Abstract
OBJECTIVES
Pancreatic adenocarcinoma is a lethal disease. Over 80% of patients are found to have metastatic disease at the time of diagnosis. Strategies to improve disease-specific outcome include identification and early detection of precursor lesions or early cancers in high risk groups. In this study we investigate whether screening at-risk relatives of familial pancreatic cancer patients is safe and has significant yield.
METHODS
We enrolled 309 asymptomatic at-risk relatives into our Familial Pancreatic Tumor Registry (FPTR) and offered them screening with MRCP followed by endoscopic ultrasound with fine needle aspiration if indicated. Relatives with findings were referred for surgical evaluation.
RESULTS
As of August 1, 2009, 109 relatives had completed at least one cycle of screening. Abnormal radiographic findings were present on initial screening in 18/109 patients (16.5%), 15 of whom underwent EUS. A significant abnormality was confirmed in 9 of 15 patients, 6 of whom ultimately had surgery for an overall diagnostic yield of 8.3% (9/109). Yield was greatest in relatives >65 years old (35% (6/17) when compared with relatives 55–65 (3% (1/31) and relatives<55 (3% (2/61).
CONCLUSIONS
Screening at-risk relatives from familial pancreatic cancer families has a significant diagnostic yield, particularly in relatives >65 years of age, confirming prior studies. MRCP as initial screening modality is safe and effective.
INTRODUCTION
Pancreatic adenocarcinoma (pancreatic cancer) is a lethal disease with 42,470 new cases and 35,240 deaths projected in the United States for 2009.1 Modern surgical management and better case selection have improved short term survival (6 and 12 month) after resection2, but the overall 5-year survival rate for all stages remains around 5.0%.3 Such poor outcomes result, in part, from the difficulty of diagnosing this disease at an early stage. In those few patients diagnosed with small (<2 cm) cancers, the 5-year survival can approach 60%.4 Identifying patients who have early, small, localized tumors at presentation, and developing a greater understanding of susceptibility and inherited risk could improve this poor overall survival rate.
Multiple risk factors for the development of pancreatic cancer have been identified, including advancing age, male gender5, tobacco use6, obesity7, a family history of pancreatic cancer8,9, certain other familial cancer syndromes10,11,12, and chronic pancreatitis13–14, both hereditary15 and acquired.
Up to 10% of pancreatic cancers in the United States are thought to have a familial origin. 16 Familial Pancreatic Cancer (FPC) is defined as pancreatic cancer which occurs in families in which there are multiple first and second degree relatives with pancreatic cancer and no known cancer syndrome. A prospective registry-based study estimated the risk of developing pancreatic cancer in healthy members of familial pancreatic cancer families to be as high as 32 fold when three first degree relatives in the family were affected with the disease.17 This added risk of PC in unaffected relatives of patients with PC in FPC families may make screening this subgroup worthwhile, perhaps improving survival by identifying premalignant precursor lesions or early stage pancreatic cancers.
Several pancreatic neoplasms have been proposed as precursor lesions in the spectrum of development of adenocarcinoma of the pancreas.18–19–20 These include intraductal papillary mucinous neoplasms (IPMNs),21 and microscopic pancreatic intraepithelial neoplasia (PanINs). IPMNs and PanINs probably develop into invasive pancreatic cancers via different pathways22. In contrast to PanINs, IPMNs can be diagnosed by cross-sectional imaging studies and endoscopic ultrasound (EUS); these modalities can thus be employed for screening.
Previous studies have evaluated the feasibility and utility of screening for pancreatic cancer in high risk relatives from FPC families using a combination of CT, EUS and endoscopic retrograde cholangiopancreaticogram (ERCP). 23–24 The yield of significant findings was in the range of 5–10%. IPMNs have been identified in these high risk screened groups.
We prospectively studied asymptomatic at-risk relatives from a prospective screening of FPC families to determine the diagnostic yield of screening for premalignant pancreatic lesions and early stage pancreatic cancer.
METHODS
Patients
In December 2002 the Familial Pancreatic Tumor Registry (FPTR) at Memorial-Sloan Kettering Cancer Center (MSKCC) was established for patients with FPC and their at-risk relatives. This registry and the screening component reported in this study have both been reviewed by the Institutional Review Board of MSKCC. Criteria for entry into the FPTR as an at-risk relative include having: one or more FDR with pancreatic cancer before age 50, two or more relatives with pancreatic cancer (one of whom is a FDR), three or more SDR with pancreatic cancer, or a known BRCA mutation with one or more relatives with pancreatic cancer. Whenever possible, the diagnosis of pancreatic cancer in affected relatives is verified through pathology reports.
The screening component of our program is offered to relatives aged 35 and over. All at-risk family members who are found to meet eligibility criteria are approached by a single experienced study assistant who reviews the risks and benefits of participation, and explains in detail the requirements for screening. If an individual agrees to participate in the registry, a detailed family history and epidemiology questionnaire is administered in person by the same study assistant. Genetic counseling is offered to all eligible family members at study entry but completion of testing is not a requirement.
The screening process involves an office visit, physical examination, and magnetic resonance cholangiopancreaticogram (MRCP) every year; computed tomography (CT) scan is an acceptable substitute if subjects are unwilling to undergo MRCP. MRCP imaging at MSKCC is performed with a l.5-T superconducting magnet (General Electric Medical Systems, Milwaukee, WI) and a torso phased array coil. Breath-hold MRCP images are obtained using a single-shot fast spin-echo sequence. This effective TE was chosen to allow depiction of both pancreaticobiliary ducts and periductal tissues. Images are obtained as contiguous 4-mm-thick slices in both axial and coronal planes, with a field of view of 26–40 cm. Field of view and numbers of slices are tailored to each patient using the minimum required to adequately image the pancreaticobiliary tree.
All studies included in this analysis were either conducted at MSKCC or images were reviewed by MSKCC physicians. MRCP was selected as initial screening modality because it is not invasive, has no associated radiation risks, and has an extremely low complication rate.25
Results of cross-sectional imaging are reviewed by a multidisciplinary team including hepatopancreatobiliary surgeons, gastroenterologists, interventional radiologists, medical and radiation oncologists, and radiologists specialized in cross-sectional imaging. After review, patients with lesions of concern (e.g. cystic lesions with a solid or nodular component, pancreatic duct dilation, pancreatitis or other lesions deemed concerning by the multidisciplinary group) are further evaluated by endoscopic ultrasound (EUS) with fine needle aspiration (FNA) for cytologic evaluation and cyst fluid CEA as indicated.
EUS examinations are performed for patients found to have pancreas abnormalities on cross sectional imaging by MRCP. All patients undergo standard video UGI endoscopy with attention to the ampulla of Vater using propofol sedation under the direction of an anesthesia team. EUS is performed by endoscopists experienced in endosonography using the GF-UM130, or GF-UE160 Olympus radial echoendoscopes and/or the GF-UC140P curvilinear array echoendoscope with switchable frequencies from 5 and 12-MHz (Olympus America Inc., Lehigh Valley, PA), according to the preference of the physician.
The pancreas is imaged from the duodenum and stomach carefully scanning through the entire pancreas from the head to the tail, to identify parenchymal or ductal changes. Video and still images are obtained and interpreted at the time of the procedure looking for specific abnormalities of the pancreas such as solid or cystic mass lesions, focal cystic dilation, ductal thickening (pancreatic duct>5 mm) or lymphadenopathy. If a significant lesion is identified, immediate fine needle aspiration (FNA) is performed using a 25 or 22 gauge needle, in the presence of a cytopathologist or cytotechnician to confirm adequacy of the specimen.
Following the procedure, patients recover in a surgical day hospital and are discharged home the same day. A follow-up telephone call is made to the patient by a registered nurse the day after the procedure to document post-procedure clinical status and identify any procedure related complications.
If the appearance of a lesion on EUS, cytology and/or carcinoembryonic antigen (CEA) levels is suspicious for a pre-malignant or malignant pancreatic lesion, surgical consultation is obtained. Surgical intervention is based on multidisciplinary review with the final decision resting with the surgeon and patient.
Statistical analysis
Descriptive statistics of the relatives who enrolled in the Familial Pancreatic Tumor Registry were compiled. Variables included gender, age, total number of relatives with pancreatic cancer, smoking history (never, past, and current, with those who smoked in the year before enrollment considered current smokers), body mass index (BMI) at the usual adult weight, race, and religion.
Our question on the age of diagnosis of the relatives with pancreatic cancer allowed for responses in 10-year age categories, up to age >=70. If a relative had pancreatic cancer and another cancer, we obtained the age at diagnosis of the first cancer, which was not necessarily the pancreatic cancer. To calculate the average age at diagnosis of the youngest relative, we selected the appropriate relative and assigned the midpoint of the age range in the questionnaire, e.g. we used 44.5 for those diagnosed between 40–49, 54.5 for those diagnosed between 50–59, etc, and 77 for those diagnosed at age >=70.
Thus, the data on mean age of the youngest affected relative is an approximation. Information was missing or could not be classified (because of multiple cancers in relatives) for 16 of the 309 relatives enrolled including 3 of the 109 who underwent surveillance. To compare the age of our study participants with that of their youngest affected relative, we classified our subjects as to whether they were younger, in the same 10-year age range, or older than the youngest affected relative.
RESULTS
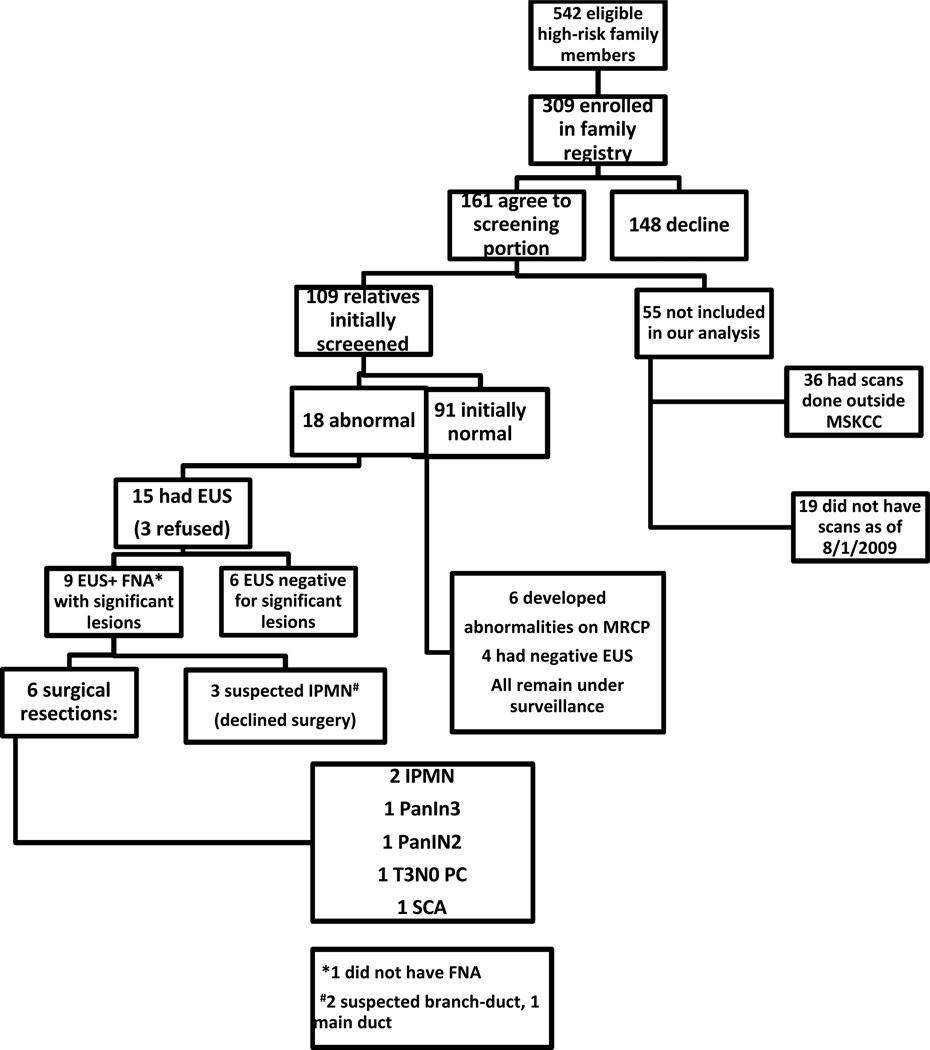
Between December 2002 and August 2009, a total of 542 eligible asymptomatic FPC family members were offered entry into our FPC registry and 309 (57%) accepted. Of these 309, 161 (52.1%) agreed to be screened, and through August 1, 2009, 109 have completed at least one cycle of screening at MSKCC (range 1–7).Demographics are shown in Table 1.
Table 1.
Demographic characteristics of at-risk FPC relatives in the MSKCC Registry and of those screened
| Total at-risk relatives | Total relatives screened | |
|---|---|---|
| Number of relatives | 309 | 109 |
| Female | 66% | 72% |
| Mean age (SD) | 50 (12.7) | 54 (11.4) |
| Age Range | 22–86 | 33–86 |
| Caucasian* | 93% | 92% |
| Graduate Degree* | 53% | 61% |
| Religion* | ||
| Catholic | 40% | 39% |
| Jewish | 26% | 33% |
| Protestant | 21% | 21% |
| Other/None | 12% | 6% |
| Smoking* | ||
| Never | 60% | 58% |
| Past | 31% | 34% |
| Current | 10% | 8% |
| Body Mass Index* | ||
| Normal | 58% | 64% |
| Overweight | 28% | 22% |
| Obese | 14% | 14% |
excludes 5 unscreened relatives who did not complete the questionnaire
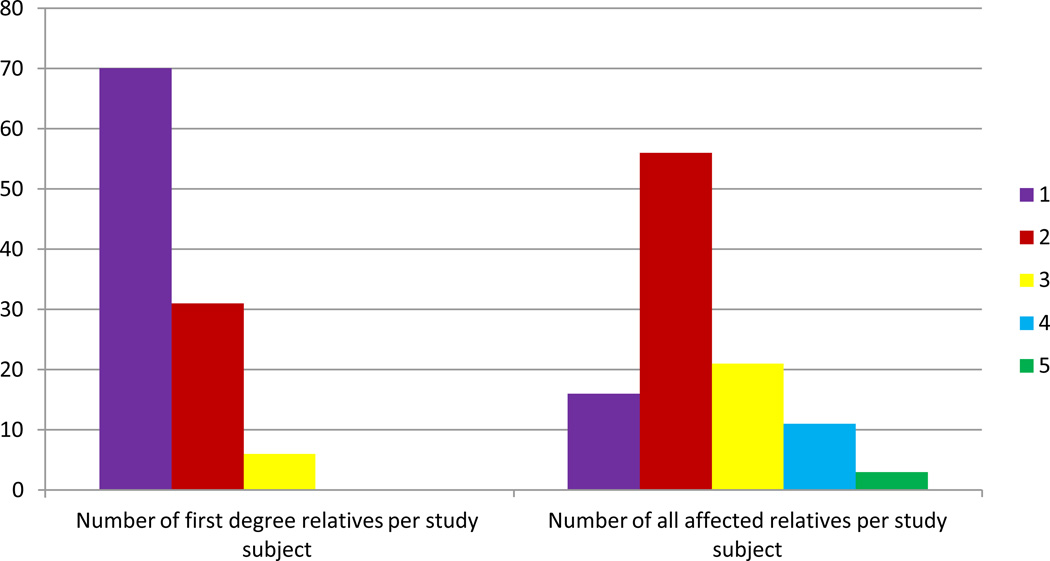
98/109 (90.0%) of initial cross-sectional imaging studies performed were MRCPs. The screened population of 109 included 78 women (mean age 54) and 31 men (mean age 53). Among the 109 screened patients, 16 (14.7%) had one affected FDR. Among these 16 relatives, nine were included because they had one affected relative diagnosed at <50 years of age. The seven other patients were included because of a known BRCA mutation and a family history of pancreatic cancer. 56//109(51.4%) had two affected relatives, 21 (19.8%) had three, 11 (10.3%) had four, and 2.8 (3%) had five affected relatives. The distribution of affected family members is seen in Figure 1.
Figure 1.
Of the 52 people without screening data, 36 had their scans performed at outside facilities and were therefore excluded from our analysis. The remaining 16 did not have scans done as of the cut-off date for the paper's analysis, 8/1/09. Of those 19, seven have had scans since 8/1/09 and 1 has a scan scheduled in the near future. Nine relatives have had blood work only (three who refused scans, four lost to follow-up, two still pending scheduling a scan). In addition, one was lost to follow-up, one was still pending scheduling a scan and one relative has subsequently declined screening. The 55 relatives not included are not demographically different from the 109 screened (same distribution of gender and age.)
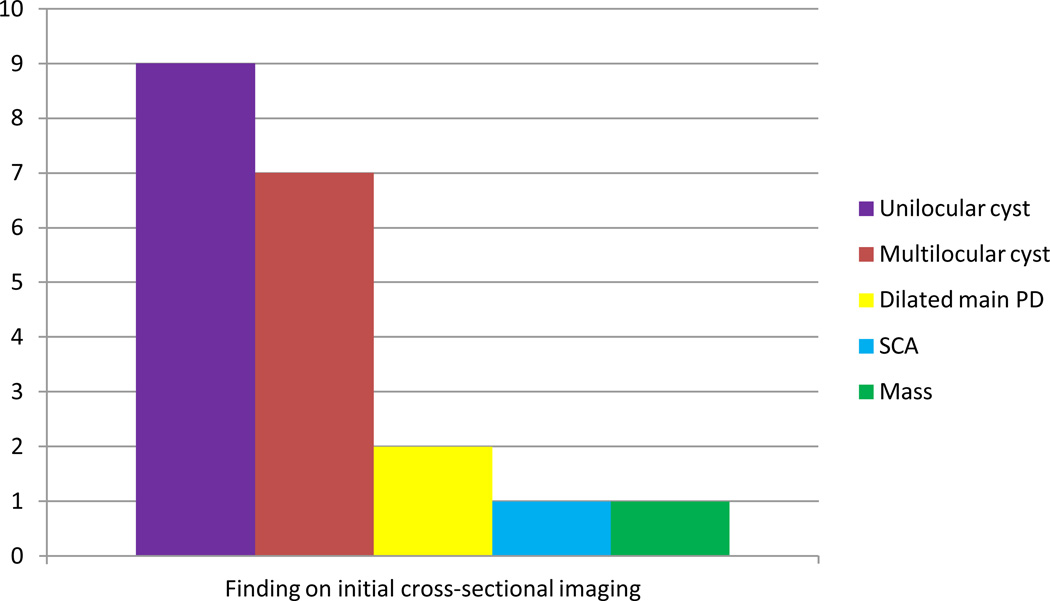
Screening results are summarized in Figure 2. Of the 109 participants who completed at least one cycle of screening as of August 1, 2009, 18 (16.5%) had a prevalent abnormal finding on initial cross sectional imaging (Figure 3). Of these 18 relatives, 15 (83.3%) underwent EUS. Of the 15 patients who underwent EUS, nine had significant findings; eight of these patients underwent FNA. The remaining six relatives underwent EUS and had lesions deemed insignificant; all continue with surveillance (Tables 2a and b).
Figure 2.
Figure 3.
Table 2.
| a. Significant prevalent lesions: findings on EUS/FNA and at surgery | ||||||
|---|---|---|---|---|---|---|
| Patient ID# | Age/ Sex |
Family history |
Findings on EUS/FNA |
Cytology/CEA | Surgical intervention |
Surgical Pathology |
| 1/23 | 67/F | 1 FDR 1SDR |
2 cystic lesions in head and body of pancreas; Mild chronic pancreatitis | Suspicious cells. Suspect MCN |
Pancreaticoduodenectomy | Multifocal IPMN of borderline malignant potential, predominately involving side branches |
| 2/325 | 86/F | 2 FDR | 3 cm cystic lesion body of pancreas | CEA 1765 Focal extracellular mucin | Distal pancreatectomy | IPMN with moderate dysplasia in background of chronic pancreatitis and PanIN2 |
| 3/645 | 58/F | 2 FDR | 2 cm mass lesion body of pancreas | Neoplastic cells represent MCN vs well-diff adenoca | Distal pancreatectomy | Mod differentiated ductal adenoca, 2.5 cm extending to peripancreatic soft tissue, +vasc and perineural invasion; nonneoplastic pancreas focal chronic pancreatitis; 0/8 + LNs: T3N0 |
| 4/776 | 55/F | 1 FDR 1 SDR BRCA+ |
1 cm cystic lesion prox body; dilated side branch of PD | Cytology negative for malignant cells | Patient declined | |
| 5/1063 | 46/F | 1 FDR 1 SDR |
1 cm septated cyst possible connection to branch PD | Mucin and glandular cells without atypia | Distal pancreatectomy | PanIN2, focal fibrosis, chronic inflammation |
| 6/1380 | 83/F | 2 FDR | 3 cm cystic lesion HOP w few thick septations; 4 cm cystic pancreatic tail lesion; PD dilation | Non diagnostic | Patient declined | |
| 7/1476 | 68/M | 1 FDR 3 SDR 1 TDR |
PD dilation 6.1 mm head, 3.6 mm in body, 3.0 mm tail | Not done | Pancreaticoduodenectomy | PanIN3, chronic pancreatitis |
| 8/1494 | 73/M | 1 FDR 1 SDR |
1 cm single cystic body lesion without septae; 1 cm single cystic head lesion; No PD duct dil | Acellular specimens; non-diagnostic | Distal pancreatectomy | Serous cystadenoma w adjacent retention cysts, PanIN-1B |
| 9/1634 | 68/F | 1 FDR 1 TDR |
1 cm hypoechoic, multicystic lesion in head; possible comm. w branch of PD | Cytology negative for malignant cells | Patient declined | |
| Patient ID# | Age/ Sex |
Family history |
Findings on EUS+/− FNA |
Cytology/CEA |
|---|---|---|---|---|
| 1/230 | 48/M | 1 FDR<50 | Normal pancreas | n/a |
| 2/241 | 56/F | 2 FDR | Normal pancreas | n/a |
| 3/724 | 73/F | 2 FDR | 1 cm simple unilocular round cyst uncinate with no internal structures | Clear thick liquid. Cytology benign. No CEA sent |
| 4/748 | 53F | 2 FDR | 5–7 mm cyst body of pancreas no communication with PD | Clear thin liquid. Cytology sparsely cellular, negative for malignancy. |
| 5/1056 | 59/F | 1 FDR 1 SDR |
3 cm microcystic mass pancreatic neck, sl dilated PD | FNA unsatisfactory specimen |
| 6/1301 | 52F | 2 FDR | 8×4mm cystic lesion; no intramural nodule or mass | Clear yellow fluid Cytology negative for malignant cells No CEA sent |
After full evaluation of imaging and EUS/FNA results by our multidisciplinary team, surgical intervention was recommended for nine patients. Six have undergone surgical resection (two pancreatoduodenectomies, four distal pancreatectomies). Pathology revealed two main duct IPMNs, one PanIN 3, one PanIN 2, one T3N0 pancreatic adenocarcinoma, and one serous cystadenoma with PanIN 1 in the specimen. Three patients had IPMN-like lesions on imaging (2/3 with only side branch changes) and EUS (FNA negative/non-diagnostic in all cases); these three patients refused surgery and remain under surveillance (Figure 2 and Table 2a). The diagnostic yield of screening was 8.3% (9/109) and 5.5% (6/109) when only surgically confirmed cases were included.
Ages of the individuals with significant findings are shown in Table 3. The range was 46 to 86 and the median age was 68. The yield from screening was highly dependent on the age of the screened relative, with those >=65 having a significantly higher yield than those under 65. There was no difference according to the age of the youngest relative with pancreatic cancer (data not shown).
Table 3.
Presence of significant lesions according to age at entry into surveillance study
| Age at entry into surveillance study | ||||||
|---|---|---|---|---|---|---|
| <55 (n=61) | 55–64 (n=31) | >=65 (n=17) | ||||
| Significant prevalent lesion | # | % | # | % | # | % |
| Yes | 2 | 3 | 1 | 3 | 6 | 35 |
| No | 59 | 97 | 30 | 97 | 11 | 65 |
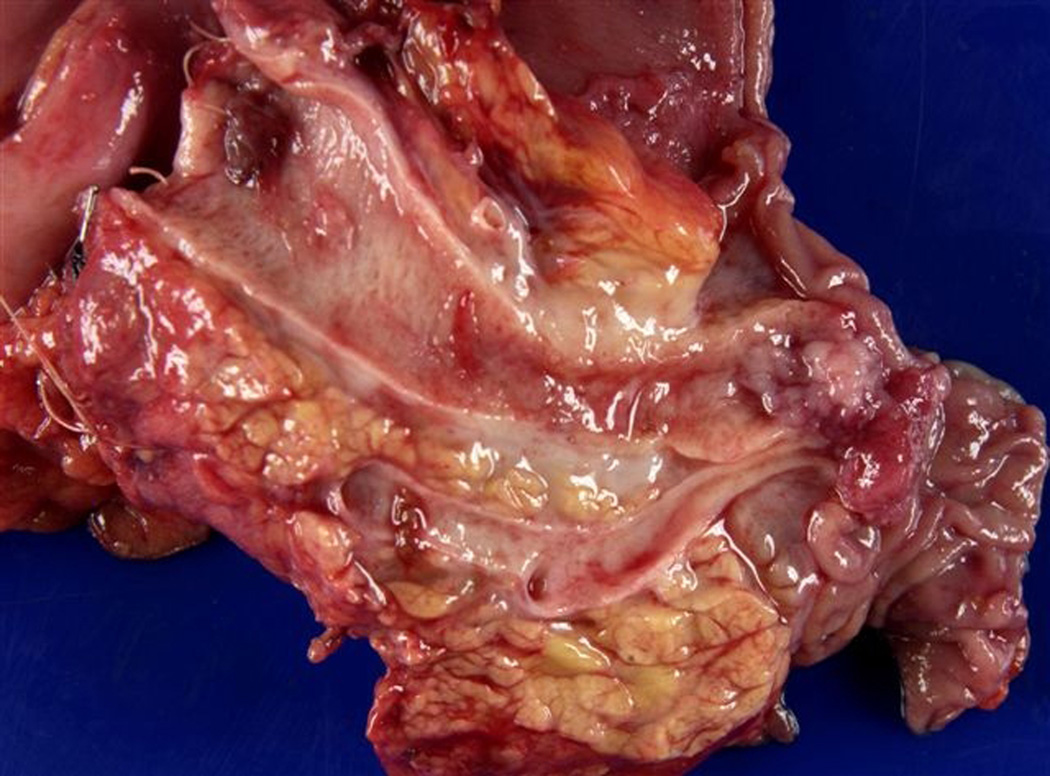
Representative images are seen in Figures 4–6.
Figure 4.
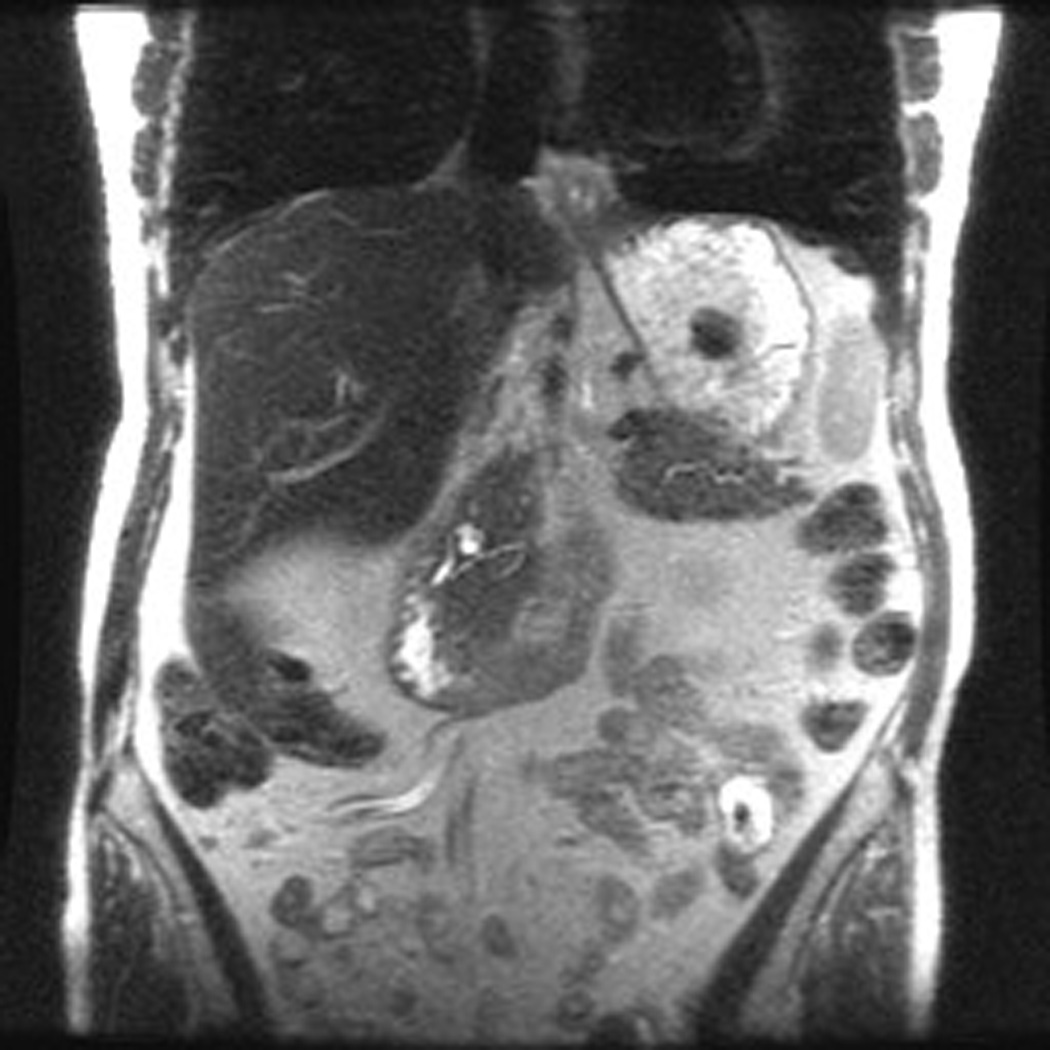
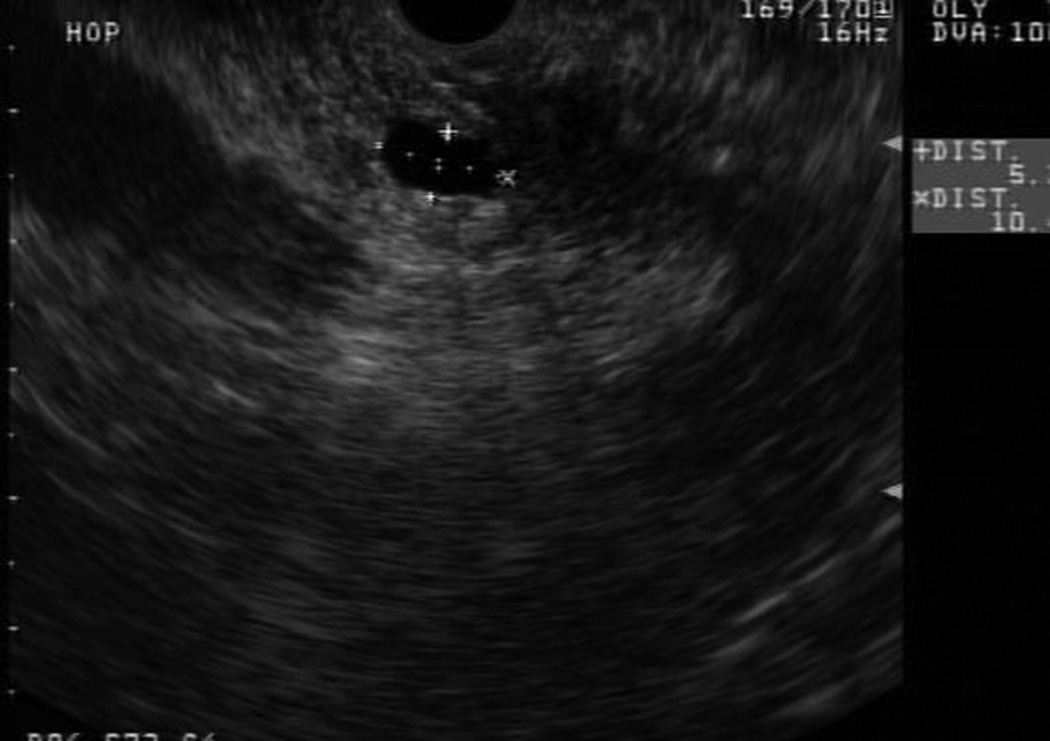
Patient 1 is a 73 year old woman with one FDR and one SDR (father diagnosed at age 70 and paternal grandmother at age 77). On initial MRCP, patient 1 was found to have a cystic pancreatic head lesion. Subsequent EUS showed two cystic lesions in the head and neck of the pancreas, communicating with branches of the PD. FNA of cyst fluid was performed, cytology from which was suspicious for malignant cells. Based on this, surgical resection was recommended, and the patient underwent a pylorus sparing pancreaticoduodenectomy. Pathology revealed multifocal IPMN of borderline malignant potential, predominately involving side branches. She recovered well from surgery and continues with surveillance of her remnant pancreas.
Six additional patients had normal MRCP initially and developed an abnormality (five uni-locular cysts, one multi-locular cyst) on repeated surveillance scanning (mean 2.5 annual cycles, range 2–4). Four of these six individuals underwent EUS and none were found to have significant findings after full multidisciplinary team review.
The remaining 85 at-risk relatives who participated in our screening program have had unremarkable cross-sectional imaging and continue to be followed with annual cross-sectional imaging.
We attempted to contact all relatives enrolled in the familial registry for interim follow-up by mailed questionnaire at two years from date of study entry; 241 of 309 total enrolled relatives reached eligibility for the two-year follow-up questionnaire. Of these 241, 187 (77.6%) completed it and none reported newly developed neoplasm. 131 of these 241 had agreed to surveillance: 84/94 (89.4%) who had scans done at MSKCC completed the interim history. 26/28 (92.9%) relatives who had scans at outside facilities completed the questionnaire. None of the 55/89 (61.8%) relatives who completed the follow-up questionnaire developed pancreatic neoplasms during this interval.
No complications related to the contrast enhanced cross-sectional imaging studies were seen in any screening participants, and no patient required IV sedation for MRCP. In addition, all EUS+/−FNA patients tolerated the procedure well and were discharged from the surgical day hospital recovery room in stable condition the same day as their procedure. Follow-up phone calls to each patient did not reveal any procedure related complications.
DISCUSSION
In our prospective, single-institution study, we screened relatives of pancreatic cancer patients who were at risk for pancreatic cancer due to their family history with MRCP. If pancreatic imaging was abnormal, EUS was performed with FNA of any suspicious lesion. Surgical consultation was obtained for patients with pancreatic lesions that were considered premalignant or malignant. As of August 2009, 109 relatives from FPC families have been screened with cross-sectional imaging at our institution. Nine individuals had initial imaging suggestive of a pancreatic precursor lesion or pancreatic cancer, and six have thus far undergone surgical resection. Our overall diagnostic yield from screening was 8.3%.
Management of high-risk individuals from pancreatic cancer families is an important issue. A number of other familial pancreatic cancer registries have been developed26and pancreatic cancer precursor lesions such as IPMNs and PanINs have been well described27. Screening programs attempting to identify these pancreatic lesions in at-risk healthy family members are underway and several have reported their findings.
Brentnall et al28 at the University of Washington studied 14 individuals from three unrelated pancreatic cancer kindreds that had two or more affected members in at least two generations. 14 patients were evaluated. Seven (50%) of these patients had ERCP findings of ectatic ducts. All seven patients were referred for pancreatectomy and found to have pancreatic duct epithelial dysplasia ranging from low to high grade. No invasive cancer was found. An additional study by the same group29 confirmed a high yield 12/43 (27.9%). Canto et. al.24 used EUS to screen 38 asymptomatic individuals from high risk families. Six patients were found to have abnormalities and underwent resection. Two significant lesions were found (one adenocarcinoma and one IPMN) for a screening yield of 5.3%. The same group published a larger prospective, controlled study25 using CT and EUS in78 high-risk FPC relatives, for an overall yield of 10.2%.
Langer et al30 published their results of screening 76 individuals from 34 familial pancreatic cancer families. No cancers were identified and only one IPMN was found for a low diagnostic yield of 1.3%. In comparison, Poley et al31 published the results of a prospective study utilizing EUS in 44 asymptomatic high risk family members. They found asymptomatic cancer in three patients (7%), and seven IPMN (16%). Their high yield likely was due to the inclusion of known carriers of FAMMM, hereditary pancreatitis, Peutz-Jehgers, p53 and BRCA 1 and2.
Our reports the largest number of healthy high-risk relatives screened at a single institution.. There is no consistent definition of FPC among centers undertaking these programs, and when we began our study we decided to be more inclusive in our definition, including those with only one first degree relative with pancreatic cancer (as long as there were also other affected relatives), and also including individuals with only one first degree relative diagnosed before the age of 50. For the latter criterion, we reasoned that genetics might play a stronger role in the etiology of pancreatic cancer in patients diagnosed at a younger age.
In our study, MRCP was selected as the initial imaging modality because it is sensitive, non-invasive, does not involve radiation exposure, and has a very low complication rate.26Although EUS has been shown to be safe and effective in evaluating the pancreas for neoplastic lesions, it requires anesthesia, and has an associated complication rate generally felt to be approximately between 0.03–0.15%32,33. CT scan was not selected because of cumulative radiation exposure anticipated from serial scans.
All of our significant lesions were found on initial screening (prevalent lesions). This is interesting and supports previous studies in which node positive cancers were found at “screening”. The utility of screening earlier than age 65 is yet unanswered, as ourstudy suggests that the highest yield was for relatives >= 65, This requires further study.
The six patients who developed abnormalities on subsequent scans—incident lesions—remain under surveillance but have not had any marked changes in their benign-appearing cystic lesions over 2–4 years of follow-up. It is hard to draw a conclusion from this as the sample size is very small.
In spite of our broader definition of “familial,” we demonstrate a similar yield of significant findings (Table 4). Although our definition of familial includes those recruited with only one first degree relative diagnosed before the age of 50, this did not result in fewer significant findings. Additionally, we utilized only cross-sectional imaging as the initial screening tool, recommending EUS only if an abnormality was found; this approach appears to be effective and safe.
Table 4.
Summary and comparison table of studies evaluating screening for pancreatic cancer
| Study/Date | Number of healthy relatives screened |
Eligibility criteria for screening |
Mean age at screening (range in years) |
Screening modality utilized |
Diagnostic yield (%) |
|---|---|---|---|---|---|
| Brentall/199928 | 14 | >2 FDR/>2 generations | 41 | EUS/ERCP/spiral CT | 50.0 |
| Kimmey/2002 29 | 43 | >2 FDR/>2 generations | n/a | EUS/ERCP | 27.9 |
| Canto/200423 | 38 | >2 FDR/>3 total affected relatives | 57 | EUS->ERCP/spiral CT | 5.3 |
| Canto/200624 | 78 | >2 FDR/>3 total affected relatives | 52 | CT/EUS->EUS/FNA +/− ERCP | 10.2 |
| Langer/200930 | 76 | >2 FDR | 60 | MRI/MRCP and EUS | 1.3 |
| Poley/200931 | 44 | >2FDR/known genetic syndrome with PC clustering | 44 | EUS | 22.7 |
| Ludwig/2010 | 109 | >1 FDR with PC< 50, >2 FDR/SDR >3 SDR Known BRCA mutation with pancreatic clustering |
54 | MRCP->EUS/FNA | 8.3 |
What the optimal initial screen should be remains up for debate. Although we found our significant yield using MRCP followed by EUS, the opposite strategy could have been employed with possibly equal effect. Our study was not designed to be comparative; i.e. our patients with MRCPs deemed “normal” did NOT undergo EUS to look for missed lesions. In addition, we had a sizable number of false positives, patients with abnormal MRCPs who on EUS had no appreciable lesion. This occurred in both the relatives with prevalent lesions as well as the incident ones. Which test to employ and in what order remains uncertain. If the greatest yield turns out to be from the initial screen, it may make sense to utilize the most sensitive test as the initial test.
In five of 109 (4.6%) study subjects with abnormal imaging, IPMNs were suspected or confirmed. This finding is similar to that of previous studies24–5,29, 31, and strengthens the evidence for IPMN as a significant pancreatic precursor lesion in FPC. The significance of IPMN in FPC however remains unclear as these may simply represent the identifiable lesions as PanIN is typically difficult to identify either radiographically or endoscopically.
Our screening program enrolled 53% of relatives who were eligible for screening. 36% of relatives eligible refused screening because they didn’t feel it would be useful. An additional 28% found screening to be prohibitively expensive. The remainder of relatives either didn’t want an invasive procedure or gave no reason for declining screening. With increased validation of a yield in the 7–10% range, pancreatic cancer screening of at-risk relatives should become more widely accepted.
In conclusion, we believe screening will become an important tool in specific populations. Although to date, the question of whether screening improves mortality remains unanswered, our study supports and strengthens the findings of previous investigators that screening at-risk relatives in FPC families has a diagnostic yield of 5–10%. Our yield of 8.3% is consistent with these previous studies, despite our large group screened, different definition of FPC, and different mode of initial screening. Questions remain regarding the optimal groups to be screened for pancreatic precursor lesions and pancreatic cancer and the best protocol for such screening. Treating precursor lesions surgically appears to be a prudent strategy but larger longitudinal, multi-institutional follow-up studies will be required to demonstrate the ultimate goal of screening: a decrease in mortality from pancreatic cancer.
What is common knowledge?
Pancreatic adenocarcinoma is a lethal disease
Earlier diagnosis results in improved survival
Certain groups are at increased risk for pancreatic cancer
Screening for pancreatic cancer is not standard of care
What is new here?
Screening relatives from high risk families for pancreatic cancer has a significant yield
MRCP is a safe and effective initial screening modality
Yield for screening is highest in relatives older than 65
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Routine screening for pancreatic screening in the general population is not recommended
-
✓
Previous studies have used EUS to screen high risk groups with success in finding pancreatic precursor lesions
WHAT IS NEW HERE
-
✓
Screening high risk relatives for pancreatic cancer with MRCP followed by EUS +/− FNA is safe and effective with a diagnostic yield of 8.5%.
-
✓
Yield of screening is similar to prior studies despite different criteria for study entry
ACKNOWLEDGMENTS
The authors would like to thank Dr Eileen O’Reilly, Sarah Caughey, and Lucia Zoercher for their helpful comments and suggestions.
Financial support: None
Footnotes
Disclosures—None of the authors has financial, professional, or personal disclosures relevant to the manuscript.
CONFLICT OF INTEREST
Guarantor of the article: Emmy Ludwig MD
Specific author contributions:
Planning & conducting study: Emmy Ludwig MD Sara H Olson PhD Jennifer Simon MA, Sharon Bayuga MPH Peter J Allen MD Robert C Kurtz MD. Collecting and interpreting data: Sara H Olson PhD Jennifer Simon MA, Sharon Bayuga MPH. Drafting and writing manuscript: Emmy Ludwig MD Robert C Kurtz MD. Participation in critical review and revision of the article: Emmy Ludwig MD, Sara H Olson PhD, Jennifer Simon MA, Sharon Bayuga MPH, Hans Gerdes MD, Mark A Schattner, Peter J Allen MD, William R. Jarnagin, Robert C Kurtz MD.
All authors approved the final draft submitted.
Potential competing interests: None
REFERENCES
- 1. [accessed May 5, 2009];American Cancer Society. web site, www.cancer.org/downloads/STT/500809 web.pdf http://www.cancer.org/docroot/stt/stt_0.asp?from=fast.
- 2.Allen PJ. Pancreatic adenocarcinoma: putting a hump in survival. J Am Coll Surg. 2007;205(4S):S76–S80. doi: 10.1016/j.jamcollsurg.2007.06.331. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG, Melbert D, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute; 2007. accessed at www.seer.cancer.gov/csr/1975_2004/ [Google Scholar]
- 4.Shimizu Y, Yasui K, Matsueda K, et al. Small carcinoma of the pancreas is curable: new computed tomography finding, pathological study and postoperative results from a single institute. J Gastroenterol Hepatol. 2005;20(10):1591–1594. doi: 10.1111/j.1440-1746.2005.03895.x. [DOI] [PubMed] [Google Scholar]
- 5.Ahlgren JD. Epidemiology and risk factors in pancreatic cancer. Semin Oncol. 1996;23:241–250. [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer: IARC Monographs of the evaluation of carcinogenic risk of chemicals to man. vol. 38. Lyon (France): IARC; 1986. pp. 279–282. Tobacco smoking. [PubMed] [Google Scholar]
- 7.Mack TM, Yu MC, Hanisch R, et al. Pancreas cancer and smoking, beverage consumption and past medical history. J Natl Cancer Inst. 1986;76:49–60. [PubMed] [Google Scholar]
- 8.Tersmette AC, Petersen GM, Offerhaus GJ, et al. Increased risk of incident pancreatic cancer among first degree relatives of patients with familial pancreatic cancer. Clin Cancer Res. 2001;7:738–744. [PubMed] [Google Scholar]
- 9.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–2638. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 10.Hruban HR, Petersen GM, Goggins M. Familial Pancreatic Cancer. Ann Oncol. 1999;10(Suppl 4):69–73. [PubMed] [Google Scholar]
- 11.Greer JB, Whitcomb DC. Role of BRCA1 and BRCA2 mutations in pancreatic cancer. Gut. 2007;56(5):601–605. doi: 10.1136/gut.2006.101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch HT, Fusaro RM. Pancreatic cancer and the familial atypical multiple mole melanoma (FAMMM) syndrome. Pancreas. 1991;6:127–131. doi: 10.1097/00006676-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Ekbom A, McLaughlin JK, Karlsson BM, et al. Pancreatitis and pancreatic cancer: a population based study. J Natl Cancer Inst. 1994;86:625–627. doi: 10.1093/jnci/86.8.625. [DOI] [PubMed] [Google Scholar]
- 14.Bansal P, Sonnenberg A. Pancreatitis is a risk factor for pancreatic cancer. Gastroenterology. 1995;109:247–251. doi: 10.1016/0016-5085(95)90291-0. [DOI] [PubMed] [Google Scholar]
- 15.Lowenfels A, Maisonneuve P, DiMango E, et al. Risk Factors for cancer in hereditary pancreatitis. Med Clin North Am. 2000;84:565–573. doi: 10.1016/s0025-7125(05)70240-6. [DOI] [PubMed] [Google Scholar]
- 16.Klein AP, Hruban RH, Brune KA, et al. Familial pancreatic cancer. Cancer J. 2001;4:266–273. [PubMed] [Google Scholar]
- 17.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–2638. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 18.Biankin AV, Kench JG, Dijkman FP, et al. Molecular pathogenesis of precursor lesions of pancreatic ductal adenocarcinoma. Pathology. 2003;35:14–24. [PubMed] [Google Scholar]
- 19.Takaori K. Current understanding of precursors to pancreatic cancer. J Hepatobiliary Pancreat Surg. 2007;14:217–223. doi: 10.1007/s00534-006-1165-6. [DOI] [PubMed] [Google Scholar]
- 20.Woo SM, Ryu JK, Lee SH, et al. Survival and prognosis of invasive intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2008;36:50–55. doi: 10.1097/MPA.0b013e31812575df. [DOI] [PubMed] [Google Scholar]
- 22.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234:313–321. doi: 10.1097/00000658-200109000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hruban RH, Wilentz RE, Kern SE. Genetic progression in the pancreatic ducts. Am J Pathol. 2000;156:1821–1825. doi: 10.1016/S0002-9440(10)65054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clinical Gastro and Hepatol. 2004;2:606–621. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 24.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: A prospective controlled study. Clinical Gastro and Hepatol. 2006;4:766–781. doi: 10.1016/j.cgh.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Tse F, Barkun JS, Romagnuolo J, et al. Nonoperative imaging techniques in suspected biliary tract obstruction. HPB (Oxford) 2006;8(6):409–425. doi: 10.1080/13651820600746867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenhalf W, Malats N, Nilsson M, Bartsch D, Neoptolemos J. International registries of families at high risk of pancreatic cancer. Pancreatology. 2008;8(6):558–565. doi: 10.1159/000159214. [DOI] [PubMed] [Google Scholar]
- 27.Singh M, Maitra A. Precursor lesions of pancreatic cancer: molecular pathology and clinical implications. Pancreatology. 2007;7(1):9–19. doi: 10.1159/000101873. [DOI] [PubMed] [Google Scholar]
- 28.Brentnall TA, Bronner MP, Byrd DR, et al. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131(4):247–255. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 29.Kimmey MB, Bronner MP, Byrd DR. Screening and surveillance for hereditary pancreatic cancer. Gastrointest Endosc. 2002;56:S82–S86. doi: 10.1016/s0016-5107(02)70092-8. [DOI] [PubMed] [Google Scholar]
- 30.Langer P, Kann PH, Fendrich V, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009;58(10):1410–1418. doi: 10.1136/gut.2008.171611. [DOI] [PubMed] [Google Scholar]
- 31.Poley JW, Kluijt I, Gouma DJ, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175–2181. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 32.Shah JN, Muthusamy VR. Minimizing complications of endoscopic ultrasound and EUS-guided fine needle aspiration. Gastrointest Endoscopy Clin N Am. 2007;17:129–143. doi: 10.1016/j.giec.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Pfau PR, Chak A. Endoscopic Ultrasound. Endoscopy. 2002;34:21–28. doi: 10.1055/s-2002-19394. [DOI] [PubMed] [Google Scholar]