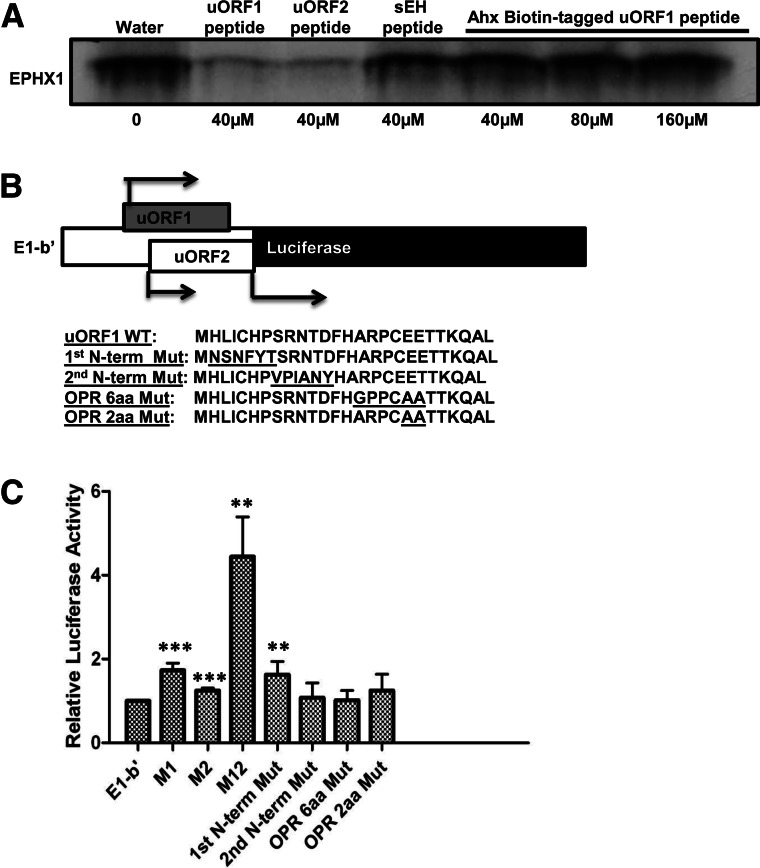
FIGURE 8.
The N-terminal amino acid sequence of E1-b′ uORF1 peptide is critical for its inhibitory function. (A) Rabbit reticulocyte lysates were programmed with full-length EPHX1 E1-b mRNA transcript, and in vitro translation occurred in the presence of [35S]-methionine with the addition of uORF peptides, control sEH peptide (40 µM, final concentration), or just water solvent and increasing concentration of Ahx-Biotin-tagged uORF1 peptide (40 µM, 80 µM, or 160 µM, final concentration). (B) Schematic showing the chimeric EPHX1-luciferase construct. E1-b′ 5′ UTR was cloned and replaced the luciferase 5′-leader sequence in the pGL3 control vector using the TK promoter. Amino acid coding sequences of wild-type E1-b′ uORF1 peptide and various mutation constructs were subsequently included: First N-terminal mutation, second N-terminal mutation, overlapping region (OPR) 6-amino-acid, and 2-amino-acid mutations. (C) Chimeric EPHX1-luciferase constructs were transfected into A549 cells. Renilla reniformis luciferase reporter (pRL-CMV) was used as an internal control. Luciferase assays were performed 24 h after transfection. M1, M2, and M12 constructs are chimeric EPHX1-luciferase constructs that contain mutated noninitiating start codons of uORF1, uORF2, or both, respectively. The data shown depict means and SD values derived from six separate experiments, each performed in triplicate. (Mutations of E1-b′ vs. E1-b′; [*] P < 0.05; [**] P < 0.01; [***] P < 0.001; Student’s t-test.)