Abstract
Background: Clinical hypomagnesemia and experimental restriction of dietary magnesium increase cardiac arrhythmias. However, whether or not circulating or dietary magnesium at usual concentrations or intakes influences the risk of cardiovascular disease (CVD), including fatal ischemic heart disease (IHD), is unclear.
Objective: We performed a systematic review and meta-analysis to investigate prospective associations of circulating and dietary magnesium with incidence of CVD, IHD, and fatal IHD.
Design: Multiple literature databases were systematically searched without language restriction through May 2012. Inclusion decisions and data extraction were performed in duplicate. Linear dose-response associations were assessed by using random-effects meta-regression. Potential nonlinear associations were evaluated by using restricted cubic splines.
Results: Of 2303 articles, 16 studies met the eligibility criteria; these studies comprised 313,041 individuals and 11,995 CVD, 7534 IHD, and 2686 fatal IHD events. Circulating magnesium (per 0.2 mmol/L increment) was associated with a 30% lower risk of CVD (RR: 0.70; 95% CI: 0.56, 0.88 per 0.2 mmol/L) and trends toward lower risks of IHD (RR: 0.83; 95% CI: 0.75, 1.05) and fatal IHD (RR: 0.61; 95% CI: 0.37, 1.00). Dietary magnesium (per 200-mg/d increment) was not significantly associated with CVD (RR: 0.89; 95% CI: 0.75, 1.05) but was associated with a 22% lower risk of IHD (RR: 0.78; 95% CI: 0.67, 0.92). The association of dietary magnesium with fatal IHD was nonlinear (P < 0.001), with an inverse association observed up to a threshold of ∼250 mg/d (RR: 0.73; 95% CI: 0.62, 0.86), compared with lower intakes.
Conclusion: Circulating and dietary magnesium are inversely associated with CVD risk, which supports the need for clinical trials to evaluate the potential role of magnesium in the prevention of CVD and IHD.
INTRODUCTION
Observational and experimental studies have shown that magnesium can exert beneficial effects on the cardiovascular system by enhancing endothelium-dependent vasodilation, improving lipid metabolism, reducing inflammation, and inhibiting platelet function (1). As a key electrolyte involved in regulation of cation flux across cardiomyocytes through direct binding and allosteric effects on potassium and calcium channels, magnesium is also required for normal cardiac electrophysiology (2). Abnormally low circulating magnesium (hypomagnesemia, <0.65 mmol/L) is a known risk factor for cardiac arrest (3). Two small randomized, controlled, crossover interventions in healthy postmenopausal women showed that restriction of dietary magnesium to less than half (101–130 mg) of the Recommended Dietary Allowance (RDA)4 induced atrial arrhythmias and supraventricular beats, which were relieved by magnesium supplementation (4, 5). Severe dietary magnesium restriction also adversely affects oxidative metabolism, glucose homeostasis, and retention and excretion of other electrolytes (4–6). Although marked reductions in magnesium concentrations or intakes produce adverse effects, whether cardiovascular disease (CVD) risk differs across the normal physiologic concentration range of circulating magnesium or dietary magnesium intake is unclear. A meta-analysis examining the associations of circulating magnesium with incident CVD and ischemic heart disease (IHD) across populations has not, to our knowledge, been previously performed. A 2005 pooled analysis of prospective cohorts found no significant association between dietary magnesium and IHD (RR: 0.87; 95% CI: 0.67, 1.10) (7); however, since that time, additional large prospective studies examining this relation have been conducted. Evaluation of both circulating and dietary magnesium is important, because circulating magnesium reflects not only diet but also gastrointestinal absorption and renal regulation, and circulating compared with dietary magnesium could differentially influence CVD risk (8). To investigate potential effects of circulating and dietary magnesium on CVD risk at usual physiologic ranges, we performed a systematic review and meta-analysis of prospective studies examining the associations of circulating magnesium and dietary magnesium with CVD, IHD, and fatal IHD. On the basis of available mechanistic evidence, we hypothesized that both circulating magnesium and dietary magnesium would be inversely associated with CVD and that associations would be strongest for fatal IHD.
METHODS
Search and screening
We followed Meta-analysis of Observational Studies in Epidemiology guidelines (9) during all stages of design, implementation, and reporting of this meta-analysis. We performed a systematic search for all prospective studies examining the association of circulating and/or dietary magnesium with CVD, IHD, or fatal IHD. Electronic searches of PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Ovid (EMBASE, AMED/AGRICOLA; http://gateway.ovid.com), the Cochrane Library (http://www.thecochranelibrary.com/view/0/index.html), Web of Knowledge (Biosis, Web of Science, ISI proceedings; http://wokinfo.com), Commonwealth Agricultural Bureau abstracts (http://www.cabdirect.org), CINAHL (http://www.ebscohost.com/academic/cinahl-plus-with-full-text/), Faculty of 1000 (http://f1000.com), and gray literature sources [Scirus (http://www.scirus.com/), the System for Information on Grey Literature in Europe (http://www.opengrey.eu/), and Epidemiology and Prevention/Nutrition, Physical Activity and Metabolism conference abstracts (http://my.americanheart.org/professional/Sessions/EPINPAM/EPINPAM_UCM_316904_SubHomePage.jsp)] were conducted without language restriction from the earliest available online indexing year to May 2012. Key search terms included magnesium, CVD, heart disease, myocardial infarction, heart attack, sudden death, sudden cardiac death (SCD), IHD, ischemic heart disease, cohort, prospective, longitudinal, case-control, incident, and incidence; full search queries for each database are available on request. Non-English records were translated into English for assessment.
Eligibility criteria
All prospective studies (cohort, nested case-control) that provided a multivariate-adjusted effect estimate with an accompanying measure of uncertainty (CI, SE, or other data to calculate variance) for circulating or dietary magnesium and incident CVD, IHD, or IHD death (including SCD) were eligible for inclusion. CVD was defined as any CVD, including cardiovascular or IHD incidence or death, and stroke or angina as part of a broader composite CVD outcome. IHD was defined as IHD incidence or death. IHD death was defined as any fatal IHD, including SCD. We excluded studies reporting stroke as a distinct outcome, because a meta-analysis of dietary magnesium and stroke was recently published (10). We also excluded studies that focused on children or that evaluated only drinking water magnesium or water hardness, dietary patterns/food groups, intracellular free magnesium, or extracellular ionized magnesium. Ionized magnesium studies were excluded because of the limited reliability of available estimates (11). Because our focus was on magnesium exposure in the normal physiologic range, we excluded studies focused on populations with disturbed mineral homeostasis (eg, patients with chronic kidney disease or heart failure). Studies presenting only crude risk estimates, ecologic studies, case reports, cross-sectional studies, retrospective case-control studies, editorials/commentaries, letters, and reviews were not eligible. For any findings published only in abstract form, we contacted the authors to determine whether results were still considered valid. When multiple manuscripts with the same cohort were published (12, 13), the analysis including the largest numbers of events was included.
Selection of articles
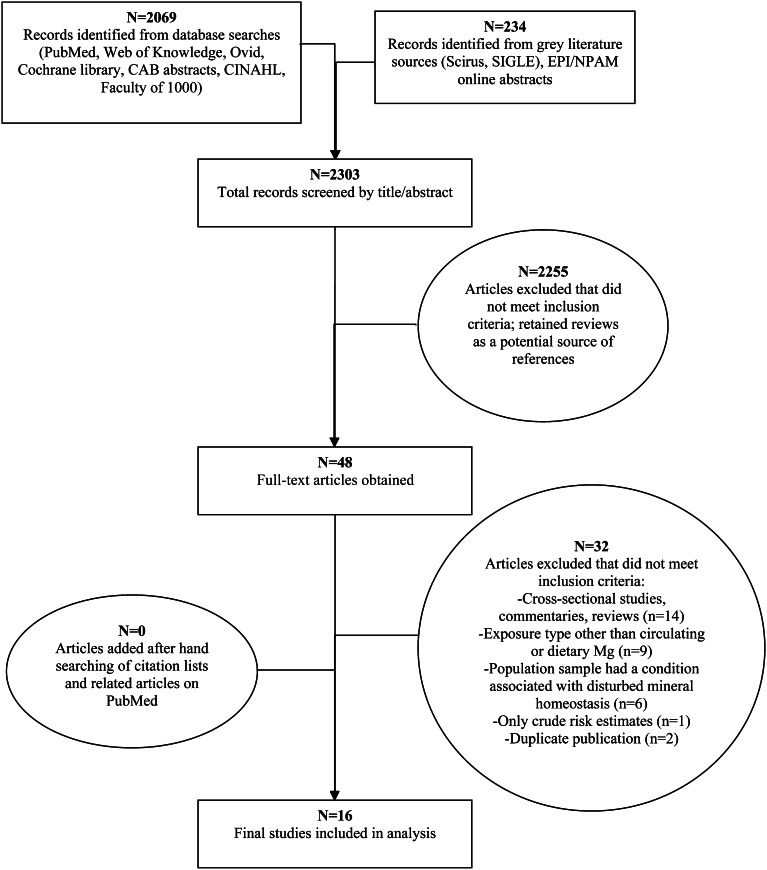
One investigator screened all identified titles and abstracts (n = 2303) for potential eligibility, including additional hand-searching of citation lists of relevant review articles (Figure 1). Among the 48 full-text articles reviewed independently and in duplicate by 2 investigators, 32 studies were excluded because of not being prospective (n = 14); not including the exposures or outcomes of interest (n = 9); focusing on a population sample with prevalent disease affecting mineral homeostasis (n = 6); reporting only crude estimates (n = 1); or being duplicate publications (n = 2). Details of these exclusions are presented as Supplemental material (see “Supplemental data” in the online issue). Citation lists and related citations in PubMed of all final included text articles were hand-searched for additional eligible studies; no new studies were identified. In sum, 16 prospective studies (7, 12–26) met the inclusion criteria and were included in the meta-analysis.
FIGURE 1.
Screening and selection of articles on circulating and dietary magnesium and risk of cardiovascular diseases. Records were identified by electronic searches of PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Web of Knowledge (http://wokinfo.com), Ovid (http://gateway.ovid.com), Cochrane library (http://www.thecochranelibrary.com/view/0/index.html), Commonwealth Agricultural Bureau abstracts (http://www.cabdirect.org), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (http://www.ebscohost.com/academic/cinahl-plus-with-full-text/), and Faculty of 1000 (http://f1000.com). Gray literature sources searched included Scirus (http://www.scirus.com/), the System for Information on Grey Literature in Europe (SIGLE) (http://www.opengrey.eu/), and Epidemiology and Prevention/Nutrition, Physical Activity and Metabolism (EPI/NPAM) conference abstracts (http://my.americanheart.org/professional/Sessions/EPINPAM/EPINPAM_UCM_316904_SubHomePage.jsp).
Data extraction
Data were independently extracted in duplicate by 2 investigators with the use of a standardized electronic form (Microsoft Excel). Information on study design, location, follow-up, age, sex, BMI, race, baseline disease (CVD, kidney disease, diabetes mellitus), and use of multivitamins and magnesium supplements was recorded. For dietary magnesium, data on the assessment method used (food-frequency questionnaire, 24-h dietary recall, other) and whether the data were energy-adjusted (yes, no) were obtained; for circulating magnesium studies, data on the assessment method used (atomic absorption spectroscopy, colorimetric assays, other) and on the blood fraction (serum, plasma) were obtained. For each category of exposure, we extracted the median exposure level, the multivariate-adjusted risk estimate and its variance measure, the number of cases, and the number of participants (nested case-control) or person-years (cohorts). Risk estimates for exposures as continuous variables (n = 2 studies) were also extracted (16, 20). If estimates for more than one multivariate model were presented, we extracted estimates with greatest adjustment for potential confounders without inclusion of potential time-varying mediators or covariates having high collinearity with magnesium (ie, dietary potassium). If the only available multivariable model presented included such variables, this was selected in preference to crude estimates or minimally adjusted models. For cohorts presenting multiple cardiovascular outcomes with shared cases, the outcome with the most cases was used for the pooled estimates for outcomes. Among 8 authors contacted for missing information (12, 16, 17, 20, 21, 23–25), we received responses from 5 authors (12, 16, 18, 23, 24) who provided unpublished data on exposure categories (eg, exposure levels, number of cases) or covariates.
Whereas no standardized quality score for observational studies exists (9), we evaluated and scored studies independently and in duplicate on a 6-point scale based on guidelines adapted from (27). The criteria (1 point each) included reporting of study participation, attrition, assessment of exposure and outcome, control of confounding, and appropriateness of the analysis for the study design. Points were summed for each study, and studies with quality scores of 0 to 3 were considered to be of lower quality, and of 4–6 of higher quality. Interrater reliability was reasonable (Cohen κ 0.72).
Statistical analysis
To maximize all data for calculation of the pooled dose-response, risk estimates were meta-analyzed with the use of the 2-step generalized least-squares trend estimation model (28). This method constructs a covariance estimate for dose-specific log RRs within each study and then estimates the dose-response relation, accounting for between- and within-study variation. Study-specific log-linear dose-response slopes are calculated for each study based on category risk estimates, SE, median exposure, number of cases, person-years of follow-up (cohorts) or number of subjects (nested case-control studies). The study-specific risk estimates are then pooled with the use of inverse-variance weighted meta-analysis to derive an overall dose-response. For studies directly reporting risk estimates for continuous exposure, these data were used and added to the analysis at the second step.
Reported HRs and ORs were assumed to approximate RRs (29). If median exposure levels in each category were not reported, values were imputed based on the reported range or obtained by direct author contact. Dose responses of circulating magnesium and dietary magnesium were standardized across studies to 0.2 mmol/L (0.486 mg/dL, 0.4 mEq/L) and 200 mg/d, respectively, based on unweighted median differences between the highest and lowest quartile category medians across all studies of 0.21 mmol/L for circulating magnesium and 190 mg/d for dietary magnesium; RRs for different increments can be calculated from our data. Nearly all studies of circulating magnesium used serum measures; the lone study using plasma (16) was pooled with these, because the serum and plasma magnesium reference ranges are similar.
Heterogeneity was quantified by using the I2 statistic (30), with >30% considered at least moderate heterogeneity. The primary analysis used a random-effects model if I2 >30%. Because random effects may overestimate the influence of small studies in the presence of heterogeneity, fixed-effects models were evaluated in secondary analyses. Prespecified sources of potential heterogeneity were explored by using meta-regression, with significance tested by the Wald test for a cross-product term (circulating or dietary magnesium multiplied by the potential source of heterogeneity < compared with ≥ median, unless otherwise noted). Sources included location (United States, other), design (cohort, nested case-control), length of follow-up, age, sex, BMI, dietary assessment method (24-h recall), circulating magnesium assessment method (atomic absorption spectroscopy, colorimetric assay), circulating magnesium fraction (serum, plasma), outcome (CVD, IHD) proportion of prevalent disease (baseline CVD, type 2 diabetes, kidney disease), and study quality score (0–3, 4–6). Power was insufficient to examine heterogeneity by using multivariable meta-regression. Publication bias was assessed by visual inspection of funnel plots and Egger's and Begg's tests (31, 32). In the presence of potential publication bias, we used Duval and Tweedie's nonparametric “trim and fill” method, which estimates the number and effect estimates of hypothetically missing studies, to adjust the pooled estimates (33). Potential nonlinear relations were examined by using restricted cubic spline models with 3 kn at the 25th, 50th, and 75th percentiles. All analyses were performed by using STATA 12 (StataCorp), with 2-tailed α = 0.05.
RESULTS
Study characteristics
The 16 identified studies provided 25 estimates of circulating or dietary magnesium and risk of CVD, including 313,041 individuals and 11,995 CVD, 7,534 IHD, and 2686 fatal IHD events (Table 1). Most studies used cohort designs; 3 estimates were derived from prospective nested case-control studies (16, 25). Two studies provided data on both circulating and dietary magnesium (12, 16).
TABLE 1.
Characteristics of 16 prospective studies providing 25 risk estimates in 313,041 individuals for circulating or dietary magnesium and risk of total CVD and IHD1
| First author | Study(country) | Cases/total individuals | Age range | Men | CVD2 | Exposure assessment3 | Disease outcome | Disease ascertainment4 | Follow-up (maximum) | Adjustment5 | Quality score6 |
| n | y | % | % | y | |||||||
| Circulating magnesium | |||||||||||
| Reunanen et al (case-control), 1996 (25) | Finnish men (Finland) | 15–69 | 100 | 22.6 | AAS (serum) | 10 (mean) | ++ | 4 | |||
| 220/507 | CVD death | ICD-8-CM codes 390.97–458.99 | |||||||||
| 160/381 | IHD death | ICD-8-CM codes 410.00–414.99 | |||||||||
| Marniemi et al, 1998 (22) | Finnish elderly (Finland) | 142/344 | ≥65 | 52.9 | 19 | AAS (serum) | CVD death | National death register; select cases confirmed at autopsy | 13 | ++ | 5 |
| Liao et al, 1998 (12) | ARIC (USA) | 223/13,922; 96/13,922 | 45–64 | 1000 | 0 | Colorimetric (serum) | IHD total | Minnesota Code, death certificates with next of kin interviews and physician questionnaires; deaths validated by autopsies | 7 | ++ | 5 |
| Ford, 1999 (18) | NHEFS (USA) | 25–74 | 40 | 0 | AAS (serum) | 19 | ++ | 5 | |||
| 2637/12,340 | IHD total | ICD-9-CM codes 410–414 | |||||||||
| 1005/12,340 | IHD death | Death certificates listing codes above | |||||||||
| Leone et al, 2006 (21) | PPS II (France) | 56/4035 | 30–60 | 100 | 1.4 | AAS (serum) | CVD death | ICD-10-CM codes I00–199 | 21 | ++ | 5 |
| Peacock et al, 2010 (13) | ARIC (USA) | 264/14,232 | 45–64 | 45 | 0 | Colorimetric (serum) | SCD | Fatal IHD cases classified by physician committee as definite or possible sudden arrhythmic death | 12 | ++ | 4 |
| Khan et al, 2010 (20)7 | FHS offspring (USA) | 554/3531 | 44.3 (mean) | 48 | 0 | Colorimetric (serum) | CVD total | Panel review of hospital, medical, and Framingham clinic visit notes by using standardized criteria | 20 | ++ | 5.5 |
| Chiuve et al (case-control), 2011 (16) | NHS (USA) | 99/390 | 44–69 | 0 | 40 | Colorimetric (plasma) | SCD | Medical records or next of kin report of death or cardiac arrest within 1 h of symptom onset, arrhythmic death as defined by Hinkle and Thaler | 16 | +++ | 6 |
| Reffelmann et al, 2011 (24) | SHIP (Germany) | 79/3910 | 20–79 | 49 | 5.8 | Colorimetric (serum) | CVD death | ICD-10-CM codes I10–I79 | 12 | ++ | 5.5 |
| Dietary magnesium | |||||||||||
| Elwood et al, 1996 (17) | Caerphilly cohort (Wales) | 45–59 | 100 | 17 | FFQ | 10 | + | 3 | |||
| 269/2172 | IHD total | ICD-9-CM codes 410–414 | |||||||||
| 96/2172 | IHD death | ICD-9-CM code 410 | |||||||||
| Liao et al, 1998 (12) | ARIC (USA) | 223/13,744; 96/13,744 | 45–64 | 1000 | 0 | FFQ | IHD total | Minnesota Code, death certificates with next of kin interviews and physician questionnaires; deaths validated by autopsies | 7 | +++ | 5 |
| Abbott et al, 2003 (14) | HHS (USA) | 548/7172 | 45–68 | 100 | 0 | 24-h recall | IHD total | ECG or cardiac enzyme evidence, SCD, heart failure or arrhythmic death in patients with IHD history, and/or autopsy findings | 15 | +++ | 5 |
| Al-Delaimy et al, 2004 (15) | HPFS (USA) | 1449/39,633 | 40–75 | 100 | 0 | FFQ | IHD total | Next of kin/coworker reports or National Death Index; confirmation with medical/autopsy reports or death certificates; SCD | 12 | +++ | 5 |
| Song et al, 2005 (7) | WHS (USA) | 39–89 | 0 | 0 | FFQ | 11 | ++ | 5.5 | |||
| 1027/35,601 | CVD total | Myocardial infarction symptoms with ECG changes or cardiac enzyme elevation; hospital record of angioplasty or coronary bypass graft; stroke determined by endpoints committee | |||||||||
| 672/35,601 | IHD total | Same as CVD incidence, excluding stroke and final CVD | |||||||||
| Kaluza et al, 2010 (19) | CSM (Sweden) | 819/23,366 | 45–79 | 100 | 0 | FFQ | CVD death | ICD-10-CM codes I00–I79 | 10 | +++ | 5 |
| Chiuve et al, 2011 (16) | NHS (USA) | 505/88,375 | 34–59 | 0 | 0 | FFQ | SCD | Medical records or next of kin report of death or cardiac arrest within 1 h of symptom onset, arrhythmic death as defined by Hinkle and Thaler | 26 | +++ | 6 |
| Otto et al, 2012 (23) | MESA (USA) | 263/5285 | 45–84 | 47 | 0 | FFQ | CVD total | Self-reported diagnoses, death certificates, autopsy reports, medical records reviewed by endpoints committee | 7 | +++ | 5 |
| Zhang et al, 2012 (26) | JACC (Japan) | 1347/35,532 | 40–79 | 0 | 0 | FFQ | CVD death | ICD-10-CM codes I01–I99 | 17 | +++ | 5.5 |
| 1343/23,083 | 100 | CVD death | Same as above | ||||||||
| 246/35,532 | 0 | IHD death | ICD-10-CM codes I20–I25 | ||||||||
| 311/23,083 | 100 | IHD death | Same as above |
AAS, atomic absorption spectrophotometry; ARIC, Atherosclerosis Risk in Communities; CM, clinical modification; CSM, Cohort of Swedish Men; CVD, cardiovascular disease; ECG, electrocardiogram; FFQ, food-frequency questionnaire; FHS offspring, Framingham Heart Study, offspring cohort; HHS, Honolulu Heart Study; HPFS, Health Professionals Follow-Up Study; ICD-CM, International Statistical Classification of Disease Clinical Modification; IHD, ischemic heart disease; JACC, Japan Collaborative Cohort Study; MESA, Multi-Ethnic Study of Atherosclerosis; NHEFS, NHANES I Epidemiologic Follow-Up Study; NHS, Nurses’ Health Study; PPS II, Paris Prospective Study II; SCD, sudden cardiac death; WHS, Women's Health Study.
Prevalent CVD at baseline.
Dietary exposure was assessed by using FFQs or 24-h dietary recalls; circulating magnesium (serum or plasma) was assessed by using colorimetric assays (colorimetric) or AAS.
The CM number indicates the revision number.
The degree of covariate adjustment is indicated by + (sociodemographic variables), ++ (sociodemographic variables and other risk factors), and +++ (sociodemographic variables, other risk factors, and dietary variables). Circulating magnesium was inversely associated with non-white race, prevalence of hypertension, diabetes, use of diuretics, and other cardiovascular drugs, with weak positive correlations with dietary magnesium intake (r ≤ 0.09) (15, 16) and serum potassium and calcium (r ≤ 0.18) (20) and inconsistent associations with BMI, smoking, lipid profiles, and glomerular filtration rate in univariate analyses (13, 16, 20, 21, 24). Two circulating magnesium studies provided estimates, including diabetes and hypertension as time-varying covariates (13, 16); models excluding these variables were used. Most dietary magnesium studies adjusted for intakes of other nutrients (12, 14–16, 19, 23, 26), including potassium (12, 14–16, 26). Few studies have reported the correlation between dietary magnesium and potassium, although r ≥ 0.91 was reported in references 20 and 26. Multivariate-adjusted estimates excluding dietary potassium were used; if the only available multivariable model presented included dietary potassium, it was selected in preference to crude estimates or minimally adjusted models. Dietary magnesium intake was also associated with intakes of energy, calcium, and fiber (r ≤ 0.75), higher educational attainment, and greater physical activity and was inversely associated with BMI, hypertension, and diabetes in univariate analyses (7, 14–16, 19, 26).
Study quality was assessed by using 6 criteria (up to 1 point per criterion), including participation (1 point if key characteristics of source population were described, including record of sampling recruitment method, period and location of recruitment, or reference to previously published study detailing source population characteristics), attrition (1 point if participants/nonparticipants did not differ by key study characteristics), exposure determination (1 point if dietary magnesium was measured by using a validated dietary assessment method; for circulating magnesium, 1 point if measured by using a published AAS or colorimetric method, with absence of evidence of the potential for hemolyzed samples or EDTA chelation), validated outcome (lack of reliance on self-report/recall), control of confounding [0.5 points for inclusion of age, sex, race/field center (if multi-ethnic cohort), BMI, smoking, alcohol, physical activity; 0.5 points for adjustment of fiber in dietary magnesium models; 0.5 points for adjustment of diabetes at baseline in circulating magnesium models], and analysis (1 point if risk estimate determination and statistical approaches were appropriate for the study design). Scores were summed; studies with scores from 0 to 3 and 4 to 6 were considered to be of lower and higher quality, respectively.
For Khan et al (20), categorical estimates were used for generalized least-squares trend, because the published continuous estimate for a 0.2-mmol/L dose (RR: 0.55; 95% CI: 0.10, 2.98) was strongly influenced by the presence of a small number of outlying hypomagnesemic individuals (serum magnesium <0.62 mmol/L) who were at markedly elevated CVD risk (RR: 1.99; 95% CI: 1.02, 3.85) compared with the reference group with normal magnesium concentrations (0.62–0.91 mmol/L).
Of the 9 studies examining circulating magnesium, 5 were from the United States and the remainder from Europe. Participants were predominately middle-aged at baseline, with a median BMI (in kg/m2) across studies of 25.8; 8 of 9 cohorts included ≤10% patients with diabetes, 4 studies excluded participants with prevalent CVD at baseline, and none reported prevalent chronic kidney disease. Circulating magnesium distributions generally fell within the normal reference range [0.75–0.96 mmol/L (34)] or the wider range used in some laboratories (0.65–1.10 mmol/L) (35). Studies used standard analytic approaches for circulating magnesium determination, atomic absorption spectroscopy, or colorimetric assays, with no record of hemolyzed samples or EDTA use.
Participants in the 9 studies evaluating dietary magnesium analyses were generally similar; most (8 of 9) excluded individuals with prevalent CVD at baseline. Dietary magnesium was most often assessed by using validated food-frequency questionnaires. The median consumption level across all studies was 289 mg—lower than the RDA of 420 mg for men and 320 mg for women >30 y (36). Most studies did not report on potential contribution of multivitamins or supplements to magnesium intake; in 2 cohorts describing such use (15, 16), <14% of participants reported taking magnesium supplements, generally contributing <100 mg total Mg/d in these participants (15).
Most studies validated outcomes by using medical records, autopsy records, and/or endpoint committees, frequently classified by using International Classification of Diseases diagnosis coding (17–19, 21, 24–26). The degree of covariate adjustment varied, with about half of studies adjusting for both sociodemographic and lifestyle variables including age, sex, race, BMI, waist circumference, smoking, alcohol, and physical activity (7, 12, 14–16, 18, 19, 21, 23). Overall, most of the studies (15 of 16) were judged to be of high quality (quality score: 4–6).
Circulating magnesium
Nine studies provided 11 estimates of circulating magnesium and incident CVD, comprising 4106 CVD, 3215 IHD, and 1528 fatal IHD events.
CVD
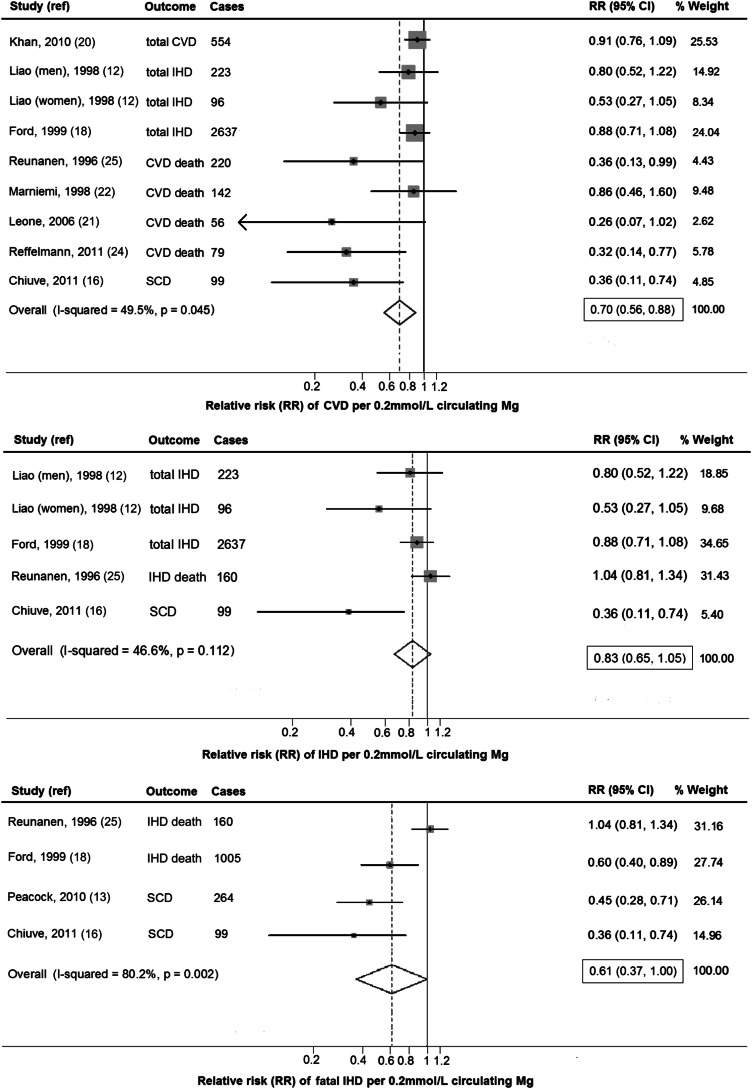
Circulating magnesium (per 0.2 mmol/L increment) was associated with a 30% lower risk of CVD (RR: 0.70; 95% CI: 0.56, 0.88; Figure 2, top), with evidence of moderate between-study heterogeneity (I2 = 49.5%). Findings were similar in secondary analyses by using fixed-effects models. In a meta-regression, study location, percentage baseline CVD, and event type (incidence compared with death) significantly modified the association between circulating magnesium and CVD (P-heterogeneity = 0.04, 0.02, and 0.02, respectively). A stronger association with lower risk was observed in non-US countries, in studies including some participants with prevalent CVD, and in studies evaluating death rather than incidence as an event type (Table 2). In a post hoc subgroup analysis, no significant difference by diuretic use was observed (P = 0.84).
FIGURE 2.
RR of CVD, IHD, and fatal IHD associated with a 0.2-mmol/L higher circulating magnesium concentration quantified by using generalized least-squares trend estimation and pooled by using a random-effects meta-analysis (n = 53,212). Circulating magnesium (per 0.2-mmol/L increment) was associated with a 30% lower risk of CVD (RR: 0.70; 95% CI: 0.56, 0.88 per 0.2 mmol/L) and a trend toward lower risks of IHD (RR: 0.83; 95% CI: 0.75, 1.05) and fatal IHD (RR: 0.61; 95% CI: 0.37, 1.00). CVD, cardiovascular disease; IHD, ischemic heart disease; ref, reference; SCD, sudden cardiac death.
TABLE 2.
RRs (95% CIs) for dietary or circulating magnesium and incidence of CVD, IHD, and fatal IHD according to prespecified potential sources of heterogeneity (n = 313,041)1
| Sources2 | Circulating magnesiumCVD (n = 9) | IHD (n = 5) | Fatal IHD (n = 4) | Dietary magnesiumCVD (n = 11) | IHD (n = 9) | Fatal IHD (n = 4) |
| Study location | ||||||
| USA | 0.83 (0.69, 0.99) | NA (only one non-US cohort) | NA | 0.87 (0.71, 1.06) | 0.82 (0.67, 0.99) | NA (only one non-US cohort) |
| Other | 0.46 (0.25, 0.84) | 0.91 (0.65, 1.28) | 0.68 (0.51, 0.90) | |||
| P-heterogeneity3 | 0.04 | 0.79 | 0.44 | |||
| Study design | ||||||
| Cohort | 0.78 (0.63, 0.96) | 0.83 (0.69, 1.00) | 0.53 (0.39, 0.71) | NA (all cohort) | NA | NA |
| Case-control | 0.36 (0.18, 0.72) | 0.68 (0.24, 1.89) | 0.68 (0.24, 1.89) | |||
| P-heterogeneity3 | 0.32 | 0.63 | 0.27 | |||
| Follow-up | ||||||
| <Median | 0.54 (0.35, 0.84) | 0.71 (0.50, 1.02) | 0.70 (0.30, 1.60) | 1.07 (0.76, 1.51) | 0.90 (0.69, 1.18) | NA (only one below median) |
| ≥Μedian | 0.82 (0.66, 1.03) | 0.88 (0.65, 1.18) | 0.55 (0.38, 0.80) | 0.81 (0.67, 0.97) | 0.72 (0.61, 0.85) | 0.35 |
| P-heterogeneity3 | 0.46 | 0.27 | 0.66 | 0.30 | 0.40 | |
| Age | ||||||
| <Median | 0.81 (0.62, 1.05) | 0.94 (0.80, 1.12) | 0.81 (0.47, 1.00) | 0.84 (0.71, 0.98) | 0.83 (0.66, 1.04) | 0.80 (0.46, 1.39) |
| ≥Μedian | 0.60 (0.42, 0.87) | 0.62 (0.40, 0.95) | 0.43 (0.28, 0.65) | 0.97 (0.69, 1.37) | 0.72 (0.57, 0.90) | 0.63 (0.41, 0.98) |
| P-heterogeneity3 | 0.95 | 0.14 | 0.25 | 0.54 | 0.55 | 0.56 |
| Sex (% male) | ||||||
| <Median | 0.81 (0.65, 1.02) | 0.47 (0.27, 0.81) | 0.55 (0.38, 0.80) | 0.93 (0.66, 1.31) | 0.83 (0.51, 1.33) | 0.56 (0.38, 0.83) |
| ≥Μedian | 0.56 (0.35, 0.88) | 0.92 (0.79, 1.07) | 0.70 (0.30, 1.60) | 0.86 (0.71, 1.05) | 0.77 (0.68, 0.87) | 0.94 (0.69, 1.28) |
| P-heterogeneity3 | 0.21 | 0.06 | 0.66 | 0.96 | 0.06 | 0.12 |
| BMI (kg/m2) | ||||||
| <Median | 0.32 (0.14, 0.72) | 0.94 (0.80, 1.12) | 0.81 (0.47, 1.39) | 0.73 (0.55, 0.98) | 0.63 (0.50, 0.81) | 0.63 (0.41, 0.98) |
| ≥Median | 0.77 (0.62, 0.95) | 0.62 (0.41, 0.95) | 0.43 (0.28, 0.65) | 1.09 (0.84, 1.42) | 0.86 (0.65, 1.13) | 0.80 (0.46, 1.39) |
| P-heterogeneity3 | 0.37 | 0.62 | 0.19 | 0.09 | 0.41 | 0.56 |
| Circulating magnesium method | ||||||
| AAS | 0.69 (0.44, 1.07) | 0.94 (0.80, 1.12) | 0.81 (0.47, 1.39) | NA | NA | NA |
| Colorimetry | 0.64 (0.44, 0.93) | 0.62 (0.40, 0.95) | 0.43 (0.28, 0.65) | |||
| P-heterogeneity3 | 0.80 | 0.14 | 0.25 | |||
| Prevalent CVD | ||||||
| No CVD | 0.87 (0.76, 0.99) | 0.83 (0.69, 1.00) | 0.81 (0.47, 1.39) | NA (only one nonzero baseline %) | NA | NA |
| >0% | 0.45 (0.28, 0.73) | 0.68 (0.24, 1.89) | 0.43 (0.28, 0.65) | |||
| P-heterogeneity3 | 0.02 | 0.63 | 0.25 | |||
| Disease type | ||||||
| CVD | 0.58 (0.35, 0.96) | NA | NA | 1.06 (0.82, 1.37) | NA | NA |
| IHD | 0.74 (0.55, 0.99) | 0.76 (0.66, 0.87) | ||||
| P-heterogeneity3 | 0.35 | 0.07 | ||||
| Event type | ||||||
| Incidence | 0.87 (0.76, 0.99) | 0.83 (0.69, 1.00) | NA | 0.88 (0.73, 1.06) | 0.83 (0.69, 0.99) | NA |
| Death | 0.45 (0.28, 0.73) | 0.68 (0.24, 1.89) | 0.89 (0.60, 1.30) | 0.61 (0.44, 0.85) | ||
| P-heterogeneity3 | 0.02 | 0.63 | 0.88 | 0.07 | ||
| Type 2 diabetes (%) | ||||||
| <Median | 0.80 (0.61, 1.05) | 0.64 (0.28, 1.47) | 0.81 (0.47, 1.39) | 1.00 (0.78, 1.30) | 0.80 (0.60, 1.06) | 0.56 (0.37, 0.83) |
| ≥Μedian | 0.50 (0.25, 0.99) | 0.85 (0.61, 1.19) | 0.43 (0.28, 0.65) | 0.79 (0.64, 0.97) | 0.73 (0.62, 0.86) | 0.94 (0.69, 1.28) |
| P-heterogeneity3 | 0.96 | 0.73 | 0.25 | 0.31 | 0.59 | 0.12 |
RRs and 95% CIs for each study were quantified by using generalized least-squares trend estimation and pooled by using random-effects meta-analysis. AAS, atomic absorption spectrophotometry; CVD, cardiovascular disease; IHD, ischemic heart disease; NA, not available.
Median values across the studies for participant characteristics were used to create binary categories. Prespecified sources of heterogeneity—including the method used to determine dietary magnesium (food-frequency questionnaire compared with 24-h recall), circulating magnesium fraction (serum compared with plasma), and quality score (0–3 compared with 4–6)—were not assessed because only one study quantified dietary magnesium with the use of a 24-h recall (14), measured circulating magnesium in plasma (16), or obtained a quality score of 0 to 3 (17). Kidney disease was also not assessed in the heterogeneity analyses, because disease prevalence was not reported in any study.
P-heterogeneity was obtained by adding a cross-product term of the main exposure and the potential source of heterogeneity (binary coded) to models.
IHD
Five studies evaluated circulating magnesium and IHD. Circulating magnesium was not significantly associated with IHD (RR: 0.83; 95% CI: 0.65, 1.05; I2 = 49.5%) (Figure 2; middle); findings were similar in secondary analyses with the use of fixed-effects models (RR: 0.88; 95% CI = 0.76, 1.02). No significant sources of between-study heterogeneity were identified, although a trend was seen toward a stronger association with lower risk in studies with mostly (≥50%) female participants (P-heterogeneity = 0.06).
Fatal IHD
Four studies comprising 27,293 unique individuals and 1528 cases evaluated circulating magnesium and fatal IHD. A trend toward lower risk was evident (RR: 0.61; 95% CI: 0.37, 1.00), with substantial between-study heterogeneity (I2 = 80.2%) (Figure 2; bottom). In secondary analyses using fixed effects, circulating magnesium was associated with a significantly lower risk of fatal IHD (RR: 0.77; 95% CI: 0.64, 0.93). Meta-regression did not identify any statistically significant sources of heterogeneity, although statistical power to identify heterogeneity was limited given only 4 studies. In a post hoc analysis restricted to studies evaluating SCD, circulating magnesium was associated with a 57% lower risk (RR: 0.43; 95% CI: 0.28, 0.65), but this was based on only 2 studies with relatively few endpoints (n = 99; n = 264 cases) (13, 16). A third cohort reported no significant association between serum magnesium and SCD (20), but this study had very few cases (n = 29 cases) and did not provide a specific risk estimate in the manuscript nor after author request.
Dietary magnesium
Eleven studies provided 14 estimates of dietary magnesium and incident CVD, including 7889 CVD, 4319 IHD, and 1158 fatal IHD events.
CVD
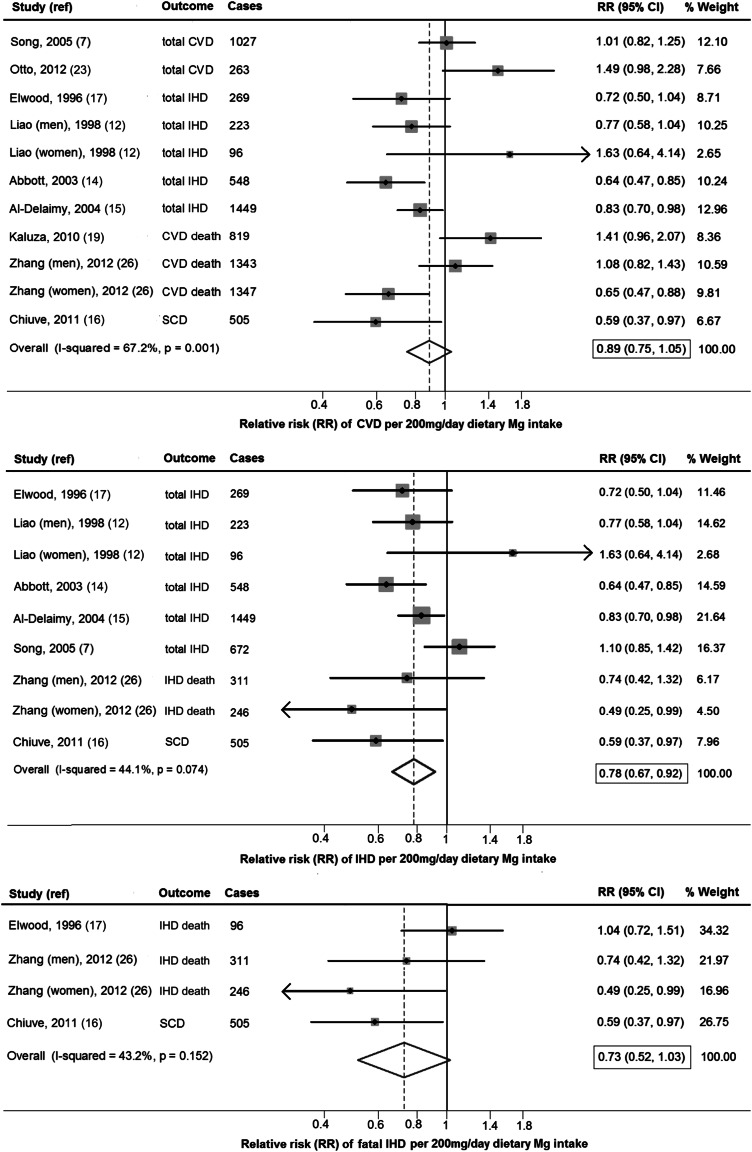
Dietary magnesium (per 200-mg/d increment) was not significantly associated with total CVD (RR: 0.89; 95% CI: 0.75, 1.05; Figure 3, top), with evidence for between-study heterogeneity (I2 = 67.7%). In secondary analyses using fixed effects, dietary magnesium was significantly associated with a lower risk of CVD (RR: 0.87; 95% CI: 0.72, 0.89) (see Supplemental Figure S1 under “Supplemental data” in the online issue). No statistically significant sources of between-study heterogeneity were identified, but trends were seen toward stronger associations with lower risk among studies with lower median BMI (<25) (P-heterogeneity = 0.09) or evaluating IHD rather than CVD (P-heterogeneity = 0.07) (Table 2).
FIGURE 3.
RR of CVD, IHD, and fatal IHD associated with a 200-mg/d higher dietary magnesium intake quantified by using generalized least-squares trend estimation and pooled by using a random-effects meta-analysis (n = 273,963). Dietary magnesium (per 200-mg/d increment) was not significantly associated with CVD (RR: 0.89; 95% CI: 0.75, 1.05) but was associated with a 22% lower risk of IHD (RR: 0.78; 95% CI: 0.67, 0.92). Dietary magnesium intake was not significantly associated with fatal IHD (RR: 0.73; 95% CI: 0.52, 1.03) with linear modeling; however, a significant nonlinear association was observed (Figure 4). CVD, cardiovascular disease; IHD, ischemic heart disease; ref, reference; SCD, sudden cardiac death.
IHD
Dietary magnesium was associated with 22% lower risk of CHD (RR: 0.78; 95% CI: 0.67, 0.92; Figure 3, middle), with moderate heterogeneity (I2 = 44.1). The pooled estimate from a fixed-effects model was similar (RR: 0.80; 95% CI: 0.72, 0.89). Trends toward stronger associations in cohorts with more men (P-heterogeneity = 0.06) and studies evaluating fatal IHD death rather than total IHD (P-heterogeneity = 0.07) were observed (Table 2).
Fatal IHD
Dietary magnesium intake (per 200-mg/d increment) was not significantly associated with fatal IHD (RR: 0.73; 95% CI: 0.52, 1.03; Figure 3, bottom) by using linear modeling; however, a significant nonlinear association was observed (Figure 4). Between-study heterogeneity was moderate (I2 = 43.2%). In a secondary fixed-effects model, dietary magnesium was significantly associated with fatal IHD (RR: 0.77; 95% CI: 0.60, 0.98). Meta-regression did not identify any statistically significant prespecified sources of heterogeneity, although the power to identify heterogeneity was limited given the number of studies.
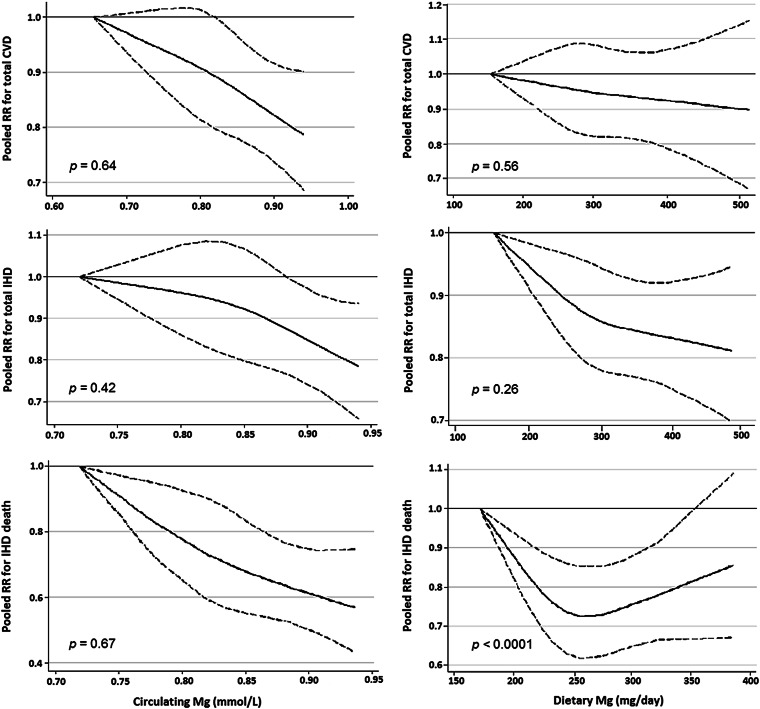
FIGURE 4.
Prospective associations between circulating and dietary magnesium and RR of CVD, IHD, and fatal IHD estimated by random-effects meta-analysis with the use of restricted cubic splines (n = 313,041). Each reference value represents the lowest median value of included studies. P values for nonlinear associations are presented. The association between dietary magnesium and fatal IHD was nonlinear (P-nonlinear < 0.001). In comparison with lower intakes, a 27% lower risk of fatal IHD was observed up to a threshold of ∼250 mg/d (RR: 0.73; 95% CI: 0.62, 0.86). CVD, cardiovascular disease; IHD, ischemic heart disease.
Nonlinear associations
We found no evidence of nonlinear associations between circulating magnesium and CVD (P = 0.64), IHD (P = 0.42), or fatal IHD (P = 0.67) or between dietary magnesium and CVD (P = 0.56) or IHD (P = 0.26) (Figure 4). These findings suggest that pooling of dose-response estimates from linear trend estimation (generalized least-squares trend) for these exposures and outcomes was appropriate. In contrast, we identified a significant nonlinear association between dietary magnesium and fatal IHD (P-nonlinearity < 0.001). Compared with lower intakes, a 27% lower risk of fatal IHD was seen up to a threshold of ∼250 mg/d (RR: 0.73; 95% CI: 0.62, 0.86) (Figure 4, bottom right).
Evaluation of publication bias
Egger's and Begg's tests suggested no evidence of publication bias for associations of circulating magnesium with CVD or fatal IHD (P > 0.05). Egger's test was significant for circulating magnesium and IHD (P = 0.01); meta-regression did not identify any statistically significant sources of heterogeneity for this outcome, but power was limited. Visual inspection of funnel plots showed asymmetry for the association of circulating magnesium with CVD (see Supplemental Figure S2 under “Supplemental data” in the online issue), explained by heterogeneity due to stronger associations (P = 0.02) in studies that included participants with prevalent CVD at baseline and that evaluated fatal IHD (see Supplemental Figure S3 under “Supplemental data” in the online issue). For dietary magnesium, no evidence of publication bias was seen for IHD or fatal IHD (P > 0.05). Egger's test was significant for dietary magnesium and CVD (P < 0.001), consistent with observed funnel plot asymmetry (see Supplemental Figure S4 under “Supplemental data” in the online issue). Duval and Tweedie's rank-based “trim and fill” method identified a “missing” small beneficial study. Addition of this hypothetical missing study (see Supplemental Figure S5 under “Supplemental data” in the online issue) did not appreciably alter the results, with a corrected pooled RR for dietary magnesium and CVD of 0.87 (95% CI: 0.74, 1.03) in comparison with the original pooled RR of 0.89 (95% CI: 0.75, 1.05).
DISCUSSION
This systematic review and meta-analysis identified significant associations of both circulating and dietary magnesium and risk of CVD events. Circulating magnesium (per 0.2-mmol/L increment) was associated with a 30% lower risk of CVD, with trends toward a lower risk of IHD and fatal IHD. Dietary magnesium was associated with a 22% lower risk of IHD and showed a nonlinear association with fatal IHD, with a 27% lower risk up to a threshold of ∼250 mg/d, compared with lower intakes. This investigation, which included a total of 313,041 individuals in whom 4106 CVD, 3215 IHD, and 1528 fatal IHD events were documented for circulating magnesium and 7889 CVD, 4319 IHD, and 1158 fatal IHD events for dietary magnesium, provides the most robust evidence to date of the associations between circulating and dietary magnesium across their usual physiologic ranges and CVD risk.
Circulating magnesium
Our finding of a significantly inverse association between circulating magnesium and incident CVD is supported by evidence from observational studies and small intervention trials showing that magnesium may improve vascular tone and endothelial function, reduce platelet aggregation, increase HDL, improve glucose homeostasis (1, 37), and lower the risk of stroke (38, 39). On the basis of the key role of magnesium in ion channel function and arrhythmias, we hypothesized that associations of circulating magnesium concentrations would be strongest for fatal IHD events. Consistent with this, we identified a trend toward a 39% lower risk of fatal IHD, and, in post hoc analyses, a 57% lower risk of SCD. Whereas the number of studies were limited for fatal IHD, these findings suggest that circulating magnesium may influence arrhythmic risk at concentrations above the clinically low range (hypomagnesemia, <0.65 mmol/L) (3). Because observational studies cannot establish causality, randomized controlled trials are needed to test this hypothesis.
Our results also indicate a need to better understand the determinants of magnesium concentrations in blood. Circulating magnesium is under close homeostatic regulation, primarily through renal reabsorption and excretion (8), although the determinants of variation within the normal physiologic range are not well understood. For example, genetic variations in single nucleotide polymorphisms may account for <2% of the variance in serum magnesium concentrations (40), and our understanding of the influence of endocrine factors on magnesium homeostasis is incomplete (41). Circulating concentrations are responsive to supplementation and long-term changes in intakes (4, 5, 37); however, the correlation between dietary intake and circulating concentrations is low (12, 16), which highlights the importance of other important regulatory and homeostatic mechanisms.
Dietary magnesium
Our meta-analysis of dietary magnesium and total IHD updates a previous pooled estimate published by Song et al in 2005 (7) by including estimates from the Japan Collaborative Cohort Study and Nurses’ Health Study cohorts and, for the first time, reports on the association between dietary magnesium and total CVD and fatal IHD. Findings from 2 small randomized controlled trials showing development of arrhythmias at low dietary magnesium intakes (4, 5) are broadly consistent with our meta-analysis result of a significantly lower risk of fatal IHD up to a dietary magnesium intake threshold of ∼250 mg/d. In these trials, restriction of dietary magnesium in healthy postmenopausal to less than half (101–130 mg) of the RDA induced atrial arrhythmias and supraventricular beats. During the magnesium-restriction phase, calcium, potassium, copper, and other nutrients were supplemented to determine the direct effect of low magnesium intake, and arrhythmias were relieved by provision of 200 mg Mg/d through supplementation.
Dietary magnesium was not significantly associated with CVD in our analysis, although a recent meta-analysis of prospective studies showed a modest significant inverse association between dietary magnesium and risk of stroke, particularly ischemic stroke (10). Taken together, these results may suggest mechanistic pathways specific to stroke that are not captured in a more heterogeneous endpoint of CVD. Evidence from large prospective studies examining the association of dietary magnesium with incident hypertension have been inconsistent (42), and additional experimental evidence is needed to elucidate the potential effects of inadequate magnesium intake, at usual consumption levels, on components of CVD risk.
Overall, our findings support the importance of adequate dietary magnesium for lowering IHD risk. Dietary magnesium intakes among most American adults are low; the estimated magnesium intake from food sources in 2005–2006 was 261 mg in women and 347 mg in men (43), which is well below the RDA (320 mg for women and 420 mg for men). In elderly Americans (>70 y), 70% of men and 80% of women consume less than the Estimated Average Requirement for magnesium (43). Because nearly all the dietary magnesium in the identified studies was from foods, our findings support recommendations to increase consumption of magnesium-rich foods rather than to take supplements. An increased consumption of magnesium-rich foods, such as whole grains, nuts, and vegetables (by 1 serving/d for whole grains and vegetables and by 2 servings/wk for nuts), has been estimated to lower the risk of cardiovascular mortality by 28% (44).
Strengths
In the absence of any large randomized controlled trials to increase circulating magnesium concentrations or magnesium intake for the prevention of cardiovascular events, our data derived from a systematic search and meta-analysis of prospective studies provide the best available evidence of how circulating and dietary magnesium may influence CVD risk. Our comprehensive search methods and contacts with authors made it unlikely that any major published report was missed. By combining all available data across all categories of exposure in each study, we increased the validity of the dose-response estimates, maximized statistical power, and were able to evaluate potential nonlinear associations. We separately evaluated CVD, IHD, and fatal IHD, which mechanistically differ and on which magnesium may plausibly have different effects. We limited our analysis to prospective cohorts or case-control studies nested within such cohorts, which greatly limited the possibility of selection bias or recall bias. We also limited our analysis to studies that used established analytic methods for measuring circulating magnesium. Disease outcomes in these studies were typically classified by using standardized algorithms and detailed records, which reduced the likelihood of misclassified outcomes. The findings were generally consistent regardless of whether random effects or fixed models were used and in various sensitivity analyses accounting for potential publication bias.
Limitations
Our findings were constrained by the availability of published or unpublished data on magnesium-CVD associations. Although we contacted many authors to obtain potential unpublished risk estimates, none were recovered. For several outcomes, the number of separate studies were limited to examine heterogeneity. Misclassification, residual confounding, and reverse causation may bias observational studies. Dietary magnesium was assessed by using food-frequency questionnaires or 24-h dietary recalls, which do not capture magnesium intake from drinking water and thereby underestimate total magnesium intake. However, this source of nondifferential misclassification would likely attenuate findings toward the null and underestimate the magnitude of the true associations. Only a few circulating magnesium studies (16, 20, 24) adjusted for glomerular filtration rate; although most participants in these cohorts were generally healthy, unrecognized differences in renal function could be a source of residual confounding for circulating magnesium. Furthermore, because of the high correlation between dietary magnesium and dietary potassium in some studies (12, 26), we cannot exclude the possibility of residual confounding by dietary potassium in our dietary magnesium analyses. The potential for reverse causation should be considered because circulating magnesium concentrations can be influenced by intake of specific medications that were not adjusted for in cohort analyses (eg, omeprazole) (45). Finally, we did not examine associations of magnesium and stroke as a distinct outcome. Circulating and dietary magnesium in some ways represent relatively distinct exposures, given the homeostatic regulation of the former and their low intercorrelation; thus, the significant associations observed for both biomarker and dietary magnesium with cardiovascular outcomes was reassuring. Nonetheless, randomized trials are needed to definitively elucidate whether magnesium is causally related to CVD risk.
Conclusions
Circulating magnesium was significantly associated with a lower risk of CVD, with trends toward a lower risk of IHD and fatal IHD. Dietary magnesium was associated with a significantly lower risk of IHD and showed a nonlinear association with fatal IHD. Our findings support the importance of dietary recommendations to increase magnesium-rich foods, including whole grains, nuts/seeds, and vegetables, which are also good sources of other nutrients. Additional experimental studies and randomized trials are needed to elucidate the roles of circulating and dietary magnesium, at usual physiologic concentrations and intakes, on CVD risk.
Supplementary Material
Acknowledgments
We thank the following authors for clarifying data in their published manuscripts and/or providing additional unpublished data: Earl Ford, Aaron Folsom, Thorston Reffelmann, Christine Albert, and Jennifer Nettleton.
The authors’ responsibilities were as follows—LD and DM: designed the research; LD, FI, JHYW, MCdOO, and SEC: conducted the research; LCD, FI, and JHYW: analyzed the data; LCD: drafted the manuscript; FI, JHYW, MCdOO, SEC, and DM: made critical revisions to the manuscript for important intellectual content; and DM: provided funding. All authors read and approved the final manuscript. DM reported ad hoc consulting fees from McKinsey Health Systems Institute (November 2011) and Foodminds (January 2012) (modest) and ad hoc travel reimbursement and/or honoraria for one-time scientific presentations on diet and cardiometabolic diseases from SPRIM (November 2009), Nutrition Impact (September 2010), the International Life Sciences Institute (12/10), Bunge (November 2011), Pollock Institute (March 2012), and Quaker Oats (April 2012) (modest). DM reported involvement in the Unilever North America Scientific Advisory Board. None of the authors reported a conflict of interest. The supporting agencies had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Abbreviations used: CVD, cardiovascular disease; IHD, ischemic heart disease; RDA, Recommended Dietary Allowance; SCD, sudden cardiac death.
REFERENCES
- 1.Shechter M. Magnesium and cardiovascular system. Magnes Res 2010;23:60–72. [DOI] [PubMed] [Google Scholar]
- 2.Mubagwa K, Gwanyanya A, Zakharov S, Macianskiene R. Regulation of cation channels in cardiac and smooth muscle cells by intracellular magnesium. Arch Biochem Biophys 2007;458:73–89. [DOI] [PubMed] [Google Scholar]
- 3.AHA (ECC Guidelines). Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care, Part 8: Advanced challenges in resuscitation: Section 1: Life-threatening electrolyte abnormalities. Circulation 2000;102:I217–22. [PubMed] [Google Scholar]
- 4.Klevay LM, Milne DB. Low dietary magnesium increases supraventricular ectopy. Am J Clin Nutr 2002;75:550–4. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen FH, Milne DB, Klevay LM, Gallagher S, Johnson L. Dietary magnesium deficiency induces heart rhythm changes, impairs glucose tolerance, and decreases serum cholesterol in postmenopausal women. J Am Coll Nutr 2007;26:121–32. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen FH, Milne DB, Gallagher S, Johnson L, Hoverson B. Moderate magnesium deprivation results in calcium retention and altered potassium and phosphorus excretion by postmenopausal women. Magnes Res 2007;20:19–31. [PubMed] [Google Scholar]
- 7.Song Y, Manson JE, Cook NR, Albert CM, Buring JE, Liu S. Dietary magnesium intake and risk of cardiovascular disease among women. Am J Cardiol 2005;96:1135–41. [DOI] [PubMed] [Google Scholar]
- 8.Arnaud MJ. Update on the assessment of magnesium status. Br J Nutr 2008;99:S24–36. [DOI] [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 10.Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. Am J Clin Nutr 2012;95:362–6. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W. Point of care testing of ionized magnesium in blood with potentiometric sensors—opportunities and challenges. Am J Biomed Sci 2011;3:301–12. [Google Scholar]
- 12.Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 1998;136:480–90. [DOI] [PubMed] [Google Scholar]
- 13.Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2010;160:464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbott RD, Ando F, Masaki KH, Tung KH, Rodriguez BL, Petrovitch H, Yano K, Curb JD. Dietary magnesium intake and the future risk of coronary heart disease (the Honolulu Heart Program). Am J Cardiol 2003;92:665–9. [DOI] [PubMed] [Google Scholar]
- 15.Al-Delaimy WK, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Magnesium intake and risk of coronary heart disease among men. J Am Coll Nutr 2004;23:63–70. [DOI] [PubMed] [Google Scholar]
- 16.Chiuve SE, Korngold EC, Januzzi JL, Jr, Gantzer ML, Albert CM. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am J Clin Nutr 2011;93:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elwood PC, Fehily AM, Ising H, Poor DJ, Pickering J, Kamel F. Dietary magnesium does not predict ischaemic heart disease in the Caerphilly cohort. Eur J Clin Nutr 1996;50:694–7. [PubMed] [Google Scholar]
- 18.Ford ES. Serum magnesium and ischaemic heart disease: findings from a national sample of US adults. Int J Epidemiol 1999;28:645–51. [DOI] [PubMed] [Google Scholar]
- 19.Kaluza J, Orsini N, Levitan EB, Brzozowska A, Roszkowski W, Wolk A. Dietary calcium and magnesium intake and mortality: a prospective study of men. Am J Epidemiol 2010;171:801–7. [DOI] [PubMed] [Google Scholar]
- 20.Khan AM, Sullivan L, McCabe E, Levy D, Vasan RS, Wang TJ. Lack of association between serum magnesium and the risks of hypertension and cardiovascular disease. Am Heart J 2010;160:715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leone N, Courbon D, Ducimetiere P, Zureik M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiol 2006;17:308–14. [DOI] [PubMed] [Google Scholar]
- 22.Marniemi J, Järvisalob J, Toikkaa T, Räihä I, Ahotupa M, Sourander L. Blood vitamins, mineral elements and inflammation markers as risk factors of vascular and non-vascular disease mortality in an elderly population. Int J Epidemiol 1998;27:799–807. [DOI] [PubMed] [Google Scholar]
- 23.de Oliveira Otto MC, Alonso A, Lee DH, Delclos GL, Bertoni AG, Jiang R, Lima JA, Symanski E, Jacobs DR, Jr, Nettleton JA. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J Nutr 2012;142:526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reffelmann T, Ittermann T, Dörr M, Völzke H, Reinthaler M, Petersmann A, Felix SB. Low serum magnesium concentrations predict cardiovascular and all-cause mortality. Atherosclerosis 2011;219:280–4. [DOI] [PubMed] [Google Scholar]
- 25.Reunanen A, Knekt P, Marniemi J, Mäki J, Maatela J, Aromaa A. Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur J Clin Nutr 1996;50:431–7. [PubMed] [Google Scholar]
- 26.Zhang W, Iso H, Ohira T, Date C, Tamakoshi A; JACC Study Group. Associations of dietary magnesium intake with mortality from cardiovascular disease: the JACC study. Atherosclerosis 2012;221:587–95. [DOI] [PubMed] [Google Scholar]
- 27.Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427–37. [DOI] [PubMed] [Google Scholar]
- 28.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006;6:40–57. [Google Scholar]
- 29.Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol 2002;55:893–9. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 31.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 33.Duval S, Tweedie R. Trim and fill: a simple funnel plot based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 34.Lowenstein FW, Stanton MF. Serum magnesium levels in the United States, 1971-1974. J Am Coll Nutr 1986;5:399–414. [DOI] [PubMed] [Google Scholar]
- 35.Tietz NW. Philadelphia, PA: WB Saunders, 1990. [Google Scholar]
- 36.Institute of Medicine (IOM). Dietary Reference Intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academies Press, 1997. [PubMed] [Google Scholar]
- 37.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in Type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med 2006;23:1050–6. [DOI] [PubMed] [Google Scholar]
- 38.Ohira T, Peacock JM, Iso H, Chambless LE, Rosamond WD, Folsom AR. Serum and dietary magnesium and risk of ischemic stroke: the Atherosclerosis Risk in Communities Study. Am J Epidemiol 2009;169:1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amighi J, Sabeti S, Schlager O, Mlekusch W, Exner M, Lalouschek W, Ahmadi R, Minar E, Schillinger M. Low serum magnesium predicts neurological events in patients with advanced atherosclerosis. Stroke 2004;35:22–7. [DOI] [PubMed] [Google Scholar]
- 40.Meyer TE, Verwoert GC, Hwang S-J, Glazer NL, Smith AV, van Rooij FJ, Ehret GB, Boerwinkle E, Felix JF, Leak TS, et al. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six loci influencing serum magnesium levels. PLoS Genet 2010;6:e1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium: an update on physiological, clinical and analytical aspects. Clin Chim Acta 2000;294:1–26. [DOI] [PubMed] [Google Scholar]
- 42.Song Y, Sesso HD, Manson JE, Cook NR, Buring JE, Liu S. Dietary magnesium intake and risk of incident hypertension among middle-aged and older US women in a 10-year follow-up study. Am J Cardiol 2006;98:1616–21. [DOI] [PubMed] [Google Scholar]
- 43.Moshfegh A, Golman J, Auhja J, Rhodes D, Lacomb R. What we eat in America, NHANES 2005-2006: usual intakes from food and water compared to 1997 Dietary Reference Intakes for vitamin D, calcium, phosphorus and magnesium. Washington, DC: US Department of Agriculture, Agricultural Research Service, 2009. [Google Scholar]
- 44.Mozaffarian D, Capewell S. United Nations’ dietary policies to prevent cardiovascular disease. BMJ 2011;343:d5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hess MW, Hoenderop JG, Bindels RJM, Drenth JPH. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther 2012;36:405–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.