Abstract
Lactococcal lactate dehydrogenases (LDHs) are coregulated at the substrate level by at least two mechanisms: the fructose-1,6-biphosphate/phosphate ratio and the NADH/NAD ratio. Among the Lactococcus lactis species, there are strains that are predominantly regulated by the first mechanism (e.g., strain 65.1) or by the second mechanism (e.g., strain NCDO 2118). A more complete model of the kinetics of the regulation of lactococcal LDH is discussed.
Lactococci are known for their homolactic metabolism, whereby more than 90% of the sugars present are converted into lactic acid. However, under certain conditions, the metabolism may shift to the production of mixed acids (acetate, ethanol, and formate). From the 1960s onward, the view that the control of this shift was modulated mainly by the intracellular concentration of fructose-1,6-biphosphate (FBP) activating both l-lactate dehydrogenase (l-LDH; EC 1.1.1.27) and pyruvate kinase (EC 2.7.1.40) was held (3, 9, 18, 19). Inorganic phosphate (Pi) was recognized as a severe inhibitor of both enzymes. Apparently, both FBP and Pi were seen to compete for the same allosteric site of LDH (9).
Recently, this metabolic model was questioned by Garrigues et al. (5), who showed that the sugar metabolism of strain NCDO 2118 was instead regulated by the NADH/NAD ratio. A high ratio inhibited glyceraldehyde 3-phosphate dehydrogenase (GAPDH; EC 1.2.1.12) and increased LDH activity. Many, but not all, researchers took up this view without reflection, creating a confusing situation. Currently, metabolic flux models based on enzyme kinetics are applied as predictive tools in metabolic engineering (see, e.g., reference 8), illustrating the importance of expressing kinetic characteristics adequately. Therefore, we undertook an investigation regarding the nature of the regulation of LDH activity among several Lactococcus lactis strains, including those frequently used in metabolic flux studies. We also examined the effects of ATP, ADP, AMP, and phosphoenolpyruvate (PEP), since Jonas et al. (9) observed strong competitive inhibition by ATP of LDH activity in L. lactis strain US3 (=NCIMB701197).
L. lactis strains (listed in Table 1) were each grown anaerobically at 30°C in pH-controlled batch cultures on glucose (10 g/liter) with SD3 medium (17). The external pH was kept at 6.5, which corresponds to an internal pH of 7.2 (12). The cultures were harvested in the late exponential phase, centrifuged at 4°C for 10 min at 5,000 × g, washed, and resuspended in triethanolamine buffer (50 mM triethanolamine [pH 7.2], 5 mM MgCl2 · 6H2O). Cell extracts (CE) were prepared by using glass beads (17). LDH activity was measured spectrophotometrically by monitoring the oxidation of NADH (340 nm; ɛ = 6,220 M−1cm−1) at 30°C. One milliliter of the standard assay mixture consisted of triethanolamine buffer (50 mM; pH 7.2), NADH (0.3 mM), FBP (10 mM), and CE (approximately 160 mg of protein/liter). The reaction was initiated by the addition of pyruvate (initial concentration, 10 mM). One unit of LDH was defined as the amount of enzyme that oxidized 1 μmol of NADH min−1. To ensure consistent reproducible values, the level of LDH activity was determined by testing the activities of four different concentrations of CE. The enzyme activity was always proportional to the concentration of the enzyme. The LDH of each strain was characterized in vitro by the addition of the compounds FBP, Pi, pyruvate, PEP, ATP, ADP, AMP, NADH, and NAD at various concentrations. Protein concentration was assayed as described by Bradford (2). Parameter estimation was carried out by application of Lineweaver-Burk plots or the least-squares method.
TABLE 1.
Influence of various compounds on the activity of lactococcal LDH
| Strain | Activation by FBP (mM)a
|
KP,0.5V (mM)b | KNAD (mM)c | NADcrit (mM)d | ATPcrit (mM)d | ADPcrit (mM)d | |
|---|---|---|---|---|---|---|---|
| KFBP,0.5V | Total | ||||||
| 65.1 | 0.550 | 10 | 9.2 | 25 | 35 | 25.4 | 18.7 |
| MG 1363 | 0.006 | 5 | 10.3 | 1.8 | 26 | 21.6 | 14.8 |
| NCIMB701197 | 0.010e | 1e | 17 | 0.54 | 13 | 14.4 | 14.9 |
| NCIMB700509 | 0.002f | 5-10f | 23 | 0.38 | 9.9 | 16.7 | 12.7 |
| NCIMB700763 | 0.002 | 8 | 31 | 0.44 | 10.3 | 13.8 | 14.0 |
| IL 1403 | 0.003 | 5 | 38 | 0.5 | 13 | 15.8 | 10.0 |
| ATCC 19435 | <0.001 | 2 | 2,400 | 2.3 | 15 | 31.4 | 13.5 |
| NCDO 2118 | <0.001 | 4.5 | 592,500 | 2.1 | 15.3 | 19.6 | 15.4 |
KFBP,0.5V, FBP concentration at which LDH was 50% activated in the absence of Pi; Total, FBP concentration at which LDH was totally activated in the absence of Pi.
KP,0.5V, phosphate concentration at which LDH was 50% inhibited, as extrapolated to 0 mM FBP.
KNAD, inhibition constant of NAD based on kinetics of competitive inhibition.
NADcrit, ATPcrit, and ADPcrit, concentrations of NAD, ATP, and ADP, respectively, at which LDH is expected to be inhibited completely based on the equation of Han and Levenspiel (6).
Taken from literature (9).
Taken from literature (3).
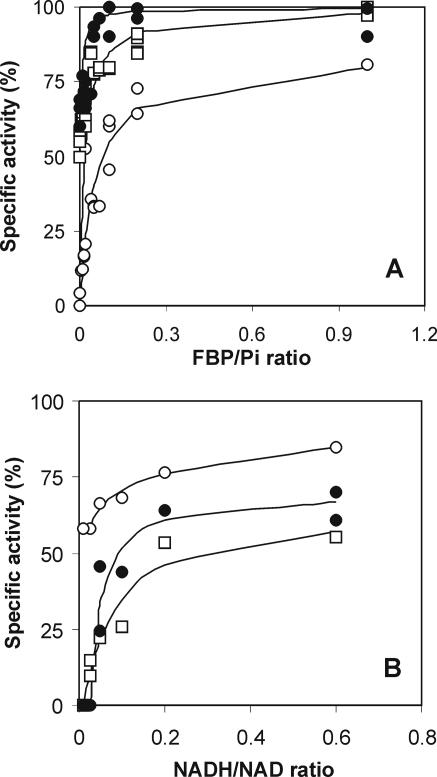
The FBP concentrations at which LDH was 50% activated (KFBP,0.5V) differed among the strains (Table 1). Strain 65.1 was found to have an LDH that was activated by substantial concentrations of FBP and was strongly inhibited by Pi (Table 1 and Fig. 1A) but was only slightly affected by the NADH/NAD ratio (Fig. 1B). Both the inhibition constant and the critical concentration of NAD were relatively high. ATP and ADP had threshold concentrations (at which they start being inhibitory) of 4 and 2 mM, respectively, for strain 65.1. These findings are in strong contrast to those for the LDHs of strains NCDO 2118 and ATCC 19435; for these strains, high activities (approximately 67% of the maximum activity) were found in the presence of FBP at the micromole level and Pi was hardly inhibitory (Table 1 and Fig. 1A). However, their LDHs were significantly regulated by the NADH/NAD ratio (Fig. 1B). For full activation, strain NCDO 2118 still required intracellular FBP concentrations of about 4 to 5 mM (Table 1), although FBP concentrations were found to be far in excess even under substrate-limiting conditions (5). Therefore, in this strain, FBP will not have a significant influence on the performance of LDH, and the results obtained in the present study support those of Garrigues et al. (5). The LDHs of strains 65.1 and NCDO 2118 were at two extremes with regard to their regulation at the substrate level. All other strains tested possessed mixtures of both regulation mechanisms (Table 1 and Fig. 1). Therefore, we conclude that the activity of lactococcal LDH is regulated by a combination of two mechanisms, the FBP/Pi ratio (the P [phosphorus] type) and the NADH/NAD ratio (the R [redox] type). The former mechanism is based on allosteric regulation (3), while the latter displayed competitive inhibition by NAD (Fig. 2), possibly at the active site. In addition, ADP and ATP, showing mixed inhibition (15), contribute to the inhibitory effect of NAD. There was inhibition by AMP, but since this compound is present in the cell at low concentrations, it was considered negligible. PEP at concentrations of up to 10 mM did not inhibit LDH in any of the strains studied.
FIG. 1.
Effect of the FBP/Pi ratio (A) and the NADH/NAD ratio (B) on the in vitro specific activity of LDH measured in extracts prepared from exponentially growing cells of strain 65.1 (○), strain NCDO 2118 (•), and strain ATCC 19435 (□). The kinetics of the NADH/NAD ratio depicted here holds for NADH concentrations of at least four times the KS,NADH value (i.e., the affinity constant for NADH); the inhibitory effect of NAD became more severe at an NADH concentration of two times the KS,NADH value.
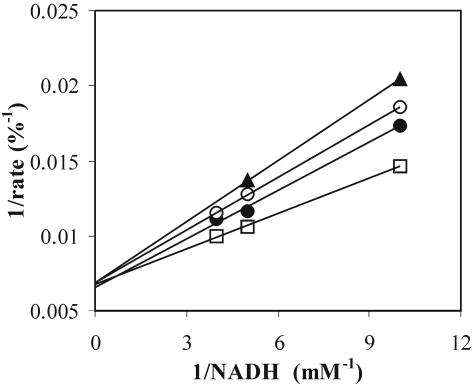
FIG. 2.
Lineweaver-Burk plot of LDH of L. lactis NCDO 2118, revealing the mechanism of competitive inhibition by NAD. NAD concentrations: □, 0 mM; •, 1.1 mM; ○, 2.1 mM; ▴, 4.3 mM.
As described previously (3, 9), FBP was seen to affect the Kms for NADH and pyruvate. Increasing the FBP concentration in the assay lowered the affinity constants of all strains tested in our study (Table 2), revealing that the allosteric mechanism is also present to some extent in the LDH of strain NCDO 2118. From the kinetics study by Crow and Pritchard (3), it can be concluded that pyruvate did not affect the Km for NADH and vice versa. The kinetics of LDH can be described by the Monod-Wyman-Changeux rate equation (10) adapted to the use of two substrates (7) and incorporating inhibition by NAD, ATP, and ADP (Table 3). Estimation of this inhibition by the kinetics according to the work of Han and Levenspiel (6) was superior to estimation by mixed-inhibition kinetics (13) (results not shown). The inhibitory effects of ATP and ADP appeared to be one of a kind and could therefore be grouped within one inhibitory term (Table 3). This conclusion was supported by the outcome of assays in which mixtures of the three inhibitors were used (data not shown). The equation could be simplified because VT,max (the maximum rate of the enzyme in the T state) was zero for most strains studied. The backward reaction of the catalytic step of LDH was not significant here, with γ/Keq (where γ is the mass-to-action ratio [lactate]/[pyruvate] and Keq is the equilibrium constant of the reaction catalyzed by LDH) being negligible when the data provided by Hoefnagel et al. (8) were used.
TABLE 2.
Values of affinity constants for pyruvate and NADH in the presence of 10 mM FBP (R state) or in the presence of 50 mM Pi (T state) as obtained in the standard assaya
| Strain | KR,Pyr (mM) | KR,NADH (mM) | VR,MAX (U/mg) | KT,PYR (mM) | KT,NADH (mM) |
|---|---|---|---|---|---|
| 65.1 | 1.4 | 0.03 | 11.5 | 18 | 0.13 |
| MG 1363 | 1.1 | 0.03 | 9.3 | 2.0 | 0.15 |
| NCIMB701197 | 1.2b | 0.04b | 9.3b | 7.7b | 0.40b |
| NCIMB700509 | 1.4c | 0.05c | 13c | 4.9c | 0.14c |
| NCIMB700763 | 0.4 | 0.10 | 10.2 | 3.4 | 0.20 |
| IL 1403 | 1.1 | 0.06 | 11.6 | 4.1 | 0.14 |
| ATCC 19435 | 2.8 | 0.03 | 11.0 | 6.6 | 0.12 |
| NCDO 2118 | 2.4 | 0.05 | 4.5 | 4.4 | 0.15 |
TABLE 3.
Variables used in VLDH = VR,max · [αPαNRn−1/(Rn+LTn)] · (1 − δ) · (1 − ɛ − η), an equation for the expression of lactococcal LDHa
| Variable | Equivalency |
|---|---|
| αP | [pyruvate]/KR,Pyr |
| cP | KR,Pyr/KT,Pyr |
| δ | [NAD]/NADcrit |
| η | [ADP]/ADPcrit |
| β | [FBP]/KFBP,0.5V |
| R | 1 + αP + αN + αPαN |
| αN | [NADH]/KR,NADH |
| cN | KR,NADH/KT,NADH |
| ɛ | [ATP]/ATPcrit |
| L | L0 · [(1 + γ)/(1 + β)]m |
| γ | [Pi]/KP,0.5V |
| T | 1 + cPαP + cNαN + cPαPcNαN |
VR,max, maximum enzyme rate in the R state; L, the equilibrium between the T and R states; KR,Pyr and KT,Pyr, affinity constants for pyruvate in the R and T states, respectively; NADcrit, ADPcrit, and ATPcrit, concentrations of NAD, ADP, and ATP, respectively, at which LDH is expected to be inhibited completely based on the equation of Han and Levenspiel (6); KFBP,0.5V, FBP concentration at which LDH was 50% activated in the absence of Pi; KR,NADH and KT,NADH, affinity constants for NADH in the R and T states, respectively; L0, equilibrium between the T state and the R state of the LDH enzyme without substrate; KP,0.5V, phosphate concentration at which LDH was 50% inhibited, as extrapolated to 0 mM FBP; m and n, number of modulator sites.
To obtain some indication of how the P and R types of LDH (of strains 65.1 and NCDO 2118, respectively) might behave under in vivo conditions, a small in silico model study was carried out. For this study, the kinetics of both types of LDH were subjected to the data for the glycolytic dynamics of resting cells of L. lactis MG1363 measured with 13C and 31P nuclear magnetic resonance as described by Neves et al. (11). These data consist of an almost complete data set for an acceleration-sustain-deceleration profile of a relatively high glycolytic flux, except for the pyruvate concentration (which was set at 1 to 3 mM during periods of high flux; see reference 5) and ADP (we considered a constant ATP-plus-ADP moiety of 8.7 mM). The mean pH of 7.2 during the maximum glycolytic flux was similar to the pH of the buffered assay mixture. The values of the parameters used in the simulation can be found in Tables 1 and 2. The lactococcal LDH enzyme is expected to consist of a tetramer (Brenda [http://www.brenda.uni-koeln.de]); hence, n = m = 4. L0, the equilibrium between the T (tense) state and the R (relaxed) state of the enzyme without substrate, was not experimentally determined. However, the simulations revealed that its value should be low (0 to 1) for the P-type LDH. This finding corresponds quite well with L0 values of glycolytic enzymes of Saccharomyces cerevisiae near pH 7 (4, 16). The activity of the R-type LDH was insensitive to L and thus to L0, which was due to a high phosphate concentration at which LDH was inhibited (KP,0.5V) and a very low KFBP,0.5V. As a consequence, for the R-type LDH, the kinetics could be further simplified by omitting the allosteric regulation. The simulations (results not shown) revealed that the P-type LDH had relatively higher activity during periods of high flux but that once the flux diminished, it was “shut down” faster than the R type. The latter finding might be part of the explanation for why strain 65.1 showed more mixed acid fermentation than strain ATCC 19435 when these strains were grown on maltose (14).
The substrate concentrations of LDH were present at near their respective Km values, meaning that the enzyme operated at far less than maximum capacity (both LDH types operated at about 10% of their maximum capacities under the given experimental conditions). Under most conditions, the FBP concentration will be far beyond its respective KFBP,0.5V value unless the flux becomes very low. In contrast to the P type, for the R type, the internal phosphate concentration never came near its respective KP,0.5V value. The concentration of NAD was about two times the inhibition constant of NAD based on kinetics of competitive inhibition (KNAD) for the R type and thus had a significant inhibiting effect on LDH activity during periods of high flux. Once detectable concentrations of NADH appeared, this type could become more active than the P type. The NAD concentration was always far below the KNAD value of the P type and thus had no effect on the regulation of this type of LDH.
The P type was sensitive to changes in the affinity constant for NADH in the R state (KR,NADH) and KP,0.5V and only relatively sensitive to changes in n, m, the concentrations of NAD, ATP, and ADP at which LDH is expected to be inhibited completely (NADcrit, ATPcrit, and ADPcrit, respectively), the affinity constant for pyruvate in the R state (KR,Pyr), and KFBP,0.5V (>0.55 mM). On the other hand, the R type was sensitive to changes in NADcrit, ATPcrit, ADPcrit, KR,Pyr, and KR,NADH.
Although both views on the regulation of lactococcal LDH (5, 9) appear to be correct, each one addresses only one type of LDH. Since the critical concentrations of ATP and ADP are quite similar, if one considers their cellular moiety to be nearly constant during growth, their inhibitory effect on LDH will have no regulatory function (likewise for GAPDH [results not shown]). However, if the size of their moiety were to undergo changes, e.g., as a function of the growth rate, then this change might very well act as a regulation mechanism. Here, we show that there are at least two types of regulation at the substrate level. These two types of regulation operate to different extents within each strain, and the overall kinetics can be described by a general equation (Table 3). Interestingly, the R-type LDH could be found in the lactoccocal strains that were isolated from plant material (strains ATCC 19435 and NCDO 2118), while the P type or a mixture of both types prevails in the typical dairy strains. We can only speculate about whether the distinction in regulation among the strains is a result of small differences in the homologies of lactococcal LDHs (15) or whether different LDH genes are expressed at different ratios (1).
Acknowledgments
The technical assistance of Therese Segerstein is greatly appreciated.
REFERENCES
- 1.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Crow, V. L., and G. G. Pritchard. 1977. Fructose 1,6-diphosphate-activated l-lactate dehydrogenase from Streptococcus lactis: kinetic properties and factors affecting activation. J. Bacteriol. 131:82-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galazzo, J. L., and J. E. Bailey. 1990. Fermentation pathway kinetics and metabolic flux control in suspended and immobilized Saccharomyces cerevisiae. Enzyme Microb. Technol. 12:162-172. [Google Scholar]
- 5.Garrigues, C., P. Loubiere, N. D. Lindley, and M. Cocaign-Bousquet. 1997. Control of the shift from homolactic acid to mixed-acid fermentation in Lactococcus lactis: predominant role in the NADH/NAD+ ratio. J. Bacteriol. 179:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han, K., and O. Levenspiel. 1988. Extended Monod kinetics for substrate, product, and cell inhibition. Biotechnol. Bioeng. 32:430-437. [DOI] [PubMed] [Google Scholar]
- 7.Hess, B., and T. Plesser. 1978. Temporal and spatial order in biochemical systems. Ann. N. Y. Acad. Sci. 316:203-213. [DOI] [PubMed] [Google Scholar]
- 8.Hoefnagel, M. H. N., M. J. C. Starrenburg, D. E. Martens, J. Hugenholtz, M. Kleerebezem, I. I. van Swam, R. Bongers, H. V. Westerhoff, and J. L. Snoep. 2002. Metabolic engineering of lactic acid bacteria, the combined approach: kinetic modeling, metabolic control and experimental analysis. Microbiology 148:1003-1013. [DOI] [PubMed] [Google Scholar]
- 9.Jonas, H. A., R. F. Anders, and G. R. Jago. 1972. Factors affecting the activity of the lactate dehydrogenase of Streptococcus cremoris. J. Bacteriol. 111:397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monod, J., J. Wyman, and J. P. Changeux. 1965. On the nature of allosteric transition: a plausible model. J. Mol. Biol. 12:88-118. [DOI] [PubMed] [Google Scholar]
- 11.Neves, A. N., R. Ventura, N. Mansour, C. Shearman, M. J. Gasson, C. Maycock, A. Ramos, and H. Santos. 2002. Is the glycolytic flux in Lactococcus lactis primarily controlled by the redox charge? J. Biol. Chem. 277:28088-28098. [DOI] [PubMed] [Google Scholar]
- 12.Poolman, B., R. M. J. Nijssen, and W. N. Konings. 1987. Dependence of Streptococcus lactis phosphate transport on internal phosphate concentration and internal pH. J. Bacteriol. 169:5373-5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roels, J. A. 1983. Energetics and kinetics in biotechnology. Elsevier Biomedical Press, Amsterdam, The Netherlands.
- 14.Sjöberg, A., I. Persson, M. Quednau, and B. Hahn-Hägerdal. 1995. The influence of limiting and non-limiting growth conditions on glucose and maltose metabolism in Lactococcus lactis ssp. lactis strains. Appl. Microbiol. Biotechnol. 42:931-938. [Google Scholar]
- 15.Swindell, S. R., H. G. Griffin, and M. J. Gasson. 1994. Cloning, sequencing and comparison of three lactococcal l-lactate dehydrogenase genes. Microbiology 140:1301-1305. [DOI] [PubMed] [Google Scholar]
- 16.Teusink, B., J. Passarge, C. A. Reijenga, E. Esgalhado, C. C. van der Weijden, M. Schepper, M. C. Walsh, B. M. Bakker, K. van Dam, H. V. Westerhoff, and J. L. Snoep. 2000. Can yeast glycolysis be understood in terms of in vivo kinetics of the constituent enzymes? Testing biochemistry. Eur. J. Biochem. 267:5313-5329. [DOI] [PubMed] [Google Scholar]
- 17.van Niel, E. W. J., K. Hofvendahl, and B. Hahn-Hägerdal. 2002. Formation and conversion of oxygen metabolites by Lactococcus lactis subsp. lactis ATCC 19435 under different growth conditions. Appl. Environ. Microbiol. 68:4350-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolin, M. J. 1964. Fructose-1,6-diphosphate requirement of streptococcal lactic dehydrogenases. Science 146:775-776. [DOI] [PubMed] [Google Scholar]
- 19.Yamada, T., and J. Carlsson. 1975. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J. Bacteriol. 124:55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]