Abstract
Successful efforts to control infectious diseases have often required the use of effective vaccines. The current global strategy for control of malaria, including elimination and eradication will also benefit from the development of an effective vaccine that interrupts malaria transmission. To this end, a vaccine that disrupts malaria transmission within the mosquito host has been investigated for several decades targeting a 25 kDa ookinete specific surface protein, identified as Pfs25. Phase 1 human trial results using a recombinant Pfs25H/Montanide ISA51 formulation demonstrated that human Pfs25 specific antibodies block parasite infectivity to mosquitoes; however, the extent of blocking was likely insufficient for an effective transmission blocking vaccine. To overcome the poor immunogenicity, processes to produce and characterize recombinant Pfs25H conjugated to a detoxified form of Pseudomonas aeruginosa exoprotein A (EPA) have been developed and used to manufacture a cGMP pilot lot for use in human clinical trials. The Pfs25-EPA conjugate appears as a nanoparticle with an average molar mass in solution of approximately 600 kDa by static light scattering with an average diameter 20 nm (range 10 to 40 nm) by dynamic light scattering. The molar ratio of Pfs25H to EPA is about 3 to 1 by amino acid analysis, respectively. Outbred mice immunized with the Pfs25-EPA conjugated nanoparticle formulated on Alhydrogel® had a 75 to 110 fold increase in Pfs25H specific antibodies when compared to an unconjugated Pfs25H/Alhydrogel® formulation. A phase 1 human trial using the Pfs25-EPA/Alhydrogel® formulation is ongoing in the United States.
Keywords: Pfs25, malaria, transmission blocking vaccine, nanoparticle, chemical-conjugate, recombinant protein
1. Introduction
Malaria vaccine research and development has been ongoing for several decades in pursuit of a vaccine that will eradicate this deadly disease. Many different potential developmental stages and antigenic targets of the malaria parasite are under investigation. The most developed investigational vaccine targets the sporozoite which is responsible for establishing a liver stage infection in order to prevent a subsequent blood stage infection and clinical disease. Early Phase 3 trial results for the investigational vaccine RTS,S formulated in AS01 reduced the incidence of clinical disease for a period of 14 months in about 50% of vaccinated infants and children [1]. Even though this is a promising outcome, a second generation, more effective malaria vaccine will be necessary to achieve the current goal of elimination and eradication [2], and such a vaccine has been identified as a “vaccine to interrupt malaria transmission” or VIMT. To this end, a vaccine component of a VIMT that would block malaria parasite infectivity in the mosquito would provide a significant advantage. Several sexual stage malaria proteins are being pursued for such a vaccine (see review [3]), including the 25 kDa Plasmodium falciparum sexual stage protein, Pfs25 [4]. Pfs25 is a surface protein attached to the surface of ookinetes by a glycosylphosphatidylinositol anchor. Based on the crystal structure of an orthologue of Pfs25 identified in P. vivax as Pvs25, Pfs25 appears as a flattened triangular shaped protein comprised of four epidermal growth factor-like domains [5]. A recombinant form of Pfs25 (Pfs25H) has been evaluated in two phase 1 human trials [6, 7]. In general, Pfs25H is a poor immunogen. In one study to overcome the poor immunogenicity, the potent water-in-oil adjuvant Montanide ISA51 was evaluated using Pvs25 or Pfs25 alone for investigational purposes [7]. Prior to the study being halted due to severe adverse events observed for the orthologous Pvs25 vaccine, individuals in the low dose group receiving recombinant Pfs25 produced Pfs25 specific antibodies that reduced mosquito infectivity, demonstrating that a transmission blocking vaccine may be attainable.
Efforts to overcome the poor immunogenicity of Pfs25H have focused on the development of a conjugate vaccine. The basis for this concept is clearly supported by commercial carbohydrate based conjugate vaccines [8]. Preclinical studies using Pfs25H conjugated to several different protein carriers have demonstrated a significant increase in antigen specific antibody titers using aluminum based adjuvants in mice [9, 10] and non-human primates [11]. Pfs25H conjugated to the outer membrane protein complex of Neisseria meningitidis not only increased the antibody concentration, but also the duration of antibodies which were biologically active [11]. Additional preclinical Pfs25 protein-protein conjugate vaccines have been produced including a self-self conjugate [10] and Pfs25H conjugated to ExoProtein A (EPA), a detoxified form of exotoxin A from Pseudomonas aeruginosa, and were shown to improve immunogenicity in mice [9, 10].
Based on the initial preclinical results in mice using Pfs25-EPA [9] and in order to better understand the potential for improving the immunogenicity and safety profile of Pfs25H in humans, an investigational chemical-conjugate vaccine was developed and manufactured at pilot-scale following current good manufacturing practices. The Pfs25-EPA conjugate was characterized biochemically and biophysically, and released as a bulk drug substrate. The processes developed led to the production of a soluble Pfs25-EPA conjugate that appeared to be nanoparticles on the order of 600,000 Da with a sphere-like shape. This new Pfs25-EPA conjugated nanoparticle vaccine significantly enhanced the Pfs25 specific antibody responses in mice when adsorbed on Alhydrogel®.
2. Materials and methods
2.1. Recombinant proteins
Pfs25H was produced in Pichia pastoris and ExoProtein A (EPA), a detoxified mutant form of Pseudomonas aeruginosa exotoxin A was produced in Escherichia coli. The recombinant proteins used here were produced in-house or at the WRAIR Biopilot Production Facility (Silver Spring, MD)under current good manufacturing practice following procedures reported by Tsai et al., [12] or Qian et al., [9] respectively.
2.2. Process development of Pfs25-EPA conjugates
Initial development used scaled-down procedures based on preset pilot-scale conditions to determine the best conditions for linker modification of Pfs25H and EPA, as well as conditions for forming the protein-protein conjugates. The thiol-modified Pfs25H (Pfs25-SH) and maleimides-modified EPA (EPA-mal) were prepared using commercially available reagents SATA and EMCS (Pierce Biotech, Rockford, IL), respectively. The detailed materials and methods for pilot-scale development are reported in supplemental materials and methods.
2.3. Analytical Characterizations
2.3.1. Determination of Pfs25H content in Pfs25-EPA
The molar ratio of Pfs25 to EPA was determined by amino acid analysis performed by W.M. Keck Facility (Yale University, New Haven, CT). The relative amounts of Pfs25H and EPA within a conjugate was calculated by a modification of a method described by Shuler et al [13]. Experimentally determined amino acid compositions were calculated for each protein component (Pfs25 and EPA) as follows, using a selected set of amino acids (Asp, Glu, Gly, Ala, Val, Met, Ile, Leu, Tyr, Phe, Arg). Nanomoles of each amino acid were converted to mole percent by normalizing the total nanomoles in the set to 100 mole percent. Amino acid composition was calculated by multiplying the mole percent obtained for each amino acid by the total number of amino acids in the set based on the known sequence.
2.3.2. Reversed phase (RP) - HPLC
Purified Pfs25-EPA was acidified with 10% (v/v) trifluoroacetic acid (TFA, Fisher Scientific) to a final concentration of 0.1% TFA (v/v) and then centrifuged at 12,000 rpm for 2 minutes. The Pfs25-EPA was analyzed on a Jupiter (Phenomenex, Torrance CA) C4 RP column (2 mm ID × 150 mm). The initial mobile phase combined 95% mobile phase A (0.1% (w/v) TFA in water) and 5% mobile phase B (0.1% (w/v) TFA in acetonitrile) and the Pfs25-EPA conjugate was eluted by increasing the mobile phase B to 100% over 38 minutes at a flow rate of 0.2 mL/min.
2.3.3. SEC-MALS-QELS-HPLC
Purified Pfs25-EPA was examined by analytical SEC using a TSK gel G4000SWxl (7.8 mm ID × 30 cm, 8μm particle size, 450 Å pore size) analytical column and a TSK gel Guard SWxl guard column with in-line multi-angle light scattering (MALS) and a quasi-elastic light scatter detection (QELS) both from Wyatt Technology (Santa Barbara, CA). Samples were prepared and analyzed essentially as described by Tsai et. al. [14]. Average molar mass was calculated using Astra software (Wyatt Technology), with the Debye fitting method.
2.3.4. Circular Dichroism
CD spectra were recorded over the wavelength range 190 – 280 nm in a 1 mm path-length quartz cuvette using a step size of 0.2 nm, a slit bandwidth of 1.0 nm and a signal averaging time of 1.0 second. Analysis of temperature on secondary structure was performed in 5°C temperature increments from 10°C to 80°C and then lowered to 20°C. Secondary structure content was calculated using the DICHROWEB web server (http://dichroweb.cryst.bbk.ac.uk) [15, 16].
2.3.5. Atomic force microscopy
AFM samples were prepared and analyzed as described [14, 17, 18]. More detailed information is contained in the Supplemental Materials and Methods.
2.3.6 Endotoxin testing
The endotoxin levels were less than 5 endotoxin units per mL or less than 5 endotoxin units per mg total protein content using the Endosafe®PTS™ (Charles River Laboratories International, Inc. Wilmington, MA).
2.4 Quantitative tryptic peptide mapping
A quantitative tryptic mapping of the Pfs25H and Pfs25-EPA was performed on a 2-dimensional nanoACQUITY UPLC® system with an online XEVO G2 quadrupole time-of-flight (Q-TOF) tandem mass spectrometer (Waters, Corp., Milford, MA) operated in the data independent alternate scanning LC/MS mode (LC/MSE). Recombinant Pfs25H and Pfs25-EPA samples of 120 μL were diluted with 50 μL 50 mM NH4HCO3 containing 0.2% RapiGest SF (Waters, p/n 186001861). Samples were digested by sequencing-grade trypsin and incubated at 37°C overnight. Before the digestion, 5 μL of 100 mM DTT was added to reduce disulfide bonds at 60°C for 30 minutes and then free cysteine residues were alkylated with 5 μL of 300 mM iodoacetamide at room temperature for 30 minutes in the dark. Trifluoroacetic acid (0.05% v/v) was used to quench the enzymatic reaction and degrade the RapiGest™ SF. The pH of the samples was adjusted to pH 10 by adding 5 μL of 1 N NH4OH for effective trapping on the 1st dimension column. Samples were spiked with 25 fmol/μL alcohol dehydrogenase before loading the nanoACQUITY UPLC®. Data acquisition was done in positive ion mode using a nanoLockSpray™ source controlled by MassLynx™ 4.1 software. Digested samples were loaded on first dimension column (300 μm × 50 mm XBridge™ BEH130 C18 5 μm) at pH 10 and eluted to a second dimension trap column (180 μm × 20 mm Symmetry® C18 5 μm) with 50% of acetonitrile and 50% 20 mM ammonium formate at pH 10 (mobile phases for first dimension pump) at a flow rate of 2 μL/minute. Eluted peptides were diluted with 0.1% formic acid (FA) in water at 20 μL/min flow on to the trap column. The peptides were eluted from the second dimension analytical column (75 μm × 100 mm HSS T3 1.8μm) with a 60 min gradient from 97% mobile phase A (0.1% FA in water) to 60% mobile phase B (0.1% FA in acetonitrile). An alternating low collision energy (5V) and elevated collision energy (ramping from 15 - 35 V) acquisition was used to acquire peptide precursor (MS) and fragmentation (MSE) data. Scan time was 1 sec. The capillary voltage was 3.6 kV, source temperature 100°C, cone voltage 45V. Sampling of the lock spray channel was performed every 30 sec. Spectra were recorded from m/z 50 to 1990. Samples were run in quadruplicate.
LC/MSE data were processed and searched using ProteinLynx Global SERVER™ (PLGS) version 2.5. Peptide/protein identifications were obtained by searching against database with Pfs25H, EPA and alcohol dehydrogenase protein sequences. The ion detection, clustering, and normalization were processed using PLGS 2.5 as described earlier [19]. Due to the parallel mode of data acquisition, all tryptic peptides that are of sufficient intensities will produce associated product ion fragmentation data that can be used for structural identification of that peptide and its corresponding protein.
2.5 Formulation and formulation stability
The antigens, Pfs25H and Pfs25-EPA, were formulated to contain 50 μg/mL Pfs25H content adsorbed to 1.6 mg/mL of aluminum hydroxide, reported here as Al2O3 (2% Alhydrogel®, Brenntag Biosector, Frederikssund, Denmark) in 4 mM PBS (154 mM sodium chloride, 2.97 mM sodium phosphate, 1.04 mM potassium phosphate, pH 7.4 ± 0.2). The PBS was mixed with Alhydrogel® by gentle vortexing (Vortex Genie 2, Scientific Industries, setting 5.5) for 1–2 minutes. Antigen was added and incubated at room temperature for 1 hour on a rotator at 20–22 rpm. After the incubation period the formulation was stored at 2–8°C. Samples of each formulation were processed for supernatants at 0 hours (directly after the formulation) and 24 hours later. At each time point, 150 μL of formulation was spun at 4000 rpm in an Eppendorf 5415D microcentrifuged for 5 minutes. Approximately 140 μL of each supernatant was transferred to a new tube and stored at −80°C until gel analysis. SDS-PAGE of the supernatants was performed on a 4–20% Tris-glycine or 3–8% Tris-acetate polyacrylamide gel (Invitrogen Corp., Carlsbad, CA) using an X-cell II Mini Cell (Invitrogen). Silver staining was used to visualize antigen in the supernatant samples.
2.6. Immunogenicity and blocking activity of Pfs25 antibodies
Mouse antisera were generated at the National Institutes of Health in compliance with guidelines of the National Institutes of Health Institutional Animal Care and Use Committee. In brief, groups of 10 CD1 female mice were immunized with 2.5 μg of Pfs25H or either 0.5 or 2.5 μg Pfs25-EPA formulated on 80 μg Alhydrogel® (Brenntag, Denmark) on days 0, 28 and bled on Days 42, 71, 98 and exsanguinated on day 125. The membrane feeding assay using pooled day 42 antisera assessing the blocking of P. falciparum NF54 parasite transmission was performed as described [20].
2.7. ELISAs
The reactivity of Pfs25H and EPA specific antibodies were measured by ELISA using mouse antisera collected on days 42, 71, 98 and 125. ELISAs were also used to assess the integrity of Pfs25H with monoclonal antibodies. These assays were performed essentially as described [7, 21]. The avidity of Pfs25 specific IgG was evaluated by ELISA as described above, except that the wash step after incubation of the antisera used wash buffers containing the addition of 3M or 6M urea. Each urea wash was incubated for a period of 5 minutes thrice.
3. Results
3.1.Preparation of Pfs25-EPA conjugates
Recombinant Pfs25H and EPA produced earlier were used to prepare the thioether conjugates. Each purified protein was modified according to the Materials and Methods and extensively dialyzed to remove free linker. Based on the conditions developed, in three independent development runs the average number of substitutions were 3.0 ± 0.3 thiol linkers to each Pfs25H and 6.6 ± 0.7maleimide linkers to EPA (data not shown).The modified proteins Pfs25H-SH and EPA-mal were mixed together using a ratio of equal mass, which approximated a molar ratio of 3 to 1, respectively. Upon completion of the incubation period, L-Cysteine Hydrochloride was added to quench the remaining unreacted thiol groups. Following this procedural step, no free thiols were detected (data not shown) (see Supplemental Materials and Methods for details).
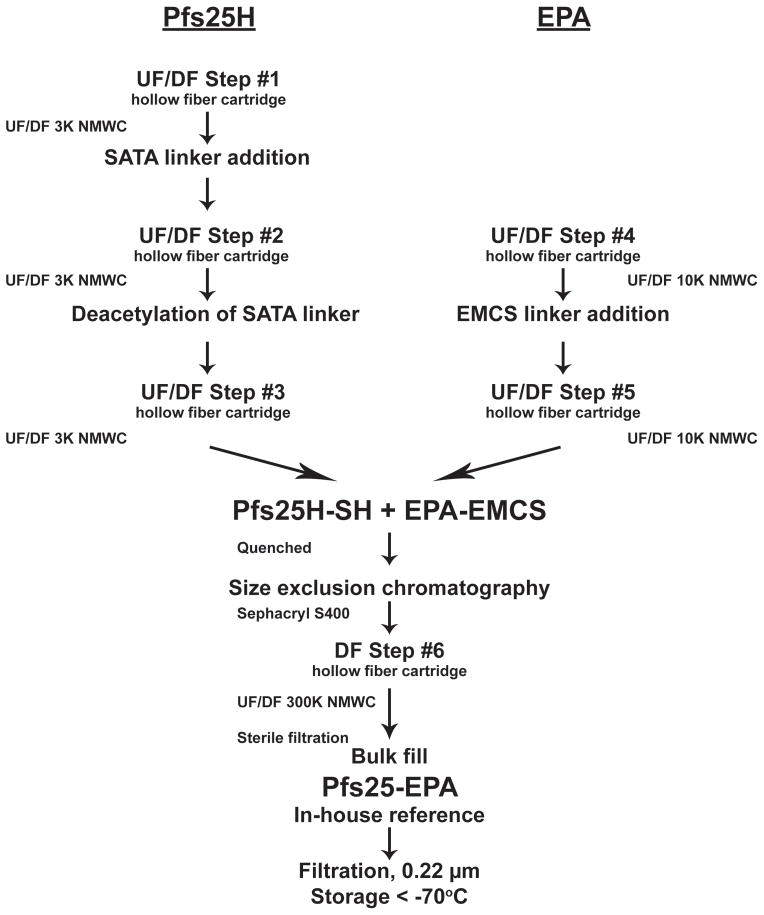
The Pfs25-EPA conjugation mixture was further processed following two strategies. The first strategy involved ultrafiltration/diafiltration (UF/DF) of the conjugate to dialyze and concentrate the Pfs25-EPA in to the final buffered solution. The second strategy involved size exclusion chromatography followed by UF. The unique differences observed between these two processes were a higher recovery without the use of the SEC column. However the predictability of producing a consistent product with an acceptable average molar mass was difficult due to the formation of larger conjugates which would constitute up to 20% of the mass of Pfs25-EPA (see Fig. 2C for comparison of lot A which contained approximately 9% of larger conjugates). These larger conjugates were removed in a controllable manner using the SEC column as determined by SEC-MALS (data not shown). Thus the latter process was selected for pilot-production (Fig. 1). The process yields for Pfs25H ranged between 25 – 45% (data not shown).
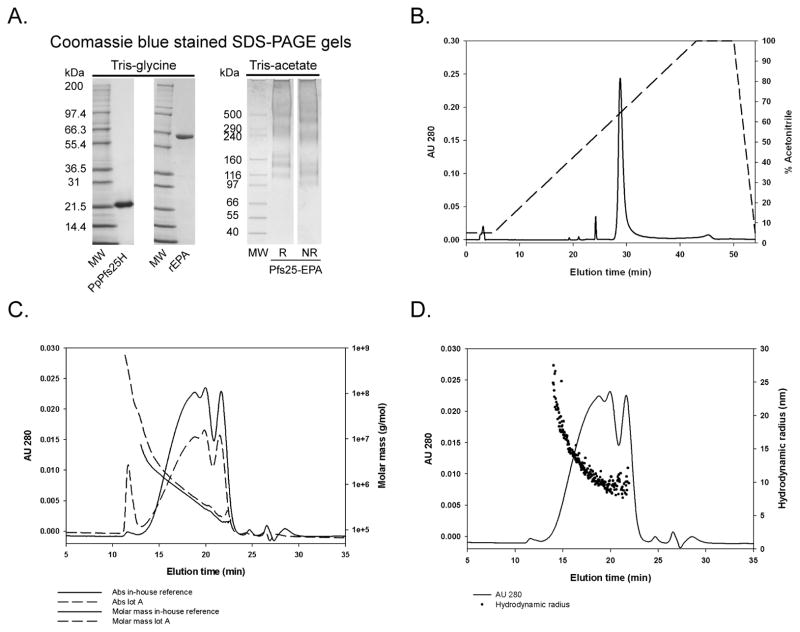
Fig. 2.
Biochemical and biophysical characterizations of in-house Pfs25-EPA. Panel A: Coomassie blue stained SDS-PAGE gel of unconjugated non-reduced Pfs25H and EPA, and reduced and non-reduced conjugated Pfs25-EPA using Tris-glycine or Tris-acetate gels, respectively; Panel B: RP-HPLC analysis of Pfs25-EPA conjugate; Panel C: analysis by analytical size exclusion-HPLC coupled with multi-angle light scattering detection (SEC-MALS) or Panel D, Quasi-elastic light or dynamic light scattering. Panel C: plot shows UV absorbance chromatogram at 280 nm (left Y axis) with plot of molar mass (right Y axis) vs. time (X axis) with (in-house reference) and without an SEC column step (lot A). Panel D: plot shows the UV absorbance chromatogram at 280 nm (left Y axis) with plot of the associated hydrodynamic radii (right Y axis) of in-house reference only vs. time (X axis).
Fig. 1.
Schematic of conjugation process initiated with bulk Pfs25H and EPA protein. Abbreviations: UF/DF, ultrafiltration/diafiltration; SATA, N-Succinimidyl S-Acetylthioaceate; EMCS, N-(ε-maleimidocaproyloxy)-succinimide ester; NMWC, nominal molecular weight cutoff.
3.2.Biochemical and biophysical analysis
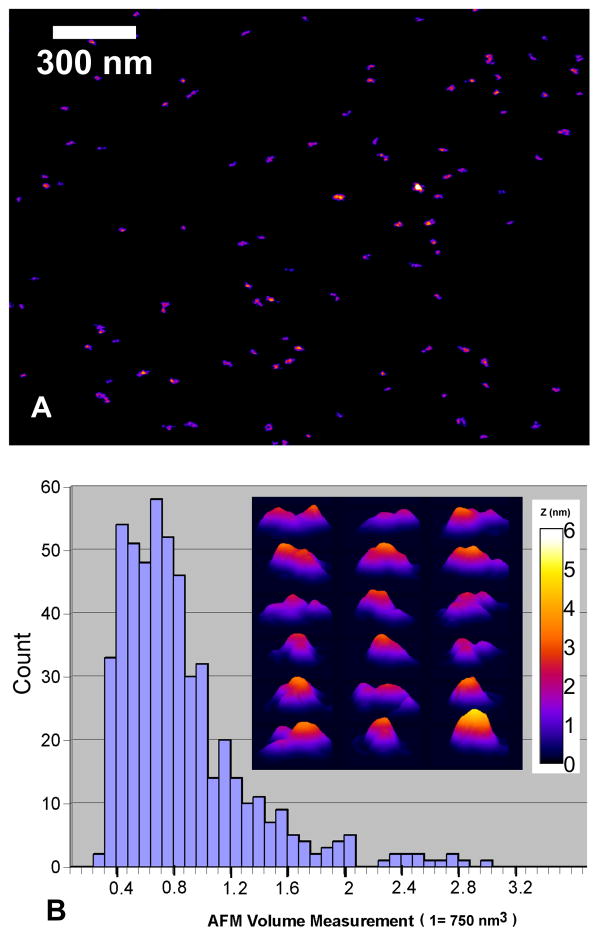
The Pfs25-EPA conjugate was analyzed biochemically and biophysically. As both proteins used in the conjugate were well-characterized recombinant proteins (Fig. 2A) [7, 9], one of which (Pfs25H) had been previously evaluated in a human phase 1 trial [7], the analytical efforts focused on understanding the properties of the conjugate. Analysis by Coomassie blue stained SDS-PAGE, performed under reducing and non-reducing conditions, showed that the chemically conjugated Pfs25-EPA formed a smear ranging from 100 kDa to greater than 500 kDa based on the molecular weight markers (Fig. 2A). A corresponding mobility shift was observed in the conjugate due to the reduction of the disulfide bonds. Analysis by RP-HPLC demonstrated the conjugate appeared as a unified chemical cross-linked protein under denaturing conditions (Fig. 2B). Under denaturing and reducing conditions, a single elution peak was also observed (data not shown). As indicated in Fig. 2C, SEC-MALS of Pfs25-EPA (in-house reference) using the final production process produced a chemical conjugate showing primarily a broad chromatographic peak with a range in molar mass from 150,000 to 1M Daltons. The average molar mass of the in-house conjugate was 550,000 Daltons which was similar to the SEC-MALS profile observed for the cGMP bulk substance with an average molar mass of 600,000 Daltons (data not shown). Dynamic light scattering demonstrated the weighted average hydrodynamic radius of Pfs25-EPA was 11.0 nm which ranged in size from 5 to 25 nm (Fig. 2D). The Pfs25-EPA conjugate by particle analysis using AFM identified particle volumes ranging from 0.4 to 3.2 (Fig. 3A & B), where a volume of 1 represents 750 nm3. Furthermore by AFM, a protein conjugate of 550,000 Daltons at the typical density of 1.4 g/ml having a sphere-like and multi-domain shape was observed with a radius of 5.6 nm while in solution the hydrodynamic radius was approximately 8.2 nm (Fig. 2D). Thus, the analysis of the shape and of molar mass distribution of the Pfs25-EPA conjugate by dynamic light scattering and AFM are consistent with each other.
Fig. 3.
AFM imaging of Pfs25-EPA conjugates. Panel A: a representative topography (with a 300 nm scale in the background) shows that the conjugates are well dispersed on the mica substrate with variable shape presentations. Panel B: a histogram of the particle volume together with a panel of representative Pfs25-EPA molecular images in three-dimensional plots (60 nm separation between each row and column) is shown as the insert with a 6-nm fire-color height scale applicable also to (A).
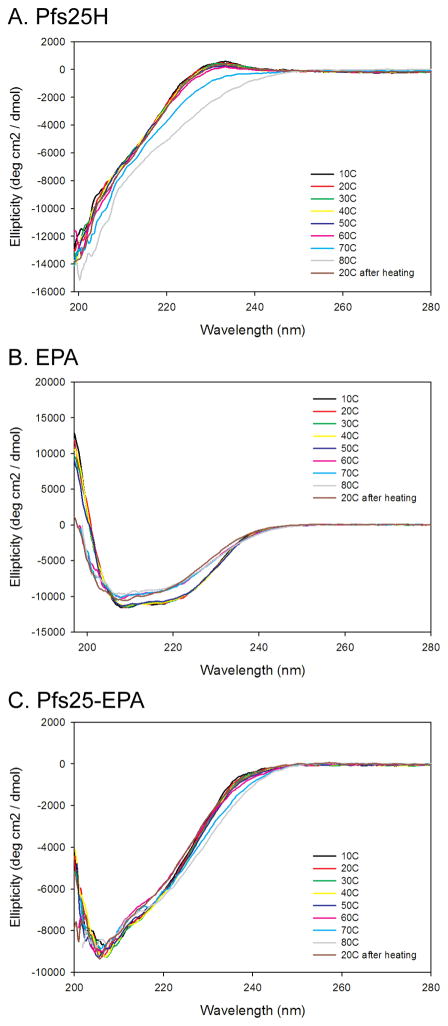
The secondary structures or ellipticity of Pfs25H, EPA and Pfs25-EPA conjugate were assessed by CD for evaluating the proteins sensitivity to heat denaturation and comparability of product composition (Fig. 4). The CD results demonstrate the stability of the unconjugated Pfs25H and EPA and the conjugate to thermal denaturation using a step gradient from 10°C through 80°C. Most importantly, the ellipticity of Pfs25H at 20°C appears similar before and after heat denaturation at 80°C, indicating the maintenance of its structural integrity (Fig. 4A). The ellipticity trace for EPA indicates that renaturation of EPA after heating is limited (Fig. 4B). It is worthy to note, that for EPA the major shift in ellipticity occurred between 50 – 60°C, indicating that the protein is thermally stable at the 20° – 25°C temperatures used throughout development. Finally, the ellipticity of Pfs25-EPA (Fig. 4C) provides an additional orthogonal method to evaluate the comparability of the in-house reference and the cGMP bulk substance which appeared to be similar (data not shown).
Fig. 4.
Far UV analysis and thermal stability of Pfs25H (A), EPA (B) and Pfs25-EPA (C). The ellipticity was plotted as a function of wavelength (nm). Spectra displayed were obtained using increments of 10°C from 10°C through 80°C including re-analysis after cooling at 20°C.
3.3. Structural integrity of Pfs25-EPA
Since the potential existed for the SATA linkers to disrupt native disulfide bond arrangements, an effort was made to characterize the structural integrity of native Pfs25H disulfide bonds within the Pfs25-EPA conjugate. To this end, a quantitative label-free mass spectrometry approach identified as LC/MSE was used [22]. We proposed that if an internal cysteine-cysteine arrangement was disrupted by disulfide exchange with a thiol linker, a cysteine thiol would be liberated which could then react with a maleimide group. In that case the detection of the associated modified peptide in the tryptic digest would not be reported due to its change in mass. Using this strategy, a quantitated reduced tryptic peptide map of Pfs25H and Pfs25-EPA was produced as described in the Material and Methods section. The peptide coverage for Pfs25H, and Pfs25-EPA was 57.2%. Of particular interest, 8 out of 17 tryptic peptides containing a total of 12 out of 22 cysteine residues were quantitated in both samples. The average sum intensity ± SD for quadruplicate runs is shown in Table 1 for 8 tryptic peptides (A – H). In addition, the single unique non-cysteine containing peptide (I) identified in both Pfs25 and Pfs25-EPA was assessed to be detected in equal amounts due to the parameters established for label-free quantitation by LC/MSE including the use of alcohol dehydrogenase as an internal spike control in order to maintain similar protein loads of various samples [22]. The capacity to quantitate an unmodified peptide such as peptide I enabled its use as an internal standard for calculating a ratio of peptides A through H as compared to peptide I (Table 1). The two sets of ratios were then used to calculate a percent of similarity between Pfs25H, and Pfs25-EPA. Overall the percent of similarity for Pfs25-EPA as compared to Pfs25H was 75% using an average of total of sum intensities (p = 0.29) (Table 1). Individual comparisons were, in general, between 70% – 90% (Table 1). The lower abundance of tryptic peptide E in Pfs25-EPA was statistically different than that for Pfs25H (p = 0.03) indicating a greater likelihood for disulfide bond rearrangement within the second EGF like domain. Tryptic peptides A and F which each contain 2 cysteines also showed an indication of possible disulfide bond rearrangement. A different lot of Pfs25-EPA was also evaluated using this approach and the overall results were similar (data not shown).
Table 1.
Quantitative tryptic peptide mapping of Pfs25H and Pfs25-EPA.
| Pfs25H | MHpa | Tryptic peptide | Pfs25H | Pfs25-EPA | % similarity of Pfs25-EPA/Pfs25Hc | p valued | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Average sum intensityb | SD | Ratio | Average sum intensityb | SD | Ratio | |||||
| A | 1963.826 | CENDLVLVNEETCEEK | 649108.3 | 99555.9 | 178.6 | 472933.8 | 35864.8 | 124.2 | 70% | 0.08 |
| B | 1128.5718 | CILDTSNPVK | 61511.5 | 9542.3 | 16.9 | 52732.3 | 3430.6 | 13.8 | 82% | 0.31 |
| C | 2182.9025 | CNLGYDMVNNVCIPNECK | 126888.5 | 19197.6 | 34.9 | 106350.5 | 24067.4 | 27.9 | 80% | 0.44 |
| D | 1519.7396 | GFLIQMSGHLECK | 20909.0 | 11179.4 | 5.8 | 19294.0 | 8676.5 | 5.1 | 88% | 0.88 |
| E | 1205.562 | IDGNPVSYACK | 72847.8 | 11200.6 | 20.0 | 44981.8 | 2267.3 | 11.8 | 59% | 0.03 |
| F | 1095.4922 | TGVCSCNIGK | 17062.0 | 2921.6 | 4.7 | 12847.5 | 980.6 | 3.4 | 72% | 0.13 |
| G | 1252.5991 | TVNKPCGDFSK | 28667.5 | 4727.9 | 7.9 | 27187.0 | 8346.5 | 7.1 | 91% | 0.84 |
| H | 921.471 | VTVDTVCK | 763.3 | 444.8 | 0.21 | 1452.0 | 390.4 | 0.38 | 182% | 0.23 |
| I | 1041.5323 | VPNVQDQNK | 3633.8 | 749.6 | 1 | 3807.5 | 1170.9 | 1 | 100% | |
|
|
||||||||||
| Average of total sum intensities | SD | Ratio | Average of total sum intensities | SD | Ratio | |||||
|
|
||||||||||
| A – H | 977757.8 | 155770.0 | 277.9 | 737778.8 | 71568.9 | 207.2 | 75% | 0.29 | ||
MHp: protonated peptide mass
Average for replicates of 4 individual runs.
% similarity of the ratios calculated for the average sum intensity or average of total sum intensities of Pfs25-EPA as compared to Pfs25H.
p value reported for paired T-test using a two-tailed distribution.
3.4. Integrity of Pfs25H epitopes
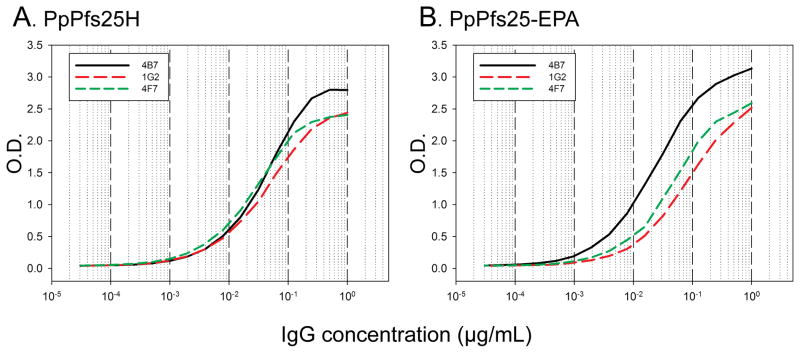
The integrity of the conformation of Pfs25H post-conjugation was assessed using Pfs25 specific mAbs that block transmission in the standard membrane feeding assay and recognize either conformation dependent or linear epitopes. In Figure 5A & B, the titration of three mAbs, two of which recognize conformation dependent epitopes (1G2, and 4F7) and one which recognizes a linear epitope (4B7) is shown against a constant quantity of Pfs25H alone or as a conjugate. The titration curves of mAbs 1G2 and 4F7 appear unchanged, while mAb 4B7 had an observable increase in reactivity by about 2-fold (initial OD1 ~0.02 μg/mL and post-conjugation OD1 ~0.01μg/mL), indicating a minor change in the conformation of Pfs25H.
Fig 5.
Analysis of the conformational integrity of Pfs25H using a panel of three Pfs25 specific functional mAbs as determined by ELISA. Equal concentrations of Pfs25H alone or within the Pfs25-EPA conjugate were plated and titration curves were determined for two conformation dependent mAbs (1G2 and 4F7) and one linear-epitope binding mAb (4B7).
3.5.Immunogenicity of Pfs25-EPA and transmission blocking activity
The immunogenicity of the Pfs25-EPA was assessed in comparison to an unconjugated Pfs25H formulated on Alhydrogel®. Analysis of the adsorption of Pfs25H and Pfs25-EPA Alhydrogel® formulations using similar concentrations as for human dosing were assessed immediately after mixing and 24 hours later by Silver stained SDS-PAGE gel analysis. Both Pfs25H and Pfs25-EPA were completely bound since no detectable soluble forms were observed as compared to a 100 – 150 ng load of each reference (Fig. 6A). The conjugated form of Pfs25-EPA increased the immunogenicity by ~75-fold using a 5-fold lower dose of Pfs25H and it was 110-fold more immunogenic at the same dose in a statistically significant manner (p<0.001) (Fig. 6). The geometric mean antibody levels achieved in outbred mice receiving 0.5 μg and 2.5 μg doses of Pfs25-EPA were 9,119 ELISA units and 13,796 ELISA units, respectively as compared to 124 ELISA units for mice receiving 2.5 μg Pfs25H alone. No significant differences were observed for Pfs25-EPA at 0.5 or 2.5 μg doses (Fig. 6B). The geometric mean antibody levels for the carrier protein, EPA, on day 42 in the 2.5 and 0.5 μg Pfs25-EPA groups were 44,200 and 39,400 ELISA units at an OD1; no antibodies were detected against EPA in the Pfs25H group (data not shown). The longevity of the Pfs25 response was assessed out to 125 days, at which time, elevated Pfs25H specific antibody responses were still observed (Fig. 6). The mouse antisera were pooled for the groups immunized with 2.5 μg of Pfs25H or Pfs25-EPA and evaluated in a membrane feeding assay to assess its capacity to block P. falciparum transmission to mosquitoes. The reduction of oocyst density and prevalence shown in Table 2 were consistent with previous observations [20], in that the inhibition of parasite development corresponded to the Pfs25H ELISA Units. The avidity of the Pfs25 specific IgG generated against Pfs25H alone or the Pfs25-EPA conjugate was assessed by ELISA on day 42 antisera. No significant difference was observed in the avidity of the IgG between the two different immunogens (data not shown).
Fig. 6.
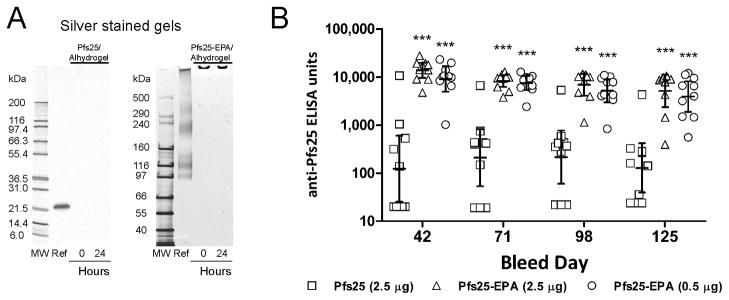
Formulation and comparative immunogenicity of unconjugated Pfs25H and conjugated Pfs25-EPA on Alhydrogel® in CD1 outbred mice. Panel A: Silver stained Tris-glycine or Tris-acetate gels with unformulated Pfs25H or Pfs25-EPA (identified as Ref., respectively) and clarified supernatants of Alhydrogel® formulations immediately after mixing (0 hrs) and 24 hours later. Panel B: The ELISA titers for individual mice are plotted with error bars representing the geometric mean and 95% confidence intervals. Asterisks denote statistically significant differences in ELISA titers between conjugated and unconjugated forms of Pfs25H at each time point (***p<0.001) as determined by Two-Way Repeated Measures ANOVA of the Log10-transformed ELISA titers.
Table 2.
Membrane feeding assay results comparing pooled immune CD1 mouse sera raised against Pfs25H or Pfs25-EPA.
| Immunogen | Sera dilutiona | ELISA Units | Activity with no human complementb
|
||||
|---|---|---|---|---|---|---|---|
| Inf/Dissc | Avg # oocysts | Median # oocysts(range) | % reduction oocysts | % reduction prevalence | |||
|
|
|||||||
| Pfs25H | 1:4 | 290 | 17/20 | 5.0 | 3 (0 – 19) | 52.6 | 15.0 |
| 1:8 | 145 | 19/20 | 11.5 | 8 (2 – 64) | 33.7 | 5.0 | |
| Pfs25-EPA | 1:4 | 2045 | 2/20 | 0.1 | 0 (0 – 1) | 99.0 | 90.0 |
| 1:8 | 1022 | 12/20 | 1.5 | 1 (0 – 5) | 91.6 | 40.0 | |
| Control | 1:4 | NAc | 20/20 | 10.5 | 8 (2 – 25) | 0 | 0 |
| 1:8 | NA | 20/20 | 17.4 | 12 (2 – 64) | 0 | 0 | |
Day 42 pooled mouse serum from mice immunized with 2.5 μg Pfs25H or conjugated Pfs25-EPA.
Percent reduction in oocyst density of test is compared to the oocyst density observed using a similar dilution of normal mouse sera (control).
Abbreviations: Inf., infected; Diss., dissected; NA, not applicable
4. Discussion
Pfs25 is a leading transmission blocking vaccine candidate for inclusion in a vaccine to block malaria parasite development within the mosquito host as part of a VIMT. Recombinant forms of Pfs25 formulated with ISA51, a water-in-oil adjuvant induced human antibodies that blocked oocyst development within the mosquito [20]. Unfortunately, the safety profile of an ISA51 formulation is unlikely to be suitable for large-scale administration of a VIMT or transmission blocking vaccine alone. A method to resolve the issue of poor immunogenicity was identified by chemically conjugating Pfs25H to a carrier molecule such as EPA [9], OMPC [23] or even itself [7]. Each of these conjugated forms enhanced the immunogenicity of a comparable soluble Pfs25H formulation by at least 4 fold in mice and in the case of the Pfs25-OMPC, an enhanced duration of Pfs25H specific antibodies was reported in rhesus monkeys [24]. Given the improved immunogenicity profile achieved by conjugating Pfs25, a scalable process was developed for producing Pfs25H conjugated to EPA. The pilot-scale process was robust and suitable for technology transfer to a contract manufacturing organization. The thioether conjugation chemistry used established methods, similar to those used in a commercial vaccine [25]. A preliminary analysis of the concentration of free linkers present during the various process steps by RP-ultra performance liquid chromatography indicated that due to the multiple UF/DF steps and SEC column, residual free linker was below the level of the sensitivity of the detection method (data not shown).
Biochemical analyses by SDS-PAGE and RP-HPLC indicate that Pfs25-EPA conjugates are formed through the formation of thio-ether linkages between Pfs25H and EPA (Fig. 2). Based on the biophysical analyses of the Pfs25-EPA by dynamic light scattering and AFM, the conjugate is shown to have sphere-like shapes composed of particles ranging from 10 to 40 nm in diameter (Fig. 3). The observation that the Pfs25-EPA conjugate enhances the immunogenicity is consistent with the general observations that nano-microparticles including viral like particles such as used in RTS,S [26] which are about 22 nm in diameter [27] improve the immunogenicity of poor immunogens [24]. A Pfs25-EPA/Alhydrogel® formulation may be considered a nano-microparticle formulation since Alhydrogel® is comprised of ~2 – 12 μm particles [23].
Analysis of the structural integrity of Pfs25H within the Pfs25-EPA conjugate using a label-free quantitative mass spectrometry approach (LC/MSE) [19, 22] to compare a quantitative tryptic peptide map showed that Pfs25H on the whole appears intact with 75% similarity in peptide abundance for the peptides containing 12 of the protein’s 22 cysteine residues, even though, the possibility that a small percentage of native disulfide bonds could have undergone rearrangement cannot be ruled out entirely. The integrity of the conformation of Pfs25H was further assessed using three Pfs25 specific mAbs [20]. Two mAbs (1G2 and 4F7) recognize a conformation dependent epitope while mAb 4B7 recognizes a cryptic linear epitope within the third epidermal growth factor like domain [28, 29] and is more reactive against denatured forms of Pfs25H as identified by immunoblot (data not shown). The analysis by ELISA was able to detect a minor change in the conjugated form of Pfs25H only with mAb 4B7 indicating a relaxing of the secondary structure within the epidermal growth factor like domain 3. However, since all three of these mAbs block oocyst development in the standard membrane feeding assay [20] this change will unlikely alter the potency of Pfs25H. A comparative immunogenicity study in outbred mice demonstrated that the Pfs25-EPA conjugate adsorbed on Alhydrogel® enhanced the immunogenicity as compared to monomeric Pfs25H using a similar formulation by ~75-fold using a 5-fold lower dose. This result was similar to our previous findings using various conjugated forms, including Pfs25-EPA [9]. Analysis of pooled Pfs25H and Pfs25-EPA mouse antisera showed that the blocking activity of parasite transmission was associated with the ELISA units tested in the standard membrane feeding assay, as previously demonstrated [20]..
In summary, a malaria transmission blocking vaccine is needed to facilitate the current elimination and eradication strategies. The present study shows the development of scalable processes for the successful pilot-scale manufacture and analytical characterization of a Pfs25-EPA conjugated nanoparticle vaccine. Pfs25-EPA formulated on Alhydrogel® enhanced the inherently poor immunogenicity of Pfs25H, while maintaining its conformational integrity. The evaluation of the safety and immunogenicity of the Pfs25-EPA vaccine in humans, which is underway (see ClinicalTrial.gov, ID number: NCT01434381) will be an important next step in the development of a transmission blocking vaccine.
Highlights.
Pfs25 is candidate for a malaria transmission blocking vaccine
A recombinant Pfs25 protein was chemically cross-linked to ExoProtein A (EPA)
The Pfs25-EPA chemical conjugate appears as a 20 nm nanoparticle
Pfs25-EPA as a nanoparticle enhanced the immunogenicity of Pfs25 in mice
Process tech transferred for cGMP pilot-scale manufacturing of Pfs25-EPA
Acknowledgments
We acknowledge the support of those within the units of the LMIV core, especially Emma Barnafo for formulation, Lynn Lambert for excellent animal husbandry, Dominique Jones for analytical support and Olga Muratova for performing the membrane feeding assays. Dr. Svetlana Kotova provided excellent technical support for the AFM studies. We thank Drs. Michael Nold (Waters Corp.) and L. Renee Olano (Research Technology Branch, NIAID, NIH) for their critical evaluation of the quantitative peptide mapping analysis. We appreciate the work of Brian Bell and Jay Wood for the successful technology transfer to the WRAIR Biopilot Production facility. This research was supported by the Intramural Research Program of the NIH, including NIAID and NIBIB.
Abbreviations
- Pfs25
Plasmodium falciparum 25 kDa sexual stage protein
- Pvs25
P. vivax 25 kDa sexual stage protein
- VIMT
Vaccine to Interrupt Malaria Transmission
- EPA
ExoProtein A
- UF/DF
ultrafiltration/diafiltration
- OMPC
Outer Membrane Protein Complex
- SATA
N-Succinimidyl S-Acetylthioaceate
- EMCS
N-(ε-maleimidocaproyloxy)-succinimide ester
- NMWC
nominal molecular weight cutoff
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. The New England journal of medicine. 2011 Nov 17;365(20):1863–75. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 2.mal ERACGoV. A research agenda for malaria eradication: vaccines. PLoS medicine. 2011;8(1):e1000398. doi: 10.1371/journal.pmed.1000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saul A. Mosquito stage, transmission blocking vaccines for malaria. Current opinion in infectious diseases. 2007 Oct;20(5):476–81. doi: 10.1097/QCO.0b013e3282a95e12. [DOI] [PubMed] [Google Scholar]
- 4.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, et al. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988 May 5;333(6168):74–6. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 5.Saxena AK, Wu Y, Garboczi DN. Plasmodium p25 and p28 surface proteins: potential transmission-blocking vaccines. Eukaryotic cell. 2007 Aug;6(8):1260–5. doi: 10.1128/EC.00060-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaslow DC. Transmission-blocking vaccines. Chemical immunology. 2002;80:287–307. doi: 10.1159/000058850. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PloS one. 2008;3(7):e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huebner RE, Nicol M, Mothupi R, Kayhty H, Mbelle N, Khomo E, et al. Dose response of CRM197 and tetanus toxoid-conjugated Haemophilus influenzae type b vaccines. Vaccine. 2004 Dec 21;23(6):802–6. doi: 10.1016/j.vaccine.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 9.Qian F, Wu Y, Muratova O, Zhou H, Dobrescu G, Duggan P, et al. Conjugating recombinant proteins to Pseudomonas aeruginosa ExoProtein A: a strategy for enhancing immunogenicity of malaria vaccine candidates. Vaccine. 2007 May 16;25(20):3923–33. doi: 10.1016/j.vaccine.2007.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubler-Kielb J, Majadly F, Wu Y, Narum DL, Guo C, Miller LH, et al. Long-lasting and transmission-blocking activity of antibodies to Plasmodium falciparum elicited in mice by protein conjugates of Pfs25. Proceedings of the National Academy of Sciences of the United States of America. 2007 Jan 2;104(1):293–8. doi: 10.1073/pnas.0609885104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Przysiecki C, Flanagan E, Bello-Irizarry SN, Ionescu R, Muratova O, et al. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proceedings of the National Academy of Sciences of the United States of America. 2006 Nov 28;103(48):18243–8. doi: 10.1073/pnas.0608545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai CW, Duggan PF, Shimp RL, Jr, Miller LH, Narum DL. Overproduction of Pichia pastoris or Plasmodium falciparum protein disulfide isomerase affects expression, folding and O-linked glycosylation of a malaria vaccine candidate expressed in P. pastoris. Journal of biotechnology. 2006 Feb 24;121(4):458–70. doi: 10.1016/j.jbiotec.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Shuler KR, Dunham RG, Kanda P. A simplified method for determination of peptide-protein molar ratios using amino acid analysis. Journal of immunological methods. 1992 Dec 8;156(2):137–49. doi: 10.1016/0022-1759(92)90020-t. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CW, Duggan PF, Jin AJ, Macdonald NJ, Kotova S, Lebowitz J, et al. Characterization of a protective Escherichia coli-expressed Plasmodium falciparum merozoite surface protein 3 indicates a non-linear, multi-domain structure. Molecular and biochemical parasitology. 2009 Mar;164(1):45–56. doi: 10.1016/j.molbiopara.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitmore L, Wallace BA. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004 Jul 1;32(Web Server issue):W668–73. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitmore L, Wallace BA. Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers. 2008 May;89(5):392–400. doi: 10.1002/bip.20853. [DOI] [PubMed] [Google Scholar]
- 17.Plassmeyer ML, Reiter K, Shimp RL, Jr, Kotova S, Smith PD, Hurt DE, et al. Structure of the Plasmodium falciparum circumsporozoite protein, a leading malaria vaccine candidate. The Journal of biological chemistry. 2009 Sep 25;284(39):26951–63. doi: 10.1074/jbc.M109.013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotova S, Prasad K, Smith PD, Lafer EM, Nossal R, Jin AJ. AFM visualization of clathrin triskelia under fluid and in air. FEBS letters. 2010 Jan 4;584(1):44–8. doi: 10.1016/j.febslet.2009.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva JC, Denny R, Dorschel CA, Gorenstein M, Kass IJ, Li GZ, et al. Quantitative proteomic analysis by accurate mass retention time pairs. Analytical chemistry. 2005 Apr 1;77(7):2187–200. doi: 10.1021/ac048455k. [DOI] [PubMed] [Google Scholar]
- 20.Cheru L, Wu Y, Diouf A, Moretz SE, Muratova OV, Song G, et al. The IC(50) of anti-Pfs25 antibody in membrane-feeding assay varies among species. Vaccine. 2010 Jun 17;28(27):4423–9. doi: 10.1016/j.vaccine.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008 Jan 10;26(2):193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva JC, Gorenstein MV, Li GZ, Vissers JP, Geromanos SJ. Absolute quantification of proteins by LCMSE: a virtue of parallel MS acquisition. Molecular & cellular proteomics: MCP. 2006 Jan;5(1):144–56. doi: 10.1074/mcp.M500230-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Harris JR, Soliakov A, Lewis RJ, Depoix F, Watkinson A, Lakey JH. Alhydrogel® adjuvant, ultrasonic dispersion and protein binding: a TEM and analytical study. Micron. 2012 Feb;43(2–3):192–200. doi: 10.1016/j.micron.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Oyewumi MO, Kumar A, Cui Z. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert review of vaccines. 2010 Sep;9(9):1095–107. doi: 10.1586/erv.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marburg S, Jorn D, Tolman RL, Arison B, Mccauley J, Kniskern PJ, et al. Bimolecular Chemistry of Macromolecules - Synthesis of Bacterial Polysaccharide Conjugates with Neisseria-Meningitidis Membrane-Protein. J Am Chem Soc. 1986 Aug 20;108(17):5282–7. [Google Scholar]
- 26.Gordon DM, McGovern TW, Krzych U, Cohen JC, Schneider I, LaChance R, et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. The Journal of infectious diseases. 1995 Jun;171(6):1576–85. doi: 10.1093/infdis/171.6.1576. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs E, Rutgers T, Voet P, Dewerchin M, Cabezon T, de Wilde M. Simultaneous synthesis and assembly of various hepatitis B surface proteins in Saccharomyces cerevisiae. Gene. 1989 Aug 15;80(2):279–91. doi: 10.1016/0378-1119(89)90292-8. [DOI] [PubMed] [Google Scholar]
- 28.Stura EA, Kang AS, Stefanko RS, Calvo JC, Kaslow DC, Satterthwait AC. Crystallization, sequence and preliminary crystallographic data for transmission-blocking anti-malaria Fab 4B7 with cyclic peptides from the Pfs25 protein of P. falciparum. Acta crystallographica Section D, Biological crystallography. 1994 Jul 1;50(Pt 4):535–42. doi: 10.1107/S0907444994001356. [DOI] [PubMed] [Google Scholar]
- 29.Stura EA, Satterthwait AC, Calvo JC, Stefanko RS, Langeveld JP, Kaslow DC. Crystallization of an intact monoclonal antibody (4B7) against Plasmodium falciparum malaria with peptides from the Pfs25 protein antigen. Acta crystallographica Section D, Biological crystallography. 1994 Jul 1;50(Pt 4):556–62. doi: 10.1107/S0907444994001782. [DOI] [PubMed] [Google Scholar]