Abstract
The aim of this study was to investigate the correlation between dental anxiety, salivary cortisol, and salivary alpha amylase (sAA) levels. Furthermore, the aim was to look into individual differences such as age, race, gender, any existing pain, or traumatic dental experience and their effect on dental anxiety. This study followed a cross-sectional design and included a convenience sample of 46. Every patient was asked to complete the Dental Anxiety Scale (DAS) and a basic demographic/dental history questionnaire. A saliva sample, utilizing the method of passive drooling, was then collected in 2-mL cryovials. Samples were analyzed for salivary cortisol and sAA levels by Salimetrics. Significant associations were observed between DAS scores and presence of pain and history of traumatic dental experience. However, no significant correlations were observed between DAS, cortisol, and sAA levels. Our study reconfirms that dental anxiety is associated with presence of pain and a history of traumatic dental experience. On the other hand, our study was the first to our knowledge to test the correlation between the DAS and sAA; nevertheless, our results failed to show any significant correlation between dental anxiety, cortisol, and sAA levels.
Key Words: Stress, Dental anxiety, Salivary cortisol, Salivary alpha amylase, Dental Anxiety Scale
Dental anxiety is a very common phenomenon and remains an obstacle for many patients to seeking proper dental care despite all the technological advances in dentistry. Multiple etiologies have been proposed in the past. Thomson et al1 suggested that even though endogenous factors (personality traits) play a role in its development, it develops mainly from exogenous (conditioning) factors. Van Wijk and Hoogstraten2 revealed that a single early traumatic experience can be the main cause of dental anxiety. Oosterink et al3 showed that a previous traumatic experience may involve pain, negative dentist remarks (NDR), and strong negative emotional responses. As a consequence, these variables act as predictors for cancelled/missed appointments, a decrease in pain threshold with increase in patient discomfort, poor compliance, increased number of emergency appointments, jeopardized patient/dentist relationship, high Decayed Missing and Filled Teeth (DMFT) index, poor oral health perception, decreased self-esteem, and decreased oral health–related quality of life.4–11 Women were found to be more affected than men, and there is a tendency for the younger age groups to have more anxiety.12
Dental anxiety was found to have a direct relationship with pain perception.13 Rhudy and Meagher14 suggested that the pain reactivity is modulated by emotional stress. In addition, Loggia et al15 revealed changes in pain pathways on neuroimaging techniques with a negative emotional state. Furthermore, Klages et al16 revealed that anxiety increases expected or experienced pain where patients with higher anxiety levels predicted a higher pain experience.
Anxiety is regarded as a form of stress and, thus, has a physiological impact on the body. Stressors can cause the activation of the autonomic nervous system (ANS), which prepares the body for the fight-or-flight reaction, and the hypothalamic-pituitary-adrenal (HPA) axis.
When the autonomic nervous system (ANS) gets activated, it causes the release of epinephrine and norepinephrine from the adrenal medulla.17 Norepinephrine was shown to increase the secretion of salivary alpha amylase (sAA) from the acinar cells of the parotid and submandibular salivary glands.18 It was suggested that the level of alpha amylase in the saliva reflects the autonomic nervous system (ANS) activity and that measuring it presents an easy, noninvasive measure of ANS activity compared to measuring the actual catecholamines in serum.18 sAA levels were shown to increase in response to various stressors like exercise, cold exposure, and hypertension, in addition to psychological stress.18 Nator et al19 also demonstrated that sAA has a definite circadian rhythm wherein its levels fluctuate during the day in a definite pattern. Because the ANS is considered a rapid response, it was suggested that it may be a better measure of stress compared to measuring the hypothalamic-pituitary-adrenal axis response.17–20
Upon activation of the hypothalamic-pituitary-adrenal axis, cortisol gets secreted from the adrenal cortex to all body fluids, including saliva. It was demonstrated in the past that salivary cortisol increases in response to stress and anxiety, and that it also presents an easy, noninvasive way of measuring stress.20 Cortisol levels in the saliva have been shown to be higher in patients with oral lichen planus.21 In addition, they were higher in patients undergoing wisdom teeth extractions and prior to urgent dental care.22 Similar to alpha amylase, cortisol has a definite circadian rhythm.
The Dental Anxiety Scale (DAS), devised by Norman Corah in 1969, is the most commonly used scale to measure dental anxiety.23 It was found to have high validity and is easy to administer; therefore, it was adopted as a measure of dental anxiety in this study.
Stress and sAA associations have been well documented and studied in the literature24–28; however, to our knowledge, no literature exists on the correlation between dental anxiety and sAA. Therefore, the aim of this study was to see if there is any correlation between dental anxiety, sAA, and salivary cortisol levels. In addition, the aim was to see if individual variations such as age, gender, race, presence of pain, or history of traumatic dental experience exhibit associations with dental anxiety. We hypothesized that dental anxiety is correlated with an increase in both alpha amylase and cortisol levels; furthermore, that presence of pain and a history of traumatic dental experience are associated with higher dental anxiety levels.
METHODS
This study took place at Tufts University School of Dental Medicine. It followed an observational, cross-sectional design. The Institutional Review Board at Tufts University approved all procedures and protocols. Informed consent was given by all participants.
A sufficient sample size resulting in 80% power and type I error rate of 5% was calculated based on previous literature. From prior literature20 a Pearson correlation coefficient equal to 0.535 yielded statistically significant results. Using the same value, with the aid of nQuery advisor version 7.0, aiming for 80% power and a type I error rate of 5% showed that a sample size of 23 would be required. In order to increase the power, we used a sample size of 50, which resulted in a power of over 90% while maintaining a type I error rate of 5% (nQuery Advisor version 7.0).
Inclusion criteria for the study entailed that participants had no prior history at Tufts Dental School and were presenting as new patients. Adult patients, age range 18–80, who were in good systemic health were considered.
Exclusion criteria included: first, patients with any preexisting conditions affecting cortisol levels, such as adrenal gland insufficiency; second, pregnant women, or women taking oral contraceptives; third, asthmatic patients who were on steroid inhalers; fourth, patients with xerostomia and those taking B-blockers, because of their effect on salivary flow; lastly, patients on antidepressants or antipsychotic medication, because of their effect on mood and mental status.
Smokers were included on the provision that they agreed not to smoke for at least 2 hours prior to sample collection. In addition, patients were asked not to consume a major meal at least 4 hours prior to sample collection, and to avoid caffeinated and alcoholic beverages at least 12 hours prior to sample collection.
Sample collection was performed between the hours of 9 am and noon to account for the diurnal rhythm for both cortisol and alpha amylase.
Patients were offered a free exam and cleaning, a value of $150, as incentive for participation, and sample collection took place over a 7-month period.
Upon participants' approval, and their meeting all inclusion/exclusion criteria and signing informed consent, they were asked to complete Corah's DAS, as well as a basic demographic/dental history questionnaire. A saliva sample was then collected utilizing the method of passive drooling, using 2-mL cryovials provided by Salimetrics. Participants were asked to allow the saliva to pool at the floor of the mouth first, before ejecting it into the cryovials through a short piece of straw. Sample collection was done over a maximum of 5 minutes, and the time and the volume of the samples were recorded. Samples were stored at −80°C until ready for analysis.
Once all samples were collected, analyses for alpha amylase and cortisol levels were done by Salimetrics. At the time of analysis, 4 samples were excluded because of evidence of blood contamination within the samples. Data collected from these 4 samples were not included in the subsequent analyses. The final sample size included in the analyses was 46.
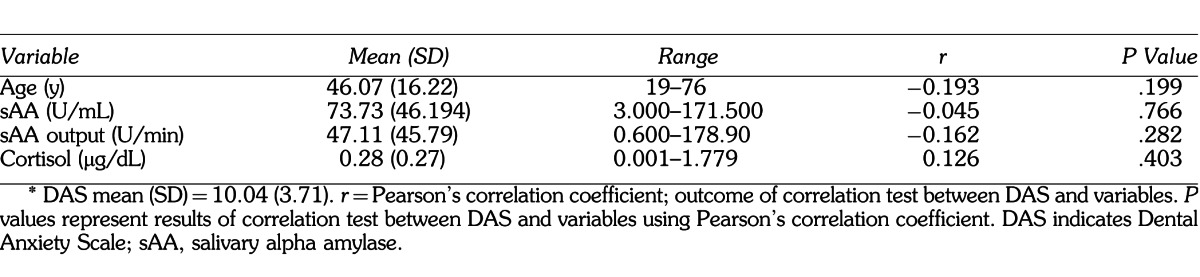
The primary outcomes in this study were sAA measured in U/mL and salivary cortisol measured in μg/dL. The DAS score was the primary predictor for the analyses done for the above salivary biomarkers; however, it was also the outcome variable for the secondary analyses. Predictors that were included in the secondary analyses, which allowed for comparisons between basic sample characteristics and dental anxiety, were age, gender, race, presence of pain, and history of traumatic dental experience. Salivary flow rate was considered a potential confounder for sAA levels; thus, sAA output (in U/min) was calculated by multiplying sAA levels with salivary flow rate for each sample. A summary of all variables and sample demographics can be found in Tables 1 and 2.
Table 1.
Summary of Sample Demographics (N = 46)†
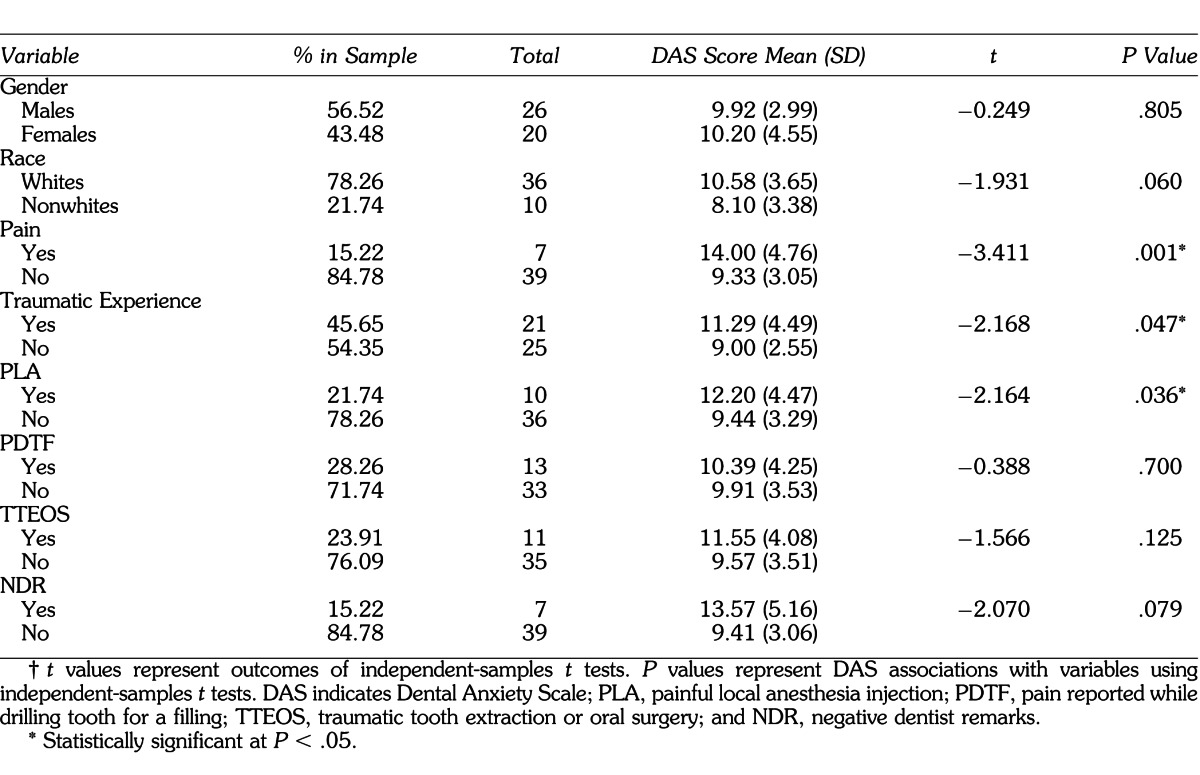
Table 2.
Summary of Continuous Variables in Sample (N = 46)*
RESULTS
All analyses were performed utilizing IBM's SPSS version 18 (SPSS, Inc). DAS associations with gender, race, presence of pain, and history of traumatic dental experience were performed using independent-samples t tests. Levene's test was utilized to test for equal variances. Pearson's correlation coefficient was used to correlate between salivary cortisol, sAA, age, and DAS. Result summaries can be found in Tables 1 through 3 and Figures 1 through 8. Some of the main analyses are described below.
Figure 1.
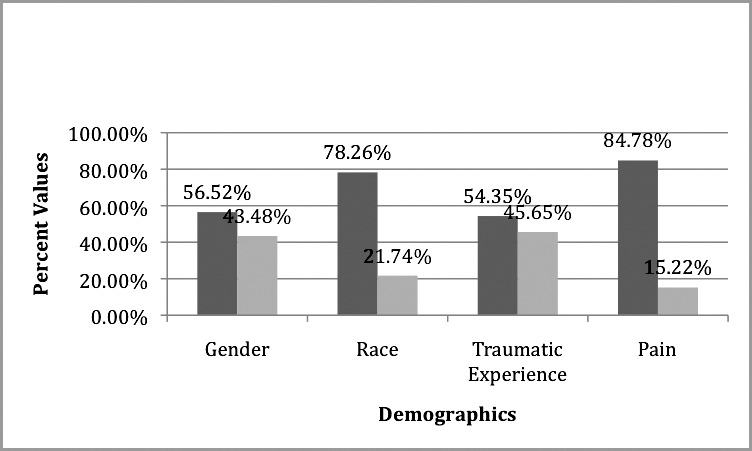
Basic demographics of sample: gender (dark gray = M, light gray = F), race (dark gray = white, light gray = nonwhite), traumatic experience (dark gray = no , light gray = yes), pain (dark gray = no, light gray = yes).
DAS and Pain
Seven subjects reported pain. Their mean DAS score was 14.00 (SD = 4.76). On the other hand, 39 subjects did not report pain, and their mean DAS score was 9.33 (SD = 3.04). An independent-samples t test was performed to test the association between DAS and presence of pain. Equal variances were tested using Levene's test, and equal variances were assumed (P = .226). A statistically significant association was observed between DAS and presence of pain (t = −3.411, P = .001). (See Table 1 and Figure 1 for summary of data.)
DAS and History of Traumatic Experience
Twenty-one subjects reported a history of traumatic dental experience. Their mean DAS score was 11.28 (SD = 4.49). On the other hand, 25 subjects did not report a traumatic dental experience, and their mean DAS score was 9.00 (SD = 2.55). An independent-samples t test was performed to test the association between DAS and history of traumatic dental experience. Equal variances were tested using Levene's test, and equal variances were not assumed (P = .015). A statistically significant association was observed between DAS and history of traumatic dental experience (t = −2.168, P = .047). (See Table 1 and Figure 1 for summary of data.)
To investigate this further, the type of traumatic experience was analyzed as follows:
DAS and Painful Local Anesthesia Injection
Ten subjects reported history of a painful local anesthesia injection. Their mean DAS score was 12.20 (SD = 4.47). On the other hand, 36 subjects did not report a painful local anesthesia injection, and their mean DAS score was 9.44 (SD = 3.29). Equal variances were tested using Levene's test, and equal variances were assumed (P = .256). An independent-samples t test was performed and a statistically significant association was observed between DAS and history of painful local anesthesia injection (t = −2.164, P = .036). (See Table 1 for summary of data.)
DAS and Pain Reported While Drilling Tooth for a Filling, Traumatic Tooth Extraction or Oral Surgery, and Negative Dentist Remarks (NDR)
Thirteen subjects reported history of pain reported while drilling tooth for a filling (PDTF), 11 reported traumatic tooth extraction or oral surgery (TTEOS), and 7 reported NDR. Their mean DAS scores were 10.39 (SD = 4.25), 11.55 (SD = 4.08), and 13.57 (SD = 5.16) respectively. On the other hand, 33 subjects did not report PDTF, 35 did not report TTEOS, and 39 did not report NDR. Their mean DAS scores were 9.91 (SD = 3.53), 9.57 (SD = 3.51), and 9.41 (SD = 3.06) respectively. Equal variances were tested using Levene's test, and equal variances were assumed for PDTF and TTEOS (P = .257, P = .709); however, they were not assumed for NDR (P = .029). Independent-samples t tests were performed; however, no statistically significant associations were observed between DAS and PDTF, TTEOS, or NDR (t = −0.388, P = .700; t = −1.566, P = .125; t = −2.070, P = .079, respectively). (See Table 1 for summary of data.)
DAS and sAA Level and sAA Output
The means for sAA level and sAA output were 73.73 U/mL (SD = 46.19) and 47.11 U/min (SD = 45.79) respectively. The mean DAS score was 10.04 (SD = 3.71). Pearson's correlation coefficient was used to test the correlation between these variables; however, no statistically significant correlations were observed (r = −0.045, P = .766, r = −0.162, P = .282 respectively). (See Table 2 and Figures 3 and 4 for summary of data.)
Figure 3.
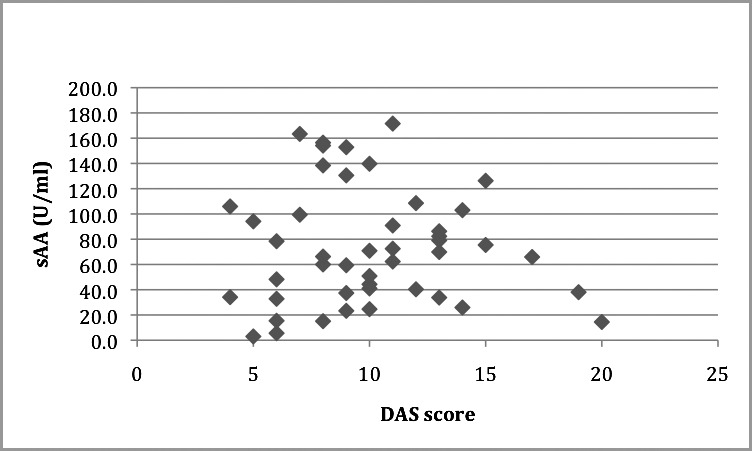
Scatter plot showing Dental Anxiety Scale (DAS) correlation with salivary alpha amylase (sAA) (U/mL). r = −0.045, P = .766.
Figure 4.
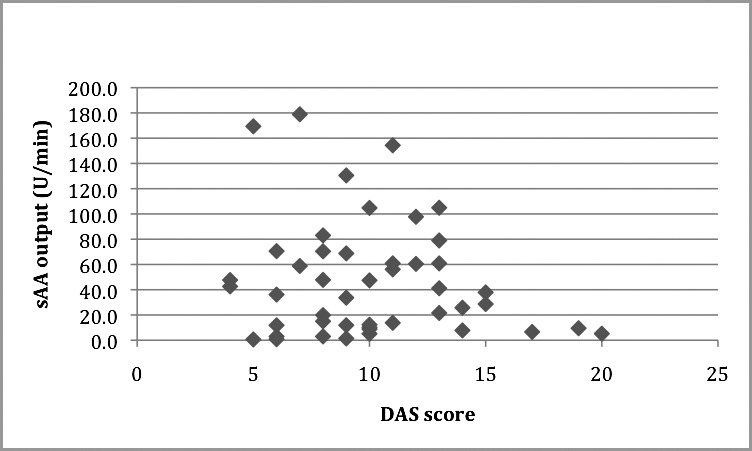
Scatter plot showing Dental Anxiety Scale (DAS) correlation with salivary alpha amylase (sAA) output (U/min). r = −0.162, P = .282.
DAS and Salivary Cortisol Levels
The mean salivary cortisol level was 0.277 μg/dL (SD = 0.273) and the mean DAS score was 10.04 (SD = 3.71). Pearson's correlation coefficient was performed to test the correlation between DAS and salivary cortisol levels; however, no significant correlation was observed (r = 0.126, P = .403). (See Table 2 and Figure 5 for summary of data.)
Figure 5.
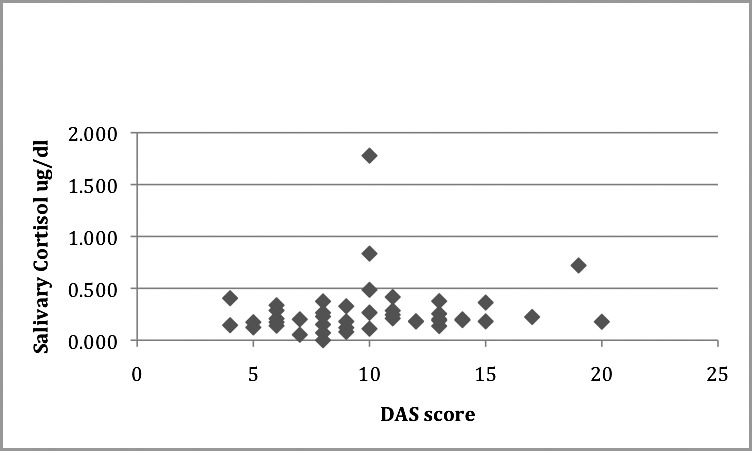
Scatter plot showing Dental Anxiety Scale (DAS) correlation with salivary cortisol (μg/dL). r = 0.126, P = .403.
sAA and sAA Output
The mean sAA was 73.730 U/mL (SD = 46.194) and the mean sAA output was 47.107 U/min (SD = 45.791). Pearson's correlation coefficient was performed to test the correlation between sAA and sAA output. A statistically significant association was observed between these variables (r = 0.630, P < .001). (See Table 3 and Figure 6 for summary of data.)
Table 3.
Summary of sAA Correlations with Salivary Variables (N = 46)
Figure 6.
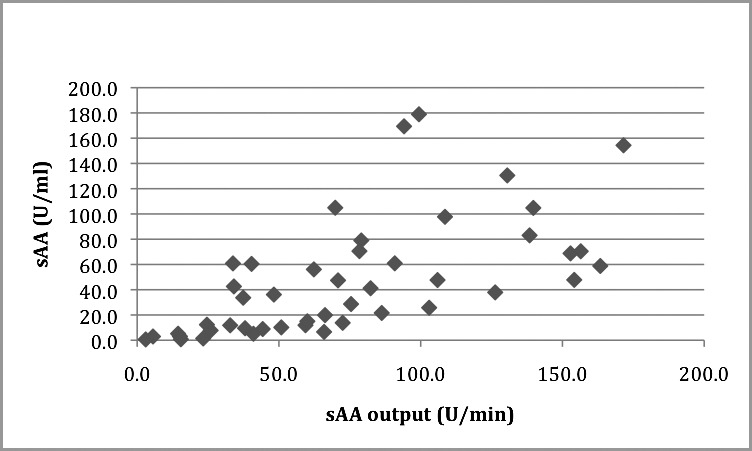
Scatter plot showing salivary alpha amylase (sAA) correlation (U/mL) with sAA output (U/min). r = 0.630, P < .001.
sAA, Flow Rate, and Volume
The mean sAA was 73.730 U/mL (SD = 46.194) and the means for flow rate and volume were 0.60 mL/min (SD = 0.48) and 1.43 mL (SD = 0.47) respectively. Pearson's correlation coefficient was performed to test the association between sAA, flow rate, and volume collected. No significant association was observed for flow rate (r = 0.126, P = .404); however, a statistically significant correlation was observed for volume (r = 0.394, P = .007). (See Table 3 and Figures 7 and 8 for summary of data.)
Figure 7.
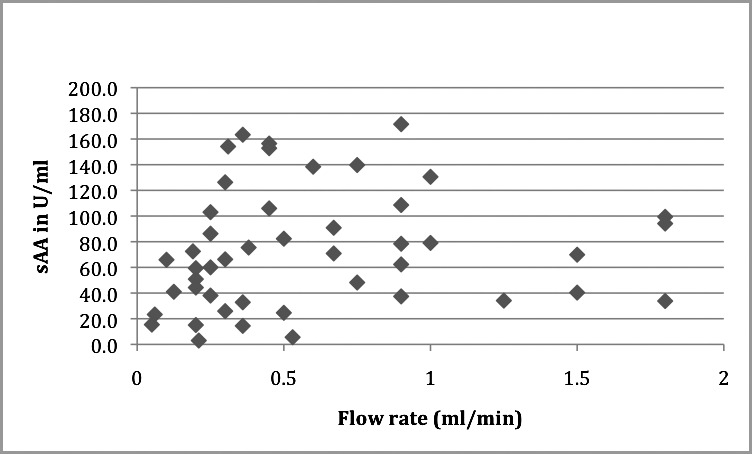
Scatter plot showing correlation between salivary alpha amylase (sAA) (U/mL) and flow rate (mL/min). r = 0.126, P= .404.
Figure 8.
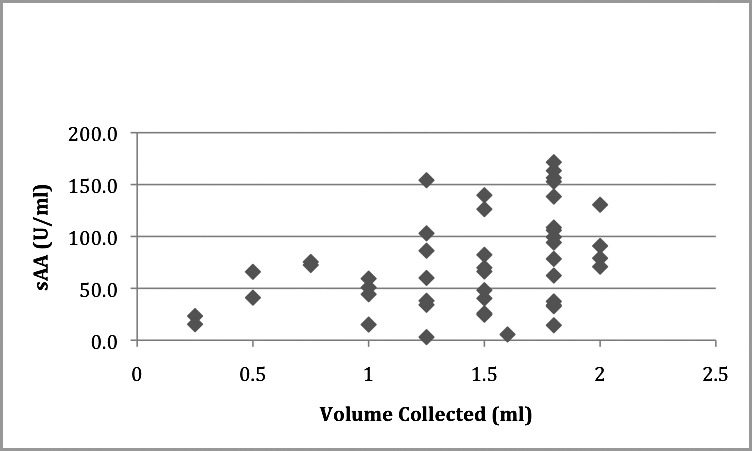
Scatter plot showing salivary alpha amylase (sAA) (U/mL) correlation with volume collected (mL). r = 0.394, P = .007.
DISCUSSION
Anxiety and Pain
In agreement with previous literature, our study results do confirm a relationship between dental anxiety and pain. In their study about perceived pain in relation to a dental hygienist treatment, Hakeberg and Cunha29 demonstrated that even though patients generally report higher anxiety towards dental treatment, it was shown that perceived pain is correlated with higher anxiety towards different aspects of a dental hygiene treatment. Their results also showed higher anxiety reported in women. Another review by Loggia et al15 demonstrated evidence that psychological factors influence pain perception. This is revealed by changes in activity in pain pathways with changes in attentional state, stress, and positive and negative emotions via neuroimaging techniques. Furthermore, Van Wijk and Makkes30 demonstrated that anxious patients report more perceived pain than nonanxious patients while receiving a local anesthesia injection, and Klages et al16 showed that patients with high anxiety report and anticipate more pain when exposed to a critical situation. Our study outcomes reconfirm the results of previous literature in which higher anxiety scores were reported in patients who had pain.
Anxiety and Previous Traumatic Dental Experience
In regards to the effect of a prior traumatic experience on dental anxiety, Agdal et al5 showed that anxious patients might experience intrusive recollection of earlier dental experiences, similar to patients with posttraumatic stress disorder. Locker et al8 also suggested that a negative dental experience is the most stated single cause of dental anxiety. In addition, Van Wijk and Hoogstaten2 demonstrated that people's expectations of pain could make them susceptible to ending up in a vicious circle of anxiety, fear of pain, and treatment avoidance. Our study results did reveal a significant correlation between dental anxiety and a prior traumatic dental experience. Considering the type of negative experiences reported, history of a painful local anesthesia injection reached statistical significance, whereas TTEOS, PDTF, and NDR did not reach statistical significance.
Anxiety, Stress, and Salivary Cortisol
Previous studies by Shah et al21 and Hill24 have demonstrated a positive correlation between stress, anxiety, and salivary cortisol levels. A study by Krueger et al31 showed that patients who have higher anxiety showed significantly higher salivary cortisol levels in an educational session compared to those who had a low dental anxiety score. In addition, a study by Koray et al32 found a positive association between state/trait anxiety scores and salivary cortisol in patients with oral lichen planus. Furthermore, in a study by Miller et al22 it was demonstrated that salivary cortisol levels in dental treatment are highest in patients undergoing tooth extraction compared to other procedures such as prophylaxis, restorative, and examination. On the contrary to all prior literature mentioned, Brand33 attempted to correlate between DAS scale score and salivary cortisol; however, no statistically significant correlation was observed. In comparison to all the studies mentioned, our study results did not show any significant correlation between the DAS score and salivary cortisol levels.
DAS, Flow Rate, and sAA
Previous literature by Nator et al,16,18, Nator and Rohleder17 Rohleder et al,34 Allwood et al,35 and Kang et al36 looked at the correlation between stress conditions, including psychological stress, and the levels of sAA. They all showed that stress causes a significant increase in sAA levels when patients were exposed to a stressful condition compared to a rest condition. In addition, Noto et al37 and Takai et al20 looked at the correlation between state/trait anxiety scoring and alpha amylase levels and found a significant correlation. Our study was the first, to our knowledge, to examine the correlation between the DAS and sAA; nevertheless, our results did not reveal any significant correlation, and there were no significant differences based on age, gender, or race.
Considering the effect of flow rate on sAA, our results were in line with other studies by Beltzer et al38 and Rohleder et al34 wherein a positive correlation was observed between the alpha amylase levels (U/mL) and the alpha amylase outputs (U/min). In the study by Rohleder et al,34 the effects of flow rate on sAA levels were examined in both baseline and stress conditions, and it was concluded that the sAA and sAA output responses to stress were significantly higher in both parameters; therefore, the stress response was the same irrespective of flow rate. In addition, our results showed a significant correlation between sAA and sAA output, which reconfirms previous findings by Rohleder et al.34
In regards to the correlation between alpha amylase level and volume of saliva collected, our study results revealed a positive correlation between sAA level and volume of saliva collected. This contradicts the results achieved by Beltzer et al,38 who demonstrated a decrease in sAA levels with the increase in volume over collection time of 5 minutes, utilizing the method of passive drooling.
Limitations
Our study design had some limitations. First, a convenience sample was utilized to achieve the sample size needed, and it consisted of new patients who had never been seen in the clinic before. We had no way of predicting their anxiety level or previous dental history, if any. Second, the patients were presenting for a regular exam appointment and few of them reported pain. Lastly, only 1 saliva sample was collected from each patient, which represented pretreatment levels of the salivary biomarkers tested. The levels could have been low because the patients had not been exposed to any dental treatment yet, and they all knew that they were not getting any dental treatment done.
Based on the results of our study, we can conclude that presence of pain and any history of traumatic dental experience are associated with patients' dental anxiety level. As far as the type of traumatic dental experience, a painful local anesthesia injection was found to be associated with the anxiety experienced by patients compared to other types of traumatic experiences. Dental anxiety, nevertheless, was not found to be associated with an increase in salivary cortisol or sAA levels, and there were no differences between gender, race, and age. Future research should aim at comparing baseline levels of these biomarkers with any changes due to different types of dental treatment, such as cleanings, extractions, and fillings, and correlate their levels to dental anxiety. In addition, the effects of volume and flow rate on the level of sAA will need to be looked into further in future research to determine whether the volume collected may be a potential confounder for alpha amylase in addition to salivary flow rate.
ACKNOWLEDGMENTS
We thank Catherine Caponigro for her help in patient recruitment; Dr Driss Zoukhri for providing storage space for the saliva samples; and Drs Daniel Oreadi and Michael Thompson.
Figure 2.
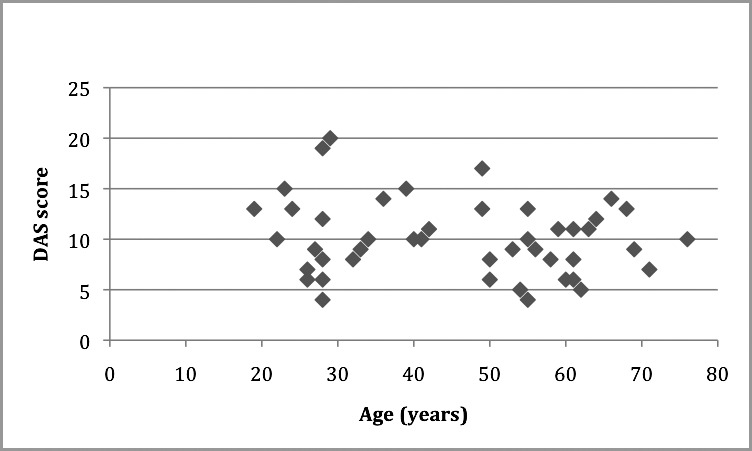
Scatter plot showing Dental Anxiety Scale (DAS) correlation with age (y). r = −0.193, P = .199.
REFERENCES
- 1.Thomson W, Broadbent J, Locker D, Poulton R. Trajectories of dental anxiety in a birth cohort. Community Dent Oral Epidemiol. 2009;37:209–219. doi: 10.1111/j.1600-0528.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Wijk A, Hoogstraten J. Experience of dental pain and fear of dental pain. J Dent Res. 2005;84:947–950. doi: 10.1177/154405910508401014. [DOI] [PubMed] [Google Scholar]
- 3.Oosterink F, De Jongh A, Hoogstraten J, Aartman I. The level of exposure–dental experiences questionnaire (LOE-DEQ): a measure of severity of exposure to distressing dental events. Eur J Oral Sci. 2008;116:353–361. doi: 10.1111/j.1600-0722.2008.00542.x. [DOI] [PubMed] [Google Scholar]
- 4.Crofts-Barnes N, Brough E, Wilson K, Beddis A, Girdler N. Anxiety and quality of life in phobic dental patients. J Dent Res. 2010;89:302–306. doi: 10.1177/0022034509360189. [DOI] [PubMed] [Google Scholar]
- 5.Agdal M, Raadal M, Skaret E, Kvale G. Oral health and its influence on cognitive behavioral therapy in patients fulfilling the diagnostic and statistical manual of mental disorders IV criteria for intraoral injection phobia. Acta Odontol Scand. 2010;2:98–105. doi: 10.3109/00016350903512792. [DOI] [PubMed] [Google Scholar]
- 6.Eitne S, Wichmann M, Paulse A, Holst S. Dental anxiety—an epidemiological study on its clinical correlation and effects on oral health. J Oral Rehabil. 2006;33:588–593. doi: 10.1111/j.1365-2842.2005.01589.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin K. Behavior-associated self reported items in patients charts as predictors of dental appointment avoidance. J Dent Educ. 2009;2:218–224. [PubMed] [Google Scholar]
- 8.Locker D, Shapiro D, Liddell A. Negative dental experiences and their relationship to dental anxiety. Community Dent Health. 1996;13:86–92. [PubMed] [Google Scholar]
- 9.Ng S, Leung W. A community study on the relationship of dental anxiety with oral health status and oral health-related quality of life. Community Dent Oral Epidemiol. 2008;36:347–356. doi: 10.1111/j.1600-0528.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 10.Vermaire J, De Jongh A, Aartman I. Dental anxiety and quality of life: the effect of dental treatment. Community Dent Oral Epidemiol. 2008;36:409–416. doi: 10.1111/j.1600-0528.2007.00416.x. [DOI] [PubMed] [Google Scholar]
- 11.Oosterink F, De Jongh A, Hoogstraten J. Prevalence of dental fear and phobia relative to other fear and phobia subtypes. Eur J Oral Sci. 2009;117:135–143. doi: 10.1111/j.1600-0722.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 12.Pohjola V, Lahti S, Vehkalahti M, Tolvanen M, Hausen H. Age-specific associations between dental fear and dental condition among adults in Finland. Acta Odontol Scand. 2008;66:278–285. doi: 10.1080/00016350802293960. [DOI] [PubMed] [Google Scholar]
- 13.Guzeldemir E, Toygar H, Cilasun U. Pain perception and anxiety during scaling in periodontally healthy subjects. J Periodontol. 2008;79:2247–2255. doi: 10.1902/jop.2008.080152. [DOI] [PubMed] [Google Scholar]
- 14.Rhudy J, Meagher M. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84:65–75. doi: 10.1016/S0304-3959(99)00183-9. [DOI] [PubMed] [Google Scholar]
- 15.Loggia M, Schweinhardt P, Bushnell C. Effect of psychological state on pain perception in the dental environment. J Can Dent Assoc. 2008;74:651–656. [PubMed] [Google Scholar]
- 16.Klages U, Kianifard S, Ulusoy O, Wehrbein H. Anxiety sensitivity as predictor of pain in patients undergoing restorative dental procedures. Community Dent Oral Epidemiol. 2006;34:139–145. doi: 10.1111/j.1600-0528.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- 17.Nater U, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34:486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Nater U, Rohleder N, Gaab J, et al. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int J Psychophysiol. 2005;55:333–342. doi: 10.1016/j.ijpsycho.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Nater U, Rohleder N, Schlotze W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 2007;32:392–401. doi: 10.1016/j.psyneuen.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Takaia N, Yamaguchib M, Aragakia T, Etoa K, Uchihashia K, Nishikawa Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch Oral Biol. 2004;49:863–968. doi: 10.1016/j.archoralbio.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Shah B, Ashok L, Sujatha G. Evaluation of salivary cortisol and psychological factors in patients with oral lichen planus. Indian J Dent Res. 2009;20:288–292. doi: 10.4103/0970-9290.57361. [DOI] [PubMed] [Google Scholar]
- 22.Miller C, Dembo J, Falace D, Kaplan A. Salivary cortisol response to dental treatment of varying stress. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:436–441. doi: 10.1016/s1079-2104(05)80123-4. [DOI] [PubMed] [Google Scholar]
- 23.Corah N. Development of a dental anxiety scale. J Dent Res. 1969;48:596. doi: 10.1177/00220345690480041801. [DOI] [PubMed] [Google Scholar]
- 24.Hill C, Walker R. Salivary cortisol determination and self-rating scales in the assessment of stress in patients undergoing the extraction of wisdom teeth. Br Dent J. 2001;191:513–515. doi: 10.1038/sj.bdj.4801220. [DOI] [PubMed] [Google Scholar]
- 25.Kanegane K, Penha S, Munhoz C, Rocha R. Dental anxiety and salivary cortisol levels before urgent dental care. J Oral Sci. 2009;51:515–520. doi: 10.2334/josnusd.51.515. [DOI] [PubMed] [Google Scholar]
- 26.Blomqvist M, Holmberg K, Lindblad F, Fernell E, Ek U, Dahllof G. Salivary cortisol levels and dental anxiety in children with attention deficit hyperactivity disorder. Eur J Oral Sci. 2007;116:1–6. doi: 10.1111/j.1600-0722.2007.00423.x. [DOI] [PubMed] [Google Scholar]
- 27.Georgelin-Gurgel M, Diemer F, Nicolas E, Hennequin M. Surgical and non-surgical endodontic treatment induced stress. J Endod. 2009;35:19–22. doi: 10.1016/j.joen.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Davis E, Granger D. Developmental differences in infant salivary alpha-amylase and cortisol responses to stress. Psychoneuroendocrinology. 2009;34:795–804. doi: 10.1016/j.psyneuen.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hakeberg M, Cunha L. Dental anxiety and pain related to dental hygienist treatment. Acta Odontol Scand. 2008;66:374–379. doi: 10.1080/00016350802415175. [DOI] [PubMed] [Google Scholar]
- 30.Van Wijk A, Makkes P. Highly anxious dental patients report more pain during dental injections. Br Dent J. 2008;205:E7. doi: 10.1038/sj.bdj.2008.583. [DOI] [PubMed] [Google Scholar]
- 31.Krueger T, Heller H, Hauffa B, Haake P, Exton M, Schedlowski M. The dental anxiety scale and effects of dental fear on salivary cortisol. Percept Mot Skills. 2005;100:109–117. doi: 10.2466/pms.100.1.109-117. [DOI] [PubMed] [Google Scholar]
- 32.Koray M, Dulger O, Ak G, et al. The evaluation of anxiety and salivary cortisol levels in patients with oral lichen planus. Oral Dis. 2003;9:298–301. doi: 10.1034/j.1601-0825.2003.00960.x. [DOI] [PubMed] [Google Scholar]
- 33.Brand HS. Anxiety and cortisol excretion correlate prior to dental treatment. Int Dent J. 1999;49:330–336. doi: 10.1111/j.1875-595x.1999.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 34.Rohleder N, Jutta M, Wolf E, Maldonado F, Kirschbaum C. The psychosocial stress-induced increase in salivary alpha-amylase is independent of saliva flow rate. Psychophysiology. 2006;43:645–652. doi: 10.1111/j.1469-8986.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- 35.Allwood M, Handwerger K, Kivlighanc K, Granger D, Stroude L. Direct and moderating links of salivary alpha-amylase and cortisol stress-reactivity to youth behavioral and emotional adjustment. Biol Psychol. 2011;88:57–64. doi: 10.1016/j.biopsycho.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang Y. Psychological stress-induced changes in salivary alpha-amylase and adrenergic activity. Nurs Health Sci. 2010;12:477–484. doi: 10.1111/j.1442-2018.2010.00562.x. [DOI] [PubMed] [Google Scholar]
- 37.Noto Y, Sato T, Kudo M, Kurata K, Hirota K. The relationship between salivary biomarkers and state-trait anxiety inventory score under mental arithmetic stress: a pilot study. Anesth Analg. 2005;101:1873–1876. doi: 10.1213/01.ANE.0000184196.60838.8D. [DOI] [PubMed] [Google Scholar]
- 38.Beltzer E, Fortunato C, Guaderrama M, Peckins M, Garramone B, Granger D. Salivary flow and alpha-amylase: collection technique, duration, and oral fluid type. Physiol Behav. 2010;1:289–296. doi: 10.1016/j.physbeh.2010.05.016. [DOI] [PubMed] [Google Scholar]


