Abstract
Background and Objective
There is a paucity of data regarding tolerability of alkaline drugs administered subcutaneously. The aim of this study was to assess the tolerability of alkaline preparations of human albumin delivered subcutaneously to healthy humans.
Methods
We compared the tolerability of neutral versus alkaline (pH 10) formulations of human albumin in ten volunteers. With an intent to minimize the time required to reach physiological pH after injection, the alkaline formulation was buffered with a low concentration of glycine (20 mmol/L). Each formulation was given at two rates: over 5 seconds and over 60 seconds. A six-point scale was used to assess discomfort.
Results
For slow injections, there was a significant difference between pH 7.4 and pH 10 injections (0.4 ± 0.2 vs 1.1 ± 0.2, mean ± SEM; p = 0.025), though the degree of discomfort at pH 10 injections was only ‘mild or slight’. For fast injections, the difference between neutral and alkaline formulations was of borderline significance. Inflammation and oedema, as judged by a physician, were very minimal for all injections, irrespective of pH.
Conclusion
For subcutaneous drug administration (especially when delivered slowly), there was more discomfort associated with alkaline versus neutral formulations of albumin, though the discomfort was mild. This study suggests that there is little discomfort and inflammation resulting from subcutaneous administration of protein drugs formulated with weak buffers at alkaline pH.
Introduction
There is a scarcity of data regarding the tolerability of subcutaneously administered drugs formulated at an alkaline pH. Although some acidic drugs (e.g. glargine insulin, pH 4) are approved for subcutaneous use in humans, we are not aware of drugs approved for subcutaneous use whose pH is markedly alkaline. Although the diuretic furosemide (pH 9) has been given by the subcutaneous route,[1,2] its approved use is restricted to the oral and intravenous routes.
Our group is interested in creating a chemically stabilized form of glucagon. We recently reported that after aging at room temperature or body temperature, acid formulations of glucagon, especially in concentrated preparations, readily form amyloid fibril aggregates that are cytotoxic to mammalian cells. However, when aged in a dilute glycine buffer at pH 10, glucagon was remarkably stable, did not form amyloid fibrils, and was not cytotoxic, even at relatively high concentrations.[3]
Since we were unaware of formal studies that have addressed tolerability of subcutaneously administered drugs at alkaline pH, we designed and carried out this study, which addresses tolerability of alkaline versus neutral preparations of human albumin delivered subcutaneously to healthy humans. Because the speed of injection has been reported to be related to intensity of pain,[4] we also compared different injection rates.
Subjects and Methods
Subjects
Ten healthy subjects of both sexes without chronic health problems were recruited from greater Portland, OR, USA. Exclusion criteria included histories of acute or chronic disease. Prior to initiating the study, the research protocol was approved by the Legacy Health System Institutional Review Board, and all subjects provided written informed consent. Ten subjects were studied. Age was limited to the 21- to 65-year range by protocol, and averaged 31.7 ±3.5 years.
Screening Visit
Each subject completed a screening visit before participating in the study. A licensed physician obtained a medical history, reviewed medications, and performed a physical examination. Women of child-bearing age were required to have a negative urine pregnancy test in order to qualify.
Preparation of Albumin Compounds
In sterile fashion, two albumin solutions were prepared, one at neutral pH (pH 7.2–7.4) and one at pH 10. For the molecular composition of both solutions, see table I. Measured osmolarity of both solutions was iso-osmolar with plasma and interstitial fluid (285–295 mOsm/L). For the basic solution, a small amount of NaOH was added to bring the pH up to 10. For the phosphate-buffered solutions, it was not necessary to titrate with acid or base to reach the neutral pH. The volume of each injected dose was 0.2 mL.
Table I.
The specific compounds and their concentrations for the neutral and alkaline formulations injected subcutaneously into normal subjects
| Compound | Concentration (g/L) |
|---|---|
| pH 10 (glycine buffer) | |
| NaCl | 7.2 |
| Glycine | 1.5 |
| NaOH | 0.00054 |
| Albumin | 0.5 |
| pH 7.2 (PBS buffer) | |
| NaCl | 8.0 |
| Na2HPO4 (anhyd) | 1.15 |
| KCl | 0.2 |
| KH2PO4 (anhyd) | 0.2 |
| Albumin | 0.5 |
anhyd = anhydrous; PBS = phosphate buffered saline.
Injection Study
While recumbent, each subject was given four subcutaneous injections by a physician, each 10 minutes apart, in each of the four quadrants of the abdomen. Each injection was one of two formulations of human serum albumin. The physician and the subject were masked as to the identity of the formulation. The order of the locations was randomized. Each injection was given at least 10 cm away from other injection sites. The injected compounds and delivery rates were:
Albumin, pH 10, given over 5 seconds.
Albumin, pH 10, given over 60 seconds.
Albumin, pH 7.4, given over 5 seconds.
Albumin, pH 7.4, given over 60 seconds.
The subject was asked to rate intensity and duration of discomfort associated with the injections. A six-point scale was used to quantify the intensity and duration of the discomfort. The ratings for intensity were: 0 = no pain, 1 = mild or slight, 2 = mild to moderate (tolerable), 3 = moderate, 4 = moderate to severe, and 5 = severe. The ratings for discomfort duration (beginning at the end of the injections) were: 0 = 0, 1 = <30 sec, 2 = from 30 sec to <5 min, 3 = from 5 to <10 min, 4 = from 10 to <30 min, 5 = 30 min or more. A five-point scale was used to quantify the time at which the discomfort started after the end of the injection: 0 = no pain, 1 = <10 sec, 2 = from 10 to <30 sec, 3 = between 30 and <60 sec, 4 = 60 sec or more.
Ten minutes after each injection, a study physician assessed the injection sites for inflammation and erythema (redness) by the Draize scale[5] (see table II). Each subject was telephoned approximately 24 hours after the injection experiment to assess whether additional concerns had arisen.
Table II.
The Draize scale for grading inflammation (erythema and oedema).[5] This scale was used to grade inflammation in the region of the subcutaneous injection of albumin at neutral and alkaline pH
| Erythema formation
|
Oedema formation
|
||
|---|---|---|---|
| Description | Score | Description | Score |
| No erythema | 0 | No oedema | 0 |
| Very slight erythema (barely perceptible) | 1 | Very slight oedema (barely perceptible) | 1 |
| Well defined erythema | 2 | Well defined oedema | 2 |
| Moderate erythema | 3 | Moderate oedema (raised approximately 1 mm) | 3 |
| Severe erythema (beet redness) to slight eschar formation | 4 | Severe oedema (raised more than 1 mm and beyond exposure area) | 4 |
Preparation of Human Serum Albumin Solutions
The human serum albumin (Buminate 25%), saline (0.9% NaCl Injection USP) and glycine (1.5% glycine irrigation USP) were all certified as sterile and purchased from Baxter Healthcare (Deerfield, IL, USA). The phosphate buffered saline (Dulbecco’s PBS) was also certified as sterile and purchased from Invitrogen (Carlsbad, CA, USA). All compounds were handled using strict aseptic technique in a certified Biological Safety Cabinet by personnel trained in proper aseptic technique.
Prior to preparation of albumin solutions, the glycine solutions were diluted to 20 mM. Titration of pH was carried out with sterile, autoclaved 1 M solutions of HCl and NaOH (Sigma-Aldrich, St Louis, MO, USA). The solutions were then filtered with a 0.2 μm/pore syringe filter. For each injection, 0.2 mL of each solution was drawn aseptically into an insulin syringe.
Data Analysis
Results are presented as mean ± standard error of the mean (SEM). Paired two-tailed Student’s t-tests were carried out to compare different study conditions. A parametric test was chosen because the distributions of experimental results were generally normal. The Pearson skewness coefficient for discomfort data for the injections at pH 10 was 0.13 for the fast injection and −0.166 for the slow injection.
Results
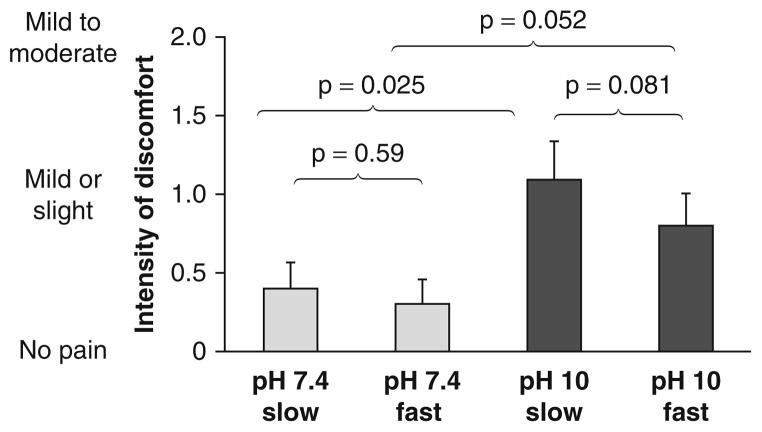
Discomfort Intensity (figure 1)
Fig. 1.
Results regarding intensity of discomfort after fast or slow subcutaneous administration of albumin at pH 7.4 or pH 10 in normal human subjects. Data are presented as mean + standard error of the mean.
In terms of discomfort intensity, for slow injections, there was a significant difference between the pH 7.4 versus pH 10 injections on the six-point scale (0.4 ± 0.2 vs 1.1 ± 0.2; p = 0.025), though the result of approximately 1 for the pH 10 injections indicates only ‘mild or slight’ discomfort. For fast injections, the difference between pH 7.4 versus pH 10 was of borderline significance (0.3 ± 0.2 vs 0.8 ± 0.2; p = 0.052).
For the pH 7.4 injections and for the pH 10 injections, the mean intensity for slow versus fast injections were not significantly different. When discomfort occurred, the subjects usually described the discomfort as burning, but also used adjectives such as itching, sharp or tingling.
Duration of Discomfort (figure 2)
Fig. 2.
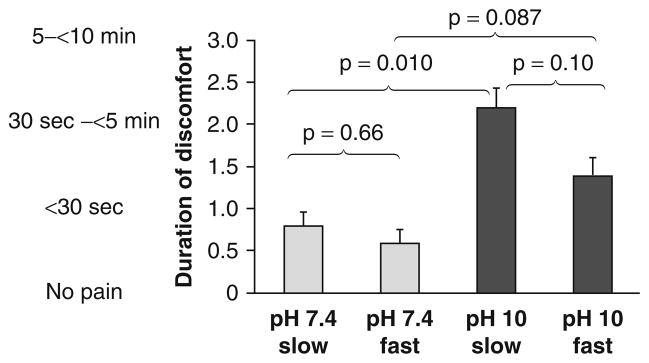
Results regarding duration of discomfort after fast or slow subcutaneous administration of albumin at pH 7.4 or pH 10 in normal human subjects. Data are presented as mean + standard error of the mean.
For slow injections, there was a significant difference between the pH 7.4 versus pH 10 formulations (0.8 ± 0.4 vs 2.2 ± 0.5; p = 0.010). For fast injections (neutral vs alkaline), the difference was not significant (0.6 ± 0.3 vs 1.4 ± 0.3; p = 0.087).
Time to Onset of Discomfort
For slow injections, there was a significant difference between the pH 7.4 versus pH 10 formulations (0.6 ± 0.3 vs 2.8 ± 0.5; p = 0.001). For fast injections (neutral vs alkaline), the difference was also significant (0.4 ± 0.2 vs 2.2 ± 0.5; p = 0.01). These results indicate that the onset of discomfort was usually less than 10 seconds for pH 7.4 injections and usually between 10 and 60 seconds for pH 10 injections.
Erythema at Injection Site
There were only three instances where there was erythema at the injection site and these were graded as slight. For slow and fast injections, for pH 7.4, the mean erythema scores were 0 and 0.1 ± 0.1 (p = 0.34). For the pH 10 solutions, the mean scores were 0.2 ± 0.1 and 0.1 ± 0.1 (p = 0.59).
Oedema at Injection Site
There were only four instances in which there was oedema at the injection site. These instances were all from the same subject, were graded as slight, and were equal under both pH and both injection speed conditions. The mean degree of oedema was 0.1 ± 0.1 for all injections, and there were no differences between different pH values or between slow versus fast injection speed.
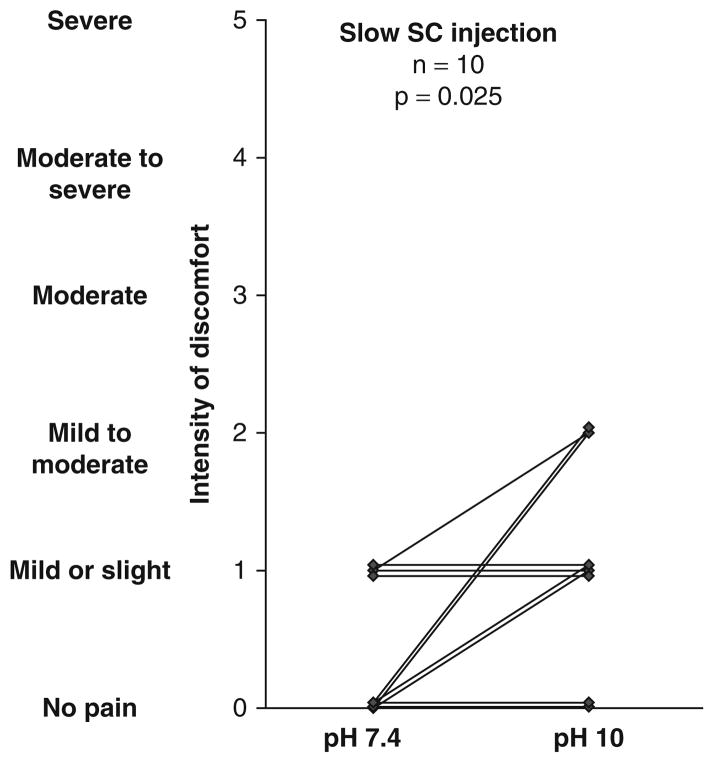
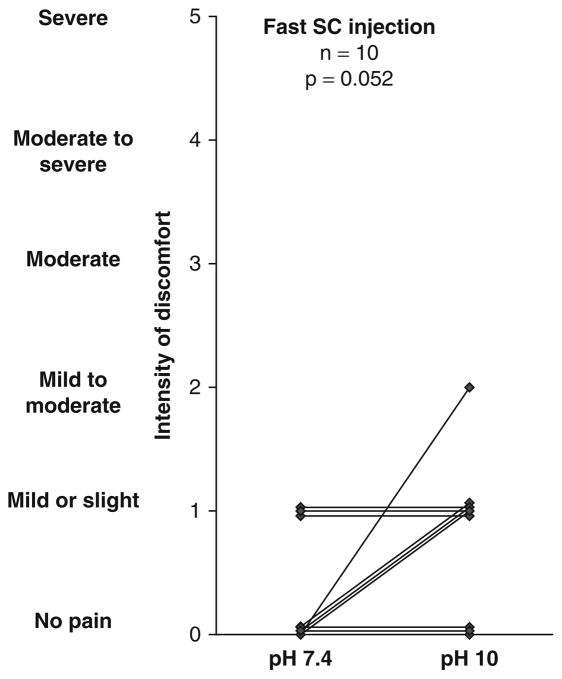
Individual Values
Individual data points for intensity and duration of discomfort are shown in figures 3 and 4.
Fig. 3.
Individual results regarding intensity of discomfort after slow subcutaneous administration of albumin at pH 7.4 or pH 10 in normal human subjects. SC = subcutaneous.
Fig. 4.
Individual results regarding intensity of discomfort after fast subcutaneous administration of albumin at pH 7.4 or pH 10 in normal human subjects. SC = subcutaneous.
Assessment by telephone one day after experiment: No subject reported any problem.
Discussion
The purpose of this study was to assess the tolerability of a highly alkaline preparation of an innocuous compound. We found that the intensity of discomfort from slow injections of human albumin at pH 10 was greater than the intensity of slow injections at pH 7.4. However, the degree of discomfort indicated was only mild. The findings for fast injections were similar, though the difference in degree of discomfort was of borderline significance. We also found that the duration of discomfort (typically between 30 sec and 5 min) was significantly greater for slow injections at pH 10 versus pH 7.4. For fast injections, the duration of discomfort was not significantly different at the two different pH levels, though a larger sample size might have shown a difference. The onset of discomfort for the injections at pH 10 occurred significantly later than for pH 7.4, for reasons that are unclear.
Inflammation and oedema, as judged by a physician, were typically absent for all injections, irrespective of pH.
There is very little published information regarding tolerability of alkaline drug formulations administered parenterally. There are two reports that suggest that furosemide given off-label by the subcutaneous route was reasonably well tolerated, despite its pH of 9, though there was often mild burning or stinging.[1,2] Acidic drugs have been reported to cause local discomfort.[4,6]
There are several reasons to formulate a drug at alkaline pH. For example, solubility of some compounds is greater at alkaline than at neutral or acid pH. Glucagon has an isoelectric point of 7.1 and is poorly soluble at neutral pH. In addition, there may be stability issues not related to solubility that favour an alkaline pH. We found that preparations of glucagon at pH 10, buffered in the same glycine solution that we used in the present study, did not form amyloid aggregates and were not cytotoxic in cultured mammalian cells. In contrast, acid preparations of glucagon such as the drugs currently marketed were cytotoxic and much less stable.[3] The currently marketed preparations are approved for use only immediately after aqueous reconstitution (at pH 3) and cannot be used for longer periods of time. Since it has been shown that small doses of glucagon given in the setting of bihormonal closed loop control to persons with type 1 diabetes mellitus are effective in averting hypoglycaemia,[7,8] stability in a portable pump over several days would be desirable.
It is quite possible that the low concentration of the glycine buffer probably helped to minimize pain. The low concentration of glycine buffer (20 mM) would be expected to allow rapid pH decline toward neutral during exposure to endogenous buffers after injection. Tolerability resulting from concentration of buffers used in drug formulations was addressed by Fransson et al.[9] They found that, after injections of acidic drugs, a weak phosphate buffer caused less discomfort than a strong phosphate buffer. Although we did not test glycine buffers of higher buffering capacity, we would predict that such preparations might well lead to greater or more prolonged discomfort due to increased duration of local alkalinity after injection.
A limitation of this study was that, due to the need for different buffers, it was not possible for the two formulations to be identical. There was some glycine in the alkaline formulation and there was phosphate and potassium in the neutral formulation (table I). The total content of salts and the osmolarity in the two formulations were similar.
Conclusion
This study demonstrated that an alkaline preparation of a common protein was tolerated with only mild discomfort. We conclude that, to the extent that these results with albumin can be generalized, there is little discomfort and inflammation resulting from subcutaneous administration of protein drugs formulated with weak buffers at alkaline pH.
Acknowledgments
We are grateful to the research subjects and to the Juvenile Diabetes Research Foundation and the Legacy Good Samaritan Foundation for financial support. With regard to potential conflicts of interest, a patent has been submitted by the authors for the use of glucagon formulated at alkaline pH in persons with diabetes.
References
- 1.Goenaga M, Millet M, Sanchez E, et al. Subcutaneous furosemide [letter] Ann Pharmacother. 2004;38:1751. doi: 10.1345/aph.1E172. [DOI] [PubMed] [Google Scholar]
- 2.Verma AK, da Silva JH, Kuhl DR. Diuretic effects of subcutaneous furosemide in human volunteers: a randomized pilot study. Ann Pharmacother. 2004;38:544–9. doi: 10.1345/aph.1D332. [DOI] [PubMed] [Google Scholar]
- 3.Ward WK, Massoud RG, Szybala CJ, et al. In vitro and in vivo evaluation of native glucagon and glucagon analog (MAR-D28) during aging: lack of cytotoxicity and preservation of hyperglycemic effect. J Diabetes Sci Technol. 2010;4:1311–21. doi: 10.1177/193229681000400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brazeau GA, Cooper B, Svetic KA, et al. Current perspectives on pain upon injection of drugs. J Pharm Sci. 1998;87:667–77. doi: 10.1021/js970315l. [DOI] [PubMed] [Google Scholar]
- 5.Draize JH, Woodward G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exper Therap. 1944;83:377–90. [Google Scholar]
- 6.Klement W, Arndt JO. Pain on i.v. injection of some anaesthetic agents is evoked by the unphysiological osmolality or pH of their formulations. Br J Anaesth. 1991;66:189–95. doi: 10.1093/bja/66.2.189. [DOI] [PubMed] [Google Scholar]
- 7.Castle JR, Engle JM, El Youssef J, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33:1282–7. doi: 10.2337/dc09-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Khatib FH, Russell SJ, Nathan DM, et al. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2:27ra27. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fransson J, Espander-Jansson A. Local tolerance of subcutaneous injections. J Pharm Pharmacol. 1996;48:1012–5. doi: 10.1111/j.2042-7158.1996.tb05892.x. [DOI] [PubMed] [Google Scholar]