell type, the GHR level present in untreated cells was considered as 100%. Data are expressed as mean ± se for each GH treatment duration relative to untreated cells for each cell type. *, P < 0.05 when comparing T47D-SCR vs. T47D-ShPRLR cells treated for the same duration with GH. Note that all samples in A were resolved on the same gels but are separated in the figure according to cell type for ease of visualization. WB, Western blot.
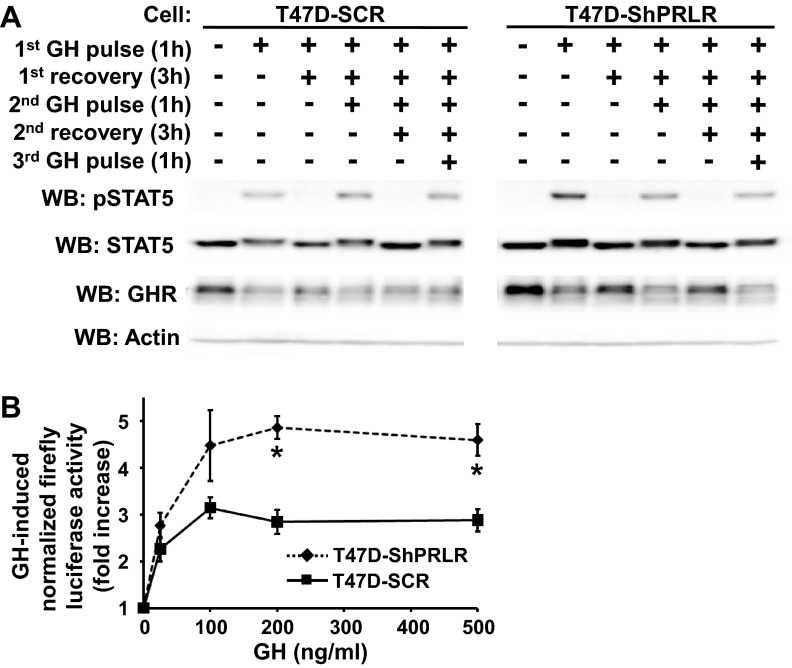
The findings above indicate that PRLR knockdown was accompanied by augmented GHR abundance (presumably associated with an increase in GHR stability), greater (but more transient) acute GH-induced STAT5 activation, and more robust acute GH-induced GHR down-regulation. In humans, as in rodents, GH is secreted in bursts, with up to 8–10 pulses per day (60–62). This pattern is believed to be important in mediation of GH action. We compared T47D-SCR vs. T47D-ShPRLR cells in terms of their responses to GH delivered in pulses, reasoning that the summed effects of transient GH exposure with interpulse removal of GH might better reveal the GH signaling consequences of PRLR silencing in breast cancer cells than might continuous GH exposure. In the experiment shown in Fig. 8A, serum-starved T47D-SCR and T47D-ShPRLR cells were each treated according to one of six regimens, as indicated, such that duration of serum-free culture was equivalent among the regimens. Cells were treated in the following ways: 1) untreated; 2) treated with GH (500 ng/ml) for a 1-h pulse before harvesting; 3) treated with a 1-h GH pulse followed by washout and a 3-h recovery period in serum-free buffer before harvesting; 4) treated with a 1-h GH pulse, followed by a 3-h recovery and a second 1-h GH pulse before harvesting; 5) treated with a first 1-h GH pulse, followed by a first 3-h recovery, a second 1-h GH pulse, and a second 3-h recovery before harvesting: and 6) treated with a first 1-h GH pulse, followed by a first 3-h recovery, a second 1-h pulse, a second 3-h recovery, and a third 1-h GH pulse before harvesting.
Fig. 8.
Pulsatile GH treatment causes enhanced STAT5-dependent luciferase reporter gene activity in T47D-ShPRLR vs. T47D-SCR cells. A, Pulsatile GH-induced STAT5 activation. Serum-starved T47D-SCR vs. T47D-ShPRLR cells were treated with GH (500 ng/ml) or vehicle, according to one of each of the following six regimens, as indicated: 1) left untreated; 2) treated with GH (500 ng/ml) for a 1-h pulse before harvesting; 3) treated with a 1-h GH pulse followed by washout and a 3-h recovery period in serum-free buffer before harvesting; 4) treated with a 1-h GH pulse, followed by a 3-h recovery and a second 1-h GH pulse before harvesting; 5) treated with a first 1-h GH pulse, followed by a first 3-h recovery, a second 1-h GH pulse, and a second 3-h recovery before harvesting: and 6) treated with a first 1-h GH pulse, followed by a first 3-h recovery, a second 1-h pulse, a second 3-h recovery, and a third 1-h GH pulse before harvesting. Detergent cell extracts were resolved by SDS-PAGE and sequentially immunoblotted with anti-pSTAT5, STAT5, anti-GHR, and antiactin. Data shown are representative of three independent experiments. Note that all samples in A were resolved on the same gels but are separated in the figure according to cell type for ease of visualization. B, GH-induced transactivation of a STAT5-dependent luciferase reporter. T47D-SCR and T47D-ShPRLR cells were transiently cotransfected with plasmids encoding a CISH element-driven firefly luciferase gene and a constitutively driven renilla luciferase gene. As detailed in Materials and Methods, cells were treated in triplicate with GH at various concentrations (25, 100, 200, or 500 ng/ml) vs. buffer alone according to the following protocol: a 1-h GH pulse, followed by washout and a 3-h recovery period, a second 1-h GH pulse, and a second 3-h recovery period. After completion of the stimulation protocol, firefly and renilla luciferase activities were measured and expressed as the GH-induced fold increase in normalized firefly luciferase activity. *, P < 0.05 when comparing T47D-SCR vs. T47D-ShPRLR cells treated with the same concentration of GH. WB, Western blot; pSTAT5, phosphorylated STAT5.
Detergent extracts of harvested cells in each condition were resolved by SDS-PAGE and sequentially immunoblotted to detect phosphorylated STAT5, total STAT5, GHR, and, as a loading control, actin. As anticipated, GHR abundance at baseline and GH-induced STAT5 tyrosine phosphorylation in response to the first GH pulse were greater in T47D-ShPRLR cells than in T47D-SCR cells. In each cell type, the first GH pulse caused some GHR down-regulation, greater in T47D-ShPRLR cells, as expected. GHR abundance increased to some degree in both cells during the recovery period but was similarly down-regulated in each with subsequent GH pulses. GH-induced STAT5 tyrosine phosphorylation was for each GH pulse of equal or greater degree in T47D-ShPRLR cells compared with T47D-SCR cells. Notably, the GH-induced tyrosine phosphorylation levels achieved in T47D-ShPRLR cells by the second GH pulse (a total of 5 h of combined GH pulses and recovery periods) or by the third GH pulse (a total of 9 h of combined GH pulses and recovery periods) were not diminished compared with those resulting from the second or third GH pulses in T47D-SCR cells. This is in distinction to the markedly decreased STAT5 tyrosine phosphorylation observed in T47D-ShPRLR cells compared with T47D-SCR cells in Fig. 6 after continuous exposure to GH for 2, 4, or 7 h. Thus, the cumulative effects of successive 1-h GH pulses with intervening 3-h recovery periods were greater in terms of STAT5 phosphorylation than were the effects of continuous GH exposures for similar overall durations.
To assess the cumulative effect of the pulse/recovery protocol on a downstream result of STAT5 activation in T47D-ShPRLR vs. T47D-SCR cells, we assessed the transactivation of the luciferase gene under the control of the STAT5-responsive element of the CISH gene (51) (Fig. 8B). T47D-ShPRLR and T47D-SCR cells were transiently cotransfected with the CISH element-driven firefly luciferase plasmid as well as a constitutively driven renilla luciferase plasmid. Each transfected cell type was then treated in replicate with GH at various concentrations (25, 100, 200, or 500 ng/ml) vs. buffer alone according to the following protocol: 1-h GH pulse, followed by washout and a 3-h recovery period, a second 1-h GH pulse, and a second 3-h recovery period. Firefly and renilla luciferase activities were measured and expressed as the GH-induced fold increase in normalized firefly luciferase activity. This GH pulse/recovery protocol produced a GH dose-responsive increase in CISH-luciferase activity in both cells that plateaued above 100 ng/ml GH. Interestingly, pulsatile GH exposure produced an approximately 2-fold greater response in the T47D-ShPRLR cells than in the T47D-SCR cells (Fig. 8B). This suggests that the cumulative transactivation effect of pulsatile GH treatment in PRLR-deficient breast cancer cells, like the acute GH-induced STAT5 activation in those cells, was markedly augmented compared with the same pulsatile GH delivery method in the T47D-SCR cells. This further bolsters the idea that both acute and cumulative sensitivity to GH (when delivered in a repeated pulsatile fashion) is enhanced when PRLR abundance is lessened.
Discussion
Like their cognate hormones, GHR and PRLR share common ancestry. The receptors reside in the same subfamily of the cytokine receptor superfamily by virtue of overall structural similarities. Likewise, some signaling cascades emanating from the ligand-engaged GHR and PRLR are shared (18–21). Studies of the biochemical and biological roles of GHR and PRLR have often focused on cells and tissues in which one or the other of the two receptors was predominantly present or in which the presence of the other was not considered when studying either GHR or PRLR. Potentially complicating our understanding of the relationship between the two receptors in humans is the knowledge that GH, traditionally known for somatogenic and metabolic effects, can also interact with PRLR and thus potentially exert lactogenic effects (27–30).
Hence, contextual issues (e.g. whether both receptors are present), the potential roles of both GH and PRL in breast cancer pathogenesis and behavior, and the ability of GH to interact with both receptors have encouraged us to investigate GH signaling in T47D human breast cancer cells that endogenously express ample GHR and PRLR (42). We previously found that both GH and PRL acutely activate JAK2/STAT5 signaling in T47D cells but that although GH engaged GHR, GH treatment more robustly led to PRLR, rather than GHR, tyrosine phosphorylation; furthermore, GHR-specific GH antagonists were largely ineffective as inhibitors of GH signaling. Most notably, we observed by coimmunoprecipitation that GHR and PRLR specifically associate in T47D cells and that the degree of their association was enhanced acutely by GH treatment. These findings raised intriguing and potentially physiologically relevant questions about GH's use of GHR vs. PRLR and about how PRLR might impact GHR availability, trafficking, and actions.
In the current work, we compared a T47D variant in which PRLR abundance was substantially reduced by stable expression of an shRNA that targets the long form of the receptor (T47D-ShPRLR cells) with control T47D in which a scrambled shRNA was stably expressed (T47D-SCR cells). Previous characterization of T47D-ShPRLR cells indicated that the PRLR level was reduced, as was the responsiveness to PRL (47). These findings were verified in the current study. Furthermore, we demonstrated that PRLR knockdown resulted in augmented abundance of the mature form of GHR that was not explained by altered levels of GHR mRNA but rather was associated with greater mature GHR stability in T47D-ShPRLR vs. T47D-SCR cells. Thus, reduction of PRLR appears to reduce mature GHR turnover and thereby raise steady-state mature GHR levels.
Acute GH responsiveness was markedly enhanced in T47D-ShPRLR cells, as evidenced by a dramatic left shift of the GH dose-response for acute STAT5 tyrosine phosphorylation, and this was accompanied by easily detected GH-induced GHR tyrosine phosphorylation in T47D-ShPRLR cells [compared with little or none detected in T47D-SCR cells or parental T47D cells (42)] and increased GH-induced JAK2 tyrosine phosphorylation in T47D-ShPRLR cells. Although a PRLR-specific antagonist inhibited GH-induced STAT5 activation in control cells, its inhibitory capacity was reduced in T47D-ShPRLR cells, suggesting that acute GH signaling in the PRLR knockdown cells was shifted toward the use of GHR rather than PRLR. Importantly, this was verified and extended by use of a GHR-specific antagonistic monoclonal antibody, anti-GHRext-mAb; this antibody substantially inhibited GH-dependent STAT5 activation in T47D-ShPRLR but not in T47D-SCR cells. Although acute GH signaling was augmented in PRLR-deficient cells, prolonged continuous exposure to GH yielded greater STAT5 phosphorylation in T47D-SCR cells. This differential time-course result was possibly explained by GH's ability to more rapidly and profoundly down-regulate GHR in the setting of PRLR deficiency. Indeed, delivery of GH to mimic a more physiological pulsatile pattern with interpulse washout of the hormone led to greater GH-induced activation of a STAT5-dependent reporter gene in T47D-ShPRLR cells. This suggests that with repeated intermittent volleys, the enhanced acute GH sensitivity observed in PRLR-deficient cells translates into enhanced integrated downstream effects.
The current findings complement our prior studies of GH signaling in breast cancer cells and enhance our appreciation for the complexity of what may at first glance appear relatively straightforward. The ability in humans of GH to use both the GHR and the PRLR whereas PRL cannot interact with GHR suggests the possibility that meaningful functions might be differentially ascribed to GH, depending on which receptor it predominantly engages (and the role of GHR-PRLR heterodimers). Discerning these functions in future studies may be facilitated by the fact that we observed GH-dependent PRLR-mediated signaling despite performing our experiments without the addition of zinc; this is relevant because added zinc in other contexts has been shown to increase human GH's affinity for the human PRLR (28). We assume that ambient zinc concentrations in our system are sufficient to allow productive GH-PRLR interaction. Utama et al. (63) also detected productive hGH-human PRLR interaction without zinc, although the affinity was augmented by zinc addition in that case.) Furthermore, comparison of T47D-SCR cells (in which GHR and PRLR are amply expressed) with T47D-ShPRLR cells (in which PRLR is present but of lesser abundance) allowed us to learn whether changing the GHR to PRLR ratio (rather than eliminating the PRLR altogether) affects GH sensitivity. Indeed, this approach was profitable in that PRL action (and therefore PRLR function) was not completely abrogated in T47D-ShPRLR (as in Fig. 4). Thus, the effects of lessened PRLR abundance, which are likely more subtle and possibly more physiologically relevant than complete PRLR loss, were revealed in this system. [Notably, inhibition of GH signaling by anti-GHRext-mAb in T47D-ShPRLR cells was incomplete; thus, the continued presence of residual PRLR could, in principle, influence GHR signaling and its sensitivity to anti-GHRext-mAb by virtue of continued heterodimerization with GHR (more below).] Such observations further embolden us in the notion that the responsiveness of cell types, including cancer cells, to GH and/or PRL may differ according to their relative GHR-PRLR abundance. To our knowledge, the current study is the first to prospectively test this notion by manipulating the GHR to PRLR ratio within the same cell type. Further studies comparing differing GHR to PRLR ratios may be warranted to address this issue further.
One striking observation in the T47D system is that GHR and PRLR specifically coimmunoprecipitate and that this association is enhanced acutely by GH (42). A number of interesting and relevant questions flow from these observations. It is first interesting to ponder whether such association between the two receptors is accounted for by heterodimerization (a GHR monomer associating with a PRLR monomer in a dimeric assemblage) vs. a higher order association (e.g. GHR-GHR homodimers associating with PRLR-PRLR homodimers). We are actively pursuing this question by attempting to map by mutagenesis the region(s) of association between GHR and PRLR and assessing the closeness of the association (hence, whether accounted for by dimerization per se vs. oligomerization) by biophysical techniques.
However, we note that GHR-PRLR association, whether dimeric or heterooligomeric, could have important effects at various levels. For example, ligand-independent homodimerization (so-called predimerization) of GHR and of PRLR has been observed (31, 34–37) and likely occurs soon after protein synthesis and perhaps early in the pathway leading to cell surface expression. If similar GHR-PRLR preheterodimerization occurs, one could envision effects of such association on the dynamics of surface GHR or PRLR expression and/or on other aspects of the ligand-independent trafficking itinerary of the receptors. Thus, we are intrigued by our findings that reduction of PRLR abundance in T47D-ShPRLR cells results in posttranslational augmentation of steady-state mature GHR levels with apparently increased mature GHR stability. Although other explanations are possible, perhaps PRLR association with GHR confers to GHR the more rapid turnover of PRLR observed in these cells; thus, reduction of PRLR (and thus of PRLR associated GHR) may enable GHR (homodimers) to achieve greater stability and increased steady-state abundance and cell surface display. Additionally, we previously showed that GHR stability and efficiency of trafficking to the cell surface are both facilitated by the receptor's association with JAK2 (53, 64, 65). Although JAK2 abundance is unaltered in T47D-ShPRLR cells compared with T47D-SCR cells, might lessening the PRLR levels allow a greater fraction of GHR to associate with JAK2 and thus exert ligand-independent effects on GHR stability and surface expression? Information concerning the mechanism(s) of the GHR-PRLR association and the consequent effects on GHR-JAK2 association may help to adjudicate among these and other possibilities.
In addition to ligand-independent effects on GHR's fate, we also found that GH-dependent GHR down-regulation, especially within 30 min of GH exposure, differed dramatically in cells with reduced PRLR (graphically presented in Fig. 7B). The markedly enhanced GH-dependent GHR down-regulation in T47D-ShPRLR cells is consistent with our findings that GH-induced GHR tyrosine phosphorylation was augmented in these cells, as was their sensitivity to inhibition of GH signaling by our GHR-specific antagonistic monoclonal antibody. We previously demonstrated several critical determinants for efficient acute GH-induced GHR down-regulation (55, 59). These include the following: the capacity of GHR to interact with JAK2 via the GHR Box 1 element and the N-terminal FERM (4.1, ezrin, radixin, muesin) domain region of JAK2; expression of a catalytically intact JAK2; a GH-dependent JAK2 activation; and tyrosine phosphorylation of GHR itself. Of note, we recently compared the wild-type GHR with a GHR mutant in which all intracellular domain tyrosine residues are changed to phenylalanines, preventing GH-induced GHR tyrosine phosphorylation but allowing GH-dependent JAK2 activation and autophosphorylation (59). The mutant GHR was severely deficient in down-regulation, despite robust and markedly prolonged GH-induced JAK2 activation; in this case, reduced GH-induced receptor down-regulation was associated with deficient GH-induced GHR internalization, a very proximal step in the down-regulation process.
Our current findings indicate that although GH does engage GHR in (parental) T47D cells [as evidenced by GH induced disulfide linkage of GHR (42)], this GH-GHR engagement is either not strong enough to cause GHR tyrosine phosphorylation and down-regulation or that the presence of PRLR actively prevents such processes from occurring. In either case, it is compelling that PRLR silencing reverses such a blockade, allows GHR to more robustly respond to GH, and/or allows greater GHR abundance and thereby favors greater GH responsiveness and down-regulation. Whether there is reciprocal impact of GHR on PRLR availability and/or function will be an important avenue to explore as well. We are also mindful that altered ligand-induced receptor down-regulation can alter downstream signaling patterns (66); thus, further examination of downstream signaling in our model may be warranted.
Along with these mechanistic and cell biological questions raised by our data, we envision potential therapeutic insights as well. To the degree that GH and PRL may be players in breast cancer pathogenesis or progression (9–12, 15–17), there has been substantial interest in the potential virtue of inhibition of the actions of either or both of these hormones. We observe that the capacity of the GHR-specific antagonist monoclonal antibody, anti-GHRext-mAb, to inhibit GH action is profound in cells that do not express PRLR (36, 42, 43., 46, 58), nearly nonexistent in T47D cells that express both receptors (see Fig. 5 and Ref. 42) and partially restored in T47D-ShPRLR cells that have reduced PRLR and increased GHR (Fig. 5). If GHR antagonists, including our monoclonal antibody, are to have therapeutic potential, our data suggest that it may be important to know not only GHR status, but also PRLR status, of cancers to be targeted. By the same token, the potential of PRLR antagonists (57) or combination therapy with GHR antagonists and PRLR antagonists for cancers such as breast cancer will be better informed by more complete understanding of the presence and consequences of the degree of coexpression of the two receptors. Studies along these lines should prove quite fruitful.
Finally, we note that the effects in our system of the duration (acute vs. several hours) of exposure to GH produced apparently disparate results in terms of the level of STAT5 dependent signals generated. However, when the typical pulsatile pattern of GH exposure seen in vivo was mimicked, we observed that repeated acute bouts of GH-induced STAT5 activation with intervening washout of the hormone yielded a summed response that was indeed greater in cells in which PRLR was reduced and acute GH signaling was augmented. This is important in several respects. First, it illustrates that standard continuous prolonged in vitro treatment that is usually used to test for GH responsiveness may be misleading. Second, it is worth considering that GH and PRL differ substantially in their circulating profiles with PRL being much less pulsatile than GH. Thus, it may be that PRLR (in terms of being a PRL effector, at least) may have evolved different mechanisms of down-regulation and reappearance when stimulated by PRL or GH than has GHR. The paradigm used in this study may therefore be of utility in examining the behavior of breast and other cancer cells in terms of GH and PRL responsiveness.
Acknowledgments
The authors appreciate helpful conversations with Drs. J. Messina, K. Zinn, Y. Gan, A. Paterson, Y. Liu, A. Buckels, and P. Berry and the generous provision of reagents by those named in the text.
This work was supported by the National Institutes of Health Grant R01DK58259 (to S.J.F.).
Parts of this work were presented at the 92nd Annual Meeting of The Endocrine Society in San Diego, CA, in June 2010 and the 94th Annual Meeting of The Endocrine Society in Houston, TX, in June 2012.
Disclosure Summary: J.X., D.S., J.J., L.D., Y.Z., H.Y., D.B., J.F.L., W.Y.C., S.Y.F., and S.J.F. have nothing to declare.
Footnotes
- anti-pY
- Antiphosphotyrosine
- CHX
- cycloheximide
- endoH
- endoglycosidase H
- GHR
- GH receptor
- h
- human
- JAK2
- Janus kinase 2
- PRL
- prolactin
- PRLR
- PRL receptor
- shRNA
- short hairpin RNA
- STAT5
- signal transducer and activator of transcription 5.
References
- 1. Huising MO, Kruiswijk CP, Flik G. 2006. Phylogeny and evolution of class-I helical cytokines. J Endocrinol 189:1–25 [DOI] [PubMed] [Google Scholar]
- 2. Teilum K, Hoch JC, Goffin V, Kinet S, Martial JA, Kragelund BB. 2005. Solution structure of human prolactin. J Mol Biol 351:810–823 [DOI] [PubMed] [Google Scholar]
- 3. Isaksson OG, Edén S, Jansson JO. 1985. Mode of action of pituitary growth hormone on target cells. Annu Rev Physiol 47:483–499 [DOI] [PubMed] [Google Scholar]
- 4. Kaplan S. 1999. Hormonal regulation of growth and metabolic effects of growth hormone. In: Kostyo J, Goodman HM, eds. Handbook of physiology. Chap 5 New York: Oxford University Press [Google Scholar]
- 5. Møller N, Jørgensen JO. 2009. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 30:152–177 [DOI] [PubMed] [Google Scholar]
- 6. Waters MJ, Hoang HN, Fairlie DP, Pelekanos RA, Brown RJ. 2006. New insights into growth hormone action. J Mol Endocrinol 36:1–7 [DOI] [PubMed] [Google Scholar]
- 7. Goffin V, Binart N, Touraine P, Kelly PA. 2002. Prolactin: the new biology of an old hormone. Annu Rev Physiol 64:47–67 [DOI] [PubMed] [Google Scholar]
- 8. Rui H. 2000. Prolactin. In: Oppenheim JJ, Feldman M, eds. Cytokine reference on-line. London: Academic Press, Harcourt; 267–283 [Google Scholar]
- 9. Swanson SM, Unterman TG. 2002. The growth hormone-deficient Spontaneous Dwarf rat is resistant to chemically induced mammary carcinogenesis. Carcinogenesis 23:977–982 [DOI] [PubMed] [Google Scholar]
- 10. Shen Q, Lantvit DD, Lin Q, Li Y, Christov K, Wang Z, Unterman TG, Mehta RG, Swanson SM. 2007. Advanced rat mammary cancers are growth hormone dependent. Endocrinology 148:4536–4544 [DOI] [PubMed] [Google Scholar]
- 11. Wang Z, Prins GS, Coschigano KT, Kopchick JJ, Green JE, Ray VH, Hedayat S, Christov KT, Unterman TG, Swanson SM. 2005. Disruption of growth hormone signaling retards early stages of prostate carcinogenesis in the C3(1)/T antigen mouse. Endocrinology 146:5188–5196 [DOI] [PubMed] [Google Scholar]
- 12. Wang Z, Luque RM, Kineman RD, Ray VH, Christov KT, Lantvit DD, Shirai T, Hedayat S, Unterman TG, Bosland MC, Prins GS, Swanson SM. 2008. Disruption of growth hormone signaling retards prostate carcinogenesis in the probasin/TAg rat. Endocrinology 149:1366–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clayton PE, Banerjee I, Murray PG, Renehan AG. 2011. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol 7:11–24 [DOI] [PubMed] [Google Scholar]
- 14. Waters MJ, Barclay JL. 2007. Does growth hormone drive breast and other cancers? Endocrinology 148:4533–4535 [DOI] [PubMed] [Google Scholar]
- 15. Clevenger CV, Furth PA, Hankinson SE, Schuler LA. 2003. The role of prolactin in mammary carcinoma. Endocr Rev 24:1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goffin V, Touraine P, Pichard C, Bernichtein S, Kelly PA. 1999. Should prolactin be reconsidered as a therapeutic target in human breast cancer? Mol Cell Endocrinol 151:79–87 [DOI] [PubMed] [Google Scholar]
- 17. Vonderhaar BK. 1999. Prolactin involvement in breast cancer. Endocr Relat Cancer 6:389–404 [DOI] [PubMed] [Google Scholar]
- 18. Campbell GS, Argetsinger LS, Ihle JN, Kelly PA, Rillema JA, Carter-Su C. 1994. Activation of JAK2 tyrosine kinase by prolactin receptors in Nb2 cells and mouse mammary gland explants. Proc Natl Acad Sci USA 91:5232–5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rui H, Kirken RA, Farrar WL. 1994. Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol Chem 269:5364–5368 [PubMed] [Google Scholar]
- 20. Clevenger CV, Kline JB. 2001. Prolactin receptor signal transduction. Lupus 10:706–718 [DOI] [PubMed] [Google Scholar]
- 21. Huang Y, Li X, Jiang J, Frank SJ. 2006. Prolactin modulates phosphorylation, signaling and trafficking of epidermal growth factor receptor in human T47D breast cancer cells. Oncogene 25:7565–7576 [DOI] [PubMed] [Google Scholar]
- 22. Boutin JM, Edery M, Shirota M, Jolicoeur C, Lesueur L, Ali S, Gould D, Djiane J, Kelly PA. 1989. Identification of a cDNA encoding a long form of prolactin receptor in human hepatoma and breast cancer cells. Mol Endocrinol 3:1455–1461 [DOI] [PubMed] [Google Scholar]
- 23. Frank SJ, Gilliland G, Kraft AS, Arnold CS. 1994. Interaction of the growth hormone receptor cytoplasmic domain with the JAK2 tyrosine kinase. Endocrinology 135:2228–2239 [DOI] [PubMed] [Google Scholar]
- 24. Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C. 1993. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237–244 [DOI] [PubMed] [Google Scholar]
- 25. VanderKuur JA, Wang X, Zhang L, Campbell GS, Allevato G, Billestrup N, Norstedt G, Carter-Su C. 1994. Domains of the growth hormone receptor required for association and activation of JAK2 tyrosine kinase. J Biol Chem 269:21709–21717 [PubMed] [Google Scholar]
- 26. DaSilva L, Howard OM, Rui H, Kirken RA, Farrar WL. 1994. Growth signaling and JAK2 association mediated by membrane-proximal cytoplasmic regions of prolactin receptors. J Biol Chem 269:18267–18270 [PubMed] [Google Scholar]
- 27. Hughes JP, Friesen HG. 1985. The nature and regulation of the receptors for pituitary growth hormone. Annu Rev Physiol 47:469–482 [DOI] [PubMed] [Google Scholar]
- 28. Cunningham BC, Bass S, Fuh G, Wells JA. 1990. Zinc mediation of the binding of human growth hormone to the human prolactin receptor. Science 250:1709–1712 [DOI] [PubMed] [Google Scholar]
- 29. Somers W, Ultsch M, De Vos AM, Kossiakoff AA. 1994. The X-ray structure of a growth hormone-prolactin receptor complex. Nature 372:478–481 [DOI] [PubMed] [Google Scholar]
- 30. Fu YK, Arkins S, Fuh G, Cunningham BC, Wells JA, Fong S, Cronin MJ, Dantzer R, Kelley KW. 1992. Growth hormone augments superoxide anion secretion of human neutrophils by binding to the prolactin receptor. J Clin Invest 89:451–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qazi AM, Tsai-Morris CH, Dufau ML. 2006. Ligand-independent homo- and heterodimerization of human prolactin receptor variants: inhibitory action of the short forms by heterodimerization. Mol Endocrinol 20:1912–1923 [DOI] [PubMed] [Google Scholar]
- 32. de Vos AM, Ultsch M, Kossiakoff AA. 1992. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 255:306–312 [DOI] [PubMed] [Google Scholar]
- 33. Cunningham BC, Ultsch M, De Vos AM, Mulkerrin MG, Clauser KR, Wells JA. 1991. Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science 254:821–825 [DOI] [PubMed] [Google Scholar]
- 34. Gent J, van Kerkhof P, Roza M, Bu G, Strous GJ. 2002. Ligand-independent growth hormone receptor dimerization occurs in the endoplasmic reticulum and is required for ubiquitin system-dependent endocytosis. Proc Natl Acad Sci USA 99:9858–9863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown RJ, Adams JJ, Pelekanos RA, Wan Y, McKinstry WJ, Palethorpe K, Seeber RM, Monks TA, Eidne KA, Parker MW, Waters MJ. 2005. Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Mol Biol 12:814–821 [DOI] [PubMed] [Google Scholar]
- 36. Yang N, Wang X, Jiang J, Frank SJ. 2007. Role of the growth hormone (GH) receptor transmembrane domain in receptor predimerization and GH-induced activation. Mol Endocrinol 21:1642–1655 [DOI] [PubMed] [Google Scholar]
- 37. Gadd SL, Clevenger CV. 2006. Ligand-independent dimerization of the human prolactin receptor isoforms: functional implications. Mol Endocrinol 20:2734–2746 [DOI] [PubMed] [Google Scholar]
- 38. Brooks AJ, Waters MJ. 2010. The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol 6:515–525 [DOI] [PubMed] [Google Scholar]
- 39. Biener E, Martin C, Daniel N, Frank SJ, Centonze VE, Herman B, Djiane J, Gertler A. 2003. Ovine placental lactogen-induced heterodimerization of ovine growth hormone and prolactin receptors in living cells is demonstrated by fluorescence resonance energy transfer microscopy and leads to prolonged phosphorylation of signal transducer and activator of transcription (STAT)1 and STAT3. Endocrinology 144:3532–3540 [DOI] [PubMed] [Google Scholar]
- 40. Herman A, Bignon C, Daniel N, Grosclaude J, Gertler A, Djiane J. 2000. Functional heterodimerization of prolactin and growth hormone receptors by ovine placental lactogen. J Biol Chem 275:6295–6301 [DOI] [PubMed] [Google Scholar]
- 41. Langenheim JF, Chen WY. 2009. Development of a novel ligand that activates JAK2/STAT5 signaling through a heterodimer of prolactin receptor and growth hormone receptor. J Recept Signal Transduct Res 29:107–112 [DOI] [PubMed] [Google Scholar]
- 42. Xu J, Zhang Y, Berry PA, Jiang J, Lobie PE, Langenheim JF, Chen WY, Frank SJ. 2011. Growth hormone signaling in human T47D breast cancer cells: potential role for a growth hormone receptor-prolactin receptor complex. Mol Endocrinol 25:597–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang N, Langenheim JF, Wang X, Jiang J, Chen WY, Frank SJ. 2008. Activation of growth hormone receptors by growth hormone and growth hormone antagonist dimers: insights into receptor triggering. Mol Endocrinol 22:978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Y, Guan R, Jiang J, Kopchick JJ, Black RA, Baumann G, Frank SJ. 2001. Growth hormone (GH)-induced dimerization inhibits phorbol ester-stimulated GH receptor proteolysis. J Biol Chem 276:24565–24573 [DOI] [PubMed] [Google Scholar]
- 45. Jiang J, Liang L, Kim SO, Zhang Y, Mandler R, Frank SJ. 1998. Growth hormone-dependent tyrosine phosphorylation of a GH receptor-associated high molecular weight protein immunologically related to JAK2. Biochem Biophys Res Commun 253:774–779 [DOI] [PubMed] [Google Scholar]
- 46. Jiang J, Wang X, He K, Li X, Chen C, Sayeski PP, Waters MJ, Frank SJ. 2004. A conformationally-sensitive GHR [growth hormone (GH) receptor] antibody: impact on GH signaling and GHR proteolysis. Mol Endocrinol 18:2981–2996 [DOI] [PubMed] [Google Scholar]
- 47. Plotnikov A, Varghese B, Tran TH, Liu C, Rui H, Fuchs SY. 2009. Impaired turnover of prolactin receptor contributes to transformation of human breast cells. Cancer Res 69:3165–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Asa SL, Digiovanni R, Jiang J, Ward ML, Loesch K, Yamada S, Sano T, Yoshimoto K, Frank SJ, Ezzat S. 2007. A growth hormone receptor mutation impairs growth hormone autofeedback signaling in pituitary tumors. Cancer Res 67:7505–7511 [DOI] [PubMed] [Google Scholar]
- 49. Bujalska IJ, Quinkler M, Tomlinson JW, Montague CT, Smith DM, Stewart PM. 2006. Expression profiling of 11β-hydroxysteroid dehydrogenase type-1 and glucocorticoid-target genes in subcutaneous and omental human preadipocytes. J Mol Endocrinol 37:327–340 [DOI] [PubMed] [Google Scholar]
- 50. Fang F, Flegler AJ, Du P, Lin S, Clevenger CV. 2009. Expression of cyclophilin B is associated with malignant progression and regulation of genes implicated in the pathogenesis of breast cancer. Am J Pathol 174:297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fang F, Antico G, Zheng J, Clevenger CV. 2008. Quantification of PRL/Stat5 signaling with a novel pGL4-CISH reporter. BMC Biotechnol 8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yi W, Kim SO, Jiang J, Park SH, Kraft AS, Waxman DJ, Frank SJ. 1996. Growth hormone receptor cytoplasmic domain differentially promotes tyrosine phosphorylation of signal transducers and activators of transcription 5b and 3 by activated JAK2 kinase. Mol Endocrinol 10:1425–1443 [DOI] [PubMed] [Google Scholar]
- 53. He K, Wang X, Jiang J, Guan R, Bernstein KE, Sayeski PP, Frank SJ. 2003. Janus kinase 2 determinants for growth hormone receptor association, surface assembly, and signaling. Mol Endocrinol 17:2211–2227 [DOI] [PubMed] [Google Scholar]
- 54. He K, Loesch K, Cowan JW, Li X, Deng L, Wang X, Jiang J, Frank SJ. 2005. Janus kinase 2 enhances the stability of the mature growth hormone receptor. Endocrinology 146:4755–4765 [DOI] [PubMed] [Google Scholar]
- 55. Deng L, He K, Wang X, Yang N, Thangavel C, Jiang J, Fuchs SY, Frank SJ. 2007. Determinants of growth hormone receptor down-regulation. Mol Endocrinol 21:1537–1551 [DOI] [PubMed] [Google Scholar]
- 56. Goffin V, Bernichtein S, Carrière O, Bennett WF, Kopchick JJ, Kelly PA. 1999. The human growth hormone antagonist B2036 does not interact with the prolactin receptor. Endocrinology 140:3853–3856 [DOI] [PubMed] [Google Scholar]
- 57. Goffin V, Bernichtein S, Touraine P, Kelly PA. 2005. Development and potential clinical uses of human prolactin receptor antagonists. Endocr Rev 26:400–422 [DOI] [PubMed] [Google Scholar]
- 58. Jiang J, Wan Y, Wang X, Xu J, Harris JM, Lobie PE, Zhang Y, Zinn KR, Waters MJ, Frank SJ. 2011. Inhibitory GH receptor extracellular domain monoclonal antibodies: three-dimensional epitope mapping. Endocrinology 152:4777–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Deng L, Jiang J, Frank SJ. 2012. Growth hormone-induced JAK2 signaling and GH receptor down-regulation: role of GH receptor intracellular domain tyrosine residues. Endocrinology 153:2311–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ionescu M, Frohman LA. 2006. Pulsatile secretion of growth hormone (GH) persists during continuous stimulation by CJC-1295, a long-acting GH-releasing hormone analog. J Clin Endocrinol Metab 91:4792–4797 [DOI] [PubMed] [Google Scholar]
- 61. Tannenbaum GS, Martin JB. 1976. Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology 98:562–570 [DOI] [PubMed] [Google Scholar]
- 62. MacLeod JN, Pampori NA, Shapiro BH. 1991. Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J Endocrinol 131:395–399 [DOI] [PubMed] [Google Scholar]
- 63. Utama FE, Tran TH, Ryder A, LeBaron MJ, Parlow AF, Rui H. 2009. Insensitivity of human prolactin receptors to nonhuman prolactins: relevance for experimental modeling of prolactin receptor-expressing human cells. Endocrinology 150:1782–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. He K, Loesch K, Cowan JW, Li X, Deng L, Wang X, Jiang J, Frank SJ. 2005. JAK2 enhances the stability of the mature GH receptor. Endocrinology 145:4755–4765 [DOI] [PubMed] [Google Scholar]
- 65. Loesch K, Deng L, Wang X, He K, Jiang J, Frank SJ. 2007. Endoplasmic reticulum-associated degradation of growth hormone receptor in Janus kinase 2-deficient cells. Endocrinology 148:5955–5965 [DOI] [PubMed] [Google Scholar]
- 66. Piazza TM, Lu JC, Carver KC, Schuler LA. 2009. SRC family kinases accelerate prolactin receptor internalization, modulating trafficking and signaling in breast cancer cells. Mol Endocrinol 23:202–212 [DOI] [PMC free article] [PubMed] [Google Scholar]