Abstract
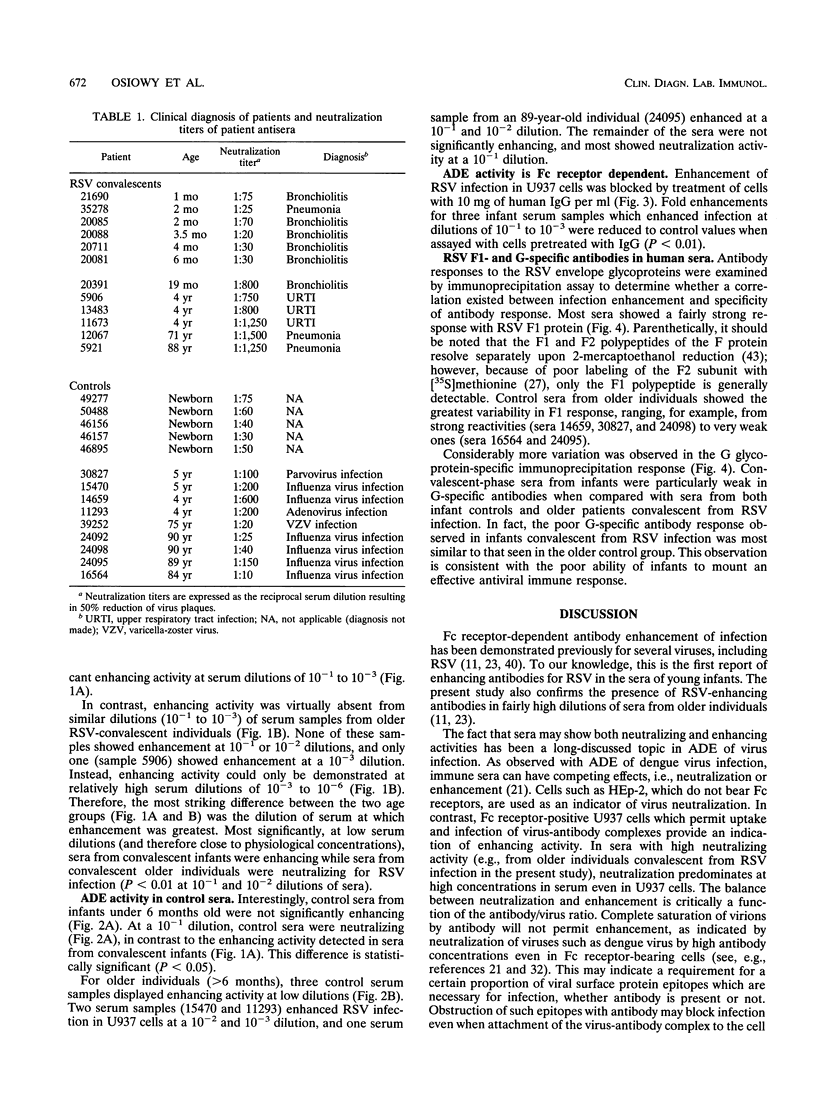
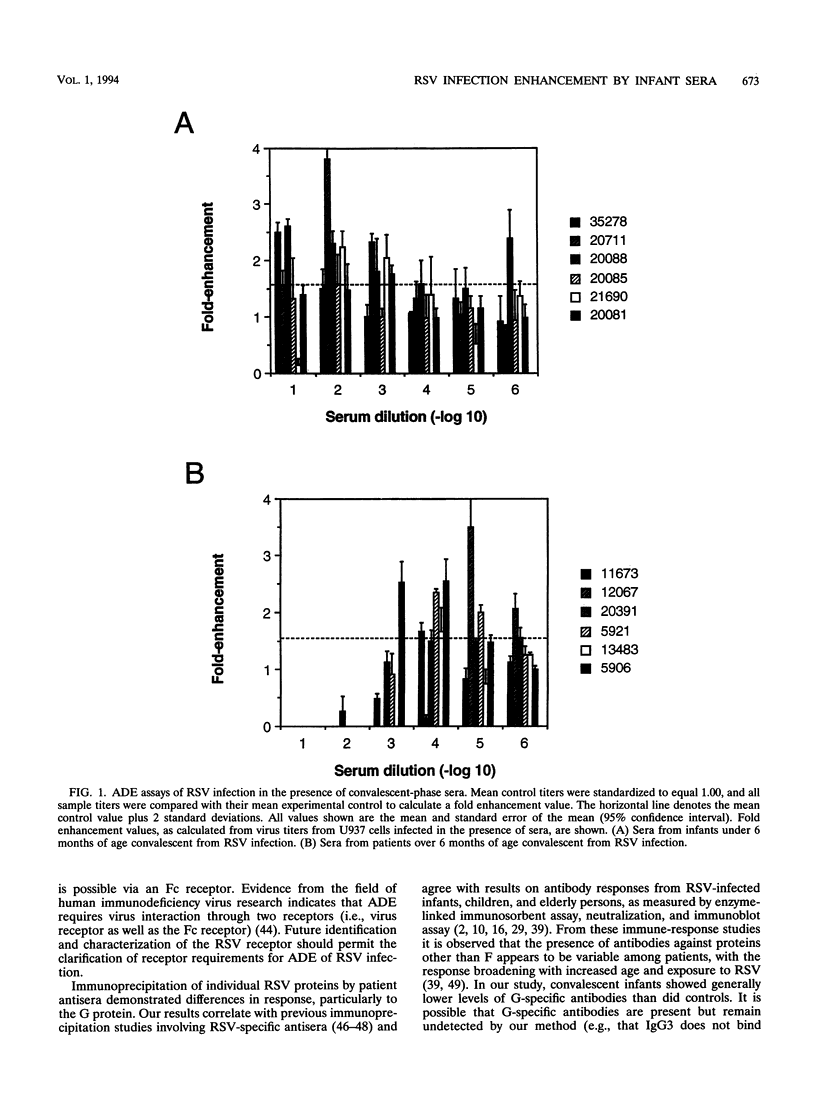
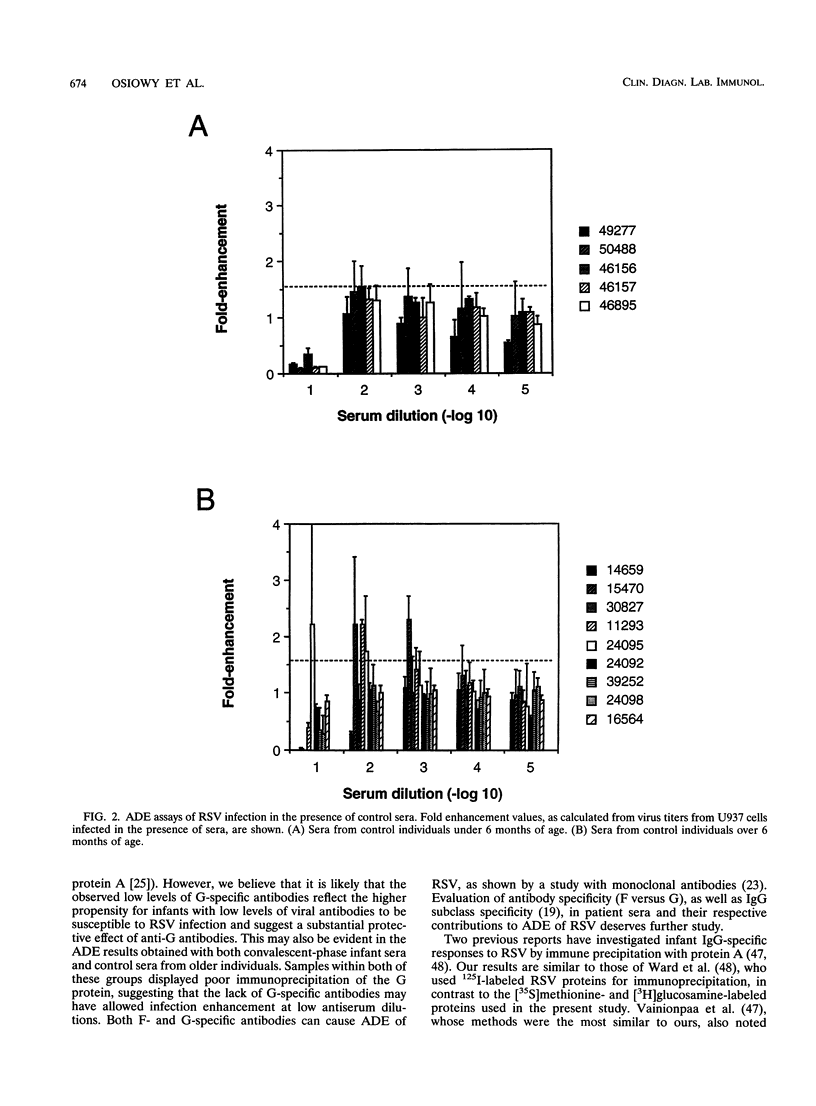
Respiratory syncytial virus (RSV) convalescent-phase sera and control sera from both infants ( < 6 months) and older individuals (1.5 to 90 years) were assayed for RSV-specific antibodies by neutralization, in vitro enhancing activity, and immunoprecipitation. Enhancement of RSV infection in U937 cells was demonstrated with convalescent-phase sera and was shown to be dependent on Fc receptors by blocking with human immunoglobulin G (P < 0.01). Convalescent-phase sera from infants enhanced infection at concentrations closer to physiological ones (10(-1) to 10(-3) dilutions of serum), while convalescent-phase sera from older individuals enhanced infection only at much lower concentrations (10(-3) to 10(-6) dilutions of serum; P < 0.01). To our knowledge, this is the first report of RSV-enhancing antibody activity in the sera of infants. The observed enhancing activity and the low neutralizing antibody levels are confined mostly to convalescent-phase sera from infants aged 0 to 6 months, suggesting that these factors may contribute to the increased severity of RSV disease frequently encountered in young infants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair B. M., Bradford H. E., Mackie D. P., McNulty M. S. Effect of macrophages and in vitro infection with parainfluenza type 3 and respiratory syncytial viruses on the mitogenic response of bovine lymphocytes. Am J Vet Res. 1992 Feb;53(2):225–229. [PubMed] [Google Scholar]
- Agius G., Dindinaud G., Biggar R. J., Peyre R., Vaillant V., Ranger S., Poupet J. Y., Cisse M. F., Castets M. An epidemic of respiratory syncytial virus in elderly people: clinical and serological findings. J Med Virol. 1990 Feb;30(2):117–127. doi: 10.1002/jmv.1890300208. [DOI] [PubMed] [Google Scholar]
- Ananaba G. A., Anderson L. J. Antibody enhancement of respiratory syncytial virus stimulation of leukotriene production by a macrophagelike cell line. J Virol. 1991 Sep;65(9):5052–5060. doi: 10.1128/jvi.65.9.5052-5060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. L., Abraham G. N. Characterization of the Fc receptor for IgG on a human macrophage cell line, U937. J Immunol. 1980 Dec;125(6):2735–2741. [PubMed] [Google Scholar]
- Becker S., Quay J., Soukup J. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J Immunol. 1991 Dec 15;147(12):4307–4312. [PubMed] [Google Scholar]
- Becker S., Soukup J., Yankaskas J. R. Respiratory syncytial virus infection of human primary nasal and bronchial epithelial cell cultures and bronchoalveolar macrophages. Am J Respir Cell Mol Biol. 1992 Apr;6(4):369–374. doi: 10.1165/ajrcmb/6.4.369. [DOI] [PubMed] [Google Scholar]
- Bernstein J. M., Hruska J. F. Respiratory syncytial virus proteins: identification by immunoprecipitation. J Virol. 1981 Apr;38(1):278–285. doi: 10.1128/jvi.38.1.278-285.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capewell A., Inglis J. M., Williamson J. Respiratory syncytial virus infection in the elderly. Br Med J (Clin Res Ed) 1984 Jan 21;288(6412):235–236. doi: 10.1136/bmj.288.6412.235-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez H. B., Keir H. M., Cash P. Immunoblot analysis of the human antibody response to respiratory syncytial virus infection. J Gen Virol. 1987 May;68(Pt 5):1267–1275. doi: 10.1099/0022-1317-68-5-1267. [DOI] [PubMed] [Google Scholar]
- Gimenez H. B., Keir H. M., Cash P. In vitro enhancement of respiratory syncytial virus infection of U937 cells by human sera. J Gen Virol. 1989 Jan;70(Pt 1):89–96. doi: 10.1099/0022-1317-70-1-89. [DOI] [PubMed] [Google Scholar]
- Glezen W. P., Paredes A., Allison J. E., Taber L. H., Frank A. L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981 May;98(5):708–715. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- Glezen W. P., Taber L. H., Frank A. L., Kasel J. A. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986 Jun;140(6):543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- Groothuis J. R., Simoes E. A., Levin M. J., Hall C. B., Long C. E., Rodriguez W. J., Arrobio J., Meissner H. C., Fulton D. R., Welliver R. C. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med. 1993 Nov 18;329(21):1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- Hall C. B., Walsh E. E., Long C. E., Schnabel K. C. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991 Apr;163(4):693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- Hendry R. M., Burns J. C., Walsh E. E., Graham B. S., Wright P. F., Hemming V. G., Rodriguez W. J., Kim H. W., Prince G. A., McIntosh K. Strain-specific serum antibody responses in infants undergoing primary infection with respiratory syncytial virus. J Infect Dis. 1988 Apr;157(4):640–647. doi: 10.1093/infdis/157.4.640. [DOI] [PubMed] [Google Scholar]
- Hohdatsu T., Nakamura M., Ishizuka Y., Yamada H., Koyama H. A study on the mechanism of antibody-dependent enhancement of feline infectious peritonitis virus infection in feline macrophages by monoclonal antibodies. Arch Virol. 1991;120(3-4):207–217. doi: 10.1007/BF01310476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holberg C. J., Wright A. L., Martinez F. D., Ray C. G., Taussig L. M., Lebowitz M. D. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol. 1991 Jun 1;133(11):1135–1151. doi: 10.1093/oxfordjournals.aje.a115826. [DOI] [PubMed] [Google Scholar]
- Hornsleth A., Bech-Thomsen N., Friis B. Detection of RS-virus IgG-subclass-specific antibodies: variation according to age in infants and small children and diagnostic value in RS-virus-infected small infants. J Med Virol. 1985 Aug;16(4):329–335. doi: 10.1002/jmv.1890160405. [DOI] [PubMed] [Google Scholar]
- Kliks S. C., Nimmanitya S., Nisalak A., Burke D. S. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988 Mar;38(2):411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- Kliks S. C., Nisalak A., Brandt W. E., Wahl L., Burke D. S. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989 Apr;40(4):444–451. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- Kontny U., Kurane I., Ennis F. A. Gamma interferon augments Fc gamma receptor-mediated dengue virus infection of human monocytic cells. J Virol. 1988 Nov;62(11):3928–3933. doi: 10.1128/jvi.62.11.3928-3933.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krilov L. R., Anderson L. J., Marcoux L., Bonagura V. R., Wedgwood J. F. Antibody-mediated enhancement of respiratory syncytial virus infection in two monocyte/macrophage cell lines. J Infect Dis. 1989 Nov;160(5):777–782. doi: 10.1093/infdis/160.5.777. [DOI] [PubMed] [Google Scholar]
- Krilov L. R., Hendry R. M., Godfrey E., McIntosh K. Respiratory virus infection of peripheral blood monocytes: correlation with ageing of cells and interferon production in vitro. J Gen Virol. 1987 Jun;68(Pt 6):1749–1753. doi: 10.1099/0022-1317-68-6-1749. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Williams R. C., Jr Differences in anti-protein A activity among IgG subgroups. J Immunol. 1969 Oct;103(4):828–833. [PubMed] [Google Scholar]
- Kurlander R. J., Batker J. The binding of human immunoglobulin G1 monomer and small, covalently cross-linked polymers of immunoglobulin G1 to human peripheral blood monocytes and polymorphonuclear leukocytes. J Clin Invest. 1982 Jan;69(1):1–8. doi: 10.1172/JCI110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. M., Hambor J., Diebold M., Galinski B. Kinetics of synthesis and phosphorylation of respiratory syncytial virus polypeptides. J Gen Virol. 1988 Feb;69(Pt 2):313–323. doi: 10.1099/0022-1317-69-2-313. [DOI] [PubMed] [Google Scholar]
- Lamprecht C. L., Krause H. E., Mufson M. A. Role of maternal antibody in pneumonia and bronchiolitis due to respiratory syncytial virus. J Infect Dis. 1976 Sep;134(3):211–217. doi: 10.1093/infdis/134.3.211. [DOI] [PubMed] [Google Scholar]
- Levine S., Dajani A., Klaiber-Franco R. The response of infants with bronchiolitis to the proteins of respiratory syncytial virus. J Gen Virol. 1988 Jun;69(Pt 6):1229–1239. doi: 10.1099/0022-1317-69-6-1229. [DOI] [PubMed] [Google Scholar]
- Mady B. J., Erbe D. V., Kurane I., Fanger M. W., Ennis F. A. Antibody-dependent enhancement of dengue virus infection mediated by bispecific antibodies against cell surface molecules other than Fc gamma receptors. J Immunol. 1991 Nov 1;147(9):3139–3144. [PubMed] [Google Scholar]
- Midulla F., Huang Y. T., Gilbert I. A., Cirino N. M., McFadden E. R., Jr, Panuska J. R. Respiratory syncytial virus infection of human cord and adult blood monocytes and alveolar macrophages. Am Rev Respir Dis. 1989 Sep;140(3):771–777. doi: 10.1164/ajrccm/140.3.771. [DOI] [PubMed] [Google Scholar]
- Morens D. M., Halstead S. B. Measurement of antibody-dependent infection enhancement of four dengue virus serotypes by monoclonal and polyclonal antibodies. J Gen Virol. 1990 Dec;71(Pt 12):2909–2914. doi: 10.1099/0022-1317-71-12-2909. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Graham B. S., Prince G. A., Walsh E. E., Chanock R. M., Karzon D. T., Wright P. F. Serum and nasal-wash immunoglobulin G and A antibody response of infants and children to respiratory syncytial virus F and G glycoproteins following primary infection. J Clin Microbiol. 1986 Jun;23(6):1009–1014. doi: 10.1128/jcm.23.6.1009-1014.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai H., Kurokawa M., Matsui S., Yamamoto T., Kuroki Y., Kishimoto C., Shiraki K. Infection enhancement of influenza A NWS virus in primary murine macrophages by anti-hemagglutinin monoclonal antibody. J Med Virol. 1992 Mar;36(3):217–221. doi: 10.1002/jmv.1890360312. [DOI] [PubMed] [Google Scholar]
- Panuska J. R., Cirino N. M., Midulla F., Despot J. E., McFadden E. R., Jr, Huang Y. T. Productive infection of isolated human alveolar macrophages by respiratory syncytial virus. J Clin Invest. 1990 Jul;86(1):113–119. doi: 10.1172/JCI114672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panuska J. R., Hertz M. I., Taraf H., Villani A., Cirino N. M. Respiratory syncytial virus infection of alveolar macrophages in adult transplant patients. Am Rev Respir Dis. 1992 Apr;145(4 Pt 1):934–939. doi: 10.1164/ajrccm/145.4_Pt_1.934. [DOI] [PubMed] [Google Scholar]
- Parrott R. H., Kim H. W., Arrobio J. O., Hodes D. S., Murphy B. R., Brandt C. D., Camargo E., Chanock R. M. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol. 1973 Oct;98(4):289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- Popow-Kraupp T., Lakits E., Kellner G., Kunz C. Immunoglobulin-class-specific immune response to respiratory syncytial virus structural proteins in infants, children, and adults. J Med Virol. 1989 Mar;27(3):215–223. doi: 10.1002/jmv.1890270307. [DOI] [PubMed] [Google Scholar]
- Porterfield J. S. Antibody-dependent enhancement of viral infectivity. Adv Virus Res. 1986;31:335–355. doi: 10.1016/s0065-3527(08)60268-7. [DOI] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Montefiori D. C., Mitchell W. M. Complement-mediated antibody-dependent enhancement of HIV-1 infection requires CD4 and complement receptors. Virology. 1990 Apr;175(2):600–604. doi: 10.1016/0042-6822(90)90449-2. [DOI] [PubMed] [Google Scholar]
- Stott E. J., Taylor G. Respiratory syncytial virus. Brief review. Arch Virol. 1985;84(1-2):1–52. doi: 10.1007/BF01310552. [DOI] [PubMed] [Google Scholar]
- Takeda A., Sweet R. W., Ennis F. A. Two receptors are required for antibody-dependent enhancement of human immunodeficiency virus type 1 infection: CD4 and Fc gamma R. J Virol. 1990 Nov;64(11):5605–5610. doi: 10.1128/jvi.64.11.5605-5610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissing W. J., van Steensel-Moll H. A., Offringa M. Severity of respiratory syncytial virus infections and immunoglobulin concentrations. Arch Dis Child. 1993 Jul;69(1):156–157. doi: 10.1136/adc.69.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi H., Honjo T., Nagai K., Chiba Y., Chiba S., Tsugawa S. Immunoglobulin A antibody response to respiratory syncytial virus structural proteins in colostrum and milk. J Clin Microbiol. 1989 Sep;27(9):1949–1951. doi: 10.1128/jcm.27.9.1949-1951.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainionpä R., Meurman O., Sarkkinen H. Antibody response to respiratory syncytial virus structural proteins in children with acute respiratory syncytial virus infection. J Virol. 1985 Mar;53(3):976–979. doi: 10.1128/jvi.53.3.976-979.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward K. A., Lambden P. R., Ogilvie M. M., Watt P. J. Antibodies to respiratory syncytial virus polypeptides and their significance in human infection. J Gen Virol. 1983 Sep;64(Pt 9):1867–1876. doi: 10.1099/0022-1317-64-9-1867. [DOI] [PubMed] [Google Scholar]
- Welliver R. C., Kaul T. N., Putnam T. I., Sun M., Riddlesberger K., Ogra P. L. The antibody response to primary and secondary infection with respiratory syncytial virus: kinetics of class-specific responses. J Pediatr. 1980 May;96(5):808–813. doi: 10.1016/s0022-3476(80)80547-6. [DOI] [PubMed] [Google Scholar]
- Yao J. S., Kariwa H., Takashima I., Yoshimatsu K., Arikawa J., Hashimoto N. Antibody-dependent enhancement of hantavirus infection in macrophage cell lines. Arch Virol. 1992;122(1-2):107–118. doi: 10.1007/BF01321121. [DOI] [PubMed] [Google Scholar]
- de Sierra T. M., Kumar M. L., Wasser T. E., Murphy B. R., Subbarao E. K. Respiratory syncytial virus-specific immunoglobulins in preterm infants. J Pediatr. 1993 May;122(5 Pt 1):787–791. doi: 10.1016/s0022-3476(06)80027-2. [DOI] [PubMed] [Google Scholar]




