Abstract
Forward and reverse signaling mediated by EphB tyrosine kinase receptors and their transmembrane ephrin-B ligands play important roles in axon pathfinding, yet little is known about the intracellular pathways involved. Here we have used growth cones from the ventral (EphB receptor-bearing) and dorsal (ephrin-B-bearing) embryonic Xenopus retina to investigate the signaling mechanisms in both forward and reverse directions. We report that unclustered, but not clustered, EphB2 ectodomains trigger fast (5–10 min) transient collapse responses in growth cones. This collapse response is mediated by low levels of intracellular cyclic GMP and requires proteasome function. In contrast, clustered, but not unclustered, ephrin-B1 ectodomains cause slow (30–60 min) growth cone collapse that depends on high cGMP levels and is insensitive to inhibition of the proteasomal pathway. Upon receptor-ligand binding, endocytosis occurs in the reverse direction (EphB2-Fc into dorsal retinal growth cones), but not the forward direction, and is also sensitive to proteasomal inhibition. Endocytosis is functionally important because blocking of EphB2 internalization inhibits growth cone collapse. Our data reveal that distinct signaling mechanisms exist for B-type Eph/ephrin-mediated growth cone guidance and suggest that endocytosis provides a fast mechanism for switching off signaling in the reverse direction.
Keywords: Eph receptor; ephrin; growth cone collapse; signaling pathway; endocytosis; proteasome; retina, Xenopus laevis
INTRODUCTION
The Eph family of receptor tyrosine kinases consists of 15 identified members that fall into two subclasses, A and B, based on sequence similarities and binding preferences for GPI-anchored ephrin-A and transmembrane ephrin-B ligands, respectively. An unusual and unique feature of Eph receptors and ephrins is their ability to initiate bidirectional signaling where a signal is propagated in both the Eph receptor-bearing cell (forward signaling) and in the ligand-expressing cell (reverse signaling). During development, Eph receptors and ephrins are highly expressed in the central nervous system and are key regulators of axon pathfinding. Their function is best characterized in the visual system where both forward and reverse signaling have been implicated in the intraretinal growth of ganglion cell axons, the divergence of ipsi- and contralateral projections at the chiasm, and the formation of the topographic retinotectal map (reviewed in Wilkinson, 2000; Mann and Holt, 2001; Knoll and Drescher, 2002; McLaughlin et al., 2003).
Stimulation of growing primary neurons with either Eph or ephrin molecules often triggers a repulsive response resulting in growth cone collapse and neurite retraction (Drescher et al., 1995; Meima et al., 1997a,b; Monschau et al., 1997; Wang and Anderson, 1997; Imondi et al., 2000; Birgbauer et al., 2001), although behaviors attributed to an attractive or permissive activity have also been observed (Holash et al., 1997; Gao et al., 2000; Zhou et al., 2001; Mann et al., 2002; Weinl et al., 2003). Recent studies have begun to identify the mechanism of Eph receptor activation and downstream signaling components that control cytoskeletal dynamics. Several pathways have been characterized for the Eph-mediated collapse response, which include interactions with the Abl family of noncytoplasmic kinases, down-regulation of the Ras-mitogen-activated protein kinase (Ras-MAPK) pathway, and differential regulation of Rho family GTPases by Ephexin (Wahl et al., 2000; Elowe et al., 2001; Shamah et al., 2001; Carter et al., 2002; reviewed in Kullander and Klein, 2002). The mechanisms of ephrin reverse signaling are still poorly understood, however. The identification of a signaling connection between ephrin-B and the Src-homology-2 (SH2) domain protein Grb4, which binds to several known cytoskeleton regulators, provides a possible link as to how ephrin-Bs transduce information leading to growth cone retraction (Cowan and Henkemeyer, 2001).
Recently studies have revealed that local protein synthesis within growth cones contributes to several aspects of axon navigation, including the mechanisms through which attractive or repulsive responses to chemotropic cues are transduced (Campbell and Holt, 2001). For example, in cultured Xenopus retinal neurons, growth cone collapse induced by the axon guidance cue Sema3A can be inhibited by protein synthesis inhibitors. Interestingly, lysophosphatidic acid (LPA), another collapse-inducing factor for retinal axons, causes a rapid rise in the level of ubiquitinated proteins in the growth cone and its collapsing activity can be blocked by inhibitors of the proteasomal pathway. This raises the intriguing possibility that different extracellular signals cause the collapse of the motile structures of the growth cone by inducing rapid and local changes in protein levels. These changes are mediated by distinct intracellular pathways that require either newly synthesized proteins or proteasome-mediated degradation. The nature of the proteins involved, however, remains to be elucidated. Moreover, the extent to which these pathways are used by other axon guidance molecules such as Eph and ephrins is not known.
In the developing visual system, B-type Ephs/ephrins are expressed in a complementary pattern with dorsal neurons expressing ephrin-B and ventral cells expressing EphB receptors (Holash et al., 1997; Birgbauer et al., 2000; Nakagawa et al., 2000; Mann et al., 2002). EphB/ephrin-B interactions play several roles in directing the growth of retinal axons. In Xenopus, EphB-bearing ventral axons are routed ipsilaterally by an ephrin-B signal localized at the chiasm (forward signaling) (Nakagawa et al., 2000), and ephrin-B-bearing dorsal axons sort themselves in their target, the optic tectum, using a gradient of EphB (reverse signaling) (Mann et al., 2002). In this article, we show that in the reverse signaling direction (dorsal axons), soluble unclustered EphB2-Fc causes fast growth cone collapse that is mediated by low cGMP and is sensitive to inhibitors of proteasomal degradation. For forward signaling (ventral growth cones), soluble clustered ephrin-B1-Fc causes slow growth cone collapse that is mediated by high cGMP and is not sensitive to inhibitors of proteasomal degradation. Endocytosis of the ectodomain only occurs in the reverse direction and pharmacological inhibition of endocytosis also blocks fast collapse. Our conclusion is that signaling in the reverse and forward directions involves distinct pathways and that the presentation of the ligand/receptor (i.e., clustered versus unclustered) influences the signaling and biological response of the receiving cell. In addition, the proteasome pathway may play a role in regulating endocytosis and the surface availability of ephrin-B1 in reverse signaling.
MATERIALS AND METHODS
Animals
Xenopus laevis embryos were raised from eggs obtained by in vitro fertilization of oocytes from adult female frogs injected with human chorionic gonadotropin hormone (Sigma). Stages were determined according to Nieuwkoop and Faber (1967).
Pharmacological Reagents
Pharmacological reagents used in this study were as follows: 20 μM Sp-cAMPS (Calbiochem; cell permeable cAMP analogue); 20 μM Rp-cAMPS (Calbiochem; inhibitor of protein kinase A); 100 μM 8-bromo-cGMP (Calbiochem; cell permeable cGMP analogue); 10 μM Rp-8-pCPT-cGMPS (Calbiochem; cell permeable inhibitor of protein kinase G); 50 μM phenylarsine oxide (PAO; Sigma; an inhibitor of phosphotyrosine phosphatases); 40 μM anisomycin (Sigma; inhibits peptidyltransferase activity on ribosomes); 25 μM cycloheximide (Sigma; inhibits the translocation reaction on ribosomes); 10 μg/mL alpha-amanitin (Calbiochem; inhibits RNA polymerase II); 50 μM N-acetyl-Leu-Leu-Norleu-Al (LnLL) (Sigma; a proteasome inhibitor); 10 μM lactacystin (Calbiochem; a specific inhibitor of the proteasome).
Retinal Explant Cultures and Collapse Assay
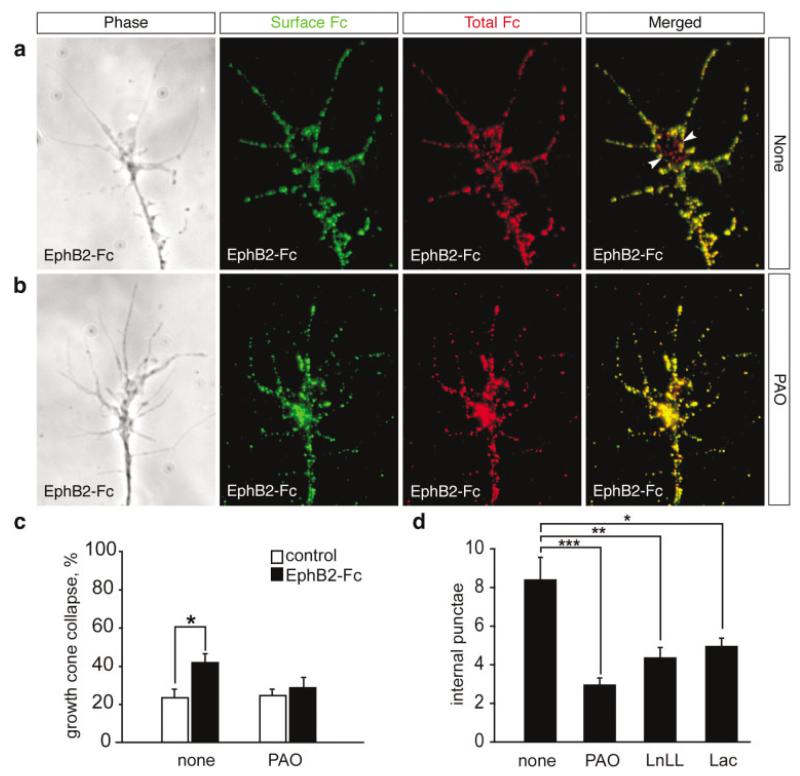
Explants from dorsal or ventral retina of stage 28–35/36 Xenopus embryos were cultured for 18–24 h as described previously (de la Torre et al., 1997). For the collapse assay, Eph-Fc and ephrin-Fc fusion proteins (R&D Systems) or Fc fragment alone (R&D Systems) were added to each culture for 5–120 min, after which cultures were fixed by adding an equal volume of ice-cold fixative (4% PFA with 15% sucrose) for 30 min. In some experiments, fusion proteins were preclustered for 1 h at room temperature with a goat antibody to the Fc fragment of human IgG (1:10; Jackson ImmunoResearch). Growth cones were examined with a 40X phase contrast objective on an inverted microscope (Nikon). Control growth cones exhibited complex profiles with multiple (5–10) long (5 μm or more) filopodia and broad lamellipodia whereas collapsed growth cones typically lacked filopodia and lamellipodia and exhibited cigar-shaped profiles [Fig. 1(a)]. For collapse quantitation, growth cones were classed as collapsed if they possessed two or fewer short (5 μm or less) filopodia. The number of collapsed and noncollapsed growth cones was counted and data are presented as percentage of collapsed growth cones ± standard error of the mean (SEM) from three to four independent experiments. Statistical analyses were carried out with a Mann-Whitney U test (StatView software), setting a significance level of p < 0.05.
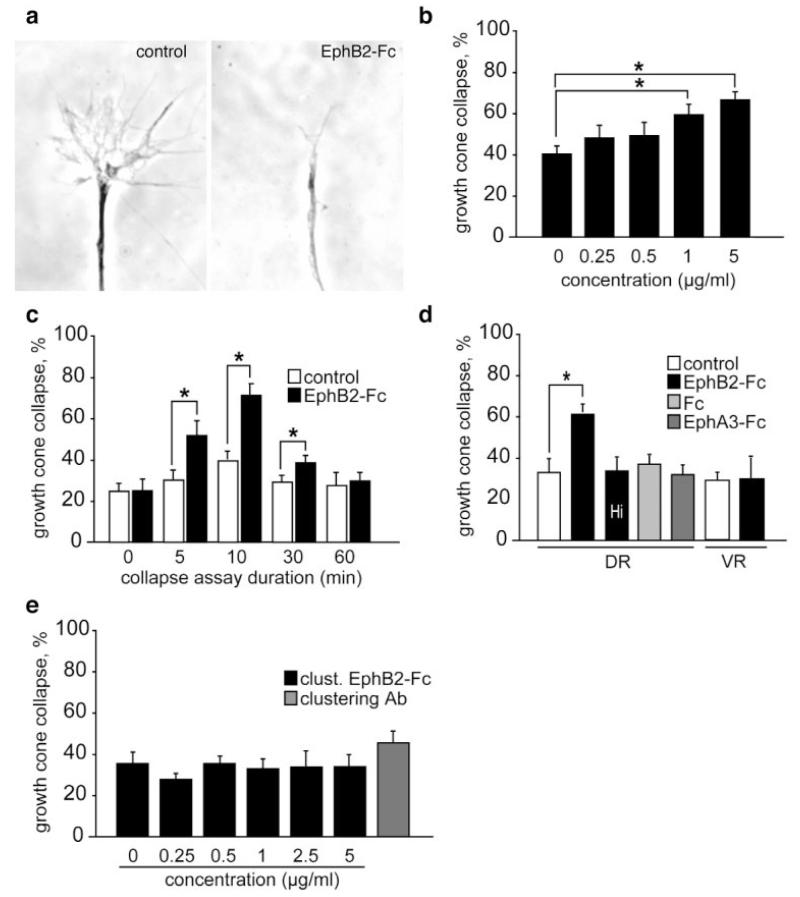
Figure 1.
Effects of EphB2 ectodomains on retinal growth cones. (a) Phase contrast images of a retinal growth cone treated with control culture medium (left panel) exhibiting noncollapsed complex morphology and a collapsed growth cone (right panel) treated with 5 μg/mL EphB2-Fc. (b) EphB2-Fc fusion protein induces collapse of Xenopus dorsal retinal growth cones in a dose-dependent manner (assayed at 10 min). (c) Time-course of response shows collapse is maximal at 10 min and has recovered by 60 min. (d) Bath-application of 5 μg/mL of heat inactivated (Hi) EphB2-Fc, Fc fragment alone, or EphA3-Fc on dorsal retinal (DR) explants does not cause growth cone collapse. Unlike dorsal axons, ventral retinal (VR) axons show no collapse response following application of 5 μg/mL EphB2-Fc fusion protein. (e) Dorsal retinal axons do not collapse after exposure to preclustered EphB2-Fc (0–5 μg/mL) or the clustering antibody (50 μg/mL). Results are percent collapse ±SEM from three to four experiments. *p < 0.05.
Immunohistochemistry
Antibodies included goat antihuman IgG Fc fragment (Jackson ImmunoResearch), Cy3-conjugated donkey antigoat Ig (Chemicon), and FITC-conjugated donkey antigoat Ig (Chemicon). To label surface-bound EphB2-Fc, cultures stimulated for 10 min with 5 μg/mL EphB2-Fc were fixed as described above, and incubated with goat antihuman IgG Fc and FITC-conjugated donkey antigoat antibody (1:1000 in PBS, 1% BSA). In a second step, samples were fixed again for 15 min, permeabilized with 0.1% Triton-X-100, and total EphB2-Fc was detected with goat antihuman IgG Fc and Cy3-conjugated donkey antigoat antibody (both antibodies diluted 1:1000 in PBS, 1% BSA, and 0.1% Triton-X-100). The merge of the two signals revealed surface-bound EphB2-Fc (yellow) and internalized EphB2-Fc (red). Growth cones were examined with a 100X PlanNeofluor objective on a Nikon inverted microscope and phase contrast and fluorescent images were captured digitally with a Quantix camera (Photometrics). Quantification of growth cones showing endocytosis of EphB2-Fc was done by counting the number of internal Cy3-positive dots in about 30 growth cones in each experimental condition. Statistical analyses were carried out with a two-tailed Student’s t test. For the detection of ephrin-B1-Fc, cultures stimulated with 5 μg/mL of preclustered ephrin-B1-Fc for 10 min were processed similarly, omitting the antihuman Fc antibody. In another set of experiments, axons treated with clustered and/or unclustered ephrin-B1-Fc were fixed and stained first with FITC-conjugated antigoat antibody that binds only to the clustering antibody, and second with goat antihuman Fc and Cy3-conjugated antigoat antibody to label both clustered and unclustered ephrin-B1-Fc. The merge of the two signals revealed clustered EphB2-Fc (yellow) and unclustered EphB2-Fc (red) bound to the cell surface. After staining, coverslips were mounted in FluorSave (Calbiochem).
RESULTS
Unclustered EphB2 Ectodomains Cause Rapid Collapse of Dorsal Retinal Growth Cones
To explore the intracellular pathways underlying growth cone collapse in reverse signaling, EphB2 ectodomains fused to the Fc tail of IgG (EphB2-Fc) were added to explant cultures of Xenopus laevis retina and the growth cones were scored for collapse. The collapse assay was used as the repulsive chemotropic assay of choice because it gives a fast, quantifiable measure of growth cone responsiveness. Growth cones with simple profiles (two short filopodia or less) were classed as “collapsed” [Fig. 1(a)]. Addition of 1–5 μg/mL of EphB2-Fc to cultures of neurons from the ephrin-B-expressing dorsal retina caused significant collapse of growth cones after 10 min [Fig. 1(b)]. A time-course study indicated that 5 μg/mL EphB2-Fc causes growth cone collapse within 5 min, the maximum response being reached 10 min after EphB2-Fc application [Fig. 1(c)]. By 30 min, growth cones start to recover and after 60 min the amount of collapse has returned to background levels [Fig. 1(c)]. Ventral retinal neurons express little or no ephrin-B and, unlike dorsal neurons, they were not affected by soluble EphB2-Fc in vitro [Fig. 1(d)].
The collapse response of dorsal retinal axons to soluble EphB2-Fc was abolished after heat inactivation (74°C for 20 min) of the fusion protein. We also verified that no change in growth cone behavior was observed when the Fc control protein or an A-type Eph receptor (EphA3-Fc) was applied to the cultures [Fig. 1(d)]. These results indicate that the observed collapsing activity is specific for the extracellular domain of the EphB2 receptor.
Next we asked whether the degree of clustering of Eph receptor was important for its activity. For this purpose, the dimeric EphB2-Fc protein was clustered into higher order oligomers with an anti-Fc antibody prior to assaying. Surprisingly, the clustered EphB2-Fc failed to induce changes in growth cone behavior at the same concentration (1–5 μg/mL) as that used with the dimeric molecule [Fig. 1(e)]. These results suggest that the degree of oligomerization of EphB receptors influences the activation of ephrin-B signaling.
Clustered Ephrin-B1 Ectodomains Cause Slow Collapse of Ventral Retinal Growth Cones
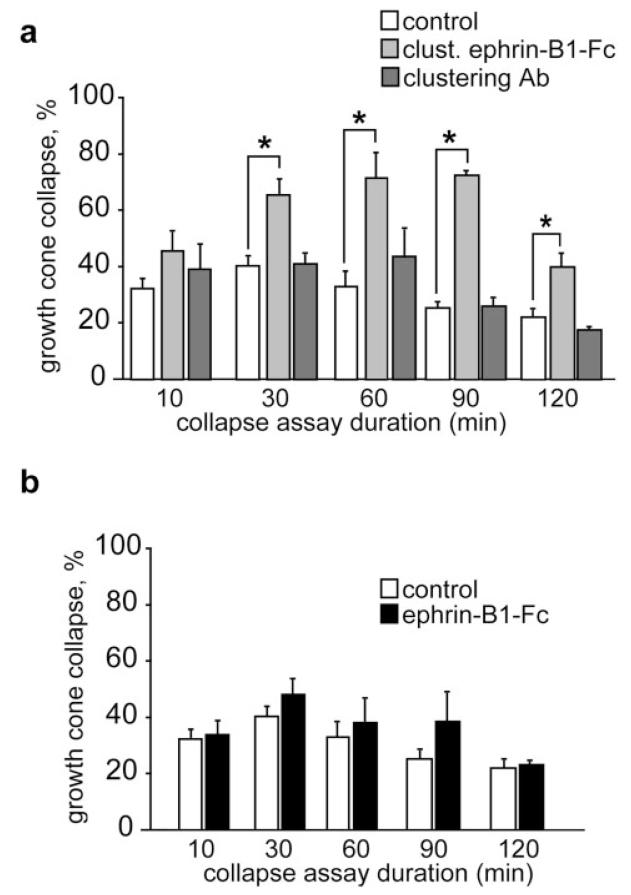
To analyze the repellent effect of ephrin-B ligands on retinal axons, the ephrin-B1-Fc chimera was used in a collapse assay with retinal explants harvested from the most ventral part of Xenopus retina that express high amounts of EphB receptors. Addition of increasing concentrations (0.5 to 5 μg/mL) of both clustered and unclustered ephrin-B1-Fc during 10 min did not cause any dramatic change in collapse compared to control conditions. With the highest concentrations of clustered ephrin-B1-Fc, however, there was a small increase in collapse rate, but this change was not significant (results not shown). This observation and previous reports on the delayed collapse response of cortical axons exposed to ephrin-B1 (Meima et al., 1997b) prompted us to study the collapse response of retinal growth cones over a longer time period. As shown in Figure 2(a), application of 5 μg/mL of clustered ephrin-B1-Fc causes a significant increase in growth cone collapse after 30 min and a maximum was reached after 60 min. The collapse response was maintained for over 90 min. In contrast, unclustered ephrin-B1-Fc at the same concentration or anti-Fc antibody alone did not show any repellent activity under the same experimental conditions [Fig. 2(b)].
Figure 2.
Effects of ephrin-B1 ectodomain on retinal growth cones. (a) Preclustered ephrin-B1-Fc (5 μg/mL) causes ventral retinal growth cone to collapse. Maximal collapse occurs at 60–90 min and drops off by 120 min. The clustering antibody (50 μg/mL) does not induce collapse. (b) Unclustered ephrin-B1-Fc fusion protein (5 μg/mL) does not induce significant levels of growth cone collapse. Results are percent collapse ±SEM from three to four experiments. *p < 0.05.
Proteasome Function Is Required for EphB2-Reverse Collapse but Not Ephrin-B1-Forward Collapse
The results described above reveal different kinetics of growth cone collapse when EphB and ephrin-B molecules were used, suggesting that these factors act on growth cone remodeling through distinct intracellular signaling pathways. To explore further the mechanism by which these factors induce repulsion, we used a pharmacological approach to manipulate intracellular pathways known to play a key role in growth cone navigation. In a recent study, it has been shown that chemotropic guidance factors, including Sema3A, netrin-1, BDNF, and LPA, act by activating local translation and/or protein degradation in growth cones (Campbell and Holt, 2001). We were interested, therefore, in examining whether translation or degradation is involved in signaling through members of the EphB family.
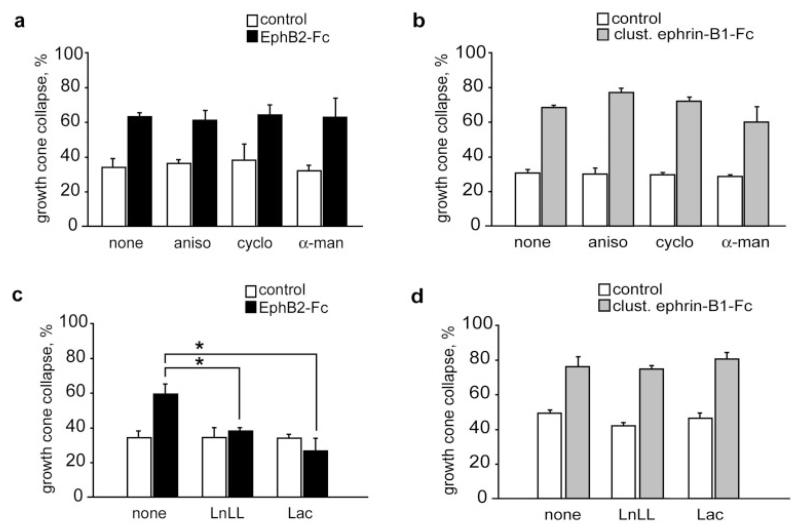
In these and subsequent experiments, collapse assays were performed with 5 μg/mL unclustered EphB2-Fc applied for 10 min on dorsal retinal axons, and 5 μg/mL clustered ephrin-B1-Fc applied for 30 min on ventral retinal axons. In the absence of any pharmacological inhibitors, comparable collapse rates were observed in both conditions (68 and 66% respectively). To determine if local protein synthesis within the growth cone is part of EphB and ephrin-B pathways, we used the protein synthesis inhibitors anisomycin and cycloheximide, and as a negative control the transcription inhibitor, α-amanitin. When added to the culture medium immediately before starting the assay, these inhibitors did not block the collapse induced by either EphB2-Fc [Fig. 3(a)] or ephrin-B1-Fc [Fig. 3(b)].
Figure 3.
EphB2-Fc-induced growth cone collapse is blocked by proteasome inhibitors. Protein synthesis and transcription inhibitors do not affect collapse induced by EphB2-Fc (a) and ephrin-B1-Fc (b). Proteasome inhibitors, LnLL and lactacystin (Lac), prevent EphB2-Fc-induced growth cone collapse (c), but do not affect ephrin-B1-Fc collapsing activity (d). aniso, anisomycin; cyclo, cycloheximide; α-man, α-amanitin. Results are percent collapse ±SEM from three to five experiments. *p < 0.05.
We then used lactacystin and LnLL, two different pharmacological inhibitors of proteasome-mediated degradation. Preincubation of the cultures with these reagents had a strong effect on growth cone behavior following EphB2-Fc application, completely blocking collapse [Fig. 3(c)]. In contrast, when the same type of experiment was performed using ephrin-B1-Fc, the proteasome inhibitors did not interfere with growth cone collapse [Fig. 3(d)]. These results indicate that EphB-receptors, but not ephrin-B ligands, induce growth cone collapse via a proteasome-dependent signaling pathway.
EphB2-Reverse and Ephrin-B1-Forward Collapse Responses Are Mediated by Opposite Levels of Intracellular Cyclic GMP
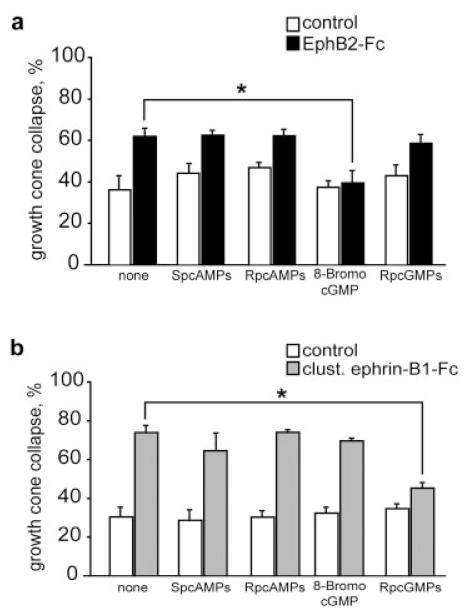
Previous studies have demonstrated that growth cone responses to diffusible axon guidance cues involve two distinct pathways that depend on changes in intracellular cAMP (group 1) or cGMP levels (group 2) (Song et al., 1997, 1998; Song and Poo, 1999; although this has recently been revised, see Nishiyama et al., 2003). To examine the role of cyclic nucleotides in EphB-collapse, four different inhibitors were bath-applied 30 min before the addition of the Fc fusion protein. Figure 4(a) shows that application of 8-bromo-cGMP, an analogue of cGMP that activates protein kinase G (PKG), abolished the collapse response of retinal growth cones exposed for 10 min to 5 μg/mL of EphB2-Fc. In contrast, application of a PKG inhibitor, Rp-8-pCPT-cGMPs, had no effect on growth cone response to EphB2-Fc. No change in growth cone behavior was observed following application of Sp-cAMPs, an analogue of cAMP, and Rp-cAMPs, an inhibitor of PKA. We also addressed the role of cyclic nucleotides in forward signaling. As shown in Figure 4(b), the PKG inhibitor Rp-cGMPs blocked the collapse response to ephrin-B1-Fc, bringing the collapse level back to control percentage. Conversely, neither 8-Br-cGMP, Sp-cAMPs, nor Rp-cAMPs prevented the collapsing effect of clustered ephrin-B1-Fc. Taken together, these results suggest that EphB2-Fc acts as a repulsive factor through a low level of intracellular cGMP, whereas the ephrin-B1-Fc signal is mediated by a high cGMP level.
Figure 4.
EphB2- and ephrin-B1-induced collapse responses are mediated by opposite levels of intracellular cGMP. (a) EphB2-Fc-induced collapse is blocked by 8-Br-cGMPs, but not by Rp-8-pCPT-cGMPs (Rp-cGMPs), Sp-cAMPs, and Rp-cAMPs. (b) Ephrin-B1-induced collapse is also dependent on cGMP levels, but in this case the response is blocked by inhibition of PKG in the presence of Rp-8-pCPT-cGMPs (Rp-cGMPs). Results are percent collapse ±SEM from three to four experiments. *p < 0.05.
EphB2-Fc but Not Ephrin-B1-Fc Is Internalized in Retinal Growth Cones
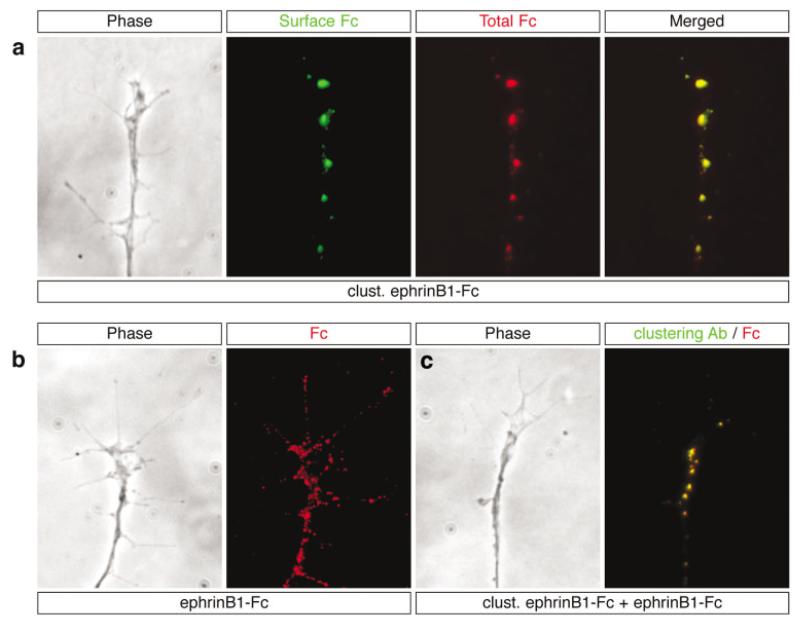
Endocytosis of receptor-ligand complexes is a common feature in signal transduction by receptor tyrosine kinases and is thought to be involved in regulating the surface availability of receptors as well as terminating signal transduction. To investigate if receptor-ligand complexes are endocytosed during forward or reverse signaling, we performed a double immunostaining procedure to selectively detect surface-bound and total Fc fusion molecules (see Materials and Methods). In growth cones stimulated with EphB2-Fc, comparison of extracellular and total EphB2-Fc labeling reveals the existence of Cy3-only (red) positive spots inside growth cones, indicating the presence of endocytosed EphB2-Fc molecules [Fig. 5(a)]. In contrast, stimulation with clustered ephrin-B1-Fc produces completely overlapping staining patterns in all growth cones analyzed [Fig. 6(a)], suggesting that forward signaling does not involve endocytosis of clustered ephrin-B1-Fc.
Figure 5.
Endocytosis in reverse signaling. (a) Double immunostaining for extracellular and total EphB2-Fc. Phase contrast image of a representative growth cone after treatment with 5 μg/mL EphB2-Fc for 10 min, and immunostaining of surface-bound EphB2-Fc (green) and total EphB2-Fc (red). The merged image shows the presence of red punctate spots (arrowheads) corresponding to endocytosed EphB2-Fc. (b) Internalized EphB2-Fc is rarely detected in the presence of 50 μM PAO, indicating that PAO blocks endocytosis of EphB2-Fc. (c) EphB2-Fc-induced collapse is prevented in stage 35 dorsal retinal growth cones by bath application of 50 μM PAO. Results are percent collapse ±SEM from four experiments. *p < 0.05. (d) Quantification of endocytosis in growth cones treated with 5 μg/mL EphB2-Fc in the absence (none) or presence of inhibitors of endocytosis (PAO) and proteasomal degradation (LnLL and lactacystin). Results are mean number of internal punctae ±SEM from about 30 growth cones. *p < 0.05; **p < 0.005; ***p < 0.001.
Figure 6.
Distribution of clustered ephrin-B1-Fc ligands on membrane surface. (a) Phase contrast image of a representative growth cone after treatment with 5 μg/mL clustered ephrinB1-Fc for 30 min, and double immunostaining for surface (green) and total (red) ephrinB1-Fc. The merged image shows complete overlap of the two signals (yellow), indicating that all the ephrinB1-Fc remains at the cell surface. The staining pattern is characterized by large raftlike patches scattered sparsely on retinal growth cones. (b) Growth cones treated with unclustered ephrinB1-Fc alone exhibit small punctate spots of labeling widely dispersed throughout the growth cone. (c) Growth cones treated sequentially with clustered ephrinB1-Fc for 30 min, followed by unclustered ephrinB1-Fc for a further 10 min, and subsequently immunostained first with a FITC-conjugated antibody to detect the clustered molecule, and second with a Cy3-conjugated antibody to label total ephrin-B1-Fc molecules. The merged image (right panel) shows complete overlap of the red/green signals (yellow), revealing that preincubation with clustered ephrin-B1-Fc causes the recruitment of virtually all endogenous receptors into large raftlike patches.
In contrast with the widely distributed pattern of small dots of label seen with the unclustered EphB2-Fc on the cell membrane [Fig. 5(a)], surface-bound clustered ephrin-B1-Fc [Fig. 6(a)] exhibited a patchy distribution of intensely fluorescent large spots scattered sparsely on the growth cones. This latter pattern of staining could reflect cross-linking of the partner receptors or an artifact derived from the detection of ephrin-B1-Fc aggregates. To investigate this, we treated the cultures sequentially, first with the clustered ephrin-B1-Fc for 30 min and second, with the unclustered ephrin-B1-Fc for 10 min. We used an FITC-conjugated antibody to detect the clustered ephrin-B-Fc and a Cy3-conjugated antibody to detect the unclustered ephrin-B-Fc. The results showed large FITC-positive ephrin-B1-Fc patches on the cell surface that overlapped completely with the Cy3 signal, with no “ectopic” spots of Cy3-labeling in between [Fig. 6(c)]. In controls performed with unclustered ephrin-B1-Fc alone, punctate staining was observed distributed all over the surface of axons, the growth cones, and fine filopodia [Fig. 6(b)]. These results indicate that the clustered ephrin-B1-Fc rapidly recruits endogenous Eph receptors into large raft-like patches in retinal neurons.
Internalization of EphB2-Fc Is Required for Growth Cone Collapse and Is Sensitive to Proteasome Inhibition
Because we have shown that unclustered EphB2-Fc is endocytosed, we wanted to examine whether clustered EphB2-Fc, which failed to induce retinal growth cone collapse, is internalized also. Interestingly, detection of clustered EphB2-Fc revealed only large plasma membrane-bound aggregates (data not shown), suggesting a possible link between EphB receptor endocytosis and growth cone collapse in reverse signaling. To ask whether EphB2-Fc internalization is needed for the collapse response, cultures of dorsal retinal growth cones were treated with PAO, an inhibitor of receptor-mediated endocytosis (Hertel et al., 1985). Immunostaining for extracellular and intracellular EphB2-Fc showed that PAO abolishes the internalization of the fusion protein [Fig. 5(b,d)]. We then performed a collapse assay using EphB2-Fc and found that the presence of PAO inhibited the collapse response [Fig. 5(c)]. Taken together, these results suggest that endocytosis of EphB-ephrin-B complex is required for growth cone collapse.
Because proteasome inhibitors were found to interfere with EphB2-induced collapse (Fig. 3), we next examined whether internalization of EphB2-Fc is dependent on the proteasome pathway. Here we found that in the presence of LnLL and lactacystin, the pool of internalized EphB2-Fc in growth cones was decreased markedly by 48 and 41%, respectively [Fig. 5(d)]. These data indicate that EphB2 reverse endocytosis is regulated at least in part by the proteasome pathway.
DISCUSSION
Here we have shown that EphB and ephrin-B molecules act as repellent factors on subpopulations of retinal growth cones. Although the morphological changes of retinal growth cones stimulated with EphB2-Fc and ephrin-B1-Fc appear qualitatively similar, the temporal dynamics of the responses are strikingly different. With ephrin-B1, collapse was slow (within 30 min of first contact) and sustained over a prolonged period of time (at least 90 min). In sharp contrast, collapse induced by EphB2 was rapid (within 5 min) and transient (about 30 min). These response dynamics appear compatible with real-time studies of growth cone behavior in cortical and retinal neurons (Drescher et al., 1995; Meima et al., 1997b; Birgbauer et al., 2001) and with the kinetics of Eph and ephrin activation. Indeed, the Eph family of receptor tyrosine kinases shows the atypical feature of a slow activation and an apparent lack of down-regulation upon ligand binding. Stimulation of ephrin-B, however, causes a rapid recruitment and activation of Src family kinases, followed by the recruitment of the phosphatase PTP-BL, a negative regulator of Src activity and ephrin-B phosphorylation, which terminates signal transduction (Palmer et al., 2002).
An important aspect of ephrin ligand function is the requirement of membrane attachment or artificial clustering to activate exogenously expressed Eph receptors (Davis et al., 1994). Moreover, stimulation with dimeric and higher oligomeric ligands induces different Eph receptor signaling complexes and biological responses in endothelial cells (Stein et al., 1998). In accordance with this idea, we report that the response of retinal growth cones depends critically on the oligomeric state of Eph and ephrin-B molecules. Our results suggest that in forward signaling, clustered ephrin-B1-Fc is required for the full biological activation of EphB receptors. Immunolabeling with anti-Fc antibodies revealed large patches of ephrin-B1-Fc at the surface of growth cones. These large patches are reminiscent of the large raft patches that arise following stimulation of ephrin-B1 with soluble EphB2 ectodomains in HEK 293 cells (Bruckner et al., 1999), suggesting that clustered ephrin-B1-Fc induces aggregation of EphB receptors, permitting transphosphorylation of tyrosine kinase domains and recruitment of signal transduction proteins into membrane microdomains. In cultures of cortical neurons, both clustered and unclustered ephrin-B1-Fc can stimulate growth cone collapse (Meima et al., 1997b). Whether this difference depends on the cell type, the level, or the nature of EphB isoforms expressed remains to be determined.
Surprisingly, in reverse signaling only unclustered EphB2-Fc, not clustered EphB2-Fc, was able to elicit growth cone collapse. One possibility is that full activation of endogenous ephrin-B by clustered EphB2-Fc initiates a cascade of signaling events that leads to an adhesive rather than a repulsive cellular response. However, in a neuronal cell line, stimulation with clustered EphB2-Fc was previously found to recruit the Grb4 adaptor protein, which induces the loss of polymerized F-actin, suggesting a repellent role for ephrin-B reverse signaling (Cowan and Henkemeyer, 2001). An alternative possibility is that, by reorganizing endogenous ephrin-B into large aggregates on the cell surface, clustered EphB2-Fc indeed sequesters ephrin-B and prevents interactions with an unknown cofactor that might be required for EphB-induced growth cone collapse. Ephrin-B1 has been shown to interact with FGF receptors when ectopically coexpressed in Xenopus embryos (Chong et al., 2000). Moreover, FGF receptors regulate retinal ganglion cell axon extension (McFarlane et al., 1996), and their function is required for retinal growth cones to respond to substrate-bound EphB receptor in vitro (F. Mann and C. E. Holt, unpublished observations). Therefore, FGF receptors could be candidate cofactors for ephrin-B-mediated growth cone collapse. Taken together, our findings raise the possibility that the degree of oligomerization, or the density of Eph and ephrin molecules at cell-to-cell contacts, could provide an important way of modulating EphB and ephrin-B function in vivo.
Upon binding to its partner receptor(s), EphB2-Fc is rapidly internalized into growth cones, consistent with the endocytosis of the EphB/ephrin-B complex. Importantly, our study provides evidence for a critical role of endocytosis in reverse ephrin-B signaling because internalization occurred with collapse-inducing unclustered EphB2-Fc but not clustered EphB2-Fc, and treatment with the phosphotyrosine phosphatase inhibitor, PAO, abolished both internalization of EphB2-Fc and growth cone collapse. How endocytosis contributes to the fast and reversible collapse response to EphB2 remains to be established. One possibility is that internalization of the Eph/ephrin complex could be necessary to allow interaction of activated ephrin-B with intracellular downstream signaling molecules at endocytic locations. A previous study has shown that endocytosis of dextran by growth cones is enhanced following stimulation with Sema3A and correlates with growth cone collapse (Fournier et al., 2000). This repulsive response can be blocked by preventing the internalization of Sema3A receptors, L1 and Neuropilin-1 (V. Castellani et al., personal communication). Taken together, these data suggest a common role of endocytosis in regulating Sema3A and EphB-induced growth cone collapse.
In vivo Eph and ephrin interactions occur at sites of cell-cell contacts, which raises the question of whether and how Eph receptors are endocytosed during ephrin-B reverse signaling. Our Xenopus culture system makes it difficult to investigate endocytosis of membrane-bound Eph receptor because mammalian cell lines expressing tagged-EphB do not survive under the culture conditions for amphibian neurons (21°C, L15 media). However, two recent studies by Klein and colleagues and by Nobes and colleagues have investigated this issue using mammalian cell lines (fibroblasts; Marston et al., in press) and primary mouse neurons (Zimmer et al., in press). They report that rapid endocytosis of EphB/ephrin-B complexes occurs at sites of cell-cell and cell-growth cone contact and that ephrin-B-mediated repulsive responses involve endocytosis of full-length EphB receptors, suggesting that this is an important mechanism for cell detachment in vivo.
Inhibitors of the proteasome have been shown to block endocytosis of ligand-activated growth hormone receptors (GHR) and the Met receptors (van Kerkhof et al., 2000; Hammond et al., 2001). Similarly, our data show that proteasome inhibitors severely reduce endocytosis of EphB2 ectodomains and abolish EphB2-mediated collapse. These results are consistent with the view that activated ephrin-B1 recruits the ubiquitin-conjugating machinery for endocytic removal from the surface. Indeed, we see rapid changes in ubiquitinylated protein levels in growth cones after only 5 min stimulation using an antibody that specifically recognizes ubiquitin-protein conjugates (FK2 antibody). After stimulation with unclustered EphB2-Fc, a 1.7-fold increase in the immunofluorescence signal in growth cones could be detected compared to unstimulated controls, whereas in the forward signaling, after stimulation with clustered ephrinB1-Fc only a 1.4-fold increase could be observed (data not shown, for Material and Methods see Campbell and Holt, 2001). Future studies will need to address which components of the pathway are ubiquitinated.
Unlike the EphB-induced fast collapse, the slow growth cone collapse triggered by ephrin-B1 in retinal neurons does not appear to involve endocytosis of ephrin-B1 ectodomain, indicating that internalization of the Eph/ephrin complex is not required to initiate Eph forward signaling. However, it might be necessary to terminate signal transduction by removal of the receptor-ligand complex from the cell surface and subsequent degradation or dissociation. The failure of retinal axons to internalize clustered ephrin-B1-Fc might account for the prolonged collapse response of the growth cones observed in our assay.
Evidence is accumulating that changes in protein levels within growth cones play an important role in several aspects of axon navigation (Campbell and Holt, 2001; Brittis et al., 2002; Ming et al., 2002). The present study shows that forward signaling through the Eph receptor is independent of rapid protein synthesis and degradation. This does not appear to be a general feature of receptor tyrosine kinases because BDNF that signals through TrkB receptor tyrosine kinase was found to depend on proteasome activity and protein synthesis (Campbell and Holt, 2001). Perhaps the slow kinetics of ephrin-B1-Fc-induced growth cone collapse implicates that the changes in growth cone cytoskeleton are in this case dependent on transport of proteins to and from the soma rather than local and rapid changes in protein levels regulated directly at the growth cone. In contrast, our results show that the proteasome inhibitors LnLL and lactacystin impaired the ability of EphB2-Fc to stimulate fast growth cone collapse in cultures of retinal explants. Proteasome-dependent cues identified so far (netrin-1, BDNF, and LPA) signal through cAMP-dependent pathways. Interestingly, we found here that EphB2 signaling can be modulated by changes in intracellular levels of cGMP or PKG activity, indicating that cGMP-dependent guidance cues can also signal through the proteasome pathway.
Over the past few years, it has become clear that forward EphB and reverse ephrin-B signaling play important roles in axon guidance during development of the visual system. Interestingly, both EphB and ephrin-B expressed at the surface of retinal ganglion cell axons are thought to transduce either repulsive or attractive information in different sites along the visual pathway. EphB and ephrin-B signaling is repulsive in the retina and at the chiasm (Birgbauer et al., 2000; Nakagawa et al., 2000; Birgbauer et al., 2001), but appears to mediate attractive behavior in the optic tectum (Hindges et al., 2002; Mann et al., 2002). This is reminiscent of the finding that Xenopus retinal ganglion cell axons change their responsiveness to netrin-1 from repulsive to attractive as they grow along the visual pathway (Shewan et al., 2002). In the latter case, both intrinsic and extrinsic mechanisms are involved in regulating the response to netrin-1. The present study suggests that the dual function of EphB and ephrin-B molecules might also be controlled by developmentally regulated intrinsic changes in cGMP levels and/or different oligomeric states of EphB and ephrin-B molecules presented at the surface of cells in the retina, chiasm, and optic tectum.
We show here that although forward and reverse signaling share a similar ability to transduce information leading to growth cone collapse, they are not interchangeable and involve distinct temporal dynamics and intracellular signaling pathways. Several mechanisms and molecules that regulate Eph and ephrin signal transduction have now been identified and a future challenge will be to determine how these pathways interconnect.
Acknowledgments
The authors thank F. Meina, D. Campbell, B. Harris, and all members of the Holt and Harris laboratories for advice and helpful discussions, and D. O’Connor for frog care. E.M. thanks specially G. Lupo for continuous encouragement and discussions. We thank Rudiger Klein, Manuel Zimmer, and Kate Nobes for sharing unpublished data and for stimulating discussions. This work was supported by a fellowship from the Human Frontier Science Program Organization (F.M.).
REFERENCES
- Birgbauer E, Cowan CA, Sretavan DW, Henkemeyer M. Kinase independent function of EphB receptors in retinal axon pathfinding to the optic disc from dorsal but not ventral retina. Development. 2000;127:1231–1241. doi: 10.1242/dev.127.6.1231. [DOI] [PubMed] [Google Scholar]
- Birgbauer E, Oster SF, Severin CG, Sretavan DW. Retinal axon growth cones respond to EphB extracellular domains as inhibitory axon guidance cues. Development. 2001;128:3041–3048. doi: 10.1242/dev.128.15.3041. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–235. doi: 10.1016/s0092-8674(02)00813-9. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg PH, Klein R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 1999;22:511–524. doi: 10.1016/s0896-6273(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Carter N, Nakamoto T, Hirai H, Hunter T. EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas) Nat Cell Biol. 2002;4:565–573. doi: 10.1038/ncb823. [DOI] [PubMed] [Google Scholar]
- Chong LD, Park EK, Latimer E, Friesel R, Daar IO. Fibroblast growth factor receptor-mediated rescue of x-ephrin B1-induced cell dissociation in Xenopus embryos. Mol Cell Biol. 2000;20:724–734. doi: 10.1128/mcb.20.2.724-734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CA, Henkemeyer M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature. 2001;413:174–179. doi: 10.1038/35093123. [DOI] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- de la Torre JR, Hopker VH, Ming GL, Poo MM, Tessier-Lavigne M, Hemmati-Brivanlou A, Holt CE. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron. 1997;19:211–224. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
- Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- Elowe S, Holland SJ, Kulkarni S, Pawson T. Down-regulation of the Ras-mitogen-activated protein kinase pathway by the EphB2 receptor tyrosine kinase is required for ephrin-induced neurite retraction. Mol Cell Biol. 2001;21:7429–7441. doi: 10.1128/MCB.21.21.7429-7441.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, Nakamura F, Kawamoto S, Goshima Y, Kalb RG, Strittmatter SM. Semaphorin3A enhances endocytosis at sites of receptor-F-actin colocalization during growth cone collapse. J Cell Biol. 2000;149:411–422. doi: 10.1083/jcb.149.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao PP, Sun CH, Zhou XF, DiCicco-Bloom E, Zhou R. Ephrins stimulate or inhibit neurite outgrowth and survival as a function of neuronal cell type. J Neurosci Res. 2000;60:427–436. doi: 10.1002/(SICI)1097-4547(20000515)60:4<427::AID-JNR1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Hammond DE, Urbe S, Vande Woude GF, Clague MJ. Down-regulation of MET, the receptor for hepatocyte growth factor. Oncogene. 2001;20:2761–2770. doi: 10.1038/sj.onc.1204475. [DOI] [PubMed] [Google Scholar]
- Hertel C, Coulter SJ, Perkins JP. A comparison of catecholamine-induced internalization of beta-adrenergic receptors and receptor-mediated endocytosis of epidermal growth factor in human astrocytoma cells. Inhibition by phenylarsine oxide. J Biol Chem. 1985;260:12547–12553. [PubMed] [Google Scholar]
- Hindges R, McLaughlin T, Genoud N, Henkemeyer M, O’Leary DD. EphB forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron. 2002;35:475–487. doi: 10.1016/s0896-6273(02)00799-7. [DOI] [PubMed] [Google Scholar]
- Holash JA, Soans C, Chong LD, Shao H, Dixit VM, Pasquale EB. Reciprocal expression of the Eph receptor Cek5 and its ligand(s) in the early retina. Dev Biol. 1997;182:256–269. doi: 10.1006/dbio.1996.8496. [DOI] [PubMed] [Google Scholar]
- Imondi R, Wideman C, Kaprielian Z. Complementary expression of transmembrane ephrins and their receptors in the mouse spinal cord: a possible role in constraining the orientation of longitudinally projecting axons. Development. 2000;127:1397–1410. doi: 10.1242/dev.127.7.1397. [DOI] [PubMed] [Google Scholar]
- Knoll B, Drescher U. Ephrin-As as receptors in topographic projections. Trends Neurosci. 2002;25:145–149. doi: 10.1016/s0166-2236(00)02093-2. [DOI] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signaling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Mann F, Holt CE. Control of retinal growth and axon divergence at the chiasm: lessons from Xenopus. Bioessays. 2001;23:319–326. doi: 10.1002/bies.1046. [DOI] [PubMed] [Google Scholar]
- Mann F, Ray S, Harris W, Holt C. Topographic mapping in dorsoventral axis of the Xenopus retinotectal system depends on signaling through ephrin-B ligands. Neuron. 2002;35:461–473. doi: 10.1016/s0896-6273(02)00786-9. [DOI] [PubMed] [Google Scholar]
- Marston DJ, Dickinson S, Nobes CD. Rac dependent trans-endocytosis of ephrins regulates Eph receptor-ephrin contact repulsion. Nat Cell Biol. 2003 doi: 10.1038/ncb1044. in press. [DOI] [PubMed] [Google Scholar]
- McFarlane S, Cornel E, Amaya E, Holt CE. Inhibition of FGF receptor activity in retinal ganglion cell axons causes errors in target recognition. Neuron. 1996;17:245–254. doi: 10.1016/s0896-6273(00)80156-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Hindges R, O’Leary DD. Regulation of axial patterning of the retina and its topographic mapping in the brain. Curr Opin Neurobiol. 2003;13:57–69. doi: 10.1016/s0959-4388(03)00014-x. [DOI] [PubMed] [Google Scholar]
- Meima L, Kljavin IJ, Moran P, Shih A, Winslow JW, Caras IW. AL-1-induced growth cone collapse of rat cortical neurons is correlated with REK7 expression and rearrangement of the actin cytoskeleton. Eur J Neurosci. 1997a;9:177–188. doi: 10.1111/j.1460-9568.1997.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Meima L, Moran P, Matthews W, Caras IW. Lerk2 (ephrin-B1) is a collapsing factor for a subset of cortical growth cones and acts by a mechanism different from AL-1 (ephrin-A5) Mol Cell Neurosci. 1997b;9:314–328. doi: 10.1006/mcne.1997.0621. [DOI] [PubMed] [Google Scholar]
- Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC, Poo MM. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- Monschau B, Kremoser C, Ohta K, Tanaka H, Kaneko T, Yamada T, Handwerker C, Hornberger MR, Loschinger J, Pasquale EB, et al. Shared and distinct functions of RAGS and ELF-1 in guiding retinal axons. Embo J. 1997;16:1258–1267. doi: 10.1093/emboj/16.6.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Brennan C, Johnson KG, Shewan D, Harris WA, Holt CE. Ephrin-B regulates the Ipsilateral routing of retinal axons at the optic chiasm. Neuron. 2000;25:599–610. doi: 10.1016/s0896-6273(00)81063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Second Edition Garland; New York: 1967. [Google Scholar]
- Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Tessier-Lavigne M, Poo M, Hong K. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- Palmer A, Zimmer M, Erdmann KS, Eulenburg V, Porthin A, Heumann R, Deutsch U, Klein R. EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase. Mol Cell. 2002;9:725–737. doi: 10.1016/s1097-2765(02)00488-4. [DOI] [PubMed] [Google Scholar]
- Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- Shewan D, Dwivedy A, Anderson R, Holt CE. Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nat Neurosci. 2002;5:955–962. doi: 10.1038/nn919. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol. 1999;9:355–363. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- Stein E, Lane AA, Cerretti DP, Schoecklmann HO, Schroff AD, Van Etten RL, Daniel TO. Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev. 1998;12:667–678. doi: 10.1101/gad.12.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof P, Govers R, Alves dos Santos CM, Strous GJ. Endocytosis and degradation of the growth hormone receptor are proteasome-dependent. J Biol Chem. 2000;275:1575–1580. doi: 10.1074/jbc.275.3.1575. [DOI] [PubMed] [Google Scholar]
- Wahl S, Barth H, Ciossek T, Aktories K, Mueller BK. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J Cell Biol. 2000;149:263–270. doi: 10.1083/jcb.149.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HU, Anderson DJ. Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron. 1997;18:383–396. doi: 10.1016/s0896-6273(00)81240-4. [DOI] [PubMed] [Google Scholar]
- Weinl C, Drescher U, Lang S, Bonhoeffer F, Loschinger J. On the turning of Xenopus retinal axons induced by ephrin-A5. Development. 2003;130:1635–1643. doi: 10.1242/dev.00386. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Eph receptors and ephrins: regulators of guidance and assembly. Int Rev Cytol. 2000;196:177–244. doi: 10.1016/s0074-7696(00)96005-4. [DOI] [PubMed] [Google Scholar]
- Zhou X, Suh J, Cerretti DP, Zhou R, DiCicco-Bloom E. Ephrins stimulate neurite outgrowth during early cortical neurogenesis. J Neurosci Res. 2001;66:1054–1063. doi: 10.1002/jnr.10029. [DOI] [PubMed] [Google Scholar]
- Zimmer M, Palmer A, Köhler J, Klein R. Contact-mediated repulsion by ephrinB ligands requires Bi-directional endocytosis. Nat Cell Biol. 2003 doi: 10.1038/ncb1045. in press. [DOI] [PubMed] [Google Scholar]