Abstract
Graft arteriosclerois (GA), also called allograft vasculopathy, is a pathologic lesion that develops over months to years in transplanted organs characterized by diffuse, circumferential stenosis of the entire graft vascular tree. The most critical component of GA pathogenesis is the proliferation of smooth muscle-like cells within the intima. When a human coronary artery segment is interposed into the infra-renal aortae of immunodeficient mice, the intimas could be expand in response to adoptively transferred human T cells allogeneic to the artery donor or exogenous human IFN-γ in the absence of human T cells. Interposition of a mouse aorta from one strain into another mouse strain recipient is limited as a model for chronic rejection in humans because the acute cell-mediated rejection response in this mouse model completely eliminates all donor-derived vascular cells from the graft within two-three weeks. We have recently developed two new mouse models to circumvent these problems. The first model involves interposition of a vessel segment from a male mouse into a female recipient of the same inbred strain (C57BL/6J). Graft rejection in this case is directed only against minor histocompatibility antigens encoded by the Y chromosome (present in the male but not the female) and the rejection response that ensues is sufficiently indolent to preserve donor-derived smooth muscle cells for several weeks. The second model involves interposing an artery segment from a wild type C57BL/6J mouse donor into a host mouse of the same strain and gender that lacks the receptor for IFN-γ followed by administration of mouse IFN-γ (delivered via infection of the mouse liver with an adenoviral vector. There is no rejection in this case as both donor and recipient mice are of the same strain and gender but donor smooth muscle cells proliferate in response to the cytokine while host-derived cells, lacking receptor for this cytokine, are unresponsive. By backcrossing additional genetic changes into the vessel donor, both models can be used to assess the effect of specific genes on GA progression. Here, we describe detailed protocols for our mouse GA models.
Keywords: Medicine, Issue 75, Anatomy, Physiology, Biomedical Engineering, Bioengineering, Cardiology, Pathology, Surgery, Tissue Engineering, Cardiovascular Diseases, vascular biology, graft arteriosclerosis, GA, mouse models, transplantation, graft, vessels, arteries, mouse, animal model, surgical techniques
Introduction
Graft arteriosclerois (GA), also called allograft vasculopathy, is a pathologic lesion that develops over months to years in transplanted organs characterized by diffuse, circumferential stenosis of the entire graft vascular tree 7. Early stages may cause eccentric and focal stenoses that are more obvious in arteries, thus more closely resembling stenoses seen in conventional atherosclerosis. The lumen loss of the graft vessels results from intimal expansion due to infiltration of host T cells and macrophages and especially to accumulation of extracellular matrix and smooth muscle-like cells originated from graft, host or both 5, 13, 19, that is inadequately compensated by outward vessel remodeling. In cardiac allografts, the most clinically significant lesions are those in the epicardial and intramyocardial coronary arteries. Ultimately, GA of the coronary arteries will cause ischemic heart failure. GA is the major cause of late cardiac graft loss. The stenoses of GA stop at the suture lines, strongly implicating the host response to graft alloantigens in its pathogenesis and leading us to classify GA as a form of chronic rejection 3. However, other forms of arterial injury may increase the risk of GA, either through increasing the net burden of injury or by intensifying and/or modulating the alloimmune response. The endothelial cell (EC) lining of graft arteries is preserved in human GA and the most superficial regions of the intima adjacent to the EC lining is the site most heavily infiltrated by host-derived IFN-γ-producing T cells and macrophages 11; in some patients GA is associated with the development of donor-specific alloantibodies that bind to graft EC 16 but the vessels show little evidence of the fibrinoid necrosis that is characteristic of acute antibody-mediated rejection 11.
The most critical component of GA pathogenesis is the proliferation of smooth muscle -like cells within the intima; if this process can be arrested, GA is unlikely to progress. Previous work from our group had shown that intimas of human coronary artery segments interposed into the infra-renal aortae of immunodeficient mice expand in response to adoptively transferred human T cells allogeneic to the artery donor and that this process could be inhibited by neutralizing human IFN-γ 18. Furthermore exogenous human IFN-γ could cause intimal (and medial) vascular smooth muscle cell (VSMC) proliferation in these arterial grafts in the absence of human T cells 15, 17. (It's important to note that human and mouse IFN-γ do not cross species, ruling out indirect effects on the mouse host in this experimental system.) These humanized mouse models have the benefit of recapitulating human T cell/vascular cell interactions and the intimal lesions are largely composed of human (i.e. graft-derived) cells, as has been observed in clinical specimens, but they do not fully recapitulate the clinical situation because they ignore the role of host macrophages and possibly other cell types involved in clinical transplant lesions. A conventional mouse model of this process could theoretically address this problem, complementing the limitations of the humanized model by involving a complete host immune system and providing the additional advantage of allowing the power of mouse genetic approaches to be applied to GA. The two most widely used mouse models involve heterotopic heart transplantation and orthotopic artery transplantation 1. The lesions that develop in the arteries of heterotopic heart grafts are largely made up of host cells, likely of bone marrow origin, whereas intimal cells of the arteries in human heart grafts are predominantly of graft origin 5, 13, 19. This is a significant distinction that has led us to develop alternative mouse models. Interposition of a mouse aorta from one strain into another mouse strain recipient is even more limited as a model for chronic rejection in humans because the acute cell-mediated rejection response in this mouse model completely eliminates all donor-derived vascular cells from the graft within two-three weeks 19. Consequently, the subsequent changes seen in the interposed vessel segment are solely a response of host cells that have repopulated the decellularized vessel scaffold, creating a highly artifactual situation of limited relevance as a model for the changes in graft vessels that occur in the clinic. We have recently developed two new mouse models to circumvent these problems 21. The first model involves interposition of a vessel segment from a male mouse into a female recipient of the same inbred strain (C57BL/6J). The second model involves interposing an artery segment from a wild type C57BL/6J mouse donor into a host mouse of the same strain and gender that lacks the receptor for IFN-γ (IFN-γR-KO) followed by administration of mouse IFN-γ (delivered via infection of the mouse liver with an adenoviral vector. Here, we describe detailed protocols and the advantages of our mouse GA models.
Protocol
Mouse allograft and syngeneic graft transplantation model
All animal studies were approved by the institutional animal care and use committees of Yale University. For allograft model, segments of thoracic aorta from male, 4-5 week old WT (C57BL/6J) or IFN-γR-KO mice were interposed into the abdominal aorta of female recipient, 8-12 week old WT using an end-to-end microsurgical anastomotic technique (see next for details). For syngeneic graft model, segments of thoracic aortae from male, 4-5 week old WT mice were interposed into the abdominal aortae of male, 8-12 week old IFN-γR1-KO using an end-to-end microsurgical anastomotic technique. At 1 week postoperatively, the animals were inoculated i.v. with Ad5.CMV-mouse IFN-γ or Ad5.CMV-LacZ (Qbiogene) at 1 x 109 PFU. Serum mouse IFN-γ levels were measured by ELISA (eBioscience) at 1 and 5 weeks after adenovirus administration. Certain animals received BrdU (Sigma-Aldrich) at 100 mg/kg s.c. for 2 weeks before sacrifice.
End-to-end microsurgical anastomotic technique (Video will be taken for this part):
1. Donor Procedure
Anesthetize the donor mice with intraperitoneal injections of ketamine (50 mg/kg) and xylazine (10 mg/kg).
After ensuring adequate anesthesia, place the donor mice under the operating microscope at X8-30 magnification on a tray in a supine position, and use Iodine Prep Pad and Alcohol Prep Pad for chest wall preparation.
Incise the anterior chest wall through the ribs and diaphragm to expose the heart.
Open the right atrium, inject 10 ml of heparinized (100 U/ml) saline solution into the left ventricle to flush the mice using a 10-ml syringe with a 25-gauge needle.
Resect the heart and lungs to expose the entire length of thoracic aorta.
Dissect the thoracic aorta carefully to minimize the direct trauma, while identify, ligate and divide the lumber branches using 11-0 suture near the aorta.
Once the thoracic aorta is free from surrounding tissue, excise the whole thoracic aorta for transplantation.
Flush the cut end of the donor aorta with heparinized (100 U/ml) saline solution, and store it in the same solution on ice (up to 6 hr) until transplantation.
2. Recipient Procedure
Inject the recipient mice with i.p. ketoprofen (5 mg/kg) for analgesia, and thenanesthetize the mice with i.p. ketamine (50 mg/kg) and xylazine (10 mg/kg). The recipient mice are deeply anesthetized within 10 minutes for a duration of 60-90 min. During surgery, the level of anesthesia is assessed every 15 min by pinching the anterior abdominal wall or foot using forceps. If the animal reacts to the noxious stimuli at anytime during surgery, an additional dose of ketamine and xylazine (1/4 or 1/3 original dose) will be administered by s.c. or i.m.
After ensuring adequate anesthesia, shave off the fur in the anterior abdominal wall using an electric shaver, clean the skin using Iodine Prep Pad and Alcohol Prep Pad, cover the eyes using ophthalmic ointment. During surgery, the following aseptic techniques will be used for the operations, including cleaning the operating table with 10% bleach, wearing sterile gloves and using sterilized microsurgery instruments(steam-sterilized instruments for the start of the operation, then hot glass bead-sterilized instruments between multiple operations, etc).
Place the recipient mice under the operating microscope at a magnification of X8-30 on a tray in a supine position.
Incise the abdominal wall at the midline from xyphoid to pubis, and spread the abdominal wall apart using a micro-retractor to expose the abdominal cavity.
Retract the intestines superiorly and wrap with gauze moistened with saline solution. The recipient mice become chilled by loss of heat through externalized intestines. It is important to maintain animal warmth. However, the heating board is not used during the surgery as modest hypothermia is of benefit in preventing spinal injury during occlusion of aortic blood flow as well as there is inefficient heat transfer with absent blood flow to the lower half of the mice body.
Move the reproductive organs inferiorly, wrap the abdomen areas of recipient mice with saline-solution-moistened gauze, and use it to retract the rectum to right side of the abdomen. The exposed tissues are irrigated periodically with saline solution during transplantation.
Dissect bluntly a segment of infrarenal aorta between the renal vessel proximally and aortic bifurcation distally and separate from inferior vena cava (IVC) carefully.
Identify and ligate all of the small branches originating from this segment of abdominal aorta using 11-0 Polyamide Monofilament suture.
Cross-clamp the isolated segment of abdominal aorta using two vascular clamps proximately 5 mm apart, one at each end.
Transect the abdominal aorta between the clamps using sharp micro-scissors to create the anastomotic sites.
Resect a small segment of abdominal aorta (up to 0.4 mm in length) from one cut end of transected recipient aorta to accommodate the donor aortic graft.
Flush the aortic segments between clamps using heparinized saline solution to remove the residual blood. The aortic segments between clamps and the donor aortic graft are irrigated periodically with heparinized (100 U/ml) saline solution (up to 40 ml/kg) during anastomosis.
Transect both sides of donor aorta with sharp micro-scissors to create a segment graft of 2.5 mm in length for transplantation.
Place the donor aortic graft in the orthotopic position, anastomose the donor graft's proximal and distal end to the recipient's proximal and distal cut end of abdominal aorta, respectively, with an end-to-end pattern using 11-0 Polyamide Monofilament suture.
For the continuous sutures, place the stay sutures at 3 and 9 o'clock positions firstly. Between two stay sutures at each side of graft, anastomose both cut edges in 3-4 stitches using the running sutures, and tie the running sutures to stay sutures with a double knot. Since both layers of closure are continuous, the risk of dehiscence is increased. The donor aortic graft should be in appropriate length to connect the recipient's proximal and distal cut end of abdominal aorta.
For the interrupted sutures, construct four anastomosis at 3 and 9 o'clock positions in both sides of graft firstly, and anastomose both cut edges in 3-4 stitches between two stay sutures at each side of graft.
Release the distal clamp to allow the retrograde flow into graft, identify the bleeding sites and make additional stitches within 3 minutes.
After ascertaining satisfactory hemostasis, release the proximal clamp.
Confirm the graft patency by presence of vigorous pulsation in the graft and the proximal adjacent segment of native abdominal aorta, in particular in the distal adjacent segment of abdominal aorta and inferior mesenteric artery. Consider thrombosis in anastomotic sites if the pulsation diminish within a few minutes after the restoration of blood flow.
Return the intestines into the abdominal cavity.
Suture close the abdominal wall using 5-0 Nylon suture in muscle layer and skin layer, respectively.
Place the recipient mice into a clean cage on the top of a heating pad, and wait 1-2 hr for the mice to regain consciousness and recover from anesthesia. It is important to maintain animal adequate warmth during recovery. Keep the mice in the warm and dry cage before animal wake from anesthesia.
Assess the motor function of hind limbs after mice recovery, and again on the second day. A successful transplantation surgery will be confirmed if no dysfunction of hind limbs is observed in recipient mice on the second day.
Administer the recipient mice with Ketoprofen in drinking water (5 mg/kg/d = 27 ug/ml in drinking water) for 48 hr. If any recipient mice suffer from pain unrelieved by the postoperative analgesics as evidenced by loss of mobility, failure to groom, abnormal bunched posture, etc, they will be euthanized. The animals will be euthanized at predefined end-points after 1-60 days.
Graft analysis
Artery grafts in allograft were procured at 4 weeks and grafts in syngeneic graft model were 6 weeks post-operatively (5 weeks after viral infection) and analyzed by standard histological techniques for Elastica-van Gieson (EVG) staining, hematoxylin and eosin (HE) staining and immunofluorescence staining. Pictures were taken using an immunofluorescence microscope system (Zeiss). Cell counting of nuclei surrounded by positive immunostaining was performed under high magnification and averaged from 5 cross-sections for each graft. The graft area measurements of the lumen (within the endothelium), intima (between the endothelium and internal elastic lamina, IEL), media (between the IEL and external elastic lamina, EEL), wall thickness (between the endothelium and external elastic lamina) and whole vessel (within the EEL) were calculated from 5 serial cross-sections, 150 μm apart for each graft, using computer-assisted image analysis and NIH Image 1.60 (http://rsbweb.nih.gov/nih-image).
Statistical analysis
All data are expressed as mean ± SEM. Two-tailed, paired t tests and a two-way ANOVA analysis were performed using the Prism software program (GraphPad Software). Differences with P<0.05 were considered to indicate statistical significance.
Representative Results
Mouse allograft arteriosclerosis (GA) model: In this model, a male donor aorta is transplanted into female recipient so that the host induces alloreactive T cell-mediated alloimmune responses against a minor Y antigen (the male-specific minor histocompatibility antigen H-Y) expressed on the graft 12, and in turn T cell-produced IFN-γ drives VSMC proliferation 20 as observed in other allograft transplantation models 2, 6, 8-10, 14. A segment of thoracic aorta from a donor male was surgically interposed into an abdominal aorta of a female recipient, and aortas were harvested at 4 weeks post-transplantation (Figure 1A). Histological analysis of artery grafts was performed by Elastica-van Gieson (EVG), H&E and immunostaining with VSMC marker SMA and pan-leukocyte marker CD45. No graft rejection was observed between C57BL/6J male mice in the aorta transplantation. Male to female transplantation induced GA, characterized by infiltration of leukocytes and neointima formation with accumulation of VSMC (Figure 1B). To determine the role of IFN-γ signaling in GA, aortas from WT or IFN-γR-KO mice were transplanted to WT female recipient. Deletion of IFN-γR in donor grafts exhibited a reduced infiltration of leukocytes and neointima formation with decreases of SMA-positive cells compared to WT (not shown). These results support a critical role for IFN-γ signaling in GA progression as previously observed in humanized mouse xenograft transplantation model 4, 15, 17 , 18.
IFN-γ-induced graft arteriosclerosis: It has been established that IFN-γalone is sufficient to induce neointima formation in a humanized mouse GA model 15, 17. Here we established an IFN-γ-mediated mouse syngeneic graft arteriosclerosis model. In this model, WT male aortas were transplanted into IFN-γR-deficient recipient mice followed by intravenously injection of replication-deficient adenovirus encoding the mouse IFN-γ transgene (Ad-IFN-γ) or the control LacZ gene (Ad-LacZ) (Figure 2A). Hepatic infection and liver transgene expression of Ad-LacZ was verified by X-gal staining in the Ad-LacZ group but not the Ad-IFN-γ groups as described previously 17. Systemic expression of IFN-γ in serum was detected at a level of 120-150 ng/ml on day 3 and retained up to 5 weeks in the Ad-IFN-γ but not in the LacZ group. Aortas were harvested at 5 weeks post-injection of adenovirus for histological analysis and morphometric assessment of artery graft intima, media, lumen and vessel area. Expression of IFN-γ, but not LacZ control, induced neointimal formation. No obvious infiltration of leukocytes was detected compared to the allograft model (Figure 2B). These data are consistent with the previous observations in humanized mouse xenograft model that IFN-γ alone is sufficient to induce neointima formation 15, 17.
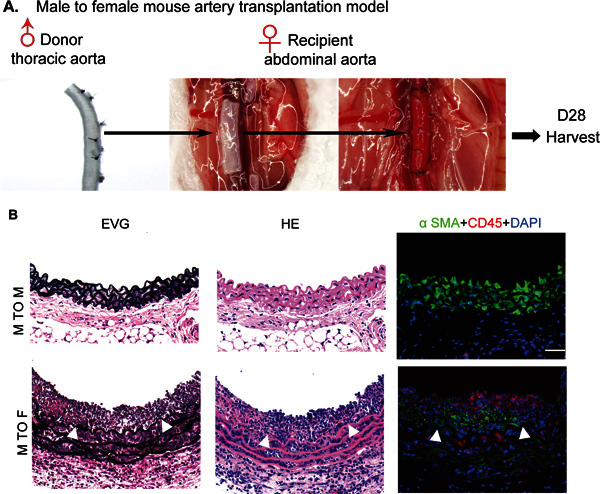 Figure 1. Illustration and scheme of mouse allograft artery transplantation model. A. An illustration of the allograft transplantation procedure. A segment of donor male thoracic aorta was surgically interposed into abdominal aorta in a female recipient. Aortas were harvested at 4 weeks post-transplantation. As a control, a segment of male donor thoracic aorta was surgically interposed into an abdominal aorta in a male recipient. B. Histological analysis of artery grafts by Elastica-van Gieson (EVG), H&E and immunostaining with anti-a-SMA and anti-CD45. Representative photomicrographs are shown. Arrowheads mark the internal elastic lamina to delineate the intima from media. Scale bar: 50 mm. No graft arteriosclerosis was observed in male to male group.
Figure 1. Illustration and scheme of mouse allograft artery transplantation model. A. An illustration of the allograft transplantation procedure. A segment of donor male thoracic aorta was surgically interposed into abdominal aorta in a female recipient. Aortas were harvested at 4 weeks post-transplantation. As a control, a segment of male donor thoracic aorta was surgically interposed into an abdominal aorta in a male recipient. B. Histological analysis of artery grafts by Elastica-van Gieson (EVG), H&E and immunostaining with anti-a-SMA and anti-CD45. Representative photomicrographs are shown. Arrowheads mark the internal elastic lamina to delineate the intima from media. Scale bar: 50 mm. No graft arteriosclerosis was observed in male to male group.
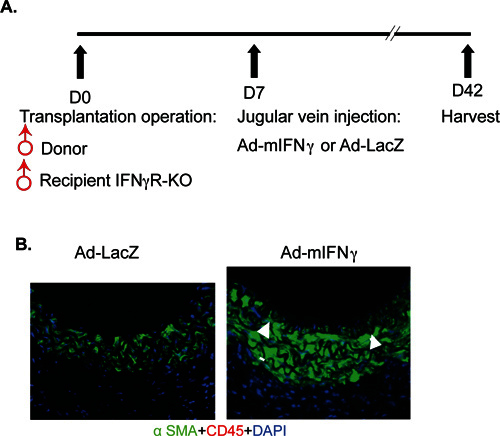 Figure 2. IFN-γ-induced mouse syngeneic artery transplantation model. A. A scheme of the IFN-γ-induced mouse syngeneic model. Male donor thoracic artery was dissected and transplanted into abdominal aorta in male recipient IFN-γR KO mice. One week post surgery, 1X109 pfu Ad-mIFN-γ or Ad-LacZ was injected into recipient mice via jugular vein. Serum was collected on day 3, 7 and 35 post-injection of viruses for IFN-γ measurement. Grafts were harvested 6 weeks for morphometric assessment. B. Histological analysis of artery grafts by immunostaining with anti-a-SMA and anti-CD45. Representative photomicrographs are shown. Arrowheads mark the internal elastic lamina to delineate the intima from media. Scale bar: 50 mm. No graft arteriosclerosis was observed in LacZ group.
Figure 2. IFN-γ-induced mouse syngeneic artery transplantation model. A. A scheme of the IFN-γ-induced mouse syngeneic model. Male donor thoracic artery was dissected and transplanted into abdominal aorta in male recipient IFN-γR KO mice. One week post surgery, 1X109 pfu Ad-mIFN-γ or Ad-LacZ was injected into recipient mice via jugular vein. Serum was collected on day 3, 7 and 35 post-injection of viruses for IFN-γ measurement. Grafts were harvested 6 weeks for morphometric assessment. B. Histological analysis of artery grafts by immunostaining with anti-a-SMA and anti-CD45. Representative photomicrographs are shown. Arrowheads mark the internal elastic lamina to delineate the intima from media. Scale bar: 50 mm. No graft arteriosclerosis was observed in LacZ group.
| Step | Problem | Possible reason | Solution |
| 2.17 | Severe bleeding | The gap (distance between two stitches) is too large, in particular of interrupted sutures. | The distance between every two stitches should be uniform during anastomosis. Add one stitch at bleeding site. |
| Only the adventitia is stitched. | Identifying and stitching the whole aortic wall | ||
| Unligated lumber branches | Ligating the lumber branches | ||
| Using too much heparin | Using up to 40 ml/kg heparinized (100 U/ml) saline solution to irrigate the surgery field during anastomosis. | ||
| 2.19 | Thrombosis | Lumen is too narrow at anastomotic site, in particular of continuous sutures. | Avoid anastomosing too many aortic wall at each stitch. The aorta size of mouse is very small. To stitch too many aortic wall during anastomosis will cause lumen narrow and thrombosis, however, to stitch too less aortic wall will result in severe bleeding. |
| The suture pass through aortic wall at the opposite side. | Identifying the aortic wall and then making stitches. If it happened, the operator should cut off the suture knot and re-stitch at this site. | ||
| Endothelial cells are injured during anastomosis. | Using the stay sutures for anastomosis. Do not hold/squeeze the whole arotic wall by forceps for anastomosis. | ||
| Heparin is not used during anastomosis. | The aorta size of mouse is very small. It is very easy for thrombosis formation in the grafts after anastomosis. Giving heparin to irrigate the surgery field is very important for reducing the thrombosis. The success rate of vessel transplantation in mice is around 50% without using heparin and 95-100% with using heparin. If the thrombosis is found within a few minutes after the restoration of blood flow, an alternative approach to remove the thrombosis is to inject 30-50 ul heparinied saline solution(100 U/ml) through IVC. Do not inject too much heparin just in case of severe bleeding. | ||
| 2.23 | Transplantation failed | Delayed bleeding and thrombosis, or others | The vessel transplantation in mice is very difficult surgery for the aorta size of mouse is very small. There are three key points for the successful surgery: 1) make sure the aortic walls of host and donor are anastomosed together without obviously gap, just in case of severe bleeding; 2) keep enough lumen space at anastomotic site, just in case of thrombosis; 3) use heparin in a right dose during anastomosis, just in case of thrombosis and severe bleeding. Following this protocol, the success rate for the author is more than 95%. |
Table 1. Troubleshooting table.
Discussion
The described protocols are focused on mouse GA models. The procedures can be applied to other graft transplantation models. These models include humanized xenograft (i.e. human coronary artery segments interposed into the infra-renal aortae of immunodeficient mice), and acute rejection mouse GA model (i.e. a mouse aorta from one genetic strain into another genetic strain recipient). Our described mouse models are more to close human GA lesions. The first model involves interposition of a vessel segment from a male mouse into a female recipient of the same inbred strain (C57BL/6J). Graft rejection in this case is directed only against minor histocompatibility antigens encoded by the Y chromosome (present in the male but not the female) and the rejection response that ensues is sufficiently indolent to preserve donor-derived smooth muscle cells for several weeks 2, 6, 8-10, 12, 14. The second model involves interposing an artery segment from a wild type C57BL/6J mouse donor into a host mouse of the same strain and gender that lacks the receptor for IFN-γ (IFN-γR-KO) followed by administration of mouse IFN-γ (delivered via infection of the mouse liver with an adenoviral vector.) There is no rejection in this case as both donor and recipient mice are of the same strain and gender but donor smooth muscle cells proliferate in response to the cytokine while host-derived cells, lacking receptor for this cytokine, are unresponsive 21. Furthermore, by backcrossing additional genetic changes into the vessel donor, both models can be used to assess the effect of specific genes on IFN-γ-driven smooth muscle cell proliferation and GA progression.
The role of IFN-γ in mouse GA models has not been well established. Our two mouse models have verified the role of IFN-γ in mouse GA. In the first model, a male donor aorta is transplanted into female recipient so that the host induces alloreactive T cell-mediated alloimmune responses against the male-specific minor histocompatibility antigen H-Y expressed on the grafts 12. Indeed, WT (C57BL/6J) male to C57BL/6J female transplantation induced GA, characterized by infiltration of leukocytes and neointima formation with VSMC accumulation. In contrast, male to male aorta transplantation does not induce GA. We determined the role of IFN-γ signaling in vascular cells by using IFN-γR-KO as donor graft transplanted to WT female recipient. Deletion of IFN-γR in donor grafts blunted leukocyte infiltration and VSMC accumulation in the neointima. Therefore, the role of IFN-γ in current mouse model is consistent with the humanized mouse xenograft transplantation model where IFN-γ signaling in graft is critical for GA progression 4, 15, 17, 18. We determined if IFN-γ alone is sufficient to induce neointima formation by creating an IFN-γ-mediated mouse syngeneic graft arteriosclerosis model. In this model, a male aorta is transplanted into a male IFN-γR-KO recipient animal in which mouse IFN-γ is then systemically expressed by intravenously injection of replication-deficient adenovirus encoding the mouse IFN-γ transgene (Ad-IFN-γ). We observed that expression of IFN-γ, but not LacZ control, induced neointimal formation. In this model, no obvious infiltration of leukocytes is detected compared to the allograft model, consistent with the previous observations in a human artery-SCID mouse transplantation model in which vascular IFN-γ signaling alone is sufficient to induce neointima formation in the absence of leukocytes 15, 17.
Disclosures
We have nothing to disclose.
Acknowledgments
This work was supported by NIH grants R01 HL109420 to WM and AHA 9320033N to LY.
References
- George JF, Pinderski LJ, Litovsky S, Kirklin JK. Of mice and men: mouse models and the molecular mechanisms of post-transplant coronary artery disease. J. Heart Lung Transplant. 2005;24:2003–2014. doi: 10.1016/j.healun.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Koulack J, McAlister VC, MacAulay MA, Bitter-Suermann H, MacDonald AS, Lee TD. Importance of minor histocompatibility antigens in the development of allograft arteriosclerosis. Clin. Immunol. Immunopathol. 1996;80:273–277. doi: 10.1006/clin.1996.0123. [DOI] [PubMed] [Google Scholar]
- Libby P, Pober JS. Chronic rejection. Immunity. 2001;14:387–397. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- Lorber MI, Wilson JH, Robert ME, Schechner JS, Kirkiles N, Qian HY, Askenase PW, Tellides G, Pober JS. Human allogeneic vascular rejection after arterial transplantation and peripheral lymphoid reconstitution in severe combined immunodeficient mice. Transplantation. 1999;67:897–903. doi: 10.1097/00007890-199903270-00018. [DOI] [PubMed] [Google Scholar]
- Minami E, Laflamme MA, Saffitz JE, Murry CE. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;112:2951–2958. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- Mitchell RN. Allograft arteriopathy: pathogenesis update. Cardiovasc. Pathol. 2004;13:33–40. doi: 10.1016/S1054-8807(03)00108-X. [DOI] [PubMed] [Google Scholar]
- Mitchell RN. Graft vascular disease: immune response meets the vessel wall. Annu Rev Pathol. 2009;4:19–47. doi: 10.1146/annurev.pathol.3.121806.151449. [DOI] [PubMed] [Google Scholar]
- Nagano H, Libby P, Taylor MK, Hasegawa S, Stinn JL, Becker G, Tilney NL, Mitchell RN. Coronary arteriosclerosis after T-cell-mediated injury in transplanted mouse hearts: role of interferon-gamma. Am. J. Pathol. 1998;152:1187–1197. [PMC free article] [PubMed] [Google Scholar]
- Nagano H, Mitchell RN, Taylor MK, Hasegawa S, Tilney NL, Libby P. Interferon-gamma deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J. Clin. Invest. 1997;100:550–557. doi: 10.1172/JCI119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisanen-Sokolowski A, Glysing-Jensen T, Koglin J, Russell ME. Reduced transplant arteriosclerosis in murine cardiac allografts placed in interferon-gamma knockout recipients. Am. J. Pathol. 1998;152:359–365. [PMC free article] [PubMed] [Google Scholar]
- Salomon RN, Hughes CCW, Schoen FJ, Payne DD, Pober JS, Libby P. Human Coronary Transplantation-Associated Arteriosclerosis - Evidence for a Chronic Immune Reaction to Activated Graft Endothelial Cells. Am. J. Pathol. 1991;138:791–798. [PMC free article] [PubMed] [Google Scholar]
- Scott DM, Ehrmann IE, Ellis PS, Chandler PR, Simpson E. Why do some females reject males? The molecular basis for male-specific graft rejection. J. Mol. Med. 1997;75:103–114. doi: 10.1007/s001090050095. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Sugiyama S, Aikawa M, Fukumoto Y, Rabkin E, Libby P, Mitchell RN. Host bone-marrow cells are a source of donor intimal smooth- muscle-like cells in murine aortic transplant arteriopathy. Nat. Med. 2001;7:738–741. doi: 10.1038/89121. [DOI] [PubMed] [Google Scholar]
- Tellides G, Pober JS. Interferon-gamma axis in graft arteriosclerosis. Circ. Res. 2007;100:622–632. doi: 10.1161/01.RES.0000258861.72279.29. [DOI] [PubMed] [Google Scholar]
- Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- Vassalli G, Gallino A, Weis M, von Scheidt W, Kappenberger L, von Segesser LK, Goy JJ. Alloimmunity and nonimmunologic risk factors in cardiac allograft vasculopathy. Eur. Heart J. 2003;24:1180–1188. doi: 10.1016/s0195-668x(03)00237-9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bai Y, Qin L, Zhang P, Yi T, Teesdale SA, Zhao L, Pober JS, Tellides G. Interferon-gamma induces human vascular smooth muscle cell proliferation and intimal expansion by phosphatidylinositol 3-kinase dependent mammalian target of rapamycin raptor complex 1 activation. Circ. Res. 2007;101:560–569. doi: 10.1161/CIRCRESAHA.107.151068. [DOI] [PubMed] [Google Scholar]
- Wang Y, Burns WR, Tang PC, Yi T, Schechner JS, Zerwes HG, Sessa WC, Lorber MI, Pober JS, Tellides G. Interferon-gamma plays a nonredundant role in mediating T cell-dependent outward vascular remodeling of allogeneic human coronary arteries. Faseb J. 2004;18:606–608. doi: 10.1096/fj.03-0840fje. [DOI] [PubMed] [Google Scholar]
- Yacoub-Youssef H, Marcheix B, Calise D, Thiers JC, Benoist H, Blaes N, Segui B, Dambrin C, Thomsen M. Chronic vascular rejection: histologic comparison between two murine experimental models. Transplant. Proc. 2005;37:2886–2887. doi: 10.1016/j.transproceed.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Yokota T, Shimokado K, Kosaka C, Sasaguri T, Masuda J, Ogata J. Mitogenic activity of interferon gamma on growth-arrested human vascular smooth muscle cells. Arterioscler. Thromb. 1992;12:1393–1401. doi: 10.1161/01.atv.12.12.1393. [DOI] [PubMed] [Google Scholar]
- Yu L, Qin L, Zhang H, He Y, Chen H, Pober J, Tellides G, Min W. AIP1 prevents graft arteriosclerosis by inhibiting IFN-γ-dependent smooth muscle cell proliferation and intimal expansion. Cir. Res. 2011;109:418–427. doi: 10.1161/CIRCRESAHA.111.248245. [DOI] [PMC free article] [PubMed] [Google Scholar]


