Abstract
The MAPKs are transducers of extracellular signals such as proinflammatory cytokines. In islet β-cells, cytokines acutely activate expression of the Nos2 gene encoding inducible nitric oxide synthase (iNOS), which ultimately impairs insulin release. Because iNOS production can also be regulated posttranscriptionally, we asked whether MAPKs participate in posttranscriptional regulatory events in β-cells and primary islets in response to cytokine signaling. We show that cytokines acutely reduce cellular oxygen consumption rate and impair aconitase activity. Inhibition of iNOS with l-NMMA or inhibition of Nos2 mRNA translation with GC7 [an inhibitor of eukaryotic translation initiation factor 5A (eIF5A) activity] reversed these defects, as did inhibition of p38 MAPK by PD169316. Although inhibition of p38 had no effect on the nuclear translocation of nuclear factor κB or the abundance of Nos2 transcripts during the immediate period after cytokine exposure, its inhibition or knockdown resulted in significant reduction in iNOS protein, a finding suggestive of a permissive role for p38 in Nos2 translation. Polyribosomal profiling experiments using INS-1 β-cells revealed that Nos2 mRNA remained associated with polyribosomes in the setting of p38 inhibition, in a manner similar to that seen with blockade of translational elongation by cycloheximide. Consistent with a role in translational elongation, p38 activity is required in part for the activation of the translational factor eIF5A by promoting its hypusination. Our results suggest a novel signaling pathway in β-cells in which p38 MAPK promotes translation elongation of Nos2 mRNA via regulation of eIF5A hypusination.
Type 1 and type 2 diabetes mellitus are characterized by the absolute and relative deficiencies in islet β-cell insulin secretion, respectively. In the months or years leading up to the development of either form of diabetes, it is thought that declining β-cell function and/or mass is triggered by elevations in circulating factors such as cytokines and free fatty acids. In the case of type 1 diabetes, our recent studies in nonobese diabetic (NOD) mice showed that the release of proinflammatory cytokines by islet-invading immune cells may lead to β-cell endoplasmic reticulum stress and insulin-secretory deficiencies, even before the development of frank diabetes (1). In this respect, treatments that specifically target the cytokine responses in the prediabetic stage might have the benefit of preventing or delaying the onset of type 1 diabetes. Similarly, in type 2 diabetes, evidence in both rodents and humans suggests that circulating proinflammatory cytokines (from adipocytes, macrophages) and glucolipotoxicity promote β-cell dysfunction and a decline in β-cell mass (2–6). Thus, intervening in the cytokine responses in the prediabetic stage of type 2 diabetes might also delay or prevent the β-cell dysfunction that leads to overt hyperglycemia.
Although many stressors have been invoked to explain the defects in β-cell insulin secretion in the prediabetic/early diabetic phases (for a review, see Ref. 7), cytokine-mediated pathways have received notable attention in recent years because of their relevance to both type 1 and type 2 diabetes. Proinflammatory cytokines, particularly the combination of TNF-α, IFN-γ, and IL1-β rapidly activate the transcription of the gene encoding inducible nitric oxide synthase (iNOS) (Nos2) via the nuclear factor κB (NFκB) pathway (8). Nitric oxide, the product of iNOS catalysis, impairs glucose oxidation and ATP production in the short term (9) and contributes to β-cell death in the longer term (10–12). Transcription of Nos2 is dependent, in part, upon the direct engagement of NFκB and interferon response factor at control elements of the Nos2 promoter (13, 14) after cytokine signaling. The activation of Nos2 transcription is followed closely by the appearance of iNOS protein in β-cells (15), suggesting that the posttranscriptional machinery to generate iNOS is either constitutively active or regulated in parallel with transcription of its gene. Several posttranscriptional processes regulate iNOS production, including Nos2 nuclear export via CRM1/exportin 1 (15, 16), Nos2 mRNA half-life (17), iNOS dimerization, and Nos2 mRNA translation at the ribosome (15). Our recent studies in islet β-cells suggest that intracellular signaling by proinflammatory cytokines (IL-1β, TNF-α, IFN-γ) may be linked to Nos2 mRNA translation by promoting a crucial posttranslational modification of eukaryotic translation initiation factor 5A (eIF5A) (15). eIF5A is the only protein known to contain the amino acid hypusine, which is synthesized posttranslationally in a two-step reaction in which the amino butyl moiety of spermidine is conjugated to the ϵ-amino group of Lys50 (18). The enzymes responsible for this modification are deoxyhypusine synthase (DHS, the rate-limiting enzyme) and deoxyhypusine hydroxylase (DHH). Without the hypusine modification, eIF5A is translationally inactive and incapable of ensuring the production of iNOS (19). The mechanism by which cytokines affect the hypusine modification of eIF5A remains unclear.
The MAPKs represent a family of kinases that is characterized by responsiveness to extracellular stimuli, such as mitogens, proinflammatory cytokines, and other stress signals (20). Some of the more prominent responses of the MAPKs, particularly in the setting of glucose and lipid metabolism, include the regulation of stress gene expression and translation, and cellular proliferation, differentiation, and apoptosis (see Ref. 21 for a review). Prior studies have shown that isoforms of p38 MAPK (henceforth referred to simply as “p38”) are particularly sensitive to cytokine stimulation and can affect transcription of Nos2 depending upon the cellular context (22–24). In rat pancreatic islets, p38 appears to promote cytokine-mediated activation of Nos2 transcription, and inhibition of p38 results in partial inhibition of nitric oxide production (25). If and how MAPKs might regulate translation of the Nos2 mRNA in the early response to proinflammatory cytokines (during the period of time in vivo when β-cells dysfunction, but before over diabetes) has not been studied. In this report, we studied in vitro the early phase of cytokine-mediated production of iNOS during a 4-h incubation period when β-cells and islets show dysfunction but not overt death (15). We show that p38 appears to control translational elongation of Nos2 mRNA via a permissive effect on the hypusination rate of the translational factor eIF5A. Our studies provide a novel link between cytokine signaling and mRNA translation via the modification of a stress-responsive translational factor.
Materials and Methods
Animals, islets, and cell lines
Wistar rats were purchased from Charles River Laboratories (Wilmington, MA) and maintained at the Indiana University Laboratory Animal Resource Center under pathogen-free conditions following protocols approved by the Institutional Animal Care and Use Committee. The cytokine-responsive rat insulinoma cell line INS-1 (832/13) was maintained as previously described (26). Rat islets were isolated from collagenase-perfused pancreata as described (27), and then hand picked and counted. Islets were allowed to recover in RPMI 1640 medium containing 11 mm glucose and supplemented with 10% fetal bovine serum and 100 U/ml penicillin/100 μg/ml streptomycin overnight before experimentation. Human islets from six cadaveric donors were obtained through the Integrated Islet Distribution Program. Upon arrival, human islets were immediately placed in RPMI culture medium as described above and then incubated overnight before experimentation. The p38α+/+ and p38α−/− mouse embryonic fibroblasts (MEFs) (a gift from Dr. A. Nebreda, Barcelona, Spain) (28) were maintained in DMEM supplemented with 10% fetal bovine serum and penicillin/streptomycin.
Antibodies
For immunoblots, antibodies against the following proteins were used: actin (691001, MP Biomedicals, Irvine, CA), HDAC1 (06-720, Upstate Biotechnology, Lake Placid, NY), DHS (sc-67161, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), NFkB p65 and phosphor-p38 (Thr180/Tyr182) (4764 and 4511, respectively, Cell Signaling Technology, Danvers, MA), iNOS and Pdx1 (06-573 and 07-696, respectively, Millipore Corp., Bedford, MA), p38 (331300, Invitrogen, Carlsbad, CA), and eIF5A (611977, BD Biosciences, Palo Alto, CA). Fluorophore-labeled secondary antibodies IRDye 800 and IRDye 700 were from Li-Cor Biosciences (Lincoln, NE).
Cytokine and inhibitor treatment
Islet or cell cultures were treated for a total of 4 h at 37 C with a cytokine cocktail (“cytomix”) containing 5 ng/ml IL-1β, 10 ng/ml TNF-α, and 100 ng/ml IFN-γ (all from ProSpec, Rehovot, Israel) as described previously (15). For inhibitor studies, the DHS inhibitor N1-guanyl-1,7-diaminoheptane (GC7) (Biosearch Technologies, Novato, CA) was used in cultures at 100 μm final concentration, the iNOS inhibitor NG-monomethyl-l-arginine (lL-NMMA, Cayman Chemical, Ann Arbor, MI) was used at 1 mm final concentration, and inhibitors to p38 and c-Jun N-terminal kinase (JNK) (PD169316 and SP600125, respectively, from Sigma, St. Louis, MO) were used in cultures at 10 μm final concentration. Cells were incubated with each inhibitor overnight (∼ 16 h) at 37 C before cytomix addition. In some polyribosomal profiling experiments, 1 μm thapsigargin (Tg) and 50 μg/ml cycloheximide (CHX) were applied for 2 h before cytomix addition.
Metabolic flux measurements
To measure the cellular metabolism of INS-1 cells, a metabolic flux analyzer (Seahorse Bioscience, Billerica, MA) was used. INS-1 cells were seeded at 50,000 cells per well in 24-well plates for 48 h before experimentation. For inhibitor studies, cells were cultured overnight with inhibitors. Cells were then treated with cytokines for 4 h, washed with XF running medium with 2 mm glucose, and incubated for 1 h in the absence of exogenous CO2. Extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) measurements were measured at 7-min intervals. Glucose (20 mm) and 1.5 μm rotenone were applied by automatic injections.
Measurement of aconitase activity
Aconitase activity was determined using an aconitase assay kit per manufacturer's instructions (Abcam, Inc., Cambridge, MA). Briefly, INS-1 cells were seeded in six-well plates at 90% confluency (∼5 × 105 cells). After overnight incubation with and without inhibitors, cells were treated with cytokines for 4 h. For the final 10 min, cells were incubated at 20 mm glucose and then harvested and lysed in 100 μl of aconitase assay buffer by repeated freeze-thawing cycles.
Measurement of iNOS stability
INS-1 cells in six-well plates were treated with or without 10 μm PD169316, then exposed to cytomix for 2 h, after which 50 μg/ml CHX was added for an additional 0, 2, or 4 h. At the end of the incubation, cells were harvested for immunoblot analysis for iNOS. Immunoblots were quantitated and normalized to actin levels using Li-Cor software (Li-Cor Biosciences) and modeled upon single-phase decay kinetics using Prism 5.0 software version 5.0 (GraphPad Software, San Diego, CA).
Cell lysate preparation and immunoblot analysis
Nuclear and cytoplasmic extracts from INS-1 cells were prepared using a rapid lysate procedure (29), and whole-cell extracts from INS-1 cells and islets were prepared as described previously (30). Immunoblot analyses of INS-1 and islet extracts were performed after resolution of protein extracts by 4–20% gradient SDS-PAGE. Immunoblots were visualized using fluorescently labeled secondary antibodies (Li-Cor Biosciences) and were quantified using Li-Cor software.
Quantitative real-time RT-PCR
Total RNA from INS-1 cells and islets were reverse transcribed and subjected to quantitative real-time RT-PCR as described previously (31). Samples were normalized to Actb message levels, except in the case of RNA from polyribosomal profiling experiments, which is instead reported as the percent of total recovered RNA. All data represent the mean of triplicate determinations from at least three independent experiments of INS-1 cells or pooled rat/human islets from three separated isolations. For amplification of rat Nos2 and Actb, the following primers were used: Nos2 forward, 5′-AGAAAACCCCAGGTGCTATTCC-3′; Nos2 reverse, 5′-TGAAAAATCTCTCCATTGCCCC-3′. Actb forward, 5′-AGGTCATCACTATTGGCAACGA-3′; Actb reverse, 5′-ACTTCATGATGGAATTGAATGTAGTT-3′. Human Nos2 and Actb primers were described previously (15).
Polyribosomal profiling (PRP) experiments
PRP experiments in INS-1 cells and MEFs proceeded as described previously (1) but with some modifications. Cells grown to 90% confluency in 100-mm petri dishes were washed twice with cold PBS containing 50 μg/ml CHX and harvested in 500 μl of lysis buffer containing 20 mm Tris-HCl (pH 7.5), 10 mm MgCl2, 100 mm NaCl, 0.4% IGEPAL, 50 U/ml recombinant ribonuclease inhibitor RNasin (Promega Corp., Madison, WI), and 50 μg/ml CHX. The cell lysates were passed through a 23-gauge needle and incubated on ice for 10 min followed by centrifugation at 13,000 × g for 10 min at 4 C. Lysate (100 μl) was preserved as the input sample to determine total mRNA levels of Nos2. Supernatant (400 μl) was then layered onto a 10–50% sucrose gradient solution containing 20 mm Tris-HCl (pH 7.5), 5 mm MgCl2, 100 mm NaCl, and 50 μg/ml CHX. The sucrose gradients were subjected to centrifugation at 4 C in a Beckman SW-41Ti rotor at 40,000 rpm for 2 h. A piston gradient fractionator (BioComp Instruments, Fredericton, Canada) was used to fractionate the gradients, and absorbance of RNA at 254 nm was recorded using an in-line UV monitor. The eluate was collected using a fraction collector, and total RNA from the fractions was purified (Rneasy; Qiagen, Chatsworth, CA), reverse transcribed, and subjected to quantitative real-time PCR.
Hypusination assay
Hypusination assays proceeded as described previously (2). Briefly, approximately 50–100 islets or 106 INS-1 cells per condition were incubated with 1.5 μCi [3H]spermidine (PerkinElmer, Boston, MA) for 4 h, and whole-cell extracts were subjected to 12% SDS-PAGE. Gels were fixed and visualized by fluorography. Hypusinated eIF5A bands were quantitated using ImageJ Software (NIH).
Small interfering RNA (siRNA) knockdown
Knockdown of p38α in INS-1 cells was achieved using the rat MAPK14 Accell si-RNA SMARTpool (Thermo Scientific, Rockford, IL; catalog no, E-080059-00-0005) or Accell control si-RNA, in accordance with manufacturer's instructions. Briefly, INS-1 cells were plated in wells of a 96-well plate and treated with the Accell reagents and si-RNAs for 72 h, and then exposed to cytomix and [3H]spermidine for an additional 4 h before harvesting for immunoblots and quantitative real-time RT-PCR.
Statistics
All data are presented as the mean ± sem. One-way ANOVA (followed by a Dunnett's post test) was used for comparisons in which two or more conditions were compared with a single control, and a Student's t test was performed in which one condition was compared with a single control. Prism software version 5.0 (GraphPad) was used for all statistical analyses. Statistical significance was defined as P < 0.05.
Results
Cytokines acutely impair β-cell oxygen consumption rate and aconitase activity via iNOS
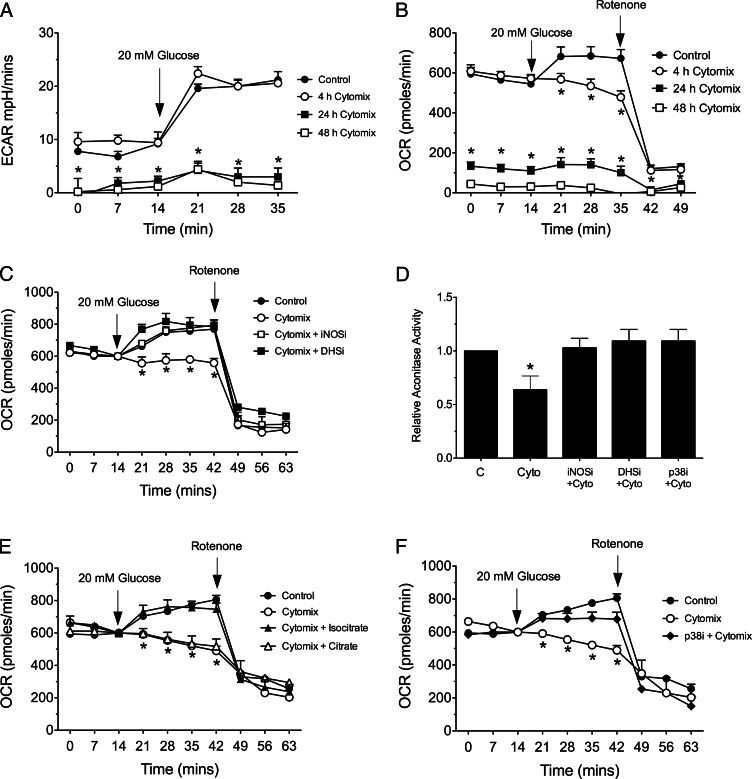
In prior studies, we showed that impairments in β-cell glucose-stimulated insulin release in NOD mice occur before the development of overt type 1 diabetes (1). To clarify the pathways leading to this dysfunction, we exposed rat INS-1 (832/13) β-cells to a mixture of cytokines (IL-1β, TNF-α, IFN-γ, “cytomix”) as seen in type 1 diabetes, and then performed metabolic flux analysis. ECAR is a reflection of the rate of anaerobic glycolysis as glucose is converted to lactate, whereas OCR primarily reflects aerobic metabolism of glucose via the tricarboxylic acid cycle and mitochondrial oxidative phosphorylation. As shown in Fig. 1, A and B, when glucose is raised from 2 to 20 mm in INS-1 cells, there is an acute increase in both ECAR and OCR, reflecting the increase in both basal anaerobic and aerobic glucose metabolism. Upon addition of rotenone (an electron transport inhibitor), complete loss in OCR was observed (Fig. 1B), indicating the dependence of oxygen consumption on the mitochondrial electron transport chain. Addition of the cytomix abolished glucose-stimulated OCR, but not ECAR, after just 4 h, and led to severe impairments in basal (nonstimulated) OCR and ECAR after 24 and 48 h (Fig. 1, A and B). These data suggest that cytokines cause early and specific impairments in aerobic glucose metabolism and are consistent with our prior studies in INS-1 cells and islets showing defective glucose-induced insulin release upon 4 h of cytomix exposure (15).
Fig. 1.
Effects of cytokines and enzyme inhibitors on glucose-induced changes in metabolic flux and aconitase activity in INS-1 (832/13) β-cells. INS-1 β-cells were plated in 24-well plates, and then exposed to cytomix at 2 mm glucose for 4, 24, or 48 h before metabolic flux measurements. At the times indicated by the arrows in each panel, glucose concentration in the medium was increased to 20 mm or 1.5 μm rotenone was added. A, ECAR during a cytomix time course; B, OCR during a cytomix time course, C, OCR after 4 h of cytomix exposure, with or without indicated enzyme inhibitors. D, Actonitase activity in cell extract after 4 h exposure to cytomix, with or without the indicated inhibitors. E, OCR after 4 h of cytomix exposure, without or without addition of 1.5 mm isocitrate or 1.5 mm citrate. F, OCR after 4 h of cytomix exposure, without or without addition of p38 inhibitor. C, Control; DHSi, 100 μm DHS inhibitor GC7; iNOSi, 1 mm iNOS inhibitor l-NMMA; p38i, 10 μm p38 inhibitor PD169316. Data represent the mean ± sem of triplicate determinations from at least three independent experiments. In all cases, *, P < 0.05 for the indicated value(s) in comparison with control by one-way ANOVA.
Cytokines cause the rapid production of inducible nitric oxide synthase (iNOS), which generates nitric oxide. As shown in Fig. 1C, a competitive inhibitor of iNOS (l-NMMA) or an inhibitor of iNOS protein translation at the ribosome [GC7 (15)] caused complete restoration of glucose-induced OCR in the presence of cytomix at 4 h, implying a role for nitric oxide in the cytokine-induced defects in aerobic cellular metabolism. Prior studies suggested that nitric oxide inhibits mitochondrial iron-containing enzymes such as aconitase and electron transport complexes by destruction of iron-sulfur centers, thereby impairing oxidative metabolism of glucose (32). In concordance with these studies, Fig. 1D shows that addition of cytomix led to a reduction in aconitase activity in INS-1 cells, an effect that was reversed upon concurrent treatment with either l-NMMA or GC7. Concurrent addition of 1.5 mm isocitrate (the resulting product of aconitase activity) restored cytomix-induced defects in OCR in INS-1 cells, whereas 1.5 mm citrate did not (Fig. 1E).
Inhibition of p38 partially blocks iNOS protein production.
MAPKs, particularly p38, have been shown to mediate effects of cytokines in islet β-cells. To determine whether p38 contributes to the early, iNOS-mediated defects after cytomix exposure, we used a specific p38 inhibitor PD169316 in metabolic flux and aconitase activity studies. As shown in Fig. 1, D and F, pretreatment of INS-1 cells with PD169316 revealed significant recovery of both aconitase activity and OCR after 4 h exposure to cytomix, results suggesting that p38 may be linked to iNOS activity.
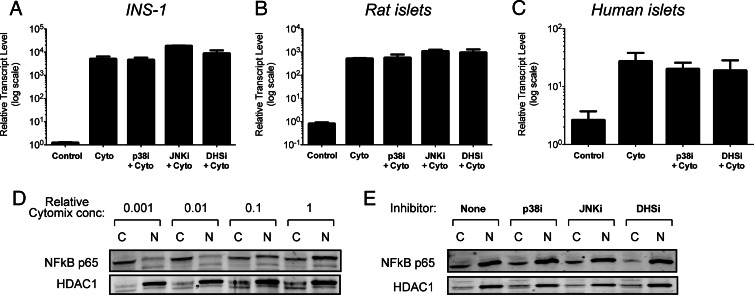
Prior studies have shown that p38 inhibition partially blocks activation of the gene encoding iNOS (Nos2) within the 24-h timeframe after cytokine exposure (33). Figure 2, A–C, shows that in the timeframe of our studies (4 h), there was no significant effect of p38 inhibition by PD169316 on the activation of Nos2 in INS-1 β-cells, rat islets, and human islets (notably, no effect of the inhibitors on Nos2 levels was observed in the absence of cytomix; data not shown). The same result was true of inhibition of the MAPK JNK by SP006125 (Fig. 2, A and B). To verify that MAPK inhibition did not affect the early signals that activate Nos2, we next performed immunoblots for NFκB (the major Nos2 activator) using cytoplasmic and nuclear extracts from INS-1 cells treated with cytomix. As shown in Fig. 2D, increasing cytomix concentrations resulted in a dose-dependent nuclear appearance of the p65 subunit of NFκB. Concurrent treatment with cytomix and either PD169316 or SP006125 did not alter the nuclear appearance of the p65 subunit of NFκB, consistent with our findings that neither p38 nor JNK affect Nos2 gene activation (Fig. 2E). Interestingly, these results parallel those seen using GC7, an inhibitor of the enzyme deoxyhypusine synthase (DHS) (Fig. 2E). DHS catalyzes the hypusination (and activation) of eIF5A, a translational factor that promotes translation of Nos2 mRNA (15, 19).
Fig. 2.
Effects of enzyme inhibitors on cytokine-induced Nos2 gene activation and NFκB nuclear translocation. INS-1 β cells (A[b]), rat islets (B[b]), and human islets (C[b]) were untreated (Control) or exposed to a standard cytomix concentration for 4 h, with or without indicated inhibitors, then cells were harvested for RNA isolation and real-time RT-PCR was performed for Nos2 message. In panels A–C, data are corrected for Actb message levels, and then normalized to Nos2 message levels in the absence of cytomix (Control). D, INS-1 cells were exposed to increasing concentrations of cytomix for 4 h, and then cytoplasmic (“C”) and nuclear (“N”) extracts were isolated and subjected to immunoblotting for NFκB p65 subunit and HDAC1 (nuclear protein control). E, INS-1 cells were exposed to a standard cytomix concentration, with or without indicated inhibitors, and then cytoplasmic (“C”) and nuclear (“N”) extracts were isolated and subjected to immunoblotting for NFκB p65 subunit and histone deacetylase (HDAC)1 (nuclear protein control). Panels A[b]–C represent the mean ± sem]r] of triplicate determinations from at least three independent experiments, and panels D and E are representative of experiments performed on three separate occasions. JNKi, 10 μ[scap]m JNK inhibitor SP600125; p38i and DHSi are as indicated in the legend to Fig. 1.
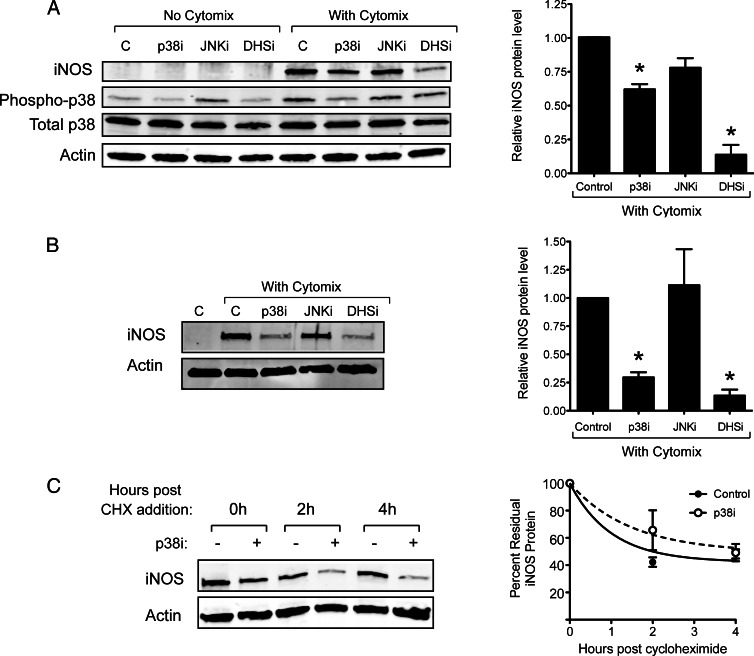
Next, we examined the effect of p38 inhibition by PD169316 on cytomix-induced iNOS protein levels by immunoblot. As shown in Fig. 3, A and B, 10 μm PD169316 attenuated cytomix-induced iNOS protein expression by approximately 40% in INS-1 β cells and by about 70% in rat islets compared with controls. Notably, higher concentrations of PD169316 (20 and 30 μm) caused even further reductions in iNOS protein levels in INS-1 cells, but we chose to use 10 μm of the drug to minimize off-target effects and to remain consistent with the concentrations used in the literature (data not shown). By contrast, inhibition of the MAPK c-Jun N-terminal kinase (JNK) with 10 μm SP600125 did not affect iNOS protein levels (Fig. 3). Whereas addition of cytomix enhanced the phosphorylation of p38 in INS-1 cells, inhibition of p38 blocked its own phosphorylation (Fig. 3A), a finding consistent with known effects of p38 inhibitors. Interestingly, inhibition of DHS also attenuated p38 phosphorylation (Fig. 3A), suggesting a possible feedback loop in which the activity of DHS may be linked to p38 activity. The results in Fig. 3, A and B, suggest that p38 activity enables a posttranscriptional process (mRNA translation or protein stability) that maintains, in part, high iNOS protein levels. The effect of p38 is likely at the level of Nos2 mRNA translation, because CHX incubation studies in INS-1 cells did not reveal any effects of p38 inhibition on iNOS protein stability (Fig. 3C).
Fig. 3.
Effects of enzyme inhibitors on cytokine-induced iNOS[b] protein levels and stability. A, INS-1 β-cells were incubated for 4 h in the presence or absence of cytomix and the indicated inhibitors, after which whole-cell extracts were harvested and immunoblots for iNOS, phospho-p38, p38, and actin are shown on the left and quantitation of immunoblots in the presence of cytokines (n = 3) is shown on the right. B, Rat islets were incubated for 4 h in the presence or absence of cytomix and the indicated inhibitors, after whichn extracts were harvested and immunoblots for iNOS and actin are shown on the left, and quantitation of the immunoblots in the presence of cytokines (n = 3) is shown on the right. C, INS-1 β cells were incubated with cytomix for 2 h, then with 50 μg/ml CHX for the indicated times to block new protein synthesis; the panel on the left shows a representative immunoblot, whereas the panel on the right shows quantitation of iNOS protein levels (normalized to actin levels) from the immunoblots (n = 3). *, P < 0.05 for the indicated value(s) in comparison with cytokine-only control by one-way ANOVA.
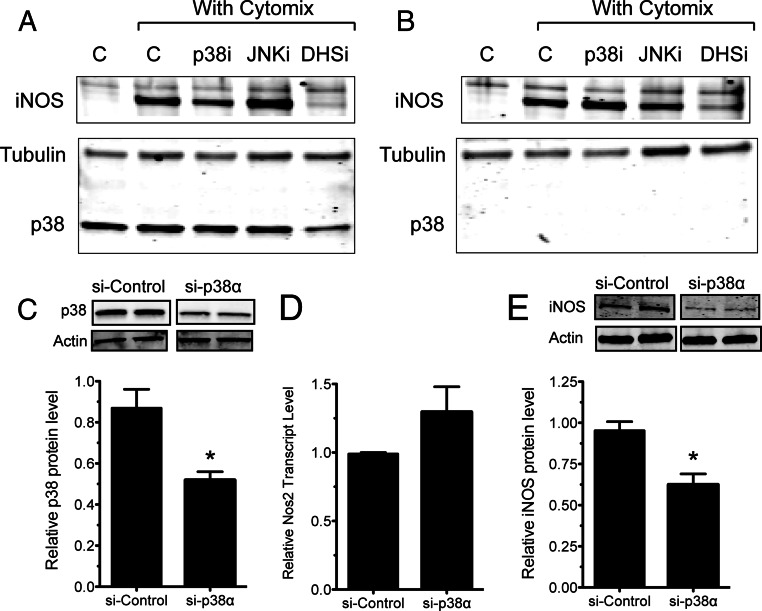
To rule out the possibility that the effect of PD169316 on iNOS protein levels was not caused by an off-target effect of the drug, we performed two additional experiments. First, we incubated p38α+/+ and p38α−/− MEFs (28) with cytomix and PD169316 and then performed immunoblots for iNOS. As shown in Fig. 4,A and B, PD169316 attenuated induction of iNOS in p38α+/+ MEFs, but not in p38α−/− MEFs, suggesting that the effect of PD169316 occurs only in the presence of p38α. Second, we performed siRNA knockdown of p38α in INS-1 cells, and then examined Nos2 mRNA and iNOS protein levels after cytomix exposure. As shown in Fig. 4C, siRNA treatment against p38 (si-p38) resulted in approximately 50% reduction in p38 protein compared with control siRNA (si-Control) by immunoblot. Figure 4, D and E, shows that exposure to cytomix caused no difference in Nos2 mRNA levels between si-Control- and si-p38-treated cells, yet si-p38 treatment attenuated iNOS protein by about 50% compared with si-Control, a result comparable to the effects observed with the p38 inhibitor PD169316. Taken together, the data in Fig. 4 suggest that the effect of PD169316 is a direct consequence of p38 inhibition.
Fig. 4.
Effect of enzyme inhibition or siRNA on cytokine-induced iNOS protein production. p38α+/+ mouse embryonic fibroblasts (MEFs) (A[b]) or p38−/− MEFs (B[b]) were incubated for 4 h in the presence or absence of cytomix and the indicated enzyme inhibitors, after which whole-cell extracts were harvested and immunoblotted for iNOS, tubulin, and p38. C[b], INS-1 β cells were treated with siRNAs indicated for 72 h, and then exposed to cytomix for 4 h, after which extracts were subjected to immunoblot analysis for p38 and actin. D[b], INS-1 extracts subjected to RT-PCR for Nos2 mRNA. E, INS-1 extracts subjected to immunoblot for iNOS and actin. In panels C and E, the top panels show two representative immunoblots and the bottom panels show quantitation of the immunoblots from three experiments. *, P < 0.05 for the indicated value(s) in comparison with si-Control by Student's t test.
p38 activity promotes translational elongation of Nos2 mRNA
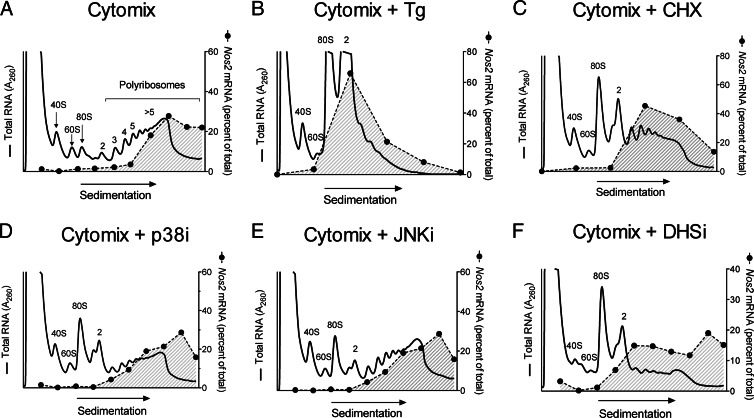
Our data in Fig. 3 suggest that p38 activity is required, in part, for the translation of Nos2 mRNA. To interrogate more directly the possibility that p38 activity is required for Nos2 translation at the ribosome, we performed PRP analysis (1, 34) using INS-1 β cells. The technique involves sedimentation of cellular RNA through a 10–50% sucrose gradient, which allows fractionation of RNA species according to their occupancy by ribosomes. INS-1 β-cells were incubated with cytomix in the absence or presence of inhibitors of translational initiation (Tg), translational elongation (CHX), p38 (PD169316), JNK (SP600125), and DHS (GC7). Figure 5 and Table 1 show, respectively, representative PRP profiles and quantitation of the polyribosome/monoribosome (P/M) distribution of RNA in INS-1 cells under the different conditions analyzed. Figure 5A shows the PRP after 4 h of cytomix incubation, identifying the positions of the 40S, 60S, 80S ribosome-associated RNA, as well as the position of polyribosome (>2 ribosomes/message)-associated RNA. Of note, the PRP in the presence of cytomix was no different than in its absence (data not shown), and Table 1 shows that total RNA and Nos2 mRNA partition primarily to polyribosomes (P/M >1). Coincubation with cytomix + Tg resulted in the dramatic enhancement in monoribosome- and diribosome-associated RNA and near-complete dissipation of polyribosome-associated RNA, with the P/M <1 (Fig. 5B and Table 1). This finding with Tg incubation indicates a block in the early initiation phase of translation, leading to accumulation of oligoribosome-associated RNAs; the loss (or run-off) in polyribosome-associated RNAs is caused by largely intact translational elongation in the face of initiation blockade. Importantly, Nos2 mRNA, which occupies primarily actively translating polyribosomes under cytomix conditions, shifts leftward toward the monoribosome-associated fractions (P/M ratio <1) in the presence of Tg (Fig. 5B, shaded region, and Table 1). By contrast, incubation with CHX leads to increases in both monoribosome- and polyribosome-associated RNAs (with a P/M ratio remaining >1), findings that reflect a block in translational elongation (Fig. 5C and Table 1); in this case, Nos2 mRNA is retained in the polyribosome fractions (P/M >1). When INS-1 cells are treated with p38 and JNK inhibitors, there remains retention of polyribosome-associated RNAs with a slight increase in monoribosome- and diribosome-associated RNAs, with P/M ratios similar to that seen with CHX alone (Fig. 5, D and E, and Table 1). Treatment with the DHS inhibitor GC7 results in a PRP similar to the MAPK inhibitors, but with even greater monoribosome and diribosome-associated RNAs and a P/M <1 but still greater than that seen with Tg (Fig. 5F and Table 1). However, similar to CHX treatment, Nos2 mRNA remains largely associated with polyribosomes in the setting of MAPK and DHS inhibition with P/M >1 in all cases (Fig. 5, D–F, shaded region, and Table 1). The findings with MAPK and DHS inhibition suggest that Nos2 mRNA is either unaffected translationally or is translationally blocked at the elongation phase.
Fig. 5.
PRP of INS-1 β-cells. INS-1 β-cells were exposed to cytomix for 4 h with or without inhibitors, and then subjected to PRP followed by real-time RT-PCR as detailed in Materials and Methods. In each panel, the solid line indicates the absorbance at 254 nm, whereas the dashed line with shading indicates the percent of total Nos2 message appearing in the indicated fraction. A[b], Cytomix alone. B[b], Cytomix plus Tg. C[b], Cytomix plus CHX. D[b], Cytomix plus p38 inhibitor PD169316. E[b], Cytomix plus JNK inhibitor SP600125. F[b], Cytomix plus DHS inhibitor GC7. Data shown are representative profiles obtained from experiments performed on three to four independent occasions. Tg, 1 μm Tg; CHX, 50 μg/ml CHX; p38i, JNKi, and DHSi are as indicated in the legends to Figs. 1 and 2. Panels indicate the positions of RNAs occupied by 40S, 60S, 80S ribosomal subunits, and the positions where RNA species are occupied by 2, 3, 4, 5, and more than 5 ribosomes (polyribosomes).
Table 1.
P/M ratios for total RNA and Nos2 mRNA in INS-1(832/13) cells
| Treatment condition | P/M (Total RNA)a | P/M (Nos2)b |
|---|---|---|
| No treatment | 3.66 ± 0.17 | N/Ac |
| Cytomix | 5 ± 1.4 | 12 ± 1.5 |
| Cytomix + Tg | 0.33 ± 0.09 | 0.54 ± 0.22 |
| Cytomix + CHX | 1.81 ± 0.28 | 3.88 ± 2.8 |
| Cytomix + p38 inhibitor | 1 ± 0.18 | 11 ± 3 |
| Cytomix + JNK inhibitor | 3.8 ± 1 | 9.9 ± 3.3 |
| Cytomix + DHS inhibitor | 0.70 ± 0.14 | 3 ± 0.21 |
Data were obtained after PRP analysis of INS-1 cells (see Fig. 5).
P/M ratios for total RNA were calculated as the ratio of the area under the curves for the polysomes region (trisomes and larger) to the monosomes region (40S, 60S, and 80S).
P/M ratios for Nos2 mRNA were calculated as the ratio of the percent of Nos2 mRNA appearing in the polysomes region (trisomes and larger) to the percent appearing in the monosomes region (40S, 60S, 80S). Data represent mean ± sem from three independent determinations.
Under “no treatment” conditions, levels of Nos2 mRNA were not detected above background in the PRP fractions. N/A, Not applicable.
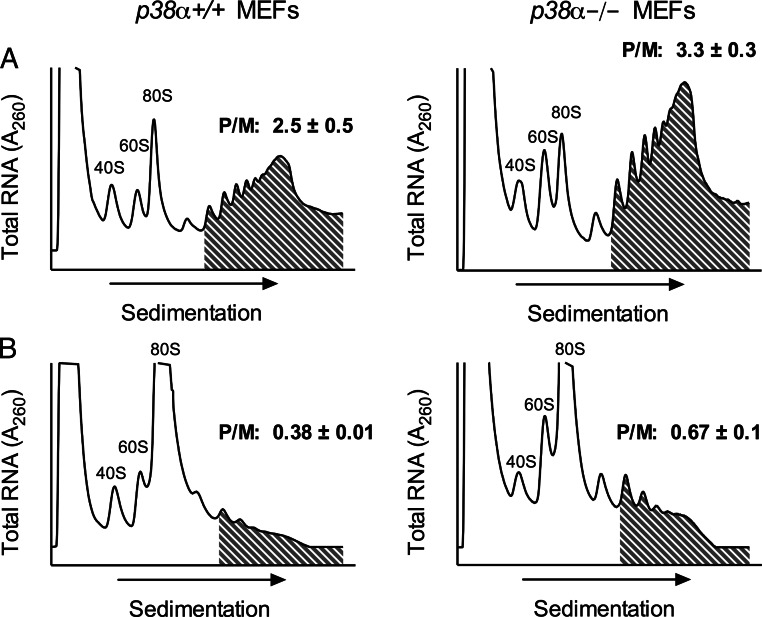
To clarify whether p38 promotes translational elongation specifically in the setting of cytokines, we next performed PRP studies using p38α+/+ and p38α−/− MEFs. As shown in Fig. 6A, p38−/− MEFs tend to exhibit greater fractional RNA within polyribosomes compared with p38+/+ MEFs (compare shaded regions in Fig. 6A) with a P/M ratio of 3.3 ± 0.3 (compared with a P/M ratio of 2.5± 0.5 in p38+/+ MEFs). When MEFs are incubated with Tg, there is a marked increase of the 80S peak and depletion of polyribosome-associated RNAs (reflecting translational initiation blockade), but the p38−/− MEFs again show relatively greater retention of polyribosome-associated RNA (P/M = 0.67 ± 0.1) compared with p38+/+ MEFs (P/M = 0.38 ± 0.01) (Fig. 6B). The results in Fig. 6 are consistent with the conclusion that the absence of p38α results in a partial defect in translational elongation, where there is relatively greater retention of polyribosome-associated RNAs.
Fig. 6.
PRP of p38α+/+ and p38α-/- mouse[b] embryonic fibroblasts. A, p38α+/+ MEFs (left panel) and p38α-/- MEFs (right panel) were exposed to cytomix for 4 h and then subjected to PRP as detailed in Materials and Methods. B, p38α+/+ MEFs (left panel) and p38α-/- MEFs (right panel) were exposed to cytomix plus 1 μm Tg for 4 h, and then subjected to PRP. Panels indicate the positions of RNAs occupied by 40S, 60S, 80S ribosomal subunits, and the shaded region indicates the polyribosome region. P/M indicates the polyribosome/monoribosome RNA ratio, as determined by area under the curve (AUC) analysis. The P/M values are statistically different in panel B by Student's t test.
P38 activity facilitates eIF5A hypusination
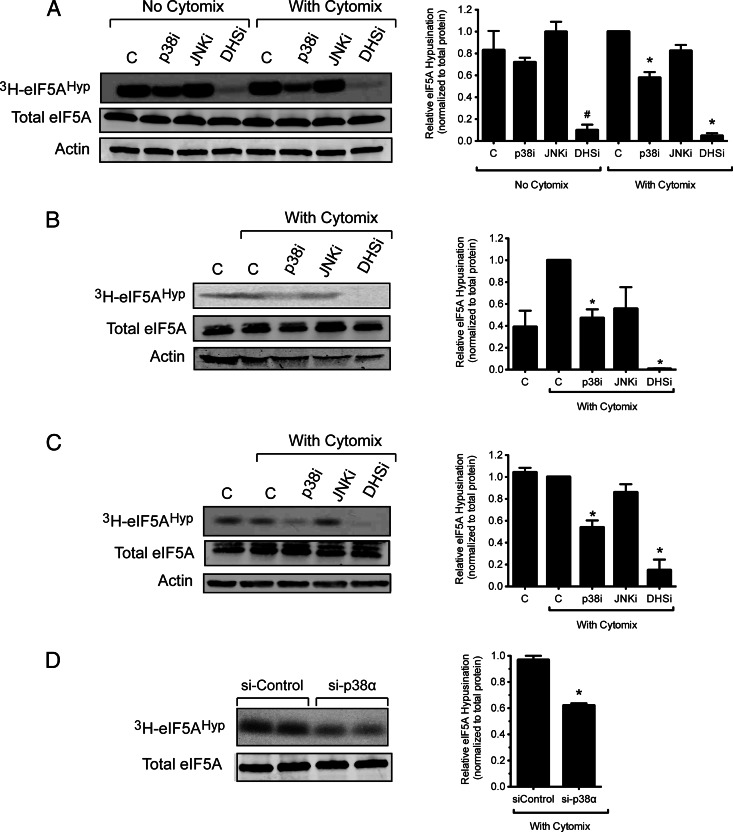
Taken together, the results presented thus far point to a role for p38 activity in Nos2 translational elongation that appears similar to that of DHS, an enzyme that is required for the function of eIF5A (15). We therefore asked whether the apparent translational effect of p38 might be related directly to the function of eIF5A. The translational elongation function of eIF5A is dependent upon the hypusination of Lys50, a reaction that is catalyzed sequentially by the enzymes DHS and DHH (18, 19). The hypusination reaction can be readily monitored by PAGE after incubating cells with the radiolabeled cofactor [3H]spermidine (2). As shown in Fig. 7, A–C, treatment of INS-1 β-cells, rat islets, and human islets with the DHS inhibitor GC7 caused the expected disruption of hypusine incorporation into eIF5A (2, 35, 36). Whereas the JNK inhibitor SP600125 had no significant effect on hypusine incorporation in any of the cell types, the p38 inhibitor PD169316 caused a significant decrease in hypusination in both the β-cell line and primary rat and human islets in the presence of cytomix (Fig. 7, A–C). Interestingly, no effect of p38 inhibition on hypusine incorporation was observed in the absence of cytomix in INS-1 cells (Fig. 7A), implying an effect of p38 that is cytokine dependent. The effects of PD169316 on hypusine incorporation were specific for p38, because PD169316 did not affect hypusine incorporation in p38α−/− MEFs (data not shown). Moreover, in the presence of cytomix, si-RNA knockdown of p38 resulted in an approximately 50% reduction in hypusine incorporation in INS-1 cells (Fig. 7D).
Fig. 7.
Effects of enzyme inhibitors or siRNA on eIF5A[b] hypusination. INS-1 β cells (A[b]), rat islets (B[b]), and human islets (C[b]) were exposed to [3H]spermidine for 4 h in the presence or absence of cytokines and the indicated enzyme inhibitors, after which whole-cell extracts were harvested and subjected to PAGE and fluorography for hypusinated eIF5A (3H-eIF5AHyp) or immunoblotting for total eIF5A and actin. D, INS-1 β-cells were treated with the siRNAs, then exposed to cytomix for 4 h, and extracts were subjected to PAGE and fluorography for 3H-eIF5AHyp or immunoblotting for total eIF5A. The panels on the left show representative fluorography and immunoblots, whereas the panels on the right show quantitation of [3H]eIF5AHyp protein levels (normalized to actin levels) from three independent experiments. *, P < 0.05 for the indicated value(s) in comparison with cytokine-only control by one-way ANOVA or Student's t test. C, control (no inhibitors); p38i, JNKi, and DHSi are as indicated in the legends to Figs. 1 and 2.
Discussion
Proinflammatory cytokines have been implicated in the pathogenesis of islet β-cell dysfunction in both type 1 and type 2 diabetes mellitus, and potential therapies targeted against cytokines or their signaling pathways have been proposed as approaches to preserve β-cell function and survival in both diseases. Most notably, recent studies in type 1 diabetic NOD mice (1) and type 1 diabetic humans (37, 38) suggest evidence for the dysfunction of β-cells in the prediabetic phase and raise the possibility that early intervention to protect β-cell function may prolong disease progression. To this end, our studies provide mechanistic insight into the rationale for use of MAPK inhibition. The MAPKs have been suggested as targets in the setting of diabetes because their actions downstream of cytokine signaling promote both transcription and translation of a host of mRNAs that contribute to the dysfunction or death of a variety of cell types that regulate metabolic homeostasis (for review, see Ref. 21). The present studies suggest a novel mechanism whereby MAPKs, particularly p38, support the translational elongation of Nos2 mRNA via hypusination of the translational factor eIF5A.
In the immediate period (hours) after exposure to proinflammatory cytokines in vitro, β-cells exhibit defects in glucose-stimulated insulin secretion well before evidence of cellular death (15). These earliest effects of cytokines have been attributed largely to the production of nitric oxide by iNOS, because inhibition of iNOS using specific small molecule inhibitors has been shown to reverse β-cell secretory defects up to 24 h after cytokine exposure (9). Early studies by Corbett and McDaniel (9) and Corbett et al. (32) suggested that the negative effects of nitric oxide on insulin secretion may result from nitrosylation of iron-containing enzymes in the mitochondria, most notably aconitase, that are necessary for ATP generation. Our studies support these findings, but additionally demonstrate that cytokines acutely (within 4 h) cause a defect in β-cell mitochondrial OCR, which can be reversed or partially reversed by inhibition of iNOS, DHS, or p38. The effects of p38 inhibition appear secondary to its effect in attenuating iNOS protein production rather than transcription, because in the timeframe studied here there were neither alterations of NFκB nuclear translocation nor Nos2 transcript levels. Notably, prior studies of Larsen et al. (25) in β-cells showed that p38 activity is required for full transcription of the Nos2 gene encoding iNOS. Reasons for the disparity between our data and those of Larsen et al. (25) could lie in 1) differences in the nature of p38 inhibitors used (PD169316 in this study vs. SB), 2) differences in experimental duration between the studies, where immediate-early (0–4 h) effects of p38 promote Nos2 translation and intermediate-late (6–24 h) effects of p38 promote both Nos2 transcription and translation, and/or 3) differences in techniques used to measure Nos2 transcript (real-time PCR vs. semiquantitative PCR). With respect to differences in the nature of inhibitor used, we recognize that use of small molecule inhibitors of p38 can have off-target/unintended effects, and therefore caution must be used in the interpretation of our data and those in the literature. Nevertheless, we believe the likelihood of off-target effects of PD169316 with respect to our data on iNOS production is low, considering that PD169316 similarly inhibits iNOS production in p38α+/+ MEFs but not in p38α−/− MEFs, and our siRNA studies in INS-1 cells support the role of p38 in iNOS production.
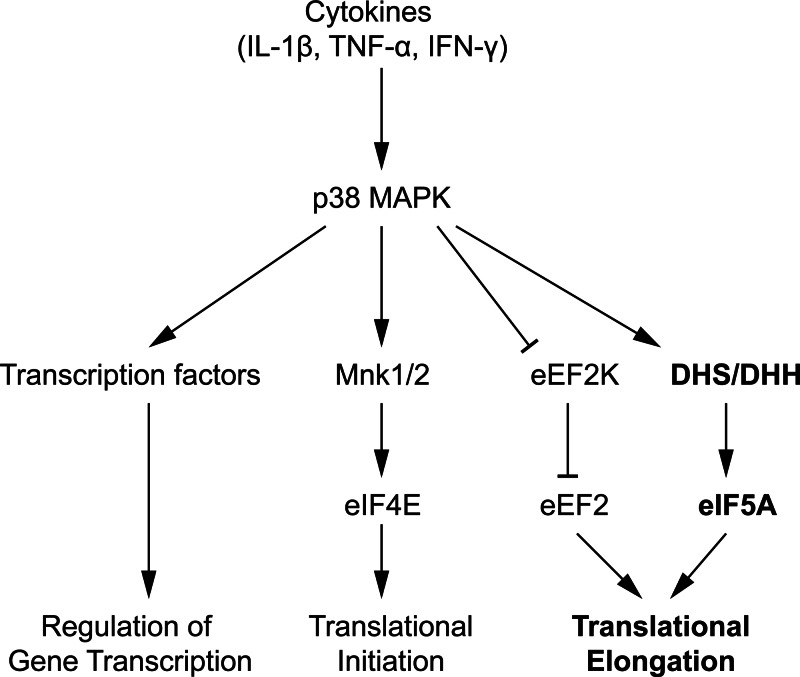
The effects of p38 on gene transcription have been well documented in the literature, as many known targets of the kinase include the gene encoding a variety of transcription factors such as the signal transducers and activators of transcription, p53, nuclear factor of activated T cells, CAAT/enhancer binding protein homologous protein, and activating transcription factor 2 among many others (for review, see Ref. 21). However, the effects of p38 on mRNA translation has received less attention. Figure 8 summarizes the major pathways by which p38 is thought to regulate mRNA translation. p38 has been shown to activate (via phosphorylation) the MAPK-interacting kinases 1 and 2 (Mnk1/2), which subsequently activate the translational initiation factor eIF4E (39–41). In this respect, it is possible that the increases we observed in the 80S ribosomal peak in the PRP upon treatment of INS-1 cells with PD169316 may reflect translational initiation blockade as a result of Mnk1/2 deactivation. p38 has also been shown to contribute to translational elongation of mRNAs. Elegant studies of Knebel et al. (42) show that the p38δ isoform promotes the activity of eukaryotic translation elongation factor 2 (eEF2) via phosphorylation (and inactivation) of its immediate upstream deactivator, eEF2 kinase. Our studies suggest that p38 may promote the translational elongation of Nos2 transcripts in response to cytokine-mediated stress. Importantly, we observed that although iNOS protein production is reduced in p38-inhibited cells, Nos2 mRNA remains situated in polyribosomes. This result would suggest that p38 inhibition results in either a reduction in iNOS protein half-life or a partial blockade in translation elongation. Because we did not observe changes in iNOS protein half-life in the 4-h period of our studies, we believe the effects observed were a result of elongation blockade. Effects on translational elongation, unlike effects on translational initiation, are more difficult to discern from standard PRP studies. To increase our sensitivity to detect a potential effect on translational elongation, we performed coincubation studies in p38α−/− MEFs using cytomix plus Tg, which causes a near-complete block in translational initiation, leaving elongation effects more clearly discernible. These studies demonstrated retention of polyribosomes in p38α−/−cells compared with p38α+/+ cells, supporting our hypothesis that p38 contributes to translational elongation in the setting of cytokine signaling.
Fig. 8.
Proposed model of p38 MAPK regulation of mRNA translation. The figure depicts pathways by which p38 in the setting of cytokine signaling has been previously shown to affect gene transcription (leftmost pathway) and mRNA translation (middle two pathways) by way of direct phosphorylation of targets (transcription factors, Mnk1/2, and eEF2K). With this study, we propose a new pathway (rightmost pathway in bold), whereby p38 regulates the activity of hypusinating enzymes (DHS and/or DHH), which subsequently control the action of eIF5A. The figure is not meant to indicate that regulation of the hypusination enzyme(s) by p38 is necessarily direct (i.e. by a phosphorylation event), because the mechanism of this regulation remains to be elucidated. eIF4E, eukaryotic translation initiation factor 4E; eEF2K, eukaryotic translation elongation factor 2 kinase.
Translational elongation at the ribosome is dependent upon a group of translational factors that is distinct from that involved in the initiation of translation (reviewed in Refs. 43 and 44). Interestingly, eIF5A was originally identified as a translational initiation factor based on early studies in vitro of methionyl-puromycin synthesis (45). However, in the intervening years, its role as an initiation factor has been questioned, and more recent studies suggest it functions primarily as an elongation factor and, this, for only a subset of cellular mRNAs that function under conditions of stress (46, 47). Nevertheless, controversy still exists in the literature as to whether eIF5A functions primarily as an initiation or elongation factor (48). In all species studied, eIF5A is uniquely posttranslationally modified at Lys50 in a two-step reaction requiring the actions of the enzymes DHS and DHH and the polyamine spermidine; this modified amino acid, known as hypusine, is required for the known translational and RNA binding functions of eIF5A (18). In recent studies we demonstrated that hypusinated eIF5A is required for nuclear export and translation of Nos2 mRNA (15). Here, we show for the first time, that p38 inhibition leads to reduced hypusination in the setting of cytokine signaling, resulting in reductions in iNOS protein that parallel effects observed with inhibition of DHS. Although both DHS and DHH contain putative MAPK phosphorylation sites, we should note that our data do not necessarily imply that p38 directly modifies (phosphorylates) either protein. Nevertheless, the reduction in DHS protein levels provides one possible explanation for why hypusination and iNOS protein are reduced upon p38 inhibition. Studies currently ongoing are evaluating the possibility that direct phosphorylation of DHS by p38 may be necessary for the activity and/or stability of DHS.
Taken together, our data suggest a pathway whereby p38 activity links, in part, the transduction of cytokine signals with activation of hypusination. Although we cannot entirely rule out the possibility that other translational elongation factors, such as eEF2, also participate in the translational elongation of Nos2 promoted by p38, it is possible that these factors are all linked closely to one another such that loss in the activity of one can affect the assembly of the full translational elongation complex. In the case of eIF5A, the factor does not appear to function as a generalized translational factor, but instead promotes the elongation of only a subset of mRNAs and only under certain conditions of stress (15, 47, 49). Thus, our observations in this study may represent a p38-linked translational pathway that is specific for Nos2 and related cytokine-induced transcripts. A limitation in these studies is the use of small molecule inhibitors, recognizing that the concern for off-target effects appear mitigated by our parallel findings in p38α−/− MEFs. Nevertheless, because such inhibitors may find their way into the clinical settings of type 1 and type 2 diabetes, our studies provide new insight into the mechanisms by which MAPK inhibition might limit the consequences of systemic inflammation on the islet β-cell.
Acknowledgments
We thank Dr. A. Nebreda (Institute for Research in Biomedicine, Barcelona, Spain) for provision of immortalized p38α+/+ and p38α−/− MEFs, and Ms. N. Stull and Ms. K. Benninger (Diabetes Center Islet Core) for expert technical assistance in the isolation of rat islets. We also thank the Integrated Islet Distribution Program for provision of human islets.
This work was supported by a grant from the Juvenile Diabetes Research Foundation, by Grant R01 DK60581 from the National Institutes of Health, and by a mentor-based grant from the American Diabetes Association (all to R.G.M.); by a predoctoral fellowship from the American Heart Association (to A.T.T.); and by Training Grant T32 DK064466 from the National Institutes of Health (to Y.N.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CHX
- Cycloheximide
- DHH
- deoxyhypusine hydroxylase
- DHS
- deoxyhypusine synthase
- ECAR
- extracellular acidification rate
- eEF2
- eukaryotic translation elongation factor 2
- eIF5A
- eukaryotic translation initiation factor 5A
- iNOS
- inducible nitric oxide synthase
- JNK
- c-Jun N-terminal kinase
- MEFs
- mouse embryonic fibroblasts
- Mnk1/2
- MAPK-interacting kinases 1 and 2
- l-NMMA
- NG-monomethyl-l-arginine
- NFκB
- nuclear factor κB
- NOD
- nonobese diabetic
- OCR
- oxygen consumption rate
- P/M
- polyribosome/monoribosome
- PRP
- polyribosomal profiling
- siRNA
- small interfering RNA
- Tg
- thapsigargin.
References
- 1. Tersey SA, Nishiki Y, Templin AT, Cabrera SM, Stull ND, Colvin SC, Evans-Molina C, Rickus JL, Maier B, Mirmira RG. 2012. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 61:818–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robbins RD, Tersey SA, Ogihara T, Gupta D, Farb TB, Ficorilli J, Bokvist K, Maier B, Mirmira RG. 2010. Inhibition of deoxyhypusine synthase enhances islet β cell function and survival in the setting of endoplasmic reticulum stress and type 2 diabetes. J Biol Chem 285:39943–39952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. 2007. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356:1517–1526 [DOI] [PubMed] [Google Scholar]
- 4. Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, Yagi N, Ohto U, Kimoto M, Miyake K, Tobe K, Arai H, Kadowaki T, Nagai R. 2012. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab 15:518–533 [DOI] [PubMed] [Google Scholar]
- 5. Fontés G, Zarrouki B, Hagman DK, Latour MG, Semache M, Roskens V, Moore PC, Prentki M, Rhodes CJ, Jetton TL, Poitout V. 2010. Glucolipotoxicity age-dependently impairs β cell function in rats despite a marked increase in β cell mass. Diabetologia 53:2369–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. 2003. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110 [DOI] [PubMed] [Google Scholar]
- 7. Ogihara T, Mirmira RG. 2010. An islet in distress: β cell failure in type 2 diabetes. J Diab Invest 1:123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lowenstein CJ, Padalko E. 2004. iNOS (NOS2) at a glance. J Cell Sci 117:2865–2867 [DOI] [PubMed] [Google Scholar]
- 9. Corbett JA, McDaniel ML. 1994. Reversibility of interleukin-1 β-induced islet destruction and dysfunction by the inhibition of nitric oxide synthase. Biochem J 299:719–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hughes KJ, Chambers KT, Meares GP, Corbett JA. 2009. Nitric oxides mediates a shift from early necrosis to late apoptosis in cytokine-treated β-cells that is associated with irreversible DNA damage. Am J Physiol Endocrinol Metab 297:E1187–E1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M. 2001. Nitric oxide-induced apoptosis in pancreatic β cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA 98:10845–10850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, T[slsw]onnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL. 2005. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-cells. Diabetes 54:452–461 [DOI] [PubMed] [Google Scholar]
- 13. Kamijo R, Harada H, Matsuyama T, Bosland M, Gerecitano J, Shapiro D, Le J, Koh SI, Kimura T, Green SJ. 1994. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science 263:1612–1615 [DOI] [PubMed] [Google Scholar]
- 14. Xie QW, Kashiwabara Y, Nathan C. 1994. Role of transcription factor NF-κ B/Rel in induction of nitric oxide synthase. J Biol Chem 269:4705–4708 [PubMed] [Google Scholar]
- 15. Maier B, Ogihara T, Trace AP, Tersey SA, Robbins RD, Chakrabarti SK, Nunemaker CS, Stull ND, Taylor CA, Thompson JE, Dondero RS, Lewis EC, Dinarello CA, Nadler JL, Mirmira RG. 2010. The unique hypusine modification of eIF5A promotes islet β cell inflammation and dysfunction in mice. J Clin Invest 120:2156–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jang BC, Sung SH, Park JG, Park JW, Suh MH, Choi IH, Yoshida M, Yoo SK, Suh SI. 2006. Leptomycin B, a metabolite of Streptomyces, inhibits the expression of inducible nitric oxide synthase in BV2 microglial cells. Int J Oncol 29:1509–1515 [PubMed] [Google Scholar]
- 17. Vodovotz Y, Bogdan C, Paik J, Xie QW, Nathan C. 1993. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor β. J Exp Med 178:605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park MH, Nishimura K, Zanelli CF, Valentini SR. 2010. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids 38:491–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maier B, Tersey SA, Mirmira RG. 2010. Hypusine: a new target for therapeutic intervention in diabetic inflammation. Discov Med 10:18–23 [PubMed] [Google Scholar]
- 20. Raman M, Chen W, Cobb MH. 2007. Differential regulation and properties of MAPKs. Oncogene 26:3100–3112 [DOI] [PubMed] [Google Scholar]
- 21. Gehart H, Kumpf S, Ittner A, Ricci R. 2010. MAPK signalling in cellular metabolism: stress or wellness? EMBO Rep 11:834–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lui P, Zeng C, Acton S, Cok S, Sexton A, Morrison AR. 2004. Effects of p38MAPK isoforms on renal mesangial cell inducible nitric oxide synthase expression. Am J Physiol Cell Physiol 286:C145–C152 [DOI] [PubMed] [Google Scholar]
- 23. Bhat NR, Zhang P, Lee JC, Hogan EL. 1998. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-α gene expression in endotoxin-stimulated primary glial cultures. J Neurosci 18:1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen C, Chen YH, Lin WW. 1999. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology 97:124–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larsen CM, Wadt KA, Juhl LF, Andersen HU, Karlsen AE, Su MS, Seedorf K, Shapiro L, Dinarello CA, Mandrup-Poulsen T. 1998. Interleukin-1β-induced rat pancreatic islet nitric oxide synthesis requires both the p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Biol Chem 273:15294–15300 [DOI] [PubMed] [Google Scholar]
- 26. Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. 2000. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49:424–430 [DOI] [PubMed] [Google Scholar]
- 27. Stull ND, Breite A, McCarthy RC, Tersey SA, Mirmira RG. 2012. Mouse islet of Langerhans isolation using a combination of purified collagenase and neutral protease. J Vis Exp 67:4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Porras A, Zuluaga S, Black E, Valladares A, Alvarez AM, Ambrosino C, Benito M, Nebreda AR. 2004. P38 α mitogen-activated protein kinase sensitizes cells to apoptosis induced by different stimuli. Mol Biol Cell 15:922–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sadowski HB, Gilman MZ. 1993. Cell-free activation of a DNA-binding protein by epidermal growth factor. Nature 362:79–83 [DOI] [PubMed] [Google Scholar]
- 30. Iype T, Francis J, Garmey JC, Schisler JC, Nesher R, Weir GC, Becker TC, Newgard CB, Griffen SC, Mirmira RG. 2005. Mechanism of insulin gene regulation by the pancreatic transcription factor Pdx-1: application of pre-mRNA analysis and chromatin immunoprecipitation to assess formation of functional transcriptional complexes. J Biol Chem 280:16798–16807 [DOI] [PubMed] [Google Scholar]
- 31. Evans-Molina C, Garmey JC, Ketchum R, Brayman KL, Deng S, Mirmira RG. 2007. Glucose regulation of insulin gene transcription and pre-mRNA processing in human islets. Diabetes 56:827–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Corbett JA, Wang JL, Sweetland MA, Lancaster JR, Jr, McDaniel ML. 1992. Interleukin 1 β induces the formation of nitric oxide by β-cells purified from rodent islets of Langerhans. Evidence for the β-cell as a source and site of action of nitric oxide. J Clin Invest 90:2384–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guan Z, Buckman SY, Springer LD, Morrison AR. 1999. Both p38alpha(MAPK) and JNK/SAPK pathways are important for induction of nitric-oxide synthase by interleukin-1β in rat glomerular mesangial cells. J Biol Chem 274:36200–36206 [DOI] [PubMed] [Google Scholar]
- 34. Teske BF, Baird TD, Wek RC. 2011. Methods for analyzing eIF2 kinases and translational control in the unfolded protein response. Methods Enzymol 490:333–356 [DOI] [PubMed] [Google Scholar]
- 35. Park MH, Wolff EC, Lee YB, Folk JE. 1994. Antiproliferative effects of inhibitors of deoxyhypusine synthase. Inhibition of growth of Chinese hamster ovary cells by guanyl diamines. J Biol Chem 269:27827–27832 [PubMed] [Google Scholar]
- 36. Umland TC, Wolff EC, Park MH, Davies DR. 2004. A new crystal structure of deoxyhypusine synthase reveals the configuration of the active enzyme and of an enzyme.NAD.inhibitor ternary complex. J Biol Chem 279:28697–28705 [DOI] [PubMed] [Google Scholar]
- 37. Keskinen P, Korhonen S, Kupila A, Veijola R, Erkkilä S, Savolainen H, Arvilommi P, Simell T, Ilonen J, Knip M, Simell O. 2002. First-phase insulin response in young healthy children at genetic and immunological risk for Type I diabetes. Diabetologia 45:1639–1648 [DOI] [PubMed] [Google Scholar]
- 38. Ferrannini E, Mari A, Nofrate V, Sosenko JM, Skyler JS, DPT-1 Study Group 2010. Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes 59:679–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fukunaga R, Hunter T. 1997. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J 16:1921–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. 1997. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J 16:1909–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang X, Flynn A, Waskiewicz AJ, Webb BL, Vries RG, Baines IA, Cooper JA, Proud CG. 1998. The phosphorylation of eukaryotic initiation factor eIF4E in response to phorbol esters, cell stresses, and cytokines is mediated by distinct MAP kinase pathways. J Biol Chem 273:9373–9377 [DOI] [PubMed] [Google Scholar]
- 42. Knebel A, Morrice N, Cohen P. 2001. A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38δ. EMBO J 20:4360–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sonenberg N, Hinnebusch AG. 2009. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136:731–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dever TE, Green R. 2012. The elongation, termination, and recycling phases of translation in eukaryotes. Cold Spring Harb Perspect Biol 4:a013706–a013706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kemper WM, Berry KW, Merrick WC. 1976. Purification and properties of rabbit reticulocyte protein synthesis initiation factors M2Bα and M2Bβ. J Biol Chem 251:5551–5557 [PubMed] [Google Scholar]
- 46. Saini P, Eyler DE, Green R, Dever TE. 2009. Hypusine-containing protein eIF5A promotes translation elongation. Nature 459:118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li CH, Ohn T, Ivanov P, Tisdale S, Anderson P. 2010. eIF5A promotes translation elongation, polysome disassembly and stress granule assembly. PLoS ONE 5:e9942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Henderson A, Hershey JW. 2011. Eukaryotic translation initiation factor (eIF) 5A stimulates protein synthesis in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 108:6415–6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kruse M, Rosorius O, Kratzer F, Bevec D, Kuhnt C, Steinkasserer A, Schuler G, Hauber J. 2000. Inhibition of CD83 cell surface expression during dendritic cell maturation by interference with nuclear export of CD83 mRNA. J Exp Med 191:1581–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]