Abstract
The rapidly growing family of transcriptional coregulators includes coactivators that promote transcription and corepressors that harbor the opposing function. In recent years, coregulators have emerged as important regulators of metabolic homeostasis, including the p160 steroid receptor coactivator (SRC) family. Members of the SRC family have been ascribed important roles in control of gluconeogenesis, fat absorption and storage in the liver, and fatty acid oxidation in skeletal muscle. To provide a deeper and more granular understanding of the metabolic impact of the SRC family members, we performed targeted metabolomic analyses of key metabolic byproducts of glucose, fatty acid, and amino acid metabolism in mice with global knockouts (KOs) of SRC-1, SRC-2, or SRC-3. We measured amino acids, acyl carnitines, and organic acids in five tissues with key metabolic functions (liver, heart, skeletal muscle, brain, plasma) isolated from SRC-1, -2, or -3 KO mice and their wild-type littermates under fed and fasted conditions, thereby unveiling unique metabolic functions of each SRC. Specifically, SRC-1 ablation revealed the most significant impact on hepatic metabolism, whereas SRC-2 appeared to impact cardiac metabolism. Conversely, ablation of SRC-3 primarily affected brain and skeletal muscle metabolism. Surprisingly, we identified very few metabolites that changed universally across the three SRC KO models. The findings of this Research Resource demonstrate that coactivator function has very limited metabolic redundancy even within the homologous SRC family. Furthermore, this work also demonstrates the use of metabolomics as a means for identifying novel metabolic regulatory functions of transcriptional coregulators.
The p160 family of steroid receptor coactivators (SRCs), which includes SRC-1 (NCoA-1), SRC-2 (glucocorticoid receptor interacting protein-1, transcriptional intermediary factor 2, NCoA2), and SRC-3 (amplified in breast-1, activator of thyroid hormone receptor, receptor-associated coactivator-3, thyroid hormone receptor activator molecule-1, CBP-interacting protein, NCoA3), has evolved as a pleiotropic coordinator of diverse physiological systems ranging from immune protection to reproduction (as reviewed in Ref. 1). Central to each of these physiological functions are the distinctive roles these coactivators play in maintenance of metabolic homeostasis. Thus, the inherent utility of the SRCs lies in their ability to coordinate the appropriate transcriptional programs to marry regulation of energy metabolism with diverse effects on tissue and systems biology.
The majority of our current knowledge of the metabolic functions of the SRCs has been garnered from the characterization of knockout (KO) and knock-in mouse models (summarized in Table 1). For example, ablation of SRC-1 has a major impact on energy expenditure and confers susceptibility to diet-induced obesity (2). SRC-1 is also an important modulator of amino acid metabolism and gluconeogenesis (3). By contrast, loss of SRC-2 provides protection against diet-induced obesity through enhanced energy expenditure (2). Moreover, we have shown that SRC-2 is essential for hepatic glucose release by regulating glucose 6-phosphatase (G6PC) gene expression (4). More recently, we have found that SRC-2 is a major sensor of whole-body energy status, serving to coordinate dietary lipid absorption with hepatic bile acid excretion through association with the energy sensor AMP-activated protein kinase (5). In skeletal muscle, SRC-2 appears to reduce oxidative capacity, in part through regulation of SRC-1 expression (6). As for SRC-3, which is essential for maintenance of white adipogenic programming, its ablation also protects against high-fat diet-induced obesity and confers a marked improvement in peripheral insulin sensitivity (6, 7). In line with these findings, SRC-3 plays an important role in control of long-chain fatty acid metabolism by regulating carnitine/acyl carnitine translocase (CACT) gene expression in skeletal muscle (8). Additionally, we have shown that alterations to the posttranslational code of SRC-3 lead to a constellation of metabolic derangements that closely resemble symptoms of the metabolic syndrome (9, 10). Collectively, these findings establish the SRCs as global regulators of metabolic homeostasis.
Table 1.
Summary of reported physiological functions for the SRCs in core metabolic tissues
| Tissue | Coactivator | Physiological function | References |
|---|---|---|---|
| Liver | SRC-1 | Controls energy balance by regulating expression of gluconeogenic genes through coactivation of C/EBPα | (3) |
| SRC-2 | Facilitates fasting glucose release through regulation of G6PC expression | (4) | |
| Controls bile acid homeostasis through regulation of BSEP expression | (5) | ||
| SRC-3 | Regulates hepatocyte proliferation and changes in the PTM code confers susceptibility to carcinogen-induced hepatic tumorigenesis | (9) | |
| (10) | |||
| WAT/BAT | SRC-1 | Loss confers susceptibility to high-fat diet induced obesity through reduced energy expenditure | (2) |
| Loss of SRC-1 and SRC-3 impacts glucose metabolism and insulin sensitivity | (28) | ||
| SRC-2 | Loss confers protection against high-fat diet induced obesity, reduced caloric intake and increased BAT activity | (2) | |
| SRC-3 | Loss impairs adipocyte differentiation and protection against high-fat diet-induced obesity | (6) | |
| Loss of SRC-1 and SRC-3 impacts glucose metabolism and insulin sensitivity | (28) | ||
| Skeletal Muscle | SRC-1 | Regulates skeletal muscle mitochondrial respiration | (26) |
| Loss of SRC-1 and SRC-3 impacts glucose metabolism and insulin sensitivity | (28) | ||
| SRC-2 | Coordinates expression of SRC-1 to control skeletal muscle metabolism | (26) | |
| SRC-3 | Controls energy expenditure through regulation of PGC-1a activity | (7) | |
| Controls long-chain fatty acid metabolism by regulating expression of CACT | (8) | ||
| Loss of SRC-1 and SRC-3 impacts glucose metabolism and insulin sensitivity | (28) | ||
| Brain | SRC-1 | Loss impairs HPA axis function | (16) |
| Loss impairs motor function via delayed development of cerebellar Purkinje cells | (13) | ||
| Controls CRH in response to glucocorticoids | (12) | ||
| SRC-2 | Loss results in adrenocortical insufficiency and neuroendocrine dysregulation | (14) | |
| Necessary for proper HPA axis function | (15) | ||
| Important for proper Sertoli cell function | (18) | ||
| SRC-3 | Acute loss disrupts reproductive behavior | (11) |
BAT, Brown adipose tissue; BSEP, bile salt export pump; HPA, hypothalamic-pituitary-adrenal; PGC-1a, peroxisomal proliferator-activated receptor-γ coactivator; PTM, posttranslational modification; WAT, white adipose tissue.
Although the SRCs continue to be appreciated as potent regulators of cellular proliferation, the recent discovery of their various metabolic roles imply that these proteins may also serve as a critical regulatory link between growth and metabolism. With the growing appreciation for the connections between metabolic derangements and a plethora of disease pathologies such as cancer, obesity, and type 2 diabetes, understanding the full metabolic repertoire of the SRCs may be relevant to the development of new and effective therapeutic strategies. Although traditional phenotyping remains useful for identifying distinct metabolic abnormalities (11–16), the combined complexities of coactivator biology and metabolism demand a more comprehensive approach for probing global metabolic functions of the SRCs. To this end, we have deployed targeted metabolomics to quantitatively evaluate the impact of individual SRCs on metabolite levels in several key metabolic organ systems (i.e. liver, heart, skeletal muscle, brain, and plasma). This study provides the first comparative metabolomic assessment of an entire family of transcriptional coregulators, revealing both tissue- and pathway-specific functions for each of the SRCs, thereby highlighting their important and emerging roles as regulators of systems metabolism.
Materials and Methods
Animal experiments
All animal experiments were performed according to protocols approved by the Animal Care Research Committee at Baylor College of Medicine. Generation of the SRC-1, SRC-2, and SRC-3 KO mice has been described previously (17–19). SRC-1 KO mice were maintained in a pure C57BL/6J genetic background, whereas SRC-2 and SRC-3 KO mice were maintained in a hybrid C57BL/6J/129 SV genetic background due to breeding issues of these two lines on a pure C57BL/6J background. Only male, age-matched littermates (10–16 wk of age) mice were used in these studies. Animals were maintained in a temperature-controlled (23 C) facility with a 12-h light, 12-h dark cycle. Mice were fed 2920X Teklad Global rodent chow (Harlan Teklad, Madison, WI), and then studied either in the ad libitum fed state or after removal of food for 24 h with free access to water. After fasting or ad libitum feeding, mice were weighed, and body temperature was measured using a digital rectal thermometer. Blood glucose was measured using a hand-held glucometer (One Touch Ultra; LifeScan, Inc., Milpitas, CA).
Plasma isolation
Whole blood was collected and transferred into a prechilled microcentrifuge tube containing 10 μl of 0.5 m EDTA (pH 8.0) for isolation of EDTA-plasma, which was transferred to a clean microcentrifuge tube and stored at −80 C.
Tissue preparation
Frozen pieces of mouse liver, heart, skeletal muscle, and whole-brain tissue were pulverized with a liquid nitrogen-chilled 3-lb sledgehammer. The resulting pulverized tissue (∼130 mg) was placed into a prechilled 14-ml tube, followed by the addition of 1.17 ml of prechilled HPLC-grade water. For cardiac tissue, frozen pieces of heart were pulverized as described above followed by the addition of prechilled 50% aqueous acetonitrile containing 0.3% formic acid. All samples were then homogenized on ice using a Polytron homogenizer. An aliquot of each homogenized tissue was used to perform a bicinchoninic acid assay to normalize the resulting metabolites to total protein. The remainder of the homogenate was used to perform metabolomic profiling as described below.
Metabolomic profiling
Methods used for metabolomics analysis have been described in detail elsewhere (8, 20, 21). Briefly, amino acids, acyl carnitines, and organic acids were measured using stable isotope dilution techniques. Amino acids and acyl carnitine species were measured using flow-injection tandem mass spectrometry (MS/MS) and sample preparation methods described previously (22). Data were acquired using a Micromass Quattro micro TM system equipped with a model 2777 auto-sampler, a model 1525 μ HPLC solvent delivery system, and a data system controlled by MassLynx 4.0 operating system (Waters Associates, Milford, MA). Organic acids were quantified using a previously described method using Trace GC Ultra coupled to a Trace DSQ MS operating under Excalibur 1.4 (Thermo Fisher Scientific, Austin, TX) (20). For each tissue, 16 amino acids and seven organic acids [trichloroacetic acid (TCA) cycle intermediates and related analytes] were measured in liver, heart, skeletal muscle, and brain. Additionally, 16 amino acids and 45 acyl carnitine metabolites were measured in EDTA-plasma. The numbers of acyl carnitine species analyzed for each tissue varied from tissue to tissue because some species of acyl carnitines are not detectable in all tissues. More specifically, 45 common acyl carnitine species were measured in liver, brain, and plasma, respectively. A total of 52 acyl carnitines were measured in heart, whereas 55 were analyzed in skeletal muscle. A complete list of the specific metabolites analyzed is listed in Supplemental Tables 1 and 2 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org. All MS analyses employed stable-isotope dilution. Addition of clusters of internal standards specific to the amino acid, organic acid, and acyl carnitine analyte modules facilitates identification of each analyte peak and provides the reference for quantifying their levels. The stable-isotope internal standards used were obtained from Isotec (St. Louis, MO), Cambridge Isotope Laboratories (Andover, MA), and CDN Isotopes (Pointe-Claire, Quebec, Canada), and a complete list of standards has been published previously (22). In addition to mass, analytes are identified on the basis of the particular MS/MS transitions monitored for each class of metabolites.
Statistical analyses
Tissue-specific MS-based metabolomic analyses of brain, liver, skeletal muscle, heart and plasma amino acids, organic acids and acyl carnitines from SRC-1, SRC-2, and SRC-3 wild-type (WT) and KO male mice (n = 5 each genotype) were analyzed using BRB ArrayTools (http://linus.nci.nih.gov/BRB-ArrayTools.html) (23) and S+ Array Analyzer (http://www.solutionmetrics.com.au/support/ArrayAnalyzer/default.html). A two-way ANOVA was performed to compare the effects of feeding (fed vs. fasted), genotype (KO vs. WT), and the interaction between feeding and genotype across each set of metabolites. To compare only the effects of genotype for fasted and for fed mice separately, an ANOVA analysis with contrasts of each set of metabolites was performed. We used the Benjamini and Hochberg method for estimation of FDR (False Discovery Rate) (24). The cut-offs for differentially expressed metabolites was FDR = 0.1. Zero values observed in the raw data were considered as missing. Metabolites with more than 10% missing data have been excluded from the analysis. Raw data values were log transformed before analysis to establish distributions closer to the Gaussian. The total number of metabolites analyzed for each tissue is as follows: plasma = 60; liver = 67; muscle = 77; brain = 67; heart = 74.
The results are presented as heat maps and Venn diagrams. Heat maps were generated using DNA-Chip (www.dchip.org) (25). Standardized z-scores of the log2-transformed values were used for heat maps of raw data with blue representing low z-score values and red representing high z-score values, respectively. The results of two-way ANOVA analyses were represented as heat maps, in which blue indicates that the Fed group is significantly lower than Fast, or KO is significantly lower than WT; red represents the significant increase of Fed vs. Fast, or KO vs. WT. Pink indicates significant statistical interactions. ANOVAs with contrasts results are represented as heat maps, in which red indicates that KO is significantly higher than WT, and blue indicates that KO is significantly lower than WT.
Graphical representations of raw data are also presented as the average of the sum (Σ) of all of a particular class of metabolite for each group of mice of a given genotype ± sem as indicated. Standard statistical comparison of different groups with Gaussian distribution was carried out using a two-tailed unpaired Student's t test. Differences of P ≤ 0.05 were considered statistically significant. For all mice studies, the experimental number used is indicated in each figure legend.
Database availability
The entire raw data sets obtained from the MS-based metabolomics analyses performed in this study will be made publicly available through the Nuclear Receptor Signaling Atlas (NURSA) data repository at http://www.nursa.org/.
Supplemental data
Supplemental Data include Supplemental Experimental Procedures, two supplemental tables, and two supplemental figures. These materials are published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org.
Results
General metabolic phenotypes and metadata summary
Work from our laboratory, and that of others, clearly has demonstrated that the SRC family of coregulators play important global roles in control of metabolism and energy balance (summarized in Table 1). These observations led us to delve deeper into metabolic phenotypes of global SRC KO mice by application of MS-based metabolomics to survey metabolites that report on pathways of fat, glucose, and amino acid metabolism in multiple tissues. Five mice of each genotype (WT and SRC-1, SRC-2, or SRC-3 global KO) were used for this purpose, and metabolites were measured in five different tissues in the ad libitum fed or 24-h fasted states. A three-dimensional principal component analysis of the entire metabolomic data set revealed only a single outlier, highlighting the overall consistency of the methodology and experimental protocols used for analysis of the various tissues tested (Supplemental Fig. 1). Metadata, which include analyses of blood glucose, body weight, and body temperature are shown for each genotype group of mice (Supplemental Fig. 2). Consistent with published data (3), SRC-1 KO mice displayed hypoglycemia in both the fed and fasted states. Fasting hypoglycemia was observed in SRC-2 KO mice as previously reported (4), whereas SRC-3 demonstrated mild hypoglycemia during fasting, again consistent with published data (7, 8) (Table 1 and Supplemental Fig. 2). All three SRC KO mouse strains had reduced body weight during ad libitum feeding compared with their respective WT littermate controls, and the SRC-3 KO mouse also showed a reduction in body weight after a 24-h fast (Supplemental Fig. 2). No differences were observed in resting body temperature of any SRC KO when compared with its WT littermate controls.
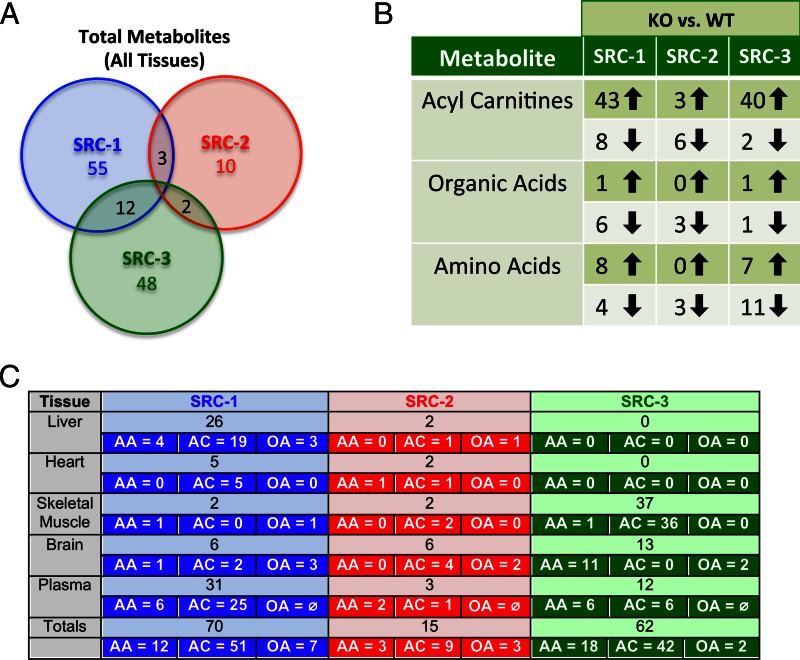
To investigate how the different SRC genotypes influence levels of amino acids, acyl carnitines, and organic acids, we performed MS analyses of four key metabolic tissues (liver, heart, skeletal muscle, and brain) and in plasma. Significant changes in metabolite concentration (increased or decreased and irrespective of feeding status) are caused in various tissues by KO of each SRC family member (Fig. 1A). Overall, a larger number of metabolites were changed significantly in SRC-1 and SRC-3 KO mice compared with SRC-2 KO mice (Fig. 1A). Remarkably, there were no metabolites changed in common across all three genotypes, whereas 12 analytes were altered in both the SRC-1 and SRC-3 KO backgrounds. Three metabolites were commonly changed in response to ablation of SRC-1 and SRC-2, whereas two metabolites were commonly changed in the absence of SRC-2 and SRC-3 (Fig. 1A). These results demonstrate minimal overlap of SRC family members with regard to regulation of major metabolites associated with protein, carbohydrate, and lipid metabolism. Also supporting this concept, KO of SRC-1 or SRC-3, but not SRC-2, caused major increases in various acyl carnitine species (Fig. 1B). Loss of SRC-3 is associated with a decrease in levels of multiple amino acids, a phenotype not observed in either SRC-1 or SRC-2 KO mice (Fig. 1B). The tissue-specific breakdown of these data reveals that ablation of each SRC has a distinct effect on global metabolism in that tissue (Fig. 1C). For example, SRC-1 ablation appears to have the most significant impact on hepatic metabolism, whereas loss of SRC-3 confers significant changes in skeletal muscle and brain metabolism. Taken together, these data highlight unique roles of individual SRCs in control of systemic metabolism.
Fig. 1.
The SRCs dynamically influence whole-body metabolism. A, Venn diagram comparison of total metabolites analyzed by MS-based metabolomics for the four major metabolic tissues, liver, heart, skeletal muscle, and brain, in addition to plasma from SRC-1, SRC-2, and SRC-3 WT and KO male mice (n = 5 each genotype). From the total metabolites analyzed from three metabolic groups (acyl carnitines, organic acids, and amino acids), the numbers of unique metabolites that changed statistically (either increased or decreased and irrespective of feeding status) by analyzing each WT group to its respective KO are indicated below the name of each coactivator (SRC-1, SRC-2, SRC-3). Overlap of any statistically changed metabolites between coactivator analyses are indicated in the intersecting regions of the Venn diagram. B, Tabular representation of the numbers and directionality of statistically altered metabolites across the three major metabolic arms (acyl carnitines, organic acids, and amino acids) as compared between WT and KO groups for each coactivator (SRC-1, SRC-2, and SRC-3). C, Summary of MS-based metabolomics analysis of amino acids (n = 16), acyl carnitines (n = 45–55), and organic acids (n =7) from SRC-1, SRC-2, and SRC-3 WT and KO mice (n = 5 each genotype). The numbers of statistically changed metabolites (either increased or decreased and irrespective of feeding status) as determined by comparing each WT with its respective littermate KO are provided for each tissue (liver, heart, skeletal muscle, brain, and plasma) for each coactivator (SRC-1, SRC-2, SRC-3). Additionally, the total numbers of statistically changed metabolites for each tissue are broken down into the three major metabolite classes (AA, amino acids; AC, acyl carnitines; OA, organic acids) analyzed for each coactivator (SRC-1, SRC-2, SRC-3). Note: For plasma, no organic acid data are represented (OA) and are therefore listed in the table as ∅. The grand totals of statistically changed metabolites for each coactivator are listed at the bottom of the table, along with a subdivision for each metabolite group (AA, amino acids; AC, acyl carnitines; OA, organic acids).
Hepatic metabolism
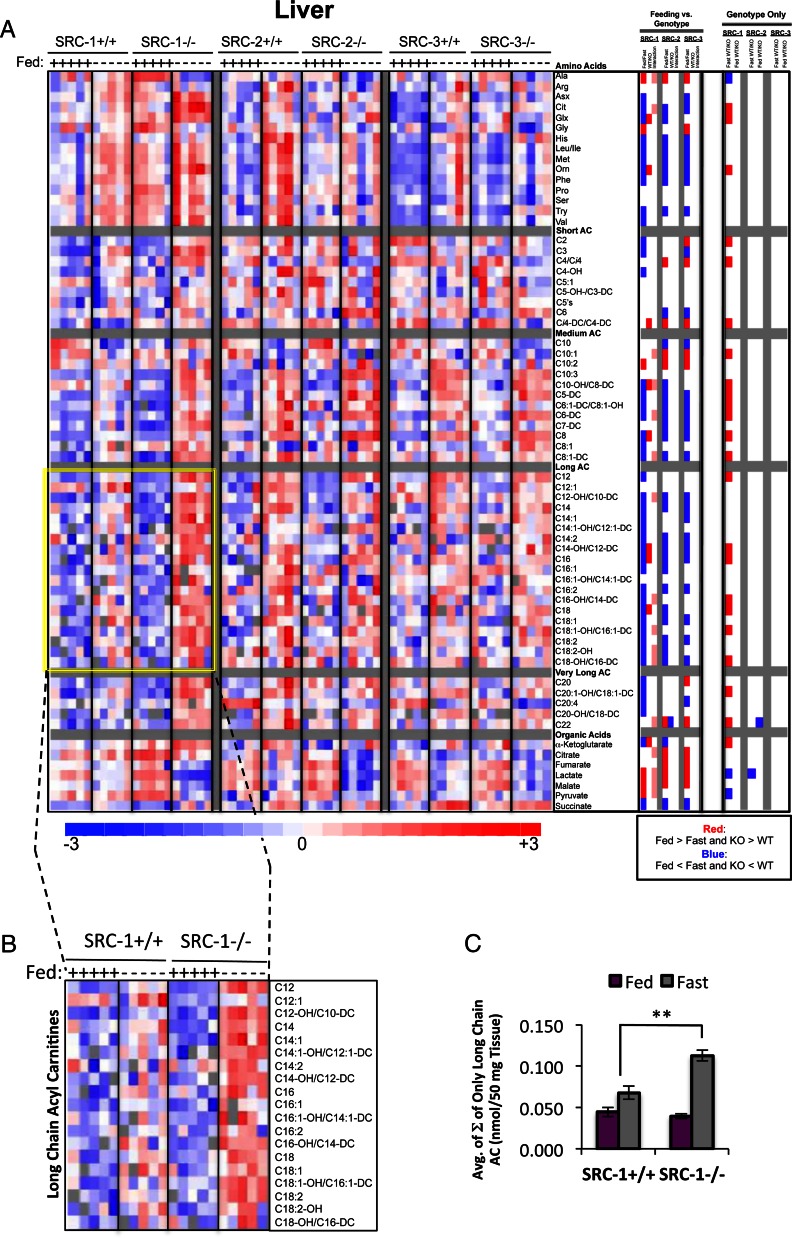
The liver has a variety of indispensable functions such as protein synthesis, lipid and carbohydrate metabolism, detoxification, and bile acid production. In this study, we were interested in characterizing the effects of ablation of individual SRC family members on hepatic amino acid, acyl carnitine, and organic acid levels. A summary of these data is presented in Fig. 1C, which highlights the numbers of total metabolites that were significantly changed (either increased or decreased and irrespective of feeding status) in each SRC KO mouse line compared with WT. Of note, selected sets of data on liver metabolites have been published elsewhere and included here only for direct comparison between all three genotypes (3). The direction of the change for significant metabolites was represented as a heat map (Fig. 2A) (Genotype Only). Whereas distinct functions for each of the SRCs have been reported in liver, our metabolomics analyses identified significant changes primarily in SRC-1 KO animals compared with WT littermates. Specifically, a total of 26 metabolites differed between SRC-1 WT and KO mice, irrespective of nutritional state. In contrast, KO of SRC-2 caused changes in only two analytes, whereas no changes were noted for SRC-3 KO (performed by ANOVA with contrasts).
Fig. 2.
Metabolomic analysis of SRC ablation on global hepatic metabolism. A, Heat map representation of z-score transformed raw data of liver-specific MS-based metabolomics analysis of amino acids (n = 16), acyl carnitines (n = 45), and organic acids (n =7) from SRC-1, SRC-2, and SRC-3 WT and KO male mice (n = 5 each genotype) fed ad libitum or after a 24-h fast as indicated. The total number of metabolites analyzed for liver equals 67, and the numbers of statistically changed metabolites (either increased or decreased and irrespective of feeding status) as determined by comparing each WT with its respective littermate KO are summarized in Fig. 1C. Note: A subset of these data was published in Ref.3. In the heat map, blue represents low z-score values and red represents high z-score values, respectively. A two-way ANOVA was performed to compare the effects of feeding (fasted vs. fed), genotype (WT vs. KO), and the interaction between feeding and genotype across each set of metabolites (“Feeding vs. Genotype” column). The results of these two-way ANOVAs were represented as a heat map, in which blue indicates that Fed is significantly lower than Fast. Blue also indicates that KO is significantly lower than WT. Red represents the significant increase of Fed vs. Fast or KO vs. WT, respectively. Pink indicates significant statistical interactions. To compare only the effects of genotype, an ANOVA with contrasts analysis of each set of metabolites was performed. These analyses were represented as a heat map, as described above (“Genotype Only” column). B, Inset of long-chain acyl carnitines from SRC-1 WT and SRC-1 KO mice, which demonstrates a more robust increase in long-chain acyl carnitines in response to fasting in the KO when compared with the WT (indicated by yellow highlight). C, Graphic representation of raw data from panel B representing the average of the sum (Σ) of only long-chain species of acyl carnitines from SRC-1 WT (n = 5) and SRC-1 KO (n = 5) mice fed ad libitum (purple bars) or after a 24-h fast (gray bars) as indicated. Data are graphed as the mean ± sem. **, P < 0.01 vs. WT mice.
We performed a two-way ANOVA of the liver metabolomics data to investigate the effect of genotype and nutritional state on the levels of different metabolites upon ablation of each of the SRCs. This analysis demonstrated that for all three genotypes, there was a significant difference in the levels of many metabolites between fed and fasted states as anticipated, because the demands for substrate availability change with nutritional state (Fig. 2A, Feeding vs. Genotype panel). Moreover, for nine acyl carnitines, five organic acids, and the two amino acids alanine and citrulline, a statistical interaction between genotype and nutritional state was noted in response to SRC-1 ablation, which was absent from the SRC-2 and SRC-3 mouse models. An ANOVA with contrast, which considers only the contribution of genotype (i.e. WT vs. KO), revealed that in the fasted state, loss of SRC-1 dramatically influenced the levels of several metabolites, especially acyl carnitines, whereas this effect was not observed in response to SRC-2 or SRC-3 ablation. Calculation of the average sum of the concentrations of long-chain acyl carnitines revealed a clear increase in these analytes in SRC-1 KO mice compared with WT littermates during fasting (Fig. 2, B and C). The fundamental change observed was a significantly higher level of these metabolites in response to switching from the fed to the fasted states, suggestive of an increased rate of flux of fatty acid through the β-oxidative pathway in liver.
Cardiac metabolism
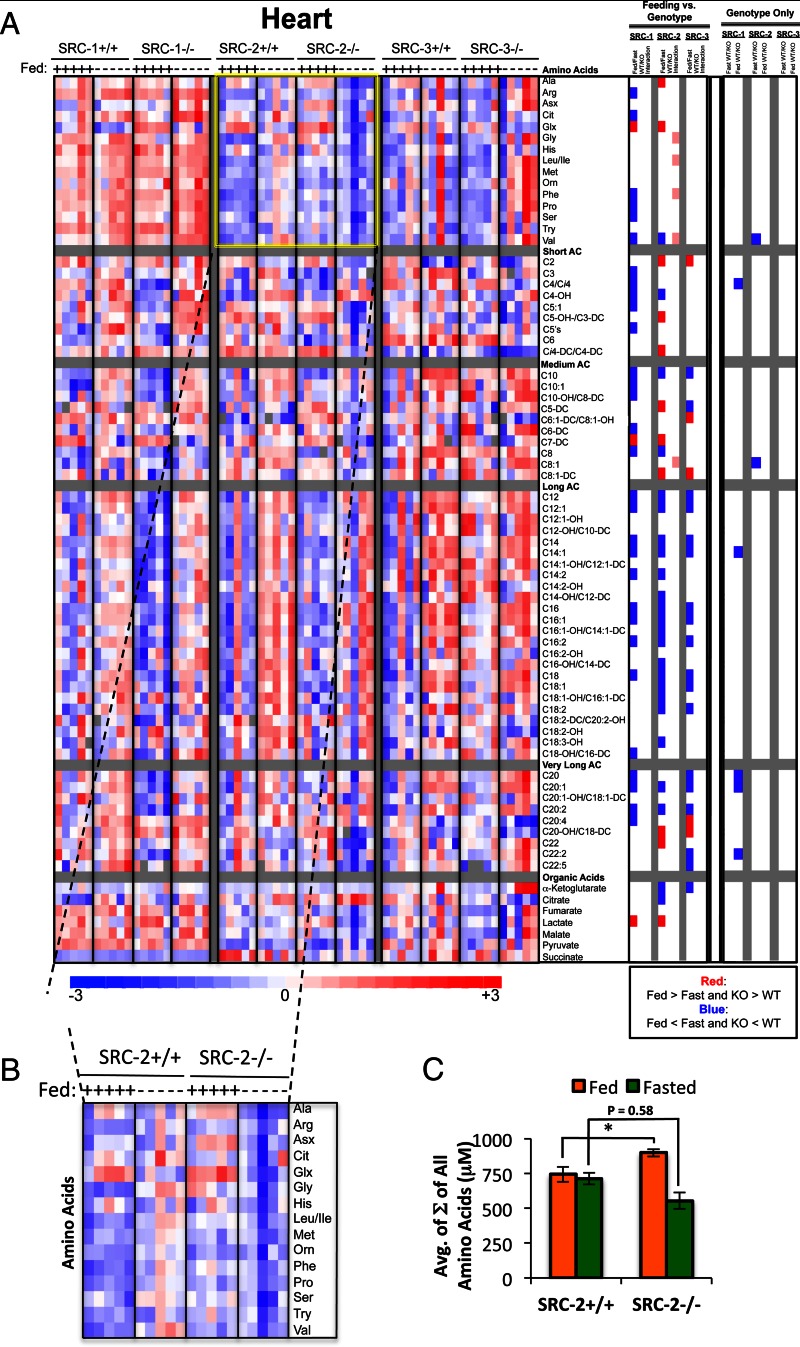
The heart, which requires a constant supply of fuel, relies mainly on the use of fatty acids for ATP production but can supplement with glucose utilization during periods of increased workload. To examine the effects of the SRC family of coactivators on adult cardiac metabolism, we measured 52 acyl carnitines (representing short, medium, long, very long, and cardiac-specific species), 16 amino acids, and seven organic acids (representing TCA cycle intermediates and associated metabolites). Using ANOVA with contrasts and a stringent FDR of 0.1, our analyses only identified a limited number of significant changes in the steady-state levels of any individual cardiac metabolites in any of the SRC KO strains compared with WT (summarized in Fig. 1C).
However, a two-way ANOVA of the cardiac metabolomics data revealed that ablation of SRC-2 significantly impacts cardiac amino acid metabolism. In fact, statistical evaluations show a significant interaction between the feeding and genotype components of our experiment that is unique to SRC-2 KO mice, with no such interactions observed for SRC-1 or SRC-3 KO mice, respectively (Fig. 3A, “Feeding vs. Genotype” panel). A closer examination of these data suggests that under fed conditions, ablation of SRC-2 results in an increase in the molar sum of all amino acids measured, whereas under fasted conditions SRC-2 KO mice display a moderate decrease in these same analytes (Fig. 3B). Whereas WT mice show no change in cardiac amino acid levels in the fasted-to-fed transition, the changes noted above in SRC-2 KO mice impart a clear change in cardiac amino acid levels in response to nutritional status. Moreover, the clear drop in cardiac amino acid levels in SRC-2 KO mice in the fasted state (Fig. 3C) suggests a unique role for SRC-2 in the maintenance of cardiac amino acid metabolism.
Fig. 3.
Metabolomic analysis of SRC ablation on global cardiac metabolism. A, Heat map representation of z-score transformed raw data for cardiac-specific MS-based metabolomics analysis of amino acids (n = 16), acyl carnitines (n = 52), and organic acids (n =7) from SRC-1, SRC-2, and SRC-3 WT and KO male mice (n = 5 each genotype) fed ad libitum or after a 24-h fast as indicated. The total number of metabolites analyzed for heart equals 74, and the numbers of statistically changed metabolites (either increased or decreased and irrespective of feeding status) as determined by comparing each WT with its respective littermate KO are summarized in Fig. 1C. In the heat map, blue represents low z-score values and red represents high z-score values, respectively. A two-way ANOVA was performed to compare the effects of feeding (fasted vs. fed), genotype (WT vs. KO), and the interaction between feeding and genotype across each set of metabolites (“Feeding vs. Genotype” column. The results of these two-way ANOVAs are represented as a heat map again in which blue indicates that Fed is significantly lower than Fast. Blue also indicates that KO is significantly lower that WT. Red represents the significant increase of Fed vs. Fast or KO vs. WT, respectively. Pink indicates statistically significant interactions. To compare only the effects of genotype, an ANOVA with contrasts analysis of each set of metabolites was performed (“Genotype Only” column). These analyses were represented as a heat map as described above. B, Inset of all amino acids analyzed from SRC-2 WT and SRC-2 KO mice, which demonstrates a dysregulation in amino acid metabolism in the SRC-2 KO in response to the feeding/fasting switch. C, Graphic representation of raw data from panel B representing the sum (Σ) of all amino acids from SRC-2 WT (n = 5) and SRC-2 KO (n = 5) mice fed ad libitum (green bars) or after a 24-h fast (green bars) as indicated. Data are graphed as the mean ± sem. *, P < 0.05 vs. WT mice.
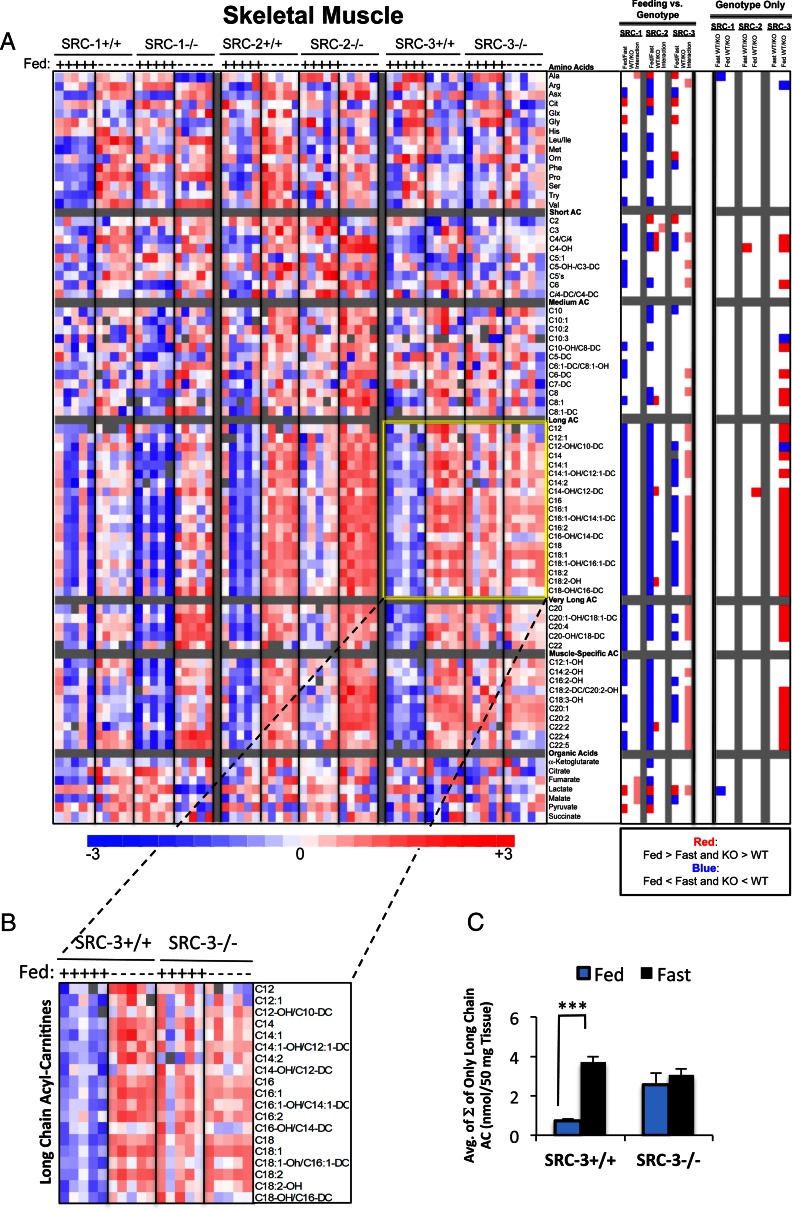
Skeletal muscle metabolism
Skeletal muscle plays a substantial role in balancing energy expenditure with caloric intake. Key metabolic regulatory roles have recently emerged for the SRC family in skeletal muscle (7, 8, 26). Using the same analytical methods described earlier, we measured the concentration of 77 skeletal muscle metabolites, comprising 55 acyl carnitines (representing short, medium, long, very long and skeletal muscle-specific species), 16 amino acids, and seven organic acids (representing TCA cycle intermediates and associated metabolites). The findings from this analysis are summarized in Fig. 1C. In contrast to what we found in the liver, 37 metabolites were significantly changed in the absence of SRC-3 (Fig. 4A), of which only two were similarly changed in SRC-2 KO mice. Moreover, only two metabolites were significantly different between either WT and SRC-1 or SRC-2 KO littermates. The heat map clearly reveals the influence of the feeding/fasting switch on broad classes of metabolites (Fig. 4A). In particular, a large increase is observed in long- and very-long-chain acyl carnitine species as well as skeletal muscle-specific acyl carnitines in the fasted compared with the ad libitum fed state, which is expected due to the increased fatty acid availability and metabolic switch to β-oxidation under this condition. Similar to what we found for SRC-1 in the liver, we observed a statistical interaction between feeding and genotype for many skeletal muscle acyl carnitines in the absence of SRC-3. A portion of the SRC-1 as well as the SRC-3 data set (highlighted inset) has been recently published (3, 8). These data are reproduced here simply to facilitate direct comparison with all three SRCs, highlighting the tissue-specific effects of SRC-3 as they pertain to skeletal muscle metabolism. These data reveal an effect of SRC-3 ablation on the long-chain acyl carnitine profile in skeletal muscle, which is clearly absent from the SRC-1 or SRC-2 KOs (Fig. 4B). A graphic representation of the average of the molar sum of long-chain acyl carnitine species demonstrates a clear effect of fasting/feeding on these analytes in WT mice, with this regulation being lost in the SRC-3 KO model (Fig. 4C). The report of a subset of these findings in a recent publication from our laboratory establishes that SRC-3 is essential for proper maintenance of long-chain fatty acid metabolism in skeletal muscle, leading to the discovery that SRC-3 controls expression of a key gene involved in long-chain fatty acid oxidation, CACT (8). Importantly, these metabolomics data provide convincing evidence that the SRCs have evolved tissue- and pathway-specific functions and show that their functions in heart and skeletal muscle are different. In the case of skeletal muscle, loss of an essential coactivator like SRC-3 cannot be overcome by SRC-1 and SRC-2.
Fig. 4.
Metabolomic analysis of SRC ablation on global skeletal muscle metabolism. A, Heat map representation of z-score transformed raw data for skeletal muscle-specific MS-based metabolomics analysis of amino acids (n = 16), acyl carnitines (n = 55), and organic acids (n =7) from SRC-1, SRC-2, and SRC-3 WT and KO male mice (n = 5 each genotype) fed ad libitum or after a 24-h fast as indicated. The total number of metabolites analyzed for skeletal muscle equals 77, and the numbers of statistically changed metabolites (either increased or decreased and irrespective of feeding status) as determined by comparing each WT with its respective littermate KO are summarized in Fig. 1C. Note: A portion of these data was published in Refs. 3 and 8. In the heat map, blue represents low z-score values and red represents high z-score values, respectively. A two-way ANOVA was performed to compare the effects of feeding (fasted vs. fed), genotype (WT vs. KO), and the interaction between feeding and genotype across each set of metabolites (“Feeding vs. Genotype” column). The results of these two-way ANOVA analyses were represented as a heat map in which blue indicates that Fed is significantly lower than Fast. Blue also indicates that KO is significantly lower that WT. Red represents the significant increase of Fed vs. Fast or KO vs. WT. Pink indicates statistically significant interactions. To compare only the effects of genotype, an ANOVA of each set of metabolites was performed (“Genotype Only” column). These analyses were z-score transformed and represented on a three-point gradient color scale as described above. B, Inset of only long-chain acyl carnitines from SRC-3 WT and SRC-3 KO mice demonstrating impairment in the feeding/fasting switch, which result in a constitutive elevation of long-chain acyl carnitines in the SRC-3 KO mice. Note: A similar representation of all the long-chain (long, very-long, and skeletal muscle-specific) acyl carnitine data for the SRC-3 WT and KO mice was reported in Ref. 8). C, Graphic representation of raw data from Fig. 4B representing the sum (Σ) of only long-chain species of acyl carnitines from SRC-3 WT (n = 5) and SRC-3 KO (n = 5) mice fed ad libitum (blue bars) or after a 24-h fast (black bars) as indicated. Data are graphed as the mean ± sem. ***, P < 0.001 vs. WT mice.
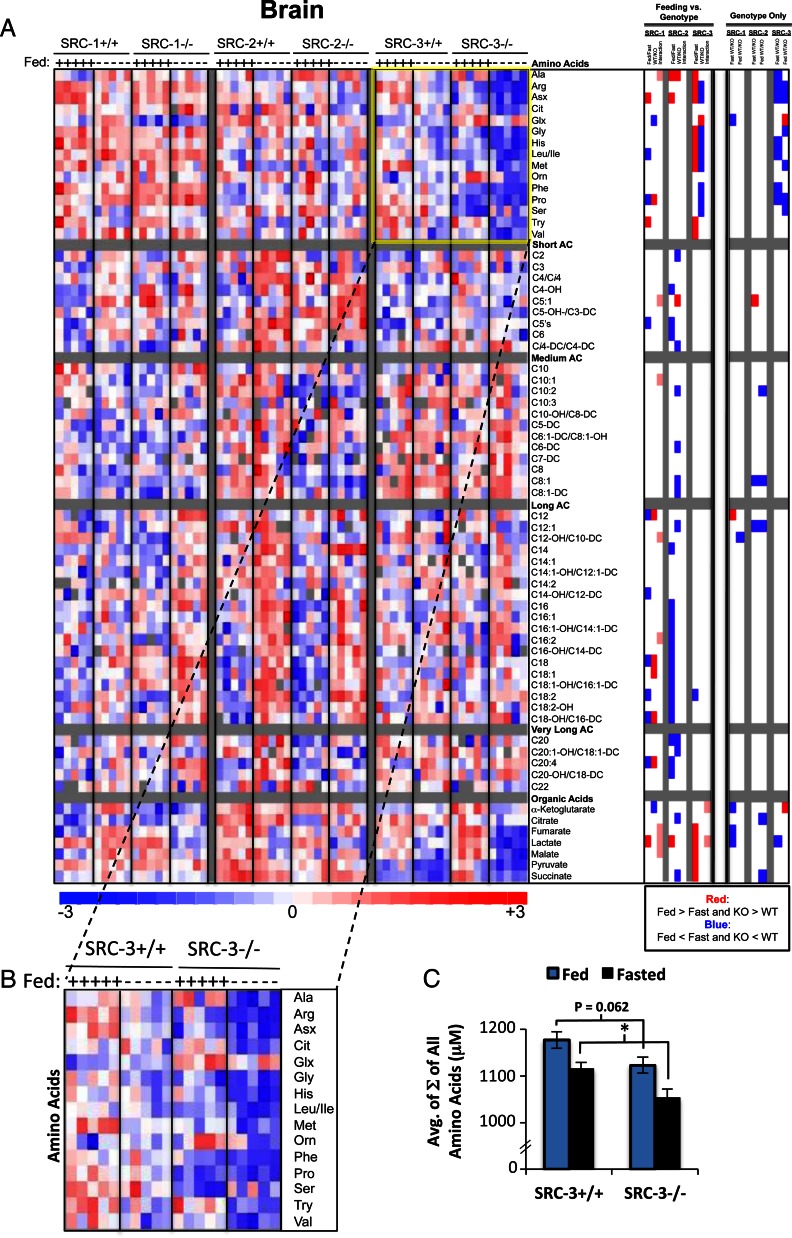
Brain metabolism
Perhaps no other tissue is as sensitive and selective with regard to metabolic fuel utilization as the brain. Under fed conditions, the mature mammalian brain utilizes glucose as its sole source of energy, whereas in fasting, ketone catabolism is used to supplement glucose as an energy source. We again used MS-based metabolomics to quantitatively evaluate the levels of 67 brain metabolites including 45 acyl carnitines, 16 amino acids, and seven organic acids. Analysis of the SRC KO lines revealed that SRC-3 has the largest influence on brain metabolism, with a total of 13 metabolites exhibiting significant changes (increased or decreased and irrespective of feeding status) in SRC-3 KO mice, compared with only six metabolites in either the SRC-1 and SRC-2 KO models (summarized in Fig. 1C). Heat map representation and ANOVA analysis with contrast (Genotype Only) suggests that ablation of SRC-3 has an overall impact on amino acid metabolism (Fig. 5A). However, a two-way ANOVA exposed a limited degree of statistical interaction. Upon closer examination, loss of SRC-3 seems to confer a decrease in amino acid levels in the brain that is independent of feeding status (Fig. 5, B and C), suggesting that SRC-3 is necessary for maintenance of brain protein metabolism.
Fig. 5.
Metabolomic analysis of SRC ablation on global brain metabolism. A, Heat map representation of z-score transformed raw data for brain-specific MS-based metabolomics analysis of amino acids (n = 16), acyl carnitines (n = 45), and organic acids (n =7) from SRC-1, SRC-2, and SRC-3 WT and KO male mice (n = 5 each genotype) fed ad libitum or after a 24-h fast as indicated. The total number of metabolites analyzed for skeletal muscle equals 67, and the numbers of statistically changed metabolites (either increased or decreased and irrespective of feeding status) as determined by comparing each WT with its respective littermate KO are summarized in Fig. 1C. In the heat map, blue represents low z-score values, and red represents high z-score values, respectively. A two-way ANOVA was performed to compare the effects of feeding (fasted vs. fed), genotype (WT vs. KO), and the interaction between feeding and genotype across each set of metabolites (“Feeding vs. Genotype” column). The results of these two-way ANOVAs were represented as a heat map in which blue indicates that Fed is significantly lower than Fast. Blue also indicates that KO is significantly lower than WT. Red represents the significant increase of Fed vs. Fast or KO vs. WT. Pink indicates statistically significant interactions. To compare only the effects of genotype, an ANOVA with contrasts analysis of each set of metabolites was performed (“Genotype Only” column). These analyses were represented as a heat map as described above. B, Inset of all amino acids analyzed from whole brain of SRC-3 WT and SRC-3 KO mice, which demonstrate a global decrease in amino acid levels in the SRC-3 KO compared with the WT mice that is irrespective of feeding status. C, Graphic representation of raw data from Fig. 5B representing the sum (Σ) of all amino acids from SRC-3 WT (n = 5) and SRC-3 KO (n = 5) mice fed ad libitum (blue bars) or after a 24-h fast (black bars) as indicated. Data are graphed as the mean ± sem. *, P < 0.05 vs. WT mice.
Plasma metabolites
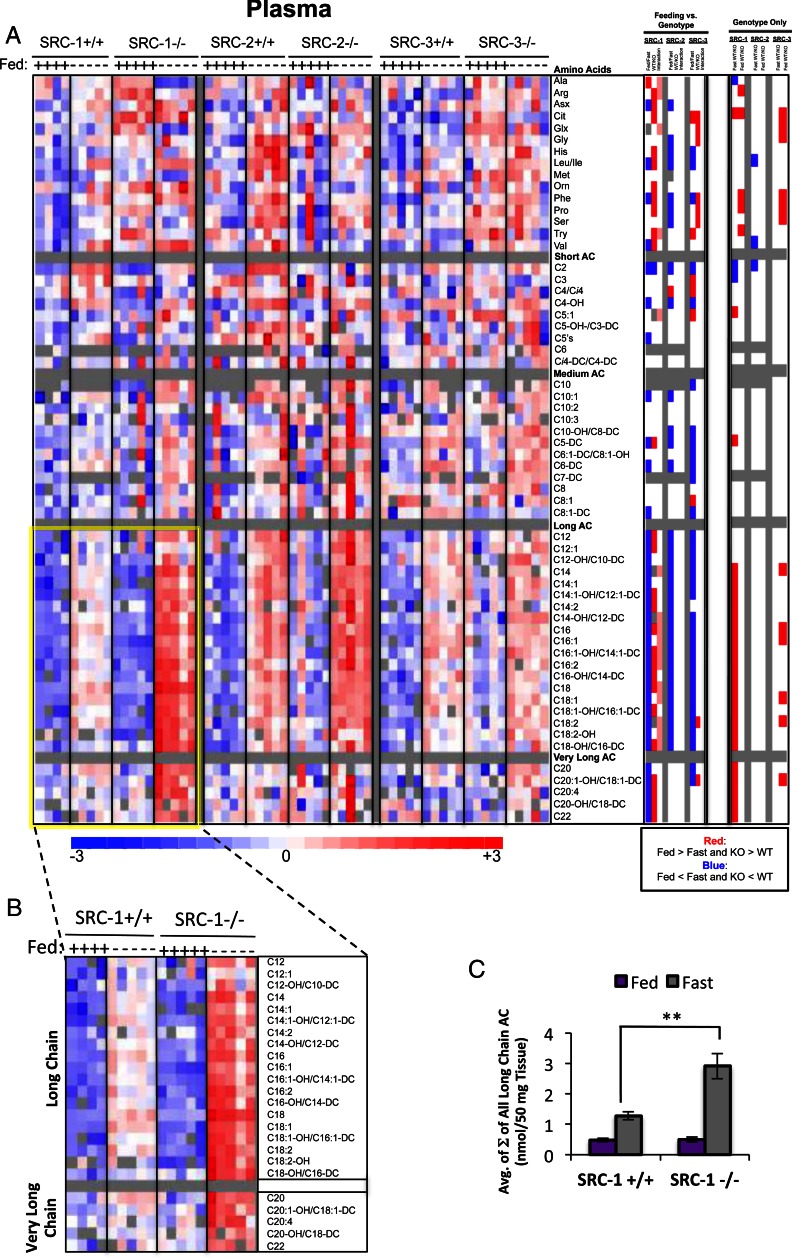
Analyses of plasma or serum are widely used in a clinical setting to identify biochemical phenotypes that correspond to clinical phenotypes. In our case, we were interested to determine how changes in the metabolic organs might be mirrored by similar biochemical changes in plasma in response to coactivator ablation. To this end, we analyzed 61 metabolites in plasma, comprising 45 acyl carnitines and 16 amino acids. Notably, selected data on plasma metabolites have also been published elsewhere (3, 8). A total of 31 metabolites differed in plasma between SRC-1 WT and KO mice, compared with only three and 12 metabolites in SRC-2 and SRC-3 KO mice, respectively (summarized in Fig. 1C). Nine metabolites changed significantly in both SRC-1 and SRC-3 KO mice, whereas only one metabolite changed in concert in plasma of SRC-1 and SRC-2 KO animals.
A two-way ANOVA demonstrated significant differences in the levels of multiple metabolites in the fed compared with the fasted states for all three SRC genotypes (Fig. 6A, “Feeding vs. Genotype” panel). More specifically, for 11 long-chain and one short-chain acyl carnitine, as well as four amino acids, there was a statistical interaction between genotype and nutritional state upon SRC-1 ablation that was not observed in the SRC-2 or SRC-3 KO models. Moreover, the ANOVA with contrast revealed that in the fasted state, loss of SRC-1 dramatically influenced the levels of several metabolites, especially acyl carnitines (Fig. 6A, “Genotype Only” panel), closely reflecting the impact of SRC-1 KO on liver metabolites (Fig. 2A, “Genotype Only” panel). Also, SRC-3 KO mice exhibited significant increases in plasma long-chain acyl carnitines in the fed state (Fig. 6A, “Genotype Only” panel) that mirrored changes described earlier for these same analytes in skeletal muscle (Fig. 4A). Six amino acids were significantly increased in plasma in the fed state in SRC-3 KO mice compared with WT littermates (Fig. 6A). Moreover, even though only three metabolites were significantly different in SRC-2 WT vs. KO mice during fasting, these metabolites were the branched-chain amino acids leucine, isoleucine, and valine, which have been recently implicated in various metabolic diseases (21, 27). Finally, loss of SRC-1 leads to a marked increase in long- and very-long-chain acyl carnitines in plasma in the fasted state (Fig. 6B), which is consistent with the liver metabolomics data discussed earlier (Fig. 2, B and C). A graphic representation of the molar sum of plasma long- and very-long-chain acyl carnitine levels in SRC-1 WT and KO mice clearly highlights the impact of SRC-1 ablation on these metabolites (Fig. 6C).
Fig. 6.
Metabolomic analysis of SRC ablation on global plasma metabolism. A, Heat map representation of z-score transformed raw data for plasma-specific MS-based metabolomics analysis of amino acids (n = 16), acyl carnitines (n = 45), and organic acids (n =7) from SRC-1, SRC-2, and SRC-3 WT and KO male mice (n = 5 each genotype) fed ad libitum or after a 24-h fast as indicated. The total number of metabolites analyzed for plasma equals 60, and the numbers of statistically changed metabolites (either increased or decreased and irrespective of feeding status) as determined by comparing each WT with its respective littermate KO are summarized in Fig. 1C. Note: A portion of these data was published in Ref. 8. In the heat map, blue indicates that Fed is significantly lower than Fast. Blue also indicates that KO is significantly lower than WT. Red represents the significant increase of Fed vs. Fast or KO vs. WT. Pink indicates statistically significant interactions. A two-way ANOVA was performed to compare the effects of feeding (fasted vs. fed), genotype (WT vs. KO), and the interaction between feeding and genotype across each set of metabolites (“Feeding vs. Genotype” column). The results of these two-way ANOVAs were z-score transformed and represented again on a three-point gradient color scale in which blue represents a decrease and red represents an increase, respectively. To compare only the effects of genotype, an ANOVA with contrasts analysis of each set of metabolites was performed (“Genotype Only” column). These analyses are represented as a heat map as described above. Note: Graphic comparisons of these amino acid data from SRC-3 WT and KO mice have been reported elsewhere (8). B, Inset of long- and very-long chain acyl carnitines from SRC-1 WT and SRC-1 KO mice, which demonstrates a more robust increase in long- and very-long chain acyl carnitines in response to fasting in the KO when compared with the WT. C, Graphic representation of raw data from panel B representing the sum (Σ) of all long- and very-long chain species of plasma acyl carnitines from SRC-1 WT (n = 5) and SRC-1 KO (n = 5) mice fed ad libitum (purple bars) or after a 24-h fast (gray bars) as indicated. Note: Selected graphic comparisons of acyl carnitines from SRC-1 WT and KO mice have been reported elsewhere (3). Data are graphed as the mean ± sem. **, P < 0.01 vs. WT mice.
Metabolic summary
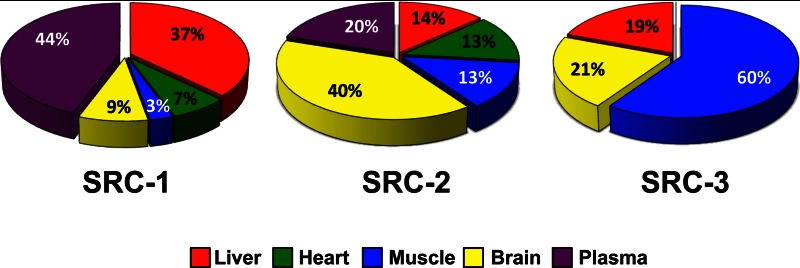
The use of quantitative metabolomics to monitor changes in tissue- and pathway-specific metabolites in response to coactivator ablation provides a clear picture of the differential roles of the SRCs on metabolic regulation. To collectively examine the tissue-specific contributions upon loss of each SRC, the number of metabolites that significantly changed was graphed as a percentage of the total changed metabolites (Fig. 7). These data beautifully illustrate that each SRC differentially controls metabolism of distinct tissues. The information provided from this Research Resource establishes the first convincing evidence for the lack of metabolic redundancy within the highly conserved SRC family of transcriptional coactivators.
Fig. 7.
Summary of the tissue-specific metabolic contributions of the SRC family. The numbers of statistically changed metabolites (either increased or decreased and irrespective of feeding status) for each metabolic tissue as determined by comparing each WT with its respective littermate KO (SRC-1, SRC-2, or SRC-3) were graphed as a percentage of the total number of statistically changed metabolites.
Discussion
Over the past decade, increasing attention has been devoted to understanding the dynamic regulatory inputs of transcriptional coactivators such as the SRCs on metabolism. Although traditional phenotyping has provided valuable clues and has expanded our working knowledge of the capacities of coregulators to influence systems metabolism, a comprehensive and quantitative metabolomics screen such as the one reported here as an outcome of the NURSA project has never been performed to evaluate the broad metabolic impact of an entire family of functionally related transcriptional coregulators. Our studies demonstrate clearly that individual coregulators function in a tissue- and pathway-specific fashion to regulate key aspects of intermediary metabolism. However, due to the use of congenic KOs to study SRC function in whole-body metabolism, it is impossible to clarify to what extent the metabolic changes we observe in various tissues are the direct result of deletion of a coactivator in that target tissue or whether these changes represent a more systemic effect of coactivator ablation. Despite this limitation, our use of quantitative metabolomics highlights the importance of the SRCs for proper regulation of systems metabolism.
To date, more than 500 different coregulator proteins have been identified, and from an evolutionary standpoint, a major issue is the extent to which different members of a coregulator family are functionally redundant. Even though proteins within the same family are comparable with regard to structural domains and transcriptional regulation, the need for a panoply of genes with similar structures and seemingly overlapping functions is not clearly understood. Previous studies in our laboratory reported similarities in metabolic phenotypes between different SRC KO mouse models (3, 4), leading us to an initial assumption that the three different SRCs possess overlapping functions and similar transcriptional targets for exerting metabolic control. In the present study, we have undertaken an unbiased and quantitative approach to compare the levels of a large number of metabolites in core metabolic organs in the absence of each of the three SRCs in two different nutritional states. An advantage of metabolic profiling is that it potentially provides a phenotypic read-out that integrates upstream variation in the genome, transcriptome, and proteome, providing the most distal and integrated view possible of the metabolic functions of the SRCs. Our metabolomics results clearly demonstrate that the absence of each individual coactivator has a very discrete impact on the metabolic landscape, even though our previous studies have identified similar gross phenotypes such as hypoglycemia and changes in energy balance shared in common among the different SRC KO models. Therefore, our study demonstrates a surprisingly limited degree of redundancy or compensation between the different SRCs with regard to regulation of fundamental pathways of lipid, amino acid, and glucose metabolism.
As mentioned earlier, both SRC-1 and SRC-2 KO mice display fasting hypoglycemia (3, 4, 28). However, the underlying molecular mechanisms that explain this phenotype clearly are different between the two SRC mouse models. In the study by Louet et al. (3), we found hypoglycemia in SRC-1 KO mice in both fasting and fed conditions. Moreover, SRC-1 KO mice showed significant increases in levels of several acyl carnitines in liver and plasma. Simultaneously, there were significant differences in the levels of several amino acids and urea cycle intermediates. Taken in the context of the hypoglycemic phenotype, it was concluded that these defects in SRC-1 KO mice were primarily due to the central role of SRC-1 as a key control factor for gluconeogenesis that functions by: 1) controlling the expression of the gluconeogenic enzymes pyruvate carboxylase and fructose 1,6-bisphosphatase and; 2) coordinating turnover of amino acids, which can serve as substrates for de novo glucose production. We also suggested that the significant changes in acyl carnitines were possibly a compensatory effect to activate fatty acid oxidation in response to hypoglycemia. One might expect to find a similar phenotype in SRC-2 KO mice because ablation of SRC-2 also leads to fasting hypoglycemia due to reduced expression of the gluconeogenic enzyme G6PC (4). However, our metabolomics data do not reveal increases in acyl carnitines in liver or plasma of SRC-2 KO mice. Whether this can be explained by the more severe hypoglycemia of SRC-1 KO mice, or possibly by a heretofore-unknown effect of SRC-1 in control of enzymes of fatty acid oxidation remains to be determined.
In addition to the studies mentioned above, our metabolomics strategy was instrumental in identifying a primary defect in long-chain acyl carnitine metabolism in skeletal muscle of SRC-3 KO mice (8). In that study, we found that SRC-3 regulates the expression of the CACT gene in skeletal muscle, a key enzyme in mitochondrial long-chain fatty acid oxidation. By comparison, regardless of whether the increased levels of acyl carnitines in SRC-1 KO mice discussed above can be explained by direct or compensatory effects, the significant elevation of urea cycle intermediates in the liver in that mouse model points to a direct regulation of urea cycle enzymes by SRC-1. Therefore, both the SRC-1 and SRC-3 studies are excellent proof-of-principle examples of how an unbiased metabolomics approach can point to pathways and tissues for further study in coactivator KO mouse models. By studying the fingerprint of a whole group of metabolites that are dependent on different genetic inputs as well as nutritional states, we have the opportunity to unravel new functions of a family of transcriptional coregulators. One can consider metabolic differences between the SRC KO models at the level of large functional groups of metabolites (i.e. amino acids, organic acids, and acyl carnitines) or in smaller subsets. An example of the latter is our finding in SRC-2 KO mice of a decrease in levels of circulating branched-chain amino acids (BCAAs) (i.e. leucine, isoleucine, and valine) during fasting conditions. As previously reported by our laboratory, SRC-2 KO mice are characterized by a phenotype resembling von Gierke's disease, which includes fasting hypoglycemia and hyperketonemia (4). From this, it follows that lower levels of leucine and isoleucine in SRC-2 KO mice may reflect their increased utilization for ketogenesis to supply the brain with a metabolic fuel that it can use in lieu of glucose. Moreover, BCAAs together with catabolic products such as glycine and C3 and C5 acyl carnitines have been associated previously with peripheral insulin sensitivity (22). It is possible that the decreased circulating levels of BCAAs in SRC-2 KO mice are due to a role of this coactivator in regulating peripheral insulin sensitivity. Further studies will be required to fully investigate this idea. Although these findings highlight specific examples of how our metabolomics approach has illuminated mechanistic details of coregulator function, the broader benefit of this strategy remains its ability to evaluate metabolism at a systems biology level.
In conclusion, we believe this study provides the first full metabolomic assessment of an entire family of transcriptional coregulators. Data presented here, together with previous publications from our laboratory, provide evidence that metabolomics can be used to define new regulatory pathways for individual coregulators such as the SRCs. Collectively, these findings highlight the potential of the SRCs to not only serve as powerful transcriptional amplifiers, but identifies this family of coactivators as master regulators of systems metabolism. Whereas this study provides an excellent proof of principle for the utility of metabolomics in identifying new functions for metabolic regulators, more importantly this Research Resource allows for integration of these metabolite data with the wealth of gene expression and proteomics data that already exist for the SRC family. Through careful integration and analysis, these data hold the potential to unveil a comprehensive picture of coactivator function that begins at the level of gene expression, culminating with changes in protein complex interactions that ultimately yield a unique metabolite signature.
Supplementary Material
Acknowledgments
We thank Rainer Lanz in the Department of Molecular and Cellular Biology at Baylor College of Medicine (Houston, TX 77030) for helpful discussions.
This work was supported by Grant P30CA125123 (to S.G.H.); Grants R01 DK058242 and R01 CA112403 (to J.X.); NURSA/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant U19 DK62434-08 (to C.B.N. and B.W.O.); NIDDK Grant PO1 HD08818-37 (to B.W.O.); and Welch Grants Q1521 and R01HD8188-38S1 (to B.Y. and B.W.O.). J.V.S. is funded by the Norwegian Cancer Society, University of Bergen, Det regionale samarbeidsorganet (Helse Vest RHF and University of Bergen), and the Norwegian Society of Endocrinology.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CACT
- Carnitine/acyl carnitine translocase
- FDR
- false discovery rate
- KO
- knockout
- MS
- mass spectrometry
- MS/MS
- tandem MS
- NCoA-1
- -2, and -3, nuclear receptor coactivator-1, -2, and 3
- SRC-1
- -2, and -3, steroid receptor coactivator 1, 2, and 3
- TCA
- tricarboxylic acid cycle
- WT
- wild-type.
References
- 1. York B, O'Malley BW. 2010. Steroid receptor coactivator (SRC) family: masters of systems biology. J Biol Chem 285:38743–38750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Picard F, Géhin M, Annicotte J, Rocchi S, Champy MF, O'Malley BW, Chambon P, Auwerx J. 2002. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell 111:931–941 [DOI] [PubMed] [Google Scholar]
- 3. Louet JF, Chopra AR, Sagen JV, An J, York B, Tannour-Louet M, Saha PK, Stevens RD, Wenner BR, Ilkayeva OR, Bain JR, Zhou S, DeMayo F, Xu J, Newgard CB, O'Malley BW. 2010. The coactivator SRC-1 is an essential coordinator of hepatic glucose production. Cell Metab 12:606–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chopra AR, Louet JF, Saha P, An J, Demayo F, Xu J, York B, Karpen S, Finegold M, Moore D, Chan L, Newgard CB, O'Malley BW. 2008. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science 322:1395–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chopra AR, Kommagani R, Saha P, Louet JF, Salazar C, Song J, Jeong J, Finegold M, Viollet B, DeMayo F, Chan L, Moore DD, O'Malley BW. 2011. Cellular energy depletion resets whole-body energy by promoting coactivator-mediated dietary fuel absorption. Cell Metab 13:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Louet JF, Coste A, Amazit L, Tannour-Louet M, Wu RC, Tsai SY, Tsai MJ, Auwerx J, O'Malley BW. 2006. Oncogenic steroid receptor coactivator-3 is a key regulator of the white adipogenic program. Proc Natl Acad Sci USA 103:17868–17873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coste A, Louet JF, Lagouge M, Lerin C, Antal MC, Meziane H, Schoonjans K, Puigserver P, O'Malley BW, Auwerx J. 2008. The genetic ablation of SRC-3 protects against obesity and improves insulin sensitivity by reducing the acetylation of PGC-1α. Proc Natl Acad Sci USA 105:17187–17192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. York B, Reineke EL, Sagen JV, Nikolai BC, Zhou S, Louet JF, Chopra AR, Chen X, Reed G, Noebels J, Adesina AM, Yu H, Wong LJ, Tsimelzon A, Hilsenbeck S, Stevens RD, Wenner BR, Ilkayeva O, Xu J, Newgard CB, O'Malley BW. 2012. Ablation of steroid receptor coactivator-3 resembles the human CACT metabolic myopathy. Cell Metab 15:752–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. York B, Yu C, Sagen JV, Liu Z, Nikolai BC, Wu RC, Finegold M, Xu J, O'Malley BW. 2010. Reprogramming the posttranslational code of SRC-3 confers a switch in mammalian systems biology. Proc Natl Acad Sci USA 107:11122–11127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu Y, Chen Q, Li W, Su X, Chen T, Liu Y, Zhao Y, Yu C. 2010. Overexpression of transcriptional coactivator AIB1 promotes hepatocellular carcinoma progression by enhancing cell proliferation and invasiveness. Oncogene 29:3386–3397 [DOI] [PubMed] [Google Scholar]
- 11. Apostolakis EM, Ramamurphy M, Zhou D, Oñate S, O'Malley BW. 2002. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Mol Endocrinol 16:1511–1523 [DOI] [PubMed] [Google Scholar]
- 12. Lachize S, Apostolakis EM, van der Laan S, Tijssen AM, Xu J, de Kloet ER, Meijer OC. 2009. Steroid receptor coactivator-1 is necessary for regulation of corticotropin-releasing hormone by chronic stress and glucocorticoids. Proc Natl Acad Sci USA 106:8038–8042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishihara E, Yoshida-Komiya H, Chan CS, Liao L, Davis RL, O'Malley BW, Xu J. 2003. SRC-1 null mice exhibit moderate motor dysfunction and delayed development of cerebellar Purkinje cells. J Neurosci 23:213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patchev AV, Fischer D, Wolf SS, Herkenham M, Götz F, Gehin M, Chambon P, Patchev VK, Almeida OF. 2007. Insidious adrenocortical insufficiency underlies neuroendocrine dysregulation in TIF-2 deficient mice. FASEB J 21:231–238 [DOI] [PubMed] [Google Scholar]
- 15. Schmidt MV, Oitzl M, Steenbergen P, Lachize S, Wurst W, Müller MB, de Kloet ER, Meijer OC. 2007. Ontogeny of steroid receptor coactivators in the hippocampus and their role in regulating postnatal HPA axis function. Brain Res 1174:1–6 [DOI] [PubMed] [Google Scholar]
- 16. Winnay JN, Xu J, O'Malley BW, Hammer GD. 2006. Steroid receptor coactivator-1-deficient mice exhibit altered hypothalamic-pituitary-adrenal axis function. Endocrinology 147:1322–1332 [DOI] [PubMed] [Google Scholar]
- 17. Qi C, Zhu Y, Pan J, Yeldandi AV, Rao MS, Maeda N, Subbarao V, Pulikuri S, Hashimoto T, Reddy JK. 1999. Mouse steroid receptor coactivator-1 is not essential for peroxisome proliferator-activated receptor α-regulated gene expression. Proc Natl Acad Sci USA 96:1585–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P. 2002. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol 22:5923–5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O'Malley BW. 2000. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci USA 97:6379–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferrara CT, Wang P, Neto EC, Stevens RD, Bain JR, Wenner BR, Ilkayeva OR, Keller MP, Blasiole DA, Kendziorski C, Yandell BS, Newgard CB, Attie AD. 2008. Genetic networks of liver metabolism revealed by integration of metabolic and transcriptional profiling. PLoS Genet 4:e1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. 2009. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, Newgard CB. 2004. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 10:268–274 [DOI] [PubMed] [Google Scholar]
- 23. Simon R, Lam AP. 2006. BRB-ArrayTools users guide, National Cancer Institute Biometric Research Branch, Bethesda, MD: In: Branch NCIBR ed. Bethesda [Google Scholar]
- 24. Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300 [Google Scholar]
- 25. Li C, Wong WH. 2001. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA 98:31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duteil D, Chambon C, Ali F, Malivindi R, Zoll J, Kato S, Geny B, Chambon P, Metzger D. 2010. The transcriptional coregulators TIF2 and SRC-1 regulate energy homeostasis by modulating mitochondrial respiration in skeletal muscles. Cell Metab 12:496–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Newgard CB. 2012. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 15:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Z, Shah OJ, Hunter T. 2012. The transcriptional coactivators p/CIP and SRC-1 control insulin resistance through IRS1 in obesity models. PLoS One 7:e36961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.