Abstract
The aircraft cabin represents a unique indoor environment due to its high surface-to-volume ratio, high occupant density and the potential for high ozone concentrations at cruising altitudes. Ozone was continuously measured and air was sampled on sorbent traps, targeting carbonyl compounds, on 52 transcontinental U.S. or international flights between 2008 and 2010. The sampling was predominantly on planes that did not have ozone scrubbers (catalytic converters). Peak ozone levels on aircraft without catalytic convertors exceeded 100 ppb, with some flights having periods of more than an hour when the ozone levels were > 75ppb. Ozone was greatly reduced on relatively new aircraft with catalytic convertors, but ozone levels on two flights whose aircraft had older convertors were similar to those on planes without catalytic convertors. Hexanal, heptanal, octanal, nonanal, decanal and 6-methyl-5-hepten-2-one (6-MHO) were detected in the aircraft cabin at sub- to low ppb levels. Linear regression models that included the log transformed mean ozone concentration, percent occupancy and plane type were statistically significant and explained between 18 and 25% of the variance in the mixing ratio of these carbonyls. Occupancy was also a significant factor for 6-MHO, but not the linear aldehydes, consistent with 6-MHO’s formation from the reaction between ozone and squalene, which is present in human skin oils.
Introduction
The aircraft cabin presents a unique environment where passengers and crew are confined in an enclosed setting for an extended time breathing air that is an approximately equal mix of that drawn in through the compression stages of the jet engines and that recycled from the cabin. Commercial aircraft typically cruise at an altitude of ~12,000 m or 39,000 ft, which results in their being in the upper troposphere or lower stratosphere at higher latitudes (> 40°). Here ozone levels range from 100s of ppb to ppm during the late spring and winter months1. Ozone from outside the aircraft will enter its cabin, resulting in elevated ozone levels in the breathing zone of the passengers and crew. In the 1960’s and 1970’s, the presence of ozone in the cabin of aircraft flying at high altitudes, particularly those flying intercontinental polar routes, was reported to cause adverse health consequences 2-5. Regulations addressing the allowable ozone levels in aircraft cabin were established starting in 1980. The 1985 regulation (FAA Federal Aviation Regulations (FARS, 14 CFR)) set a maximum value of 0.25 ppm, sea level equivalent, and a value of 0.10 ppm for any 3 hour interval during a flight 6. The concentration limits of the FARS exceed the current US EPA National Ambient Air Quality Standard (NAAQS) for ground level ozone of 0.075 ppm over an 8 hour averaging time, which is higher than the EPA Clean Air Science Advisory Committee recommendation for the NAAQS human-health based standard of between 0.060 and 0.070. This was a recent change from the previous 1-hour NAAQS standard of 0.12 ppm.
In response to the 1985 FARS standard for ozone, catalysts to remove ozone were incorporated in the environmental control systems of some aircraft – predominantly on long range aircraft that flew polar routes where the maximum ozone levels were encountered 7. Recent measurements of ozone in aircraft cabins have shown that planes with catalytic convertors have reduced ozone levels. However, many aircraft do not have systems to remove ozone, and mean ozone levels exceeding 100 ppb over the course of the flight can occur 8, 9. Furthermore, while ozone converters are rated to decompose 90 to 98% of the ozone present in the air stream when first installed, they can be poisoned by water, sulfur containing compounds and contaminants present in small leaks from fuel or hydraulic systems. Such poisoning decreases their effectiveness to remove ozone, resulting in increased cabin ozone levels even in aircraft with ozone converters.
In addition to the direct impact of ozone on passengers and crew, it is also important to consider the impact of ozone derived products on the cabins occupants. A number of studies in both chambers and actual indoor environments have shown that ozone reacts with common indoor air contaminants (e.g., the cleaning/scenting agents d-limonene and α-pinene) to form formaldehyde, other aldehydes, ketones and organic acids (as reviewed in references 10 and 11 10,11). In studies conducted in a simulated B-767 aircraft cabin when either skin-oil soiled T-shirts 12 or “passengers” 13 were present, adding ozone to the air at concentrations of 60-70 ppb resulted in the production of a series of saturated and unsaturated aldehydes with from three to ten carbon atoms, carboxylic acids and other products that are less common in indoor air, such as 6-methyl-5-hepten-2-one (6-MHO), geranyl acetone and 4-oxopentanal (4-OPA) 12, 13. Similar products were identified when ozone and 2 occupants were simultaneously present in a simulated office setting 14. Additional experiments reported in the latter paper identified reactions between ozone and squalene, a major constituent of skin oil, as the source of 6-MHO, geranyl acetone and 4-OPA, while reactions between ozone and several unsaturated fatty acids found in human skin oil were identified as the major sources of the decanal measured in these studies. Other than formaldehyde, which is both a carcinogen and a respiratory and ocular irritant15, the potential adverse health effects of many of these compounds have not been extensively studied. In recent investigations, Anderson et al.16, 17 have found that exposure to 4-OPA is capable of inducing inflammatory cytokine expression in pulmonary epithelial cells15 and is an irritant and sensitizer based on dermal and pulmonary exposures using a murine model16. Additionally, several studies have suggested that symptom reports of airway irritation from the products of ozone reacting with unsaturated hydrocarbons cannot be explained solely from the production of formaldehyde, acetaldehyde, organic acids or ultrafine particles 18-21.
The aim of the present study was to simultaneously monitor the levels of both ozone and selected ozone-derived products in the cabin of various types of commercial aircraft during both transcontinental (U.S.) and transoceanic flights. The targeted oxidation products included saturated aldehydes with between 6 and 10 carbon atoms, as well as 6- MHO. (Unfortunately, the analytical methods did not permit quantification of 4-OPA.) The measurements reported in the present paper are the next logical step after the studies conducted earlier in a simulated aircraft cabin. An actual aircraft cabin is far more complex than a simulated one; the temperature, relative humidity and air exchange rates on these flights span a larger range than was used for the simulated flights. Additionally, in the present study the carryon luggage, the diverse passengers (clothed in a variety of apparel and using different personal care products) and even the materials used in the cabins of the different aircraft represent sources of pollutants that were not present in the simulated aircraft. In brief, this study better defines the oxidation products, and resultant mixing ratios, which passengers and crew are exposed to on actual flights. It also helps to elucidate the role that ozone plays in generating many of the products. These are important steps in evaluating the potential adverse effects associated with exposures to ozone and ozone derived products on commercial aircraft.
Methods
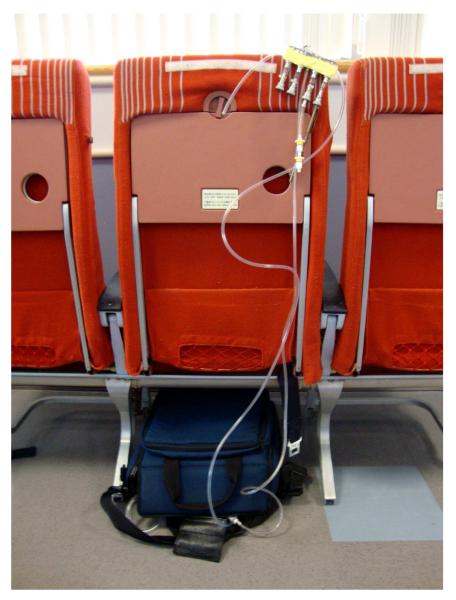
Samples were collected from commercial flights after securing permission from the airlines. Flights were predominantly transcontinental US flights taken during the winter and spring of 2008 to 2010 along with several transoceanic flights. Ozone was measured using a 2B Technology Model 205 Dual Beam ozone analyzer which uses absorption of UV light at 254 nm to quantify the ozone concentration. An internal temperature and pressure sensor is present in the absorption cell to compute the mixing ratio. It uses a particle filter to protect the absorption cell. A Teflon® lined polyethylene tube placed at breathing zone height was connected to the inlet of the analyzer and the instrument was placed under the airline seat in front of the technician operating it on board the aircraft (Figure 1). It was turned on when the aircraft reached cruising altitude and approved electronic devices were permitted to be used. One minute mean ozone concentrations were stored in the instrument’s memory until subsequently downloaded.
Figure 1.
Sampling system for the ozone monitor, sorbent tubes and pumps on/under an aircraft seat.
Air samples for the ozone by-products were collected on sorbent tubes that contained three sequential layers of absorbents (Tenax TA, Carboxen 569 and Carbosieve III, purchased from Supelco, Sigma-Aldrich, St. Louis, MO). A nominal air flow sampling rate of 20 cc/min was used resulting in 3 to 10 L of sampled air, depending upon the duration of the flight. A constant flow BGI OMNI pump set to an overall flow of 4 L/min was used with the flow split to sample with multiple sorbent tubes. In addition, the multiple bed adsorbent tube was attached to a low flow splitter to achieve flow rates of 20cc/min. Ozone scrubbers were prepared generally following the procedure outlined in Coleman et al. 22 using stainless steel tubes containing KI held in place by glass wool plugs. An ozone scrubber was attached upstream of each sorbent tube to remove ozone, which could oxidize compounds sorbed on the trap. The sorbent trap was thermally desorbed into a GC/MS for analysis (Perkin Elmer ATD400 coupled to an Agilent 5980/5971A). Based on flow checks conducted on the sampling pump before and after flights, we judge the uncertainty in the sampling rate to be < 10%. At low levels, the reproducibility of the measured mixing ratios was approximately 20%.
Quality Assurance/Quality Control
The 2B Technology ozone meter was calibrated by the manufacturer and the calibration was verified at EPA laboratories once during the course of the study and periodically compared to the readings of an API Ozone Monitor Model 101A across a range of ozone concentrations to verify the response.
The exact flow rate of the BGI OMNI pump was measured on the ground before and after a flight with a DryCal®, and was verified during the flight using a rotameter. The volume was corrected for temperature and pressure using a nominal aircraft cabin temperature of 298°K and pressure of 78.25 kPa. The selected pressure, an average of that for equivalent effective altitudes between 1800m and 2400m, is the value typically maintained in commercial aircraft for passenger comfort.
Calibration standards were prepared using a static gas bulb approach. A mixture of the target analytes was prepared by injecting between 10 and 50 l of a mixture of the pure compounds in the desired ratios into a 2.0 L spherical glass bulb containing several glass beads to facilitate mixing when the bulb was shaken and capped with a Mininert® valve. The bulb was placed in an oven at 90°C for at least one hour during which all of the liquid evaporated. A second, lower concentration 2.0 L spherical glass bulb was then prepared by transferring 1mL of the gaseous standard using a gas tight syringe, capping the bulb with a Mininert® valve and storing the bulb in a 90°C oven until individual standards were prepared. Individual standards were prepared by injecting 50 l and 2.0 mL using gas tight syringes of the low concentration gas standard into a heated 125ml gas sampling bulb through which ultra-pure nitrogen gas was flowing at a rate of 150 to 200 ml/min and to which the adsorbent trap was attached. A five minute transfer time was used to transfer the entire gas standard to the sampling trap. The adsorbent traps were then analyzed in a manner identical to samples by thermal desorption GC/MS. Six point calibration curves were prepared with R2 > 0.98 acceptance criteria. Using an external standard, the curve was verified daily to within ± 20% of the target value prior to running samples. The low concentration bulb was prepared fresh every few days or if the external standard was outside the target value range. If the response of a new calibration bulb standard was not acceptable, a new calibration curve was prepared. The target compounds were hexanal, heptanal, octanal, nonanal, decanal, 6-MHO, t-2-heptenal, and t-2-octenal. All standards were maximum purity available (>92%-99%) and purchased from Sigma-Aldrich (St. Louis, MO) or Alfa Aesar (Ward Hill, MA) (nonanal).
Data Analysis
The general descriptive statistics, the distribution of the compounds mixing ratios and linear regression models were run using SPSS (Version 19.0). The ozone concentrations were log transformed prior to running the linear regression analyses as its concentrations were consistent with a log normal distribution. Linear regression models were run for each compound, with the compound as the dependent variable and the log transformed ozone concentration (mean and peak of each flight), percent occupancy, plane type, volume of the plane, and number of seats as the independent variables. The plane type was treated as three separate dummy variables on a Boeing 737 (1 for 737 and 0 for the other two), on a Boeing 747 (1 for 747 and 0 for the other two), on a Boeing 757 (1 for 757 and 0 for the other two) and on a Boeing 777 (having all three dummy variables as (0)). The volume of the planes was estimated from the cabin width and length given in the technical information for each plane (Web site http://www.boeing.com/commercial/airports/plan_manuals.html accessed 8-28-12), assuming that the cabin’s interior was a circle with half the width being the radius. The number of seats was also taken from the technical information for a given plane, although we recognize that the exact configuration can vary across different airlines. A significance of 0.05 was used for considering a model fit or a variable’s contribution significant.
Results and Discussion
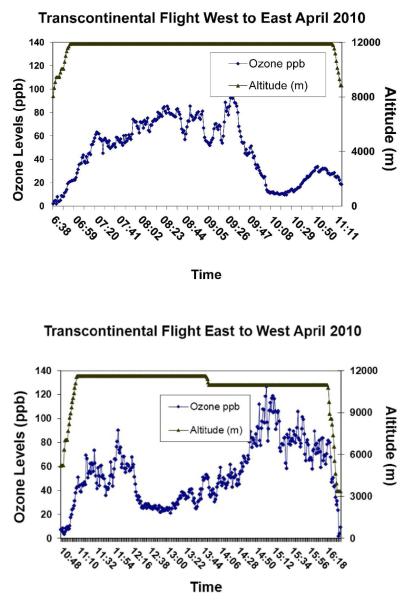
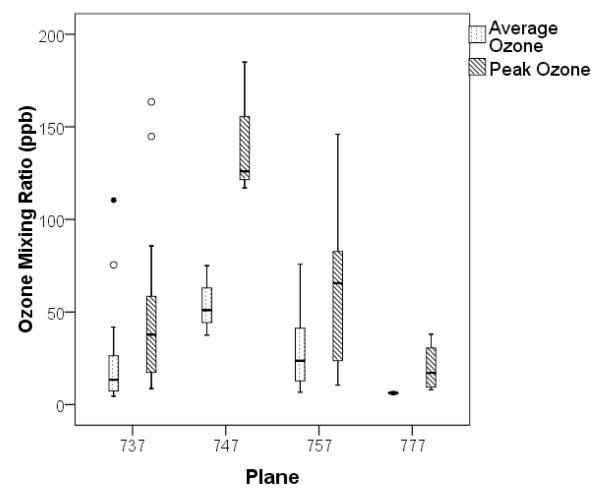
Continuous ozone measurements and air samples for ozone by-products were collected on 36 transcontinental US and 14 transoceanic flights on Boeing 737, 747, 757 or 777 aircraft between October 2008 and June 2010. The Boeing 737 and 757 aircraft were not equipped with catalysts to remove ozone from the ventilation air while the Boeing 747 and 777 were. Maintenance records for the catalytic convertors in service on the flights taken were not available. The flights were in the northern hemisphere with cruise altitudes above 9100 meters (30,000 feet). Most were selected to be during the winter and spring on routes that flew at higher latitudes to increase the likelihood that the aircraft encountered high external ozone levels. An example of the variations in ozone concentrations, along with flight altitude, on both the west-to-east and east-to-west legs of a transcontinental flight are shown in Figure 2. The summary statistics for the flights’ mean and peak ozone concentrations, broken down by plane type, are displayed in Figure 3 as box plots.
Figure 2.
Examples of one minute ozone concentrations measured during two individual transcontinental flights in April 2010 on planes without catalytic convertors (altitude profile displayed on right y-axis).
Figure 3.
Box and whisker plot of mean and peak ozone concentrations measured while at cruising altitude according to aircraft type. The airline and flight routes selected were flown by Boeing aircraft. The line in the box is the median, the box represent the 25th and 75th quartiles, the whiskers the upper and low bounds of the distribution calculated as 1.5 times the interquartile range away from the 25th and 75th quartile and the circles suspect outliers (open circle) and extreme outliers (filled in circle) based on the distribution
During the spring and summer in both 2009 and 2010 the aircraft cabin ozone levels were elevated on most transcontinental and international flights on aircraft without ozone convertors, while flights taken in late fall had lower ozone levels (< 20ppb), consistent with previous studies 8. Flights taken on Boeing 777s in March and April 2010 had some of the lowest ozone levels (Figure 3) even though they were flown at high altitudes over the ocean during the spring – scenarios with expectedly high ozone outside the cabin when at cruise altitude1. The Boeing 777 is a relatively new aircraft that is equipped with ozone converters. The Boeing 747s that were part of this study also had ozone convertors, yet on two of the three flights taken in May 2010 the cabin ozone levels were similar to those measured on planes without a convertor. The 747s were older aircraft with presumably older ozone converters that had been repeatedly serviced. The measured ozone levels within the cabin of these aircraft suggests that the ozone convertors were no longer efficiently removing ozone, indicating that a more stringent maintenance or test schedule for ozone convertors is needed on at least some aircraft.
The summary statistics for selected ozone by-products are given in Table 1. These include the first reported mixing ratios for 6-MHO and the C6- to C10-aldehydes in the cabin of commercial aircraft. These compounds can be produced from reactions of ozone and various unsaturated hydrocarbons. The primary source of 6-MHO is reaction of ozone with squalene 12, 13, which is typically 10 to 12% of human skin oil by weight23. The primary source of decanal is the reaction of ozone with various unsaturated fatty acids found in human skin oil 14; chief among these is cis-hexadec-6-enoic acid, which is typically 5 to 6% of skin oil by weight23. The levels of 6-MHO are similar to or slightly smaller than those measured in a simulated aircraft cabin, which reached steady state mixing ratios of ~ 4 ppb at ozone concentrations of 60 - 70 ppb and air exchange rates comparable to those of the commercial aircraft included in this study (see Figure 5, bottom in reference 13). The studies in the simulated aircraft also reported steady-state mixing ratios of the sum C4- to C8- aldehydes to be 1 to 4 ppb, nonanal levels of about 2 ppb and decanal of levels of about 3 ppb. The mixing ratios of these same saturated aldehydes measured on the commercial aircraft included in this study (Table 1) are in the same range. Unsaturated C7- to C9-aldehydes were also detected in the range of 0.1 to 0.5 ppb in the simulated aircraft study. These compounds were identified in several samples collected in the present study. However, the levels were near the detection limit, which was in the tenths of ppb depending on the volume of air sampled, and could not be adequately quantified.
Table 1.
Mixing Ratios (ppb) of Carbonyls Measured During Flight (n=52)
| Compound | Mean±Standard Deviation |
Median | Maximum | Number above detection limit |
|---|---|---|---|---|
| Hexanal | 3.1±1.9 | 2.8 | 8.4 | 44 |
| Heptanal | 0.90±0.74 | 0.77 | 4.0 | 40 |
| 6-methyl-5- hepten-2-one |
1.8±2.7 | 0.73 | 13. | 49 |
| Octanal | 1.0±1.0 | 0.75 | 4.5 | 44 |
| Nonanal | 2.8±2.8 | 1.9 | 14. | 47 |
| Decanal | 2.2±2.8 | 1.6 | 12 | 44 |
The larger scatter in the mixing ratios of ozone by-products measured in the aircraft cabin compared to the simulated aircraft likely reflect the greater scatter in ozone concentrations in the actual aircraft as well as differences in occupant densities, air exchange rates, non-human sources, temperature and relative humidity. In addition, it is not clear how the varying ozone concentrations that occur within the aircraft (Figure 2) affect production of ozone by-products compared to the relatively constant ozone concentrations that were used in the mock aircraft. Several other factors can impact the mixing ratios of the compounds produced from ozone reactions within the cabin. These include the amount of unsaturated organic compound precursors, the surface area to volume ratio, mixing of air within the aircraft cabin and other processes that can scavenge ozone. The amount of precursor compounds, which includes squalene and unsaturated fatty acids in skin oil, would partially depend on the density of people on the aircraft or percent occupancy for a particular aircraft type. Unoccupied seats could be soiled with skin oils from previous passengers, but the surface levels resulting from soiling would be expected to be lower than those on people themselves. Finally, long chain aldehydes and 6-MHO precursors (e.g., geranyl acetone) are anticipated to continue to desorb from human skin after their initial formation. Hence, the short-term history of passenger’s exposure to ozone (e.g. ozone levels in the city where the airplane was boarded) may be another parameter to consider when investigating mixing ratios of these carbonyls in aircraft cabins.
To evaluate the role of the ozone levels and other factors that might contribute to the formation of the ozone by-products within aircraft cabins a series of linear mixed models were run for each ozone by-product. In the regression models the ozone by-product was the dependent variable, and log transformed mean ozone or peak ozone concentration, percent occupancy, volume of the plane, number of seats and a dummy variable for aircraft type were the independent variables. The ozone concentration was log transformed since its distribution was consistent with a log normal distribution. The percent occupancy for each flight was either estimated from a visual examination of the number of empty seats on the plane or from information provided by the flight crew on the actual number of passengers and crew, coupled with the seating capacity. The aircraft type influences the air exchange rate and mixing of air within the aircraft, as well as the surface area to volume ratio. The results of the linear regression analyses for the log transformed mean ozone concentration, percent occupancy and plane type, using only the byproduct mixing ratios that were above the detection limit, are given in Table 2. The volume of the plane and the number of seats were not significant in the tested models and are not included in Table 2.
Table 2.
Linear Regression Analysis of Ozone By-Products
| Adjusted R2 | Significance | N | ||||
|---|---|---|---|---|---|---|
| Hexanal | 0.179 | .027 | 44 | |||
| Variable | Constant | Dummy737 | Dummy757 | Dummy747 | Log transformedAveOzone |
%Occupancy |
| Coefficient | −.311 | .124 | −.047 | .238 | −.059 | |
| Significance | .296 | .200 | .654 | .826 | .182 | .685 |
| Adjusted R2 | Significance | N | ||||
| 6-MHO | 0.249 | .003 | 49 | |||
| Variable | Constant | Dummy737 | Dummy757 | Dummy747 |
Log
transformedAveOzone |
%Occupancy |
| Coefficient | .329 | −.218 | −.313 | .497 | .326 | |
| Significance | .009 | .173 | .419 | .110 | .003 | .020 |
| Adjusted R2 | Significance | N | ||||
| Heptanal | 0.231 | .015 | 40 | |||
| Variable | Constant | Dummy737 | Dummy757 | Dummy747 | Log transformedAveOzone |
%Occupancy |
| Coefficient | .509 | .136 | .020 | .369 | −.059 | |
| Significance | .859 | .036 | .616 | .928 | .052 | .702 |
| Adjusted R2 | Significance | N | ||||
| Octanal | 0.208 | .014 | 45 | |||
| Variable | Constant | Dummy737 | Dummy757 | Dummy747 |
Log
transformedAveOzone |
%Occupancy |
| Coefficient | .450 | −.020 | −.178 | .416 | .061 | |
| Significance | .326 | .074 | .941 | .393 | .019 | .675 |
| Adjusted R2 | Significance | N | ||||
| Nonanal | 0.222 | .008 | 47 | |||
| Variable | Constant | Dummy737 | Dummy757 | Dummy747 |
Log
transformedAveOzone |
%Occupancy |
| Coefficient | .500 | −.014 | −.168 | .360 | .243 | |
| Significance | .080 | .046 | .959 | .407 | .033 | .092 |
| Adjusted R2 | Significance | N | ||||
| Decanal | 0.193 | .020 | 44 | |||
| Variable | Constant | Dummy737 | Dummy757 | Dummy747 |
Log
transformedAveOzone |
%Occupancy |
| Coefficient | .429 | .017 | −.153 | .370 | −.037 | |
| Significance | .714 | .081 | .950 | .484 | .046 | .806 |
Bolded entries: significant at the 0.05 level.
First row for each compound lists adjusted R2, overall significance of the regression analysis and number of measurements used in the regression analysis; row labeled “Coefficient” lists coefficients for the independent variables in the model, while row labeled “Significance” lists significance for the variable in the given column.
The overall models which included log transformed mean ozone, percent occupancy and aircraft type (as dummy variables) were all statistically significant (<0.05) explaining 18%, 25%, 23%, 21%, 22% and 19% of the variance for hexanal, 6-MHO, heptanal, octanal, nonanal and decanal, respectively. “Log transformed mean ozone” was a statistically significant parameter in the mathematic model for 6-MHO, octanal, nonanal and decanal and just below significance (0.052) for heptanal. This indicates that ozone-initiated chemistry contributed to the mixing ratios of these aldehydes measured on the different flights when controlling for plane type, which alters the surface area to volume ratio and ventilation rate. While the overall regression model for hexanal was predictive, no individual variables were specifically identified as statistically important. Hexanal is anticipated to have sources within the aircraft other than ozone-initiated chemistry. The regression model for 6-MHO also had “percent occupancy” as a statistically significant parameter, consistent with the source of that compound being almost exclusively reactions of ozone with squalene from human skin oil, and, hence, a function of the density of people on the aircraft. “Percent occupancy” was not a statistically significant parameter in the overall regression model for decanal, which is also produced from ozone reacting with constituents of human skin oil. This was likely due to the fact that there are other sources of decanal, including other sources of unsaturated fatty acids that react with ozone to produce decanal, present on the various aircraft. The high variability in measured decanal mixing ratios (see Table 1; the standard deviation is more than 6 times the mean and the maximum mixing ratio is roughly 50 times the mean) is consistent with this hypothesis.
Linear regression analyses were also conducted using the “log transformed peak ozone concentration” rather than the “log transformed mean ozone concentration”. The results for the peak ozone concentrations were similar (not shown), but slightly less of the variance was explained and the significances were slightly lower than when using the mean ozone values. While the peak and mean concentrations were highly correlated, these results suggest that mean concentration is a better predictor of by-product formation.
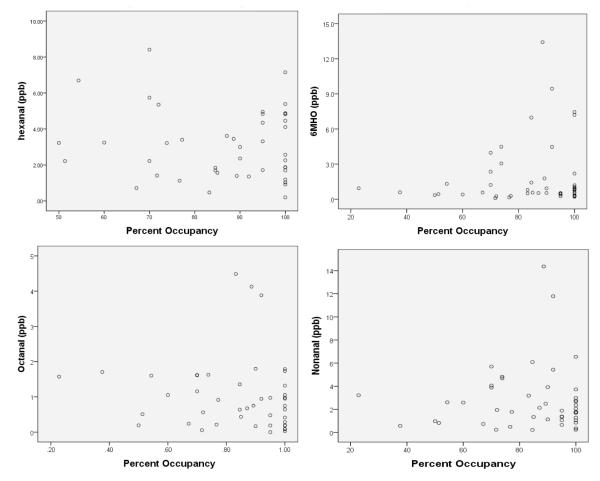
Figure 4 presents scatter plots of hexanal, 6-MHO, octanal and nonanal mixing ratios measured on individual flights versus the percent occupancy for those flights. Visually, a rough trend can be discerned for increasing 6-MHO levels with increasing occupancy. Such a visual trend is absent in the hexanal plot. The plots for octanal and nonanal lie somewhere between those for 6-MHO and hexanal in terms possible correlations. (Heptanal’s plot is similar to hexanal’s and decanal to octanal’s; these plots are not shown.) These plots are consistent with results from the linear regression models (see above).
Figure 4.
Scatter plots of hexanal, 6-MHO, octanal and nonanal mixing ratios measured on individual flights versus the percent occupancy for those flights.
The presence of 6-MHO, even on flights with relatively low ozone levels, indicates that there likely is no threshold for the ozone levels in terms of the production of carbonyls in enclosed environments. It should also be noted that ozone levels measured in the cabin are “residual” ozone levels (i.e., the ozone that remains after ozone has been consumed in gas phase and surface reactions). Some of the surface reactions that consume ozone include ozone/squalene reactions that produce 6-MHO.
As noted in the introduction, the analytical methods used on these flights were not appropriate for quantifying 4-OPA. However, given the levels of 6-MHO measured in the aircraft cabins, formation of 4-OPA can be inferred. Ozone reacts with both 6-MHO and geranyl acetone to form 4-OPA. These are fast reactions 24 – fast enough to compete with the rate at which the cabin air was being replaced with outside air on the flights surveyed. Using proton transfer reaction-mass spectrometry (PTR-MS), 4-OPA has been quantified both in experiments in a simulated aircraft cabin 12, 13 and in a simulated office 14. The measured levels of 6-MHO in these previous studies were comparable to those we measured for 6-MHO, so 4-OPA would also be expected to be present at levels similar to those measured in the simulated aircraft environment. The formation and expected presence of 4-OPA is especially noteworthy given recent toxicology studies 16, 17. It would be valuable to develop an analytical procedure capable of routinely monitoring 4- OPA in commercial aircraft to better define the exposures of passengers and crew to this potentially irritating dicarbonyl.
Acknowledgments
This work was funded by the U.S. Federal Aviation Administration (FAA) Office of Aerospace Medicine through the Air Transportation Center of Excellence for Airliner Cabin Environment Research (ACER), Cooperative Agreement 04-C-ACE-UMDNJ, 07- C-RITE-UMDNJ. Although the FAA has sponsored this project, it neither endorses nor rejects the findings of this research. This research was supported in part by the NIEHS sponsored Center for Environmental Exposure and Disease, Grant #P30ES005022. Harvard NIEHS Center for Environmental Health (grant number ES000002). We also thank Shahnaz Alimokhtari, Seema Bhangar and Mordecai Weisel for assistance in collection of the air samples.
References
- 1.Bhangar S, Nazaroff WW. Atmospheric ozone levels encountered by commercial aircraft on transatlantic routes. Environ Res Lett. 2013;8(1) [Google Scholar]
- 2.Bennett G. Ozone contamination of high altitude aircraft cabins. Aerospace Medicine. 1962;33:969–73. [PubMed] [Google Scholar]
- 3.Young WA, Shaw DB, Bates DV. Presence of ozone in aircraft flying at 35,000 feet. Aerospace Medicine. 1962;33:311–8. [PubMed] [Google Scholar]
- 4.van Heusden S, Mans LG. Alternating measurement of ambient and cabin ozone concentrations in commercial jet aircraft. Aviation Space & Environmental Medicine. 1978;49(9):1056–61. [PubMed] [Google Scholar]
- 5.Daubs J. Flight crew exposure to ozone concentrations affecting the visual system. American Journal of Optometry & Physiological Optics. 1980;57(2):95–105. doi: 10.1097/00006324-198002000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Committee on Airliner Cabin Air Quality National Research Council, The Airliner Cabin Environment:Air Quality and Safety. The National Academies Press; 1986. p. 303. [PubMed] [Google Scholar]
- 7.Hocking MB, Hocking D. Air Quality in Airplane Cabins And Similar Enclosed Spaces, Volume 4. Vol. 4. Springer-Verlag; Berlin Heidelberg, Germany: 2005. [Google Scholar]
- 8.Bhangar S, Cowlin SC, Singer BC, Sextro RG, Nazaroff WW. Ozone levels in passenger cabins of commercial aircraft on North American and transoceanic routes. Environmental Science & Technology. 2008;42(11):3938–43. doi: 10.1021/es702967k. [DOI] [PubMed] [Google Scholar]
- 9.Spengler JD, Ludwig S, Weker RA. Ozone exposures during trans-continental and trans-Pacific flights. Indoor Air. 2004;14(Suppl 7):67–73. doi: 10.1111/j.1600-0668.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- 10.Weschler CJ. Chemistry in indoor environments: 20 years of research. Indoor Air. 2011;21(3):205–18. doi: 10.1111/j.1600-0668.2011.00713.x. [DOI] [PubMed] [Google Scholar]
- 11.Weschler CJ. Chemical reactions among indoor pollutants: what we’ve learned in the new millennium. Indoor Air. 2004;14(Suppl 7):184–94. doi: 10.1111/j.1600-0668.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- 12.Wisthaler A, Tamas G, Wyon DP, Strom-Tejsen P, Space D, Beauchamp J, Hansel A, Mark TD, Weschler CJ. Products of ozone-initiated chemistry in a simulated aircraft environment. Environmental Science & Technology. 2005;39(13):4823–32. doi: 10.1021/es047992j. [DOI] [PubMed] [Google Scholar]
- 13.Weschler CJ, Wisthaler A, Cowlin S, Tamas G, Strom-Tejsen P, Hodgson AT, Destaillats H, Herrington J, Zhang J, Nazaroff WW. Ozone-initiated chemistry in an occupied simulated aircraft cabin. Environmental Science & Technology. 2007;41(17):6177–84. doi: 10.1021/es0708520. [DOI] [PubMed] [Google Scholar]
- 14.Wisthaler A, Weschler CJ. Reactions of ozone with human skin lipids: sources of carbonyls, dicarbonyls, and hydroxycarbonyls in indoor air. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(15):6568–75. doi: 10.1073/pnas.0904498106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golden R. Identifying an indoor air exposure limit for formaldehyde considering both irritation and cancer hazards. Critical Reviews in Toxicology. 2011;41(8):672–721. doi: 10.3109/10408444.2011.573467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson SE, Franko J, Jackson LG, Wells JR, Ham JE, Meade BJ. Irritancy and allergic responses induced by exposure to the indoor air chemical 4- oxopentanal. Toxicological Sciences. 2012;127(2):371–81. doi: 10.1093/toxsci/kfs102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson SE, Jackson LG, Franko J, Wells JR. Evaluation of dicarbonyls generated in a simulated indoor air environment using an in vitro exposure system. Toxicological Sciences. 2010;115(2):453–61. doi: 10.1093/toxsci/kfq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiedler N, Laumbach R, Kelly-McNeil K, Lioy P, Fan Z-H, Zhang J, Ottenweller J, Ohman-Strickland P, Kipen H. Health effects of a mixture of indoor air volatile organics, their ozone oxidation products, and stress. Environmental Health Perspectives. 2005;113(11):1542–8. doi: 10.1289/ehp.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkins CK, Clausen PA, Wolkoff P, Larsen ST, Hammer M, Larsen K, Hansen V, Nielsen GD. Formation of strong airway irritants in mixtures of isoprene/ozone and isoprene/ozone/nitrogen dioxide. Environmental Health Perspectives. 2001;109(9):937–41. doi: 10.1289/ehp.01109937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolkoff P, Clausen P, Larsen K, Hammer M, Larsen S, Nielsen G. Acute airway effects of ozone-initiated d-limonene chemistry: importance of gaseous products. Toxicol Lett. 2008;181(3):171–176. doi: 10.1016/j.toxlet.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Wolkoff P, Wilkins CK, Clausen PA, Nielsen GD. Organic compounds in office environments - sensory irritation, odor, measurements and the role of reactive chemistry. Indoor Air. 2006;16(1):7–19. doi: 10.1111/j.1600-0668.2005.00393.x. [DOI] [PubMed] [Google Scholar]
- 22.Coleman BK, Destaillats H, Hodgson AT, Nazaroff WW. Ozone consumption and volatile byproduct formation from surface reactions with aircraft cabin materials and clothing fabrics. Atmos Environ. 2008;42(4):642–654. [Google Scholar]
- 23.Nicolaides N. Skin Lipids: Their Biochemical Uniqueness. Science. 1974;186(4158):19–26. doi: 10.1126/science.186.4158.19. [DOI] [PubMed] [Google Scholar]
- 24.Fruekilde P, Hjorth J, Jensen NR, Kotzias D, Larsen B. Ozonolysis at vegetation surfaces: A source of acetone, 4-oxopentanal, 6-methyl-5-hepten-2-one, and geranyl acetone in the troposphere. Atmos Environ. 1998;32(11):1893–1902. [Google Scholar]