Abstract
Background: Available data have indicated independent direct relations of dietary animal protein and meat to the blood pressure (BP) of individuals.
Objective: In this study, we aimed to assess whether BP is associated with the intake of dietary amino acids higher relatively in animal than in vegetable protein (alanine, arginine, aspartic acid, glycine, histidine, lysine, methionine, and threonine).
Design: The study was a cross-sectional epidemiologic study that involved 4680 persons aged 40–59 y from 17 random population samples in the People's Republic of China, Japan, the United Kingdom, and the United States. BP was measured 8 times at 4 visits; dietary data (83 nutrients and 18 amino acids) were from four 24-h dietary recalls and two 24-h urine collections.
Results: Dietary glycine and alanine (the percentage of total protein intake) were considered singly related directly to BP; with these 2 amino acids together in regression models (from model 1, which was controlled for age, sex, and sample, to model 5, which was controlled for 16 possible confounders), glycine, but not alanine, was significantly related to BP. Estimated average BP differences associated with a 2-SD higher glycine intake (0.71 g/24 h) were 2.0–3.0-mm Hg systolic BP (z = 2.97–4.32) stronger in Western than in East Asian participants. In Westerners, meat was the main dietary source of glycine but not in East Asians (Chinese: grains/flour and rice/noodles; Japanese: fish/shellfish and rice/noodles).
Conclusion: Dietary glycine may have an independent adverse effect on BP, which possibly contributes to direct relations of animal protein and meat to BP.
INTRODUCTION
Available data have indicated that animal protein and particularly red meat intake relate directly to blood pressure (BP)6, whereas vegetable protein intake and vegetarian eating patterns relate inversely to BP (1, 2). Animal protein and vegetable protein differ in their predominant amino acids. In animal protein, alanine, arginine, aspartic acid, glycine, histidine, lysine, methionine, and threonine predominate (expressed as the percentage of total protein); in vegetable protein, cysteine, glutamic acid, phenylalanine, proline, and serine predominate (1). The intake of one of these latter 5 amino acids, glutamic acid, had a significant independent inverse relation to BP in analyses on 4680 individuals aged 40–59 y in the population-based International Study on Macro/Micronutrients and Blood Pressure (INTERMAP) (3). The INTERMAP hypothesized that a higher intake of one or more amino acids predominant in animal protein is associated with higher BP.
SUBJECTS AND METHODS
Basic premises, population samples, field methods
Basic INTERMAP scientific and methodologic premises are as follows: multiple nutrients have small independent influences on the BP of individuals that, in combination, summate as sizable effects (4), and to detect the effect of single nutrients on the BP of individuals, standardized high-quality data are needed of large population samples. Accordingly, INTERMAP surveyed 4680 men and women aged 40–59 y from 17 population samples in Japan (4 samples), the People's Republic of China (3 samples), the United Kingdom (2 samples), and the United States (8 samples) (4). Participants were randomly selected from population lists arrayed into 4 age and sex strata. Each participant attended 4 visits; visits 1 and 2 were on consecutive days, and visits 3 and 4 were on consecutive days an average of 3 wk later. BP was measured twice per visit with a random zero sphygmomanometer and averaged. Measurements of height, weight, and data on daily alcohol consumption over the previous 7 d were obtained at 2 visits. Dietary data were collected at each visit by an in-depth multipass 24-h recall (4–6). Questionnaire data were obtained on multiple possible confounders. Each participant provided 2 24-h urine collections, which were start and end timed at the research center; measurements included urinary volume, electrolytes, creatinine, and urea (a biomarker of total protein intake) (4, 5, 7).
Individuals were excluded if they did not attend all 4 visits, their diet data were considered to be incomplete or inaccurate, their 24-h energy intakes were <2092 kJ/d or >20,920 kJ/d for women or >33,472 kJ/d for men, 2 urine collections were not available, or other data were incomplete or indicated a protocol violation (total 215 people).
Ethics
The study received institutional ethics committee approval at each site; all participants gave written informed consent.
Statistical methods
Food data of individuals were converted into nutrients (83 nutrients and 18 amino acids) with the use of country-specific tables on the nutrient composition of foods, which were standardized across countries by the Nutrition Coordinating Center, University of Minnesota (4–6). For nutrients that supplied energy, the intake was calculated as the percentage of total energy; for others nutrients, the intake was calculated per 1000 kJ and also as amounts per 24 h; in addition, amino acid intake was also calculated as the percentage of total protein. Urinary values per 24 h were calculated as urinary concentrations multiplied by volumes standardized to 24 h. Measurements per person were averaged for BP and nutrients across the 4 visits and, for urinary excretions, across the 2 collections. For descriptive statistics, means ± SDs and numbers and percentages were calculated by country and study wide. Reliability, as a measure of possible regression dilution bias (8, 9) for amino acid and BP variables, was estimated as observed univariate regression coefficient expressed as a percentage of the theoretical true coefficient from the formula:
The ratio was the intraindividual variance:interindividual variance calculated from means of the first and second 2 visits to account for higher correlations between values on consecutive days (9–12).
To identify possible confounders, associations between nutrients were explored by using a partial correlation adjusted for the population sample, age, and sex, pooled across countries, and weighted by sample size. A multiple linear regression was used to examine relations of each of the dietary amino acids (g/d, percentage of kJ, and percentage of total protein) to systolic blood pressure (SBP) and diastolic blood pressure (DBP). Adjustment for confounders was done sequentially with 9 models (3–15 covariates) (Table 1) without and with height and weight (1–4). These latter 2 variables, considered together, are often associated with sizable reductions in the strength of nutrient-BP relations when included in multiple linear regression analyses (1, 8, 12). This incidence is probably related to their high-order temporal stability, the great accuracy of their measurement, and their frequent correlation with nutrient intakes and BP (ie, their being actual confounders). Hence, it is relevant to evaluate nutrient-BP relations without and with control for them.
TABLE 1.
Estimated mean differences in blood pressure and dietary glycine intake from foods higher by 2 SDs by using multiple regression analyses1
| Systolic blood pressure |
Diastolic blood pressure |
|||||||
| Not adjusted for height and weight |
Adjusted for height and weight |
Not adjusted for height and weight |
Adjusted for height and weight |
|||||
| Models | Difference | z score | Difference | z score | Difference | z score | Difference | z score |
| mm Hg | mm Hg | mm Hg | mm Hg | |||||
| Main analyses (n = 4680): glycine expressed as the percentage of total protein | ||||||||
| 1 | 3.842 | 8.48 | 2.692 | 6.20 | 1.882 | 6.15 | 1.212 | 4.08 |
| 2 | 3.612 | 8.02 | 2.592 | 5.99 | 1.82 | 5.95 | 1.21 | 4.07 |
| 3 (urinary sodium, urinary potassium, alcohol) | 3.002 | 6.55 | 2.03 | 4.60 | 1.50 | 4.78 | 0.89 | 2.94 |
| 4 (PUFA, SFA, cholesterol) | 3.152 | 6.66 | 2.20 | 4.83 | 1.64 | 5.07 | 1.05 | 3.36 |
| 5a (phosphorus) | 2.81 | 5.75 | 1.93 | 4.12 | 1.41 | 4.22 | 0.86 | 2.67 |
| 5b (magnesium) | 2.91 | 6.09 | 2.09 | 4.56 | 1.54 | 4.73 | 1.03 | 3.25 |
| 5c (calcium) | 2.72 | 5.07 | 1.80 | 3.50 | 1.30 | 3.55 | 0.73 | 2.06 |
| 5d (iron) | 3.02 | 6.38 | 2.14 | 4.68 | 1.57 | 4.86 | 1.02 | 3.25 |
| 5e (fiber) | 3.002 | 6.31 | 2.17 | 4.73 | 1.57 | 4.83 | 1.05 | 3.34 |
| 5f (animal protein) | 2.932 | 5.90 | 2.222 | 4.66 | 1.472 | 4.33 | 1.032 | 3.14 |
| 5g (vegetable protein) | 2.952 | 5.94 | 2.222 | 4.68 | 1.472 | 4.35 | 1.032 | 3.13 |
| 5h (total protein) | 3.402 | 7.02 | 2.472 | 5.30 | 1.772 | 5.34 | 1.192 | 3.70 |
| 5i (glutamic acid) | 3.092 | 5.55 | 2.162 | 4.03 | 1.41 | 3.71 | 0.82 | 2.24 |
| 5j (red meat) | 2.89 | 5.68 | 2.102 | 4.29 | 1.45 | 4.18 | 0.942 | 2.81 |
| Sensitivity analyses | ||||||||
| Adjusted also for total energy (kJ/d) (n = 4680) | ||||||||
| 4 | 3.142 | 6.64 | 2.20 | 4.84 | 1.64 | 5.07 | 1.06 | 3.37 |
| 5a (phosphorus) | 2.84 | 5.81 | 1.96 | 4.17 | 1.41 | 4.22 | 0.86 | 2.65 |
| Glycine expressed as g/d (instead percentage of total protein), adjusted also for total energy (kJ/d) (n = 4680) | ||||||||
| 4 | 1.58 | 2.30 | 0.54 | 0.82 | 0.74 | 1.56 | 0.08 | 0.17 |
| 5a (phosphorus) | 2.88 | 3.88 | 1.41 | 1.98 | 1.44 | 2.84 | 0.52 | 1.06 |
| Glycine expressed as the percentage of kJ (instead of the percentage of total protein) (n = 4680) | ||||||||
| 4 | 1.05 | 2.21 | 0.42 | 0.92 | 0.53 | 1.63 | 0.15 | 0.46 |
| 5a (phosphorus) | 2.22 | 4.29 | 1.23 | 2.47 | 1.21 | 3.41 | 0.60 | 1.73 |
| Censored normal regression adjusting for antihypertensive treatment (n = 4680) | ||||||||
| 4 | 3.772 | 6.93 | 2.552 | 4.95 | 2.142 | 5.82 | 1.362 | 3.90 |
| 5a (phosphorus) | 3.282 | 5.86 | 2.15 | 4.06 | 1.832 | 4.85 | 1.122 | 3.11 |
| Nonhypertensive persons (n = 3671) | ||||||||
| 4 | 1.742 | 4.23 | 1.13 | 2.86 | 0.93 | 3.02 | 0.54 | 1.81 |
| 5a (phosphorus) | 1.52 | 3.57 | 0.93 | 2.27 | 0.79 | 2.50 | 0.42 | 1.36 |
| Nonintervened persons (n = 2238)3 | ||||||||
| 4 | 2.08 | 2.94 | 1.56 | 2.28 | 1.16 | 2.36 | 0.84 | 1.77 |
| 5a (phosphorus) | 1.60 | 2.21 | 1.16 | 1.66 | 0.89 | 1.77 | 0.59 | 1.22 |
| Excluding persons with high day-to-day variability in nutrient intake or blood pressure (n = 3473) | ||||||||
| 4 | 2.97 | 5.32 | 2.01 | 3.72 | 1.40 | 3.71 | 0.84 | 2.30 |
| 5a (phosphorus) | 2.60 | 4.51 | 1.74 | 3.13 | 1.21 | 3.11 | 0.72 | 1.90 |
Model 1 was controlled for sample, age, and sex. Model 2 was controlled as for model 1 and for special diet (yes or no), supplement intake (yes or no), cardiovascular disease or diabetes diagnosis (yes or no), physical activity (medium plus heavy; h/d), and family history of high blood pressure (yes, no, or unknown). Model 3 was controlled as for model 2 and for urinary sodium and urinary potassium (mmol/24 h) and 14-d alcohol (g/d). Model 4was controlled as for model 3 and for cholesterol (mg/1000 kJ) and total SFA and total PUFA (percentage of kJ). Models 5a–5j were controlled as for model 4 and for each stipulated nutrient (expressed per 1000 kJ or as the percentage of kJ). Nonintervened individuals did not consume a special diet, did not consume nutritional supplements, were not diagnosed with cardiovascular disease or diabetes, and were not taking medication for high bold pressure, cardiovascular disease, or diabetes. Nonhypertensive persons had systolic blood pressure <140 mm Hg and diastolic blood pressure <90 mm Hg and were not taking an antihypertensive medication. z value = regression coefficient ÷ SE; z value ≥1.96: uncorrected P ≤ 0.05; z value ≥2.58: uncorrected P ≤ 0.01; z value ≥3.29: uncorrected P ≤ 0.001. Two-SD higher glycine intake was 0.89% for the percentage of total protein, 2.39 for g/d, and 0.31% for the percentage of total kilocalories.
Test for cross-country heterogeneity was significant, P < 0.05.
Regressions were not adjusted for special diet, supplement use, or diagnosis of cardiovascular disease or diabetes.
Regression models were fitted by country and coefficients pooled across countries, which were weighted by the inverse of variance, to estimate overall associations. The cross-country heterogeneity of coefficients was tested by using the chi-square test; interactions were assessed for age and sex. Departures from linearity were tested with quadratic terms. Regression coefficients were expressed as millimeters of mercury for 2-SD difference in amino acid intake (4 within-country SDs pooled and weighted by sample size). Statistical significance is presented as z values; equivalent P values are footnoted. Sensitivity analyses included censored normal regression to adjust for a potential antihypertensive treatment bias (13). Adjusted mean SBP and DBP by country-specific quartiles of each amino acid (percentage of total protein) were calculated by using ANOVA and plotted.
Analyses were conducted with SAS version 9.1 software (SAS Institute Inc).
RESULTS
Descriptive statistics
See Table S1 under “Supplemental data” in the online issue for country-specific characteristics of participants. With animal protein intake much lower for Chinese participants than for others, absolute Chinese intakes of amino acids predominant in animal protein were correspondingly lower. As grams per day and percentage of kilojoules, average values tended to be similar for persons from Japan, the United Kingdom, and the Unites States. For the percentage of total protein, intakes were similar across the 4 countries.
Univariate estimates of the reliability of intakes of 8 amino acids were generally similar, ∼50–65% of the theoretical coefficient (see Table S2 under “Supplemental data” in the online issue), similar for men and women, and with a tendency for higher reliability estimates for Chinese than for people from the other countries. BP-reliability estimates were high in the range of 91–92% for SBP and 90–93% for DBP across the countries and high for all sex and country subgroups.
Partial correlations
As the percentage of total protein, partial correlations with each other of pairs of the 8 amino acids predominant in animal protein were positive (range: 0.10–0.83) and direct also with animal protein and red meat (see Table S3 under “Supplemental data” in the online issue). Their correlations were mostly inverse with the 5 amino acids predominant in vegetable protein, and inverse also with total carbohydrate, sugars, starch, and fiber. Their correlations were mostly low-order with dietary lipids, minerals, urinary electrolytes, weight, and BMI. As grams per day or the percentage of kilojoules, partial correlations were of a high order (≥0.86) for pairs of these amino acids (see Tables S4 and S5 under “Supplemental data” in the online issue).
Multiple linear regression analyses: individual amino acids and BP
Glycine
There was a consistent positive robust glycine-BP relation, which tended to be strongest with glycine expressed as the percentage of total protein (Table 1). This relation prevailed in all multivariate models, including those with glycine expressed as the percentage of total protein, grams per day, and percentage of kilojoules, with control for dietary protein (animal, vegetable, and total), glutamic acid, and meat. With glycine intake higher by 2 SDs (0.89% of total protein), the average SBP was higher by 2.8 mm Hg in multivariate model 5a without control for weight and height and 1.9 mm Hg with control for these variables; across all multivariate models (models 4 to 5j), the average SBP was higher by 1.8–3.4 mm Hg (z = 3.50–7.02), and the average DBP was higher by 0.7–1.8 mm Hg (z = 2.06–5.34).
Tests for age and sex interactions and nonlinearity yielded nonsignificant results (see Table S6 under “Supplemental data” in the online issue). Some cross-country heterogeneity tests were significant (Table 1), which was attributable to the Japanese data (ie, a low-order, nonsignificant, inverse relation of glycine to BP) (see Table S7 under “Supplemental data” in the online issue). Despite no significant interaction terms, the direct relation of glycine to DBP was larger for women than men and larger at ages 50–59 than 40–49 y (Table 2).
TABLE 2.
Estimated mean differences in blood pressure and dietary glycine intake (percentage of total protein) from foods higher by 2 SDs by using multiple linear regression analyses by sex and age group1
| Systolic blood pressure |
Diastolic blood pressure |
|||||||
| Not adjusted for height and weight |
Adjusted for height and weight |
Not adjusted for height and weight |
Adjusted for height and weight |
|||||
| Models | Difference | z score | Difference | z score | Difference | z score | Difference | z score |
| mm Hg | mm Hg | mm Hg | mm Hg | |||||
| Men (n = 2359) | ||||||||
| 5a (phosphorus) | 2.53 | 3.73 | 1.77 | 2.71 | 1.08 | 2.20 | 0.51 | 1.08 |
| 5b (magnesium) | 2.55 | 3.84 | 1.86 | 2.92 | 1.10 | 2.28 | 0.58 | 1.24 |
| 5c (calcium) | 2.54 | 3.40 | 1.69 | 2.35 | 1.23 | 2.27 | 0.60 | 1.15 |
| 5d (iron) | 2.77 | 4.21 | 2.01 | 3.17 | 1.19 | 2.49 | 0.62 | 1.35 |
| 5e (fiber) | 2.64 | 3.99 | 1.93 | 3.03 | 1.17 | 2.43 | 0.65 | 1.39 |
| 5f (animal protein) | 2.59 | 3.72 | 1.99 | 2.98 | 0.99 | 1.96 | 0.56 | 1.16 |
| 5g (vegetable protein) | 2.60 | 3.75 | 2.00 | 2.99 | 0.98 | 1.95 | 0.55 | 1.13 |
| 5i (glutamic acid) | 2.97 | 3.81 | 2.04 | 2.72 | 0.95 | 1.68 | 0.28 | 0.52 |
| 5j (red meat) | 2.58 | 3.64 | 1.93 | 2.82 | 1.05 | 2.03 | 0.55 | 1.12 |
| Women (n = 2321) | ||||||||
| 5a (phosphorus) | 2.692 | 3.82 | 1.81 | 2.68 | 1.702 | 3.70 | 1.252 | 2.80 |
| 5b (magnesium) | 2.862 | 4.17 | 2.012 | 3.05 | 1.942 | 4.33 | 1.502 | 3.44 |
| 5c (calcium) | 2.57 | 3.33 | 1.69 | 2.28 | 1.432 | 2.85 | 0.992 | 2.02 |
| 5d (iron) | 2.902 | 4.25 | 1.992 | 3.03 | 1.932 | 4.33 | 1.472 | 3.37 |
| 5e (fiber | 2.992 | 4.38 | 2.122 | 3.23 | 1.962 | 4.40 | 1.522 | 3.50 |
| 5f (animal protein) | 2.862 | 4.04 | 2.122 | 3.11 | 1.862 | 4.04 | 1.482 | 3.28 |
| 5g (vegetable protein) | 2.882 | 4.07 | 2.122 | 3.13 | 1.882 | 4.06 | 1.482 | 3.29 |
| 5i (glutamic acid) | 2.952 | 3.69 | 2.11 | 2.75 | 1.872 | 3.58 | 1.402 | 2.77 |
| 5j (red meat) | 2.902 | 3.99 | 2.04 | 2.92 | 1.832 | 3.87 | 1.362 | 2.96 |
| 40–49 y old (n = 2365) | ||||||||
| 5a (phosphorus) | 2.632 | 4.07 | 1.76 | 2.91 | 1.13 | 2.43 | 0.56 | 1.27 |
| 5b (magnesium) | 2.682 | 4.25 | 1.83 | 3.09 | 1.222 | 2.68 | 0.66 | 1.51 |
| 5c (calcium) | 2.452 | 3.44 | 1.65 | 2.47 | 1.112 | 2.16 | 0.57 | 1.16 |
| 5d (iron) | 2.762 | 4.39 | 1.81 | 3.05 | 1.20 | 2.65 | 0.59 | 1.35 |
| 5e (fiber) | 2.712 | 4.30 | 1.82 | 3.06 | 1.23 | 2.69 | 0.64 | 1.48 |
| 5f (animal protein) | 2.542 | 3.83 | 1.772 | 2.86 | 1.082 | 2.27 | 0.592 | 1.31 |
| 5g (vegetable protein) | 2.542 | 3.84 | 1.74 | 2.81 | 1.072 | 2.24 | 0.562 | 1.23 |
| 5i (glutamic acid) | 2.772 | 3.74 | 1.63 | 2.34 | 1.05 | 1.97 | 0.31 | 0.61 |
| 5j (red meat) | 2.43 | 3.61 | 1.55 | 2.46 | 0.93 | 1.93 | 0.37 | 0.80 |
| 50–59 y old (n = 2315) | ||||||||
| 5a (phosphorus) | 2.95 | 3.93 | 2.23 | 3.03 | 1.68 | 3.47 | 1.18 | 2.46 |
| 5b (magnesium) | 3.04 | 4.16 | 2.42 | 3.37 | 1.83 | 3.86 | 1.37 | 2.93 |
| 5c (calcium) | 3.06 | 3.73 | 2.21 | 2.74 | 1.58 | 2.98 | 1.00 | 1.92 |
| 5d (iron) | 3.17 | 4.38 | 2.51 | 3.52 | 1.92 | 4.09 | 1.43 | 3.09 |
| 5e (fiber) | 3.15 | 4.33 | 2.53 | 3.53 | 1.88 | 3.99 | 1.42 | 3.06 |
| 5f (animal protein) | 3.24 | 4.30 | 2.74 | 3.70 | 1.84 | 3.76 | 1.44 | 3.00 |
| 5g (vegetable protein) | 3.28 | 4.35 | 2.77 | 3.75 | 1.86 | 3.81 | 1.47 | 3.05 |
| 5i (glutamic acid) | 3.20 | 3.78 | 2.58 | 3.10 | 1.65 | 3.02 | 1.18 | 2.19 |
| 5j (red meat) | 3.28 | 4.21 | 2.69 | 3.52 | 1.98 | 3.93 | 1.50 | 3.03 |
Models 5a–5j were controlled for sample, age, sex, special diet (yes or no), supplement intake (yes or no), cardiovascular disease or diabetes diagnosis (yes or no), physical activity (medium plus heavy; h/d), family history of high blood pressure (yes, no, or unknown), urinary sodium and urinary potassium (mmol/24-h), 14-d alcohol (g/d), cholesterol (mg/1000 kJ), total SFA and total PUFA (percentage of kJ), and each stipulated nutrient (expressed per 1000 kJ or as the percentage of kJ). Two-SD higher glycine intake was 0.89% for the percentage of total protein, 2.39 for g/d, and 0.31% for the percentage of total kilocalories. z value = regression coefficient ÷ SE; z value ≥1.96: uncorrected P ≤ 0.05; z value ≥2.58: uncorrected P ≤ 0.01; z value ≥3.29: uncorrected P ≤ 0.001.
Test for cross-country heterogeneity was significant, P < 0.05
Alanine
There was a significant independent positive relation of dietary alanine (percentage of total protein) to BP (Table 3). Tests for age and sex interactions and nonlinearity were nonsignificant (see Table S6 under “Supplemental data” in the online issue); cross-country heterogeneity was recorded for several models, which was attributable to inverse relations of alanine to BP for Japanese and Chinese participants (see Table S8 under “Supplemental data” in the online issue).
TABLE 3.
Estimated mean differences in blood pressure and dietary alanine intake from foods higher by 2 SDs by using multiple regression analyses1
| Systolic blood pressure |
Diastolic blood pressure |
|||||||
| Not adjusted for height and weight |
Adjusted for height and weight |
Not adjusted for height and weight |
Adjusted for height and weight |
|||||
| Models | Difference | z score | Difference | z score | Difference | z score | Difference | z score |
| mm Hg | mm Hg | mm Hg | mm Hg | |||||
| Main analyses (n = 4680): alanine expressed as the percentage of total protein | ||||||||
| 1 | 3.282 | 6.22 | 1.932 | 3.82 | 1.412 | 4.06 | 0.622 | 1.84 |
| 2 | 3.012 | 5.75 | 1.812 | 3.60 | 1.352 | 3.90 | 0.632 | 1.87 |
| 3 (urinary sodium, urinary potassium, alcohol) | 2.372 | 4.50 | 1.32 | 2.61 | 1.022 | 2.91 | 0.38 | 1.13 |
| 4 (PUFA, SFA, cholesterol) | 2.372 | 4.20 | 1.322 | 2.43 | 1.182 | 3.15 | 0.532 | 1.46 |
| 5a (phosphorus) | 2.70 | 4.59 | 1.61 | 2.83 | 1.36 | 3.45 | 0.67 | 1.75 |
| 5b (magnesium) | 2.72 | 4.55 | 1.75 | 3.04 | 1.51 | 3.76 | 0.90 | 2.32 |
| 5c (calcium) | 1.62 | 2.64 | 0.65 | 1.10 | 0.71 | 1.74 | 0.11 | 0.27 |
| 5d (iron) | 2.282 | 4.03 | 1.29 | 2.37 | 1.132 | 3.00 | 0.52 | 1.42 |
| 5e (fiber) | 2.36 | 4.08 | 1.352 | 2.42 | 1.182 | 3.05 | 0.532 | 1.41 |
| 5f (animal protein) | 1.942 | 3.11 | 1.272 | 2.12 | 0.852 | 2.07 | 0.432 | 1.08 |
| 5g (vegetable protein) | 1.982 | 3.19 | 1.282 | 2.16 | 0.862 | 2.11 | 0.422 | 1.06 |
| 5h (total protein) | 2.682 | 4.60 | 1.632 | 2.91 | 1.342 | 3.46 | 0.682 | 1.82 |
| 5i (glutamic acid) | 2.312 | 2.75 | 0.992 | 1.23 | 0.76 | 1.35 | −0.06 | −0.11 |
| 5j (red meat) | 1.812 | 2.94 | 0.97 | 1.64 | 0.83 | 2.05 | 0.29 | 0.74 |
| Sensitivity analyses | ||||||||
| Adjusted also for total energy (kJ/d) (n = 4680) | ||||||||
| 4 | 2.372 | 4.20 | 1.312 | 2.42 | 1.182 | 3.15 | 0.522 | 1.45 |
| 5a (phosphorus) | 2.73 | 4.63 | 1.62 | 2.85 | 1.37 | 3.46 | 0.66 | 1.73 |
| Alanine expressed as g/d (instead of the percentage of total protein), adjusted also for total energy (kJ/d) (n = 4680) | ||||||||
| 4 | 0.88 | 1.20 | −0.13 | −0.19 | 0.47 | 0.93 | −0.19 | −0.40 |
| 5a (phosphorus) | 2.92 | 3.48 | 1.17 | 1.45 | 1.62 | 2.83 | 0.51 | 0.91 |
| Alanine expressed as the percentage of kJ (instead of the percentage of total protein) (n = 4680) | ||||||||
| 4 | 0.37 | 0.75 | −0.18 | −0.39 | 0.18 | 0.52 | −0.17 | −0.50 |
| 5a (phosphorus) | 2.12 | 3.65 | 0.97 | 1.74 | 1.22 | 3.06 | 0.50 | 1.29 |
| Censored normal regression adjusting for antihypertensive treatment (n = 4680) | ||||||||
| 4 | 2.882 | 4.51 | 1.61 | 2.66 | 1.532 | 3.66 | 0.732 | 1.84 |
| 5a (phosphorus) | 3.19 | 4.77 | 1.85 | 2.92 | 1.73 | 3.89 | 0.86 | 2.05 |
| Nonhypertensive persons (n = 3671) | ||||||||
| 4 | 1.57 | 3.35 | 0.91 | 2.02 | 0.83 | 2.40 | 0.39 | 1.18 |
| 5a (phosphorus) | 1.94 | 3.89 | 1.19 | 2.48 | 1.07 | 2.92 | 0.57 | 1.61 |
| Nonintervened persons (n = 2238)3 | ||||||||
| 4 | 0.77 | 0.98 | 0.40 | 0.53 | 0.43 | 0.83 | 0.17 | 0.35 |
| 5a (phosphorus) | 1.30 | 1.55 | 0.86 | 1.06 | 0.78 | 1.39 | 0.44 | 0.81 |
| Excluding persons with high day-to-day variability in nutrient intake or blood pressure (n = 3473) | ||||||||
| 4 | 2.71 | 4.01 | 1.66 | 2.54 | 1.25 | 2.79 | 0.62 | 1.43 |
| 5a (phosphorus) | 2.96 | 4.22 | 1.90 | 2.80 | 1.45 | 3.10 | 0.80 | 1.77 |
Model 1 was controlled for sample, age, and sex. Model 2 was controlled as for model 1 and for special diet (yes or no), supplement intake (yes or no), cardiovascular disease or diabetes diagnosis (yes or no), physical activity (medium plus heavy; h/d), and family history of high blood pressure (yes, no, or unknown). Model 3 was controlled as for model 2 and for urinary sodium and urinary potassium (mmol/24 h) and 14-d alcohol (g/d). Model 4 was controlled as for model 3 and for cholesterol (mg/1000 kJ) and total SFA and total PUFA (percentage of kJ). Models 5a–5j were controlled as for model 4 and for each stipulated nutrient (expressed per 1000 kJ or as the percentage of kJ). Nonintervened individuals did not consume a special diet, did not consume nutritional supplements, were not diagnosed with cardiovascular disease or diabetes, and were not taking medication for high bold pressure, cardiovascular disease, or diabetes. Nonhypertensive persons had systolic blood pressure <140 mm Hg and diastolic blood pressure <90 mm Hg and were not taking an antihypertensive medication. z value = regression coefficient ÷ SE; z value ≥1.96: uncorrected P ≤ 0.05; z value ≥2.58: uncorrected P ≤ 0.01; z value ≥3.29: uncorrected P ≤ 0.001. Two-SD higher alanine intake was 0.85% for the percentage of total protein, 2.62 for g/d, and 0.34% for the percentage of total kilocalories. There were no significant interactions with sex or age.
Test for cross-country heterogeneity significant, P < 0.05.
Regressions were not adjusted for special diet, supplement use, or diagnosis of cardiovascular disease or diabetes.
Arginine, aspartic acid, histidine, lysine, methionine, and threonine
These amino acids were not consistently, significantly related to BP (see Tables S9–S14 under “Supplemental data” in the online issue).
Multiple regression analyses: glycine and alanine paired, glycine paired with each of the other amino acids, and glycine plus all 7 other amino acids
Glycine plus alanine
With dietary glycine and alanine (percentage of total protein) together in multivariate models, the glycine-SBP relation remained significantly positive (Figure 1, Table 4), the alanine-BP relation was inverse, low-order, and nonsignificant (Table 4). The glycine-BP relation again prevailed in models controlled also for protein (animal, vegetable, and total) and meat. Although there was no significant cross-country heterogeneity, the glycine-BP relation was stronger for Western persons (see Tables S15 and S16 under “Supplemental data” in the online issue).
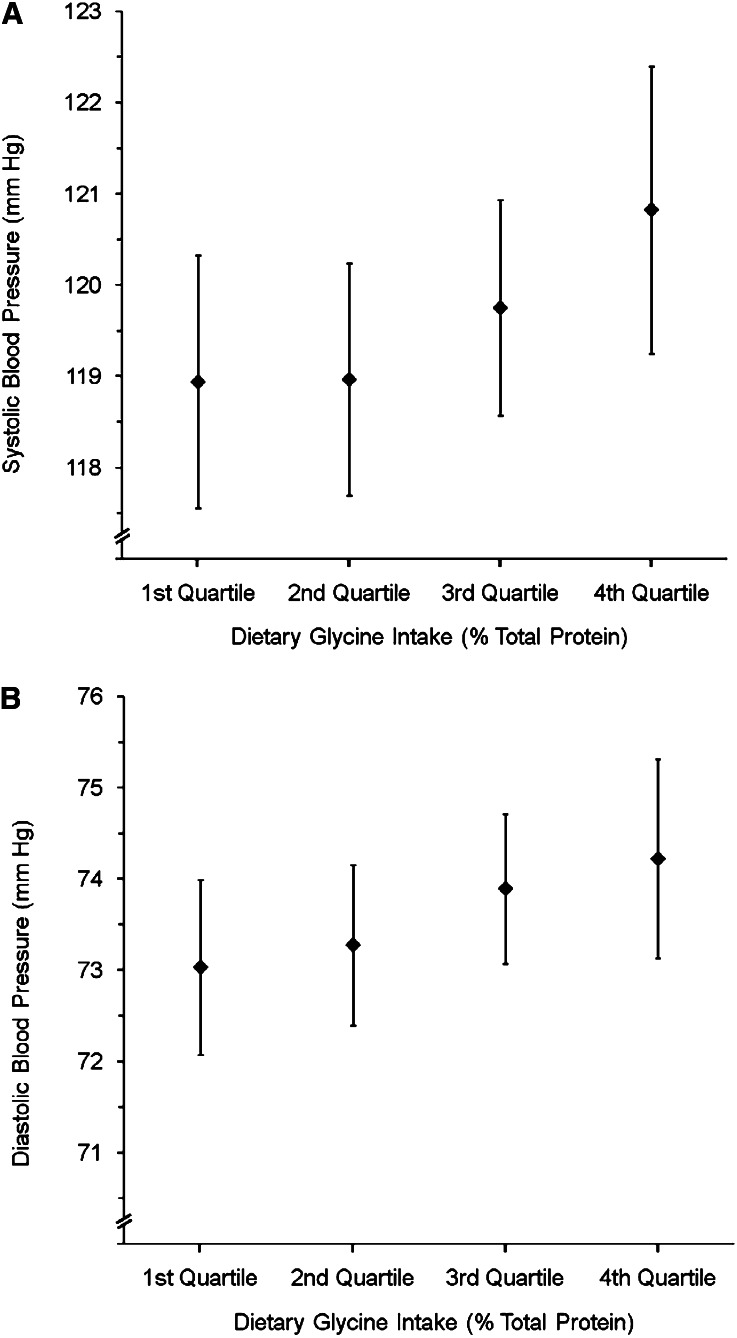
FIGURE 1.
Mean (95% CI) systolic blood pressure (mm Hg) (A) and diastolic blood pressure (mm Hg) (B) by quartiles of dietary glycine intake (percentage of total protein) on the basis of country-specific quartiles [country-specific quartile cutoffs for dietary glycine intake (percentage of total protein) were as follows: for Japan, 4.07 (25th percentile), 4.24 (50th percentile), and 4.88 (75th percentile); for People's Republic of China, 3.89, 4.13, and 4.68, respectively; for the United Kingdom, 3.82, 4.02, and 4.28, respectively; and for the United States, 3.89, 4.20, and 4.51, respectively], pooled across countries, and adjusted for model 4 covariates and dietary alanine [adjusted for country, age, sex, special diet, supplement intake, cardiovascular disease or diabetes diagnosis, physical activity, family history of high blood pressure, 24-h urinary sodium and potassium, 14-d alcohol, cholesterol, total SFA, total PUFA, magnesium, and alanine (see Table 1 footnote 1 for units)] for all 4680 participants. P-trend for A = 0.01; P-trend for B = 0.02.
TABLE 4.
Estimated mean differences in blood pressure and dietary glycine and alanine intake (percentage of total protein) from foods higher by 2 SDs considered jointly in multiple regression analyses1
| Systolic blood pressure |
Diastolic blood pressure |
|||||||
| Not adjusted for height and weight |
Adjusted for height and weight |
Not adjusted for height and weight |
Adjusted for height and weight |
|||||
| Models and amino acids | Difference | z score | Difference | z score | Difference | z score | Difference | z score |
| mm Hg | mm Hg | mm Hg | mm Hg | |||||
| 4 (PUFA, SFA, cholesterol) | ||||||||
| Glycine | 2.97 | 4.26 | 2.36 | 3.52 | 1.42 | 3.02 | 1.01 | 2.22 |
| Alanine | −0.39 | −0.51 | −0.91 | −1.25 | −0.04 | −0.09 | −0.39 | −0.83 |
| 5a (phosphorus) | ||||||||
| Glycine | 2.54 | 3.58 | 2.02 | 2.97 | 1.14 | 2.38 | 0.79 | 1.71 |
| Alanine | 0.62 | 0.76 | −0.09 | −0.12 | 0.54 | 1.02 | 0.06 | 0.12 |
| 5b (magnesium) | ||||||||
| Glycine | 2.76 | 3.93 | 2.21 | 3.28 | 1.30 | 2.73 | 0.92 | 2.01 |
| Alanine | 0.14 | 0.16 | −0.38 | −0.47 | 0.40 | 0.71 | 0.06 | 0.10 |
| 5c (calcium) | ||||||||
| Glycine | 2.74 | 3.84 | 2.14 | 3.12 | 1.23 | 2.55 | 0.83 | 1.78 |
| Alanine | −0.43 | −0.57 | −0.96 | −1.31 | −0.10 | −0.21 | −0.45 | −0.95 |
| 5d (iron) | ||||||||
| Glycine | 2.94 | 4.20 | 2.35 | 3.51 | 1.40 | 2.96 | 1.00 | 2.20 |
| Alanine | −0.36 | −0.47 | −0.87 | −1.18 | −0.04 | −0.08 | −0.37 | −0.79 |
| 5e (fiber) | ||||||||
| Glycine | 2.90 | 4.11 | 2.36 | 3.50 | 1.40 | 2.93 | 1.05 | 2.29 |
| Alanine | −0.49 | −0.62 | −1.07 | −1.39 | −0.15 | −0.29 | −0.57 | −1.13 |
| 5f (animal protein) | ||||||||
| Glycine | 2.87 | 4.05 | 2.25 | 3.32 | 1.28 | 2.66 | 0.88 | 1.90 |
| Alanine | −0.22 | −0.28 | −0.58 | −0.76 | −0.02 | −0.04 | −0.28 | −0.58 |
| 5g (vegetable protein) | ||||||||
| Glycine | 3.03 | 4.32 | 2.37 | 3.53 | 1.46 | 3.08 | 1.02 | 2.24 |
| Alanine | −0.86 | −1.11 | −1.20 | −1.61 | −0.36 | −0.72 | −0.60 | −1.25 |
| 5h (total protein) | ||||||||
| Glycine | 2.95 | 4.19 | 2.36 | 3.51 | 1.36 | 2.86 | 0.98 | 2.13 |
| Alanine | −0.12 | −0.16 | −0.65 | −0.88 | 0.08 | 0.16 | −0.28 | −0.59 |
| 5i (glutamic acid) | ||||||||
| Glycine | 2.94 | 4.20 | 2.35 | 3.50 | 1.37 | 2.88 | 0.97 | 2.13 |
| Alanine | −0.53 | −0.67 | −1.13 | −1.48 | −0.08 | −0.15 | −0.49 | −0.98 |
| 5j (red meat) | ||||||||
| Glycine | 2.96 | 4.23 | 2.36 | 3.52 | 1.42 | 3.00 | 1.02 | 2.24 |
| Alanine | −0.60 | −0.77 | −1.03 | −1.38 | −0.18 | −0.35 | −0.47 | −0.98 |
Model 4 was controlled for sample, age, sex, special diet (yes or no), supplement intake (yes or no), cardiovascular disease or diabetes diagnosis (yes or no), physical activity (medium plus heavy; h/d), family history of high blood pressure (yes, no, or unknown), urinary sodium and urinary potassium (mmol/24 h), 14-d alcohol (g/d), cholesterol (mg/1000 kJ), and total SFA and total PUFA (percentage of kJ). Models 5a–5j were controlled as for model 4 and for each stipulated nutrient (expressed per 1000 kJ or as the percentage of kJ). Two-SD higher glycine intake was 0.89% for the percentage of total protein, 2.39 for g/d, and 0.31% for the percentage of total kilocalories. Two-SD higher alanine intake was 0.85% for the percentage of total protein, 2.62 for g/d, and 0.34% for the percentage of total kilocalories. z value = regression coefficient ÷ SE; z value ≥1.96: uncorrected P ≤ 0.05; z value ≥2.58: uncorrected P ≤ 0.01; z value ≥3.29: uncorrected P ≤ 0.001. No significant cross-country heterogeneity was detected, P < 0.05.
Glycine paired with each of the other 6 amino acids
Findings were similar to the foregoing for glycine (the percentage of total protein) paired with each of the other 6 amino acids predominant in animal protein (see Tables S15 and S16 under “Supplemental data” in the online issue).
Glycine with all 7 other amino acids
With glycine and all 7 other amino acids predominant in animal protein in the same model (model 4, see Table S15 and S16 under “Supplemental data” in the online issue), glycine remained significantly related to BP, with SBP differences of +3.9 and +2.8 mm Hg without and with height and weight in the model, respectively. Again, the glycine-SBP relation (but not the glycine-DBP relation) was stronger for Western than for East Asian participants but significant for Westerners only (see Tables S15 and S16 under “Supplemental data” in the online issue). Of the 7 other amino acids in the model, aspartic acid (percentage of total protein) was also related significantly to BP, with SBP differences of +2.2 and +2.6 mm Hg for a 2-SD higher aspartic acid intake (without and with height and weight in the model, respectively) (see Tables S15 and S16 under “Supplemental data” in the online issue).
Glycine with glutamic acid
Because of previous analyses that showed that dietary glutamic acid (ie, the most common amino acid, especially in vegetable products) was related inversely to BP (3), multivariate models on the dietary glycine-BP relation also encompassed glutamic acid as a covariate. Again, with glycine expressed either as the percentage of total protein or percentage of total kilojoules, the overall glycine-BP relation remained strong and significant as did the glutamic acid-BP relation expressed as the percentage of kilojoules; however, the glutamic acid-BP relation expressed as the percentage of total protein became of low order and nonsignificant (see Table S17 under “Supplemental data” in the online issue). Both relations (ie, glycine-BP and glutamic acid-BP relations), tended to be stronger for Westerners than East Asians.
Multiple regression analyses: glycine and the direct relation of dietary animal protein and red meat to BP
Dietary glycine (percentage of total protein) had a substantial influence on the direct relation of dietary animal protein and red meat to SBP and DBP. Thus, in model 4 (without weight and height), the estimated effect on SBP of red meat intake higher by 2 SDs was +1.6 mm Hg without glycine in the model and was reduced to 0.4 mm Hg with the addition to the model of glycine (percentage of total protein) (corresponding regression coefficients for red meat were 0.0353 and 0.0092 with z scores of 3.4 and 0.8) (data not tabulated).
Food sources of glycine
Food sources of glycine varied across East Asian and Western samples (Table 5); for UK and US participants, meat was the predominant source; Japanese and Chinese participants ingested a small percentage of their glycine from meat; for Japanese, fish and shellfish were the largest source, and pasta, rice, and noodles were the second large source; for Chinese, grains and flour were the largest source, and pasta, rice, and noodles were the second large source.
TABLE 5.
Food groups supplying most dietary glycine by country
| Japan (n = 1145) |
People's Republic of China (n = 839) |
United Kingdom (n = 501) |
United States (n = 2195) |
||||||||
| Food source | g/d | % | Food source | g/d | % | Food source | g/d | % | Food source | g/d | % |
| Fish and shellfish | 1.16 | 33.5 | Grains and flour | 0.77 | 28.1 | Meat | 1.61 | 47.2 | Meat | 1.87 | 51.7 |
| Pasta, rice, and noodles | 0.60 | 17.5 | Pasta, rice, and noodles | 0.63 | 23.1 | Bread, rolls, and biscuits | 0.48 | 14.0 | Bread, rolls, and biscuits | 0.25 | 6.9 |
| Meat | 0.60 | 17.4 | Meat | 0.46 | 16.7 | Vegetables and beans | 0.24 | 6.9 | Fish and shellfish | 0.23 | 6.3 |
| Vegetarian meat substitutes | 0.25 | 7.3 | Vegetables and beans | 0.29 | 10.5 | Milk and cheese | 0.23 | 6.8 | Milk and cheese | 0.22 | 6.2 |
| Vegetables and beans | 0.20 | 5.8 | Cakes, puddings, cookies, and other sweet snacks | 0.14 | 5.1 | Fish and shellfish | 0.19 | 5.6 | Vegetables and beans | 0.21 | 5.9 |
| Eggs | 0.16 | 4.5 | Fish and shellfish | 0.13 | 4.7 | Cakes, puddings, cookies, and other sweet snacks | 0.15 | 4.5 | Grains and flour | 0.13 | 3.5 |
| Bread, rolls, and biscuits | 0.10 | 3.0 | Vegetarian meat substitutes | 0.08 | 2.8 | Cereals | 0.11 | 3.1 | Pasta, rice, and noodles | 0.12 | 3.2 |
| Condiments and seasonings | 0.07 | 2.1 | Eggs | 0.08 | 2.8 | Eggs | 0.06 | 1.8 | Eggs | 0.11 | 3.1 |
| Milk and cheese | 0.07 | 2.1 | Nuts and seeds | 0.07 | 2.7 | Soup, gravy, and sauces | 0.05 | 1.6 | Nuts and seeds | 0.09 | 2.5 |
| Cakes, puddings, cookies, and other sweet snacks | 0.06 | 1.7 | Alcoholic beverages | 0.03 | 1.0 | Pasta, rice, and noodles | 0.05 | 1.4 | Cereals | 0.07 | 1.9 |
| Soup, gravy, and sauces | 0.06 | 1.6 | — | — | — | Fruits | 0.04 | 1.2 | Cakes, puddings, cookies, and other sweet snacks | 0.06 | 1.7 |
| — | — | — | — | — | — | Other dairy products | 0.04 | 1.2 | Savory snacks | 0.05 | 1.5 |
| — | — | — | — | — | — | Nuts and seeds | 0.04 | 1.0 | Condiments and seasonings | 0.04 | 1.1 |
DISCUSSION
The main finding in this study on relations to BP of 8 dietary amino acids predominant in animal protein was that there was a robust, consistent, independent direct relation to BP of dietary glycine expressed as the percentage of total protein and percentage of kilojoules and as the absolute amount as grams per day. Glycine accounted substantially for the direct relation to BP of meat and animal protein; results were stronger for Western than East Asian participants; meat was the main dietary source of glycine for Westerners but not for East Asians.
These findings complemented those from our previous INTERMAP report on the inverse relation to BP of dietary glutamic acid, which is the amino acid predominant in vegetable protein; and those in our INTERMAP publications on the inverse relation to BP of vegetable protein intake and direct relation to BP of animal protein and red meat intakes (1–3).
To the best of our knowledge, this is the first article on relations to BP of dietary amino acids predominant in animal protein. In the Dietary Approaches to Stop Hypertension and the Optimal Macronutrient Intake Trial to Prevent Heart Disease feeding trials, the total protein intake, particularly of vegetable and dairy protein, was increased as part of several dietary interventions that together lowered BP; a modest reduction in meat consumption may have contributed to this outcome; the multiple nutritional modifications in these trials make it impossible to delineate separate BP effects of the change in intake of individual nutrients (14–16). Earlier literature that reported lower BP in vegetarian than omnivorous populations did not deal with specific nutrients; more-recent articles from observational studies and clinical trials have not reported on the amino acids considered here (1). In a metabolome-wide analysis of INTERMAP urine specimens, urinary alanine concentrations differed significantly between countries; the multiple regression of urinary alanine excretion on BP revealed a significant positive association (17). In 3 East Asian studies (18–20), inverse relations were reported to SBP of the urinary ratio of sulfate to urea (index of dietary sulfur–containing amino acids from animal protein) and also of serum phenylalanine and serine and overnight urinary cysteine and 24-h urinary 3-methylhistidine (a marker of animal protein intake). These articles reported no dietary BP data.
It remains to be determined whether the observed direct relation between dietary glycine and BP is reproducible. Limited experimental data indicated an inverse (not direct) effect in sugar-fed rats (21) and the offspring of rats given a glycine supplement for the duration of pregnancy (22). Biological mechanisms for the direct association we observed are not currently forthcoming; possible mechanisms cited for the inverse effect in experimental models included evidence that glycine may reduce oxidative stress and increase the availability of nitric oxide and other agents that modulate vascular tone or endothelial function (23).
The glycine-BP relation was generally stronger with glycine expressed as the percentage of total protein than as grams per day or the percentage of kilojoules. This effect may have been because glycine as the percentage of total protein correlated less strongly with other variables, which may have possibly confounded analyses than when glycine was expressed otherwise.
The bias toward the null for exposure-BP associations induced by reduced BPs of treated hypertensive participants is a concern for all studies that involve such individuals (12, 13). Glycine-BP associations were quantitatively similar in main analyses and models adjusted for the antihypertensive treatment effect.
Limitations of our findings included their cross-sectional nature, but they were the only population-based data available; an effect-size underestimation because of the limited reliability in nutrient measurements (a regression-dilution bias), despite multiple, standardized, high-quality measurements; the ability to control only partially (albeit considerably) for high-order collinearity in dietary variables, which was less of a problem in analyses with amino acids expressed as a percentage of total dietary protein than when expressed otherwise; the uncertainty as to generalizability to persons <40 and >59 y of age; and the apparent small effect size. The last limitation, which was anticipated by the INTERMAP (4), must be kept in perspective; with small independent influences of multiple nutrients (as observed in the INTERMAP) (1–3, 24–29) combined effects become substantial (ie, improved nutrition is capable of preventing or reducing unfavorable BP levels for most people, per findings of the Dietary Approaches to Stop Hypertension and the Optimal Macronutrient Intake Trial to Prevent Heart Disease feeding trials) (14–16). Also, long-term BP effects of habitual eating patterns, from infancy into older age, may be greater as has been indicated by data on salt intake and BP (30). Moreover, a small reduction of the population average SBP (eg, 2 mm Hg) was estimated to result in mortality rates lower by 6% for stroke and 4% for coronary heart disease (30, 31). Finally, eating patterns that are based mainly on foods with predominantly vegetable (not animal) protein, which are lower in glycine, higher in glutamic acid, ω-3 and ω-6 PUFAs, calcium, magnesium, phosphorus, iron, and other micronutrients, and low or moderate in fats, saturated fats, cholesterol, refined sugars, caloric density, salt, and alcohol, have multiple favorable influences on BP, serum lipids, cardiovascular disease risk, and general health.
In conclusion, we recorded an independent direct relation of dietary glycine to BP with control for multiple possible confounders. Glycine may account, at least in part, for previously reported direct relations of animal protein intake and meat to BP.
Supplementary Material
Acknowledgments
We thank all INTERMAP staff at local, national, and international centers for their invaluable efforts; see reference 4 for a partial listing of these colleagues, who conducted the study at its several centers.
The authors’ responsibilities were as follows—JS and PE: designed the study and had primary responsibility for the final content of the manuscript; IJB and QC: performed statistical analyses; QC, JS, and MLD: wrote the manuscript; KM, NO, and LZ: contributed to the critical revision of the manuscript; and all authors: read and approved the final manuscript. All authors had full access to the data and take responsibility for their integrity. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: BP, blood pressure; DBP, diastolic blood pressure; INTERMAP, International Study of Macro/Micronutrients and Blood Pressure; SBP, systolic blood pressure.
REFERENCES
- 1.Elliott P, Stamler J, Dyer AR, Appel L, Dennis B, Kesteloot H, Ueshima H, Okayama A, Chan Q, Garside DB, et al. Association between protein intake and blood pressure: the INTERMAP Study. Arch Intern Med 2006;166:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzoulaki I, Brown IJ, Chan Q, Van Horn L, Ueshima H, Zhao L, Stamler J, Elliott P. Relation of iron and red meat intake to blood pressure: cross sectional epidemiological study. BMJ 2008;337:a258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stamler J, Brown IJ, Daviglus ML, Chan Q, Kesteloot H, Ueshima H, Zhao L, Elliott P. Glutamic acid, the main dietary amino acid, and blood pressure. The INTERMAP Study (International Collaborative Study of Macronutrients, Micronutrients and Blood Pressure). Circulation 2009;120:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamler J, Elliott P, Dennis B, Dyer AR, Kesteloot H, Liu K, Ueshima H, Zhou B. INTERMAP: background, aims, design, methods, and descriptive statistics (non-dietary). J Hum Hypertens 2003;17:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dennis B, Stamler J, Buzzard M, Conway R, Elliott P, Moag-Stahlberg A, Okayama A, Okuda N, Robertson C, Robinson F, et al. INTERMAP: the dietary data--process and quality control. J Hum Hypertens 2003;17:609–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schakel SF, Dennis BH, Wold AC, Conway R, Zhao L, Okuda N, Okayama A, Moag-Stahlberg A, Robertson C, Van Heel N, et al. Enhancing data on nutrient composition of foods eaten by the participants in the INTERMAP Study in China, Japan, the United Kingdom, and the United States. J Food Compost Anal 2003;16:395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyer A, Elliott P, Chee D, Stamler J. Urinary biochemical markers of dietary intake in the INTERSALT study. Am J Clin Nutr 1997;65(suppl):1246S–53S. [DOI] [PubMed] [Google Scholar]
- 8.Liu K. Measurement error and its impact on partial correlation and multiple linear regression analyses. Am J Epidemiol 1988;127:864–74. [DOI] [PubMed] [Google Scholar]
- 9.Grandits GA, Bartsch GE, Stamler J. Method issues in dietary data analysed in the Multiple Risk Factor Intervention Trial. Am J Clin Nutr 1997;65(suppl):211S–27S. [DOI] [PubMed] [Google Scholar]
- 10.Dyer AR, Shipley M, Elliott P. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT Study. I. Estimates of reliability. The INTERSALT Cooperative Research Group. Am J Epidemiol 1994;139:927–39. [DOI] [PubMed] [Google Scholar]
- 11.Dyer AR, Elliott P, Shipley M. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT Study. II. Estimates of electrolyte-blood pressure associations corrected for regression dilution bias. The INTERSALT Cooperative Research Group. Am J Epidemiol 1994;139:940–51. [DOI] [PubMed] [Google Scholar]
- 12.Dyer AR, Liu K, Sempos CT. Nutrient data analysis techniques and strategies Berdanier CD, Dwyer J, Feldman EB. eds. Handbook of nutrition and food. Boca Raton, FL: CRC Press, 2007:93–103. [Google Scholar]
- 13.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med 2005;24:2911–35. [DOI] [PubMed] [Google Scholar]
- 14.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 15.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, III, Simons-Morton DG, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 16.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, III, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids. Results of the OmniHeart randomized trial. JAMA 2005;294:2455–64. [DOI] [PubMed] [Google Scholar]
- 17.Holmes E, Loo R-L, Stamler J, Bictash M, Yap IKS, Chan Q, Ebbels T, De Iorio M, Brown IJ, Veselkov KA, et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 2008;453:396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamori Y, Kihara M, Nara Y, Ohtaka M, Horie R, Tsunematsu T, Note S. Hypertension and diet: multiple regression analysis in a Japanese farming community. Lancet 1981;1:1204–5. [DOI] [PubMed] [Google Scholar]
- 19.Zhou B, Zhang X, Zhu A, Zhao L, Zhu S, Ruan L, Zhu L, Liang S. The relationship of dietary animal protein and electrolytes to blood pressure: a study on three Chinese populations. Int J Epidemiol 1994;23:716–22. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Ikeda K, Yamori Y. Inverse relationship between urinary markers of animal protein intake and blood pressure in Chinese: results from the WHO Cardiovascular Diseases and Alimentary Comparison (CARDIAC) Study. Int J Epidemiol 2002;31:227–33. [DOI] [PubMed] [Google Scholar]
- 21.El Hafidi M, Perez I, Zamora J, Soto V, Carvajal-Sandoval G, Banos G. Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am J Physiol Regul Integr Comp Physiol 2004;287:R1387–93. [DOI] [PubMed] [Google Scholar]
- 22.Jackson AA, Dunn RL, Marchand MC, Langley-Evans SC. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin Sci 2002;103:633–9. [DOI] [PubMed] [Google Scholar]
- 23.El Hafidi M, Perez I, Banos G. Is glycine effective against elevated blood pressure? Curr Opin Clin Nutr Metab Care 2006;9:26–31. [DOI] [PubMed] [Google Scholar]
- 24.Ueshima H, Stamler J, Elliott P, Chan Q, Brown IJ, Carnethon M, Daviglus ML, He K, Moag-Stahlberg A, Rodriguez BL, et al. Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure. INTERMAP study. Hypertension 2007;50:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott P, Kesteloot H, Appel LJ, Dyer AR, Ueshima H, Chan Q, Brown IJ, Zhao L, Stamler J. Dietary phosphorus and blood pressure: INTERMAP study. Hypertens 2008;51:669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miura K, Stamler J, Nakagawa H, Elliott P, Ueshima H, Chan Q, Brown IJ, Tzoulaki I, Saitoh S, Dyer AR, et al. Relationship of dietary linoleic acid to blood pressure: the International Study of Macro-Micronutrients and Blood Pressure. Hypertension 2008;52:408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown IJ, Elliott P, Robertson CE, Chan Q, Daviglus ML, Dyer AR, Huang C-C, Rodriguez BL, Sakata K, Ueshima H, et al. Dietary starch intake of individuals and their blood pressure: the international study of macronutrients and micronutrients and blood pressure. J Hypertens 2009;27:231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakurai M, Stamler J, Miura K, Brown IJ, Nakagawa H, Elliott P, Ueshima H, Chan Q, Tzoulaki I, Dyer AR, et al. Relationship of dietary cholesterol to blood pressure: the INTERMAP study. J Hypertens 2011;29:222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown IJ, Stamler J, Van Horn L, Robertson CE, Chan Q, Dyer AR, Huang C-C, Rodriguez BL, Zhao L, Daviglus ML, et al. Sugar-sweetened beverage, sugar intake of individuals and their blood pressure: International Study of Macro/Micronutrients and Blood Pressure. Hypertension 2011;57:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stamler J, Rose G, Stamler R, Elliott P, Dyer A, Marmot M. INTERSALT Study findings. Public health and medical care implications. Hypertension 1989;14:570–7. [DOI] [PubMed] [Google Scholar]
- 31.Stamler J. The INTERSALT Study: background, methods, findings, and implications. Am J Clin Nutr 1997;65(suppl):626S–42S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.