Abstract
The mammalian circadian clock influences the timing of many biological processes such as the sleep/wake cycle, metabolism, and cell division. Environmental cues such as light exposure can influence the timing of this system through the posttranslational modification of key components of the core molecular oscillator. We have previously shown that DNA damage can reset the circadian clock in a time-of-day-dependent manner in the filamentous fungus Neurospora crassa through the modulation of negative regulator FREQUENCY levels by PRD-4 (homologue of mammalian Chk2). We show that DNA damage, generated with either the radiomimetic drug methyl methane sulfonate or UV irradiation, in mouse embryonic fibroblasts isolated from PER2::LUC transgenic mice or in the NIH3T3 cell line, elicits similar responses. In addition to induction of phase advances, DNA damage caused a decrease in luciferase signal in PER2::LUC mouse embryonic fibroblast cells that is indicative of PER2 degradation. Finally, we show that the activity of the BMAL1 promoter is enhanced during DNA damage. These findings provide further evidence that the DNA damage-mediated response of the clock is conserved from lower eukaryotes to mammals.
Keywords: circadian, DNA damage, phase-response curve, Per2, BMAL1, luciferase
An evolutionarily conserved molecular feedback loop regulates the circadian clock in organisms ranging from fungi to mammals. This transcription-translation feedback loop consists of a positive and a negative arm made up of proteins that serve to regulate gene expression with a period of approximately 24 h. Components of this loop include the negative regulator Frequency (FRQ) in the fungus Neurospora crassa and the Period (PER) and Cryptochrome (CRY) proteins in mammals (Dunlap, 1999; Ko and Takahashi, 2006). Environmental cues, such as light exposure, lead to modification of these core components so that the molecular clock can entrain to the daily variation of a given environmental stimulus.
DNA damage activates specific signal transduction pathways in response to the type of DNA insult, which then leads to events such as cell cycle arrest, DNA repair, and/or apoptosis (Bartek et al., 2007; Lukas et al., 2004). Several of these pathways involve the ATM-mediated activation of Chk2 in mammals (PRD-4 in Neurospora), which primarily serves to arrest cellular division (Lukas et al., 2004).
Early work by Sweeney showed that the circadian clock in Gonyaulax can be reset by UV irradiation (Sweeney, 1963). Remarkably, unlike other known resetting cues, only phase advances were evident, with the largest occurring in the early evening. We have shown that DNA damage can also reset the circadian clock in a time-of-day-dependent manner in Neurospora (Pregueiro et al., 2006). DNA damage induced by the radiomimetic drug methyl methane sulfonate (MMS) at various times in the circadian cycle leads to only phase advances in the subjective day. Maximal advances coincide with de novo FRQ synthesis and minimal advances occur during the subjective night when FRQ is mature. These phase advances occur through the direct interaction of PRD-4/Chk2 kinase with FRQ, which then phosphorylates FRQ and converts hypophosphorylated FRQ to a hyperphosphorylated state. This change in phosphorylation leads to FRQ degradation and thus shifts the phase of the clock to a time of the day when FRQ levels are low.
The connection between DNA damage and the circadian clock has also been investigated in mammals (reviewed in Chen-Goodspeed and Lee, 2007; Gery and Koeffler, 2007; Kondratov and Antoch, 2007). Work in irradiated Per2 null mice showed that these animals are more susceptible to tumors in the absence of this core clock component than their wildtype counterparts (Fu et al., 2002). More recently, PER1 has been shown to interact with the DNA damage response (DDR) kinases Checkpoint Kinase 2 (Chk2) and Ataxia Telangiectasia Mutated (ATM) after treatment of cell cultures with ionizing radiation (IR) (Gery et al., 2006), and the phosphorylation of PER1 by Chk2 has also been shown in the human embryonic kidney 293T cell line (Matsuoka et al., 2007). From this work it is clear that the circadian clock and its molecular machinery have evolved to respond to DNA damage, to perhaps prevent the exposure of clock-controlled responses from UV insult.
While we were completing this work, a complementary study appeared showing that IR-mediated DNA damage can also reset the mammalian clock in Rat-1 fibroblasts and in mice; as was the case in Gonyaulax and Neurospora, only phase advances were evident (Oklejewicz et al., 2008). That study provided strong evidence that this response is mediated through ATM as phase advances were ameliorated in IR-treated ATM-deficient primary human cells, as well as in IR-treated RAT-1 fibroblasts that were preheated with an ATM-specific small molecule inhibitor or with caffeine, a known inhibitor of ATM activity. We report here a similar response to DNA damage in mouse embryonic fibroblast (MEF) cells. UV and MMS treatment of serum-shocked MEFsisolated from PER2::LUC knock-in mice, which express a PER2/LUCIFERASE fusion protein from the endogenous Per2 locus (Yoo et al., 2004), results in only phase advances that change in magnitude depending on the status of PER2. The largest advances occur during de novo PER2 synthesis, minimal advances when PER2 is mature, and DNA damage causes an immediate decrease in PER2::LUC levels, which is indicative of degradation. Finally, we observed enhanced levels of BMAL1 promoter activity after DNA damage in NIH3T3 MEF cells transiently transfected with a BMAL1 promoter construct driving luciferase. Our data, together with Sweeney’s work in Gonyaulax, as well as other recent findings in Rat-1 fibroblasts and mice, suggest that this property of the clock is evolutionarily conserved from lower eukaryotes to mammals.
MATERIALS AND METHODS
Animals and Cell Lines
PER2::LUC knock-in mice were kindly donated by Dr. Joseph Takahashi. PER2::LUC MEF fibroblasts were generated from homozygous breeders. Mouse embryos were isolated at embryonic day 14 (E14) and the cells isolated from the individual embryos were immortalized by passaging them for approximately 1 month. Individual PER2::LUC lines were serum shocked in 50% horse serum for 2 h (Balsalobre et al., 1998) and assessed for circadian rhythmicity via realtime bioluminescence monitoring (see below). A line that demonstrated the greatest amplitude and longest sustained rhythm (5–7 days) with a period of approximately 23.5 h (sin fit damped as calculated by the LumiCycle analysis software package) was chosen for this study. PER2::LUC and NIH3T3 cells were passaged in DMEM (Invitrogen, Carlsbad, CA) containing phenol red, 10% FBS, and 1% penicillin/streptomycin.
Real-Time Bioluminescence Monitoring and DNA Damage
Cells were grown to 100% confluence in 35-mm tissue-culture dishes and synchronized via serum shock. The cells were then washed with 1× PBS and replaced with 2 mL of assay medium (L15,10% FBS, 1% penicillin/streptomycin, 10 mM HEPES, and 0.1 mM luciferin). Phenol red-free L15 medium (Invitrogen) was used in place of DMEM as L15 is specifically formulated for cells grown in a non-CO2-equilibrated environment. The tissue-culture dishes were sealed with silicone grease and glass coverslips and then transferred to the LumiCycle (Actimetrics, Wilmette, IL). In some cases, the dishes were synchronized, transferred to the LumiCycle, and then resynchronized prior to DNA damage studies to ensure good rhythm quality.
For DNA damage studies involving MMS, the dishes were removed at the appropriate time after serum shock and 1 mL of medium was transferred to a 15-mL conical tube and stored in a 37 °C water bath. This was done to avoid resetting the clock via a medium change as was previously observed in Rat-1 fibroblasts (Izumo et al., 2006). MMS (Sigma, St. Louis, MO) or an equivalent volume of vehicle control (assay medium) was then added to give a final concentration of 1 mM to the remaining 1 mL of medium and the dishes were transferred back to the LumiCycle for 2 h. The dishes were then removed, the medium was replaced with the 1 mL of the original conditioned medium, and the dishes were then resealed and transferred back to the LumiCycle for data acquisition. All treatment times indicated reflect the time of release from MMS treatment. Data analyses were performed using the LumiCycle Analysis software program (Actimetrics). The phase of treated cultures was compared with vehicle controls as well as untreated controls to be certain that the manipulations were not undergoing substantial phase shifts. Phase shifts were calculated by comparing the 5th peak after MMS treatment with the peak of the vehicle control.
For DNA damage studies involving UV, the PER2::LUC cells were plated and synchronized as described above. Cells were removed from the LumiCycle at the appropriate time after serum shock, 2 mL of assay medium was removed and stored at 37 °C, and the cells were either irradiated with UV via a Stratalinker 1800 (Stratagene, La Jolla, CA) at a dose of 20 J/m2 or left untreated (mock control). UV irradiation by this method is an established way to induce DNA damage in NIH3T3 cells in culture (Lee et al., 2006; Sanchez-Prieto et al., 2000; Wu and Yung, 2002). The prewarmed conditioned medium was then added back and the cells were transferred back to the LumiCycle for data acquisition. Phase shifts were calculated by comparing the 4th peak after UV treatment with the peak of the mock treated control. Time point zero is designated as the time when the cells were released from serum shock.
Transfections
NIH3T3 cells were plated in 35-mm tissue-culture dishes (Corning) and transfected with a BMAL1 promoter construct driving the expression of a destabilized luciferase (pBMALl-dLuc), which was kindly provided by Dr. John Hogenesch. Transfections were performed with Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. Bioluminescence assays were performed as described above with the exception that the cells were transfected 2 to 3 days prior to synchronization to ensure that the cells were at 100% confluence, and phase shifts were calculated by comparing the 3rd peak after MMS treatment with the peak of the vehicle control.
RESULTS
DNA Damage Induces Phase Advances in PER2::LUC MEF Cells
In Neurospora, DNA damage via the radiomimetic drug MMS generates an atypical phase-response curve (PRC) where only phase advances are evident (Pregueiro et al., 2006). DNA damage induces a maximum phase advance when de novo FRQ synthesis occurs and a decrease in the magnitude of these advances occurs as FRQ matures. In this context, we set out to determine whether this response by the clock to DNA damage is conserved in the mammalian system. To assess this, an immortalized MEF cell line was generated from the PER2::LUC knock-in mouse line as described in Materials and Methods. In this MEF cell line, PER2 levels climb from approximately 12–14 to approximately 22–26 h after serum shock. As PER2 plays a logically analogous role in the mammalian clock to that of FRQ, we reasoned that DNA damage at the phase of minimal PER2 expression might lead to large phase advances of the clock and these advances should decrease over time as PER2 matures. Thus, by this logic, MMS pulses starting at approximately 12 to 14 h after serum shock were expected to initially yield maximum advances that would decrease in magnitude through approximately 24 to 26 h.
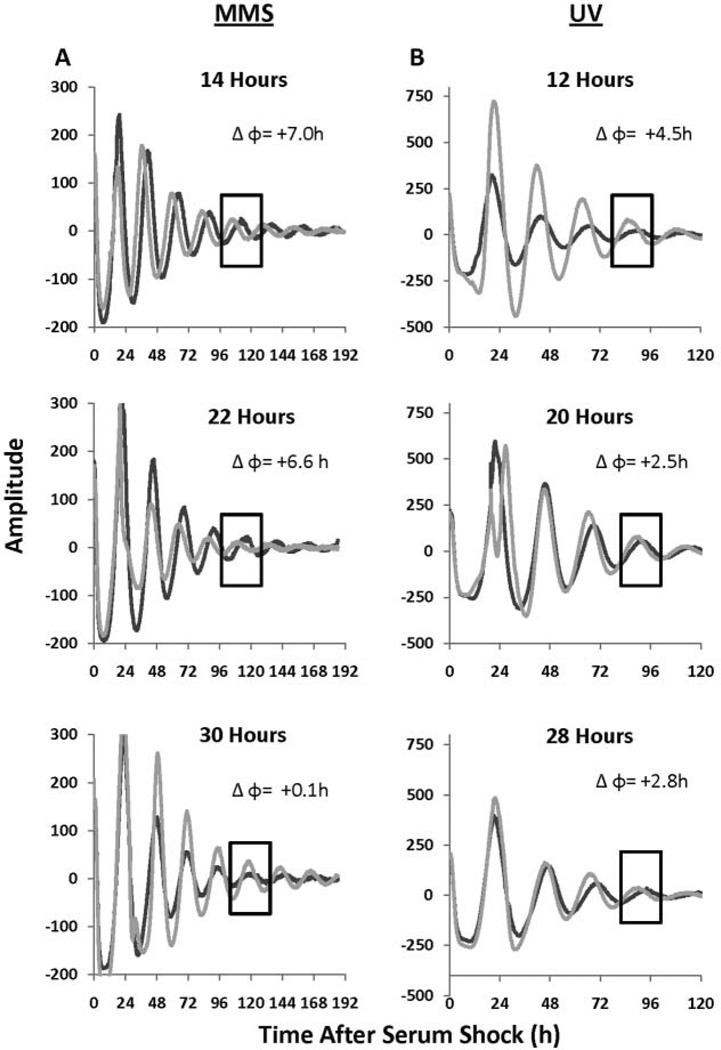
To test this, confluent PER2::LUC MEF cells were treated with either 1 mM MMS to induce DNA damage, or a vehicle control, for 2 h at various times after synchronization. A concentration of 1 mM MMS has been shown to induce DNA damage in the MEF cell line NIH3T3 (Kuo et al., 1997). As expected, DNA damage induced only phase advances that were persistent several days after MMS treatment (Fig. 1A). Interestingly, steady-state advances occurred even when a transient delay was initially observed (Fig. 1A, 30-h treatment).
Figure 1.
Methyl methane sulfonate (MMS) and UV-mediated DNA damage-induced phase shifts in PER2::LUC mouse embryonic fibroblast (MEF) cells. (A) Treatment with either 1 mM MMS or a vehicle control was performed for 2 h and released at 14, 22, and 30 h after serum shock, and the rhythms were followed for 7 days. DNA damage with 1 mM MMS yielded a phase change (Δφ) of 7.0, 6.6, and 0.1 h, respectively, as is evident by comparing treated cultures and controls in the boxed region. The black line indicates the treatment control and the gray line indicates 1 mM MMS treatment. (B) Treatment with 20 J/m2 of UV at 12, 20, and 28 h after serum shock was performed and the rhythms were followed approximately 5 days. DNA damage with UV yielded a Δφ of 4.5,2.5, and 2.8 h, respectively, as can be seen by comparing treated and control cultures within the boxed region. The black line indicates the mock treatment control and the gray line indicates UV treatment. The data have been smoothed and baseline subtracted to emphasize the rhythms.
UV light also induced phase advances in the PER2::LUC MEF cell line, establishing that this response can occur independently of how the DNA damage was induced (Fig. 1B). This dose of UV was chosen based on previous work in NIH3T3 cells (see Materials and Methods) and preliminary dose-response experiments where a range of 1 J/m2 to 50 J/m2 was tested (data not shown). A dose of 20 J/m2 was selected because this resulted in little to no cell death and did not eliminate rhythmicity, while still causing a change in clock phase. This experiment was performed twice, with Figure 1B representing the experiment with the higher quality rhythms.
DNA Damage Induces an Acute Decrease of Luciferase Activity in PER2::LUC MEF Cells
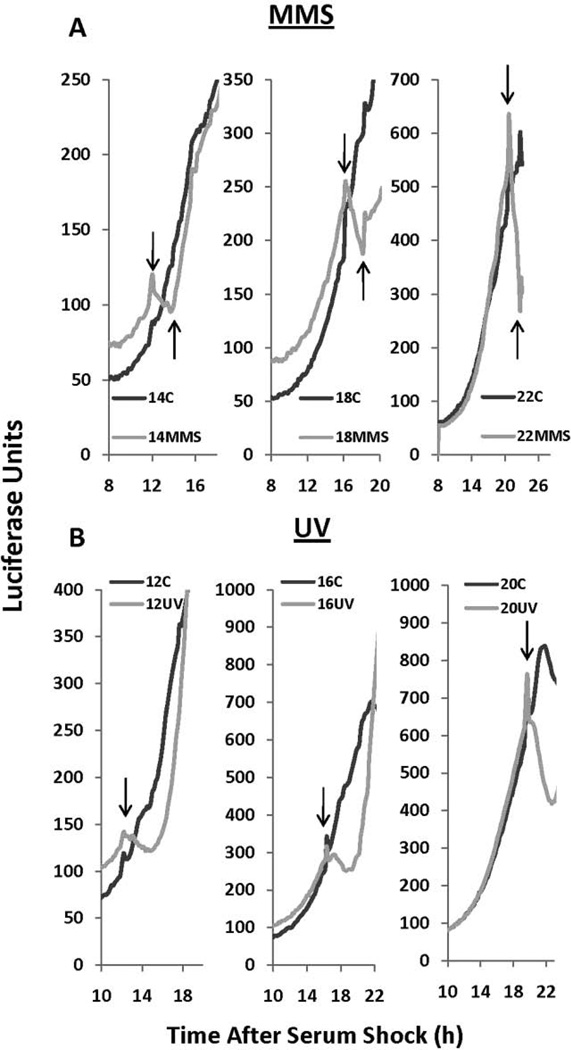
One major benefit to following the circadian clock in PER2::LUC MEF cells is the ability to track PER2 protein levels in real time, as opposed to following the transcriptional activation of a clock promoter or periodic assessments via Western blot analysis. In Neurospora, DNA damage induces the largest phase advances during the phase of the clock when FRQ is initially expressed, and this resetting is mediated by PRD4/ CHK2-dependent phosphorylation and subsequent degradation of FRQ. Similarly, a decrease in PER2::LUC levels, as measured by luciferase activity, is seen during MMS treatment at 14, 18, and 22 h after serum shock, which is the phase of the clock when PER2 levels are increasing (Fig. 2A). Similarly, a decrease in luciferase activity at 12, 16, and 20 h was observed immediately following UV treatment (Fig. 2B). These data indicate that PER2 protein degradation is triggered by factors that damage DNA, with the magnitude of the reduction dependent on the status of PER2. This is consistent with our hypothesis that the negative arm of the clock is impacted at phases of the clock when PER2 levels are increasing.
Figure 2.
Methyl methane sulfonate (MMS) and UV-mediated DNA damage causes an acute decrease in luciferase levels in PER2::LUC cells. (A) An acute decrease in luciferase levels was observed during 1 mM MMS treatment at 14 (left), 18 (middle), and 22 (right) h after serum shock, when PER2::LUC levels are increasing. Onset of MMS treatment is indicated by the downward arrow, and offset of MMS treatment is indicated by the upward arrow. (B) UV treatment (20 J/m) at 12,16, and 20 h after synchronization also causes an acute decrease in PER2::LUC levels. The black line indicates the treatment control and the gray line indicates either MMS (A) or UV (B) treatment. Raw data are replotted with no smoothing or background subtractions so that acute responses can be reported in real time.
DNA Damage Yields an Atypical PRC in PER2::LUC MEF Cells
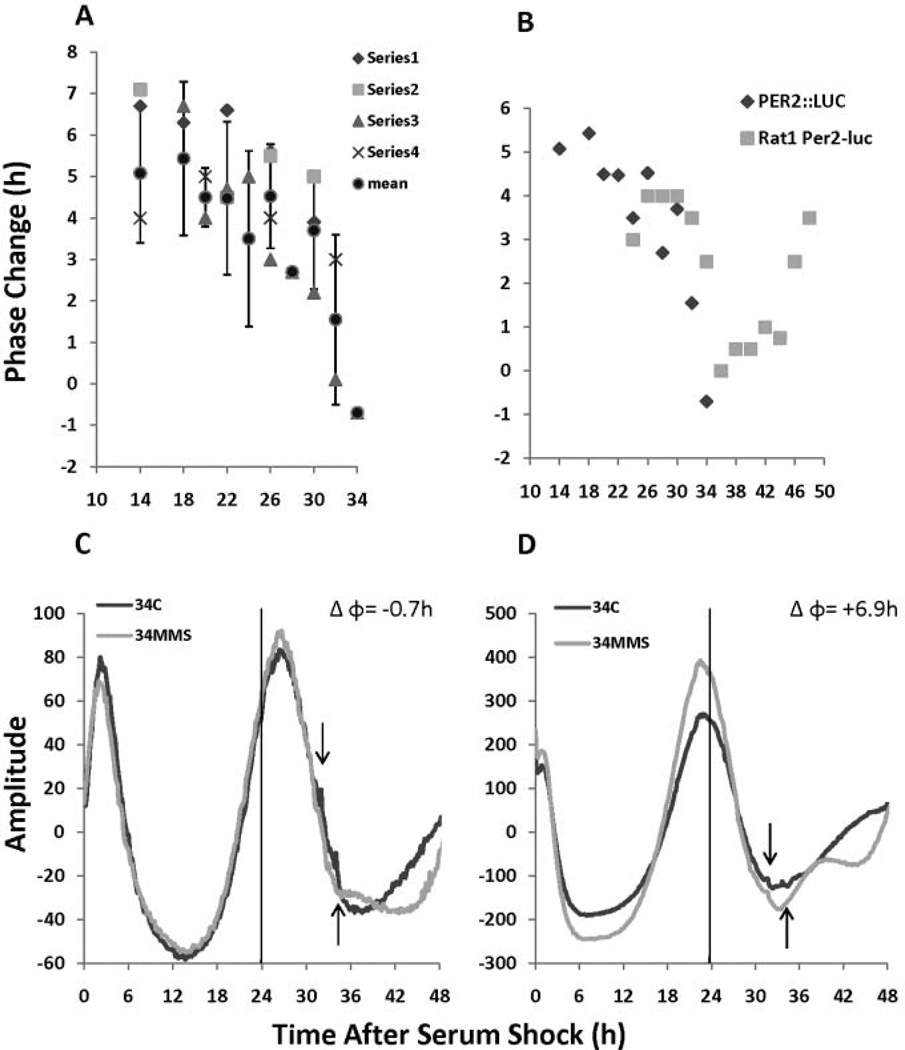
Treatment with 1 mM MMS at various time points spanning most of the first cycle of PER2 expression yields maximum phase advances when PER2 is initially expressed as demonstrated in a representative PRC (Fig. 3A), consistent with DNA damage-mediated PRC data in Rat-1 fibroblasts (Oklejewicz et al., 2008; Fig. 3B). The timing of the decreased sensitivity to DNA damage is very similar from 24 to 30 hours after synchronization, but sensitivity to DNA damage in PER2::LUC cells decreases more rapidly (by about 2 h) at 32 and 34 h in MEFs compared with the RAT-1 cells. This minor inconsistency may be due to a number of different variables such as methods of synchronization, methods of DNA damage, or in the differing cell lines used to follow the clock.
Figure 3.
DNA damage-induced phase-response curves (PRCs) in PER2::LUC mouse embryonic fibroblast (MEF) cells. (A) A representative PRC in which the phase shift is plotted as a function of when methyl methane sulfonate (MMS) treatment was given; the diamond, square, triangle, and × are the Δφ for each individual experiment, and the circle is the mean ± SD. The mean Δφ and standard deviation are as follows: 14: 5.8 ± 1.6 h, 18: 5.4 ± 1.9 h, 20:4.5 ± 0.7 h, 22: 4.48 ± 1.8 h, 24: 3.5 ± 2.1 h, 26: 4.5 ± 1.25 h, 28: 2.8 h ± N/A, 30: 3.7 ± 1.4 h, 32:1.55 ± 2.05 h, and 34: −0.7 ± N/A. MMS treatment at various points over the first cycle of PER2::LUC expression yields maximum phase advances at phases when PER2::LUC levels are increasing (see text for details). (B) Comparison of published DNA damage-induced PRC data from RAT-1 cells (gray boxes, replotted from Oklejewicz et al., 2008) with PER2::LUC MEF cells (black diamonds). The data points shown represent the mean values from each PRC experiment for each individual time point. (C and D) DNA damage 34 h after serum shock results in a variable phase response, where the magnitude of the phase change is dependent on the phase of PER2 expression. In panel C, PER2 levels are declining during DNA damage, which results in a Δφ of −0.7 h, whereas DNA damage during the beginning of the next cycle of PER2 results in a Δφ of +6.9 h. Raw data with no smoothing or background subtraction are shown.
One property of the PER2::LUC MEF cell line we observed was variability of the timing of the first peak of PER2, where the peak expression of PER2 during the first cycle occurred between 22 and 26 h after synchronization (Fig. 3C and D). This variability, in some cases, resulted in MMS causing large advances when slight advances were anticipated. For instance, in the separate experiments depicted in Figure 3C and D, MMS was given between 32 and 34 h after serum shock in both cases. Typically this is a time when PER2 levels are still declining (as shown in Fig. 3C), but in the experiment represented in Figure 3D, PER2 expression happened earlier after clock synchronization. As a consequence, MMS treatment occurred during the beginning of the 2nd cycle of PER2 resulting in a large phase advance. This “exception to the rule” further supports the observation that maximal advances occur during de novo PER2 synthesis.
DNA Damage Leads to Phase Advances in NIH3T3 cells and Induces BMAL1 promoter Activity
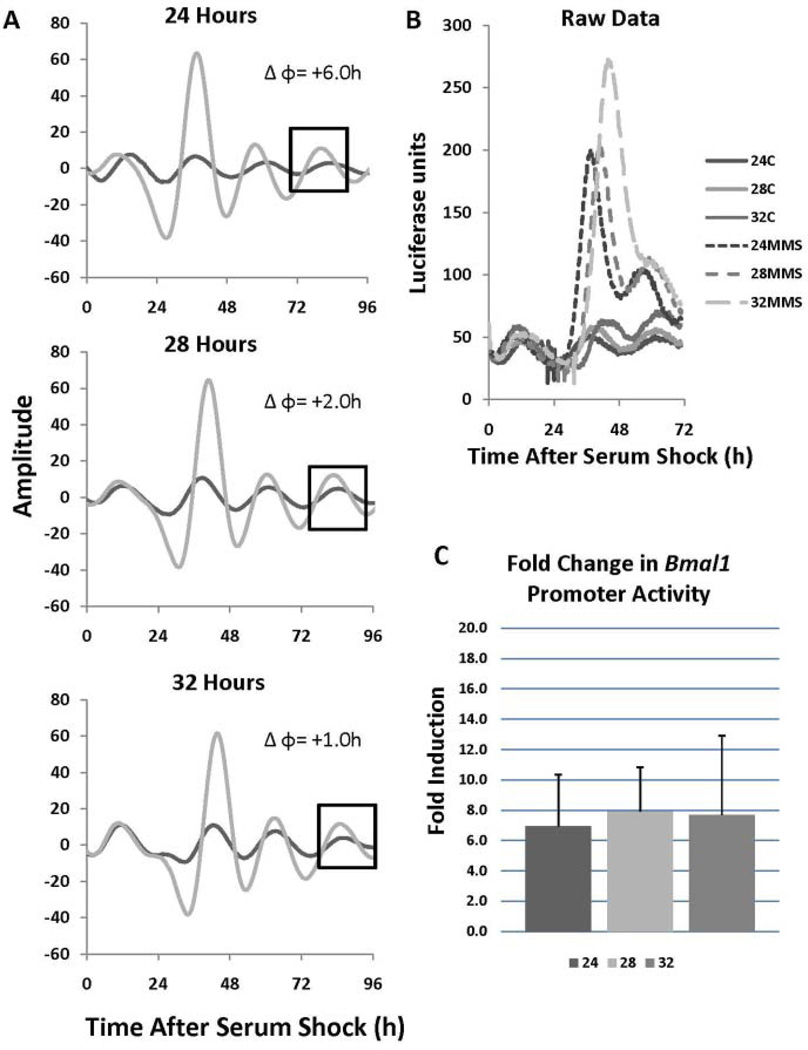
For purposes of comparison and to more fully understand the molecular basis of the resetting response, MMS treatments were carried out at various phases in synchronized NIH3T3 cells transiently transfected with a mouse BMAL1 promoter construct driving luciferase expression (Sato et al., 2006). As with the PER2::LUC cells, only phase advances were observed with the largest occurring at 24 h after serum shock, and diminishing at 28 and 32 h after serum shock when PER2 levels decrease (Fig. 4A). Although a full PRC was not generated, these data are consistent with what was observed in the PER2::LUC MEF line. In addition to phase advances, we consistently observed a substantial enhancement of BMAL1 promoter activity to levels much higher than that observed in the control treated cells, and this increase occurred irrespective of when the pulse was given (Fig. 4B and C). These data are consistent with previous findings in mice that showed that BMAL1 is a late responder to IR-mediated DNA damage (Fu et al., 2002).
Figure 4.
DNA damage induces BMAL1 promoter activity. (A) NIH3T3 cells were transiently transfected with a mouse BMAL1 promoter construct driving luciferase expression, synchronized via serum shock, treated with 1 mM methyl methane sulfonate (MMS) for 2 h, and released at 24, 28, and 32 h after synchronization. The data were baseline subtracted and smoothed. The black box highlights the cycle where the phase change was calculated. The dark line indicates a treatment control and the gray line indicates MMS treatment. (B) Raw data traces from panel A illustrates MMS treatment induces BMAL1 promoter activity to significantly higher levels than the treatment controls. Induction of BMAL1 promoter activity occurred at all time points tested. (C) Fold induction from panel B, which was calculated from the first peak after MMS treatment; n = 4.
DISCUSSION
Our initial surprise at the unexpected observation of an all-advance PRC to MMS in Neurospora (Pregueiro et al., 2006) has now given way to a relative sense of inevitability as additional all-advance PRCs to DNA damage in different systems have emerged or been rediscovered. It is noteworthy that all the full PRCs available (Gonyaulax: Sweeney, 1963; Neurospora: Pregueiro et al, 2006; Rat-1 fibroblasts: Oklejewicz et al, 2008; and, finally, PER2::LUC MEFs and NIH3T3 cells: this study) report only advances, and in the systems where the molecular underpinnings of the rhythms are somewhat understood (Neurospora and mammalian cells), the phasing is mostly consistent with larger advances seen when negative elements in the loop are being synthesized. Dose- and phasedependent phase shifting with UV light has also been reported for Paramecium (Ehret, 1960); delays were reported there, but treatments were reported from only 1 phase and the rhythm was followed only for 2 cycles, so it may be that, as seen here in Figures 1 and 2, initial delays could have resolved into advances had the experiment been continued. The phase of maximum sensitivity reported for Gonyaulax is different from that seen in Neurospora or the mammalian cell lines, but it is hard to assign significance to this absent more information as to the phases at which clock-critical proteins are made in that system.
Experimental evidence has shown that PER1 interacts with Chk2 and ATM (Gery et al., 2006) and that it is phosphorylated during DNA damage (Matsuoka et al., 2007). DNA damage with MMS or UV irradiation causes a decrease in luciferase signal in PER2::LUC MEF cells, which is indicative of PER2 degradation, and we observe the greatest advances during the phase of the clock when PER2 levels are increasing. Consistent with our findings, IR-mediated DNA damage in whole mice induced a phase advance of behavioral rhythms that were much stronger at CT6 compared with IR irradiation at CT22 (Oklejewicz et al., 2008). CT6 corresponds to the time of day when PERI and PER2 are initially expressed in the SCN, compared with CT22 when PER1 and PER2 levels are decreasing (Godinho et al., 2007). That this response is maintained in the SCN, which is less likely than peripheral tissues to be exposed to DNA damaging agents, reinforces the notion that this is a core property of the clock.
That PER2 appears to be degraded was of interest given that the molecular connection between the DDR and the clock in mammals has only been demonstrated for PER1. The mammalian oscillator contains many negative arm components (PER1, PER2, CRY1, and CRY2), and it may be that degradation of several of these components, for instance PER1 and PER2, is needed for proper resetting of the clock during DNA damage. In addition to the PER1/ATM interaction previously referenced, inhibition of ATM activity via an ATM specific inhibitor and caffeine leads to amelioration of DNA damage-induced phase advances in Rat-1 fibroblasts (Oklejewicz et al., 2008), which reinforces the importance of this pathway in the clock’s response to DNA damage. It stands to reason that the ATM/Chk2 pathway is most likely responsible for the degradation of PER2, but it is presently unclear exactly if or how this pathway is involved.
The magnitude of the reduction in luciferase signal increases as PER2 matures, which is a bit surprising because one might expect the greatest effect on PER2 to transpire early on when the largest phase advances occur. A simple explanation for this is that there is simply less PER2 at this time, and therefore little degradation would be expected. Another possibility is that as PER2 matures and becomes progressively phosphorylated it is more sensitive to degradation, as is the case for FRQ in Neurospora (Pregueiro et al., 2006). Therefore, PER2 expressed early in the cycle, for example, at 12 or 14 h after serum shock, might be more resistant to degradation because it is more sparsely phosphorylated and thus less primed for degradation, as opposed to later in the cycle when PER2 is more highly phosphorylated.
Finally, we show that BMAL1 promoter activity is induced after DNA damage independently of when the damage occurs. It has been shown in Rat-1 cells that there is no induction of BMAL1 transcription up to 4 h after DNA damage (Oklejewicz et al., 2008) and that BMAL1 is a late responder to DNA damage (Fu et al., 2002). Our data are in agreement with both studies inasmuch as we do not see induction of the BMAL1 promoter to levels higher than the treatment control until 7 to 8 h after DNA damage.
In addition to a nonfunctional circadian oscillator, BMAL1 knockout mice present many physiological abnormalities, such as premature aging (Kondratov et al., 2006). This suggests a clear role for this gene in processes other than control of the molecular clock. It has been shown that WEE1 kinase is clock controlled and transactivated directly by BMAL1 (Matsuo et al., 2003). WEE1 controls the timing of mitosis entry through the phosphorylation of cyclin-dependent kinases (Kellogg, 2003). DNA damage transiently induces WEE1 in yeast and leads to cell cycle arrest at the G2 phase (Raleigh and O’Connell, 2000). Furthermore, recent work has shown that the cell cycle inhibitor protein p21 is a clock-controlled gene that is regulated by the positive arm proteins ROR and Rev-erb, which also control the expression of BMAL1 (Grechez-Cassiau et al., 2008).
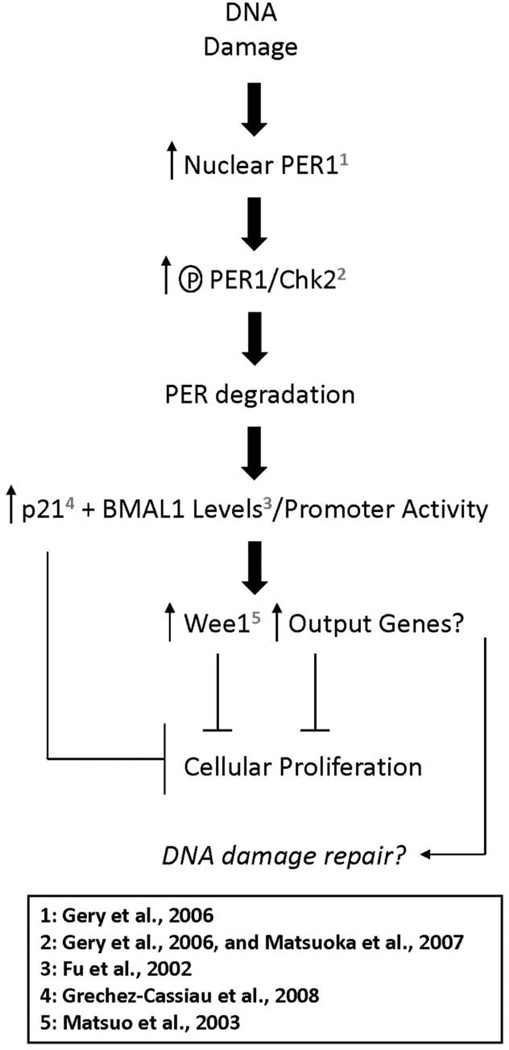
Figure 5 presents a flow diagram that summarizes our results, places them in context with related studies, and offers a plausible molecular explanation for the DNA damage-related clock response. IR-mediated DNA damage causes increased PER1 nuclear localization (Gery et al., 2006). PER1 phosphorylation is then increased through direct association with ATM/CHK2 (Gery et al., 2006, and Matsuoka et al., 2007). It is presently unknown whether PER2 or any of the other negative arm proteins (the CRYs, for instance) behave in a similar manner. Phosphorylation of PERs then potentially leads to their degradation. Our data suggest a reduction in PER2 levels; however, the status of other negative arm proteins, such as PER1, under these conditions is presently unknown. Loss of these PER proteins potentially causes the increased BMAL1 promoter activity we observed and consequently an increase in the levels of BMAL1 mRNAas was previously observed in IR-treated mice (Fu et al., 2002). Increased BMAL1 could potentially coincide with p21 induction as they are both controlled by RORs and Rev-erbs (Grechez-Cassiau et al., 2008). Additionally, an increase in BMAL1 may lead to an increase in WEE1 levels, inasmuch as this kinase has been shown to be a clock-controlled gene directly activated by BMAL1 (Matsuo et al., 2003), or in other output genes involved in the cessation of cellular proliferation or DNA damage repair.
Figure 5.
Processes underlying the DNA damage-related clock response. Points and processes addressed and proteins examined in previous studies are numbered as marked. See the text for a more detailed discussion.
These data support past findings that the components of the core molecular clock may play a role in DNA damage responses and offer new insight as to how this occurs in the mammalian system. However, the biological relevance of the DNA damage-mediated resetting of the clock remains unclear. Further study is needed to determine the true significance of the conserved clock response to DNA damage and our findings provide clues toward this endeavor.
ACKNOWLEDGMENTS
The authors thank John Hogenesch, Julie Baggs, and all of the other members of the Hogenesch lab for the pBMAL1-dLuc construct and for their help in launching our real-time bioluminescence experiments. Thanks to Carl H. Johnson and his lab for the useful discussion with regard to the work presented here. We also thank Giles Duffield and Nathan Watson for their help with the generation of the PER2::LUC MEF cell line. Finally, we thank Eric DuFour for his help in maintaining our mouse colony. This work was supported by the National Institutes of Health grant GM083336 (to J.J. L. and J.C.D.) and grant GM034985 (to J.C.D). We also acknowledge the support of the Norris Cotton Cancer Center at Dartmouth Medical School.
REFERENCES
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26:7773–7779. doi: 10.1038/sj.onc.1210881. [DOI] [PubMed] [Google Scholar]
- Chen-Goodspeed M, Lee CC. Tumor suppression and circadian function. J Biol Rhythms. 2007;22:291–298. doi: 10.1177/0748730407303387. [DOI] [PubMed] [Google Scholar]
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Ehret CF. Action spectra and nucleic acid metabolism in circadian rhythms at the cellular level. Cold Spring Harb Symp Quant Biol. 1960;25:149–158. doi: 10.1101/sqb.1960.025.01.014. [DOI] [PubMed] [Google Scholar]
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- Gery S, Koeffler HP. The role of circadian regulation in cancer. Cold Spring Harb Symp Quant Biol. 2007;72:459–464. doi: 10.1101/sqb.2007.72.004. [DOI] [PubMed] [Google Scholar]
- Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22:375–382. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Godinho SI, Maywood ES, Shaw L, Tucci V, Barnard AR, Busino L, Pagano M, Kendall R, Quwailid MM, Romero MR, et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316:897–900. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- Grechez-Cassiau A, Rayet B, Guillaumond F, Teboul M, Delaunay F. The circadian clock component BMAL1 is a critical regulator of p21WAF1/CIP1 expression and hepatocyte proliferation. J Biol Chem. 2008;283:4535–4542. doi: 10.1074/jbc.M705576200. [DOI] [PubMed] [Google Scholar]
- Izumo M, Sato TR, Straume M, Johnson CH. Quantitative analyses of circadian gene expression in mammalian cell cultures. PLoS Comput Biol. 2006;2:e136. doi: 10.1371/journal.pcbi.0020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DR. Weel-dependent mechanisms required for coordination of cell growth and cell division. J Cell Sci. 2003;116:4883–4890. doi: 10.1242/jcs.00908. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Kondratov RV, Antoch MP. Circadian proteins in the regulation of cell cycle and genotoxic stress responses. Trends Cell Biol. 2007;17:311–317. doi: 10.1016/j.tcb.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo ML, Chou YW, Chau YP, Huang TS. Resistance to apoptosis induced by alkylating agents in v-Ha-ras-transformed cells due to defect in p53 function. Mol Carcinog. 1997;18:221–231. [PubMed] [Google Scholar]
- Lee MH, Lee SW, Lee EJ, Choi SJ, Chung SS, Lee JI, Cho JM, Seol JH, Baek SH, Kim KI, et al. SUMO-specific protease SUSP4 positively regulates p53 by promoting Mdm2 self-ubiquitination. Nat Cell Biol. 2006;8:1424–1431. doi: 10.1038/ncb1512. [DOI] [PubMed] [Google Scholar]
- Lukas J, Lukas C, Bartek J. Mammalian cell cycle checkpoints: Signalling pathways and their organization in space and time. DNA Repair (Amst) 2004;3:997–1007. doi: 10.1016/j.dnarep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–259. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- Oklejewicz M, Destici E, Tamanini F, Hut RA, Janssens R, van der Horst GT. Phase resetting of the mammalian circadian clock by DNA damage. Curr Biol. 2008;18:286–291. doi: 10.1016/j.cub.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Pregueiro AM, Liu Q, Baker CL, Dunlap JC, Loros JJ. The Neurospora checkpoint kinase 2: A regulatory link between the circadian and cell cycles. Science. 2006;313:644–649. doi: 10.1126/science.1121716. [DOI] [PubMed] [Google Scholar]
- Raleigh JM, O’Connell MJ. The G(2) DNA damage checkpoint targets both Weel and Cdc25. J Cell Sci 113. 2000;(Pt 10):1727–1736. doi: 10.1242/jcs.113.10.1727. [DOI] [PubMed] [Google Scholar]
- Sanchez-Prieto R, Rojas JM, Taya Y, Gutkind JS. A role for the p38 mitogen-acitvated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res. 2000;60:2464–2472. [PubMed] [Google Scholar]
- Sato TK, Yamada RG, Ukai H, Baggs JE, Miraglia LJ, Kobayashi TJ, Welsh DK, Kay SA, Ueda HR, Hogenesch JB. Feedback repression is required for mammalian circadian clock function. Nat Genet. 2006;38:312–319. doi: 10.1038/ng1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney BM. Resetting the biological clock in Gonyaulax with ultraviolet light. Plant Physiol. 1963;38:704–708. doi: 10.1104/pp.38.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MH, Yung BY. UV stimulation of nucleophosmin/B23 expression is an immediate-early gene response induced by damaged DNA. J Biol Chem. 2002;277:48234–48240. doi: 10.1074/jbc.M206550200. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]