Abstract
Patients frequently experience anterior cruciate ligament (ACL) injuries but current ACL reconstruction strategies do not restore the native biomechanics of the knee, which can contribute to the early onset of osteoarthritis in the long term. To design more effective treatments, investigators must first understand normal in vivo knee function for multiple activities of daily living (ADLs). While the 3D kinematics of the human knee have been measured for various ADLs, the 3D kinetics cannot be directly measured in vivo. Alternatively, the 3D kinetics of the knee and its structures can be measured in an animal model by simulating and applying subject-specific in vivo joint motions to a joint using robotics. However, a suitable biomechanical surrogate should first be established. This study was designed to apply a simulated human in vivo motion to human knees to measure the kinetics of the human knee and ACL. In pursuit of establishing a viable biomechanical surrogate, a simulated in vivo ovine motion was also applied to human knees to compare the loads produced by the human and ovine motions. The motions from the two species produced similar kinetics in the human knee and ACL. The only significant difference was the intact knee compression force produced by the two input motions.
Keywords: Anterior cruciate ligament, Biomechanics, Activities of daily living, Ovine, Surrogate
INTRODUCTION
Osteoarthritis is a prevalent clinical problem following anterior cruciate ligament (ACL) injury, even after ACL reconstruction.18,23,40 Among other factors, the chronic degeneration of the knee joint has been attributed to the failure of current ACL reconstructions to restore the native joint biomechanics.4 The ACL serves as the primary restraint to anterior tibial translation and also exhibits 3D forces and moments during daily activities.6,9,12,19,25,30,33,34 Following ACL reconstruction, surgeons currently use clinical laxity tests such as the Lachman test to determine the reconstruction efficacy. While this clinical test can at best quantify the relative restoration of anterior tibial translation, the test does not address the other degrees of freedom (DOF) of knee laxity. Additionally, ACL reconstructions have been shown to inadequately restore rotational stability in the knee joint.2,11 These abnormal knee motions shift joint contact regions and cause wear to thinner regions of articular cartilage which are not accustomed to higher or frequent loading.4 Therefore, the abnormal knee biomechanics resulting from ACL injury and uncorrected by ACL reconstruction may contribute to an the early onset of osteoarthritis.
To design more effective ACL reconstructions, investigators require design parameters based on normal in vivo knee biomechanics for multiple activities of daily living (ADLs). However, the 3D in vivo forces and moments of the normal intact knee, as well as those for just the ACL have not yet been determined for any ADL. Walking is the most common dynamic ADL in the general population. While knee kinematics have been measured in humans during walking,3,16,22 the joint and tissue forces are impossible to directly measure in vivo. Investigators have attempted to measure tissue forces in the human knee during passive flexion/extension motions using implanted force sensors.32 However, ACLs from human subjects cannot be isolated to calibrate the sensors using physiologic loading conditions. Investigators have also estimated knee joint kinetics from inverse dynamics calculations using kinematic and ground reaction force measurements,26,38,39 as well as knee models which include muscle contributions.21,37,38 Unfortunately, these methods require many assumptions and produce highly variable estimates. Another group has directly measured in vivo joint contact forces in knee arthroplasty patients using an instrumented total knee replacement.10 However, these results may not reflect normal joint loading in a healthy population. While the in vivo 3D forces and moments of the knee joint cannot be directly measured in humans, an animal model can be used to simulate the subject-specific 3D kinematics and measure the corresponding 3D knee and ACL kinetics using robotics. Investigators can also use an animal model to investigate the effects of injury and healing, as well as to evaluate the functional efficacy of ACL reconstructions.
The ovine stifle joint has been widely used in orthopaedic research. Investigators have used the ovine model to study the biological, structural, and mechanical effects of injury (including the progression of osteoarthritis), as well as to evaluate surgical treatments.5,17,24,27,28,35 The ovine stifle joint consists of the same major soft tissue structures found in the human knee and is a valid surgical model for the human cruciate ligaments.1,31 In addition, the ovine stifle joint allows for sensors or marker systems to be rigidly fixed to the tibia and femur to accurately measure the 6 deg of freedom (DOF) kinematics of the tibiofemoral joint.15,36 However, there are biomechanical differences between the ovine stifle joint and human knee. The range of flexion exhibited by the ovine stifle joint during gait is approximately half that of the human knee.22,36 The human knee is at or near full extension at heel-strike and flexes to about 60° during swing whereas the ovine stifle joint never achieves the straight-leg orientation that the human knee exhibits at heel-strike.22,36 Instead, the ovine stifle joint is flexed near 43° at hoof strike and reaches near 77° of flexion during swing.22,36 Also, the magnitudes and directions of ACL force significantly differ between human and ovine when subjected to the same anterior tibial loads.43
Knee biomechanics investigations using the ovine stifle joint could provide valuable insight for better understanding the normal function of the human knee, as well as the effects of injury and repair. The long term goal of this research is to minimize joint degeneration after ACL injury by designing more effective ACL reconstructions. To achieve this goal, we plan to use an animal surrogate to establish design criteria and evaluation benchmarks for traditional and tissue-engineered ACL reconstructions based on normal ACL function during ADLs.8 To gain a better understanding of human knee and ACL function during an ADL, we first apply a simulated human in vivo motion to human knees to measure the 3D kinetics of the human knee and ACL. In pursuit of establishing a viable biomechanical surrogate, we also apply a simulated ovine in vivo motion to human knees to compare the knee and ACL loads produced by the human and ovine motions, focusing on the direction of effect. If the simulated motions from the two different species challenge the human knee/ACL similarly, we would proceed to use the ovine model to establish design criteria for ACL reconstructions and to evaluate ACL reconstructions in vivo. The ability to measure knee kinematics for the normal, injured, and surgically-repaired ovine surrogate and to apply these motions to both ovine and human knees would allow investigators to predict the biomechanical effects of various injuries and surgical treatments for the human knee.
MATERIALS AND METHODS
Experimental Design
Six human knees (no pairs) were tested using a 6° of freedom (DOF) robot. A Kuka robot (KR210; Kuka Robotics Corp., Clinton Township, MI) equipped with a 6-axis load cell (Theta Model; ATI Industrial Automation, Apex, NC) was used to reproduce a 6 DOF ovine in vivo walking motion measured by Tapper et al.36 and a 6 DOF human in vivo walking motion measured by Lafortune et al.22 Each human knee was subjected to 10 cycles of the ovine gait path and 10 cycles of the human gait path while recording the 3D joint forces and moments. Next, the bony interaction and all soft tissue except the ACL were removed from each joint, leaving the ACL as the only structure transmitting force and moments across the joint. The testing procedures were then repeated for the ACL condition to acquire 3D ACL forces and moments. The intact joint and ACL kinetics were analyzed to determine the differences in joint kinetics produced by the human and ovine motions.
Joint Preparation
Human cadaveric lower limbs (4 female, 2 male, age: 83 ± 4 (SEM) years) were stored at −20 °C until the day prior to testing. All human knees were screened for soft tissue injuries and joint degeneration. Each knee was dissected free of all muscles and tendons, leaving the knee joint capsule, the collateral and cruciate ligaments, and both menisci intact. The tibia was then potted in a custom tibial fixture. The detailed joint preparation, including the use of the custom tibial fixture to establish the tibial joint coordinate system13 has been reported previously.7
Robot Setup
The tibial fixture was attached to the robot and aligned to the robot axes, and the tibial joint center point (JCP) was digitized using a 3D coordinate measurement machine (CMM; Faro Digitizer Model #F04L2; FARO Technologies Inc., Lake Mary, FL). We first attached the tibial fixture to the load cell (attached to the robot end effector) and adjusted the fixture to align the tibial joint coordinate system with the robot and load cell axes. A point on the tibial ACL insertion site (between the tibial spines) was digitized as our tibial JCP. We input the 3D tibial JCP coordinates into our robot software and load cell software so that all rotations and translations were imposed about this point and all forces and moments were recorded about this point. Note that the remaining portion of the setup procedure depended on the motion which was being simulated, due to the different starting positions for the human and ovine motions.
For the human motion, we attached the femur to the base fixture and adjusted the joint position to the starting position for the human motion. The robot arm was adjusted to guide the hanging femur onto the femoral base fixture. The forces and moments were zeroed in the load cell software prior to securing the femur to the base fixture. The flexion angle was measured with the CMM and adjusted to 45°, where small translations were used to minimize the forces and moments to <5 N and <1 Nm, respectively. This flexion angle corresponds to a point during swing where we assume joint loads to be minimal. A portion of the human gait path was applied to move the joint to 20° of flexion, which corresponds to the maximum flexion angle during stance of the human gait path. At this flexion angle, the anatomical points required to establish the tibial and femoral joint coordinate systems13 were marked. These points were then digitized and the 3D coordinates were input into a custom MATLAB program which output the 6 DOF position of the tibial JCP with respect to the femoral JCP and calculated the 3 rotations required to match the rotational positions reported by Lafortune et al.22 at mid-stance. The robot was adjusted to achieve these rotations. This process was repeated until each rotational position was validated to within ±0.5°. The robot was then adjusted to achieve the anterior-posterior and medial–lateral displacements reported by Lafortune et al.22 at mid-stance. Subsequently, 500 N of force in compression was applied to the joint. This magnitude was determined from average knee compression forces from unpublished data acquired by Hewett et al. We then applied 10 cycles of the human gait path, which reduced the force in compression. The process of applying 500 N was repeated until the joint maintained a compression force of 500 ± 10 N after 10 cycles.
For the ovine motion, we attached the femur to the base fixture and adjusted the joint position to the starting position for the ovine motion. The robot arm was adjusted to guide the hanging femur onto the femoral base fixture. The forces and moments were zeroed in the load cell software prior to securing the femur to the base fixture. The flexion angle was measured with the CMM and adjusted to 60.5°, where small translations were used to minimize the forces and moments to <5 N and <1 Nm, respectively. This flexion angle was selected as our ovine starting point because we hypothesized that the joint is minimally loaded at the midpoint of joint flexion during swing since there is an absence of ground reaction forces.
Robot Testing
All tests were performed at room temperature. The intact human knee was subjected to two different simulated motions, each with a different starting position. At the ovine starting position, we applied the first set of 10 cycles of the ovine gait path to precondition the joint, followed by another 10 cycles to record the forces and moments for the intact knee. We then adjusted the robot position to the starting position of the human motion, where we applied 10 cycles of the simulated human gait path to precondition the joint, followed by another 10 cycles to measure the forces and moments for the intact knee. All soft tissue and the distal portions of the femoral condyles were removed to attain the ACL condition. Another 10 cycles of the human gait path were again applied to record the ACL forces and moments. We then adjusted the robot position to the ovine starting position and applied 10 cycles of the ovine gait path to record the human ACL forces and moments for the ovine motion. Finally, the ACL was removed and the forces and moments were recorded for the bone-only conditions of both the ovine and human motions.
Data Analysis
The forces and moments recorded for the bone-only condition were subtracted from the joint and ACL kinetic measurements to eliminate the effect of tibial weight and robot inertia. The 8th and 9th cycles of each 10 cycle test were averaged, normalized to consecutive heel strikes, and used for analysis to eliminate initial cycle effects. For each intact joint and ACL, forces and moments were analyzed in the tibial reference frame based on the knee joint coordinate system.13 All forces and moments were analyzed for the stance and swing phases of gait.
Statistical Analysis
All force and moment data was normal and heteroscedastic. For both the intact and ACL conditions, a repeated measures analysis of variance (ANOVA) was performed for the stance and swing phases across all directions of force and moment with the motion as a fixed factor. Post hoc comparisons were performed using a Tamhane correction. The significance level for all comparisons was set at p < 0.05.
RESULTS
Intact Joint
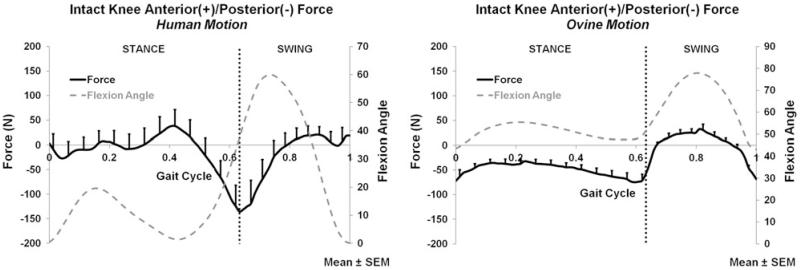
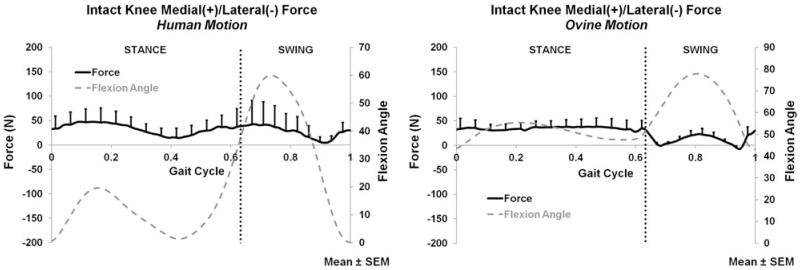
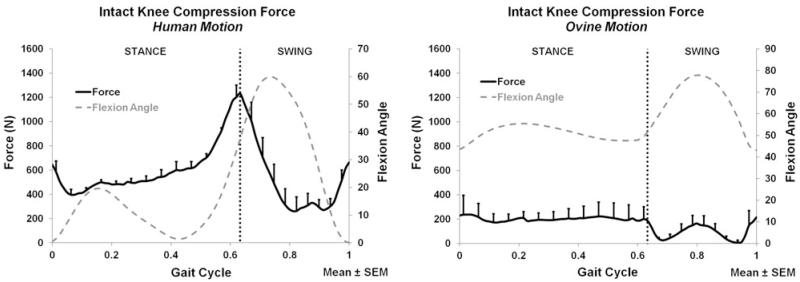
The intact knee force and moment measurements (average and peak) for both the applied human and ovine motions are listed in Table 1. The anterior–posterior (A-P), medial–lateral (M-L), compression–distraction (C-D) forces and corresponding flexion angle are plotted for the entire gait cycle in Figs. 1, 2, and 3, respectively.
TABLE 1.
Intact knee forces and moments produced by the human and ovine motions.
| Human motion |
Ovine motion |
|||||
|---|---|---|---|---|---|---|
| Avg. ± SEM | Direction | Peak | Avg. ± SEM | Direction | Peak | |
| Stance phase | ||||||
| Forces (N) | 12.6 ± 31.2 | Posterior | 135 | 49.0 ± 10.3 | Posterior | 74.8 |
| 33.6 ± 23.5 | Medial | 47.8 | 34.6 ± 15.3 | Medial | 38.1 | |
| 609 ± 45.8 | Compression | 1.23 × 103 | 202 ± 91.7 | Compression | 238 | |
| Moments (N–m) | 10.7 ± 6.95 | Adduction | 18.1 | 9.29 ± 3.26 | Adduction | 10.2 |
| 2.22 ± 2.26 | Extension | 12.4 | 3.59 ± 0.866 | Extension | 4.35 | |
| 2.09 ± 1.89 | Internal | 5.24 | 1.56 ± 0.654 | Internal | 2.34 | |
| Swing phase | ||||||
| Forces (N) | 23.2 ± 29.7 | Posterior | 135 | 0.711 ± 8.03 | Posterior | 71.3 |
| 27.8 ± 30.6 | Medial | 42.6 | 13.4 ± 8.66 | Medial | 34.6 | |
| 522 ± 115 | Compression | 1.23 × 103 | 98.1 ± 60.2 | Compression | 215 | |
| Moments (N–m) | 6.72 ± 5.06 | Adduction | 17.6 | 2.85 ± 2.20 | Adduction | 9.94 |
| 6.52 ± 2.90 | Extension | 21.4 | 1.45 ± 1.24 | Extension | 4.35 | |
| 0.584 ± 0.852 | Internal | 2.33 | 0.0392 ± 0.297 | Internal | 2.29 | |
FIGURE 1.
Anterior/posterior force of the intact knee for the human motion (left) and ovine motion (right); N = 6.
FIGURE 2.
Medial/lateral force of the intact knee for the human motion (left) and ovine motion (right); N = 6.
FIGURE 3.
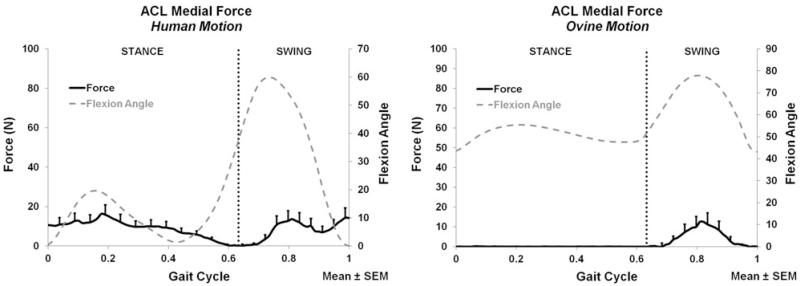
Compression force of the intact knee for the human motion (left) and ovine motion (right); N = 6.
During both stance and swing phases, only C-D forces (Fig. 3) significantly differed between the human and ovine applied motions. The A-P (Fig. 1) and M-L (Fig. 2) forces, and all directions of moments did not significantly differ between the two motions during stance or swing. During stance, the only significant difference was the larger compression force produced by the human motion (p < 0.016); Fig. 3. During swing, the human motion produced a significantly larger compression force than the ovine motion (p < 0.017); Fig. 3.
ACL
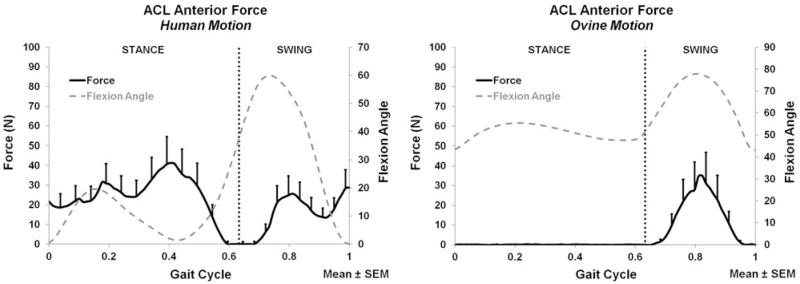
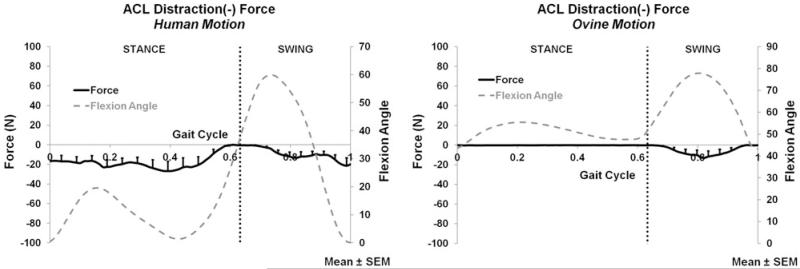
The ACL force and moment measurements (average and peak) for both the applied human and ovine motions are listed in Table 2. The A-P, M-L, C-D forces and corresponding flexion angle are plotted for the entire gait cycle in Figs. 4, 5, and 6, respectively. During both stance and swing phases, the human and ovine motions did not produce significant force or moment differences.
TABLE 2.
ACL forces and moments produced by the human and ovine motions.
| Human motion |
Ovine motion |
|||||
|---|---|---|---|---|---|---|
| Avg. ± SEM | Direction | Peak | Avg. ± SEM | Direction | Peak | |
| Stance phase | ||||||
| Forces (N) | 24.0 ± 8.45 | Anterior | 41.4 | 0.0480 ± 0.0964 | Anterior | 0.256 |
| 8.78 ± 2.95 | Medial | 16.3 | 0.0327 ± 0.0636 | Medial | 0.224 | |
| 17.5 ± 6.28 | Distraction | 26.8 | 0.175 ± 0.0657 | Distraction | 0.345 | |
| Moments (N–m) | 0.445 ± 0.154 | Adduction | 0.868 | 0.0136 ± 0.0191 | Adduction | 0.071 |
| 1.56 ± 0.593 | Flexion | 2.82 | Neglible load (<0.01 N–m) | |||
| 0.104 ± 0.0405 | External | 0.176 | Neglible load (<0.01 N–m) | |||
| Swing phase | ||||||
| Forces (N) | 15.1 ± 5.94 | Anterior | 28.9 | 12.6 ± 6.58 | Anterior | 35.0 |
| 7.95 ± 3.31 | Medial | 14.3 | 4.25 ± 2.52 | Medial | 12.6 | |
| 9.38 ± 3.49 | Distraction | 21.37 | 5.30 ± 2.65 | Distraction | 13.0 | |
| Moments (N–m) | 0.420 ± 0.199 | Adduction | 0.857 | 0.197 ± 0.217 | Adduction | 0.662 |
| 1.02 ± 0.410 | Flexion | 1.94 | 0.877 ± 0.475 | Flexion | 2.38 | |
| 0.0805 ± 0.0456 | External | 0.143 | 0.0778 ± 0.0598 | External | 0.216 | |
FIGURE 4.
Anterior ACL force for the human motion (left) and ovine motion (right); N = 6.
FIGURE 5.
Medial ACL force for the human motion (left) and ovine motion (right); N = 6.
FIGURE 6.
Distraction ACL force for the human motion (left) and ovine motion (right); N = 6.
DISCUSSION
This study investigated the 3D kinetics of the human intact knee and ACL using simulated in vivo motions. To our knowledge, this is the first study to measure the 3D intact knee and ACL forces and moments using this methodology. The intact knee and ACL loads reported in this study coincide with findings from previous studies which implemented different methods to estimate these loads. Since we are unable to conduct controlled in vivo investigations of injuries and healing in human subjects, a biomechanical surrogate is needed. By applying both human and ovine simulated in vivo motions to the human knee, we began to explore the ovine stifle joint as a biomechanical surrogate for the human knee. While the compression-distraction (C-D) force significantly differed between motions for the intact knee, there were no other significant differences between the intact knee or ACL kinetics produced by the human and ovine motions. The method used to apply the human motion may have contributed to the significantly higher compression force produced by the human motion. Since the simulated ovine and human kinematics produced similar intact knee and ACL kinetics, we will proceed to use the ovine surrogate to investigate the biomechanical effects of various knee injuries and repair strategies (e.g. ACL reconstructions).
The human joint compression forces for the simulated human motion are comparable to some previous findings. The peak compression forces reported in this study are comparable to some previous estimates of human tibiofemoral contact forces. Several studies applied inverse dynamics approaches to estimate joint compression forces of 2.0–3.0 BW, with some estimates as high as 6.0 BW, for the normal human knee joint during level walking.14,26,41 Alternatively, D’Lima et al.10 used instrumented total knee replacements to measure compression forces of 1.8–2.5 BW for treadmill walking. Our average human knee compression forces (Human–Human condition) peaked at 1232 ± 112 (SEM) N which would correspond to ≈2.0 BW in a 63 kg individual. Unfortunately, we had no knowledge of the actual body weights. Thus, we applied 500 N of compression force to each human knee prior to applying the human motion, based on average knee compression forces from unpublished data acquired by Hewett et al. In comparison to a previous study in which Thambyah et al.39 used inverse dynamics techniques to calculate an average compression force of 779 N at mid-stance and a peak compression magnitude of 2075 N, we applied only 500 N of compression force at mid-stance and reached a peak compression magnitude of only 1232 N. For future studies, we will investigate using a higher compression force at the mid-stance starting position for the human motion.
The restraining roles of the human ACL reported in this study are similar to those previous findings6,9,12,19,25,30,33,34 but the ACL loads reported in this study are lower than those previously reported for human gait using computational modeling. For both input motions, the ACL restrained anterior, medial, and distraction displacements, as well as adduction, external, and flexion rotations. It is not possible to directly compare our results to previous ACL biomechanics studies which were conducted using simulations of clinical tests rather than actual in vivo motions. However, the directions of anatomical displacements and rotations restrained by the ACL in this study coincide with previous findings that the human ACL serves as a major restraint to anterior tibial translation9,25,33 and a secondary restraint to medial tibial translation, joint abduction/adduction, and joint flexion.6,12,19,30,34 Shelburne et al.33 used knee computational models to estimate ACL kinetics during walking in young healthy subjects. They reported an estimated peak ACL load of 303 N which is much higher than the peak resultant ACL load of 52 N (averaged across subjects) which we measured during stance by applying the human motion to the human ACL. A limitation of this study which could have contributed to lower forces in the intact human knees and ACLs was the use of cadaveric knees from elderly subjects. The in vivo human motion which was simulated in this study was an average of motions acquired from young healthy subjects.22 However, the simulated motion was applied to older knees which exhibit reduced biomechanical properties compared to the knees of young healthy subjects.20,42 Although all joints were screened for soft tissue damage and signs of osteoarthritis, some cadaveric ACLs were minimally loaded when subjected to the human motion and thereby increased the deviation among samples.
This study had other limitations. The methods used to apply the ovine and human motions differed. The best method for each motion was selected based on the in vivo data that was available. The ovine motion was initiated during swing, where joint forces and moments were assumed minimal. Alternatively, the human motion was initiated at mid-stance, where 500 N was applied to the joint. Another limitation was that we applied average in vivo motions in this study. Ideally, the subject-specific kinematics for each joint should be recorded in vivo and applied to the same joint since the joint morphology and biomechanics will vary among subjects. Using an animal model, this methodology would allow for the measurement of 3D kinetics that would be more indicative of in vivo forces and moments. Finally, the limitations of our robot methodology including testing at room temperature have been reported previously.7
The results of this study suggest that although there are biomechanical and morphological differences between the human knee and ovine model, the walking kinematics of the two species produce similar loads in the human knee. There are differences between the knee kinematics of the two species during gait, notably the considerably smaller range of flexion in the ovine joint.36 Although the ovine stifle joint contains all of the major soft tissue structures of the human knee, there are differences in joint size and morphology between the ovine stifle and human knee joints.1,29 However, the 3D kinetic measurements suggest that there are similarities in the knee kinetics produced by the motions of the different species. With the exception of compression-distraction forces, the ovine and human motions did not produce significantly different forces and moments in the human intact knee.
These findings have important implications for designing future studies. Since the normal ovine and human motions produced similar human knee kinetics, applying ovine in vivo motions recorded for injury and repaired conditions to human knees may provide helpful parameters for the development of treatments for the human knee. We have also begun to measure and simulate the subject-specific 3D kinematics of normal and injured ovine knees to measure the corresponding 3D joint and ACL kinetics. Applying subject-specific kinematics should provide more accurate estimates of in vivo joint and ACL forces. Using ovine stifle joint kinematics for normal, injury, and repaired conditions, we plan to establish design criteria and evaluation benchmarks for traditional and tissue-engineered ACL reconstructions with the long term goal of minimizing joint degeneration after ACL injury.
ACKNOWLEDGMENTS
This work was partially supported by NIH grants EB004859 and AR056660. The authors do not have any conflicts of interest.
REFERENCES
- 1.Allen MJ, Houlton JEF, Adams SB, Rushton N. The surgical anatomy of the stifle joint in sheep. Vet. Surg. 1998;27:596–605. doi: 10.1111/j.1532-950x.1998.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 2.Amis AA, Bull AMJ, Lie DTT. Biomechanics of rotational instability and anatomic anterior cruciate ligament reconstruction. Oper. Tech. Orthopaed. 2005;15:29–35. [Google Scholar]
- 3.Andriacchi TP, Dyrby CO. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J. Biomech. 2005;38:293–298. doi: 10.1016/j.jbiomech.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J. Bone Joint Surg. A. 2009;91:95–101. doi: 10.2106/JBJS.H.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appleyard RC, Burkhardt D, Ghosh P, Read R, Cake M, Swain MV, Murrell GAC. Topographical analysis of the structural, biochemical and dynamic biomechanical properties of cartilage in an ovine model of osteoarthritis. Osteoarthr. Cartilage. 2003;11:65–77. doi: 10.1053/joca.2002.0867. [DOI] [PubMed] [Google Scholar]
- 6.Berchuck M, Andriacchi TP, Bach BR, Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J. Bone Joint Surg. A. 1990;72:871–877. [PubMed] [Google Scholar]
- 7.Boguszewski DV, Shearn JT, Wagner CT, Butler DL. Investigating the effects of anterior tibial translation on anterior knee force in the porcine model: Is the porcine knee ACL dependent? J. Orthopaed. Res. 2011;29:641–646. doi: 10.1002/jor.21298. [DOI] [PubMed] [Google Scholar]
- 8.Butler DL, Shearn JT, Juncosa N, Dressler MR, Hunter SA. Functional tissue engineering parameters toward designing repair and replacement strategies. Clin. Orthop. Relat. Res. 2004;427:S190–S199. doi: 10.1097/01.blo.0000144858.65450.d2. [DOI] [PubMed] [Google Scholar]
- 9.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J. Bone Joint Surg. A. 1980;62:259–270. [PubMed] [Google Scholar]
- 10.D’Lima DD, Steklov N, Fregly BJ, Banks SA, Colwell CW., Jr. In vivo contact stresses during activities of daily living after knee arthroplasty. J. Orthopaed. Res. 2008;26:1549–1555. doi: 10.1002/jor.20670. [DOI] [PubMed] [Google Scholar]
- 11.Georgoulis AD, Ristanis S, Chouliaras V, Moraiti C, Stergiou N. Tibial rotation is not restored after ACL reconstruction with a hamstring graft. Clin. Orthop. 2007;(454):89–94. doi: 10.1097/BLO.0b013e31802b4a0a. [DOI] [PubMed] [Google Scholar]
- 12.Gollehon DL, Torzilli PA, Warren RF. The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J. Bone Joint Surg. A. 1987;69:233–242. [PubMed] [Google Scholar]
- 13.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: Application to the knee. J. Biomech. Eng. 1983;105:136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 14.Harrington IJ. A bioengineering analysis of force actions at the knee in normal and pathological gait. Bio-Med. Eng. 1976;11:167–172. [PubMed] [Google Scholar]
- 15.Herfat ST, Shearn JT, Bailey DL, Greiwe RM, Galloway MT, Gooch C, Butler DL. Effect of surgery to implant motion and force sensors on vertical ground reaction forces in the ovine model. J. Biomech. Eng. 2011;133 doi: 10.1115/1.4003322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am. J. Sports Med. 1999;27:699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- 17.Hunt P, Scheffler SU, Unterhauser FN, Weiler A. A model of soft-tissue graft anterior cruciate ligament reconstruction in sheep. Arch. Orthop. Trauma Surg. 2005;125:238–248. doi: 10.1007/s00402-004-0643-z. [DOI] [PubMed] [Google Scholar]
- 18.Jomha NM, Borton DC, Clingeleffer AJ, Pinczewski LA. Long term osteoarthritic changes in anterior cruciate ligament reconstructed knees. Clin. Orthop. 1999;(358):188–193. [PubMed] [Google Scholar]
- 19.Kanamori A, Woo SL, Ma CB, Zeminski J, Rudy TW, Li G, Livesay GA. The forces in the anterior cruciate ligament and knee kinematics during a simulated pivot shift test: a human cadaveric study using robotic technology. Arthroscopy. 2000;16:633–639. doi: 10.1053/jars.2000.7682. [DOI] [PubMed] [Google Scholar]
- 20.Kempson GE. Relationship between the tensile properties of articular cartilage from the human knee and age. Ann. Rheum. Dis. 1982;41:508–511. doi: 10.1136/ard.41.5.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.S Kuster, M., Wood GA, Stachowiak GW, Gächter A. Joint load considerations in total knee replacement. J. Bone Joint Surg. B. 1997;79:109–113. doi: 10.1302/0301-620x.79b1.6978. [DOI] [PubMed] [Google Scholar]
- 22.Lafortune MA, Cavanagh PR, Sommer HJ, III, Kalenak A. Three-dimensional kinematics of the human knee during walking. J. Biomech. 1992;25:347–357. doi: 10.1016/0021-9290(92)90254-x. [DOI] [PubMed] [Google Scholar]
- 23.Lohmander LS, Östenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 24.Lu Y, Markel MD, Nemke B, Wynn S, Graf B. Comparison of single-versus double-tunnel tendon-to-bone healing in an ovine model: a biomechanical and histological analysis. Am. J. Sports Med. 2009;37:512–517. doi: 10.1177/0363546508327543. [DOI] [PubMed] [Google Scholar]
- 25.Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee: the contributions of the supporting structures. A quantitative in vitro study. J. Bone Joint Surg. A. 1976;58:583–594. [PubMed] [Google Scholar]
- 26.Morrison JB. The mechanics of the knee joint in relation to normal walking. J. Biomech. 1970;3:51–61. doi: 10.1016/0021-9290(70)90050-3. [DOI] [PubMed] [Google Scholar]
- 27.Munirah S, Samsudin OC, Chen HC, Sharifah Salmah SH, Aminuddin BS, Ruszymah BHI. Articular cartilage restoration in load-bearing osteochondral defects by implantation of autologous chondrocyte-fibrin constructs: an experimental study in sheep. J. Bone Joint Surg. B. 2007;89:1099–1109. doi: 10.1302/0301-620X.89B8.18451. [DOI] [PubMed] [Google Scholar]
- 28.Oakley SP, Lassere MN, Portek I, Szomor Z, Ghosh P, Kirkham BW, Murrell GAC, Wulf S, Appleyard RC. Biomechanical, histologic and macroscopic assessment of articular cartilage in a sheep model of osteoarthritis. Osteoarthr. Cartilage. 2004;12:667–679. doi: 10.1016/j.joca.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Osterhoff G, Löffler S, Steinke H, Feja C, Josten C, Hepp P. Comparative anatomical measurements of osseous structures in the ovine and human knee. Knee. 2011;18:98–103. doi: 10.1016/j.knee.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Piziali RL, Seering WP, Nagel DA, Schurman DJ. The function of the primary ligaments of the knee in anterior-posterior and medial-lateral motions. J. Biomech. 1980;13:777–784. doi: 10.1016/0021-9290(80)90239-0. [DOI] [PubMed] [Google Scholar]
- 31.Radford WJP, Amis AA, Stead AC. The ovine stifle as a model for human cruciate ligament surgery. Vet. Comp. Orthopaed. Traumatol. 1996;9:134–139. [Google Scholar]
- 32.Roberts CS, Cumming JF, Grood ES, Noyes FR. In vivo measurement of human anterior cruciate ligament forces during knee extension exercises. Trans. 40th Orthopaedic Research Society. 1994:15–84. [Google Scholar]
- 33.Sakane M, Livesay GA, Fox RJ, Rudy TW, Runco TJ, Woo SL. Relative contribution of the ACL, MCL, and bony contact to the anterior stability of the knee. Knee Surg. Sports Traumatol. Arthrosc. 1999;7:93–97. doi: 10.1007/s001670050128. [DOI] [PubMed] [Google Scholar]
- 34.Seering WP, Piziali RL, Nagel DA, Schurman DJ. The function of the primary ligaments of the knee in varus-valgus and axial rotation. J. Biomech. 1980;13:785–794. doi: 10.1016/0021-9290(80)90240-7. [DOI] [PubMed] [Google Scholar]
- 35.Tapper JE, Fukushima S, Azuma H, Sutherland C, Marchuk L, Thornton GM, Ronsky JL, Zernicke R, Shrive NG, Frank CB. Dynamic in vivo three-dimensional (3D) kinematics of the anterior cruciate ligament/medial collateral ligament transected ovine stifle joint. J. Orthopaed. Res. 2008;26:660–672. doi: 10.1002/jor.20557. [DOI] [PubMed] [Google Scholar]
- 36.Tapper JE, Fukushima S, Azuma H, Thornton GM, Ronsky JL, Shrive NG, Frank CB. Dynamic in vivo kinematics of the intact ovine stifle joint. J. Orthopaed. Res. 2006;24:782–792. doi: 10.1002/jor.20051. [DOI] [PubMed] [Google Scholar]
- 37.Taylor WR, Heller MO, Bergmann G, Duda GN. Tibio-femoral loading during human gait and stair climbing. J. Orthopaed. Res. 2004;22:625–632. doi: 10.1016/j.orthres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Taylor WR, Poepplau BM, König C, Ehrig RM, Zachow S, Duda GN, Heller MO. The medial-lateral force distribution in the ovine stifle joint during walking. J. Orthopaed. Res. 2011;29:567–571. doi: 10.1002/jor.21254. [DOI] [PubMed] [Google Scholar]
- 39.Thambyah A, Pereira BP, Wyss U. Estimation of bone-on-bone contact forces in the tibiofemoral joint during walking. Knee. 2005;12:383–388. doi: 10.1016/j.knee.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: A study of radiographic and patient relevant outcomes. Ann. Rheum. Dis. 2004;63:269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winby CR, Lloyd DG, Besier TF, Kirk TB. Muscle and external load contribution to knee joint contact loads during normal gait. J. Biomech. 2009;42:2294–2300. doi: 10.1016/j.jbiomech.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 42.Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am. J. Sports Med. 1991;19:217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- 43.Xerogeanes JW, Fox RJ, Takeda Y, Kim H, Ishebashi Y, Carlin GJ, Woo SL. A Functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann. Biomed. Eng. 1998;26:345–352. doi: 10.1114/1.91. [DOI] [PubMed] [Google Scholar]