Abstract
Background: Body mass index (BMI) and percentage body fat (%BF) are widely used to assess adiposity. These indexes fail to account for independent contributions of fat mass (FM) and lean body mass (LBM) to body weight, which vary according to age, sex, pubertal status, and population ancestry in the pediatric population.
Objective: The objective was to develop pediatric reference curves for fat mass index (FMI) and lean body mass index (LBMI) and evaluate the effects of population ancestry and LBM on measures of excess adiposity (BMI, %BF, and FMI).
Design: Sex-specific FMI and LBMI reference curves relative to age for children and adolescents aged 8–20 y were generated from cross-sectional body-composition data measured by dual-energy X-ray absorptiometry from NHANES.
Results: The mean LBMI z score was higher in blacks (males: 0.26; females: 0.45) than in whites (males: −0.07; females: −0.09) and Mexican Americans (males: 0.05; females: −0.09). The positive predictive value of overweight by BMI to identify excess adiposity defined by FMI was lower in blacks (males: 35.9%; females: 30.3%) than in whites (males: 65.4%; females: 52.2%) and Mexican Americans (males: 73.3%; females: 68.3%). Participants classified as having excess adiposity by FMI but normal adiposity by %BF had significantly higher BMI, LBMI, and height z scores than did those classified as having excess adiposity by %BF but normal adiposity by FMI.
Conclusions: Relative to FMI, the prevalence of excess adiposity is overestimated by BMI in blacks and underestimated by %BF in individuals with high LBM. The use of FMI and LBMI improves on the use of %BF and BMI by allowing for the independent assessment of FM and LBM.
See corresponding editorial on page 1.
INTRODUCTION
The metabolic and cardiovascular complications of obesity are often severe and lifelong. Early identification of at-risk individuals is essential for the successful prevention and treatment of obesity-related diseases (1). BMI [calculated as body mass (kg)/height (m)2] is widely used to identify individuals with excess adiposity (2). Children with a BMI between the 85th and 95th percentiles are defined as overweight and those with BMI ≥95th percentile as obese (3). BMI is easily measured; however, it is limited by its failure to distinguish between fat mass (FM)5 and lean body mass (LBM).
The use of BMI as a surrogate of adiposity is especially problematic in the pediatric population, because the relative contributions of FM and LBM to body weight vary by age, sex, pubertal status, and population ancestry. Annual increases in BMI from midchildhood onward are largely because of increases in LBM rather than to increases in FM (4, 5), and differences in BMI percentiles indicate differences in FM only for high percentiles of BMI (6). Body composition differs by population ancestry as well, because blacks have a higher LBM than do whites (7–10). The failure of BMI to account for the independent contributions of FM and LBM may lead to misclassification of adiposity status when applied to individuals (11).
Studies in pediatric populations have used percentage body fat (%BF) to illustrate deficiencies in BMI as a surrogate of adiposity across population ancestry groups (12–14). However, the use of %BF as the gold standard of adiposity is an incomplete solution that does not consider height, body proportions, and LBM (15). Van Itallie et al (16) proposed the use of compartment-specific indexes normalized to height [FM index (FMI) and fat-free mass (FFM) index (FFMI)] as superior measures of nutritional status after illustrating the inadequacy of BMI, absolute FM, and %BF in a study comparing measures of body composition in healthy men with men undergoing experimental semistarvation. Recently, reference data were published for FMI and FFMI in British children by using the 4-compartment model (17); however, there are currently no reference data for FMI and LBM index (LBMI) in US children. LBM excludes the contribution of bone mass to FFM and is therefore a preferred measure because it is more tissue specific. Recently published data for lean mass/height2 in children (18) include bone mineral content and therefore represent FFM/height2 (TL Kelly, personal communication, 2012).
The goals of this study were to develop sex-specific reference data for FMI and LBMI relative to age in children and adolescents aged 8–20 y by using dual-energy X-ray absorptiometry (DXA) data from NHANES and to describe relations between FMI, LBMI, %BF, and BMI. We hypothesized that 1) blacks would have a higher LBM than would nonblacks and 2) FMI would identify individuals with excess adiposity yet normal %BF because of the independent effects of LBM and FM.
SUBJECTS AND METHODS
Study sample
Cross-sectional whole-body DXA data on children and adolescents from 1999 to 2004 NHANES were used. NHANES is an annual survey conducted by the National Center for Health Statistics (NCHS) that uses a complex, multistage probability sampling method including oversampling of non-Hispanic blacks, Mexican Americans, low-income whites, and adolescents aged 12–19 y to produce reliable statistics (19). The survey included a household interview and a detailed examination obtained in mobile examination centers. Approval for NHANES 1999–2004 was obtained from the NCHS Institutional Review Board (IRB); a waiver of IRB oversight for the use of this existing, de-identified, and publically available data were obtained from the IRB at The Children's Hospital of Philadelphia.
DXA and anthropometric measurements
Whole-body DXA scans were obtained by using a Hologic QDR 4500A fan beam densitometer (Hologic Inc) in eligible participants aged ≥8 y. All DXA scans were reviewed and analyzed by the University of California, San Francisco, Radiology department by using Hologic Discovery Software, version 12.1 (Hologic Inc). Females were excluded from the DXA evaluation if they had a positive pregnancy test at the time of the examination or if they stated that they were pregnant. DXA scans were not obtained in females aged 8–17 y in 1999 because NCHS IRB approval had not yet been obtained. Multiple imputation of missing data was performed by the NCHS to address the potential biases of nonrandom missing DXA data. Full details of the methods and rationale for multiple imputation are described in the NHANES DXA technical documentation files (19). The sample used to generate FMI and LBMI reference curves contained imputed data for 10% of males and 13.5% of females.
Age was calculated in months as reported at the time of examination. US Census Bureau classifications for race and ethnicity were ascertained by participant self-report at the time of the interview. Height (cm) and weight (kg) were obtained by using standard procedures (20) and were used to calculate BMI (kg/m2). Sex-specific BMI z scores for age were calculated by using the 2000 CDC reference data (21). FMI and LBMI were calculated from DXA-measured body-composition data as fat or LBM [(kg)/height (m)2]. LBM excluded bone mineral content. Whole-body %BF was calculated as total-body FM (kg)/total body mass (kg) × 100. A prior multicenter analysis of DXA body-composition data showed an overestimation of LBM and underestimation of FM by Hologic QDR 4500A fan-beam densitometers (22). Accordingly, NHANES DXA body-composition data for FM and LBM were adjusted by the NCHS such that LBM was decreased by 5% and FM increased by an equivalent amount (in kg) to maintain total body mass (23).
Generation of FMI and LBMI reference curves
We used data from 8961 (3766 female) participants aged 8–25 y to generate reference curves for FMI and LBMI. Our sample included fewer females than males because the publically available NHANES DXA body-composition data in the 1999–2000 release cycle do not include data for females aged 8–17 y (23). The 21–25 y age range was included to eliminate a truncation effect on the reference curves that was observed when only data for participants 8–20 y of age were used. Curves were generated respective to age by using the lambda-mu-sigma (LMS) method (LMSchartmaker Pro version 2.54; Cole and Pan 2011) separately for males and females (24). The LMS method is widely used for the generation of reference percentiles because it addresses the heteroscedasticity and skewness frequently present in growth data. The optimal power to obtain normality is summarized by a smooth line (L). Trends in the mean (M) and CV (S) are similarly smoothed. The resulting L, M, and S curves contain the information to generate any centile curve and to convert measurements (even extreme values) into exact z scores by using the following equation:
where X is the body-composition measure of interest. Overall goodness-of-fit of models were assessed by using visual inspection and evaluation of the Q statistic—a plot of standardized residuals compared with df used to fit the curve (25).
Evaluation of FMI compared with BMI and %BF
We compared FMI with BMI and %BF in 7095 (2890 female) participants aged 8–19 y; 28 participants (13 females) with DXA body-composition data were excluded from analyses because of missing data for weight (and therefore BMI). This age range was selected because it allowed for the characterization of %BF by using published reference data for %BF derived from NHANES (26). Analyses were limited to non-Hispanic whites, non-Hispanic blacks, and Mexican Americans because there were too few participants of other population ancestry groups to allow for reliable estimates.
BMI status for participants was determined by using the 2000 CDC BMI growth charts and was categorized as normal weight (BMI <85th percentile), overweight (85th to 95th percentile), or obese (BMI ≥95th percentile) per current expert recommendations (3). There is no universally accepted gold standard for the definition of excess adiposity using DXA body-composition data in children and adolescents. The 75th–85th percentile for %BF has previously been shown to correspond with excess adiposity in children and adolescents (13), and the 75th percentile for %BF has been used as the criteria for identifying excess adiposity in a study of dyslipidemia in NHANES (27). Given this precedent, we defined excess adiposity by FMI as a FMI greater than or equal to the sex- and age-specific 75th percentile with the use of our newly created reference data for FMI and excess adiposity by %BF as %BF greater than or equal to the age- and sex-specific 75th percentile with the use of published reference data for %BF (26).
The prevalence of high adiposity was then determined in participants categorized as normal, overweight, or obese by BMI. The prevalence of high adiposity is the positive predictive value (PPV) that a participant in a given BMI classification has excess adiposity as defined by FMI. The PPV of each BMI categorization for males and females was then compared for non-Hispanic whites, non-Hispanic blacks, and Mexican Americans. Characteristics of participants classified as having excess adiposity by FMI but normal adiposity by %BF were then compared with those classified as having excess adiposity by %BF but normal adiposity by FMI.
Statistical analysis
All statistical analyses were conducted with Stata 12 (StataCorp). All analyses were performed by using sample weights to account for the complex sample design as recommended by the NCHS (28) and included imputed data. Reference curves for FMI and LBMI were generated for each of the 5 imputations, and the output data including L, M, S, and centile values were then averaged to create final reference curves and z scores. Two-sample t tests were used to compare means; chi-square analysis was to compare proportions. Statistical significance was defined by using a 2-sided P value <0.05 for all analyses.
RESULTS
FMI and LBMI reference curves
Descriptive information for the samples used to create FMI and LBMI reference curves and for comparisons of FMI with %BF is provided in Table 1. Males accounted for 56% of the sample used to create reference curves because the publically available NHANES 1999–2000 data set does not contain DXA body-composition data for females <18 y of age. The percentage of participants used to create reference curves classified as overweight or obese by BMI was 39.5% for males and 37.5% for females.
TABLE 1.
Descriptive characteristics of NHANES participants used to create FMI and LBMI reference curves and in analyses of FMI compared with %BF1
| Reference curve creation(8–25 y of age) |
Comparison with %BF(8–19 y of age) |
|||
| Males | Females | Males | Females | |
| Sample size (n) | 5195 | 3766 | 4205 | 2890 |
| Ancestry group (%)2 | ||||
| Non-Hispanic white | 61.8 | 62.5 | 69.9 | 71.3 |
| Non-Hispanic black | 14.5 | 14 | 17.1 | 16.7 |
| Mexican American | 12.2 | 11 | 13 | 12 |
| Other | 11.5 | 12.5 | — | — |
| Age (y) | 16.7 ± 0.13 | 17.5 ± 0.2 | 13.4 ± 0.1 | 13.7 ± 0.1 |
| BMI (kg/m2) | 23.3 ± 0.1 | 23.8 ± 0.2 | 21.8 ± 0.1 | 22.4 ± 0.2 |
| BMI status (%) | ||||
| Overweight4 | 20.3 | 18.3 | 16.4 | 16.4 |
| Obese5 | 19.2 | 19.2 | 17.8 | 17.7 |
DXA data from NHANES participants aged 8–25 y were used for reference curve creation to avoid a truncation effect; the analyses comparing FMI and %BF used data from NHANES participants aged 8–19 y to match the age range for which %BF reference data are available. DXA, dual-energy X-ray absorptiometry; FMI, fat mass index; LBMI, lean BMI; %BF, percentage body fat.
Weighted estimate (all such values).
Mean ± SE (all such values).
Defined as BMI ≥85th and ≤94th percentile for participants aged 8–20 y or BMI (in kg/m2) ≥25 and <30 for participants aged 21–25 y.
Defined as BMI ≥95th percentile for participants aged 8–20 y or BMI (in kg/m2) ≥30 in participants aged 21–25 y.
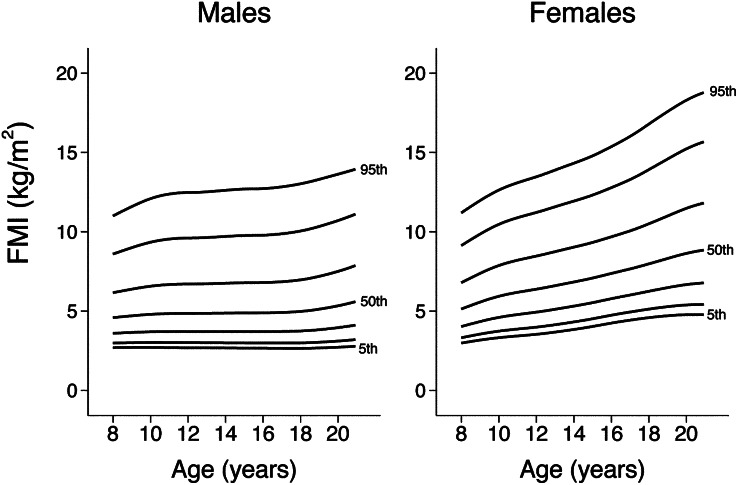
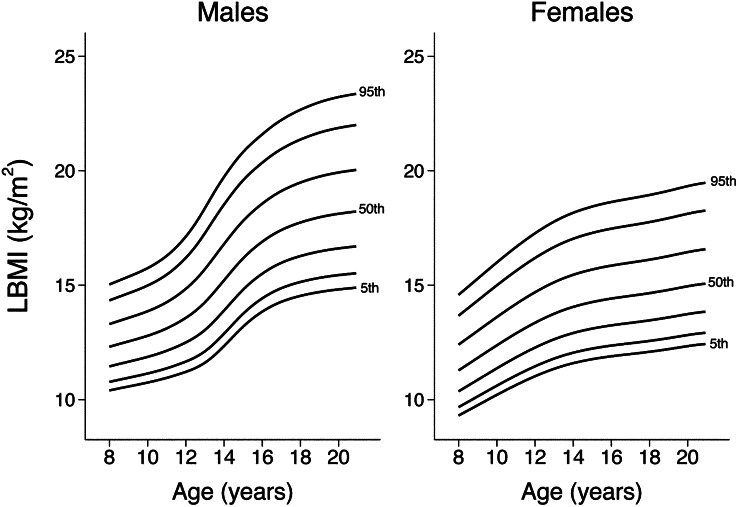
Smoothed reference percentiles for FMI and LBMI for males and females aged 8–20 y are shown in Table 2 and Table 3. In addition to the 50th percentile “M,” each table also provides the L and S values, which can be used to calculate z scores for individuals. Growth curves providing the 5th, 10th, 25th, 50th, 75th, 90th, and 95th centiles for FMI and LBMI in males and females are shown in Figure 1 and Figure 2. Finely scaled versions of FMI and LBMI growth curves are available elsewhere (see Supplemental Figures 1–4 under “Supplemental data” in the online issue).
TABLE 2.
Age- and sex-specific reference percentiles for FMI in children and adolescents aged 8–20 y1
| Males |
Females |
|||||||||||||||||
| M |
M |
|||||||||||||||||
| Age | L | S | 5th | 10th | 25th | 50th | 75th | 90th | 95th | L | S | 5th | 10th | 25th | 50th | 75th | 90th | 95th |
| 8.0–8.49 y | −0.727 | 0.393 | 2.7 | 3.0 | 3.6 | 4.6 | 6.2 | 8.6 | 11.0 | −0.557 | 0.384 | 3.0 | 3.3 | 4.0 | 5.1 | 6.8 | 9.1 | 11.2 |
| 8.5–8.99 y | −0.709 | 0.401 | 2.7 | 3.0 | 3.6 | 4.6 | 6.3 | 8.8 | 11.3 | −0.513 | 0.388 | 3.1 | 3.4 | 4.2 | 5.4 | 7.1 | 9.5 | 11.6 |
| 9.0–9.49 y | −0.690 | 0.408 | 2.7 | 3.0 | 3.7 | 4.7 | 6.4 | 9.0 | 11.6 | −0.469 | 0.391 | 3.2 | 3.6 | 4.3 | 5.6 | 7.4 | 9.9 | 12.0 |
| 9.5–9.99 y | −0.673 | 0.415 | 2.7 | 3.0 | 3.7 | 4.8 | 6.5 | 9.2 | 11.9 | −0.431 | 0.394 | 3.3 | 3.7 | 4.5 | 5.8 | 7.6 | 10.2 | 12.3 |
| 10.0–10.49 y | −0.656 | 0.421 | 2.7 | 3.0 | 3.7 | 4.8 | 6.6 | 9.3 | 12.1 | −0.400 | 0.396 | 3.3 | 3.7 | 4.6 | 5.9 | 7.9 | 10.5 | 12.6 |
| 10.5–10.99 y | −0.643 | 0.426 | 2.7 | 3.0 | 3.7 | 4.8 | 6.6 | 9.5 | 12.3 | −0.378 | 0.398 | 3.4 | 3.8 | 4.7 | 6.1 | 8.1 | 10.7 | 12.9 |
| 11.0–11.49 y | −0.633 | 0.430 | 2.7 | 3.0 | 3.7 | 4.8 | 6.7 | 9.5 | 12.4 | −0.364 | 0.399 | 3.4 | 3.9 | 4.8 | 6.2 | 8.2 | 10.9 | 13.1 |
| 11.5–11.99 y | −0.626 | 0.433 | 2.7 | 3.0 | 3.7 | 4.9 | 6.7 | 9.6 | 12.4 | −0.356 | 0.399 | 3.5 | 3.9 | 4.9 | 6.3 | 8.3 | 11.1 | 13.3 |
| 12.0–12.49 y | −0.623 | 0.434 | 2.7 | 3.0 | 3.7 | 4.9 | 6.7 | 9.6 | 12.5 | −0.353 | 0.399 | 3.5 | 4.0 | 4.9 | 6.4 | 8.5 | 11.2 | 13.5 |
| 12.5–12.99 y | −0.622 | 0.434 | 2.7 | 3.0 | 3.7 | 4.9 | 6.7 | 9.6 | 12.5 | −0.355 | 0.398 | 3.6 | 4.1 | 5.0 | 6.5 | 8.6 | 11.4 | 13.7 |
| 13.0–13.49 y | −0.618 | 0.435 | 2.7 | 3.0 | 3.7 | 4.9 | 6.7 | 9.6 | 12.5 | −0.361 | 0.396 | 3.7 | 4.1 | 5.1 | 6.6 | 8.7 | 11.6 | 13.9 |
| 13.5–13.99 y | −0.613 | 0.437 | 2.7 | 3.0 | 3.7 | 4.9 | 6.7 | 9.7 | 12.6 | −0.370 | 0.394 | 3.8 | 4.2 | 5.2 | 6.7 | 8.9 | 11.8 | 14.1 |
| 14.0–14.49 y | −0.607 | 0.439 | 2.7 | 3.0 | 3.7 | 4.9 | 6.8 | 9.7 | 12.6 | −0.382 | 0.392 | 3.8 | 4.3 | 5.3 | 6.8 | 9.0 | 11.9 | 14.3 |
| 14.5–14.99 y | −0.600 | 0.441 | 2.7 | 3.0 | 3.7 | 4.9 | 6.8 | 9.7 | 12.7 | −0.397 | 0.389 | 3.9 | 4.4 | 5.4 | 7.0 | 9.2 | 12.1 | 14.5 |
| 15.0–15.49 y | −0.596 | 0.443 | 2.7 | 3.0 | 3.7 | 4.9 | 6.8 | 9.8 | 12.7 | −0.413 | 0.386 | 4.0 | 4.5 | 5.5 | 7.1 | 9.3 | 12.3 | 14.8 |
| 15.5–15.99 y | −0.594 | 0.443 | 2.7 | 3.0 | 3.7 | 4.9 | 6.8 | 9.8 | 12.7 | −0.430 | 0.383 | 4.1 | 4.6 | 5.7 | 7.2 | 9.5 | 12.5 | 15.1 |
| 16.0–16.49 y | −0.591 | 0.444 | 2.7 | 3.0 | 3.7 | 4.9 | 6.8 | 9.8 | 12.7 | −0.447 | 0.381 | 4.2 | 4.7 | 5.8 | 7.4 | 9.7 | 12.8 | 15.4 |
| 16.5–16.99 y | −0.585 | 0.446 | 2.7 | 3.0 | 3.7 | 4.9 | 6.8 | 9.8 | 12.8 | −0.460 | 0.379 | 4.3 | 4.8 | 5.9 | 7.5 | 9.9 | 13.0 | 15.7 |
| 17.0–17.49 y | −0.574 | 0.449 | 2.7 | 3.0 | 3.7 | 4.9 | 6.9 | 9.9 | 12.8 | −0.468 | 0.379 | 4.4 | 4.9 | 6.0 | 7.7 | 10.1 | 13.3 | 16.0 |
| 17.5–17.99 y | −0.559 | 0.452 | 2.7 | 3.0 | 3.7 | 4.9 | 6.9 | 10.0 | 12.9 | −0.469 | 0.380 | 4.5 | 5.0 | 6.1 | 7.8 | 10.3 | 13.6 | 16.4 |
| 18.0–18.49 y | −0.540 | 0.456 | 2.7 | 3.0 | 3.7 | 5.0 | 7.0 | 10.0 | 13.0 | −0.460 | 0.383 | 4.6 | 5.1 | 6.3 | 8.0 | 10.5 | 13.9 | 16.8 |
| 18.5–18.99 y | −0.517 | 0.460 | 2.7 | 3.0 | 3.8 | 5.0 | 7.1 | 10.2 | 13.2 | −0.443 | 0.386 | 4.7 | 5.2 | 6.4 | 8.1 | 10.7 | 14.3 | 17.2 |
| 19.0–19.49 y | −0.489 | 0.464 | 2.7 | 3.0 | 3.8 | 5.1 | 7.2 | 10.3 | 13.3 | −0.418 | 0.391 | 4.7 | 5.3 | 6.5 | 8.3 | 11.0 | 14.6 | 17.6 |
| 19.5–19.99 y | −0.456 | 0.468 | 2.7 | 3.1 | 3.9 | 5.2 | 7.3 | 10.5 | 13.5 | −0.386 | 0.396 | 4.8 | 5.3 | 6.6 | 8.5 | 11.2 | 14.9 | 18.0 |
| 20.0–20.49 y | −0.419 | 0.472 | 2.7 | 3.1 | 4.0 | 5.3 | 7.5 | 10.7 | 13.6 | −0.351 | 0.401 | 4.8 | 5.4 | 6.7 | 8.6 | 11.5 | 15.2 | 18.3 |
| 20.5–20.99 y | −0.376 | 0.475 | 2.8 | 3.2 | 4.0 | 5.5 | 7.7 | 10.9 | 13.8 | −0.317 | 0.407 | 4.8 | 5.4 | 6.7 | 8.8 | 11.7 | 15.5 | 18.6 |
Smoothed L, M, and S curves for FMI were generated by using equivalent df values of 3, 5, and 4 in males and of 4, 5, and 4 in females, respectively. FMI, fat mass index; L (lambda), optimal power to obtain normality; M (mu), median; S (sigma), CV.
TABLE 3.
Age- and sex-specific reference percentiles for LBMI in children and adolescents aged 8–20 y1
| LBMI (kg/m2) | ||||||||||||||||||
| Males |
Females |
|||||||||||||||||
| M |
M |
|||||||||||||||||
| Age | L | S | 5th | 10th | 25th | 50th | 75th | 90th | 95th | L | S | 5th | 10th | 25th | 50th | 75th | 90th | 95th |
| 8.0–8.49 y | −0.932 | 0.111 | 10.4 | 10.8 | 11.5 | 12.3 | 13.3 | 14.3 | 15.0 | −1.299 | 0.133 | 9.3 | 9.7 | 10.4 | 11.3 | 12.4 | 13.7 | 14.6 |
| 8.5–8.99 y | −0.932 | 0.112 | 10.5 | 10.9 | 11.6 | 12.4 | 13.4 | 14.5 | 15.2 | −1.299 | 0.133 | 9.5 | 9.9 | 10.6 | 11.6 | 12.7 | 14.0 | 14.9 |
| 9.0–9.49 y | −0.932 | 0.113 | 10.6 | 11.0 | 11.7 | 12.5 | 13.6 | 14.6 | 15.4 | −1.299 | 0.133 | 9.8 | 10.1 | 10.9 | 11.8 | 13.0 | 14.3 | 15.3 |
| 9.5–9.99 y | −0.932 | 0.114 | 10.7 | 11.0 | 11.8 | 12.7 | 13.7 | 14.8 | 15.6 | −1.299 | 0.133 | 10.0 | 10.4 | 11.1 | 12.1 | 13.3 | 14.7 | 15.6 |
| 10.0–10.49 y | −0.932 | 0.115 | 10.7 | 11.1 | 11.9 | 12.8 | 13.9 | 15.0 | 15.7 | −1.299 | 0.133 | 10.2 | 10.6 | 11.4 | 12.4 | 13.6 | 15.0 | 16.0 |
| 10.5–10.99 y | −0.932 | 0.117 | 10.8 | 11.3 | 12.0 | 12.9 | 14.0 | 15.2 | 16.0 | −1.299 | 0.133 | 10.4 | 10.8 | 11.6 | 12.6 | 13.9 | 15.3 | 16.3 |
| 11.0–11.49 y | −0.932 | 0.119 | 11.0 | 11.4 | 12.1 | 13.1 | 14.3 | 15.5 | 16.3 | −1.299 | 0.133 | 10.6 | 11.1 | 11.8 | 12.9 | 14.2 | 15.6 | 16.7 |
| 11.5–11.99 y | −0.932 | 0.123 | 11.1 | 11.5 | 12.3 | 13.3 | 14.5 | 15.8 | 16.7 | −1.299 | 0.133 | 10.8 | 11.3 | 12.1 | 13.1 | 14.4 | 15.9 | 17.0 |
| 12.0–12.49 y | −0.932 | 0.127 | 11.2 | 11.7 | 12.5 | 13.6 | 14.8 | 16.2 | 17.1 | −1.299 | 0.133 | 11.0 | 11.5 | 12.3 | 13.4 | 14.7 | 16.2 | 17.3 |
| 12.5–12.99 y | −0.932 | 0.132 | 11.4 | 11.9 | 12.7 | 13.9 | 15.2 | 16.7 | 17.7 | −1.299 | 0.133 | 11.2 | 11.6 | 12.5 | 13.6 | 14.9 | 16.4 | 17.5 |
| 13.0–13.49 y | −0.932 | 0.137 | 11.6 | 12.1 | 13.0 | 14.3 | 15.7 | 17.3 | 18.4 | −1.299 | 0.133 | 11.3 | 11.8 | 12.6 | 13.8 | 15.1 | 16.7 | 17.8 |
| 13.5–13.99 y | −0.932 | 0.140 | 11.9 | 12.5 | 13.4 | 14.7 | 16.2 | 17.9 | 19.1 | −1.299 | 0.133 | 11.5 | 11.9 | 12.8 | 13.9 | 15.3 | 16.9 | 18.0 |
| 14.0–14.49 y | −0.932 | 0.140 | 12.3 | 12.9 | 13.9 | 15.2 | 16.8 | 18.5 | 19.7 | −1.299 | 0.133 | 11.6 | 12.1 | 12.9 | 14.1 | 15.5 | 17.0 | 18.2 |
| 14.5–14.99 y | −0.932 | 0.139 | 12.8 | 13.3 | 14.4 | 15.7 | 17.3 | 19.1 | 20.3 | −1.299 | 0.133 | 11.7 | 12.1 | 13.0 | 14.2 | 15.6 | 17.2 | 18.3 |
| 15.0–15.49 y | −0.932 | 0.137 | 13.2 | 13.8 | 14.8 | 16.2 | 17.8 | 19.6 | 20.8 | −1.299 | 0.133 | 11.8 | 12.2 | 13.1 | 14.3 | 15.7 | 17.3 | 18.4 |
| 15.5–15.99 y | −0.932 | 0.135 | 13.5 | 14.1 | 15.2 | 16.6 | 18.2 | 20.0 | 21.2 | −1.299 | 0.133 | 11.8 | 12.3 | 13.2 | 14.3 | 15.8 | 17.4 | 18.5 |
| 16.0–16.49 y | −0.932 | 0.133 | 13.8 | 14.4 | 15.5 | 16.9 | 18.6 | 20.4 | 21.6 | −1.299 | 0.133 | 11.9 | 12.4 | 13.2 | 14.4 | 15.9 | 17.5 | 18.6 |
| 16.5–16.99 y | −0.932 | 0.133 | 14.1 | 14.7 | 15.8 | 17.2 | 18.9 | 20.7 | 21.9 | −1.299 | 0.133 | 11.9 | 12.4 | 13.3 | 14.5 | 15.9 | 17.5 | 18.7 |
| 17.0–17.49 y | −0.932 | 0.133 | 14.3 | 14.9 | 16.0 | 17.4 | 19.1 | 20.9 | 22.2 | −1.299 | 0.133 | 12.0 | 12.5 | 13.4 | 14.5 | 16.0 | 17.6 | 18.8 |
| 17.5–17.99 y | −0.932 | 0.133 | 14.4 | 15.0 | 16.1 | 17.6 | 19.3 | 21.2 | 22.5 | −1.299 | 0.133 | 12.0 | 12.5 | 13.4 | 14.6 | 16.0 | 17.7 | 18.9 |
| 18.0–18.49 y | −0.932 | 0.133 | 14.5 | 15.1 | 16.3 | 17.7 | 19.5 | 21.4 | 22.7 | −1.299 | 0.133 | 12.1 | 12.6 | 13.5 | 14.7 | 16.1 | 17.8 | 18.9 |
| 18.5–18.99 y | −0.932 | 0.134 | 14.6 | 15.2 | 16.4 | 17.9 | 19.6 | 21.5 | 22.8 | −1.299 | 0.133 | 12.1 | 12.6 | 13.5 | 14.7 | 16.2 | 17.8 | 19.0 |
| 19.0–19.49 y | −0.932 | 0.134 | 14.7 | 15.3 | 16.5 | 18.0 | 19.7 | 21.7 | 23.0 | −1.299 | 0.133 | 12.2 | 12.7 | 13.6 | 14.8 | 16.3 | 17.9 | 19.1 |
| 19.5–19.99 y | −0.932 | 0.134 | 14.8 | 15.4 | 16.5 | 18.1 | 19.8 | 21.8 | 23.1 | −1.299 | 0.133 | 12.3 | 12.8 | 13.7 | 14.9 | 16.4 | 18.0 | 19.2 |
| 20.0–20.49 y | −0.932 | 0.135 | 14.8 | 15.4 | 16.6 | 18.1 | 19.9 | 21.9 | 23.2 | −1.299 | 0.133 | 12.3 | 12.8 | 13.7 | 15.0 | 16.4 | 18.1 | 19.3 |
| 20.5–20.99 y | −0.932 | 0.135 | 14.9 | 15.5 | 16.7 | 18.2 | 20.0 | 21.9 | 23.3 | −1.299 | 0.133 | 12.4 | 12.9 | 13.8 | 15.0 | 16.5 | 18.2 | 19.4 |
Smoothed L, M, and S curves for LBMI were generated by using equivalent df of 1, 6, and 5 in males and 1, 6, and 1 in females. L (lambda), optimal power to obtain normality; LBMI, lean body mass index; M (mu), median; S (sigma), CV.
FIGURE 1.
Reference curves for FMI in males and females; 5th, 10th, 25th, 50th, 75th, 90th, and 95th centiles are shown. FMI, fat mass index.
FIGURE 2.
Reference curves for LBMI in males and females; 5th, 10th, 25th, 50th, 75th, 90th, and 95th centiles are shown. LBMI, lean BMI.
Population ancestry differences in body-compartment z scores
Significant population ancestry group differences existed in sex-specific FMI and LBMI z scores for age, as shown in Table 4. Among males, non-Hispanic blacks had significantly higher (P < 0.0001) LBMI z scores (0.26) than non-Hispanic whites (−0.07) and Mexican Americans (0.05) and significantly lower (P < 0.0001) FMI z scores (−0.27) than whites (0.02) and Mexican Americans (0.26). Among females, non-Hispanic blacks had significantly higher (P < 0.0001) LBMI z scores (0.45) than those in non-Hispanic whites (−0.09) and Mexican Americans (−0.09), but there was no difference in FMI z scores in non-Hispanic blacks (0.04) compared with those in non-Hispanic whites (−0.04) and Mexican Americans (0.13) (P = 0.19 and 0.11, respectively).
TABLE 4.
Population ancestry group differences in body-compartment z scores in NHANES participants aged 8–19 y1
| Males |
Females |
|||||
| LBMI z score | FMI z score | BMI z score | LBMI z score | FMI z score | BMI z score | |
| Non-Hispanic white | −0.07 ± 0.04 | 0.02 ± 0.04 | 0.45 ± 0.05 | −0.09 ± 0.05 | −0.04 ± 0.04 | 0.45 ± 0.05 |
| Non-Hispanic black | 0.26 ± 0.03 | −0.27 ± 0.03 | 0.49 ± 0.03 | 0.45 ± 0.03 | 0.04 ± 0.03 | 0.77 ± 0.03 |
| Mexican American | 0.05 ± 0.03 | 0.26 ± 0.03 | 0.66 ± 0.04 | −0.09 ± 0.04 | 0.13 ± 0.05 | 0.56 ± 0.05 |
All values are means ± SEs (all such values); n = 7095. The LBMI z score was higher in non-Hispanic blacks than in non-Hispanic whites (males and females: P < 0.0001) and Mexican Americans (males and females: P < 0.0001) and in Mexican Americans than in non-Hispanic whites (P = 0.04). FMI z score was higher in Mexican Americans than in non-Hispanic whites (males: P < 0.0001; females: P = 0.02) and non-Hispanic blacks (males: P < 0.0001) and in non-Hispanic whites than in non-Hispanic blacks (males: P < 0.0001). BMI z score was higher in Mexican Americans than in non-Hispanic whites and blacks in males (P < 0.01) and higher in non-Hispanic blacks than in whites and Mexican Americans in females (P < 0.01). Chi-square analyses were used to determine significance. FMI, fat mass index; LBMI, lean BMI.
Population ancestry differences in the PPV of BMI to identify excess adiposity
The PPV of the currently recommended BMI classifications for overweight and obese to identify excess adiposity as defined by either FMI or %BF ≥75th percentile is shown separately for males and females in non-Hispanic blacks, non-Hispanic whites, and Mexican Americans in Table 5. Significant population ancestry differences in the PPV to identify excess adiposity were found in both sexes and all BMI classifications, but were most dramatic in participants classified as overweight by BMI.
TABLE 5.
Variation in PPV of BMI to identify adiposity classified by FMI or %BF ≥75th percentile by population ancestry group in NHANES participants aged 8–19 y1
| PPV of overweight and obese by BMI to identify adiposity as defined by FMI >75th percentile |
PPV of overweight and obese by BMI to identify adiposity as defined by %BF >75th percentile |
|||
| Overweight by BMI | Obese by BMI | Overweight by BMI | Obese by BMI | |
| Males | 62.1 (54.9, 68.8) | 98.0 (95.8, 99.1) | 41.9 (37.1, 46.9) | 86.9 (84.1, 89.2) |
| Non-Hispanic white | 65.4 (55.7, 74.0) | 98.1 (93.7, 99.4) | 44.7 (38.3, 51.2) | 87.4 (82.3, 91.2) |
| Non-Hispanic black | 35.9 (28.7, 43.8)2 | 96.9 (95.3, 98.0) | 21.3 (15.0, 29.4)2 | 79.2 (72.8, 84.5) |
| Mexican American | 73.3 (66.5, 79.1) | 99.0 (97.3, 99.6) | 49.7 (43.6, 55.8) | 92.6 (90.3, 94.4) |
| Females | 50.6 (43.5, 57.6) | 99.7 (98.5, 99.9) | 41.2 (35.7, 46.9) | 89.2 (84.0, 92.3) |
| Non-Hispanic white | 52.2 (43.0, 61.3) | 100.0 | 43.8 (36.1, 51.8) | 93.0 (86.3, 96.5) |
| Non-Hispanic black | 30.3 (23.4, 38.3)3 | 98.7 (94.0, 99.7) | 19.2 (14.8, 24.5)2 | 79.0 (72.1, 84.5) |
| Mexican American | 68.3 (61.1, 74.7) | 100.0 | 55.7 (47.0, 64.1) | 90.4 (82.3, 95.0) |
Values are PPVs; 95% CIs in parentheses. n = 7095. Chi-square analyses were used to determine significance. PPV is the prevalence of high adiposity (as defined by FMI or %BF) in a given BMI classification. FMI, fat mass index; PPV, positive predictive value; %BF, percentage body fat.
Significantly different from non-Hispanic whites and Mexican Americans, P < 0.0001.
Significantly different from non-Hispanic whites and Mexican-Americans, P < 0.001.
In males classified as overweight by BMI, the PPV of having excess adiposity as defined by FMI was 35.9% in non-Hispanic blacks. This was significantly lower than the PPV of 65.4% seen in non-Hispanic whites and 73.3% in Mexican Americans (P < 0.0001). The PPV in non-Hispanic whites did not differ significantly from that in Mexican Americans (P = 0.13). In males classified as obese by BMI, non-Hispanic blacks had a lower PPV at 96.9% compared with 99% in Mexican Americans (P = 0.03). No statistically significant differences in PPV were found between obese non-Hispanic whites and non-Hispanic blacks or Mexican Americans.
In females classified as overweight by BMI, the PPV for identifying adiposity as defined by FMI was found to be significantly lower at 30.3% in non-Hispanic blacks than the value of 52.2% in non-Hispanic whites and 68.3% in Mexican Americans (P < 0.001). The PPV was significantly higher in Mexican Americans than in non-Hispanic whites (P < 0.03). In females classified as obese, the PPV of having excess adiposity in non-Hispanic white and Mexican American females was 100% compared with 98.7% in non-Hispanic blacks.
Significant population ancestry differences in the PPV of BMI to identify excess adiposity were also seen when %BF was used to define adiposity. Interestingly, the PPVs of both overweight and obesity by BMI were considerably lower when %BF was used to identify adiposity as compared with FMI. In males, the PPV of overweight by BMI for identifying adiposity defined by %BF was 21.3%, 44.7%, and 49.7% in non-Hispanic blacks, non-Hispanic whites, and Mexican Americans, respectively, compared with 35.9%, 65.4%, and 73.3% when adiposity was defined by FMI. Similar patterns were seen for obese by BMI and in females (Table 5).
Comparison of participants classified as having excess adiposity by FMI and %BF
We investigated whether the characteristics of individuals classified as having excess adiposity by FMI differed from those classified by %BF in participants aged 8–19 y (ages for which there are %BF reference data). Overall, 23.4% were classified as having excess adiposity by both measures, and 70.4% were classified as not having excess adiposity by either measure. There was discordance in the classification of excess adiposity in the remaining 6.2% of the sample; 4.9% of participants were classified as having excess adiposity by FMI only and 1.3% by %BF only.
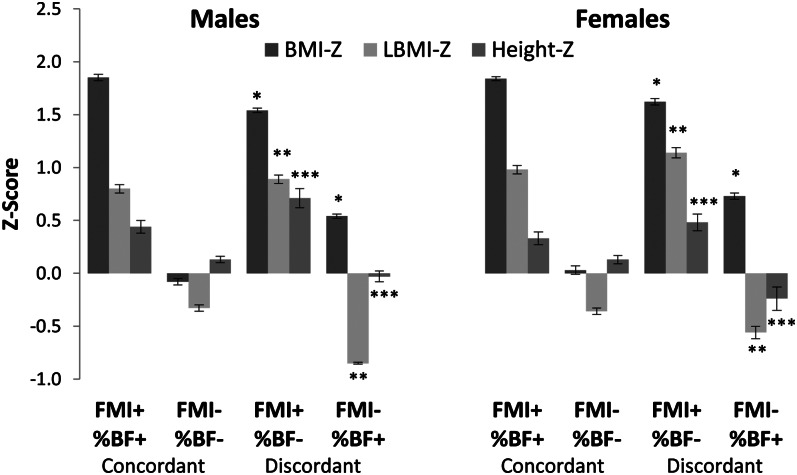
As shown in Figure 3, significant differences were seen in both male and female participants discordantly classified by FMI and %BF. Male participants classified as having excess adiposity by FMI but normal adiposity by %BF were found to have higher BMI z scores (1.54 compared with 0.54; P < 0.001), LBMI z scores (0.89 compared with −0.85; P < 0.001) and height z scores (0.71 compared with −0.03; P < 0.001) compared with those classified as having excess adiposity by %BF but normal adiposity by FMI. Similar findings were seen in females; participants classified as having excess adiposity by FMI but normal adiposity by %BF had higher BMI z scores (1.62 compared with 0.73; P < 0.001), LBMI z scores (1.14 compared with −0.56; P < 0.001), and height z scores (0.48 compared with −0.24; P < 0.001) than did those classified as having excess adiposity by %BF but normal adiposity by FMI. Non-Hispanic blacks were also found to account for a higher percentage of those classified as having excess adiposity by FMI but normal adiposity by %BF in both males and females (males: 16.5% compared with 4.7%, P = 0.02; females: 32.8% compared with 4.5%, P < 0.001). Females classified as having excess adiposity by FMI but normal by %BF also were found to be younger (13.8 ± 0.3 y compared with 15.2 ± 0.3 y; P = 0.04). No age difference was found in males.
FIGURE 3.
Mean (±SE) BMI-Z, LBMI-Z, and height-Z in NHANES participants concordantly and discordantly classified as having excess adiposity by FMI and %BF. BMI-Z (*), LBMI-Z (**), and height-Z (***) were all significantly greater in male and female participants classified as having excess adiposity by FMI but normal adiposity by %BF than in those classified as having excess adiposity by %BF but normal adiposity by FMI (P < 0.001). Two-sample t tests were used to determine significance. n = 7095. BMI-Z, BMI z score; height-Z, height z score; FMI, fat mass index; LBMI-Z, lean BMI z score; %BF, percentage body fat.
Reference percentiles for FMI and LBMI were also generated by using unadjusted body-composition data as measured with Hologic QDR 4500A densitometers (ie, lean mass was increased to the preadjustment level, and FM was decreased by an equivalent amount such that total mass remained the same) and are available elsewhere (see Supplemental Tables S1 and S2 under “Supplemental data” in the online issue). The analyses reported above were repeated by using unadjusted reference data, and the same population ancestry differences in body composition and PPV of BMI to identify excess adiposity were observed.
DISCUSSION
We generated nationally representative reference curves and percentiles for FMI and LBMI in children and adolescents aged 8–20 y by using NHANES DXA body-composition data. The use of these percentiles and z scores provides more accurate assessments of adiposity than do BMI and %BF by allowing for the independent assessment of FM and LBM compartments.
The reference curves for FMI and LBMI indicate important sex- and age-specific differences. Unlike males, females exhibit an age-related increase in FMI at all levels of adiposity (percentiles). In contrast with FMI, LBMI values are consistently greater in males than in females. The age-related increase in LBMI percentiles was steeper in males than in females, especially between the ages of 11 and 16 y—consistent with rapid accrual of LBM during male puberty. In females, the age-related increase was greatest between 8 and 12 y of age; the change in slope at ∼12 y of age corresponds with the median age of menarche (29). Previous studies have estimated FMI in pediatric populations; however, comparisons with our data should be performed with caution because of differences in the method used to estimate FM, sample size and characteristics, and study design (6, 30, 31).
Significant differences between population ancestry groups were identified in the ability of overweight by BMI to identify excess adiposity as defined by FMI. Most dramatic was the markedly lower PPV of overweight by BMI in non-Hispanic blacks than in non-Hispanic whites and Mexican Americans—a finding that has been reported previously when %BF was used to define excess adiposity (12–14). Body-composition differences by population ancestry group have been reported: non-Hispanic black children and adolescents have greater LBM (7), lower total and visceral adipose tissue (32, 33), greater LBM density (8), and greater limb-to-trunk proportions (34) compared with whites. To what extent these differences in body composition are responsible for the observed variation in cardiometabolic risk by population ancestry group (35–37) is unclear and warrants future study. The use of FMI and LBMI may improve the investigation of cardiometabolic risk by allowing for the independent evaluation of FM and LBM.
The PPV of BMI to identify excess adiposity was lower when %BF was used to define excess adiposity compared with FMI. It is possible that this is partly attributable to the presence of height2 in the denominators of both BMI and FMI, which may inflate their association as compared with %BF (which does not contain height or a quadratic term). However, our data indicate that %BF underestimates the prevalence of excess adiposity by misclassifying individuals with both high FM and high LBM as normal. Careful analysis of the characteristics of participants classified as having excess adiposity by FMI but normal by %BF confirms that this population has high LBMI, BMI, and height z scores. This subgroup with high FM and high LBM would be missed if %BF was used as a screening tool. Obese children and adolescents have previously been shown to have high LBM for height (38). High LBM may not be protective against the development of cardiometabolic disease because studies in adults have found that FFMI is positively associated with a higher odds of the metabolic syndrome and dyslipidemia (39, 40). It is also possible that individuals classified as having excess adiposity by %BF but normal adiposity by FMI could be at risk of cardiometabolic disease as a result of LBM deficits; however, these individuals would be identified if both FMI and LBMI were used.
Interestingly, females classified as having excess adiposity by FMI but normal adiposity by %BF were younger, on average, than those classified as having excess adiposity by %BF but normal by FMI. This may represent females who underwent early pubertal maturation and accrued LBM in sufficient quantities so as to be misclassified by %BF. Accurate assessment of body composition in early-maturing individuals is important because early pubertal maturation is a risk factor for the development of excess adiposity (41) and the metabolic syndrome (42). These hypotheses are speculative, however, because NHANES does not contain pubertal status.
The ability to assess FMI and LBMI independently and simultaneously will likely prove to be especially useful for children with chronic diseases. FM and LBM may be affected differently in chronic disease, and a normal BMI may conceal deficits in lean mass (43). For example, cachectic obesity is defined as LBM deficits in the setting of FM excess (44) and has been identified in many conditions, including survivors of pediatric allogeneic hematopoietic stem cell transplantation, juvenile rheumatoid arthritis, end-stage renal disease, and Crohn disease (45–48).
This study had a number of potential limitations. We used cross-sectional data from NHANES, which does not allow for the longitudinal assessment of FM and LBM accrual in individuals. However, this is the only source of DXA body-composition data large enough to create nationally representative reference curves in children and adolescents. Our FMI and LBMI reference curves were based on the entire sample of 1999–2004 NHANES participants aged 8–25 y and represent contemporary US youth. In contrast, the current CDC BMI reference curves excluded more recent NHANES data to avoid the influence of population-wide increases in excess body weight (21). There is no currently accepted gold standard for the definition of excess adiposity. We defined excess adiposity as an FMI ≥75th percentile to allow for direct comparisons with previous studies, which used this cutoff to define excess adiposity by %BF (13, 27). This cutoff is not biologically based, and further research is needed to identify thresholds for FMI and LBMI that relate to health and disease. Finally, estimates of FM by DXA have been shown to differ from estimates provided by 4-compartment models (often considered the gold standard for the assessment of FM), and the relation between these estimates have been shown to differ by DXA manufacturer and among individuals (17, 49–51). DXA is more readily available and easier to use, however, which makes it a more practical approach than the 4-compartment model to assess body composition.
In conclusion, we present the first reference data for FMI and LBMI in children and adolescents drawn from a large representative sample of the US population. Non-Hispanic blacks of both sexes had a significantly greater LBM than did nonblacks. The failure of BMI and %BF to account for the independent contributions of FM and LBM led to an overdiagnosis of excess adiposity among non-Hispanic blacks when BMI was used and to an underdiagnosis of excess adiposity among individuals with high LBM when %BF was used. Thus, the use of FMI and LBMI improves on the use BMI and %BF by allowing for the independent assessment of FM and LBM. Future studies are needed to determine which body-composition index or combination of indexes will provide the most accurate assessment of cardiometabolic risk and nutritional status.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—DRW, RHM, MBL, and BSZ: designed the research and edited and contributed to the final draft of the manuscript; DRW and BSZ: conducted the research; DRW and RHM: conducted the statistical analysis; DRW: wrote the first draft of the manuscript; and BSZ: had primary responsibility for the final content of the manuscript. No conflicts of interest were declared.
Footnotes
Abbreviations used: DXA, dual-energy X-ray absorptiometry; FFM, fat-free mass; FFMI, fat-free mass index; FM, fat mass; FMI, fat mass index; IRB, Institutional Review Board; LBM, lean body mass; LBMI, lean BMI; LMS, lambda-mu-sigma; NCHS, National Center for Health Statistics; PPV, positive predictive value; %BF, percentage body fat.
REFERENCES
- 1.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i–xii, 1–253. [PubMed] [Google Scholar]
- 2.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics 2007;120(suppl 4):S193–228. [DOI] [PubMed] [Google Scholar]
- 4.Wells JC. A Hattori chart analysis of body mass index in infants and children. Int J Obes Relat Metab Disord 2000;24:325–9. [DOI] [PubMed] [Google Scholar]
- 5.Maynard LM, Wisemandle W, Roche AF, Chumlea WC, Guo SS, Siervogel RM. Childhood body composition in relation to body mass index. Pediatrics 2001;107:344–50. [DOI] [PubMed] [Google Scholar]
- 6.Demerath EW, Schubert CM, Maynard LM, Sun SS, Chumlea WC, Pickoff A, Czerwinski SA, Towne B, Siervogel RM. Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics 2006;117:e487–95. [DOI] [PubMed] [Google Scholar]
- 7.Nelson DA, Barondess DA. Whole body bone, fat and lean mass in children: comparison of three ethnic groups. Am J Phys Anthropol 1997;103:157–62. [DOI] [PubMed] [Google Scholar]
- 8.Schutte JE, Townsend EJ, Hugg J, Shoup RF, Malina RM, Blomqvist CG. Density of lean body mass is greater in blacks than in whites. J Appl Physiol 1984;56:1647–9. [DOI] [PubMed] [Google Scholar]
- 9.Ellis KJ, Shypailo RJ, Abrams SA, Wong WW. The reference child and adolescent models of body composition. A contemporary comparison. Ann N Y Acad Sci 2000;904:374–82. [DOI] [PubMed] [Google Scholar]
- 10.Foster BJ, Platt RW, Zemel BS. Development and validation of a predictive equation for lean body mass in children and adolescents. Ann Hum Biol 2012;39:171–82. [DOI] [PubMed] [Google Scholar]
- 11.Ellis KJ, Abrams SA, Wong WW. Monitoring childhood obesity: assessment of the weight/height index. Am J Epidemiol 1999;150:939–46. [DOI] [PubMed] [Google Scholar]
- 12.Dugas LR, Cao G, Luke AH, Durazo-Arvizu RA. Adiposity is not equal in a multi-race/ethnic adolescent population: NHANES 1999-2004. Obesity (Silver Spring) 2011;19:2099–101. [DOI] [PubMed] [Google Scholar]
- 13.Flegal KM, Ogden CL, Yanovski JA, Freedman DS, Shepherd JA, Graubard BI, Borrud LG. High adiposity and high body mass index-for-age in US children and adolescents overall and by race-ethnic group. Am J Clin Nutr 2010;91:1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman DS, Wang J, Thornton JC, Mei Z, Pierson RN, Jr, Dietz WH, Horlick M. Racial/ethnic differences in body fatness among children and adolescents. Obesity (Silver Spring) 2008;16:1105–11. [DOI] [PubMed] [Google Scholar]
- 15.Wells JC. A critique of the expression of paediatric body composition data. Arch Dis Child 2001;85:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Itallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body's fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr 1990;52:953–9. [DOI] [PubMed] [Google Scholar]
- 17.Wells JC, Williams JE, Chomtho S, Darch T, Grijalva-Eternod C, Kennedy K, Haroun D, Wilson C, Cole TJ, Fewtrell MS. Body-composition reference data for simple and reference techniques and a 4-component model: a new UK reference child. Am J Clin Nutr 2012;96:1316–26. [DOI] [PubMed] [Google Scholar]
- 18.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS ONE 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics. National Health and Nutrition Examination Survey: technical documentation for the 1999-2004 dual energy X-ray absorptiometry (DXA) multiple imputation data files. 2008. Available from: http://www.cdc.gov/nchs/nhanes/dxx/dxa.htm (cited 1 August 2012).
- 20.National Center for Health Statistics. National Health and Nutrition Examination Survey: body composition procedures manual. 2008. Available from: http://www.cdc.gov/nchs/data/nhanes/bc.pdf (cited 1 August 2012).
- 21.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data 2000;Jun 8:1–27. [PubMed] [Google Scholar]
- 22.Schoeller DA, Tylavsky FA, Baer DJ, Chumlea WC, Earthman CP, Fuerst T, Harris TB, Heymsfield SB, Horlick M, Lohman TG, et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr 2005;81:1018–25. [DOI] [PubMed] [Google Scholar]
- 23.National Center for Health Statistics. National Health and Nutrition Examination Survey: NHANES 1999-2000 data documentation. 2008. Available from: http://www.cdc.gov/nchs/data/nhanes/dxa/dxx.pdf (cited 28 December 2012).
- 24.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr 1990;44:45–60. [PubMed] [Google Scholar]
- 25.Pan H, Cole TJ. A comparison of goodness of fit tests for age-related reference ranges. Stat Med 2004;23:1749–65. [DOI] [PubMed] [Google Scholar]
- 26.Ogden CL, Li Y, Freedman DS, Borrud LG, Flegal KM. Smoothed percentage body fat percentiles for U.S. children and adolescents, 1999–2004. Natl Health Stat Report 2011;Nov 9:1–7. [PubMed] [Google Scholar]
- 27.Lamb MM, Ogden CL, Carroll MD, Lacher DA, Flegal KM. Association of body fat percentage with lipid concentrations in children and adolescents: United States, 1999-2004. Am J Clin Nutr 2011;94:877–83. [DOI] [PubMed] [Google Scholar]
- 28.National Center for Health Statistics. National Health and Nutrition Examination Survey: analytic and reporting guidelines. 2006. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/analytical_guidelines.htm (cited 1 August 2012).
- 29.Chumlea WC, Schubert CM, Roche AF, Kulin HE, Lee PA, Himes JH, Sun SS. Age at menarche and racial comparisons in US girls. Pediatrics 2003;111:110–3. [DOI] [PubMed] [Google Scholar]
- 30.Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, Dietz WH, Horlick M. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes (Lond) 2005;29:1–8. [DOI] [PubMed] [Google Scholar]
- 31.Eissa MA, Dai S, Mihalopoulos NL, Day RS, Harrist RB, Labarthe DR. Trajectories of fat mass index, fat free-mass index, and waist circumference in children: Project HeartBeat! Am J Prev Med 2009;37:S34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harsha DW, Frerichs RR, Berenson GS. Densitometry and anthropometry of black and white children. Hum Biol 1978;50:261–80. [PubMed] [Google Scholar]
- 33.Goran MI, Nagy TR, Treuth MS, Trowbridge C, Dezenberg C, McGloin A, Gower BA. Visceral fat in white and African American prepubertal children. Am J Clin Nutr 1997;65:1703–8. [DOI] [PubMed] [Google Scholar]
- 34.Wagner DR, Heyward VH. Measures of body composition in blacks and whites: a comparative review. Am J Clin Nutr 2000;71:1392–402. [DOI] [PubMed] [Google Scholar]
- 35.Libman IM, LaPorte RE, Becker D, Dorman JS, Drash AL, Kuller L. Was there an epidemic of diabetes in nonwhite adolescents in Allegheny County, Pennsylvania? Diabetes Care 1998;21:1278–81. [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association. Type 2 diabetes in children and adolescents. Diabetes Care 2000;23:381–9. [DOI] [PubMed] [Google Scholar]
- 37.Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab 2003;88:2534–40. [DOI] [PubMed] [Google Scholar]
- 38.Leonard MB, Shults J, Wilson BA, Tershakovec AM, Zemel BS. Obesity during childhood and adolescence augments bone mass and bone dimensions. Am J Clin Nutr 2004;80:514–23. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Rennie KL, Gu W, Li H, Yu Z, Lin X. Independent associations of body-size adjusted fat mass and fat-free mass with the metabolic syndrome in Chinese. Ann Hum Biol 2009;36:110–21. [DOI] [PubMed] [Google Scholar]
- 40.Schubert CM, Rogers NL, Remsberg KE, Sun SS, Chumlea WC, Demerath EW, Czerwinski SA, Towne B, Siervogel RM. Lipids, lipoproteins, lifestyle, adiposity and fat-free mass during middle age: the Fels Longitudinal Study. Int J Obes (Lond) 2006;30:251–60. [DOI] [PubMed] [Google Scholar]
- 41.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of menarcheal age to obesity in childhood and adulthood: the Bogalusa Heart Study. BMC Pediatr 2003;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic Syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the Bogalusa Heart Study. Int J Obes Relat Metab Disord 2003;27:1398–404. [DOI] [PubMed] [Google Scholar]
- 43.Wells JC. Body composition in childhood: effects of normal growth and disease. Proc Nutr Soc 2003;62:521–8. [DOI] [PubMed] [Google Scholar]
- 44.Roubenoff R, Kehayias JJ. The meaning and measurement of lean body mass. Nutr Rev 1991;49:163–75. [DOI] [PubMed] [Google Scholar]
- 45.Mostoufi-Moab S, Ginsberg JP, Bunin N, Zemel BS, Shults J, Thayu M, Leonard MB. Body composition abnormalities in long-term survivors of pediatric hematopoietic stem cell transplantation. J Pediatr 2012;160:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burnham JM, Shults J, Dubner SE, Sembhi H, Zemel BS, Leonard MB. Bone density, structure, and strength in juvenile idiopathic arthritis: importance of disease severity and muscle deficits. Arthritis Rheum 2008;58:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foster BJ, Kalkwarf HJ, Shults J, Zemel BS, Wetzsteon RJ, Thayu M, Foerster DL, Leonard MB. Association of chronic kidney disease with muscle deficits in children. J Am Soc Nephrol 2011;22:377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thayu M, Denson LA, Shults J, Zemel BS, Burnham JM, Baldassano RN, Howard KM, Ryan A, Leonard MB. Determinants of changes in linear growth and body composition in incident pediatric Crohn's disease. Gastroenterology 2010;139:430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sopher AB, Thornton JC, Wang J, Pierson RN, Jr, Heymsfield SB, Horlick M. Measurement of percentage of body fat in 411 children and adolescents: a comparison of dual-energy X-ray absorptiometry with a four-compartment model. Pediatrics 2004;113:1285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Der Ploeg GE, Withers RT, Laforgia J. Percent body fat via DEXA: comparison with a four-compartment model. J Appl Physiol 2003;94:499–506. [DOI] [PubMed] [Google Scholar]
- 51.Wong WW, Hergenroeder AC, Stuff JE, Butte NF, Smith EO, Ellis KJ. Evaluating body fat in girls and female adolescents: advantages and disadvantages of dual-energy X-ray absorptiometry. Am J Clin Nutr 2002;76:384–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.