Abstract
The eye is a peripheral outpost of the central nervous system (CNS) where the retinal ganglion cells (RGCs) reside. RGC axons navigate to their targets in a remarkably stereotyped and error-free manner and it is this process of directed growth that underlies the complex organization of the adult brain. The RGCs are the only retinal neurons to project into the brain and their peripheral location makes them an unusually accessible population of projection neurons for experiments involving in vivo gene transfer, anatomical tracing, transplantation and in vitro culture. In this paper, we review recent findings that have contributed to our understanding of some of the guidance decisions that axons make in the developing visual system. We look at two choice points in the pathway, the optic nerve head (onh) and the midline chiasm, and discuss evidence that supports the idea that key molecules in guiding axon growth at these junctures are netrin-1 (onh) and ephrin-B (chiasm). In the optic tectum where RGC axon terminals are arrayed in topographic order, we present experimental evidence to suggest that in the dorso-ventral dimension, the B-type ephrins and Eph receptors are of prime importance, possibly through attractive interactions. This complements the anterior-posterior topographic mapping known to be mediated through A-type ephrin/Eph repulsive interactions. An emerging theme is that guidance molecules such as ephrin-B and netrin-1 have complex patterns of restricted expression in the pathway and play multiple and changing roles in axon guidance.
Keywords: retina, axon guidance, netrin, Eph receptor, ephrin
Introduction
Vision depends on the elaboration of appropriate functional connections between the retina and visual processing centres in the brain. During development, newly generated retinal ganglion cells (RGC) send axons that exit the eye through a circular region in the centre of the retina, the optic disc, and bundle together to form the optic nerves. RGC axons cross the ventral midline of the hypothalamus, at the optic chiasm, and extend dorsally in the optic tracts. In some vertebrate species, a proportion of RGC axons do not cross the midline and remain on the ipsilateral side of the brain. As a result, the optic tracts carry visual information from both eyes, a feature essential for binocular vision and depth perception.
Once they reach their principal target structure, the tectum (superior colliculi in Mammals), RGC axons stop and form synaptic connections with their target cells. Visual projections are organized such that two adjacent RGCs in the retina are connected to two adjacent points in the target field. This topographic arrangement allows the formation of a continuous image of the visual field onto the surface of the target structure.
Which are the molecules and mechanisms that guide migrating RGC axons during the establishment of this stereotyped pattern of projection? The selection of the appropriate pathway by RGC growth cones occurs stepwise in response to a succession of different guidance cues expressed in their local environment. The past decades have seen significant advances in the identification of these signals, and the present review focuses on two families of molecules, the netrin and ephrin/Eph families, for which important functions have been attributed. We present here evidence from in vivo experiments that netrin and ephrin/Eph molecules control RGC axon navigation at various choice points along the visual pathway, and discuss recent advances in the understanding of the signal transduction mechanisms underlying retinal growth cone guidance. We confine our discussion mainly to work done in the Xenopus visual system but it should be noted that much important experimental work has been done in other experimental systems such as zebrafish, mouse and chick (Fricke et al., 2001, Hutson and Chien, 2002, McLaughlin et al., 2003a, McLaughlin et al., 2003b).
Netrin-1 guides visual axons out of the eye
One of the first pathfinding tasks of visual axons is to exit the eye through the optic disk, at the retina-optic nerve junction (Fig. 1). RGC axons initially extend along the vitreal surface of the neural retina and follow a centripetal route towards the centre of the retina. Once they reach the optic disk, growth cones make a 90° turn to dive deep in the retina and exit the eye through the optic nerve head. Within the retina, RGC axons grow in close contact with Müller glial cell endfeet and with the vitreal basal lamina, both of which have been shown to express cell adhesion and extracellular matrix molecules, such as NCAM, L1 and chondroitin-sulfate proteoglycans that might stimulate axon growth and provide guidance signal to RGC axons (for review see Stuermer and Bastmeyer, 2000). It remains unclear, however, whether additional signals such as chemoattractants contribute to direct the navigation of RGC axons towards the optic disk. The search for candidate molecules involved in intraretinal pathfinding led to a focus on netrin-1, a laminin-related secreted protein, produced by glial cells at the optic disk and optic nerve head in fish (Lauderdale et al., 1997, Strahle et al., 1997), frogs (de la Torre et al., 1997) and rodents (Deiner et al., 1997), see Fig. 1. Netrin-1 was first reported to function as a long-range chemoattractive cue for commissural axons in the spinal cord (Kennedy et al., 1994, Serafini et al., 1994, Shirasaki et al., 1996). In the retina, however, it is unlikely that netrin-1 acts at long-range to attract RGC axons, since no alteration of the optic disk-directed growth of RGC axons was observed in netrin-1 deficient mice (Deiner et al., 1997). In this mutant, however, although RGC axons can reach the optic disk, they fail to enter the optic nerve head and instead grow aberrantly in other regions of the retina. This failure of many RGC axons to exit the eye results in a smaller optic nerve and hypo-pigmented streaks at the surface of the retinal pigmented epithelium, probably formed by ectopic axons growing between pigmented cells (Deiner et al., 1997). Thus netrin-1 appears to act at a short-range to attract RGC growth cones into the optic nerve head. This effect is mediated by signaling involving the netrin-1 receptor, ‘deleted in colorectal cancer’ (DCC) expressed on RGC axons, since netrin-1 and DCC knock out mice exhibit similar retinal abnormalities.
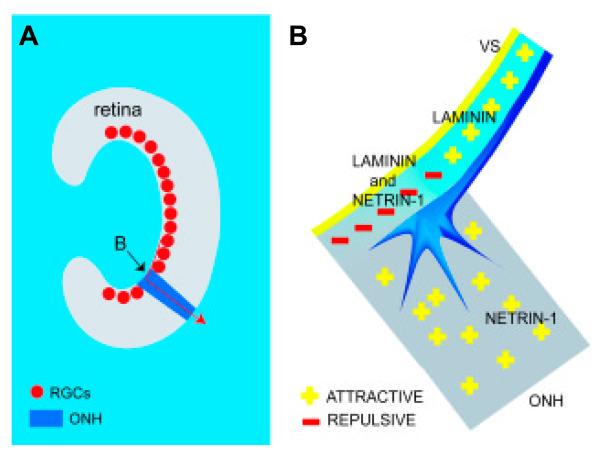
Fig. 1. Netrin-1 helps to guide the growth of axons out of the eye.
(A) The arrangement of retinal ganglion cells (RGCs; red) along the vitreal surface of the retina (grey) where laminin is located. Axons grow across the vitreal surface and make a sharp bend at the entrance to the optic nerve head (ONH, dark blue; arrow at B) where netrin-1 is localized. (B) A growth cone entering the ONH where it encounters laminin and netrin-1 on the vitreal surface and netrin-1 only in the ONH. It is hypothesised that the laminin and netrin-1 signals combine to repel axons away from the surface and force axons into the netrin-1-rich ONH, and so out of the eye.
Consistent with the proposed role of netrin-1 as a short-range chemoattractant, recombinant netrin-1 causes attractive turning of Xenopus retinal growth cone in vitro, an effect that can be blocked with antibodies to DCC (de la Torre et al., 1997). In addition, this response can be modulated by the level of cytosolic cyclic nucleotides (Ming et al., 1997, Song and Poo, 1999). For instance, it was shown that, in the presence of a cAMP analogue that activates protein kinase A (PKA), netrin-1-mediated attraction of retinal growth cones is converted into a repulsive activity (de la Torre et al., 1997). Thus, the behaviour of RGC growth cones in response to netrin-1 might be dependent on other concomitant signals that modulate the level of intracellular cAMP. This is the case, for example, for the extracellular matrix protein laminin-1 which is able to abrogate the netrin-1-induced cAMP rise in cultured RGC axons. As a result, laminin-1 changes netrin-1 attraction into repulsion through a mechanism that involves β1 integrin receptor expressed on RGC axons (Höpker et al., 1999).
In the developing retina, laminin-1 is abundant in the vitreal basal lamina along which RGC axons navigate. Thus laminin-1 and netrin-1 are co-expressed at the vitreal surface of the optic disk, whereas only netrin-1 but not laminin-1 is found deeper in the optic nerve head (Fig. 1B). The addition of a mimetic peptide of laminin to a Xenopus retinal cup grown in vitro induces the failure of some RGC axon fascicules to leave the eye through the optic disk (Höpker et al., 1999). These results are consistent with the idea that the restricted co-expression of laminin-1 and netrin-1 at the entrance to the optic nerve head results in a repulsive signal that serves to ‘push’ the growth cone away from the retinal surface and grow deep into the attractive netrin-1-rich/laminin-1-poor optic nerve head (Fig. 1B).
Developmental changes in netrin-1 responsiveness in RGC axons
Netrin-1 is expressed in the optic disk/nerve head but also further along the visual pathway (see summary diagram in Fig. 4) where it governs RGC axon guidance, as shown by the abnormal course of visual axons within the ventral hypothalamus of netrin-1- and DCC-deficient mice (Deiner and Sretavan, 1999). Analysis of netrin-1 expression in the brain of Xenopus embryos revealed that netrin-1 expression domains correspond to regions devoid of visual afferents that flank the trajectory of RGC axons (Shewan et al., 2002). This suggests that, although netrin-1 can attract RGC axons in the optic disk, it might function later on as a repellent and tell visual axons where not to go. Consistent with this idea, the in vitro response of RGC axons to a gradient of netrin-1 critically depends on the developmental stages at which axons are assayed. This was shown in a “whole pathway” explant preparation, where RGC axons are challenged with netrin-1 at various points in their journey along the visual pathway (Shewan et al., 2002). Unlike young axons that have not yet exited the eye and show attraction towards a source of netrin-1, RGC axons that have grown through the optic nerve head are insensitive to netrin-1. Moreover, after crossing the optic chiasm, RGC axons extending in the dorsal optic tract are strongly repelled by netrin-1. This later result supports the idea that netrin-1 functions as a repellent in the distal part of the visual pathway and helps to constrain the growth of RGC axons into the appropriate trajectory.
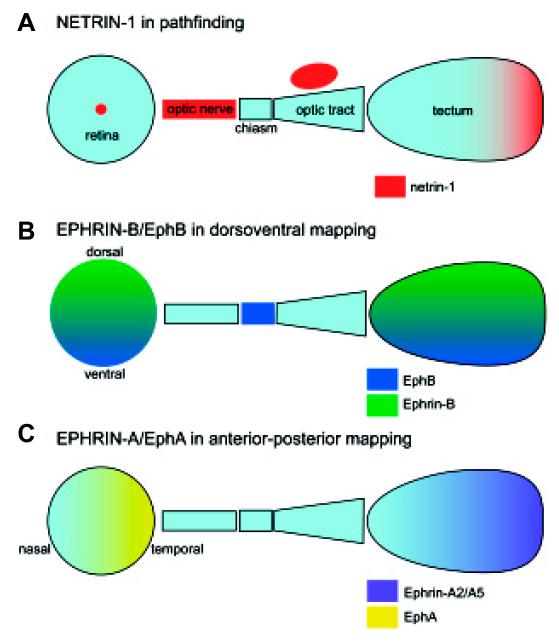
Fig. 4. Summary of distribution and role of netrin-1, A- and B-type ephrins and receptors in the developing visual pathway.
Eye: dorsoventral axis is top to bottom, nasotemporal axis left to right. Tectum: mediolateral (mammals) and dorsoventral (frogs) is top to bottom, anterior-posterior axis is left to right.
This developmental change in netrin-1 responsiveness can be in part explained by intrinsic changes in RGC growth cones, since RGC axons aged in vitro without having experienced the in vivo pathway also exhibit a repulsive response to netrin-1 (Shewan et al., 2002). However, in cultures of retinal explants harvested at different developmental stages, growth cones either show an attractive or a repulsive behaviour but never exhibit a neutral response to netrin-1, as some axons do in the “whole pathway” preparation. Thus extrinsic factors present in the visual pathway might modulate the progressive maturational change that occurs in RGC growth cones response to netrin-1.
The age-related intrinsic change in netrin-1 responsiveness could, in theory, be mediated by a temporal change in netrin-1 receptor expression. In other systems, netrin-1-induced attraction requires DCC whereas repulsion is initiated by a receptor complex involving DCC and UNC-5 (Hong et al., 1999). However, in situ hybridization in Xenopus retina failed to detect UNC-5 mRNA in RGC (Anderson and Holt, 2002) implying that UNC-5 does not mediate the repulsive responses to netrin-1 in the retina. Another potential receptor for netrin-1 is the membrane-associated adenosine A2b receptor, a G-protein-coupled receptor that induces cAMP production upon binding to netrin-1 (Corset et al., 2000). A2b is proposed to be part of a receptor complex for netrin-1 that associates with DCC (Corset et al., 2000). Interestingly, high A2b immunoreactivity is detected in young RGC growth cones and decreases in older growth cones, in a way that parallels a decrease of cytosolic cAMP in old RGC growth cone. The addition of both a cAMP analogue and an A2b specific agonist to cultures of old retinal axons can block the netrin-1-induced repulsion of older growth cones, whereas A2b antagonists used in cultures of young axons can convert netrin-1-mediated attraction to repulsion (Shewan et al., 2002). Taken together, these results suggest that the age-dependant change in netrin-1 responsiveness could be induced by a change in A2b receptor expression in RGC axons. Further investigations will be necessary to understand how the down-regulation of A2b downstream led to growth cone repulsion and whether it acts directly by regulating the amount of cytosolic cAMP.
EphB and ephrin-B molecules are involved in intraretinal axon guidance
Eph receptors and ephrins represent another family of guidance molecule for which recent studies have provided evidence of important roles in developing visual projections. The Eph family of receptor tyrosine kinases comprises 15 identified members that interact with a second family of cell surface molecules, the ephrins. With a few exceptions, A-type Eph receptors interact with glycosylphosphatidylinositol anchored ephrins (ephrin-A) and B-type Eph receptors bind transmembrane ephrins (ephrin-B) (Nomenclature committee, 1997). Eph receptors and ephrins have the important property of being able to initiate bi-directional signaling, where a signal is propagated in both the Eph receptor-bearing cell (‘forward signaling’) and in the ligand-expressing cell (‘reverse signaling’) (for review see (Kullander and Klein, 2002).
Eph receptors and ephrins are known to be the largest class of molecules that exhibit polarized expression patterns in the retina. In general, A-type receptors and ligands are expressed in opposite nasal-temporal gradients and B-type receptors and ligands show complementary distributions across the dorsal-ventral axis (see Fig. 4). This applies to fish (Brennan et al., 1997), amphibians (Mann et al., 2002), birds (Connor et al., 1998, Holash et al., 1997) and mammals (Birgbauer et al., 2000, Marcus et al., 1996) and marsupials (Vidovic and Marotte, 2003, Vidovic et al., 1999). The presence of both Eph receptors and their ephrin ligands suggest that these molecules might function to regulate intraretinal axon growth. Evidence for such a role has come from the study of mice deficient for two genes encoding the EphB2 and EphB3 receptors (Birgbauer et al., 2000). During RGC axon pathfinding to the disk, both receptors are initially distributed uniformly through the retina and later become differentially expressed along the dorso-ventral axis of the retina, with higher expression levels detected in the ventral part of the retina. Labelling of RGCs using the lipophilic dye, DiI, was used to show that RGC axons orient and grow normally towards the optic disk in the EphB2/EphB3 double mutant, but as they approach the centre of the retina, a small proportion of axons bypass the optic disk and extend abnormally into the opposite side of the retina. Several lines of evidence suggest that EphB molecules act in this system as guidance cues through a “reverse signaling” mechanism rather than functioning as traditional signaling receptors. First, the placing of DiI crystals into either the dorsal or the ventral retina shows that, although EphB2 and EphB3 are predominantly expressed in ventral retina, RGC axons from dorsal retina are the most severely affected. Second, partial rescue of the observed phenotype was obtained in mice which express a mutant EphB2 which lacks a cytoplasmic domain, suggesting that EphB2 functions in this system in a kinase-independent manner. These data led to propose a model in which ephrin-B-expressing RGC axons from the dorsal retina extending towards the optic disk encounter increasing amounts of EphB2 and EphB3 receptors that prevent dorsal axons from growing into the opposite side of the eye (Birgbauer et al., 2000, Birgbauer et al., 2001).
However, in mice deficient for EphB2 and EphB3, as well as mice lacking netrin-1, abnormal RGC axon behaviours are often only seen close to the optic disk suggesting that the mechanisms that direct the centripetal growth of RGC axons are mediated by other factors. Interestingly, two other Eph receptors, EphB5 and EphA7, distribute in centroperipheral gradients in chick retina, suggesting a possible role in directing the convergent growth of RGC axon the centre of the retina (Sefton et al., 1997). However, experimental proof of such a function is still lacking.
EphB and ephrin-B control axon divergence at the optic chiasm
The optic chiasm is an important choice point along the visual pathway where RGC axons have to decide whether or not to cross the ventral midline of the brain. The degree of decussation of RGC axons at the optic chiasm depends on the type of animal. Fish and birds have no binocular vision and only crossed projections. In most mammals, all RGCs located in the temporal retina send axons ipsilaterally, whereas in species with less binocular vision uncrossed projections arise from a subpopulation of RGCs in the ventrotemporal (VT) region of the retina.
A key issue is to identify the nature of the molecular mechanisms that control the routing of RGC axons at the optic chiasm. Early studies in mouse have shown that the optic chiasm is the source of guidance molecules for crossed and uncrossed axons (for review see (Mason and Sretavan, 1997), but the first identification of the nature of the molecular actors that regulate axon divergence at the chiasm come from studies in the amphibians. During embryonic development in Xenopus laevis, all RGC axons from the two optic nerves cross each other at the optic chiasm. It is only during metamorphosis that the first ipsilateral projections start to develop from the VT retina, in order to subserve the acquisition of binocular vision in young froglets. The search for candidate molecules specifically expressed in the binocular part of the retina led to a focus on Eph receptors. High levels of B-type Eph receptors are expressed in the VT region of Xenopus retina through development and the corresponding ephrin-B ligand is detected at the optic chiasm of metamorphosing animals but not in premetamorphic embryos (Nakagawa et al., 2000); see Fig. 2). In addition, precocious expression of ephrin-B ligand at the chiasm of premetamorphic embryos is sufficient to misroute some axons from the VT retina ipsilaterally (Nakagawa et al., 2000). Taken together, these results suggest that ephrin-B ligand forms a repulsive ‘barrier’ at the chiasm that repels the subpopulation of EphB-expressing fibers into the ipsilateral tract (Fig. 2). Does the same ephrin-B-based mechanism work in other vertebrates? This idea is supported by the fact that in chick embryos, which lack permanent ipsilateral projections, EphB binding sites (ie. ephrin-B) cannot be detected at the ventral midline of the diencephalon (Nakagawa et al., 2000). On the other hand, one ephrin ligand, ephrin-B2, is specifically expressed in radial glia cells at the chiasm of mouse embryos in a pattern that correlates with the temporal development of ipsilateral projections in mice (Williams et al., 2003). A recent study provides evidence that ephrin-B2 is necessary for the development of ipsilateral projections, since inhibition of ephrin-B2 activity by soluble EphB4-Fc completely abolished the formation of ipsilateral projections in a semi-intact preparation of the visual pathway (Williams et al., 2003).
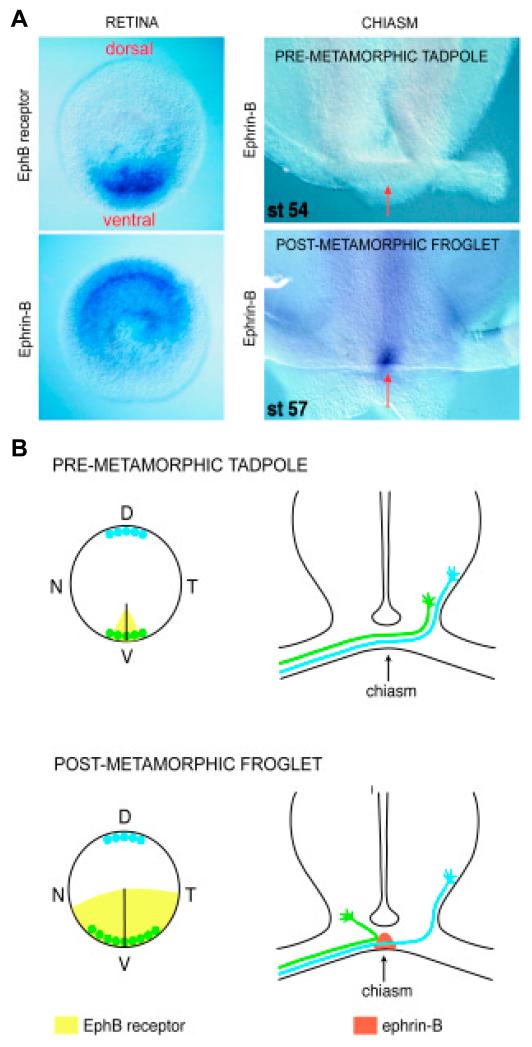
Fig. 2. Ephrin-B mediates divergent axon choice at the optic chiasm.
(A) Shows the distribution of ephrin-B and EphB receptor in the embryonic retina (left two panels) and the chiasm of pre- and post- and metamorphic Xenopus (right two panels). Ephrin-B is expressed in a high-dorsal to low-ventral gradient in the retina while the EphB receptor is expressed in an opposing high-ventral to low-dorsal gradient. Ephrin-B is not expressed at the chiasm until metamorphosis which coincides with the initiation of the ipsilateral projection. A subpopulation of ventral EphB-expressing cells project ipsilaterally at metamorphosis. Photomicrographs adapted from Nakagawa et al., 2000 and Mann et al., 2002. (B) Model of repulsive axon guidance at the chiasm. See text for details. Diagram adapted from Mann and Holt, 2001.
Another important question is to understand how ipsilaterally projecting RGCs are specified to respond to ephrin-B repulsive activity at the optic chiasm. This issue is complicated by the fact that in mouse only a small proportion (about 3%) of RGCs in the VT retina send axons ipsilaterally. Among the putative receptors for ephrin-B2, EphB1 was the most likely candidate, since its expression is restricted to some RGCs in mouse VT retina, unlike EphB2 and EphB3 which show a widespread expression (Williams et al., 2003). Anterograde and retrograde labelling of visual projections in EphB1 null mutant revealed a strong reduction in the number of ipsilaterally projecting RGC axons that instead project aberrantly to the contralateral side of the brain (Williams et al., 2003). Recently the zinc-finger transcription factor, Zic2, was found to be expressed exclusively in RGCs with uncrossed projections and the extent of Zic2 expression in different vertebrates correlates with the degree of binocularity (Herrera et al., 2003). Gain and loss of function experiments in mouse demonstrate that expression of Zic2 is required for axons to be repelled at the chiasm and to project ipsilaterally. These findings raise the possibility that Zic2 could directly regulate the expression of EphB1 in subsets of growing RGC axons.
Topographic mapping of visual projection depends on Eph family molecules
Topographic mapping of RGC axons in their target occurs along two orthogonal axes: the nasal-temporal axis of the retina maps along the posterior-anterior axis of the optic tectum and the dorsal-ventral retinal axis along the ventral-dorsal tectal axis (lateral-medial (L-M) in mammals; see summary diagrams in Figs. 3,4). The search for candidate molecules involved in setting-up topographic mapping of visual projections has first involved the A-type subclass of Eph and ephrins, which are expressed in opposite gradients along the A-P axis of the retina and tectum, respectively. A large amount of evidence from both in vitro and in vivo experiments have led to a model in which the graded repulsion of EphA-expressing RGC axons and ephrin-A in the target prevent temporal axons from terminating in the ligand-rich posterior part of the tectum (for review see (Drescher et al., 1997, McLaughlin et al., 2003a).
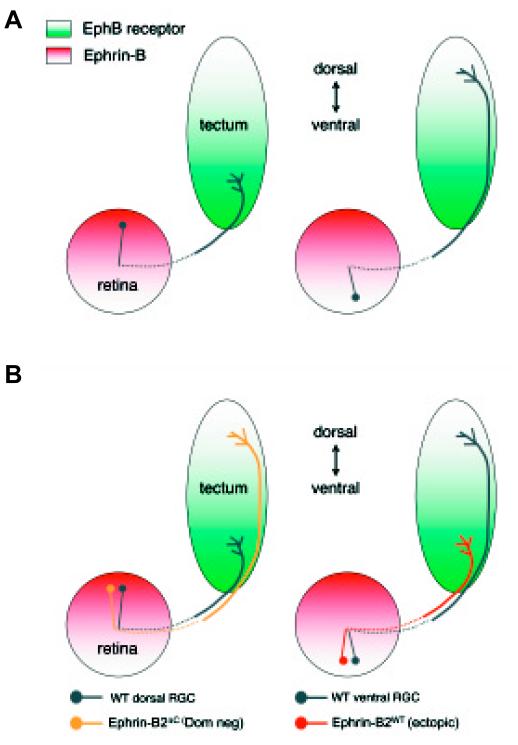
Fig. 3. Topographic mapping along the dorso-ventral axis involves attractive ephrin/EphB interactions.
(A) The normal distribution of B-type ephrins and receptors in the retina and tectum. Regions of high ephrin-B expression (dorsal retina) project to regions of high receptor expression (ventral tectum) indicative of matching, attractive interactions. (B) Inhibition of EphB receptor function in dorsal retina causes axons to map significantly further dorsally (like ventral cells) and ectopic expression of EphB in ventral RGCs causes them to map ventrally (like dorsal cells) instead of dorsally. Summary of data from Mann et al., 2002.
The mechanisms involved in D-V patterning have been discovered more recently. Due to the polarized expression of B-type Eph molecules in the D-V axis of developing retina in many vertebrate species, EphB and ephrin-B were proposed to be choice candidates for such a function. In chick and mouse, gradients of ephrin-B1 ligand were detected in the tectum and superior colliculi respectively (Braisted et al., 1997, Hindges et al., 2002). Unlike in the A-P axis, ephrin-B is expressed in the dorsal tectum (medial SC) where ventral RGC expressing high levels of EphB receptors terminate, suggesting that attractive rather than repulsive interactions between Eph and ephrin are involved in mapping along this axis. In mouse, RGC axons enter their target structure, form interstitial branches at appropriate A-P positions that extend along the latero-medial (L-M) axis towards their appropriate topographic target region. In double mutants for EphB2 and EphB3 receptors, injection of DiI into the VT retina of postnatal animal revealed ectopic termination of RGC afferents in regions lateral to the appropriate target zone along the L-M axis. In contrast, dorsal axons terminate in their appropriate region (Hindges et al., 2002). The same aberrant termination zone of ventral RGCs axons were observed in mutants for kinase-inactivated EphB2, suggesting that forward rather than reverse EphB signaling is most probably involved in L-M targeting. Consistent with the hypothesis of an attractant effect between B-type Eph and ephrin, these results indicate that signaling through EphB receptors is required for RGC axons to form branches that extent dorsally in the SC toward region of high ephrin-B expression.
In lower vertebrates such as frogs, B-type Eph receptors and ephrins have also been shown to play an important role in D-V patterning. In the tectum of Xenopus embryos, a D-V gradient of EphB1 can be detected, with a maximum expression level in the ventral part of tectum where ephrin-B expressing dorsal RGC axons terminate (Mann et al., 2002). EphB and ephrin-B function was disrupted in vivo by applying the extracellular domain of the EphB2 receptor to the optic pathway in an “exposed brain preparation”. This treatment causes targeting errors of retinal axons which terminate in a more dorsal position in the tectum, leaving the ventral tectum (normally innervated by ephrin-B-expressing RGCs) completely devoid of retinal afferents (Mann et al., 2002). Taken together, these findings suggest that ephrin-B “reverse signaling” in dorsal RGC axons might be important for the recognition of D-V positional cues. To address this idea directly, a dominant negative ephrin-B ligand that lacks a cytoplasmic domain was expressed by targeted lipofection into dorsal retinal precursors. Analysis of D-V topography shows that transfected axons project significantly more dorsally in the tectum (Fig. 3). In contrast, missexpression of full-length ephrin-B ligand in ventral RGCs causes them to terminate more ventrally in their target (Fig. 3) (Mann et al., 2002). These results demonstrate that the cytoplasmic tail of ephrin-Bs is important for appropriate D-V mapping and support the idea of an attractive mechanism by which EphB1 receptor in the tectum provides topographic guidance cues for ephrin-B-expressing RGC axons.
Mechanisms of Eph and ephrin bifunctional activity
The studies discussed above have contributed significantly to our understanding of Eph receptors and ephrin in vivo functions during RGC axon development. However, the mechanisms by which these molecules control growth cone behaviour remain poorly understood. In vivo gain and loss of function experiments suggest that Eph/ephrin activation leads to growth cone responses that can be attributed to both repulsion (in retina and optic chiasm) and attraction (in tectum and superior colliculi). Similarly, dual function of B-type Eph molecules has been observed in vitro: dorsal retinal neurons adhere and grow preferentially on EphB substrate (Holash et al., 1997, Mann et al., 2002) but are repelled by soluble EphB added to the culture medium (Birgbauer et al., 2001, Mann et al., 2003).
Which mechanisms underlie the bifunctional action of Eph and ephrin? As shown previously for netrin-1, changes in cytosolic cyclic nucleotides might modulate Eph/ephrin signaling. For example, the repulsive response to soluble EphB relies on low levels of cGMP (Mann et al., 2003). Another factor known to influence Eph/ephrin responsiveness is the degree of clustering. As both receptor and ligands are membrane-anchored molecules, soluble forms of Eph and ephrin require to be artificially oligomerized in order to activate their binding partner in vitro (Davis et al., 1994). In endothelial cells, different oligomeric forms of ephrin-B1-Fc induce distinct cell responses mediated by the recruitment of distinct downstream signaling complexes (Stein et al., 1998). Moreover, in culture of retinal explants, clustered (multimeric) ephrin-B1-Fc fusion protein induces growth cone collapse whereas unclustered (dimeric) ephrin-B1-Fc does not. In addition, unclustered but not clustered EphB2-Fc stimulates retinal growth cone retraction (Mann et al., 2003). How does this relate to the in vivo situation? The above data suggest that signals or adaptor proteins that control the degree oligomerisation might modulate whether Eph and ephrin act as attractants or repellents. Alternatively, the differences in the density of Eph/ephrin expression might be critical to determine growth cone response. This latter idea is supported by recent experiments where growing visual axons are confronted by ectopic ephrin-B1 expressed in the chick tectum: high ephrin-B1 expression attracts RGC axon interstitial branches, whereas lower expression causes branch repulsion (McLaughlin et al., 2003b).
Another proposed mechanism that modulates repulsion versus adhesion involves extracellular proteolytic cleavage of ephrin ectodomain. Upon binding to cognate Eph receptors, ephrin ligands are cleaved from the cell surface by the Kuzbanian metalloprotease, and this processing is thought to be important for axon growth cones to break adhesive contacts with an ephrin-expressing cell (Hattori et al., 2000). In principle, Eph/ephrin interactions occurring in the absence of metalloprotease activity would prevent growth cone retraction and result in sustained adhesive behaviour. This mechanism has been described for A-type Eph and ephrin and it is not known whether it occurs in the B-subclass of Eph and ephrin molecules, although proteolytically-released forms of ephrin-B ligands exist in vitro (Nicola et al.,1996). Instead, in cell lines and primary neuronal cultures, the entire EphB-ephrin-B complex is removed from the cell surface by endocytose (Mann et al., 2003, Marston et al., 2003, Zimmer et al., 2003). In cultures of retinal cells, endocytosis occurs during ‘reverse signaling’: internalization of EphB receptor occurs in dorsal retinal growth cones and appears to be necessary for growth cone collapse, since an inhibitor of endocytosis blocks this response. In primary cultures of mouse hippocampal neurons, it has been show that the intracellular domain of EphB receptors is important for endocytosis and subsequent growth cone collapse (Zimmer et al., 2003). In addition, previous studies reported that ephrin-A5 mediates repulsion through activation of a full-length EphA7 and adhesion through a truncated EphA7 receptor that lacks a cytoplasmic domain (Holmberg et al., 2000). Since truncated forms of EphB receptors have been detected in mouse neural system (Ciossek et al., 1995), it would be interesting to determine their exact localization and examine how they could possibly determine EphB/ephrin-B activity.
Protein synthesis and degradation in visual axon navigation
Recent studies have revealed that several aspects of growth cone navigation, including the mechanisms by which external signals are transduced within the growth cone, might be regulated by rapid and local changes in protein levels. For example, in Xenopus retinal axons, netrin-1-induced growth cone turning can be blocked with specific translation inhibitors and proteasomal inhibitors (Campbell and Holt, 2001). EphB-induced collapse, however, does not depend on protein synthesis, but can be blocked with inhibitors of the proteasomal pathway which also inhibit EphB internalisation into retinal growth cones (Mann et al., 2003). In contrast, growth cone repulsion stimulated by ephrin-B is not affected by blocking protein synthesis or degradation (Mann et al., 2003). The nature of the proteins involved in mediating growth cone steering is not yet known and efforts are now being made to identify the mRNAs present in the axons and growth cones.
In Xenopus embryos, in situ hybridization methods have revealed strong positive signals for mRNAs of various B-type Eph/ephrin molecules in the optic nerve suggesting that translation regulatory mechanisms might control the levels of EphB and ephrin-B protein expression at the growth cone (Mann et al., 2002). Evidence for such a mechanism come from the study of spinal commissural neurons in which EphA2 mRNA is translated in the distal part of the axons arriving at the ventral midline (Brittis et al., 2002). Controlled translation could be involved in regulating the localized increase of EphB1 in ipsilateral axons that approach the chiasm midline. In addition, mRNA translation could control the subcellular localisation of EphB receptors, a mechanism that would explain why interstitial branches but not main visual axons are sensitive to ephrin-B1 ligand in their target structure (Hindges et al., 2002, McLaughlin et al., 2003b).
Conclusion
Netrin and Eph/ephrin molecules belong to the growing list of guidance molecules that have been shown to contribute to the accurate development of visual projections. Although they play distinct roles, they share the ability to exert bi-functional activity on growing RGC axons, they are reused along the visual pathway and their role is conserved among vertebrate species.
Acknowledgements
The authors were supported by grants from the Wellcome Trust, MRC and HSFP.
Abbreviations used in this paper
- CNS
central nervous system
- onh
optic nerve head
- RGC
retinal ganglion cell
References
- ANDERSON RB, HOLT CE. Expression of unc-5 in the developing Xenopus visual system. Mech Dev. 2002;118:157–60. doi: 10.1016/s0925-4773(02)00215-0. [DOI] [PubMed] [Google Scholar]
- BIRGBAUER E, COWAN CA, SRETAVAN DW, HENKEMEYER M. Kinase independent function of ephb receptors in retinal axon pathfinding to the optic disc from dorsal but not ventral retina. Development. 2000;127:1231–41. doi: 10.1242/dev.127.6.1231. [DOI] [PubMed] [Google Scholar]
- BIRGBAUER E, OSTER SF, SEVERIN CG, SRETAVAN DW. Retinal axon growth cones respond to ephb extracellular domains as inhibitory axon guidance cues. Development. 2001;128:3041–8. doi: 10.1242/dev.128.15.3041. [DOI] [PubMed] [Google Scholar]
- BRAISTED JE, MCLAUGHLIN T, WANG HU, FRIEDMAN GC, ANDERSON DJ, O’LEARY DD. Graded and lamina-specific distributions of ligands of ephb receptor tyrosine kinases in the developing retinotectal system. Dev Biol. 1997;191:14–28. doi: 10.1006/dbio.1997.8706. [DOI] [PubMed] [Google Scholar]
- BRENNAN C, MONSCHAU B, LINDBERG R, GUTHRIE B, DRESCHER U, BONHOEFFER F, HOLDER N. Two eph receptor tyrosine kinase ligands control axon growth and may be involved in the creation of the retinotectal map in the zebrafish. Development. 1997;124:655–64. doi: 10.1242/dev.124.3.655. [DOI] [PubMed] [Google Scholar]
- BRITTIS PA, LU Q, FLANAGAN JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–35. doi: 10.1016/s0092-8674(02)00813-9. [DOI] [PubMed] [Google Scholar]
- CAMPBELL DS, HOLT CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–26. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- CIOSSEK T, LERCH MM, ULLRICH A. Cloning, characterization, and differential expression of mdk2 and mdk5, two novel receptor tyrosine kinases of the eck/eph family. Oncogene. 1995;11:2085–95. [PubMed] [Google Scholar]
- CONNOR RJ, MENZEL P, PASQUALE EB. Expression and tyrosine phosphorylation of eph receptors suggest multiple mechanisms in patterning of the visual system. Dev Biol. 1998;193:21–35. doi: 10.1006/dbio.1997.8786. [DOI] [PubMed] [Google Scholar]
- CORSET V, NGUYEN-BA-CHARVET KT, FORCET C, MOYSE E, CHEDOTAL A, MEHLEN P. Netrin-1-mediated axon outgrowth and camp production requires interaction with adenosine A2b receptor. Nature. 2000;407:747–50. doi: 10.1038/35037600. [DOI] [PubMed] [Google Scholar]
- DAVIS S, GALE NW, ALDRICH TH, MAISONPIERRE PC, LHOTAK V, PAWSON T, GOLDFARB M, YANCOPOULOS GD. Ligands for eph-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–9. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- DE LA TORRE JR, HOPKER VH, MING GL, POO MM, TESSIER-LAVIGNE M, HEMMATI-BRIVANLOU A, HOLT CE. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor dcc. Neuron. 1997;19:1211–24. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
- DEINER MS, KENNEDY TE, FAZELI A, SERAFINI T, TESSIER-LAVIGNE M, SRETAVAN DW. Netrin-1 and dcc mediate axon guidance locally at the optic disc: Loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–89. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- DEINER MS, SRETAVAN DW. Altered midline axon pathways and ectopic neurons in the developing hypothalamus of netrin-1- and dcc-deficient mice. J Neurosci. 1999;19:9900–12. doi: 10.1523/JNEUROSCI.19-22-09900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRESCHER U, BONHOEFFER F, MULLER BK. The eph family in retinal axon guidance. Curr Opin Neurobiol. 1997;7:75–80. doi: 10.1016/s0959-4388(97)80123-7. [DOI] [PubMed] [Google Scholar]
- FRICKE C, LEE JS, GEIGER-RUDOLPH S, BONHOEFFER F, CHIEN CB. Astray, a zebrafish roundabout homolog required for retinal axon guidance. Science. 2001;292:507–10. doi: 10.1126/science.1059496. [DOI] [PubMed] [Google Scholar]
- HATTORI M, OSTERFIELD M, FLANAGAN JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289:1360–5. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- HERRERA E, BROWN L, ARUGA J, RACHEL RA, DOLEN G, MIKOSHIBA K, BROWN S, MASON CA. Zic2 patterns binocular vision by specifying the uncrossed retinal projection. Cell. 2003;114:545–57. doi: 10.1016/s0092-8674(03)00684-6. [DOI] [PubMed] [Google Scholar]
- HINDGES R, MCLAUGHLIN T, GENOUD N, HENKEMEYER M, O’LEARY DD. Ephb forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron. 2002;35:475–87. doi: 10.1016/s0896-6273(02)00799-7. [DOI] [PubMed] [Google Scholar]
- HOLASH JA, SOANS C, CHONG LD, SHAO H, DIXIT VM, PASQUALE EB. Reciprocal expression of the eph receptor cek5 and its ligand(s) in the early retina. Dev Biol. 1997;182:256–69. doi: 10.1006/dbio.1996.8496. [DOI] [PubMed] [Google Scholar]
- HOLMBERG J, CLARKE DL, FRISEN J. Regulation of repulsion versus adhesion by different splice forms of an eph receptor. Nature. 2000;408:203–6. doi: 10.1038/35041577. [DOI] [PubMed] [Google Scholar]
- HONG K, HINCK L, NISHIYAMA M, POO MM, TESSIER-LAVIGNE M, STEIN E. A ligand-gated association between cytoplasmic domains of unc5 and dcc family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–41. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- HöPKER VH, SHEWAN D, TESSIER-LAVIGNE M, POO M, HOLT C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- HUTSON LD, CHIEN CB. Pathfinding and error correction by retinal axons: The role of astray/robo2. Neuron. 2002;33:205–17. doi: 10.1016/s0896-6273(01)00579-7. [DOI] [PubMed] [Google Scholar]
- KENNEDY TE, SERAFINI T, DE LA TORRE JR, TESSIER-LAVIGNE M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–35. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- KULLANDER K, KLEIN R. Mechanisms and functions of eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–86. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- LAUDERDALE JD, DAVIS NM, KUWADA JY. Axon tracts correlate with netrin-1a expression in the zebrafish embryo. Mol Cell Neurosci. 1997;9:293–313. doi: 10.1006/mcne.1997.0624. [DOI] [PubMed] [Google Scholar]
- MANN F, MIRANDA E, WEINL C, HARMER E, HOLT CE. B-type eph receptors and ephrins induce growth cone collapse through distinct intracellular pathways. J Neurobiol. 2003;57:323–36. doi: 10.1002/neu.10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANN F, RAY S, HARRIS W, HOLT C. Topographic mapping in dorsoventral axis of the Xenopus retinotectal system depends on signaling through ephrin-b ligands. Neuron. 2002;35:461–73. doi: 10.1016/s0896-6273(02)00786-9. [DOI] [PubMed] [Google Scholar]
- MARCUS RC, GALE NW, MORRISON ME, MASON CA, YANCOPOULOS GD. Eph family receptors and their ligands distribute in opposing gradients in the developing mouse retina. Dev Biol. 1996;180:786–9. doi: 10.1006/dbio.1996.0347. [DOI] [PubMed] [Google Scholar]
- MARSTON DJ, DICKINSON S, NOBES CD. Rac-dependent transendocytosis of ephrinbs regulates eph-ephrin contact repulsion. Nat Cell Biol. 2003;5:879–88. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- MASON CA, SRETAVAN DW. Glia, neurons, and axon pathfinding during optic chiasm development. Curr Opin Neurobiol. 1997;7:647–53. doi: 10.1016/s0959-4388(97)80084-0. [DOI] [PubMed] [Google Scholar]
- MCLAUGHLIN T, HINDGES R, O’LEARY DD. Regulation of axial patterning of the retina and its topographic mapping in the brain. Curr Opin Neurobiol. 2003a;13:57–69. doi: 10.1016/s0959-4388(03)00014-x. [DOI] [PubMed] [Google Scholar]
- MCLAUGHLIN T, HINDGES R, YATES PA, O’LEARY DD. Bifunctional action of ephrin-b1 as a repellent and attractant to control bidirectional branch extension in dorsal-ventral retinotopic mapping. Development. 2003b;130:2407–18. doi: 10.1242/dev.00467. [DOI] [PubMed] [Google Scholar]
- MING GL, SONG HJ, BERNINGER B, HOLT CE, TESSIER-LAVIGNE M, POO MM. Camp-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–35. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA S, BRENNAN C, JOHNSON KG, SHEWAN D, HARRIS WA, HOLT CE. Ephrin-b regulates the ipsilateral routing of retinal axons at the optic chiasm. Neuron. 2000;25:599–610. doi: 10.1016/s0896-6273(00)81063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEFTON M, ARAUJO M, NIETO MA. Novel expression gradients of eph-like receptor tyrosine kinases in the developing chick retina. Dev Biol. 1997;188:363–8. doi: 10.1006/dbio.1997.8638. [DOI] [PubMed] [Google Scholar]
- SERAFINI T, KENNEDY TE, GALKO MJ, MIRZAYAN C, JESSELL TM, TESSIER-LAVIGNE M. The netrins define a family of axon outgrowth-promoting proteins homologous to c. Elegans unc-6. Cell. 1994;78:409–24. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- SHEWAN D, DWIVEDY A, ANDERSON R, HOLT CE. Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nat Neurosci. 2002;5:955–62. doi: 10.1038/nn919. [DOI] [PubMed] [Google Scholar]
- SHIRASAKI R, MIRZAYAN C, TESSIER-LAVIGNE M, MURAKAMI F. Guidance of circumferentially growing axons by netrin-dependent and -independent floor plate chemotropism in the vertebrate brain. Neuron. 1996;17:1079–88. doi: 10.1016/s0896-6273(00)80241-x. [DOI] [PubMed] [Google Scholar]
- SONG HJ, POO MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol. 1999;9:355–63. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- STEIN E, LANE AA, CERRETTI DP, SCHOECKLMANN HO, SCHROFF AD, VAN ETTEN RL, DANIEL TO. Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev. 1998;12:667–78. doi: 10.1101/gad.12.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAHLE U, FISCHER N, BLADER P. Expression and regulation of a netrin homologue in the zebrafish embryo. Mech Dev. 1997;62:147–60. doi: 10.1016/s0925-4773(97)00657-6. [DOI] [PubMed] [Google Scholar]
- STUERMER CA, BASTMEYER M. The retinal axon’s pathfinding to the optic disk. Prog Neurobiol. 2000;62:197–214. doi: 10.1016/s0301-0082(00)00012-5. [DOI] [PubMed] [Google Scholar]
- VIDOVIC M, MAROTTE LR. Analysis of ephb receptors and their ligands in the developing retinocollicular system of the wallaby reveals dynamic patterns of expression in the retina. Eur J Neurosci. 2003;18:1549–58. doi: 10.1046/j.1460-9568.2003.02882.x. [DOI] [PubMed] [Google Scholar]
- VIDOVIC M, MAROTTE LR, MARK RF. Marsupial retinocollicular system shows differential expression of messenger rna encoding epha receptors and their ligands during development. J Neurosci Res. 1999;57:244–54. doi: 10.1002/(SICI)1097-4547(19990715)57:2<244::AID-JNR10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- WILLIAMS SE, MANN F, ERSKINE L, SAKURAI T, WEI S, ROSSI DJ, GALE NW, HOLT CE, MASON CA, HENKEMEYER M. Ephrin- b2 and ephb1 mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39:919–35. doi: 10.1016/j.neuron.2003.08.017. [DOI] [PubMed] [Google Scholar]
- ZIMMER M, PALMER A, KOHLER J, KLEIN R. Ephb-ephrinb bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol. 2003;5:869–78. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]