Summary
Because most patients with cancer are aged and because immunological functions are altered during aging, it is important to account for aging-associated immunological alterations in the design of new cancer immunotherapies. We thus compared immune populations in young and aged mice and found that B7-DC+ (PD-L2/CD273) B cells, a minor population in young mice, were significantly increased in aged mice. Induction of both Th1 and Th17 cells was significantly augmented by B7-DC+ B cells from aged mice, and this effect was blocked with anti-B7-DC antibodies in vitro and in vivo. Moreover, retardation of tumor growth in aged mice was largely B7-DC dependent. Tumor growth in young mice was significantly inhibited by immunization with B7-DC+ B cells from aged mice owing to increased induction of tumor antigen-specific cytotoxic T lymphocytes. These data indicate that B7-DC+ B cells could play an important role in aging-associated cancer immunopathology as well as in other aging-associated diseases and further suggest that B7-DC+ B cells have potential for future cancer immunotherapy.
Keywords: B7-DC, PD-L2, co-stimulation, B cell, cytotoxic T lymphocyte, antitumor immunity, Th17
Introduction
Immunological functions are significantly altered during aging (Khatami, 2009; Lustgarten, 2009; Shaw et al., 2010). For example, the supply of new naïve T cells from thymus is significantly decreased owing to thymic involution in aged individuals. Moreover, antigen-presenting and T-cell priming functions of dendritic cells (DCs) are significantly decreased compared with young individuals and are associated with deficiencies in the up-regulation of costimulatory molecules on aged DCs. Therefore, the immune system in aged individuals responds poorly to new antigens, helping explain why they are generally more susceptible than young individuals to new pathogens. Furthermore, it has been reported that immunosuppressive populations such as regulatory T cells (Tregs) and myeloid-derived suppressor cells are increased with age although total leukocyte numbers are decreased in aged individuals (Nishioka et al., 2006; Grizzle et al., 2007; Lages et al., 2008). These populations contribute to impaired immune responses by suppressing T-cell-mediated immunity through cell–cell contact and various soluble factors. Although these findings suggest that it is important to design immune therapies for aged individuals that account for these significant age-associated changes, our knowledge of these changes is incomplete.
For example, cancer immunotherapy holds promise because of its potential specificity with minimal side effects. However, clinical responses in clinical trials of cancer immunotherapies to date generally been only modest. Although more than 90% of patients with cancer are middle aged or older, most preclinical cancer work have been done in young mice, presumably due to convenience, time, and cost. Consequently, important information regarding aged individuals is rarely taken into consideration in designing cancer immunotherapy.
Based on these considerations and the importance of co-stimulatory and co-inhibitory molecules in regulating immunity, we sought to characterize the effects of aging on the expression and functional consequences of some of these molecules in various immune cell subsets. Co-stimulatory and co-inhibitory molecules are particularly important for the control of T-cell-mediated immunity (Chen, 2004). After naïve T-cell priming by professional antigen-presenting cells (APCs), especially DCs, the quality of T-cell function is determined by the character of the interactions between co-receptors and co-ligands. B7-DC (CD273/PD-L2) and B7-H1 (CD274/ PD-L1) bind the same inhibitory receptor, programmed cell death (PD)-1 (CD279), which is inducible on activated T and B cells and plays an essential role in suppressing autoimmunity and inflammation (Okazaki & Honjo, 2007). More important, the PD-1/B7-H1 pathway plays an important role in tumor evasion and T-cell exhaustion in chronic infection and cancer (Barber et al., 2006; Day et al., 2006; Blackburn et al., 2009).
The physical binding between B7-DC and PD-1 was confirmed in vitro (Latchman et al., 2001; Tseng et al., 2001). However, the functional consequences of B7-DC binding to PD-1 have not been fully established. Ample evidence supports the idea that in peripheral tissues, B7-H1, which is expressed ubiquitously, is a dominant ligand for PD-1-mediated inhibition. In contrast, many reports suggest that B7-DC supports PD-1-independent T-cell proliferation and Th1 immunity in vitro and in vivo, including the generation of tumor antigen-specific cytotoxic T lymphocytes (CTLs), which is beneficial to anticancer immune responses (Tseng et al., 2001; Shin et al., 2003, 2005; Matsumoto et al., 2004, 2008; Okazaki & Honjo, 2007; Tsushima et al., 2007; Ishiwata et al., 2010). Because B7-DC expression is restricted and it is normally inside DCs and macrophages, not on their surfaces, induction of peripheral tolerance by PD-1 is most likely independent of B7-DC. We showed that in young B7-DC knock out (KO) mice, T-cell proliferation was diminished, the Th1 response was decreased, tumor antigen-specific CTL generation was impaired, and tumor growth was facilitated. These observations demonstrate the importance of B7-DC signals in T-cell proliferation and Th1 type T-cell-mediated immunity in antitumor immunity.
In this study, we compared immune populations between young and aged mice and found that many reported aging-associated immune alterations are present, but not significant. However, one significant and unexpected difference was a consistent increase in B7-DC+ B cells in lymphoid organs of aged mice. We found that B7-DC+ B cells in aged mice induced significant Th17 and Th1 cells in vitro and in vivo and that these contributed to antitumor immunity. These results provide a new perspective on the age-related role of co-signaling molecule expression on B cells in the regulation of cancer immunity.
Results
B7-DC+ B cells increase significantly in aged mice
To evaluate the immunological alterations in aging, we compared immune cell populations between young and aged mice. Young mice were 3–5 months old (equivalent to 13–16 years in humans), and aged mice were more than 24 months old (equivalent to older than 65 years in humans). Total leukocyte numbers were decreased in spleens and lymph nodes and increased in bone marrow (BM) of aged mice, although these changes did not achieve statistical significance (Fig. S1). In the general population of T cells, B cells, and myeloid cells, both CD4+ T cells and CD8+ T cells were decreased in spleens and lymph nodes of aged mice whereas they were increased in BM (Fig S2–S8). PD-1+ cells were increased significantly in the spleen, lymph node, and BM of aged mice. The significant increase in PD-1+ T cells in aged mice was previously reported as representing T cells that were hypo-proliferative with reduced cytokine production following stimulation (Channappanavar et al., 2009; Shimada et al., 2009; Shimatani et al., 2009; Lages et al., 2010). We also observed an increase in plasmacytoid DCs (CD11b−CD11c+B220+ cells) in spleens of aged mice (Fig S2 and S3).
Although B7-DC expression was originally thought to be restricted to DCs and macrophages (Tseng et al., 2001; Yamazaki et al., 2002), we show that this is not so. We found a significant increase in B7-DC+B220+CD19+ B cells representing an average of 5.5% of total leukocytes in spleen, 2.8% in lymph node, 2.7% in BM, and 0.8% in peripheral blood of aged mice (Fig. 1A and Fig. S9). This population was a minor population in young mice representing an average of ≤ 1% of total leukocytes in spleen and < 0.5% in lymph node, BM, and peripheral blood. Although the total number of B cells was not significantly altered in aged mice (Fig S2–S8), B7-DC+ B cells were increased and comprised more than 10% of total B cells (Fig. 1B). Consequently, in aged mice, the total number of B7-DC+ B cells reached more than four times the number in spleen, eight times the number in lymph node and 10 times the number in BM vs. young mice (3.8 ± 1.8 × 106 vs. 0.9 ± 0.46 × 106 in spleen, 4.3 ± 2.0 × 105 vs. 0.52 ± 0.18 × 105 in lymph node and 6.3 ± 3.6 × 105 vs. 2.1 ± 0.34 × 105 in BM, Fig. 1C). We next investigated the kinetics of the increase in B7-DC+ B cells. In spleen, B7-DC+ B cells progressively increased until 18 months of age after which age numbers plateaued. In BM, the kinetics were slower; B7-DC+ B cells began to increase after 18 months of age (Fig. 1D).
Fig. 1.
Significant increase in B7-DC+ B cells in aged mice. (A) Representative flow cytometry analysis of spleen, lymph node (LN), bone marrow (BM), and peripheral blood (Blood) cells of young (3–5 months old) and aged (24–26 months old) mice. Numbers are percent gated events. (B, C) Summary of the percentage (B) and total number (C) of B7-DC+ B cells from the indicated sources (10–15 mice per group) are shown. B cells are defined here as CD3−CD19+B220+CD11c− cells. (D) The kinetics of the appearance (percentage and total number) of B7-DC+ B cells in the spleen and BM of naïve mice (5–15 mice per group).
B7-DC+ B cells express an activated phenotype suggesting a unique B-cell subset
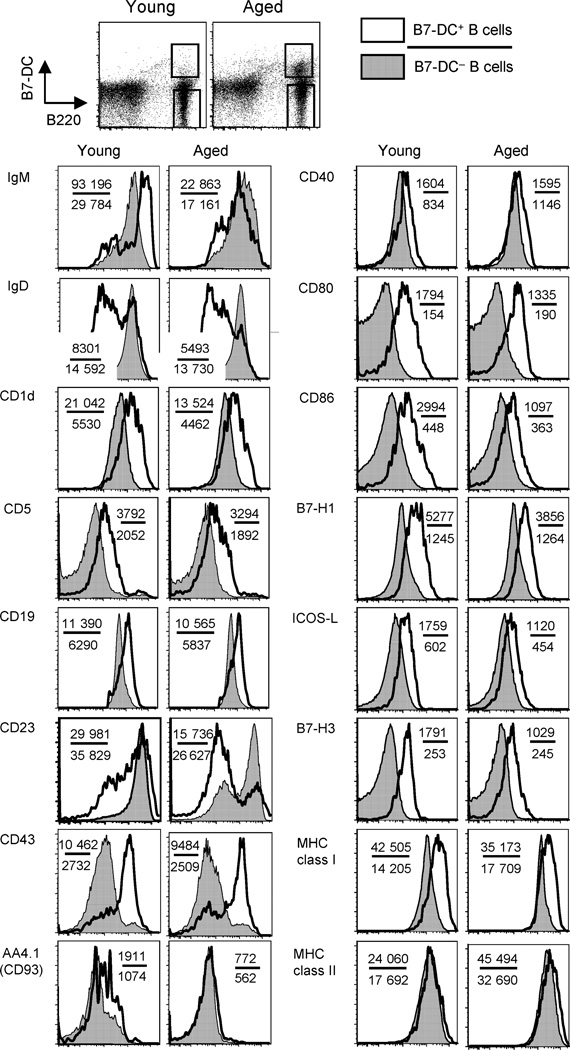
The phenotype of B7-DC+ B cells was IgMhigh, IgDlow, CD1d+, CD5+, CD19high, CD23low, CD43+, and AA4.1 (CD93)− (Fig. 2), corresponding to mature B cells, and partially to B-1 B cells, marginal zone B cells and regulatory B cells (Pillai & Cariappa, 2009). Unlike B-1 B cells, they are prevalent outside the peritoneum. They differ from marginal zone B cells in CD5 and CD43 expression and from regulatory B cells in CD43 expression. The expression of the co-signaling molecules CD80, CD86, B7-H1, ICOS-L, and B7-H3 were slightly up-regulated along with major histocompatibility complex (MHC) class I in B7-DC+ B cells compared with B7-DC− B cells; however, almost no differences were observed in CD40 or MHC class II. These observations indicated that B7-DC+ B cells express an activated phenotype and are not easily classified into existing B-cell subsets. Moreover, there were no significant differences in expression of any of these molecules between B7-DC+ B cells from young and aged mice, suggesting a simple age-related increase in number. We further observed increased B7-DC+ B cells with this phenotype in aged Balb/C and Ames mice (data not shown), demonstrating that these cells are not peculiar to the C57BL/6 background. Finally, we confirmed that B-1 cells in the mouse peritoneum express B7-DC consistent with a prior report (Zhong et al., 2007); however, the total number of this population in naïve young mice is small (~8000 cells per mouse) and was unchanged in aged mice (data not shown).
Fig. 2.
Flow cytometric phenotype of spleen B7-DC+ B cells from young and aged mice. Expression profiles of surface molecules on B7-DC+ or B7-DC− B cells were examined. Open histograms indicate analyses of the B7-DC+ B cells; closed histograms indicate B7-DC− B cells. Results are representative of five experiments with similar results. Numbers in the panels indicates mean fluorescence intensity of each molecule in B7-DC+ B cells (upper figure) and B7-DC− B cells (lower figure).
IL-4-independent control of B cell B7-DC expression differs from DC or macrophage control
B7-DC expression is strongly induced in myeloid cells by IL-4 and IL-13 (Tseng et al., 2001; Yamazaki et al., 2002; Loke & Allison, 2003; Matsumoto et al., 2008). We cultured single-cell suspensions of spleen from young and aged mice ± recombinant IL-4. As reported, B7-DC expression was significantly enhanced on conventional DCs (CD11c+B220−) and macrophages (CD11b+Gr-1 −) from both young and aged mice (Fig. S10). In addition, B7-DC was slightly increased on plasmacytoid DCs (CD11c+B220+) and granulocytes (CD11b+Gr-1+). However, B7-DC expression was not induced on B cells (CD11c−B220+) in either young or aged mice. In fact, the B7-DC+ fraction in CD11c−B220+ cells of aged mice was diminished by overnight culture with recombinant IL-4, indicating that the regulation of B7-DC expression on B cells in aged mice was strikingly different from the regulation of B7-DC expression on DCs and macrophages.
Aged B7-DC+ B cells are not regulatory and induce Th1 and Th17 immunity in vitro
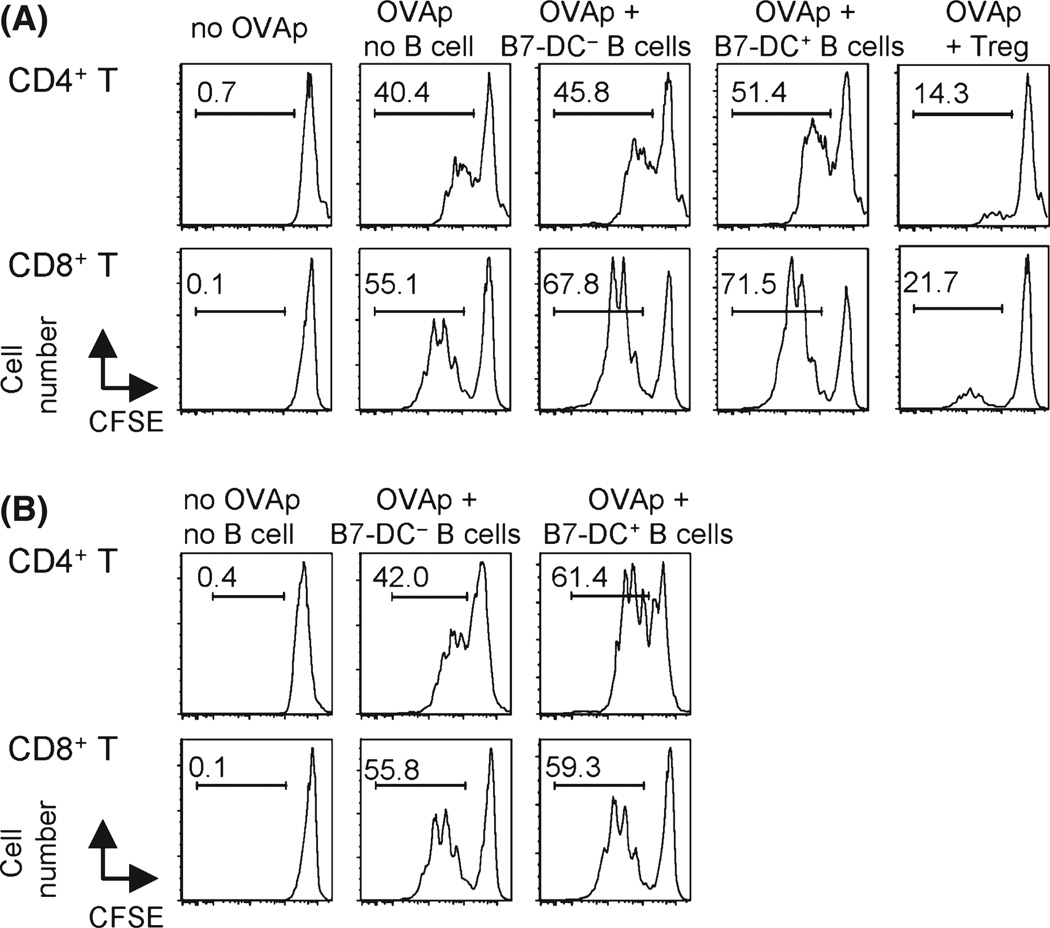
Based on phenotype, we first suspected that B7-DC+ B cells were regulatory B cells with immunosuppressive function through IL-10 production (Vitale et al., 2010). However, B7-DC+B cells from aged mice did not suppress T-cell proliferation in vitro; on the contrary, they augmented T-cell proliferation (Fig. 3A). Suppressive function was not observed in B7-DC+ B cells from young mice, and IL-10 was not produced by B7-DC+ B cells from either young or aged mice (not shown). Therefore, we conclude that B7-DC+ B cells are not regulatory B cells.
Fig. 3.
B7-DC+ B cells do not suppress, but moderately augment, antigen-specific T-cell proliferation in vitro. (A) In vitro suppression assay. Single-cell suspensions of the whole spleen from OT-II or OT-I mice were cultured with or without B7-DC+ or B7-DC− B 0cells or CD4+CD25+ Tregs from naïve aged mice in the presence of cognate OVA-specific peptide (OVAp). (B) In vitro proliferation assay. OT-II or OT-I T cells (CD4+ or CD8+ OVA specific, respectively) were sorted by flow cytometry after magnetic purification, carboxyfluorescein diacetate succinimidyl ester (CFSE) labeled, and cultured with or without sorted B7-DC+ or B7-DC− B cells from aged mice in the presence or absence of the respective OVAp. T-cell proliferation was measured by CFSE dilution. The data are representative of five independent experiments with similar results. OVA, ovalbumin.
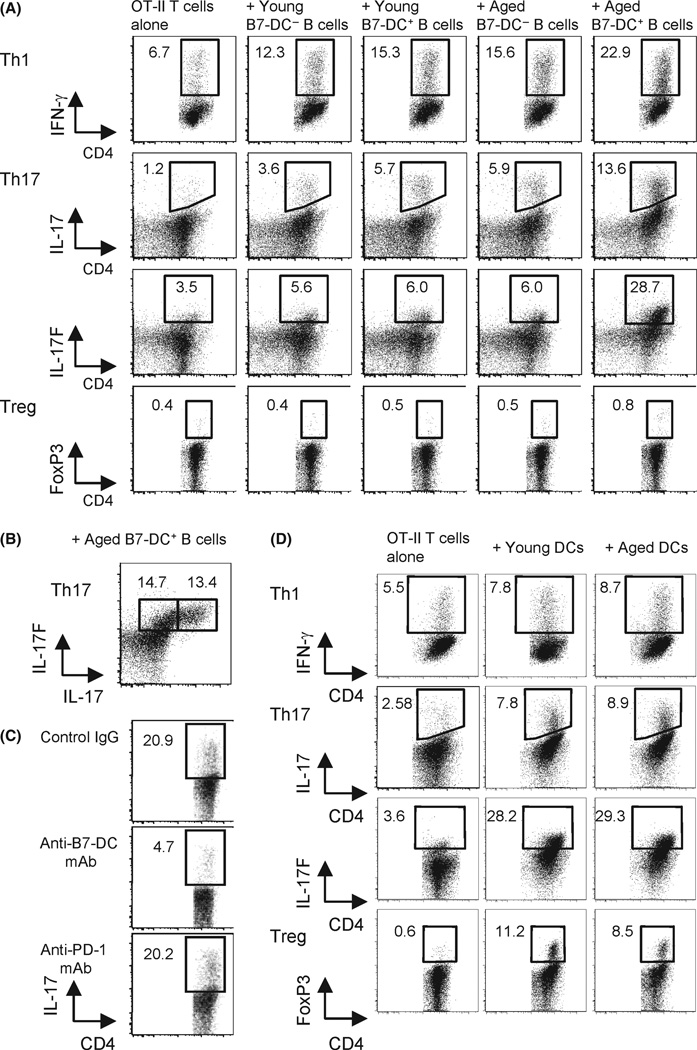
We then evaluated the possibility that B7-DC+ B cells functioned as APCs. An in vitro proliferation assay testing antigen-specific immunity to ovalbumin (OVA) was performed by culturing purified CD8+ or CD4+ T cells from OT-I or OT-II mice whose transgenic T cells recognize OVA in the context of MHC class I or class II, respectively, along with sorted B7-DC− B or B7-DC+ B cells from aged mice in the presence of cognate OVA peptide (Fig. 3B). These transgenic mice allow us to carry out focused studies of antigen-specific T-cell-mediated immune responses. We found that B7-DC+ B cells supported antigen-specific CD4+ T-cell proliferation slightly better than B7-DC− B cells; however, the difference was not significant and was similar to that of B7-DC+ DCs from young mice (Shin etal., 2003). The most conspicuous function of B7-DC expression in young mice was to enhance interferon (IFN)-γ-producing CD4+ Th1 T cells and Th1-mediated immunity in vitro and in vivo (Shin et al., 2005; Matsumoto et al., 2008). We therefore tested the quality of CD4+ helper T cells stimulated by B7-DC+ B cells. The generation of Th1 and Th17 cells was increased in OVA-specific CD4+ OT-II T cells that were cultured with B7-DC+ B cells in the presence of cognate OVA peptide (Fig. 4A). These increases were more significant in T cells stimulated by B7-DC+ B cells from aged vs. young mice (IFN-γ: 46.7% vs. 24.3%, IL-17: 130.5% vs. 58.3%, IL-17F: 378.3% vs. 7.1% increase, respectively). Approximately half the induced Th17 cells produced both IL-17A and IL-17F and half produced mainly IL-17F (Fig. 4B). Induction of Th17 was strongly inhibited in the presence of anti-B7-DC mAb (TY25) but not anti-PD-1 mAb (RMP1-14) (Fig. 4C), indicating that induction was dependent on B7-DC and independent of PD-1 expression. The significant induction of Th17 T cells by B7-DC+ B cells from aged mice was unexpected as B7-DC expression on DCs does not induce Th17 immunity (Ishiwata et al., 2010). In addition, neither B7-DC+ B cells nor B7-DC− B cells preferentially induce Tregs in vitro (Fig. 4A). By contrast, DCs from young or aged mice mediated no significant differences in Th1, Th17 (or Treg) induction (Fig. 4D).
Fig. 4.
B7-DC+ B cells from aged mice augmented Th1 and Th17 induction in vitro. (A) Purified naïve OT-II ovalbumin (OVA)-specific CD4+ T cells were incubated with or without B7-DC+ or B7-DC− B cells sorted from naïve young or aged mice in Th1, Th17, or Treg polarizing conditions for 5 days in the presence of OVA peptide. Intracellular cytokine expression was detected by flow cytometry. Numbers are percent gated events. (B) Components of Th17 cells induced by B7-DC+ B cells from aged mice in (A). (C) Inhibition of Th17 induction. OVA-specific OT-II CD4+ T cells were incubated with B7-DC+ B cells from aged mice under Th17 polarizing conditions. Anti-B7-DC blocking mAb (TY25) or anti-PD-1 blocking mAb (RMP1-14) was added to the culture during polarization. (D) Purified naïve OVA-specific OT-II CD4+ T cells were incubated with or without DCs isolated from naïve young or aged mice in Th1, Th17, or Treg polarizing conditions in vitro for 5 days in the presence of OVA peptide. Intracellular cytokine expression was detected by flow cytometry. These data are representative of five independent experiments with similar results.
Aged B7-DC+ B cells augment Th1 and Th17 polarization in vitro
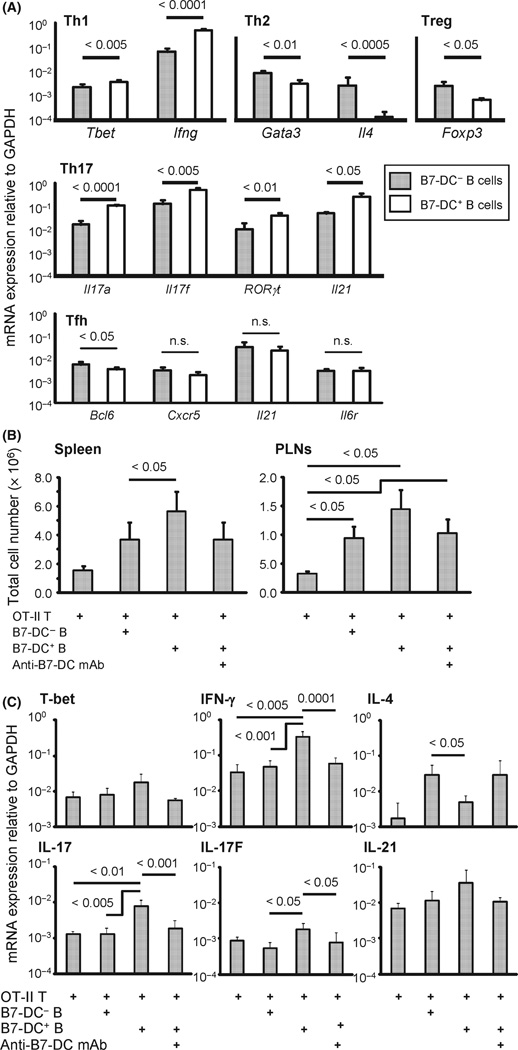
To examine Th polarization further, we sorted naïve CD4+ T cells from OT-II mice and cultured them with B7-DC+ or B7-DC− B cells under Th1, Th2, Treg, Th17, or follicular helper T-cell (Tfh)-skewing conditions in the presence of cognate OVA peptide and examined the expression of transcriptional factors, cytokines, and cytokine and chemokine receptors that are relatively specific for particular Th cells by real-time PCR (Fig. 5A). We found that mRNA expression levels of Th1 (T-bet, IFN-γ)- and Th17-associated (RORγt, IL-17, IL-17F, and IL-21) transcriptional factors and cytokines were significantly increased in CD4+ T cells cultured with B7-DC+ B cells compared with CD4+ T cells cultured with B7-DC− B cells. The expression of Th2 (Gata-4, IL-4)- and Treg-associated (Foxp3) molecules were significantly decreased. In addition, purely Tfh-associated factors were not up-regulated. Transfer of specific T-cell subsets into T-cell-deficient Rag1 KO mice allows detailed studies of T-cell functions in vivo. We first transferred CD4+ OT-II cells into Rag1 KO mice along with B7-DC+ or B7-DC− B cells from aged mice and showed that the number of germinal center cells (a Tfh function) was not altered (Fig. S11). Therefore, we conclude that B7-DC+ B cells from aged mice can enhance Th1- and Th17-associated molecules to induce Th1- and Th17-mediated immunity, but do not affect Tfh immunity. This series of in vitro and in vivo data indicated that aging-related increases in B7-DC+ B cells might contribute to Th1- and Th17-mediated immune responses in a B7-DC-dependent manner.
Fig. 5.
Gene expression profile of CD4+ T cells stimulated by B7-DC+ B cells from aged mice. (A) Purified and sorted na¨ıve ovalbumin (OVA)-specific CD4+ OT-II T cells were incubated with B7-DC+ or B7-DC− B cells sorted from naïve aged mice in Th1, Th17, Treg, or Tfh polarizing conditions in vitro for 5 days in the presence of OVA peptide. CD4+ T cells were then purified by the combination of magnetic selection and flow cytometric sorting and the expression level of messenger RNA (mRNA) for Th1 (T-bet, IFN-γ), Th2 (GATA3, IL-4), Treg (FoxP3), Th17 (IL-17, IL-17F, RORγt) and Tfh (IL-21, Bcl-6, CXCR5, and IL-6 receptor (IL-6R)) was measured by real-time quantitative PCR (n = 5 per group) using primer sets described in Methods relative to GAPDH mRNA expression. (B) Purified and sorted naïve CD4+ OT-II T cells were transferred into RAG-1 knock out mice. Six weeks later, OVA peptide–pulsed B7-DC+ or B7-DC− B cells sorted from naïve aged mice were injected i.v. After an additional 5 days with or without the administration of anti-B7-DC blocking mAb (TY25), the spleen and peripheral lymph nodes (PLNs) were extracted, and the cells were stained with anti-CD3, anti-CD4, and anti-Thy1.1 mAbs. Total cell number of CD4+Thy1.1+ T cells is shown (n = 5 per group). (C) In the same experiment as (B), CD3+CD4+Thy1.1+ T cells were sorted from a portion of the spleen cells, and gene expression of the same molecules in (A) was measured by real-time quantitative PCR. Only gene sets that had a difference between groups are shown. Each panel is from one of the three representative experiments. IFN, interferon.
In vivo function of B7-DC+ B cells
Based on these in vitro and in vivo results, we hypothesized that B7-DC+ B cells from aged mice would enhance CD4+ T-cell proliferation and induce Th1 and Th17 cells in vivo. To test this hypothesis, we adoptively transferred OVA-specific CD4+ OT-II T cells into Rag1 KO mice and then immunized them with OVA peptide-pulsed B7-DC+ or B7-DC− B cells. Five days after immunization, adoptively transferred OT-II T cells in the spleen and lymph nodes were recovered for study (Fig. 5B). Proliferation of OT-II T cells from mice immunized with B7-DC+ B cells was significantly greater than in mice immunized with B7-DC− B cells. This enhancement was dependent on B7-DC expression as administration of anti-B7-DC blocking mAb (clone TY25) significantly reduced it. These results suggest that B7-DC+ B cells have a greater ability to promote in vivo antigen-specific CD4+ T-cell proliferation than B7-DC− B cells. The quality of these CD4+ T cells was also evaluated by qPCR. Consistent with in vitro data, production of IFN-γ (Th1 cytokine), IL-17 and IL-17F (Th17 cytokines) and IL-21 (Tfh cytokine) was enhanced, and IL-4 (Th2 cytokine) production was decreased in OT-II T cells from mice immunized with B7-DC+ B cells compared with OT-II T cells from mice immunized with B7-DC− B cells (Fig. 5C). The Th1 polarization transcription factor T-bet was slightly elevated in OT-II T cells from mice immunized with B7-DC+ B cells, but the difference was not statistically significant. As the expression of other factors was not altered between groups, we conclude that B7-DC+ B cells from aged mice have the potential specifically to induce Th1 and Th17 immunity and to decrease Th2 in vivo. These data are consistent with previous reports showing that B7-DC (on DCs) in young mice enhances Th1 and decreases Th2 polarization in vivo (Shin et al., 2005; Matsumoto et al., 2008; Ishiwata et al., 2010).
Because a significant phenotype of B7-DC KO mice is accelerated tumor growth after tumor challenge (Shin et al., 2005), and in the light of this previously unrecognized population of B7-DC+ B cells in aged mice that enhanced Th1 and Th17 cell induction in vitro and in vivo (Fig 4A and Fig 5A,C), we tested the function of B7-DC+ B cells in antitumor immunity further. The contribution of Th17-mediated immunity in antitumor immune responses appears to depend on the specific setting. In some conditions, Th17 cells can enhance anticancer immunity and increase tumor-infiltrating lymphocytes, which correlates with improved prognosis in patients with cancer (Tesmer et al., 2008; Martin-Orozco et al., 2009; Murugaiyan & Saha, 2009; Zou & Restifo, 2010). We thus hypothesized that B7-DC+ B cells could retard tumor growth. There is a general agreement that transplanted tumors grow more slowly in aged mice than in young mice (Itzhaki et al., 2000; Kaptzan et al., 2004; Kubera et al., 2009; Leibovici et al., 2009). We also confirmed that the growth of mouse colon tumor (MC38) was significantly delayed in aged mice compared to young mice (Fig. 6A). Surprisingly, the administration of anti-B7-DC monoclonal antibody (mAb) abrogated the retardation of tumor growth in aged mice, resulting in an acceleration of tumor growth to the rate seen in young mice receiving anti-B7-DC mAb. This result suggests that the antitumor effect of B7-DC signals in aged mice is more prominent than in young mice and that the retardation of tumor growth in aged mice is dependent on B7-DC+ cells. Moreover, the administration of cognate OVA peptide-pulsed B7-DC+ B cells from aged mice strongly inhibited the tumor growth of OVA-producing mouse melanoma (B16/ OVA) (Fig. 6B). This difference in antitumor effect between mice that receive peptide-pulsed B7-DC+ B cells and B7-DC− B cells was not because of differential B-cell proliferation or antigen acquisition (Fig S12 and S13). In fact, B-cell proliferation was significantly better in B cells from young vs. aged mice; however, no significant difference was observed between B7-DC+ B cells and B7-DC− B cells (Fig. S12). This antitumor effect was adaptive immunity dependent because tumor growth was not suppressed by the same treatment in Rag1 KO mice lacking T and B cells (not shown). Moreover, this antitumor effect of peptide-charged B7-DC+ B cells was abrogated by administration of anti-B7-DC blocking antibody (Fig. 6B). Further, the transfer of B7-DC+ DCs from aged mice failed to restore antitumor immunity in B7-DC KO mice (Fig. S14), indicating that the antitumor effect was induced in a B-cell B7-DC-dependent manner. Further supporting B-cell B7-DC-dependent effects, tumor regression in mice receiving peptide-pulsed B7-DC− B cells was not significantly different from mice receiving no treatment or from mice treated with B7-DC+ B cells plus anti-B7-DC antibody. Correlating with the inhibition of tumor growth, the generation of antigen-specific CD8+ CTLs was significantly increased in mice receiving antigen-pulsed-B7-DC+ cells compared to mice receiving B7-DC− cells (Fig. 6C,D). This enhanced induction of CTLs was also abrogated by the administration of anti-B7-DC-blocking mAb. In aggregate, these data are consistent with B7-DC+ B cells from aged mice having strong potential to induce antitumor immunity because they induce tumor antigen-specific CTLs in vivo through a mechanism involving enhanced Th1 and Th17 induction.
Fig. 6.
B7-DC+ B cells from aged mice have potential to regress tumor growth. (A) MC38 cells were injected s.c. in young or aged mice with or without the administration of anti-B7-DC blocking mAb (αB7-DC mAb: clone TY25) or control IgG (Ctr Ig) (8–10 mice per group). Tumor size was measured 16 days after tumor challenge. (B) B16/OVA cells were injected s.c. young mice on day 0. One-half million B7-DC+ or B7-DC− B cells sorted from naïve aged mice were injected i.v. on day 4, 6, 8, and 10 (two million cells per mouse in total) with or without the administration of anti-B7-DC blocking mAb (TY25) or control IgG. Tumor size was monitored three times a week until day 22. (C, D) B16/OVA-bearing mice in (B) were sacrificed on day 22 and a single-cell suspension from the spleens and lymph nodes were prepared for staining with CD8 and OVA peptide-MHC class I-pentamer. Representative flow cytometry panels of indicated groups (5–10 mice per group) (C) and graphical summary (D) are shown. OVA, ovalbumin.
Discussion
We now demonstrate the phenotype and function of a novel immune cell population: extraperitoneal B7-DC+ B cells. In young mice, the expression of B7-DC is low and restricted primarily to DCs and macrophages, in which cells it is induced by IL-4 (Yamazaki et al., 2002; Loke & Allison, 2003; Shin et al., 2005). However, B7-DC+ B cells in aged mice were prevalent in lymphoid organs and were observed in the three mouse strains studied (C57BL/6, Balb/c and Ames). The in vitro regulation of B7-DC expression in B cells in aged mice differs from the regulation in DCs and macrophages in young mice. B7-DC expression on B-1 cells in the mouse peritoneum has been reported, and the possibility that these cells were involved in the pathogenesis of lupus was suggested (Zhong et al., 2007, 2009). We also confirmed that B-1 cells in mouse peritoneum express B7-DC and then showed that this population did not increase with age as did the peripheral tissue B7-DC+ B cells that we now describe.
Likewise, the flow cytometry phenotype and functional properties of these age-associated B7-DC+ B cells do not clearly fit into currently defined B-cell subsets. Interestingly, the surface phenotypes of B7-DC+ B cells from both young and aged mice were similar, and they both resembled activated mature B cells, but by contrast, their functions were different. Aged extra-peritoneal B7-DC+ B cells induced both Th1 and Th17 immunity whereas young extra-peritoneal B7-DC+ B cells induced neither. Thus, these age-related B7-DC+ B cells could contribute to Th1- and Th17-mediated diseases such as certain autoimmune disorders or Alzheimer disease. Regarding origins, these data are consistent with the concept that extra-peritoneal B7-DC+ B cells differ from peritoneal B-1 B cells based on their increase with age whereas peritoneal B-1 B cells do not and based on their functional differences. Alternatively, peritoneum could be a site of B7-DC+ B-cell development from which they seed other tissues with age and also undergo functional changes. Further work is required to resolve these issues.
The importance of B7-DC expression on germinal center cells in the generation of long-lived plasma cells was previously noted (Good-Jacobson et al., 2010). As we showed that B7-DC+ B cells from aged mice induced the expression of the Tfh-associated factor IL-21 that contributes to germinal center formation, but did not promote germinal center formation itself, age-associated B7-DC+ B cells appear to differ with respect to germinal center formation potential vs. other B7-DC+ cells.
Because we previously identified a significant role for B7-DC in generating Th1-dependent immunity such as antitumor immune responses (Shin et al., 2005), we suspected that B7-DC+ B cells would have significant potential to suppress tumor growth. This was indeed the case as mice adoptively transferred with B7-DC+ B cells demonstrated significant control of tumor growth, and some even rejected growing tumors. This effect was B7-DC dependent as anti-B7-DC mAb abrogated it. We found that the antitumor effect was adaptive immunity dependent and mediated by augmented tumor-specific CTLs. Further, better tumor-specific immunity mediated by aged B7-DC+ B cells was not because of superior antigen capture or proliferation. Finally, we could not demonstrate that DC B7-DC signals were tumor protective. We infer from all these data that aged B7-DC+ B cells generate Th1- and Th17-mediated immunity, contributing to the antitumor effect. B7-DC− B cells did not exhibit an ability to inhibit tumors, indicating the possibility that B7-DC− B cells are not easily converted into B7-DC+ B cells in vivo, consistent with our present in vitro work and other prior in vitro work (Zhong et al., 2007).
To reconcile the age-associated increase in tumor susceptibility with age and the age-associated increase in B7-DC+ B cells that can augment antitumor immunity, it could be that they are important in defense against growing tumors, but are not significant mediators of cancer immune surveillance that prevents unapparent tumors from growing into clinically apparent cancers. These B7-DC+ B cells could also play immunopathologic roles in other diseases. For example, the induced Th17 cells could exaggerate inflammation that contributes to aging-related diseases such as rheumatoid arthritis or Alzheimer disease. Thus, it is not yet clear whether the increase in B7-DC+ B cells is net beneficial or detrimental to the overall health of aged individuals.
In summary, we identified an aging-associated increase in a novel immune population of extra-peritoneal B7-DC+ B cells that demonstrated strong Th1 and Th17 cell induction and enhanced generation of tumor antigen-specific CTLs, which efficiently mediated tumor regression. Further investigations into the mechanisms of their origin and function will help clarify the physiological and pathological importance of B7-DC+ B cells in aging.
Experimental procedures
Mice
C57BL/6 and BALB/c mice were obtained from NCI (Frederick, MD, USA). OT-I and OT-II mice were obtained from Taconic (Hudson, NY, USA). Thy1.1 C57BL/ 6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). All mice were maintained under specific pathogen-free conditions in accordance with the institutional guidelines of the University of Texas Health Science Center at San Antonio. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio (UTHSCSA). Ames mice were the generous gift from Dr. Arlan Richardson (Barshop Institute, UTHSCSA, San Antonio, TX, USA).
Reagents
Antibodies were from reliable commercial vendors. Recombinant cytokines were purchased from R&D systems (Minneapolis, MN, USA). MHC class I- and MHC class II-specific OVA peptides (OVA257–264 and OVA323–339) were purchased from Genscript (Piscataway, NJ, USA). Carboxyfluorescein diacetate succinimidyl ester (CFSE) was purchased from Invitrogen (Carlsbad, CA, USA). Whole T cells, OT-I CD8+ T cells, OT-II CD4+ T cells, and Tregs were purified using magnetic isolation kits from Miltenyi Biotec (Auburn, CA, USA) or sorted by FACS Aria (BD Biosciences, San Diego, CA, USA). Pro5 H2Kb/SIINFEKL Pentamer was purchased from Proimmune (Bradenton, FL, USA).
T-cell proliferation and suppression assays
In vitro T-cell suppression and proliferation assays were performed as previously described (Shin et al., 2005; Tomihara et al., 2010). For in vitro suppression assays, purified whole spleen cells from naïve OVA peptide–specific T-cell receptor transgenic OT-I or OT-II mice were stimulated with specific peptide in the presence or absence of B7-DC+ or B7-DC− B cells or Tregs. T-cell suppression was calculated by the ratio of CFSE dilution or thymidine incorporation, and suppression against the positive control group. For the in vitro proliferation assay, magnetically purified CD4+ or CD8+ T cells from the spleen of OT-I or OT-II mice were cultured with or without magnetically purified CD11c+ DCs or sorted B7-DC+ or B7-DC− B cells. For the in vivo proliferation assay, OT-II CD4+ T cells (Thy1.1+) were adoptively transferred to Thy1.2+ RAG1 KO mice. Some mice were immunized with OVA peptide-pulsed B7-DC+ or B7-DC− B cells in the presence or absence of anti-B7-DC blocking mAb, and transferred T cells were detected and sorted by flow cytometry 5 days after the cell immunization.
Induction of Th1, Th2, Th17, Tfh cells, and Tregs and intracellular cytokine staining
In vitro induction of Th1, Th2, Th17, Tfh cells, and Tregs was performed according to published methods (Shin et al., 2005; Nurieva et al., 2008; Ishiwata et al., 2010). Briefly, CD4+ OT-II T cells were magnetically purified or sorted and cultured for 5 days with freshly isolated DCs, BM-derived DCs or B7-DC+ or B7-DC− B cells and OVA peptide in the presence of mouse IL-12 and anti-mouse IL-4 mAb for Th1 generation, with mouse IL-4 and anti-mouse IFN-γ mAb for Th2 generation, human TGFβ and mouse IL-6 and IL-23 and anti-mouse IL-4 and IFN-γ mAbs for Th17 generation, human TGFβ and anti-mouse IL-4 and IFN-γ mAbs for Treg generation, or mouse IL-6, and anti-mouse IL-4, IFN-γ and TGF-β (1D11) mAbs for Tfh. Four hours before the end of culture, phorbol 12-myristate 13-acetate, and ionomycin (Sigma-Aldrich, St. Louis, MO, USA) were added for re-stimulation with monensin (eBioscience, San Diego, CA, USA). After surface molecules were stained, cells were fixed and permeabilized and intracellular cytokines were stained with fluorescent conjugated antibody for flow cytometry analysis.
Quantification of Th-specific molecules
Following in vitro Th induction or in vivo Th cell stimulation, CD4+ T cells were sorted, and mRNA was extracted. Reverse-transcribed cDNA synthesized from mRNA was used for the template in real-time qPCR assays. Primers for qPCR were designed according to previous publications (Fontenot et al., 2003; Harrington et al., 2005; Kang et al., 2006; Nurieva et al., 2008; Nizri et al., 2009).
Subcutaneous tumor models
One-half million MC38 or B16/OVA cells were administered subcutane-ously to naïve mice on day 0. Some mice received 0.25 mg of anti-B7-DC blocking mAb or control IgG i.p. every 3 days starting on day 3 after tumor challenge. Mice that received B16/ OVA were injected i.v. on day 4, 6, 8 and 10 with B7-DC− or B7-DC+ B cells (two million cells per mouse in total) obtained from the spleens of aged mice with or without OVA peptide conjugation. Tumor size was measured every other day. On day 22, mice were sacrificed, and cells from spleen and lymph nodes were analyzed by flow cytometry.
Statistical analysis
Data are expressed as mean ± SEM. Student′s t test or ANOVA was performed, where appropriate, with two-tailed P < 0.05 considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Alan Richardson for supplying mice; Dr. Sara M. Ludwig, Dr. Srilakshmi Pandeswara and Ms Aijie Liu for technical assistance. This work was supported by funding from CTRC, The Voelcker Foundation, The Holly Beach Public Library Association, AG036613, CA54174, a University Research Council Grant, and the Texas STAR Program.
Footnotes
Author contributions
K. Tomihara: flow cytometry analysis, cell sorting, in vitro proliferation and Th cell induction assay, qPCR, in vivo experiments. Takako Shin: animal colony management, primer design, cell line management, flow cytometry analysis, cell sorting. V. J. Hurez: flow cytometry analysis, data analysis manuscript preparation. H. Yagita: blocking antibody generation and production, manuscript preparation. D. M. Pardoll: data analysis, statistical analysis, manuscript preparation. B. Zhang: experiment design, data analysis, manuscript preparation. T. J. Curiel: experimental design, data analysis, manuscript preparation. Tahiro Shin: experiment design, data analysis, statistical analysis, manuscript preparation.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1–S8 Immunological comparison between young and aged mice using flow cytometry. Cell numbers (S1, S3, S5, and S8) and representative flow cytometry panels (S2, S4, S6, and S7) in spleen (S1, S2, and S3), lymph node (S1, S4, and S5), peripheral blood (S6) and bone marrow (S1, S7, and S8). Flow cytometry panels are representative of more than five independent experiments with similar results. Data shown in S1, S3, S5, and S8 are the summary of each independent experiment (5–15 mice per group).
Fig. S9 Significant increase in B7-DC+ B cells in aged mice.
Fig. S10 B7-DC expression in B cells and myeloid cells from young and aged mice assessed after overnight culture of whole spleen single-cell suspensions in the presence or absence of recombinant mouse IL-4. CD11c+B220−: myeloid DCs, CD11c−B220+: B cells, CD11c+B220+: plasma-cytoid DCs, CD11b+Gr-1+: granulocytes, CD11b+Gr-1−: macrophages, CD11b−Gr-1+: plasmacytoid DCs and granulocytes.
Fig. S11 Germinal center formation.
Fig. S12 Comparison of proliferation between B7-DC+ and B7-DC− B cells from young or aged mice.
Fig. S13 Comparison of antigen acquisition between B7-DC+ and B7-DC− B cells and DCs.
Fig. S14 B7-DC+ cells but not B7-DC+ DCs from aged mice have potential to regress tumor growth.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R, Twardy BS, Krishna P, Suvas S. Advancing age leads to predominance of inhibitory receptor expressing CD4 T cells. Mech. Ageing Dev. 2009;130:709–712. doi: 10.1016/j.mad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eich-baum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grizzle WE, Xu X, Zhang S, Stockard CR, Liu C, Yu S, Wang J, Mountz JD, Zhang HG. Age-related increase of tumor susceptibility is associated with myeloid-derived suppressor cell mediated suppression of T cell cytotoxicity in recombinant inbred BXD12 mice. Mech. Ageing Dev. 2007;128:672–680. doi: 10.1016/j.mad.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Ishiwata K, Watanabe N, Guo M, Tomihara K, Brumlik MJ, Yagita H, Pardoll D, Chen L, Shin T. Costimulator B7-DC attenuates strong Th2 responses induced by Nippostrongylus brasiliensis. J. Immunol. 2010;184:2086–2094. doi: 10.4049/jimmunol.0804051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki O, Skutelsky E, Kaptzan T, Siegal A, Michowitz M, Sinai J, Huszar M, Nafar S, Leibovici J. Macrophage-recognized molecules of apoptotic cells are expressed at higher levels in AKR lymphoma of aged as compared to young mice. Adv. Exp. Med. Biol. 2000;479:251–262. doi: 10.1007/0-306-46831-X_22. [DOI] [PubMed] [Google Scholar]
- Kang HS, Beak JY, Kim YS, Petrovich RM, Collins JB, Grissom SF, Jetten AM. NABP1, a novel RORgamma-regulated gene encoding a single-stranded nucleic-acid-binding protein. Biochem. J. 2006;397:89–99. doi: 10.1042/BJ20051781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptzan T, Skutelsky E, Itzhaki O, Sinai J, Michowitz M, Yossipov Y, Schiby G, Leibovici J. Age-dependent differences in the efficacy of cancer immunotherapy in C57BL and AKR mouse strains. Exp. Gerontol. 2004;39:1035–1048. doi: 10.1016/j.exger.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Khatami M. Inflammation, aging, and cancer: tumoricidal versus tumorigenesis of immunity: a common denominator mapping chronic diseases. Cell Biochem. Biophys. 2009;55:55–79. doi: 10.1007/s12013-009-9059-2. [DOI] [PubMed] [Google Scholar]
- Kubera M, Grygier B, Arteta B, Urbanska K, Basta-Kaim A, Budziszewska B, Leskiewicz M, Kolaczkowska E, Maes M, Szczepanik M, Majewska M, Lason W. Age-dependent stimulatory effect of desipramine and fluoxetine pretreatment on metastasis formation by B16F10 melanoma in male C57BL/6 mice. Pharmacol. Rep. 2009;61:1113–1126. doi: 10.1016/s1734-1140(09)70174-4. [DOI] [PubMed] [Google Scholar]
- Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J. Immunol. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lages CS, Lewkowich I, Sproles A, Wills-Karp M, Chougnet C. Partial restoration of T-cell function in aged mice by in vitro blockade of the PD-1/PD-L1 pathway. Aging Cell. 2010;9:785–798. doi: 10.1111/j.1474-9726.2010.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- Leibovici J, Itzhaki O, Kaptzan T, Skutelsky E, Sinai J, Michowitz M, Asfur R, Siegal A, Huszar M, Schiby G. Designing ageing conditions in tumour microenvironment-a new possible modality for cancer treatment. Mech. Ageing Dev. 2009;130:76–85. doi: 10.1016/j.mad.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustgarten J. Cancer, aging and immunotherapy: lessons learned from animal models. Cancer Immunol. Immunother. 2009;58:1979–1989. doi: 10.1007/s00262-009-0677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Inoue H, Nakano T, Tsuda M, Yoshiura Y, Fukuyama S, Tsushima F, Hoshino T, Aizawa H, Akiba H, Pardoll D, Hara N, Yagita H, Azuma M, Nakanishi Y. B7-DC regulates asthmatic response by an IFN-gamma-dependent mechanism. J. Immunol. 2004;172:2530–2541. doi: 10.4049/jimmunol.172.4.2530. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Fukuyama S, Eguchi-Tsuda M, Nakano T, Matsumoto T, Matsumura M, Moriwaki A, Kan-o K, Wada Y, Yagita H, Shin T, Pardoll DM, Patcharee R, Azuma M, Nakanishi Y, Inoue H. B7-DC induced by IL-13 works as a feedback regulator in the effector phase of allergic asthma. Biochem. Biophys. Res. Commun. 2008;365:170–175. doi: 10.1016/j.bbrc.2007.10.156. [DOI] [PubMed] [Google Scholar]
- Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J. Immunol. 2009;183:4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J. Immunol. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- Nizri E, Irony-Tur-Sinai M, Lory O, Orr-Urtreger A, Lavi E, Brenner T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J. Immunol. 2009;183:6681–6688. doi: 10.4049/jimmunol.0902212. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr. Opin. Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Hayashi M, Nagasaka Y, Ohno-Iwashita Y, Inomata M. Ageassociated up-regulation of a negative co-stimulatory receptor PD-1 in mouse CD4+ T cells. Exp. Gerontol. 2009;44:517–522. doi: 10.1016/j.exger.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Shimatani K, Nakashima Y, Hattori M, Hamazaki Y, Minato N. PD-1+ memory phenotype CD4+ T cells expressing C /EBPalpha underlie T cell immunodepression in senescence and leukemia. Proc. Natl. Acad. Sci. U.S.A. 2009;106:15807–15812. doi: 10.1073/pnas.0908805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin T, Kennedy G, Gorski K, Tsuchiya H, Koseki H, Azuma M, Yagita H, Chen L, Powell J, Pardoll D, Housseau F. Cooperative B7-1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J. Exp. Med. 2003;198:31–38. doi: 10.1084/jem.20030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin T, Yoshimura K, Shin T, Crafton EB, Tsuchiya H, Housseau F, Koseki H, Schulick RD, Chen L, Pardoll DM. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J. Exp. Med. 2005;201:1531–1541. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol. Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomihara K, Guo M, Shin T, Sun X, Ludwig SM, Brumlik MJ, Zhang B, Curiel TJ. Antigen-specific immunity and cross-priming by epithelial ovarian carcinoma-induced CD11b(+)Gr-1(+) cells. J. Immunol. 2010;184:6151–6160. doi: 10.4049/jimmunol.0903519. [DOI] [PubMed] [Google Scholar]
- Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsushima F, Yao S, Shin T, Flies A, Flies S, Xu H, Tamada K, Pardoll DM, Chen L. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale G, Mion F, Pucillo C. Regulatory B cells: evidence, developmental origin and population diversity. Mol. Immunol. 2010;48:1–8. doi: 10.1016/j.molimm.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11 /V(H)12 and phosphatidylcholine binding. Eur. J. Immunol. 2007;37:2405–2410. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
- Zhong X, Lau S, Bai C, Degauque N, Holodick NE, Steven SJ, Tumang J, Gao W, Rothstein TL. A novel subpopulation of B-1 cells is enriched with autore-activity in normal and lupus-prone mice. Arthritis Rheum. 2009;60:3734–3743. doi: 10.1002/art.25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.