Abstract
Adaptive CD4 T-cell responses are important in the pathogenesis of chronic Helicobacter pylori gastritis. However, the gastric antigen-presenting cells that induce these responses have not yet been identified. Here we show that dendritic cells (DCs) are present in the gastric mucosa of healthy subjects but are more prevalent and more activated in the gastric mucosa of H. pylori -infected subjects. H. pylori induced gastric DCs isolated from noninfected subjects to express increased levels of CD11c, CD86 and CD83, and to secrete proinflammatory cytokines, particularly interleukin (IL)-6 and IL-8. Importantly, gastric DCs pulsed with live H. pylori, but not control DCs, mediated T-cell secretion of interferon-γ. The ability of H. pylori to induce gastric DC maturation and stimulate gastric DC activation of Th1 cells implicates gastric DCs as initiators of the immune response to H. pylori.
INTRODUCTION
The gastric mucosa is the most frequent site of mucosal inflammation in humans due to the high incidence of colonization by Helicobacter pylori, which infects 50% of the world’s population. H. pylori infection invariably causes chronic inflammation, which may progress to peptic ulcer disease or gastric adenocarcinoma or lymphoma1,2. However, the immunological mechanisms specific to the gastric mucosa that initiate the host response to H. pylori have drawn little investigative attention. In particular, gastric dendritic cells (DCs) and their role in early H. pylori pathogenesis have not yet been characterized.
Several lines of evidence point to the likely role of gastric DCs in the pathogenesis of H. pylori gastritis. First, gastric inflammation in human and murine H. pylori infection is caused primarily by Th1 cells through interferon (IFN)-γ secretion3–9. Second, gastric DCs have been identified in human subjects infected with H. pylori 10,11, and DCs were shown to recruit to the gastric mucosa in experimental Helicobacter infection in mice12,13. Third, gastric tissue levels of the DC cytokine interleukin (IL)-12 are elevated during human H. pylori infection, consistent with the dependence of Th1 polarization on DC cytokine secretion14. Fourth, H. pylori induces maturation, activation, and inflammatory cytokine secretion by human monocyte-derived DCs (MoDCs) and murine bone marrow DCs (BM-DCs) in vitro, “licensing” the DCs for T-cell priming15–18. Consistent with these findings, major histocompatibility complex-II-deficient mice that lack functional DCs did not develop immunity to H. pylori 19, and adoptive transfer of H. pylori -pulsed BM-DCs induced protective Th1 responses in recipient mice20. Despite the above observations, the presence of DCs in healthy gastric mucosa has been questioned12,13,21, and the well-documented presence of DCs in the intestinal mucosa has raised the suggestion that intestinal Peyer’s patches are an induction site for the adaptive response to H. pylori 22,23.
Here we provide the first definitive identification of DCs in human gastric mucosa. DCs were present in the gastric mucosa of healthy subjects but were more prevalent and more activated in the gastric mucosa of H. pylori -infected subjects. We also show that isolated human gastric DCs exposed to live H. pylori rapidly matured, phagocytosed the bacteria, released proinflammatory cytokines, and triggered T-cell IFN-γ secretion. These novel findings implicate gastric DCs in the induction of the Th1 response to H. pylori.
RESULTS
Isolation and characterization of HLA-DR+ DCs from normal human gastric mucosa
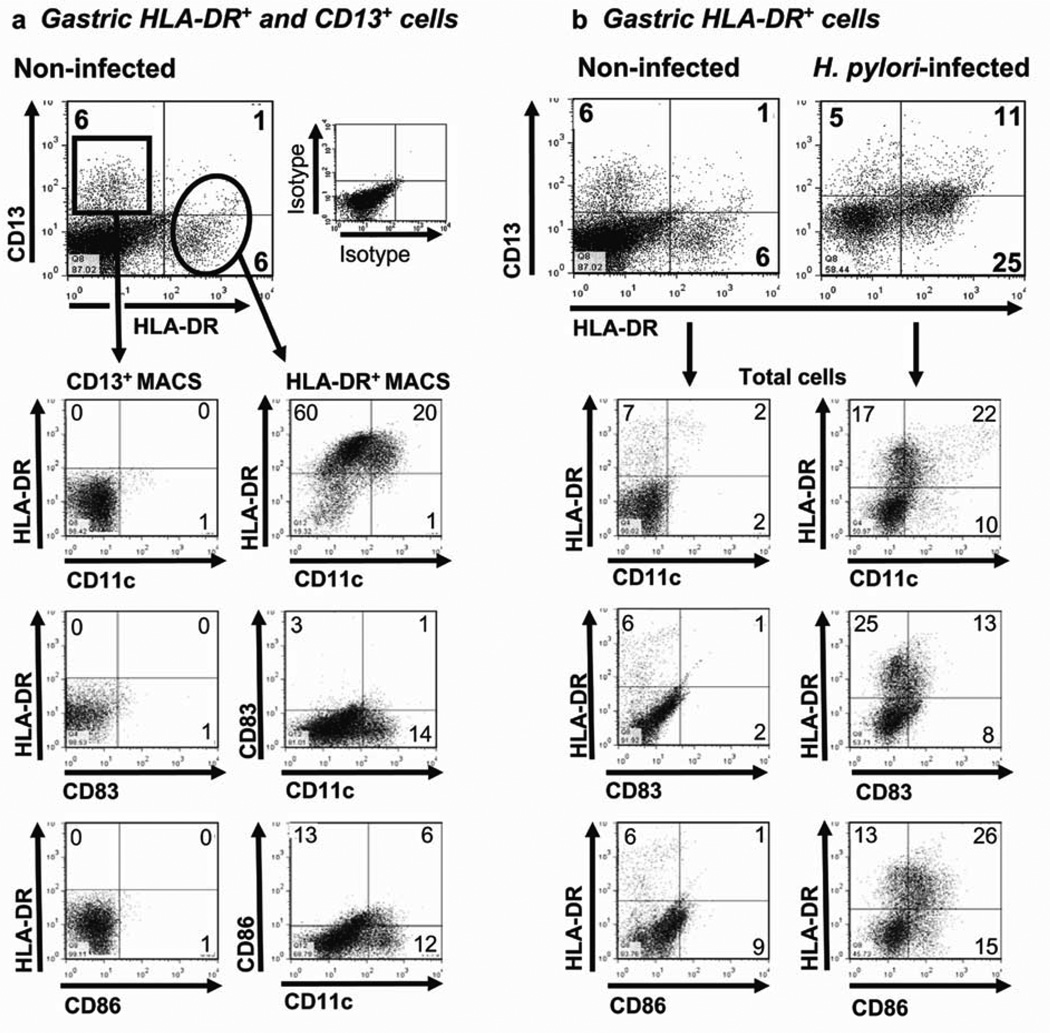
To determine the function of gastric DCs and other antigen-presenting cells (APCs) in human H. pylori infection, we first isolated and characterized cells from normal human gastric mucosa, using a modification of our previously published protocol24,25. Digestion of gastric mucosa from noninfected subjects undergoing gastric bypass surgery yielded mixed cell suspensions containing CD45+ leukocytes (15 ± 5%) and glandular epithelial cell populations. From surgically obtained normal gastric tissue, we routinely recovered 108.1 ± 6.3×106 total cells per g gastric tissue (n =10). On the basis of expression of the major histocompatibility complex class II molecule HLA-DR, which is required for antigen-presentation, and CD13 (aminopeptidase N), which we previously identified as a marker for human intestinal macrophages26,27, we detected two putative APC populations in suspensions of gastric mucosal cells: an HLA-DRhigh/CD13low population (4.8 ± 0.8%), referred to as HLA-DR+ DCs, and an HLA-DR−/CD13+ population (4.7 ± 1.1%), referred to as CD13+ cells (Figure 1a). An HLA-DRmed/CD13+ population similar to intestinal macrophages26,27 was not present in gastric cell suspensions. HLADR+ DC and CD13+ cell populations contained less than 1.5% CD3+ T cells, CD20+ B cells, and CD117+ mast cells.
Figure 1.
Phenotype of gastric mucosal cells from non-infected and H. pylori -infected subjects. (a) CD13+ and HLA-DR+ cells isolated from the gastric mucosa of a representative noninfected subject and enriched by magnetic antibody cell sorting (MACS) separation (n =10). (b) Phenotypic characterization of HLA-DR+ DCs among cells isolated from a representative, noninfected subject (n =4) and an H. pylori -infected subject (n =4). Numbers in the quadrants indicate percentage of cells in the respective quadrant.
Both HLA-DR+ DC and CD13+ cell populations had a heterogeneous phenotype. The gastric HLA-DR+, but not CD13+, population contained cells that expressed the DCassociated molecules CD11c, DC-SIGN, and CD206 (Figure 1a; Table 1). The low expression of CD80, CD83 and CD86 suggested that freshly isolated gastric DCs were largely immature, irrespective of CD11c expression (Figure 1a). The HLA-DR+/CD14+ cells likely represented recently recruited monocytes/monocytic DC precursors28. Gastric HLA-DR+ DCs were >99% CD45+ and did not express CD1a, the plasmacytoid DC marker CD123, or the natural killer cell marker CD56. Among the cells that expressed CD13, a myeloid marker29,30, 46.7 ± 0.2% also expressed the granulocytemonocyte colony stimulating factor (GM-CSF) receptor CD116, another myeloid marker, 15.7 ± 6.5% expressed CCR3, an eosinophil marker, and 8.5 ± 4.1% expressed CD56, an natural killer cell marker. In addition, the CD13+ population contained 47.1 ± 7.0% CD45− cells, likely epithelial cells, indicating that CD13 alone is not a suitable selection marker for gastric APCs. In control experiments, mononuclear cells isolated from the gastric mucosa of noninfected, nonobese organ transplant donors displayed a similar phenotype (data not shown), indicating that the findings presented here are not the consequence of obesity.
Table 1.
Phenotype of gastric HLA DR+ DCs, CD13+ cells and MoDCs from noninfected and H. pylori-infected subjects
| % HLA DR+a |
% CD13+ |
% MoDCb |
|||
|---|---|---|---|---|---|
| H. pylori status | Non-infected | Infected | Non-infected | Infected | Non-infected |
| n | 4 | 3 | 3 | 3 | 4 |
| CD11cc | 18.6 ± 1.8%d | 46.6 ± 5.0%*** | 5.2 ± 2.9% | 6.9 ± 2.6% | 96.7 ± 3.0% |
| CD14 | 14.4 ± 2.8 | 22.3 ± 2.1 | 4.1 ± 1.6 | 9.8 ± 2.6 | 2.8 ± 0.6 |
| CD80 | 2.8 ± 0.6 | 12.8 ± 8.1 | 1.6 ± 0.7 | 0.9 ± 0.4 | 15.2 ± 5.8 |
| CD83 | 2.0 ± 0.3 | 6.3 ± 2.6* | 2.3 ± 1.1 | 4.4 ± 1.3 | 5.9 ± 2.1 |
| CD86 | 7.5 ± 0.9 | 20.3 ± 3.9** | 2.4 ± 1.5 | 5.2 ± 4.2 | 59.7 ± 11.8 |
| CD206 | 14.5 ± 4.3 | 5.8 ± 0.0e | 2.7 ± 2.0 | 0.3 ± 0.0e | 86.2 ± 9.7 |
| DC-SIGN | 14.1 ± 6.3 | 25.1 ± 10.1 | 3.1 ± 1.6 | 5.6 ± 1.7 | 91.5 ± 3.9 |
Abbreviation: MoDC, monocyte-derived dendritic cells.
Gastric mucosal cell subsets were enriched by MACS.
Immature MoDCs (day 6) were used as controls.
Surface markers were analyzed by FACS, with cells gated as either HLA DRhigh or CD13+ cells.
Mean ± s.e.m.
n = 1
P<0.001;
P<0.01;
P<0.05
H. pylori infection enhances the prevalence and activation of DCs in human gastric mucosa
Studies in mice indicate that H. pylori infection causes an influx of DCs into the gastric mucosa12,13,31. To determine whether human H. pylori infection similarly increases the prevalence of gastric DCs, we isolated cells from gastric biopsies of subjects determined by serology, rapid urease CLO test, and histopathology to be actively infected with H. pylori. From H. pylori -infected subjects, we routinely recovered 4.6 ± 0.33×106 total cells per 10 biopsies. As shown in Figure 1b, the gastric mononuclear cell suspension from a representative noninfected subject contained fewer HLA-DR+ cells (7%) than the suspension from an H. pylori -infected subject (36%). Among three noninfected and three H. pylori -infected subjects, the percentage of gastric HLA-DR+ cells was significantly lower in the noninfected than the infected subjects (4.8 ± 0.8% vs. 34.9 ± 3.9%, P =0.001). The mononuclear cell population in the representative noninfected subject (Figure 1b) contained similar numbers of CD13+ (HLA-DR−) cells compared to the H. pylori -infected subject (6 vs. 5%). These findings were confirmed in three noninfected and three infected subjects (Figure 1b; 5.5 ± 0.8 and 4.7 ± 1.1%, P >0.05). Gastric epithelial cells, which can express HLA-DR during H. pylori infection32, were excluded from the DC population by gating on HLA-DRhigh/CD45+ cells. Importantly, H. pylori infection resulted in a more activated phenotype in the gastric HLADR+ DCs, reflected in increased expression of CD11c (P <0.001), CD83 (P =0.02), CD86 (P =0.002), CD80, DC-SIGN, and CD14 compared with cells isolated from noninfected subjects (Figure 1b; Table 1).
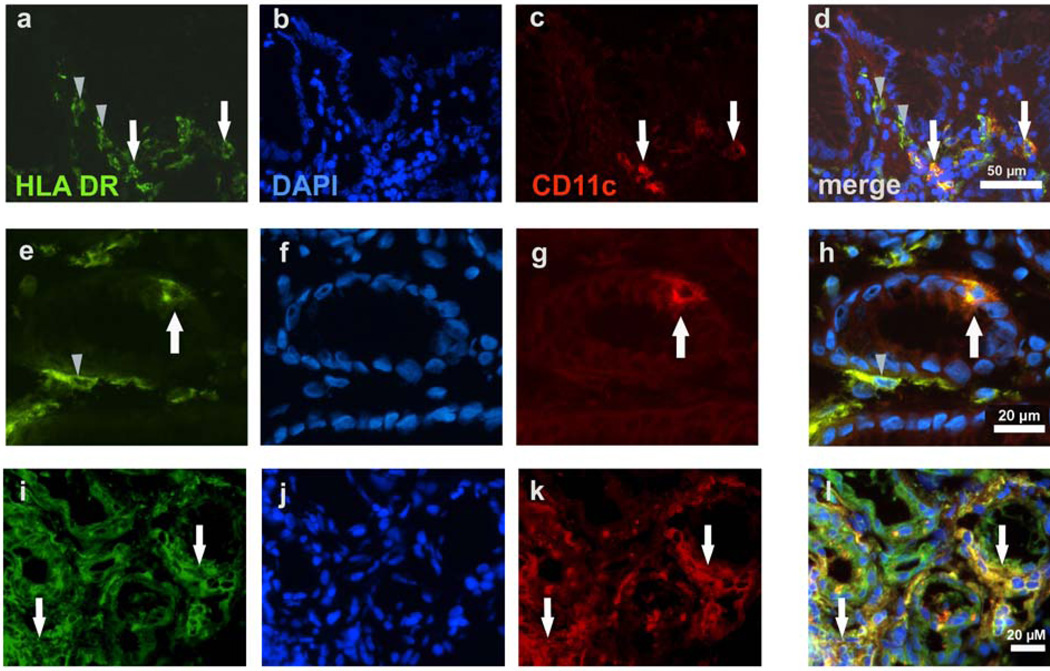
The increased prevalence of HLA-DR+ DCs in gastric cell suspensions from H. pylori infected subjects compared with noninfected subjects was corroborated by microscopic analysis of tissue sections. In noninfected subjects, low numbers of HLA-DR+ DCs were distributed throughout the gastric lamina propria, and a proportion of the cells co-expressed CD11c (Figure 2a-d). Interestingly, HLA-DR+ DCs that co-expressed CD11c also were detected in the glandular epithelium near the mucosal surface (Figure 2e-h), a location that strategically positions the cells for the uptake of antigen that comes in contact with the apical surface of the epithelium. Importantly, all HLA-DR+ cells coexpressed the mononuclear phagocyte marker HAM56, confirming the designation of these cells as APCs (data not shown). In H. pylori -infected subjects, the prevalence of HLA-DR+ DCs was substantially increased (Figure 2i-l); dense aggregates of DCs were located between the gastric glands and a higher proportion of gastric HLA-DR+ DCs coexpressed CD11c. These observations were quantified by digital image analysis using ImageJ software (Table 2; Supplementary Figure 1). In H. pylori -infected subjects, significantly higher area fractions of gastric lamina propria (with epithelial cells excluded) were positive for HLA-DR (P =0.03) and for CD11c (P =0.01) than in noninfected subjects, indicating that a greater number of these cells was present in the infected tissue. CD11c and HLA-DR colocalization as determined by the Manders’ colocalization coefficients33,34 (Table 2) and intensity scatter plots (Supplementary Figure 1) was significantly increased in H pylori -infected compared to noninfected subjects (M1: P =0.003 for overlap of CD11c with HLA-DR; M2: P =0.01 for overlap of HLA-DR with CD11c).
Figure 2.
Immunohistochemical identification of dendritic cells (DCs) in the gastric mucosa of noninfected and H. pylori -infected subjects. (a-d) Gastric mucosa from a noninfected subject contains HLA-DR+ cells in which a small proportion are HLADR+/ CD11c+. (e-h) Gastric mucosa from a noninfected subject shows gastric glands with an intraepithelial HLA-DR+/CD11c+ cell. (i-l) Gastric mucosa from an H. pylori infected subject contains many HLA-DR+ cells in which a large proportion are HLADR+/ CD11c+. Note the increased HLA-DR expression in gastric epithelial cells in the infected subject (i-l) compared with epithelial cells in the noninfected subject (a-h). (a,e,i) HLA-DR-FITC; (b,f,j) 46-diamidino-2-phenyl indole (DAPI) nuclear stain; (c,g,k) CD11c-TXRD (red); (d,h,l) merge. White arrows indicate HLA-DR+/CD11c+ DCs; gray arrowheads indicate HLA-DR+/CD11c− cells, likely immature DCs. Results are representative of four noninfected and four H. pylori -infected subjects.
Table 2.
Digital image analysis of HLA-DR and CD11c-labeled gastric tissue sections from noninfected and H. pylori-infected subjects
| H. pylori statusa | n | % HLA-DR+ areab | % CD11c+ areab | M1 (CD11c on HLA-DR)c | M2 (HLA-DR on CD11c)c |
|---|---|---|---|---|---|
| Non-infected | 5 | 9.03 ± 1.21 | 2.61 ± 0.77 | 0.09 ± 0.01 | 0.06 ± 0.01 |
| Infected | 4 | 21.18 ± 4.91 | 14.73 ± 4.15 | 0.46 ± 0.09 | 0.37 ± 0.10 |
| P valued | 0.03 | 0.01 | 0.003 | 0.01 |
H. pylori status of tissue donors was determined by serological analysis.
Percentage of HLA-DR+ (green) or CD11c+ (red) pixels of total pixels in gastric lamina propria, determined using the ImageJ “analyze particles” tool.
Manders’ colocalization coefficients M1 and M233. M1, proportion of red signal overlapping with a signal in the green channel. M2, proportion of green signal overlapping with a signal in the red channel.
Paired Student’s t-test.
H. pylori activates isolated human gastric HLA-DR+ DCs but not CD13+ cells
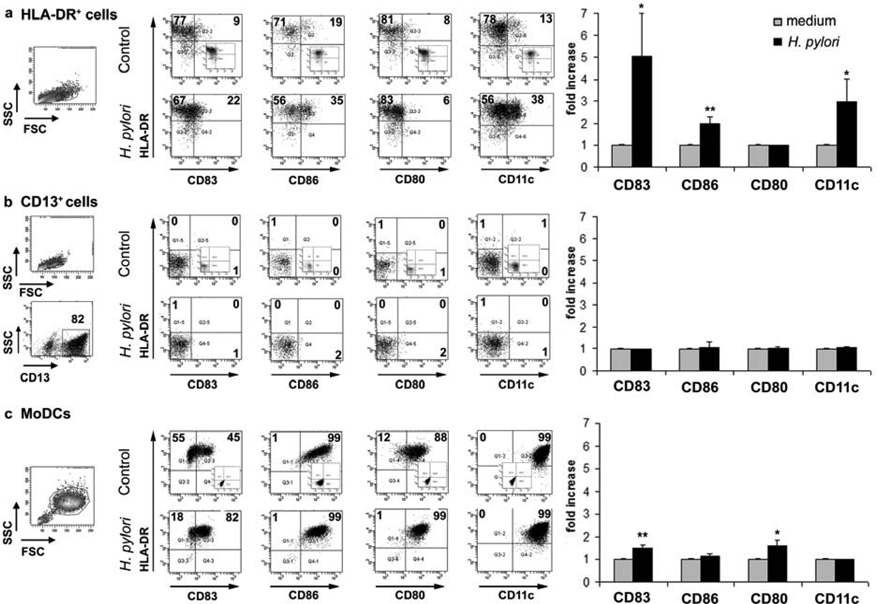
Having shown that H. pylori infection in vivo is associated with an accumulation of more mature DCs in the gastric mucosa, we investigated whether gastric HLA-DR+ cells isolated from noninfected subjects would mature in response to H. pylori stimulation in vitro. Compared with control HLA-DR+ DCs cultured in medium alone, gastric HLA-DR+ DCs exposed to an optimal concentration of live H. pylori for 15 h showed increases in the proportion of CD83+, CD86+, CD11c+, but not CD80+, DCs (Figure 3a), with concentration-dependent effects occurring between an multiplicity of infection (MOI) of 5–20 (data not shown). The increase in the proportion of these DC maturation and activation markers was significant for three separate donors (Figure 3a, right panel). Moreover, after H. pylori stimulation in vitro, CD11c expression correlated with DC maturation, reflected in a significantly higher geometric mean expression of CD83 on CD11c+ DCs compared with the CD11c− subset (P <0.05; data not shown). In contrast, H. pylori did not significantly alter the very low expression of CD83, CD86, CD80 or CD11c on gastric CD13+ cells (Figure 3b, left and right panels), suggesting that gastric CD13+ cells are not equipped to initiate T-cell activation. Importantly, HLA-DR expression was also not induced on CD13+ cells after H. pylori exposure (Figure 3b). Following exposure to H. pylori, increased proportions of MoDCs expressed CD83 and CD80, similar to previously reported studies15,35 (Figure 3c). All MoDCs expressed high levels of CD86 and CD11c expression, irrespective of H. pylori exposure (Figure 3c, left panels). The increased expression of CD83 and CD86 by cultured but non-stimulated gastric DCs in Figure 3a compared with the freshly isolated DCs in Figure 1a and Table 1 is due to spontaneous DC maturation in culture, as previously reported for human colonic DCs28.
Figure 3.
Maturation of gastric HLA-DR+ dendritic cells (DCs) and monocyte-derived DCs (MoDCs) in response to H. pylori. (a) HLA-DR+ DCs and (b) CD13+ cells isolated from the gastric mucosa of an H. pylori -negative donor, and (c) MoDCs generated from H. pylori -negative donor monocytes were exposed to live H. pylori (strain 60190, multiplicity of infection 20) or medium alone (control) for 15 h, followed by fluorescenceactivated cell sorting (FACS) analysis. Numbers in dot plots indicate percentage of cells in respective quadrants. Insets show isotype-matched controls. Bar graphs on the right show mean fold increase ± s.e.m. (n =3) in the expression of the indicated markers, calculated as f(x) = % stained cells (sample)/% stained cells (medium control); * P ≤0.05, ** P ≤0.01; unpaired Student’s t -test.
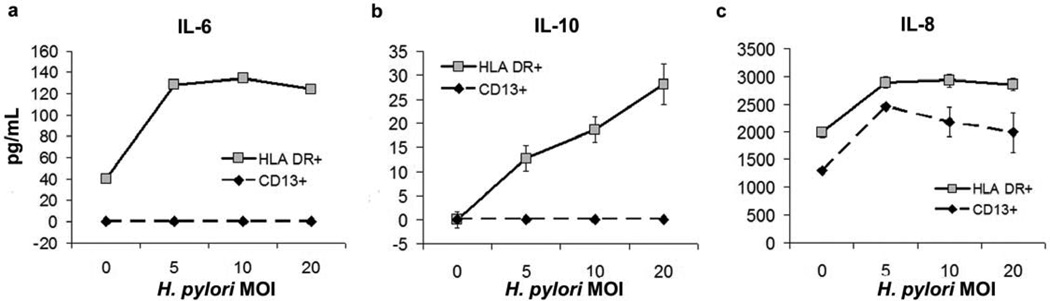
Cytokines secreted by pathogen-stimulated DCs impact T-cell lineage development during T-cell priming35. In cultures of gastric HLA-DR+ cells, the addition of H. pylori increased the concentration-dependent secretion of IL-6, IL-10 and IL-8 (Figure 4), but not IL-12p70 (data not shown). H. pylori also induced increased IL-6, IL-8 and IL-10, but not IL-12p35 or p40 gene expression in gastric HLA-DR+ DCs, as determined by quantitative RT-PCR analysis (data not shown). Consistent with the inability of H. pylori to activate gastric CD13+ cells, H. pylori did not induce secretion of IL-6 or IL-10 by gastric CD13+ cells, although IL-8 secretion was induced (Figure 4). An equivalent number of MoDCs produced >20-fold higher levels of IL-6, IL-10, and IL-8, as well as IL- 12p70 (data not shown).
Figure 4.
H. pylori -induced cytokine secretion by gastric dendritic cells (DCs). Culture supernatants of gastric HLA-DR+ DCs or CD13+ cells obtained from H. pylori -negative donors and stimulated with H. pylori in vitro were collected after 15 h, and secretion of (a) IL-6, (b) IL-10 and (c) IL-8 was determined by enzyme-linked immunosorbent assay. Data are from a representative experiment (n =3, using cells from separate subjects); values correspond to mean ± s.d.
Gastric DCs take up and process H. pylori bacteria and soluble antigen
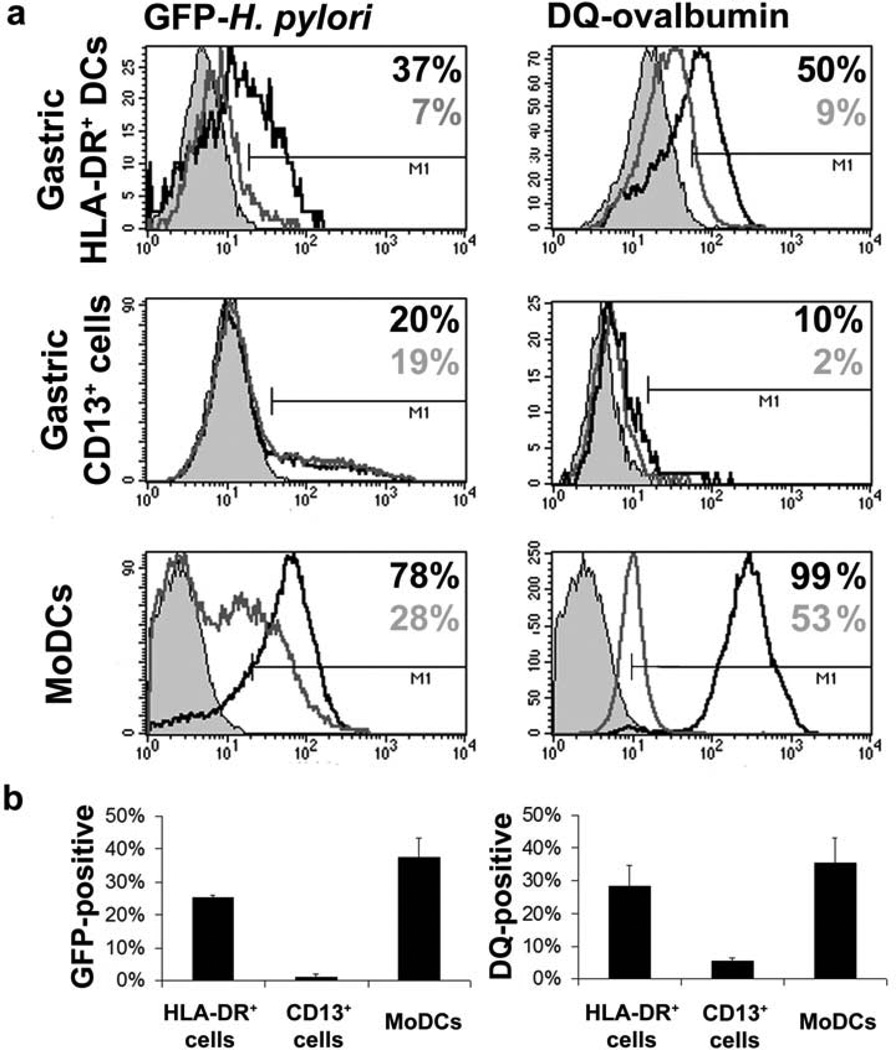
Because antigen uptake and processing is a prerequisite for antigen presentation, we next analyzed the capacity of gastric HLA-DR+ DCs to take up H. pylori and soluble antigen, using green fluorescent protein (GFP)-labeled live H. pylori bacteria and DQ-ovalbumin. DQ-ovalbumin is a self-quenched ovalbumin conjugate that is internalized through the mannose receptor by clathrin-mediated endocytosis and shows bright green fluorescence on proteolytic cleavage in endosomal compartments, thereby allowing analysis of antigen processing. Gastric HLA-DR+ DCs phagocytosed GFP- H. pylori and endocytosed DQ-ovalbumin, indicated by increased DC fluorescence after 30 min incubation at 37°C but not at 4°C (Figure 5a,b). Gastric CD13+ cells displayed poor phagocytic activity for GFP- H. pylori and reduced endocytic activity for DQ-ovalbumin, and MoDCs avidly took up both GFP- H. pylori and DQ-ovalbumin.
Figure 5.
Antigen uptake and processing by gastric HLA-DR+ dendritic cells (DCs), CD13+ cells and monocyte-derived DCs (MoDCs). Magnetic antibody cell sortingenriched gastric HLA-DR+ DCs or CD13+ cells or 5-day-old MoDCs from H. pylori negative donors were incubated for 30 min in the presence of GFP- H. pylori (multiplicity of infection 10; left panels) or DQ-ovalbumin (10 µg/mL; right panels) at 37 or 4°C. (a) Solid grey histograms correspond to cells incubated in medium alone, open grey histograms to cells along with GFP- H. pylori or DQ-ovalbumin at 4°C, and open black histograms to cells along with GFP- H. pylori or DQ-ovalbumin at 37°C. (b) Percentage of cells positive for GFP- H. pylori (left panel) and DQ-ovalbumin (right panel) expressed as mean ± s.e.m. of 2–3 independent experiments; values corrected for nonspecific binding at 4°C.
H. pylori -pulsed human gastric HLA-DR+ DCs induce T-cell IFN-γ secretion
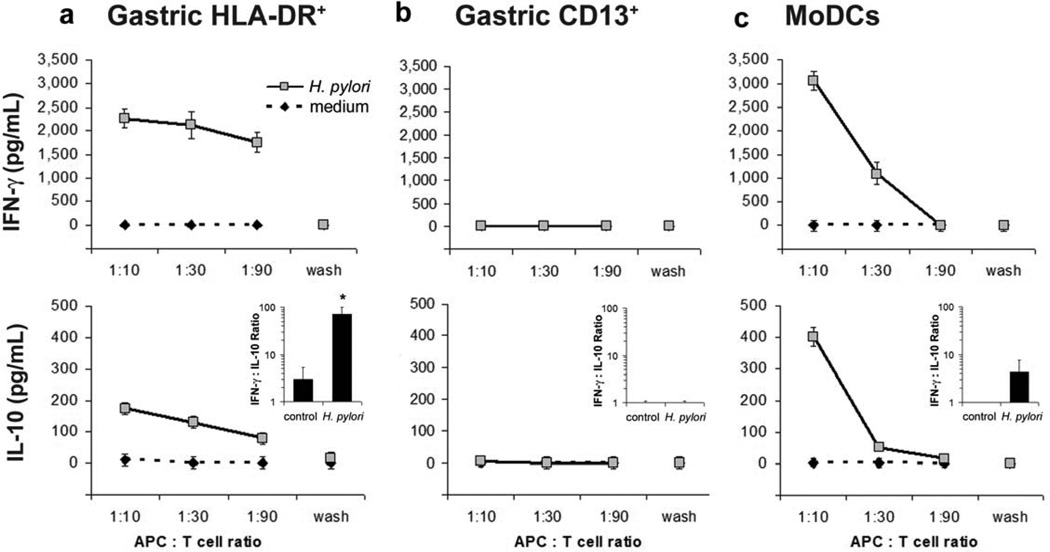
Because exposure to H. pylori induced the maturation and activation of isolated gastric HLA-DR+ cells, and HLA-DR+ DCs efficiently took up H. pylori, we next determined whether H. pylori -pulsed gastric DCs had the capacity to prime T cells. Gastric HLADR+ DCs purified by fluorescence-activated cell sorting (FACS) were pulsed with H. pylori for two h, washed, and co-cultured for 3 days with autologous blood CD4+ T cells obtained from H. pylori -negative donors. H. pylori -pulsed gastric HLA-DR+ DCs induced the T cells to secrete high levels of IFN-γ and low levels of IL-10 (Figure 6). Importantly, the ratio of IFN-γ to IL-10 increased significantly (71 ± 29-fold at an APC/T cell ratio of 1:10, n =3, P ≤0.05, unpaired Student’s t -test) when T cells were cultured with H. pylori pulsed DCs in contrast to culture with untreated DCs, indicating a preferential induction of Th1 cells (Figure 6, insets). Pulsed gastric CD13+ cells were unable to induce detectable T-cell cytokine production, whereas MoDCs induced T-cells to secrete levels of IFN-γ and IL-10 similar to those induced by gastric DCs. Together, these findings implicate gastric mucosal HLA-DR+ DCs as key inducers of the Th1 response characteristic of H. pylori -associated inflammation.
Figure 6.
Gastric HLA-DR+ dendritic cells (DCs) drive a Th1-predominant response. (a) Gastric HLA-DR+ DCs, (b) gastric CD13+ cells or (c) monocyte-derived DCs (MoDCs) derived from cells from healthy subjects were pulsed with H. pylori, multiplicity of infection 20 for 2 h, washed twice, and co-cultured with autologous blood CD4+ T cells for 3 days. Supernatants from the second wash (“wash”) were used as controls to exclude direct effects of residual bacteria on the T cells. Concentrations of interfreron (IFN)-γ and interleukin (IL)-10 in culture supernatants were determined by enzymelinked immunosorbent assay. Data are representative of three (gastric HLA-DR+ DCs) or two (gastric CD13+ cells and MoDCs) experiments with cells from separate subjects; values correspond to mean ± s.e.m.. Insets in the lower panels show the ratio of IFN-γ to IL-10 for two or three experiments (mean ± s.e.m.) at an antigen-presenting cell (APC)/T-cell ratio of 1:10; * P <0.05, unpaired Student’s t -test.
DISCUSSION
We report here the first characterization and isolation of DCs from human gastric mucosa and provide evidence that gastric DCs induce the adaptive T-cell response to H. pylori. Our analysis of gastric tissue sections and isolated gastric mucosal cells showed that HLA-DR+ DCs are indeed present in noninflamed gastric mucosa, whereas previously their presence had been a matter of debate12,13,21. Importantly, gastric DCs were more prevalent, mature, and activated in H. pylori -infected subjects, corroborating earlier reports of HLA-DR+ cells in inflamed gastric mucosa10,11.
Stimulation of a primary T-cell response requires antigen uptake and presentation in the context of co-stimulatory molecules and appropriate cytokine signals by mature DCs36,37. H. pylori colonizes the mucous layer of the gastric mucosa, but some bacteria or bacterial antigens cross the epithelial barrier, enabling tissue DCs to sample bacterial antigens38–40. As we show, isolated human gastric DCs were indeed able to phagocytose and process H. pylori bacteria in vitro. In this connection, we identified a unique subset of HLA-DR+/CD11c+ DCs within the gastric epithelial layer that may play a role in luminal antigen uptake similar to the role of transepithelial DC extensions in the mucosa of the small intestine41,42.
H. pylori -induced DC maturation has previously been described for MoDCs15–17 and BM-DCs18,31. Extending those findings, our study is the first to show that human gastric DCs undergo increased CD83, CD86, and CD11c expression in response to H. pylori infection in vivo and H. pylori exposure in vitro. H. pylori also induced secretion of IL-6 by isolated gastric DCs, as previously demonstrated for murine BM-DCs12,18 and human MoDCs43. In response to H. pylori, both myeloid cells and epithelial cells secrete IL-8, which contributes to gastric inflammation by recruiting neutrophils and monocytes to the gastric lamina propria17,43–45. In this connection, we show that both HLA-DR+ and CD13+ gastric cells secreted IL-8 in response to H. pylori. The bacterial factors that induce gastric DC activation have not yet been identified, but TLR2, 4 and 9 likely are involved, as H. pylori treatment of BM-DCs from MyD88−/−, TLR2/4−/− and TLR 2/4/9−/− mice fails to induce DC activation18,46.
The development of H. pylori -induced gastritis is dependent on CD4 T-cells, specifically IFN-γ-producing Th1 cells3,6,9,47. In humans, IFN-γ sustains mucosal inflammation and may promote disease progression to gastric ulcer47–49. Here, purified gastric HLA-DR+ DCs, as well as control MoDCs, from noninfected donors pulsed with H. pylori in vitro induced a Th1 response with strong secretion of IFN-γ, implicating gastric HLA-DR+ DCs as a determinant of gastric T-cell lineage development.
Surprisingly, we were unable to detect mRNA or protein for the Th1-promoting cytokine IL-12 by H. pylori -pulsed gastric DCs, although MoDCs secreted high levels of IL-12, as previously reported15. The apparent inability of gastric HLA-DR+ DCs to produce IL-12 raises the possibility that gastric DCs induce T-cell IFN-γ secretion through macrophage inhibitory factor, a protein that has recently emerged as a Th1- stimulating cytokine in H. pylori gastritis50. Also, the previously reported IL-12 secretion by H. pylori -pulsed gastric DCs in DC and T-cell co-cultures may have been augmented by T-cell-derived IFN-γ through a positive feedback loop51. Others have shown that T cells isolated from H. pylori -infected gastric mucosa and re-stimulated with H. pylori secreted IFN-γ, IL-4 and IL-1049. T regulatory cells (Tregs) are crucial for limiting inflammatory damage in H. pylori gastritis, and insufficient IL-10 secretion by CD4+CD25+ Tregs in H. pylori -infected adults is associated with an increased frequency of peptic ulcer disease49. In this connection, we have shown that a potent Treg response appears to reduce gastric pathology and limit ulceration in children6. Although our study showed that H. pylori induced substantially higher levels of IFN-γ than IL-10, indicating a predominant Th1 response, gastric DCs pulsed with H. pylori did induce T cells to secrete low levels of IL-10, raising the possibility that gastric DCs also may induce Tregs.
In summary, we present the first identification and characterization of DCs in the mucosa of the human stomach. The activation of human gastric DCs by H. pylori enables these cells to induce potent T-cell IFN-γ secretion, consistent with the Th1 response characteristic of H. pylori -driven gastritis. Our findings implicate gastric DCs as key initiators of the immune response to H. pylori and offer new insight into the early events in the pathogenesis of H. pylori infection.
METHODS
Patients and tissue samples
Gastric tissue specimens were obtained with institutional review board approval from healthy human subjects undergoing elective gastric bypass surgery for treatment of obesity (bypass samples) and from H. pylori -infected subjects undergoing diagnostic esophagogastroduodenoscopy (biopsy samples). Additional tissue specimens were obtained from noninfected, nonmorbidly obese transplant donors. Gastric samples (1 cm ×10 cm) from bypass donors were excised from the lesser curvature of the gastric body and transferred to the laboratory in sterile Endopouch™ Retrievers™ (Ethicon, Cincinnati, OH). Biopsy samples from endoscoped patients were obtained from the lesser curvature of the gastric body and antrum and transferred to the laboratory in sterile RPMI 1640 medium immediately after sampling. Serological analysis for H. pylori was performed on all patients before tissue sampling, and active H. pylori infection of endoscoped patients was confirmed by rapid urease CLO test. All samples were processed within 1 h of sampling. A section of each specimen was frozen in Tissue-Tek OCT compound (Sakura, Torrance, CA) for histological analysis, and the remaining tissue was processed for cell isolation. Functional assays were performed with cells isolated from gastric tissue from H. pylori negative patients undergoing gastric bypass surgery.
Monoclonal antibodies
The following monoclonal anti-human antibodies were used for immunohistology and flow cytometry: CD1a (clone HI149), CD3 (HIT3a), CD11c (Bly6), CD13 (WM15), CD14 (M5E2), CD20 (2H7), CD56 (B159), CD80 (BB1), CD83 (HB15e), CD86 (FUN-1), CD116 (M5D12), CD123 (7G3), HLA-DR (L243), DC-SIGN (19.2), CD206 (mannose receptor, DCN46) and CCR3 (5E8). Anti-CD45 was obtained from Miltenyi-Biotec (Auburn, CA), and all other antibodies and appropriate isotype controls from Becton Dickinson (San Jose, CA).
Immunofluorescence histology and image analysis
Frozen tissue sections (5 µm) were fixed for 10 min in acetone at −20°C and blocked with DakoCytomation (Carpinteria, CA) protein block. After washing in DPBS/0.05% Tween 20, monoclonal antibodies were added at predetermined optimum concentrations for 2 h at room temperature. Bound antibodies were detected with appropriate isotype-specific secondary antibodies labeled withfluorescein isothiocyanate or Texas Red (both from Southern Biotechnologies, Birmingham, AL), as previously described52. Cell nuclei were labeled with 46-diamidino-2-phenyl indole. Sections were mounted, sealed, and stored at 4°C until analysis by fluorescent microscopy (Nikon Eclipse T2000-U, equipped with a CoolSnap ES digital camera and NIS Elements BR2.30 software, Nikon, Tokyo, Japan).
Digital image analysis was performed using ImageJ 1.42q software. Density of red (CD11c) and green (HLA-DR) cells in the lamina propria was determined as percent positive pixels per area using the “analyze particles” tool. Colocalization of red and green pixels was determined using the PSC colocalization plugin53 (intensity scatter plots) and the JaCOP plugin34 (Mander’s colocalization coefficients M1 and M2). Images were thresholded to exclude background staining, and regions of interest were set to exclude surface and glandular epithelial cells. Three or more independent areas from 4 – 5 slides per group were analyzed.
Isolation of gastric mucosal cells
Gastric lamina propria cells were isolated from surgical specimens following the protocol established in our laboratory for the isolation of intestinal macrophages24,25, with slight modifications. Briefly, excess mucus was removed by blotting tissue samples on Whatman filter paper, after which the muscle layer and submucosa were separated from the mucosa by mechanical dissection. The remaining mucosa was washed twice for 20 min at 37°C in a shaking water bath in Hank’s balanced salt solution (Mediatech Inc., Manassas, VA) supplemented with 0.2 mg/mL DTT (Sigma, St. Louis, MO), after which the surface epithelium was removed by incubating the tissue three times for 30 min in Hank’s balanced salt solution containing 1.25 mM EDTA and 0.2 mg/mL DTT. The tissue then was minced and transferred to a solution containing 0.5 U/mL collagenase L (Sigma), 0.2 mg/mL DNAse (Sigma), 20 mM Hepes (Mediatech), and antibiotics in RPMI (Mediatech). To isolate cells from biopsy specimens, we treated biopsies with collagenase solution (0.5 U/mL) as above without prior removal of the surface epithelium. After three collagenase treatments as above, cells were collected by centrifugation (10 min, 4°C, 400 g ), and resuspended in RPMI 1640 supplemented with 10% heat-inactivated human AB serum (Mediatech). Debris and aggregates were removed from the cell suspensions by sedimentation on ice for 30 min.
HLA-DR+ and CD13+ cells were enriched up to 80% purity by positive magnetic bead selection (MACS; Miltenyi-Biotec) using PE-labeled primary monoclonal antibodies and anti-PE microbeads together with LS separation columns and a VarioMACS® magnet. For T-cell stimulation assays, we purified gastric HLA-DR+ DCs or CD13+ cells to >95% by FACS sorting (FACSVantage DiVa, Becton Dickinson). FACS-sorted cells were >95% viable as determined by propidium iodide exclusion.
Generation of monocyte-derived DCs
Monocyte-derived DCs were generated from blood CD14+ monocytes isolated by MACS by culturing the monocytes in complete medium (RPMI 1640, 10% heat-inactivated human AB serum and antibiotics) supplemented with rhGM-CSF (25 ng/mL) and rhIL-4 (7 ng/mL) both from R&D Systems (Minneapolis, MN). Nonadherent cells were harvested as MoDCs by vigorous pipetting after 5 days of culture.
Preparation of H. pylori bacteria and DC stimulation
The CagA+, VacA s1m1 H. pylori strain 60190 was kindly provided by G Perez-Perez (New York University School of Medicine). Bacteria were grown at 36°C under semi-anaerobic conditions on Brucella agar plates, 5% horse blood (Becton Dickinson) for 3 days. Colonies were harvested and suspended in warm Brucella broth supplemented with 10% fetal calf serum, and bacterial concentrations were determined by spectrophotometry at 600 nm based on a standard curve generated using the LIVE/DEAD BacLight Bacterial Viability and Counting Kit (Molecular Probes, Eugene, OR). To determine the response of gastric DCs and CD13+ cells to H. pylori, we incubated freshly isolated cells in duplicate (or triplicate, cell number permitting) in complete medium supplemented with 25 ng/mL rhGM-CSF on 24-well plates for 2 h before adding H. pylori at an MOI of 5 – 20 for 15 h. Cells then were harvested, and surface marker expression was determined by FACS. Cytokine concentrations for IL-6, IL-10, IL-8, and IL-12p70 in culture supernatants were determined using human Quantikine® ELISA kits (R&D Systems), following the manufacturer’s instructions. Purified gastric HLA-DR+ or CD13+ cells were not adherent to culture plates.
Antigen uptake assays
The ability of enriched gastric DCs, CD13+ cells and MoDCs obtained from H. pylori –negative subjects to take up particulate or soluble antigen was studied using GFP-labeled H. pylori (Strain M6, MOI 10, kind gift from John Y Kao, University of Ann Arbor, MI) and DQ™ ovalbumin (10 µg/mL, Molecular Probes). Freshly isolated cells were placed in pre-warmed, serum-free medium and incubated at 37°C for 2 h, after which GFP- H. pylori or DQ-ovalbumin were added to the cell suspensions for another 30 min at 37°C to allow antigen uptake. Control cultures were incubated at 4°C. Cells then were washed in ice cold phosphate-buffered saline/1% fetal calf serum/0.09% Na-azide (Becton Dickinson), fixed in Cellfix, and analyzed by flow cytometry.
T-cell stimulation assay
Autologous CD4+ lymphocytes obtained from H. pylori -negative subjects were enriched from peripheral blood mononuclear cells using the MACS system. Freshly isolated, FACS-purified gastric HLA-DR+ DCs, CD13+ cells or MoDC were incubated for 2 h at 37°C, then pulsed with H. pylori (MOI 10) for another 2 h, followed by two washes. Co-cultures of APCs and CD4+ T-cells were prepared in duplicate in 96-well flat bottom plates in 200 µL complete medium. Supernatants from the second wash were added to control T-cell cultures to exclude direct effects of residual free H. pylori on the T cells. After three days, cell-free supernatants were collected for analysis of cytokine production (IL-10, IFN-γ and TGF-β) by enzyme-linked immunosorbent assay.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the National Institutes of Health (DK-54495, AI-83539, AI- 78217, DK-47322, DK-084063, RR-20136 and the Mucosal HIV and Immunobiology Center, DK-64400) and the Research Service of the Veterans Administration. We also thank G Perez-Perez (New York University School of Medicine) and John Y Kao (University of Ann Arbor) for providing H. pylori bacteria, and Donna Crabb and Lynn Duffy (University of Alabama at Birmingham) for preparing H. pylori cultures.
Footnotes
SUPPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/mi
DISCLOSURE
The authors declared no conflicts of interest.
REFERENCES
- 1.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 2.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 3.Bamford KB, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 4.Kraft M, et al. IFN-gamma synergizes with TNF-alpha but not with viable H. pylori in up-regulating CXC chemokine secretion in gastric epithelial cells. Clin. Exp. Immunol. 2001;126:474–481. doi: 10.1046/j.1365-2249.2001.01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JM, Kim JS, Jung HC, Song IS, Kim CY. Up-regulation of inducible nitric oxide synthase and nitric oxide in Helicobacter pylori -infected human gastric epithelial cells: possible role of interferon-gamma in polarized nitric oxide secretion. Helicobacter. 2002;7:116–128. doi: 10.1046/j.1083-4389.2002.00068.x. [DOI] [PubMed] [Google Scholar]
- 6.Harris PR, et al. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology. 2008;134:491–499. doi: 10.1053/j.gastro.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T, Kita M, Ohno T, Iwakura Y, Sekikawa K, Imanishi J. Role of tumor necrosis factor-alpha and interferon-gamma in Helicobacter pylori infection. Microbiol. Immunol. 2004;48:647–654. doi: 10.1111/j.1348-0421.2004.tb03474.x. [DOI] [PubMed] [Google Scholar]
- 8.Smythies LE, Waites KB, Lindsey JR, Harris PR, Ghiara P, Smith PD. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J. Immunol. 2000;165:1022–1029. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 9.Smythies LE, Chen JA, Lindsey JR, Ghiara P, Smith PD, Waites KB. Quantitative analysis of Helicobacter pylori infection in a mouse model. J. Immunol. Methods. 2000;242:67–78. doi: 10.1016/s0022-1759(00)00215-5. [DOI] [PubMed] [Google Scholar]
- 10.Sarsfield P, Jones DB, Wotherspoon AC, Harvard T, Wright DH. A study of accessory cells in the acquired lymphoid tissue of Helicobacter gastritis. J. Pathol. 1996;180:18–25. doi: 10.1002/(SICI)1096-9896(199609)180:1<18::AID-PATH624>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, et al. Localization of antigen-presenting cells in Helicobacter pylori-infected gastric mucosa. Pathol. Int. 2002;52:265–271. doi: 10.1046/j.1440-1827.2002.01347.x. [DOI] [PubMed] [Google Scholar]
- 12.Kao JY, et al. Helicobacter pylori -secreted factors inhibit dendritic cell IL-12 secretion: a mechanism of ineffective host defense. Am. J Physiol Gastrointest. Liver Physiol. 2006;291:G73–G81. doi: 10.1152/ajpgi.00139.2005. [DOI] [PubMed] [Google Scholar]
- 13.Nishi T, et al. Involvement of myeloid dendritic cells in the development of gastric secondary lymphoid follicles in Helicobacter pylori -infected neonatally thymectomized BALB/c mice. Infect. Immun. 2003;71:2153–2162. doi: 10.1128/IAI.71.4.2153-2162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeshima E, et al. Helicobacter pylori -induced interleukin-12 p40 expression. Infect. Immun. 2009;77:1337–1348. doi: 10.1128/IAI.01456-08. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Kranzer K, et al. Induction of maturation and cytokine release of human dendritic cells by Helicobacter pylori. Infect. Immun. 2004;72:4416–4423. doi: 10.1128/IAI.72.8.4416-4423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guiney DG, Hasegawa P, Cole SP. Helicobacter pylori preferentially induces interleukin 12 (IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect. Immun. 2003;71:4163–4166. doi: 10.1128/IAI.71.7.4163-4166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hafsi N, et al. Human dendritic cells respond to Helicobacter pylori promoting NK cell and Th1-effector responses in vitro. J. Immunol. 2004;173:1249–1257. doi: 10.4049/jimmunol.173.2.1249. [DOI] [PubMed] [Google Scholar]
- 18.Rad R, et al. Toll-Like Receptor-Dependent Activation of Antigen-Presenting Cells Affects Adaptive Immunity to Helicobacter pylori. Gastroenterology. 2007;133:150–163. doi: 10.1053/j.gastro.2007.04.071. [DOI] [PubMed] [Google Scholar]
- 19.Pappo J, Torrey D, Castriotta L, Savinainen A, Kabok Z, Ibraghimov A. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect. Immun. 1999;67:337–341. doi: 10.1128/iai.67.1.337-341.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang M, Berndt BE, Eaton KA, Rathinavelu S, Pierzchala A, Kao JY. Helicobacter pylori -pulsed dendritic cells induce H. pylori -specific immunity in mice. Helicobacter. 2008;13:200–208. doi: 10.1111/j.1523-5378.2008.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ninomiya T, Matsui H, Akbar SM, Murakami H, Onji M. Localization and characterization of antigen-presenting dendritic cells in the gastric mucosa of murine and human autoimmune gastritis. Eur. J. Clin. Invest. 2000;30:350–358. doi: 10.1046/j.1365-2362.2000.00629.x. [DOI] [PubMed] [Google Scholar]
- 22.Nagai S, et al. Role of Peyer's patches in the induction of Helicobacter pylori -induced gastritis. Proc. Natl. Acad. Sci. USA. 2007;104:8971–8976. doi: 10.1073/pnas.0609014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiriya K, et al. Essential role of Peyer's patches in the development of Helicobacter -induced gastritis. Int. Immunol. 2007;19:435–446. doi: 10.1093/intimm/dxm008. [DOI] [PubMed] [Google Scholar]
- 24.Smith PD, et al. Isolation and purification of CD14-negative mucosal macrophages from normal human small intestine. J. Immunol. Methods. 1997;202:1–11. doi: 10.1016/s0022-1759(96)00204-9. [DOI] [PubMed] [Google Scholar]
- 25.Smythies LE, Wahl LM, Smith PD. Isolation and purification of human intestinal macrophages. Curr. Protoc. Immunol. 2006;7.6B:1–9. doi: 10.1002/0471142735.im0706bs70. [DOI] [PubMed] [Google Scholar]
- 26.Smythies LE, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith PD, et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J. Immunol. 2001;167:2651–2656. doi: 10.4049/jimmunol.167.5.2651. [DOI] [PubMed] [Google Scholar]
- 28.Bell SJ, et al. Migration and maturation of human colonic dendritic cells. J. Immunol. 2001;166:4958–4967. doi: 10.4049/jimmunol.166.8.4958. [DOI] [PubMed] [Google Scholar]
- 29.Rosenzwajg M, Tailleux L, Gluckman JC. CD13/N-aminopeptidase is involved in the development of dendritic cells and macrophages from cord blood CD34+ cells. Blood. 2000;95:453–460. [PubMed] [Google Scholar]
- 30.Shipp MA, Look AT. Hematopoietic differentiation antigens that are membrane-associated enzymes: cutting is the key! Blood. 1993;82:1052–1070. [PubMed] [Google Scholar]
- 31.Drakes ML, Czinn SJ, Blanchard TG. Regulation of murine dendritic cell immune responses by Helicobacter felis antigen. Infect. Immun. 2006;74:4624–4633. doi: 10.1128/IAI.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Archimandritis A, Sougioultzis S, Foukas PG, Tzivras M, Davaris P, Moutsopoulos HM. Expression of HLA-DR, costimulatory molecules B7-1, B7-2, intercellular adhesion molecule-1 (ICAM-1) and Fas ligand (FasL) on gastric epithelial cells in Helicobacter pylori gastritis influence of H. pylori eradication. Clin. Exp. Immunol. 2000;119:464–471. doi: 10.1046/j.1365-2249.2000.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manders EM, Stap J, Brakenhoff GJ, van Driel R, Aten JA. Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J. Cell Sci. 1992;103(Pt 3):857–862. doi: 10.1242/jcs.103.3.857. [DOI] [PubMed] [Google Scholar]
- 34.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell P, et al. Chronic exposure to Helicobacter pylori impairs dendritic cell function and inhibits Th1 development. Infect. Immun. 2007;75:810–819. doi: 10.1128/IAI.00228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123:326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito T, et al. Helicobacter pylori invades the gastric mucosa and translocates to the gastric lymph nodes. Lab Invest. 2008;88:664–681. doi: 10.1038/labinvest.2008.33. [DOI] [PubMed] [Google Scholar]
- 39.Dubois A, Boren T. Helicobacter pylori is invasive and it may be a facultative intracellular organism. Cell Microbiol. 2007;9:1108–1116. doi: 10.1111/j.1462-5822.2007.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mai UE, Perez-Perez GI, Allen JB, Wahl SM, Blaser MJ, Smith PD. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J. Exp. Med. 1992;175:517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rescigno M, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 42.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kranzer K, et al. Impact of Helicobacter pylori virulence factors and compounds on activation and maturation of human dendritic cells. Infect. Immun. 2005;73:4180–4189. doi: 10.1128/IAI.73.7.4180-4189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tummuru MK, Sharma SA, Blaser MJ. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol. Microbiol. 1995;18:867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- 45.Ogura K, et al. Interleukin-8 production in primary cultures of human gastric epithelial cells induced by Helicobacter pylori. Dig. Dis. Sci. 1998;43:2738–2743. doi: 10.1023/a:1026671815512. [DOI] [PubMed] [Google Scholar]
- 46.Rad R, et al. Extra- and intracellular pattern recognition receptors cooperate in the recognition of Helicobacter pylori. Gastroenterology. 2009;136:2247–2257. doi: 10.1053/j.gastro.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 47.O'Keeffe J, Moran AP. Conventional, regulatory, and unconventional T cells in the immunologic response to Helicobacter pylori. Helicobacter. 2008;13:1–19. doi: 10.1111/j.1523-5378.2008.00559.x. [DOI] [PubMed] [Google Scholar]
- 48.D'Elios MM, Amedei A, Benagiano M, Azzurri A, Del Prete G. Helicobacter pylori T cells and cytokines: the "dangerous liaisons". FEMS Immunol. Med. Microbiol. 2005;44:113–119. doi: 10.1016/j.femsim.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Robinson K, et al. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008;57:1375–1385. doi: 10.1136/gut.2007.137539. [DOI] [PubMed] [Google Scholar]
- 50.Wong BL, et al. Essential role for macrophage migration inhibitory factor in gastritis induced by Helicobacter pylori. Am. J. Pathol. 2009;174:1319–1328. doi: 10.2353/ajpath.2009.080708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trinchieri G. Interleukin-12 and interferon-gamma. Do they always go together? Am. J Pathol. 1995;147:1534–1538. [PMC free article] [PubMed] [Google Scholar]
- 52.Bimczok D, Sowa EN, Faber-Zuschratter H, Pabst R, Rothkötter HJ. Site-specific expression of CD11b and SIRPalpha (CD172a) on dendritic cells: implications for their migration patterns in the gut immune system. Eur. J. Immunol. 2005;35:1418–1427. doi: 10.1002/eji.200425726. [DOI] [PubMed] [Google Scholar]
- 53.French AP, Mills S, Swarup R, Bennett MJ, Pridmore TP. Colocalization of fluorescent markers in confocal microscope images of plant cells. Nat. Protoc. 2008;3:619–628. doi: 10.1038/nprot.2008.31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.