Abstract
Background: Animal studies have shown that vitamin K treatment reduced vascular calcification, but human data are limited.
Objective: We determined the association between vitamin K status and coronary artery calcium (CAC) progression in the Multi-Ethnic Study of Atherosclerosis by using a case-cohort design.
Design: Serum phylloquinone (vitamin K1) was measured in 296 participants with extreme CAC progression and 561 randomly selected participants without extreme CAC progression; all subjects had baseline and follow-up CAC measures (mean follow-up: 2.5 y). A serum vitamin K1 concentration was considered low at <1.0 nmol/L (the distribution median). Outcomes were replicated by using post hoc per-protocol analyses of a vitamin K1 supplementation trial.
Results: The OR (95% CI) for extreme CAC progression for subjects with low serum vitamin K1 compared with subjects without extreme CAC progression was 1.34 (0.94, 1.90; NS) when adjusted for demographics and confounders. A significant interaction between low vitamin K1 and antihypertension medication use was detected (P = 0.016). Hypertension medication users with low serum vitamin K1 were more likely to have extreme CAC progression than were medication users without extreme CAC progression [OR (95% CI): 2.37 (1.38, 4.09)]. In replication, baseline antihypertensive medication users in the supplementation group had less CAC progression than did those in the control group [adjusted mean ± SEM of the 3-y CAC change was +5 ± 20 Agatston units (AU) in the vitamin K1 group (n = 40) and +44 ± 13 AU in the placebo group (n = 49); P < 0.01].
Conclusions: Although the point estimate of our primary analysis suggests low serum vitamin K1 is associated with greater CAC progression, the difference was NS. Low serum vitamin K1 was significantly associated with CAC progression in antihypertension medication users, which, to our knowledge, is a novel finding conditionally replicated by using an independent sample. Intervention trials are needed to determine whether improving serum vitamin K1 reduces CAC progression, especially in hypertensive individuals. This trial was registered at clinicaltrials.gov as NCT00183001.
INTRODUCTION
Coronary artery calcification is a common manifestation of cardiovascular disease (CVD)4. Although a single coronary artery calcium (CAC) measurement independently predicts future cardiac events and mortality independent of established risk factors (1, 2), CAC progression has been shown to better predict future fatal and nonfatal cardiac events (3, 4). In the Multi-Ethnic Study of Atherosclerosis (MESA), which is a population-based study of atherosclerosis progression, risk factors for CAC progression included age, male sex, white race-ethnicity, BMI, blood pressure, triglycerides, smoking status, treated hypertension, lipid-lowering medication use, and type 2 diabetes (5). Approximately 5% of MESA participants were characterized as having more CAC progression than was predicted on the basis of identified risk factors (5). This result suggested that in some individuals, CAC progression is related to factors yet to be identified.
A preventive role for vitamin K against CAC progression has been proposed on the basis of its role in activating matrix gla protein (MGP), which is a calcification inhibitor in vascular tissue. In mice, a targeted deletion of the MGP gene results in rapid and complete arterial calcification, which results in death by 6 wk (6). For MGP to function, vitamin K is required as an enzymatic cofactor to γ-carboxylate the protein. It has been suggested that a reduction in the functional (carboxylated) MGP, rather than the amount of total MGP, may increase risk of vascular calcification (7). In addition to MGP, other vitamin K–dependent proteins in cardiovascular tissue, such as the gla-rich protein, may also influence atherosclerosis progression (8).
Although animal and in vitro data support a role of vitamin K in the protection against vascular calcification (7, 9), data from human observational studies are limited because the majority of such studies have relied on self-reported vitamin K intake to estimate vitamin K status. The mixed results (10–12) may stem partially from the difficulty in estimating nutrient status by using self-reported dietary measures (13, 14). In contrast, nutritional biomarkers are considered more-objective measures of nutrient status (15). Phylloquinone (vitamin K1) is the primary circulating form of vitamin K. Circulating vitamin K1 has been validated against food-frequency questionnaires (FFQs) and is considered a general indicator of vitamin K nutritional status (16). In a randomized controlled trial, vitamin K1 supplementation reduced CAC progression in older adults who had preexisting CAC but did not influence the development of new CAC (17), which suggested that vitamin K may be relevant to CAC progression. Therefore, we tested the hypothesis that low vitamin K status is associated with greater CAC progression by comparing serum vitamin K1 concentrations of MESA participants with extreme CAC progression to participants without extreme CAC progression over an average 2.5 y of follow-up.
SUBJECTS AND METHODS
The MESA is an ongoing observational study that examines the prevalence and progression of subclinical CVD in a multiethnic cohort. The MESA cohort (n = 6814) was recruited in 2000–2002 from 6 US communities, including Forsyth County, NC; northern Manhattan and the Bronx, NY; Baltimore County, MD; St Paul, MN; Chicago and Maywood, IL; and Los Angeles County, CA. The cohort of participants is 38% non-Hispanic white, 28% African American, 22% Hispanic, and 12% Chinese American, all of whom were free of clinically diagnosed CVD at baseline. The study design and methods of the MESA have been described in detail (18). The MESA was approved by the institutional review boards at all participating centers. All participants gave written informed consent.
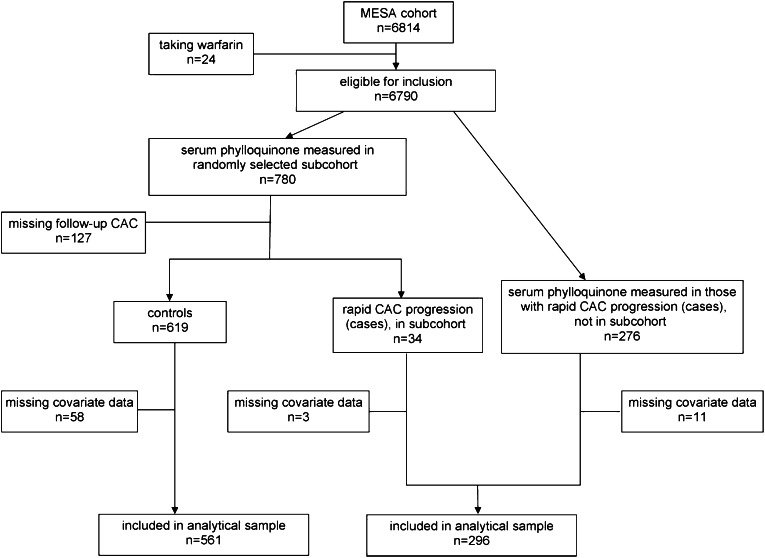
This substudy of the MESA used a case-cohort design (19). Participant selection is shown schematically in Figure 1. Because warfarin is a vitamin K antagonist, warfarin users (n = 24) were excluded. Serum vitamin K1 was measured in 780 randomly chosen MESA participants from stored samples taken at the baseline visit. These 780 participants did not differ from MESA participants who did not have serum vitamin K1 measured in terms of age, sex, race-ethnicity, BMI, triglycerides, total cholesterol, physical activity, vitamin K1 intake, education, season (of blood draw), smoking status, hypertension, type 2 diabetes, or kidney function (all P > 0.14). A total of 127 of 780 participants had missing follow-up CAC measures primarily because they did not attend the follow-up clinic visit and, thus, were not eligible. Compared with participants with follow-up measures, participants without follow-up CAC measures were less likely to graduate from high school and had lower baseline CAC (P < 0.001) but did not significantly differ in age, sex, race-ethnicity, BMI, triglycerides, total cholesterol, physical activity, vitamin K1 intake, education, season (of blood draw), smoking status, hypertension, type 2 diabetes, or kidney function (all P > 0.13).
FIGURE 1.
Participant selection for the MESA case-cohort study. CAC, coronary artery calcium; MESA, Multi-Ethnic Study of Atherosclerosis.
A participant was considered to have extreme progression if they had prevalent CAC at baseline that progressed more than was predicted according to risk factors identified in the MESA by Kronmal et al (5). Because the subcohort was chosen randomly, 34 subjects were subsequently identified as having extreme CAC progression. Serum vitamin K1 was also measured in 276 additional MESA participants previously identified as having extreme CAC progression (5), which left a total of 310 cases eligible for inclusion. The final sample for this analysis included 296 extreme progressors (cases) and 561 noncases for whom dietary intake and other covariate data were available. The 72 participants (6.8%) with missing covariate data were more likely to be black, currently smoke, and not have graduated from high school (all P ≤ 0.01 on the basis of chi-square analyses), and they participated in less-intentional physical activity and had higher systolic blood pressure and less CAC at baseline (all P ≤ 0.02 on the basis of Wilcoxon's 2-sample test).
Coronary calcium
The CAC measurement in the MESA has been thoroughly described (20). Briefly, CAC was measured with either electron-beam computed tomography (at Chicago, IL; Los Angeles, CA; and New York, NY) or with multidetector computed tomography (at Baltimore, MD; Forsyth County, NC; and St Paul, MN). Each participant was scanned twice consecutively. All scans were read by a radiologist or cardiologist, who was blinded to all clinical and demographic information, at a central reading center (Los Angeles Biomedical Research Institute, Harbor-University of California, Los Angeles, Torrance, CA) and were quantified by using the method of Agatston [Agatston score (AS)] (21). Results of the 2 scans were averaged for each participant (20). All participants were scanned at their baseline visit. Follow-up scans were obtained on one-half of MESA participants (chosen at random) at the second follow-up visit (2002–2004), whereas the other one-half of participants had follow-up scans at the third follow-up visit (2004–2005) (5, 18).
Serum vitamin K1
At the baseline MESA examination, fasting serum was collected and frozen at −70°C. Serum vitamin K1 was measured in thawed samples by using reversed-phase HPLC followed by fluorometric detection (22) at the Vitamin K Laboratory at the USDA Human Nutrition Research Center on Aging at Tufts University. This laboratory currently participates in the international vitamin K external quality-assurance scheme (23). The lower limit of detection for circulating vitamin K1 by using the sample volume available was 0.1 nmol/L. Samples with vitamin K1 concentrations below the lower limit of detection were entered as 0.05 nmol/L (22, 24). Low and high control specimens had average values of 0.56 and 3.15 nmol/L, with a total (intraassay plus interassay) CV of 15.2% and 10.9%, respectively (24).
Race-ethnicity
Race-ethnicity was self-reported as white, Chinese, African American, or Hispanic.
Covariates
Height and weight were measured without shoes, and BMI was calculated as weight divided by the square of height. Triglycerides, cholesterol, glucose, and creatinine were measured from fasting plasma samples (25). The estimated glomerular filtration rate (eGFR) was calculated by using the Chronic Kidney Disease Epidemiology Collaboration formula (26), and impaired kidney function was defined as an eGFR <60 mL/min (27). Physical activity was measured by using a physical activity survey, as described previously (28). The total intentional physical activity included walking for exercise, sports, dancing, and conditioning exercises (28). Medical history and medication use, smoking status, and demographic information were collected by using standardized questionnaires. The highest level of education was categorized as less than high school, a high school graduate, or a college graduate or further. At the baseline visit, the dietary intake of participants over the previous 12 mo was assessed by using a modified 120-item Block FFQ. The MESA FFQ was patterned after the FFQ used in the Insulin Resistance Atherosclerosis Study, which has been validated in non-Hispanic white, non-Hispanic black, and Hispanic individuals (29).The FFQ was modified to also include unique Chinese foods and culinary practices to accommodate the MESA subject population, as previously described (30). The Nutrition Data Systems for Research database (Nutrition Coordinating Center University of Minnesota; http://www.ncc.umn.edu/products/ndsr.html) was used to estimate dietary vitamin K1 intake by multiplying the number of servings of a particular food times the vitamin K1 content per serving size of that food (31). A similar strategy was used to estimate the total energy intake.
Statistical analyses
Participants characteristics of the extreme CAC progression group and control group were compared by using the chi-square test (categorical outcomes), Student's t test (for normally distributed continuous outcomes), or the Wilcoxon rank-sum test (for continuous outcomes that did not normalize after ln transformation). In the randomly chosen subcohort, differences in participant characteristics were examined across categories of serum vitamin K1 (<1.0 and ≥1 nmol/L) adjusted for triglycerides and lipid-lowering medication use by using multiple linear regression for continuous outcomes or logistic regression for categorical outcomes.
Logistic regression was used to assess the association between low serum vitamin K1 and extreme CAC progression by using a robust variance estimation that accounted for the case-cohort design (32). The exposure was considered dichotomously by using <1.0 nmol/L as the cutoff. This concentration corresponded to the overall median of our distribution, but it was chosen as a threshold a priori because it corresponded to concentrations that are achieved when dietary intakes meet the current recommendations (90 μg/d for women and 120 μg/d for men) (33, 34) and was consistent with the threshold associated with lower disease risk in another population-based study (35). We also examined the association by using serum vitamin K1 as a continuously measured exposure. A series of multivariable logistic regression models were subsequently used to adjust for potential confounders associated with vitamin K status or CAC progression. Vitamin K1 is transported on triglyceride-rich lipoproteins (36) and may, therefore, be affected by lipid-lowering medication use (which included statins, bile-acid sequestrants, fibrates, and niacin/nicotinic acid), and thus, those covariates were adjusted for first (model 1). Model 2 further adjusted for demographic characteristics and CVD comorbidities [age, sex, race, study site, education, total cholesterol, diabetes, kidney function (eGFR <60 mL/min), blood pressure and hypertension medication). Because serum vitamin K1 can reflect generally healthy lifestyles, the final model (model 3) was further controlled for energy-adjusted vitamin K1 intake [an indicator of a healthy diet (37)], and physical activity. Season was also included as a covariate because seasonal differences in serum vitamin K1 have been reported (16). Our main analysis included 857 participants for whom all covariate data were available. To assess the robustness of our findings to missing covariate information, we also ran models 1 and 2 by excluding vitamin K1 intake and physical activity (covariates with missing data) to include the participants who were missing these covariates. Because the 72 participants with missing data were missing dietary intake and physical activity data, we did not run model 3 in this additional analysis.
Because extreme progression can only occur in individuals with existing CAC, we subsequently restricted the analysis to participants with detectable CAC at the baseline visit (n = 538). We also tested whether the association between low serum vitamin K1 and extreme CAC progression was consistent across demographics [sex, race, age (<65 y, the median age at the baseline visit, and ≥65 y)] and comorbid conditions [hypertension (dichotomized according to antihypertension medication use on the basis of an earlier analysis of CAC progression in the MESA) (5)], diabetes (fasting glucose concentration ≥126 mg/dL or use of oral hypoglycemia medication or insulin), impaired kidney function (eGFR <60 mL/min), and lipid-lowering medication use] by entering interaction terms into the fully adjusted models. The OR (95% CI) for extreme CAC progression for subjects who reported consumption of less than the current recommended adequate intake of dietary vitamin K1 compared with subjects who reported consumption of recommended amounts of dietary vitamin K1 (90 μg/d for women and 120 μg/d for men) (33) was also estimated.
To verify that our results were not related to the case-cohort sampling approach, we performed a similar analysis by using only participants randomly selected in the subcohort (n = 592). Annualized CAC progression was categorized as an AS increase of <100 or ≥100 Agatston units (AU)/y. Logistic regression was used to determine the association between CAC progression ≥100 AU/y and serum vitamin K1. The analysis was repeated by using the randomly selected participants with detectable CAC at baseline (n = 273). In participants without detectable CAC at baseline (n = 319), logistic regression was also used to determine the association between serum vitamin K1 <1.0 nmol/L and CAC development (defined as an AS >0 AU at follow-up; n = 50).
Replication study
Because we detected a significant but not hypothesized interaction between serum vitamin K1 and hypertension (defined according to medication use), we sought to confirm our observation by using data from a randomized trial that reported the effect of 3 y vitamin K1 supplementation (500 μg/d) on CAC progression in 388 community-dwelling older adults (mean ± SD age: 68 ± 6 y; 61% women) (17). Methodologic details of this trial have been well described (17, 38). We used multiple linear regression with the 3-y change in CAC as the outcome to determine the effect of vitamin K1 supplementation on CAC progression in an intent-to-treat analysis stratified according to baseline hypertension treatment. In an earlier analysis, we showed the effect of vitamin K1 supplementation was significant in participants who adhered to the treatment (predefined as subjects who took ≥85% of supplements on the basis of a direct pill count) and in participants who adhered to the treatment and had an AS >10 AU at baseline (17). Thus, we similarly restricted the current analyses. This trial was registered at clinicaltrials.gov as NCT00183001.
Analyses were carried out with SAS software (version 9.2; SAS Institute Inc). P < 0.05 was considered statistically significant for main effects, and P < 0.10 was considered significant for interaction terms.
RESULTS
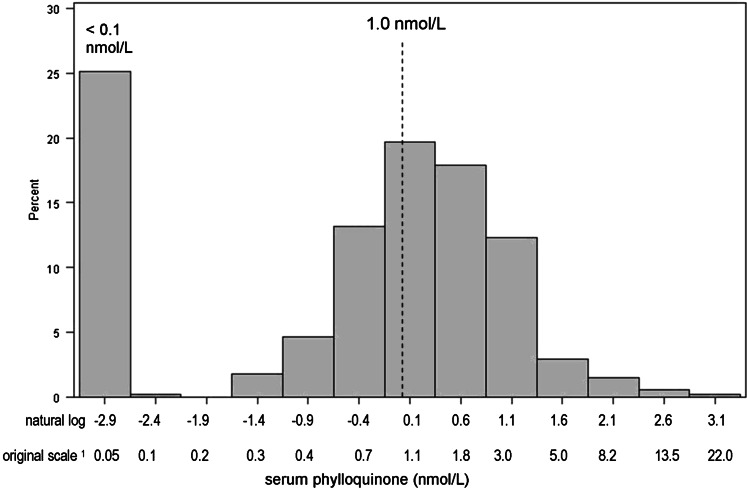
Characteristics of MESA participants included in this analysis are shown in Table 1. Participant mean ± SD age was 64 ± 10 y. Overall, 55% of subjects were men, and 42% of subjects were white. Participants identified with extreme CAC progression were older, more likely to be white, had more CAC and higher blood pressure at the baseline visit, and were more likely to be diabetic and take lipid-lowering and antihypertensive medications than were control subjects. However, the median (±IQR) serum vitamin K1 concentrations did not differ between cases (0.9 ± 1.7 nmol/L) and control subjects (1.1 ± 1.6 nmol/L) (P = 0.24). Subjects with extreme progression tended to have a lower ratio of serum vitamin K1 to triglycerides than that of controls at baseline, although the difference was not significant (median ± IQR: 0.6 ± 1.2 compared with 0.8 ± 1.2 nmol/mmol; P = 0.08). The mean follow-up time was 2.7 ± 0.7 y in extreme CAC progressors and 2.4 ± 0.8 y in controls (P < 0.001). Twenty-five percent of participants had serum vitamin K1 concentrations <0.1 nmol/L, and thus, the (ln) transformed distribution appeared bimodal (Figure 2). Characteristics of the randomly chosen subcohort are shown in Table 2 according to categories of serum vitamin K1. Subjects with higher serum vitamin K1 concentrations were more likely to be of Chinese descent, had lower BMI, higher triglycerides, were less likely to smoke, and participated in more intentional physical activity.
TABLE 1.
Characteristics of MESA participants with extreme CAC progression and control subjects1
| Extreme CAC progression (n = 296) | Control subjects (n = 561) | P2 | |
| Age (y)3 | 69 ± 9 | 61 ± 10 | <0.001 |
| Sex, F [n (%)] | 88 (30) | 306 (55) | <0.001 |
| Race-ethnicity [n (%)] | 0.02 | ||
| White | 146 (49) | 211 (38) | |
| African American | 64 (22) | 141 (25) | |
| Hispanic | 55 (19) | 131 (23) | |
| Chinese | 31 (10) | 78 (13) | |
| Education [n (%)] | 0.62 | ||
| Less than high school | 58 (20) | 95 (17) | |
| High school graduate | 128 (43) | 253 (45) | |
| College graduate | 110 (37) | 213 (38) | |
| Baseline CAC | |||
| AS (AU)4 | 764 ± 968 | 0 ± 62 | <0.0015 |
| AS category [n (%)] | <0.001 | ||
| 0 AU | 0 (0) | 319 (57) | |
| 1–400 AU | 64 (22) | 211 (38) | |
| >400 AU | 232 (78) | 31 (6) | |
| Yearly CAC increase (AS)4 | 181 ± 140 | 0 ± 12 | <0.0015 |
| BMI (kg/m2)3 | 28.7 ± 5.1 | 28.3 ± 5.4 | 0.33 |
| Triglycerides (mg/dL)4 | 125 ± 88 | 110 ± 82 | 0.05 |
| Total cholesterol (mg/dL)3 | 189 ± 38 | 193 ± 33 | 0.11 |
| Systolic blood pressure (mm Hg)3 | 135 ± 22 | 125 ± 21 | <0.001 |
| Diastolic blood pressure (mm Hg)3 | 74 ± 10 | 72 ± 10 | 0.01 |
| Type 2 diabetes [n (%)] | 84 (28) | 63 (11) | <0.001 |
| Impaired kidney function (GFR <60 mL/min) [n (%)] | 56 (19) | 64 (11) | 0.003 |
| Lipid-lowering medication [n (%)] | 86 (29) | 94 (17) | <0.001 |
| Hypertension [n (%)]6 | 199 (67) | 237 (42) | <0.001 |
| Antihypertension medication [n (%)] | 173 (58) | 196 (35) | <0.001 |
| In antihypertension medication users7 | |||
| Thiazide diuretics | 54 (31) | 61 (31) | 0.98 |
| ACE inhibitors | 76 (44) | 68 (35) | 0.07 |
| β-blockers | 45 (26) | 54 (28) | 0.66 |
| Other | 53 (31) | 56 (29) | 0.63 |
| Current smoker [n (%)] | 34 (11) | 71 (13) | 0.62 |
| Intentional physical activity (MET-min/wk)48 | 19.1 ± 31.5 | 13.8 ± 31.3 | <0.001 |
| Serum vitamin K14 | 0.9 ± 1.7 | 1.1 ± 1.6 | 0.245 |
| Vitamin K1 intake (μg/d)4 | 87 ± 95 | 97 ± 107 | 0.33 |
| Energy adjusted (μg/d)9 | 93 ± 4 | 98 ± 2 | 0.20 |
ACE, angiotensin-converting enzyme; AS, Agatston score; AU, Agatston units; CAC, coronary artery calcium; GFR, glomerular filtration rate; MESA, Multi-Ethnic Study of Atherosclerosis; MET-min, metabolic equivalent task minutes.
Categorical outcomes were compared by using the chi-square test; continuous outcomes were compared by using Student's t test unless indicated otherwise.
Values are means ± SDs.
Values are medians ± IQRs.
Comparison was based on Wilcoxon's rank-sum test because the measure did not achieve a normal distribution after ln transformation.
Defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or use of an antihypertension medication.
In antihypertension medication users, the total percentage is >100% because some participants reported taking more than one type of medication.
Total intentional physical activity per week (sum of walking for exercise, sports, dancing, and conditioning).
Values are geometric least-squares means ± SEMs.
FIGURE 2.
Distribution of serum vitamin K1 in the MESA (n = 857). 1Midpoints of the ln scale were back-transformed to obtain the original scale. MESA, Multi-Ethnic Study of Atherosclerosis.
TABLE 2.
Characteristics of MESA participants in a randomly chosen subcohort (n = 592) according to serum vitamin K1 concentrations1
| <1.0 nmol/L (n = 282) | ≥1.0 nmol/L (n = 310) | P | |
| Age (y)2 | 61 ± 1 | 62 ± 1 | 0.40 |
| Sex, F [n (%)] | 153 (54) | 161 (52) | 0.75 |
| Race-ethnicity [n (%)] | <0.001 | ||
| White | 118 (42) | 109 (35) | |
| African American | 75 (27) | 76 (25) | |
| Hispanic | 80 (29) | 54 (19) | |
| Chinese | 9 (3) | 71 (23) | |
| Education [n (%)] | 0.09 | ||
| Less than high school | 53 (19) | 45 (46) | |
| High school graduate | 126 (45) | 146 (47) | |
| College graduate | 103 (37) | 119 (39) | |
| Baseline AS category [n (%)] | 0.41 | ||
| AS = 0 AU | 88 (60) | 158 (51) | |
| AS = 1–400 AU | 93 (30) | 121 (39) | |
| AS >400 AU | 28 (10) | 31 (10) | |
| BMI (kg/m2)2 | 29.0 ± 0.3 | 27.7 ± 0.3 | 0.003 |
| Triglycerides (mg/dL)3 | 99 ± 4 | 125 ± 4 | <0.001 |
| Total cholesterol (mg/dL)2 | 191 ± 2 | 194 ± 2 | 0.25 |
| Systolic blood pressure (mm Hg)2 | 124 ± 1 | 127 ± 1 | 0.15 |
| Diastolic blood pressure (mm Hg)2 | 71 ± 1 | 73 ± 1 | 0.03 |
| Diabetes [n (%)] | 22 (15) | 37 (12) | 0.23 |
| Lipid-lowering medication [n (%)] | 16 (11) | 62 (20) | 0.11 |
| Impaired kidney function (GFR <60 mL/min) [n (%)] | 39 (14) | 35 (11) | 0.35 |
| Antihypertension medication use [n (%)] | 90 (32) | 121 (39) | 0.07 |
| Among antihypertension medication users [n (%)]4 | |||
| Thiazide diuretics | 36 (40) | 31 (26) | 0.03 |
| ACE inhibitors | 36 (40) | 38 (31) | 0.20 |
| β-blockers | 18 (20) | 38 (31) | 0.07 |
| Other | 25 (27) | 36 (30) | 0.75 |
| Current smoker [n (%)] | 40 (14) | 33 (11) | 0.04 |
| Intentional physical activity (MET-min/wk)3 | 9 ± 1 | 13 ± 1 | 0.002 |
| Vitamin K1 intake (μg/d) (energy adjusted)3 | 82 ± 5 | 114 ± 5 | <0.001 |
| Season [n (%)] | 0.16 | ||
| January to March | 81 (37) | 82 (26) | |
| April to June | 77 (27) | 111 (36) | |
| July to September | 59 (21) | 60 (19) | |
| October to December | 65 (23) | 57 (18) |
Differences in continuous outcomes were analyzed by using general linear regression adjusted for triglycerides and lipid-lowering medication use (unless analyzed on that outcome). Differences in categorical outcomes were analyzed by using logistic regression adjusted for triglycerides and lipid-lowering medication use (unless analyzed on that outcome). P values are for the overall Wald's chi-square test. ACE, angiotensin-converting enzyme; AS, Agatston score; AU, Agatston units; GFR, glomerular filtration rate; MESA, Multi-Ethnic Study of Atherosclerosis; MET-min, metabolic equivalent task minutes.
Values are least-squares means ± SEMs adjusted for triglycerides and lipid medication use.
Values are least-squares geometric means ± SEMs adjusted for triglycerides and lipid medication use.
In antihypertension medication users, the total percentage is >100% because some participants reported taking more than one type of medication.
Case-cohort results
Results of the primary analysis of the association between serum vitamin K1 and extreme CAC progression according to the case-cohort design are shown in Table 3. Unadjusted OR (95% CI) for extreme CAC progression in subjects with serum vitamin K1 concentrations <1.0 nmol/L compared with subjects with serum vitamin K1 concentrations ≥1.0 nmol/L was 1.16 (0.88, 1.53) (P = 0.29). In the fully adjusted model, the OR (95% CI) was 1.34 (0.94, 1.90) (P = 0.11) for subjects with serum vitamin K1 concentrations <1.0 nmol/L compared with subjects with serum vitamin K1 concentrations ≥1.0 nmol/L. When we repeated our analyses to include the 72 participants with missing covariate data in models 1 and 2, associations between serum vitamin K1 and extreme CAC progression were not appreciably changed. In the 538 participants with CAC at baseline, the fully adjusted OR (95% CI) for extreme CAC progression for subjects with serum vitamin K1 concentrations <1.0 nmol/L was 1.27 (0.86, 1.86; NS).
TABLE 3.
Association between low serum vitamin K1 and CAC progression in MESA participants (n = 857)1
| Subjects with serum vitamin K1 concentrations <1.0 nmol/L compared with subjects with serum vitamin K1 concentrations ≥1.0 nmol/L | Estimated per 1.7-nmol/L (the IQR) decrease in serum vitamin K1 | |
| Unadjusted | 1.16 (0.88, 1.53) | 1.04 (0.90, 1.19) |
| Model 1 | 1.33 (0.99, 1.78) | 1.11 (0.96, 1.29) |
| Model 2 | 1.33 (0.94, 1.89) | 1.09 (0.90, 1.33) |
| Model 3 | 1.34 (0.94, 1.90) | 1.10 (0.90, 1.34) |
| In MESA participants with prevalent CAC at baseline (n = 538) | ||
| Unadjusted | 1.31 (0.95, 1.81) | 1.10 (0.95, 1.31) |
| Model 1 | 1.46 (1.04, 2.04) | 1.20 (1.00, 1.44) |
| Model 2 | 1.25 (0.85, 1.83) | 1.12 (0.90, 1.39) |
| Model 3 | 1.27 (0.86, 1.86) | 1.12 (0.89, 1.40) |
| In MESA participants taking antihypertension medication (n = 369)2 | ||
| Unadjusted | 1.71 (1.14, 2.55) | 1.11 (0.91, 1.35) |
| Model 1 | 1.88 (1.23, 2.86) | 1.17 (0.94, 1.44) |
| Model 2 | 2.06 (1.22, 3.47) | 1.12 (0.83, 1.50) |
| Model 3 | 2.37 (1.38, 4.09) | 1.13 (0.84, 1.54) |
| In MESA participants with prevalent CAC taking antihypertension medication (n = 282)3 | ||
| Unadjusted | 1.53 (0.97, 2.39) | 1.03 (0.84, 1.26) |
| Model 1 | 1.71 (1.06, 2.76) | 1.10 (0.84, 1.41) |
| Model 2 | 1.74 (0.99, 3.07) | 1.05 (0.77, 1.42) |
| Model 3 | 1.98 (1.10, 3.57) | 1.05 (0.77, 1.44) |
All values are ORs; 95% CIs in parentheses. Analyses were based on logistic regression by using a robust variance estimation that accounted for the case-cohort design (32) adjusted for follow-up time and as follows: model 1 was adjusted for triglycerides and lipid-lowering medication use; model 2 was adjusted as for model 1 and for age, race-ethnicity, sex, BMI, systolic blood pressure, hypertension medication use (unless stratified on that variable), diabetes, kidney function (glomerular filtration rate <60 mL/min), total cholesterol, study site, and education; and model 3 was adjusted as for model 2 and for energy-adjusted vitamin K1 intake, physical activity, and season. CAC, coronary artery calcium; MESA, Multi-Ethnic Study of Atherosclerosis.
Stratified analysis was based on P-interaction between a low serum vitamin K1 concentration (defined as <1.0 nmol/L) and hypertension (defined as individuals taking antihypertensive medication in all participants) = 0.016
Stratified analysis was based on P-interaction between low serum vitamin K1 concentration (defined as <1.0 nmol/L) and hypertension (defined as individuals taking antihypertensive medication in participants with prevalent CAC) = 0.10.
Other population-based studies showed vitamin K2 (menaquinone) intake, but not vitamin K1 intake, to be associated with vascular calcification (10, 11). Although dietary vitamin K2 intake data are not available in the MESA, we examined the association between vitamin K1 intake and CAC progression. The fully adjusted OR (95% CI) for having extreme CAC progression for subjects who reported consumption of less than the recommended amount of dietary vitamin K1 (n = 181) compared with subjects who reported consumption of adequate amounts of dietary vitamin K1 (n = 676) was 1.00 (0.69, 1.44; NS).
Interactions
In the case-cohort analysis, we detected a significant interaction between low serum vitamin K1 (defined as <1.0 nmol/L) and hypertension (defined as subjects taking an antihypertensive medication) (P-interaction = 0.016) such that the fully adjusted OR (95% CI) for extreme CAC progression for participants who were taking antihypertensive medication (n = 369) with serum vitamin K1 concentrations <1.0 nmol/L was 2.37 (1.38, 4.09) (Table 3), whereas for participants who were not taking an antihypertensive medication (n = 488) with serum vitamin K1 concentrations <1.0 nmol/L, the fully adjusted OR (95% CI) for extreme CAC progression was 0.79 (0.47, 1.30; NS). When the analysis was restricted to subjects with detectable CAC at baseline, the interaction between low serum vitamin K1 (defined as <1.0 nmol/L) and hypertension (defined as participants who were taking an antihypertensive medication) was P = 0.10. In subjects with prevalent CAC, the OR (95% CI) for extreme CAC progression in antihypertensive medication users (n = 282) with serum vitamin K1 concentrations <1.0 nmol/L was 1.98 (1.10, 3.57) (Table 3), and for subjects who were not taking an antihypertensive medication (n = 256) with serum vitamin K1 concentrations <1.0 nmol/L, the fully adjusted OR (95% CI) for extreme CAC progression was 1.21 (0.75, 1.96; NS). Because some antihypertension medications are associated with changes in serum calcium, we explored whether additional adjustment for serum calcium altered our results in the hypertensive participants. Although the magnitude of point estimates was somewhat attenuated, the significance was unchanged [the calcium-adjusted OR (95% CI) was 2.20 (1.26, 3.82) for all treated hypertensives and 1.86 (1.02, 3.38) for treated hypertensives with prevalent CAC]. The association between low serum vitamin K1 and extreme CAC progression did not differ by any demographic characteristic evaluated (sex P-interaction = 0.33; race-ethnicity P-interaction = 0.23; age P-interaction = 0.61) or according to the other comorbidities (type 2 diabetes P-interaction = 0.45; eGFR <60 mL/min P-interaction = 0.89; lipid-lowering medication use P-interaction = 0.35) (see Supplementary Figure 1 under “Supplemental data” in the online issue).
Subcohort results
In the 592 randomly selected participants, 79% of subjects with an AS increase ≥100 AU/y were considered to have extreme CAC progression. The median (range) CAC increase in the 37 participants with a CAC increase ≥100 AU/y was 139 AU/y (0–651 AU/y) compared with a median (range) CAC increase of 0 AU/y (0–93 AU/y) in the 555 individuals with an annual CAC increase <100 AU/y. The OR (95% CI) for having a CAC increase ≥100 AU/y in subjects with serum vitamin K1 concentrations <1.0 compared with ≥1.0 nmol/L was 1.62 (0.70, 3.76; NS), adjusted for triglycerides, lipid-lowering medication use, age, race-ethnicity, sex, BMI, systolic blood pressure, hypertension medication use, diabetes, kidney function (eGFR <60 mL/min), total cholesterol, study site, education, energy-adjusted vitamin K1 intake, physical activity, and season. With restriction of the analysis to the 273 subcohort participants with baseline CAC, the fully adjusted OR (95% CI) for having a CAC increase ≥ 100AU/y for subjects with serum vitamin K1 concentrations <1.0 nmol/L was 2.02 (0.84, 4.88; NS). In the 319 participants without detectable CAC at baseline, the fully adjusted OR (95% CI) for CAC development for subjects with serum vitamin K1 concentrations <1.0 nmol/L was 1.10 (0.55, 2.20; NS).
Replication study results
See Supplementary Table 1 under “Supplemental data” in the online issue for participant characteristics according to baseline antihypertension medication use. In the intent-to-treat analysis, the effect of vitamin K1 supplementation on CAC progression did not differ according to baseline antihypertension medication use (P-interaction = 0.24) (Table 4). In per-protocol analyses, when nonadherent participants were excluded, the interaction between baseline antihypertension medication use and vitamin K1 supplementation was significant (P = 0.05). In adherent participants who were taking antihypertension medication at baseline, the adjusted mean (±SEM) 3-y change in CAC in subjects who were randomly assigned to vitamin K1 supplementation (n = 40) was 6 ± 15 AU compared with 63 ± 13 AU for participants in the placebo group (n = 49) (between-group difference: P = 0.007) (Table 4). In adherent participants with prevalent CAC at baseline, the magnitude of the between-group difference in the 3-y change in CAC in participants who were taking an antihypertensive medication was higher (vitamin K1 supplementation: 5 ± 20 AU; placebo: 93 ± 19 AU; P = 0.004). In adherent participants who were not taking antihypertensive medications (n = 222), the 3-y change in CAC did not differ between the vitamin K1–supplemented group and placebo group in any analysis (all between-group difference: P ≥ 0.63) (Table 4).
TABLE 4.
Three-year change in AS according to vitamin K1 supplementation stratified by hypertension treatment status in community-dwelling adults1
| Antihypertensive medication use |
No antihypertensive medication use |
P-interaction between vitamin K1 supplementation and baseline use of HT treatment | P-main effect of vitamin K1 supplementation on CAC progression4 | |||||
| Vitamin K1 | Placebo | Between-group difference 23 | Vitamin K1 | Placebo | Between-group difference P value24 | |||
| All participants | ||||||||
| n | 50 | 63 | — | 150 | 125 | — | — | — |
| Baseline AS5 | 107 ± 284 | 66 ± 240 | 0.81 | 11 ± 137 | 26 ± 139 | 0.63 | — | — |
| Unadjusted6 | 23 ± 14 | 45 ± 12 | 0.24 | 28 ± 7 | 32 ± 7 | 0.65 | 0.36 | 0.26 |
| Model 16 | 25 ± 13 | 44 ± 12 | 0.28 | 29 ± 6 | 34 ± 7 | 0.80 | 0.39 | 0.36 |
| Model 26 | 18 ± 13 | 49 ± 12 | 0.09 | 28 ± 6 | 33 ± 7 | 0.57 | 0.24 | 0.34 |
| All participants with baseline AS >10 | ||||||||
| n | 34 | 43 | — | 77 | 69 | — | — | — |
| Baseline AS5 | 154 ± 388 | 185 ± 400 | 0.67 | 116 ± 273 | 116 ± 272 | 0.88 | — | — |
| Unadjusted6 | 33 ± 20 | 65 ± 18 | 0.22 | 48 ± 12 | 56 ± 13 | 0.64 | 0.43 | 0.09 |
| Model 16 | 34 ± 19 | 64 ± 17 | 0.26 | 48 ± 11 | 55 ± 12 | 0.69 | 0.47 | 0.12 |
| Model 26 | 22 ± 20 | 74 ± 17 | 0.06 | 45 ± 23 | 58 ± 12 | 0.46 | 0.24 | 0.12 |
| Adherent participants | ||||||||
| n | 40 | 49 | — | 122 | 100 | — | — | — |
| Baseline AS5 | 111 ± 239 | 66 ± 230 | 0.63 | 11 ± 167 | 42 ± 189 | 0.33 | — | — |
| Unadjusted6 | 15 ± 15 | 58 ± 14 | 0.04 | 27 ± 7 | 31 ± 8 | 0.75 | 0.15 | 0.03 |
| Model 16 | 17 ± 14 | 54 ± 13 | 0.06 | 29 ± 7 | 29 ± 8 | 0.93 | 0.09 | 0.05 |
| Model 26 | 6 ± 15 | 63 ± 13 | 0.007 | 28 ± 7 | 31 ± 8 | 0.77 | 0.05 | 0.04 |
| Adherent participants with baseline AS >10 | ||||||||
| n | 29 | 33 | — | 63 | 59 | — | — | — |
| Baseline AS5 | 181 ± 40 | 154 ± 362 | 0.97 | 137 ± 290 | 139 ± 303 | 0.77 | — | — |
| Unadjusted6 | 18 ± 20 | 81 ± 19 | 0.03 | 47 ± 13 | 49 ± 13 | 0.86 | 0.08 | 0.03 |
| Model 16 | 22 ± 20 | 78 ± 18 | 0.04 | 49 ± 13 | 47 ± 13 | 0.91 | 0.11 | 0.03 |
| Model 26 | 5 ± 20 | 93 ± 19 | 0.004 | 44 ± 13 | 52 ± 14 | 0.63 | 0.08 | 0.02 |
AS, Agatston score; CAC, coronary artery calcium; HT, hypertension.
Baseline AS was compared by using the Wilcoxon's rank-sum test.
Change in CAC was compared by using general linear regression adjusted as follows: model 1 was adjusted for baseline AS, and model 2 was adjusted as for model 1 and for age, sex, BMI, statin use, triglycerides, cholesterol, diabetes, physical activity, and smoking.
Main effect of vitamin K1 supplementation on CAC progression has been published previously (17).
Values are medians ± IQRs.
Values are least-squares means ± SEMs.
DISCUSSION
We hypothesized that vitamin K status would be associated with CAC because 1) animal studies showed that vitamin K antagonism with warfarin (which interrupts the vitamin K–dependent carboxylation of MGP) led to extreme arterial calcification in rats (39), and existing calcification was reversed by diets high in vitamin K (9), and 2) in vitro experiments showed that MGP synthesized by vascular smooth-muscle cells in the absence of vitamin K remained undercarboxylated and had a reduced capacity to function as a calcification inhibitor (7). The mechanistic evidence and the outcomes of an earlier randomized trial (17) suggested that vitamin K may be more relevant to CAC progression than to plaque development. Because the association between vitamin K status and CAC progression has not been well examined in population-based studies, the primary goal of this case-comparison study was to test whether serum vitamin K1 was associated with CAC progression in the MESA. Although low serum vitamin K1 was associated with 34% higher odds of extreme CAC progression, our primary result was not significant.
Hypertension (defined as the use of an antihypertensive medication) was identified as a risk factor for CAC progression in MESA (5). In our study, hypertension treatment modified the association between serum vitamin K1 and extreme CAC progression, such that participants who were taking antihypertensive medication were more than twice as likely to have extreme CAC progression if they had a serum vitamin K1 concentration <1.0 nmol/L, whereas serum vitamin K1 was not associated with CAC progression in subject who were not taking antihypertensive medication. Because this observation was not motivated by an a priori hypothesis, we sought to replicate it by using data from a randomized trial of vitamin K1 supplementation and showed, in a per protocol analysis, that vitamin K1 supplementation reduced CAC progression in participants who were taking antihypertension but not in participants who were not taking antihypertension medication. The results of this post hoc, secondary analysis reflected those of the primary analysis (17) in that the effect of vitamin K1 supplementation was significant when nonadherent participants were excluded. Although the percentage of adherent participants did not differ between treatment groups, adherence may have been influenced by unmeasured factors, which may have introduced some bias to these results. When hypertension was defined according to Joint National Committee criteria [systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or the use of antihypertensive medication (40)], the interaction between low serum vitamin K1 and hypertension in the MESA was attenuated. It is plausible that hypertension defined by using blood pressure measurements taken on a single day may have misclassified some participants so that treated participants represented true hypertension (41). Conversely, the hypertension treatment may, itself, have influenced the association between vitamin K status and CAC. The most common antihypertensive medications in both studies were angiotensin-converting enzyme (ACE) inhibitors and thiazide diuretics. In the MESA, 39% of treated hypertensives were taking ACE inhibitors, and 32% of treated hypertensives were taking thiazide diuretics. In the supplementation study, 45% of treated hypertensives were taking ACE inhibitors, and 48% of treated hypertensives were taking thiazide diuretics. Thiazide diuretics promote a positive calcium balance, which was shown to upregulate MGP expression in vascular smooth muscle in rodent studies (42). In recent in vivo and ex vivo experiments, angiotensin II–receptor antagonists reversed the reduction in MGP expression in vascular tissue and exacerbated calcification caused by angiotensin II (43). It is possible that medications that interfere with angiotensin II activity may lead to increased MGP expression. In either case, without sufficient vitamin K, the increased MGP would not be carboxylated and, therefore, would not be able to inhibit calcification, which suggested that vitamin K may complement hypertension medications with respect to vascular calcification. Because we were insufficiently powered to stratify our analyses according to hypertension medication class, it will be important to clarify if the association between vitamin K and CAC progression differs in patients treated with different types of hypertension medications.
Consistent with our finding, Dutch observational studies, in which vitamin K1 intakes were 2-fold more than what is currently recommended in the United States, did not find vitamin K1 intake to be associated with arterial calcification (10, 11). However, there is more than one form of vitamin K. Vitamin K1, which is present in green leafy vegetables and vegetable oils, is the primary dietary form in US diets. In the MESA, the OR (95% CI) for extreme CAC progression did not differ between participants who reported the consumption of recommended amounts of vitamin K1 and subjects who reported the consumption of less than recommended amounts of vitamin K1. Vitamin K2 is a class of compounds present in limited amounts in meat, certain dairy products, and fermented soybean products. Food-composition data for vitamin K2 are incomplete in most nutrient databases, and the FFQ used in the MESA did not estimate vitamin K2 intake. This lack may have misclassified some individuals with respect to the overall vitamin K intake in MESA. Vitamin K2, which is not thought to be an important dietary contributor to vitamin K intake in the United States, is generally undetectable in circulation (44). The Dutch studies showed that higher vitamin K2 intakes, which were driven by fermented cheeses, were associated with less calcification (10, 11). Although it is plausible vitamin K2 is more relevant to CAC, it is also plausible that other food compounds in these cheeses were associated with CVD, for which the vitamin K2 intake was a marker.
This study had several strengths and certain limitations to consider. The case-cohort design was a derivation of the case-control study, but because the serum vitamin K1 was obtained before case-status identification, the temporal relation between exposure and outcome was known (19). Our subcohort was selected at random, and cases were ascertained over time on the basis of the rate of CAC progression. All of our cases had prevalent CAC at baseline (evidence of subclinical CVD). We also subsequently limited our control group to include individuals with subclinical CVD as well. The primary analysis was powered a priori to detect an OR of 1.45, and thus, we had little chance of detecting an effect smaller than that. With consideration of the number of hypotheses tested, we could not rule out chance as an explanation for our results. When serum vitamin K1 was considered as a continuously measured exposure, its association with CAC progression was attenuated compared with when it was dichotomized. It is plausible that there is a threshold effect for circulating vitamin K1 with respect to CAC, as has been reported for other nutrients and disease outcomes (45). However, when we added a quadratic term of serum vitamin K1 to our models, it was not statistically significant (P ≥ 0.16). We showed that the association between serum vitamin K1 and CAC progression differed between participants who reported taking antihypertensive medication and participants who did not, which was a finding that we were able to replicate with qualification in an independent sample. To the best of our knowledge, no other study has determined the association between vitamin K status and CAC progression by using objectively measured biomarkers instead of self-reported intakes, which are prone to multiple biases (46, 47). However, no single vitamin K biomarker is considered a gold-standard measure of status. We measured circulating vitamin K1 because it is indicative of the overall status, and that measure was shown to correspond to vitamin K nutritional intake in an earlier population-based study (16). Other functional biomarkers exist, which include measuring the circulating concentrations of undercarboxylated vitamin K–dependent proteins, such as MGP (48). It will be important to explore additional measures of vitamin K status in future studies to obtain additional information on how vitamin K may influence the calcification process. Warfarin is commonly used to treat some types of heart disease, some of which may be related to calcification (49). Because warfarin is a vitamin K antagonist, warfarin users were not eligible for inclusion in the current study, and our results are not applicable to patients taking warfarin. Because of the low prevalence of warfarin use, we are unable to comment on whether warfarin, through a reduction in vitamin K activity, would relate to CAC progression on its own. Although CAC progression has been associated with increased risk of clinical cardiac events and mortality (3, 4), the relevance of low serum vitamin K1 to clinical CVD remains to be determined.
In conclusion, although the point estimate of our primary analysis suggests a serum vitamin K1 concentration <1.0 nmol/L could be associated with greater CAC progression, it was NS. We unexpectedly showed that hypertensives (defined according to medication use) were significantly more likely to have extreme CAC progression if they had low serum vitamin K1 than were persons who were not taking antihypertension medication. Because 20% of adults in the Untied States are treated for hypertension (50), and we estimated 50% of US adults have low vitamin K status (on the basis of a serum vitamin K1 concentration <1.0 nmol/L), future mechanistic and clinical studies are needed to understand why vitamin K appears to be more relevant to CAC progression in persons being treated for hypertension and to determine whether vitamin K's role in CAC progression differs according to specific classes of hypertension medications.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—MKS and SBK: designed the study, analyzed data, and drafted the manuscript; MEM and HC: contributed to the statistical analyses and writing of the manuscript; SLB, GLB, MC, and RPT: contributed to the design of the analyses, interpretation of data, and writing of the manuscript; SLB: was responsible for laboratory analyses; and all authors: read and approved the final version of the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: ACE, angiotensin-converting enzyme; AS, Agatston score; AU, Agatston units; CAC, coronary artery calcium; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FFQ, food-frequency questionnaire; MESA, Multi-Ethnic Study of Atherosclerosis; MGP, matrix gla protein.
REFERENCES
- 1.Ostrom MP, Gopal A, Ahmadi N, Nasir K, Yang E, Kakadiaris I, Flores F, Mao SS, Budoff MJ. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol 2008;52:1335–43. [DOI] [PubMed] [Google Scholar]
- 2.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology 2003;228:826–33. [DOI] [PubMed] [Google Scholar]
- 3.Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010;3:1229–36. [DOI] [PubMed] [Google Scholar]
- 4.Zeb I, Budoff MJ. MESA: the NIH-sponsored study that validates atherosclerosis imaging for primary prevention. Curr Atheroscler Rep 2011;13:353–8. [DOI] [PubMed] [Google Scholar]
- 5.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2007;115:2722–30. [DOI] [PubMed] [Google Scholar]
- 6.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997;386:78–81. [DOI] [PubMed] [Google Scholar]
- 7.Schurgers LJ, Spronk HM, Skepper JN, Hackeng TM, Shanahan CM, Vermeer C, Weissberg PL, Proudfoot D. Post-translational modifications regulate matrix Gla protein function: importance for inhibition of vascular smooth muscle cell calcification. J Thromb Haemost 2007;5:2503–11. [DOI] [PubMed] [Google Scholar]
- 8.Viegas CS, Cavaco S, Neves PL, Ferreira A, Joao A, Williamson MK, Price PA, Cancela ML, Simes DC. Gla-rich protein is a novel vitamin K-dependent protein present in serum that accumulates at sites of pathological calcifications. Am J Pathol 2009;175:2288. –98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schurgers LJ, Spronk HM, Soute BA, Schiffers PM, DeMey JG, Vermeer C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood 2007;109:2823–31. [DOI] [PubMed] [Google Scholar]
- 10.Beulens JW, Bots ML, Atsma F, Bartelink ML, Prokop M, Geleijnse JM, Witteman JC, Grobbee DE, van der Schouw YT. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis 2009;203:489. –93. [DOI] [PubMed] [Google Scholar]
- 11.Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr 2004;134:3100–5. [DOI] [PubMed] [Google Scholar]
- 12.Villines TC, Hatzigeorgiou C, Feuerstein IM, O'Malley PG, Taylor AJ. Vitamin K1 intake and coronary calcification. Coron Artery Dis 2005;16:199–203. [DOI] [PubMed] [Google Scholar]
- 13.Thompson FE, Byers T. Dietary assessment resource manual. J Nutr 1994;124:2245S–317S. [DOI] [PubMed] [Google Scholar]
- 14.Miller TM, Abdel-Maksoud MF, Crane LA, Marcus AC, Byers TE. Effects of social approval bias on self-reported fruit and vegetable consumption: a randomized controlled trial. Nutr J 2008;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prentice RL, Sugar E, Wang CY, Neuhouser M, Patterson R. Research strategies and the use of nutrient biomarkers in studies of diet and chronic disease. Public Health Nutr 2002;5:977–84. [DOI] [PubMed] [Google Scholar]
- 16.McKeown NM, Jacques PF, Gundberg CM, Peterson JW, Tucker KL, Kiel DP, Wilson PW, Booth SL. Dietary and nondietary determinants of vitamin K biochemical measures in men and women. J Nutr 2002;132:1329–34. [DOI] [PubMed] [Google Scholar]
- 17.Shea MK, O'Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SL. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr 2009;89:1799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 19.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 1986;73:1–11. [Google Scholar]
- 20.Carr JJ, Nelson JC, Wong ND, Nitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 22.Davidson KW, Sadowski JA. Determination of vitamin K compounds in plasma or serum by high-performance liquid chromatography using postcolumn chemical reduction and fluorimetric detection. Methods Enzymol 1997;282:408–21. [DOI] [PubMed] [Google Scholar]
- 23.Card DJ, Shearer MJ, Schurgers LJ, Harrington DJ. The external quality assurance of phylloquinone (vitamin K(1)) analysis in human serum. Biomed Chromatogr 2009;23:1276–82. [DOI] [PubMed] [Google Scholar]
- 24.Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson-Hughes B, Gundberg CM, Cupples LA, Wilson PW, Kiel DP. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab 2004;89:4904–9. [DOI] [PubMed] [Google Scholar]
- 25.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2005;111:1313–20. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van LF, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–47. [DOI] [PubMed] [Google Scholar]
- 28.Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2009;169:444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol 1999;9:314–24. [DOI] [PubMed] [Google Scholar]
- 30.Nettleton JA, Rock CL, Wang Y, Jenny NS, Jacobs DR. Associations between dietary macronutrient intake and plasma lipids demonstrate criterion performance of the Multi-Ethnic Study of Atherosclerosis (MESA) food-frequency questionnaire. Br J Nutr 2009;102:1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKeown NM, Rasmussen HM, Charnley JM, Wood RJ, Booth SL. Accuracy of phylloquinone (vitamin K-1) data in 2 nutrient databases as determined by direct laboratory analysis of diets. J Am Diet Assoc 2000;100:1201–4. [DOI] [PubMed] [Google Scholar]
- 32.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol 1999;52:1165–72. [DOI] [PubMed] [Google Scholar]
- 33.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press, 2001. [PubMed] [Google Scholar]
- 34.Booth SL, O'Brien-Morse ME, Dallal GE, Davidson KW, Gundberg CM. Response of vitamin K status to different intakes and sources of phylloquinone-rich foods: comparison of younger and older adults. Am J Clin Nutr 1999;70:368–77. [DOI] [PubMed] [Google Scholar]
- 35.Neogi T, Booth SL, Zhang YQ, Jacques PF, Terkeltaub R, Aliabadi P, Felson DT. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum 2006;54:1255–61. [DOI] [PubMed] [Google Scholar]
- 36.Lamon-Fava S, Sadowski JA, Davidson KW, O'Brien ME, McNamara JR, Schaefer EJ. Plasma lipoproteins as carriers of phylloquinone (vitamin K1) in humans. Am J Clin Nutr 1998;67:1226–31. [DOI] [PubMed] [Google Scholar]
- 37.Braam L, McKeown N, Jacques P, Lichtenstein A, Vermeer C, Wilson P, Booth S. Dietary phylloquinone intake as a potential marker for a heart-healthy dietary pattern in the Framingham Offspring cohort. J Am Diet Assoc 2004;104:1410–4. [DOI] [PubMed] [Google Scholar]
- 38.Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, Dawson-Hughes B. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab 2008;93:1217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol 1998;18:1400–7. [DOI] [PubMed] [Google Scholar]
- 40.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- 41.Perry HM, Jr, Miller JP. Difficulties in diagnosing hypertension: implications and alternatives. J Hypertens 1992;10:887–96. [PubMed] [Google Scholar]
- 42.Mendoza FJ, Martinez-Moreno J, Almaden Y, Rodriguez-Ortiz ME, Lopez I, Estepa JC, Henley C, Rodriguez M, Aguilera-Tejero E. Effect of calcium and the calcimimetic AMG 641 on matrix-Gla protein in vascular smooth muscle cells. Calcif Tissue Int 2011;88:169–78. [DOI] [PubMed] [Google Scholar]
- 43.Jia G, Stormont RM, Gangahar DM, Agrawal DK. Role of matrix Gla protein in angiotensin II-induced exacerbation of vascular stiffness. Am J Physiol Heart Circ Physiol 2012;303:H523. –32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Booth SL, Suttie JW. Dietary intake and adequacy of vitamin K. J Nutr 1998;128:785–8. [DOI] [PubMed] [Google Scholar]
- 45.Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol 2008;624:55–71. [DOI] [PubMed] [Google Scholar]
- 46.Jenab M, Slimani N, Bictash M, Ferrari P, Bingham SA. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet 2009;125:507–25. [DOI] [PubMed] [Google Scholar]
- 47.Potischman N. Biologic and methodologic issues for nutritional biomarkers. J Nutr 2003;133(suppl 3):875S–80S. [DOI] [PubMed] [Google Scholar]
- 48.Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewe RB, Brandenburg VM, Bekers O, Vermeer C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost 2010;104:811. –22.; [DOI] [PubMed] [Google Scholar]
- 49.Torn M, Cannegieter SC, Bollen WL, van der Meer FJ, van der Wall EE, Rosendaal FR. Optimal level of oral anticoagulant therapy for the prevention of arterial thrombosis in patients with mechanical heart valve prostheses, atrial fibrillation, or myocardial infarction: a prospective study of 4202 patients. Arch Intern Med 2009;169:1203–9. [DOI] [PubMed] [Google Scholar]
- 50.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 2010;303:2043–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.