Summary
Background
The Patient's Anastrozole Compliance to Therapy (PACT) program is a large randomized study designed to assess whether the provision of educational materials (EM) could improve compliance with aromatase inhibitor therapy in postmenopausal women with early, hormone receptor-positive breast cancer.
Patients and Methods
The PACT study presented a large, homogeneous dataset. The baseline analysis included patient demographics and initial treatments and patient perceptions about treatment and quality of life.
Results
Overall, 4,923 patients were enrolled at 109 German breast cancer centers/clinics in cooperation with 1,361 office-based gynecologists/oncologists. 4,844 women were randomized 1:1 to standard therapy (n = 2,402) or standard therapy plus EM (n = 2,442). Prior breast-conserving surgery and mastectomy had been received by 76% and 24% of the patients, respectively. Radiotherapy was scheduled for 85% of the patients, adjuvant chemotherapy for 38%. Reflecting the postmenopausal, hormone-sensitive nature of this population, only 285 patients (7%) had received neoadjuvant chemotherapy.
Conclusions
A comparison with epidemiological data from the West German Breast Center suggests that the patients in the PACT study are representative of a general postmenopausal early breast cancer population and that the findings may be applicable to ‘real-world’ Germany and beyond. Compliance data from PACT are eagerly anticipated.
Keywords: Breast cancer, Compliance, Aromatase inhibitors, Breast-conserving surgery, Mastectomy
Zusammenfassung
Hintergrund
Das Programm Patienten-Compliance bei Anastrozoltherapie (PACT) ist eine große randomisierte Studie, die evaluieren soll, ob die regelmäßige Bereitstellung von Informationsmaterialien (EM) die Compliance unter einer adjuvanten Aromataseinhibitor-Therapie bei postmenopausalen Patientinnen mit frühem, hormonrezeptor-positivem Mammakarzinom verbessern könnte.
Patientinnen und Methoden
PACT stellt ein großes, homogenes Patientinnenkollektiv dar. Die hier dargestellte Baseline-Analyse beschreibt Demographie und Erstbehandlung sowie die Patientinnen-Selbsteinschätzung in Hinsicht auf Therapie und Lebensqualität.
Ergebnisse
Insgesamt wurden 4923 Patientinnen an 109 deutschen Brustzentren und Kliniken in Kooperation mit 1361 niedergelassenen Gynäkologen und Onkologen in die Studie eingeschlossen. 4844 Patientinnen wurden 1:1 zu Standardtherapie (n = 2402) bzw. Standardtherapie mit zusätzlichen EM (n = 2442) randomisiert. 76% der Patientinnen erhielten vor Studieneinschluss eine brusterhaltende Therapie und 24% eine Mastektomie. Eine Strahlentherapie war für 85% der Patientinnen geplant und eine Chemotherapie für 38%. Wie in dieser postmenopausalen Krankenpopulation mit endokrin empfindlichen Tumoren zu erwarten war, erhielten nur 285 Patientinnen (7%) eine neoadjuvante Chemotherapie.
Schlussfolgerungen
Der Vergleich mit den epidemiologischen Daten des Westdeutschen Brustzentrums zeigt, dass die Patientengruppe der PACT-Studie repräsentativ für die allgemeine postmenopausale Patientenpopulation ist. Dies legt nahe, dass die Ergebnisse der PACT-Studie sehr gut auf die reale Behandlungssituation in Deutschland und darüber hinaus übertragbar sein werden. Die Ergebnisse zur Compliance in der PACT-Studie werden daher dringend erwartet.
Introduction
Breast cancer is the most frequently diagnosed cancer in women worldwide. In 2008, 23% of the total new cancer cases and 14% of cancer deaths were attributable to breast cancer, and approximately half of all cases are thought to occur in developed countries [1]. In Germany, about 71,660 new cases of breast cancer are diagnosed each year [2] and the overall breast cancer mortality rate is 24.6 per 100,000 women [2].
For postmenopausal women with hormone receptor-positive early breast cancer, adjuvant endocrine therapy is the recommended treatment since it has been shown to reduce the risk of recurrence and to increase overall survival [3, 4]. Third-generation aromatase inhibitors (AIs) are widely recommended in these patients for first-line endocrine treatment [5] where there is node-positive disease [6] or following 2–3 years of tamoxifen treatment [5, 7]. In the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial, first-line treatment with the non-steroidal AI anastrozole (Arimidex®, AstraZeneca, Wedel, Germany) was shown to be superior to tamoxifen treatment in terms of both efficacy and safety, at a median follow-up of 68 months [8]. These benefits have been shown to be maintained after treatment completion in an analysis at 10 years [9].
In order to achieve the same benefits of adjuvant endocrine therapy in the clinic as those observed in clinical trial settings, long-term adherence to treatment (at least 5 years) is required [10]. Robust data are not widely available concerning the compliance with adjuvant endocrine therapy in breast cancer; however, findings from a study in the USA indicated that poor compliance rates were observed with endocrine therapy versus radiotherapy or chemotherapy [11]. While limited data regarding treatment compliance with adjuvant endocrine therapy suggest that compliance may be an issue in women with breast cancer [12, 13, 14], these findings are not from prospective studies in real-life clinical settings. Furthermore, while data are available on the practice of prescribing AIs in Germany [15, 16], these do not accurately capture whether the medication was actually taken correctly.
We designed the Patient's Anastrozole Compliance to Therapy (PACT) program to prospectively evaluate the extent of compliance, and potential reasons for non-compliance, in postmenopausal women with early breast cancer receiving anastrozole as initial adjuvant therapy. To our knowledge, PACT comprises the largest homogeneous dataset evaluating compliance and quality of life in endocrine-responsive early breast cancer. In this paper, we report the PACT study design and methodology and present the patient baseline data.
Patients and Methods
Aims
The primary study objective was to assess whether provision of additional educational materials (EM) in the form of motivational letters and informational brochures could be used to enhance compliance in postmenopausal women with early breast cancer receiving anastrozole.
Patients
Patients > 18 years were eligible for inclusion in this study if they had confirmed hormone receptor-positive (estrogen receptor (ER) and/or progesterone receptor (PgR)) primary breast cancer, were postmenopausal (menses > 1 year ago), had undergone bilateral oophorectomy or had serum follicle-stimulating hormone, luteinizing hormone and plasma estradiol levels in the postmenopausal range. As this was a non-interventional study, the treating physician determined the postmenopausal status in line with the inclusion criteria. Women meeting these criteria with no menses for ≥ 1 year, either as a result of natural biological age or chemotherapy, could be included in the study if the treating physician judged that menses would not return. Patients had had to be scheduled by the investigator for adjuvant endocrine treatment with anastrozole after the usual treatment for breast cancer and had to have the ability to read and understand German. Patients were excluded if they had moderate or severe hepatic dysfunction or severe renal dysfunction (creatinine clearance < 20 ml/min), if they were receiving concomitant therapy known to affect the sex hormone status or were taking tamoxifen.
The study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of Ulm and by all appropriate local ethics committees. All patients provided written informed consent.
Study Design
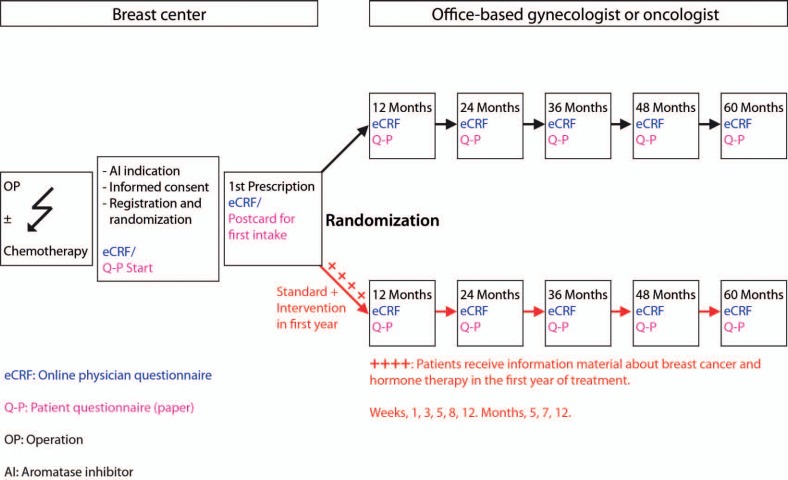
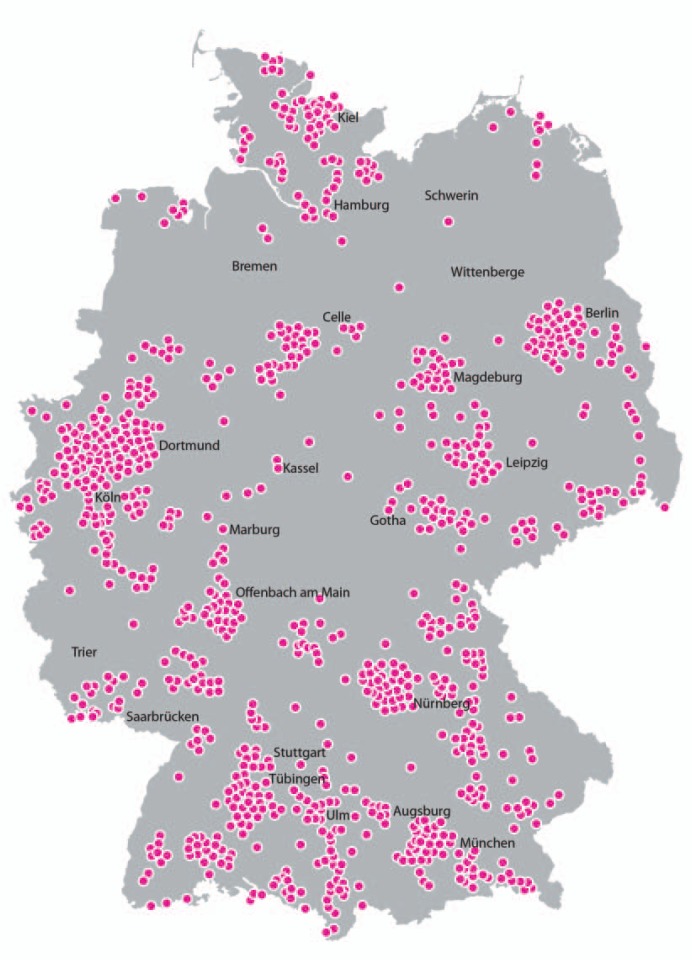
PACT is a prospective, multicenter, nationwide, randomized, open, parallel-group, non-interventional study (fig. 1). Specialized clinics (breast centers) collaborated with office-based gynecologists or oncologists for further recruitment of patients. In total, 109 breast centers/clinics and 1,361 gynecological and oncological practices in Germany were active in recruiting and treating patients in the study (fig. 2).
Fig. 1.
Study design and information about educational materials. Starter set: letter, brochures ‘Breast cancer and endocrine therapy’ and ‘Social information’, diary/notebook reminder stickers, pill box. Week 1: letter, brochure ‘Side effects of aromatase inhibitors’. Week 3: letter, brochure ‘Self-reflection: a personal experience’, lip balm. Week 5: letter, brochure ‘Early recognition and treatment’, fitness band with exercise suggestions. Week 8: letter, brochure ‘Communication with friends and family, and your caring physician’. Week 12: letter, brochure ‘Coping with fears’, CD with relaxation music. Month 5: letter, brochure ‘Breast cancer and sexuality’. Month 7: letter, brochure ‘Exercise and diet’, sports towel. Month 12: letter, optional registration for a reminder service, handbag mirror.
Fig. 2.
Distribution of PACT study centers (n = 109) throughout Germany.
Patients were randomized on a 1:1 basis to receive standard therapy (adjuvant endocrine therapy (anastrozole 1 mg once daily)) or standard therapy plus EM. The EM comprised 9 letters and brochures sent to the patient by mail during the first year of therapy, which were accompanied by gift items of low monetary value (7-day tablet box, lip care stick, fitness band including exercise tips, CD with relaxation music, towel, pocket mirror). The materials were designed with the aim of motivating the participants to take their medication on a regular basis, by discussing issues likely to be of particular relevance at specific points in time during therapy. The materials were developed with the assistance of 5 patient advocates who had all been affected by breast cancer and were active in the sphere of patient advocacy. These patrons were authors and a communications trainer, and/or were involved with national breast cancer foundations and societies.
During the study, the patients were permitted to receive any further investigations and treatments deemed necessary by their investigators according to current standards of care. As this was an in-practice evaluation study, the decision to treat with anastrozole was required to be taken, and documented, independently (tumor board or treating physician) prior to consideration of offering participation in the PACT program. The treating physician was required to follow local therapy guidelines and standard practice. Patient registration and informed consent were obtained following tumor board decision, and all patients were informed about anastrozole prior to being told about the study.
Study Endpoints
Primary endpoints were to assess and compare the differences in compliance rates and persistence rates after 12 months of adjuvant endocrine therapy between the standard and standard-plus-EM arms. Secondary endpoints were: assessment and comparison of the persistence rates and compliance rates with adjuvant endocrine therapy between the two arms after 24 months of therapy; assessment of time to treatment discontinuation and reasons for treatment discontinuation; identification of factors influencing and correlating with the persistence and compliance rate (including quality of life); disease-free survival after 12 and 24 months of adjuvant endocrine treatment and association of this clinical endpoint with compliance rates; and assessment of treatment tolerability and toxicity.
Statistical Analysis
The compliance rate was defined as the percentage of patients being ‘compliant’ to anastrozole therapy at 12 months. Patients were classed as compliant if they scored 80–100% when questioned about their daily intake of anastrozole and if all prescription information (documented by the investigator) was consistently available for the individual treatment period. The persistence rate was defined as the percentage of patients with a persistent use of anastrozole at 12 months. A patient was classified as a persistent user of anastrozole when the case report form (CRF) documentation, independent of the evaluation of compliance, supported the intake of anastrozole during the full 12-month period. All other cases (e.g. premature discontinuation from study or anastrozole therapy) resulted in a non-persistent classification.
The patients’ self-reported health-related quality of life and disease-related symptoms were assessed at baseline and after 12 and 24 months, or at treatment termination, using the European Organization for the Research and Treatment of Cancer Quality of Life C30 Core Questionnaire (EORTC QLQ-C30) and the EORTC Breast Cancer-Specific Quality of Life Questionnaire (EORTC QLQ-BR23). For the general health status and quality of life, the scores were from ‘1’ for ‘very poor’ to ‘7’ for ‘excellent’. Social, cognitive, sexual and other functioning scales ranged from ‘1’ for ‘very poor’ to ‘4’ for ‘excellent’. Scores of ‘1’ were calibrated to equate to ‘0’ (very poor) and scores of ‘7’ for health status and quality of life and ‘4’ for all other questions were equated to ‘100’ (excellent). Symptoms reported by patients were also assessed using the EORTC QLQ-C30 and -BR23 German questionnaires. The degree of various symptoms patients had experienced prior to study start was rated from 1 (absence of symptoms) to 4 (very strong symptoms). These scores were calibrated to ‘0’ for no symptoms and ‘100’ for very strong symptoms.
To determine the sample size of the study, the following assumptions were applied. Fisher's exact test (two-sided) was selected for determination of the differences in compliance rate and persistence rate between the two intervention groups. By implementing Fisher's exact test with α = 0.05 and assuming a compliance rate in patients receiving standard therapy of 50%, a sample size of 4,674 patients (2,337 patients per arm) was considered sufficient. This was based on the assumption that approximately 10% of all enrolled patients would discontinue anastrozole therapy during the first year and would therefore not be included in the intent-to-treat population.
This paper will present descriptive data from the baseline survey. The study is registered with ClinicalTrials.gov (NCT00555867).
Baseline Results
Patient and Tumor Characteristics
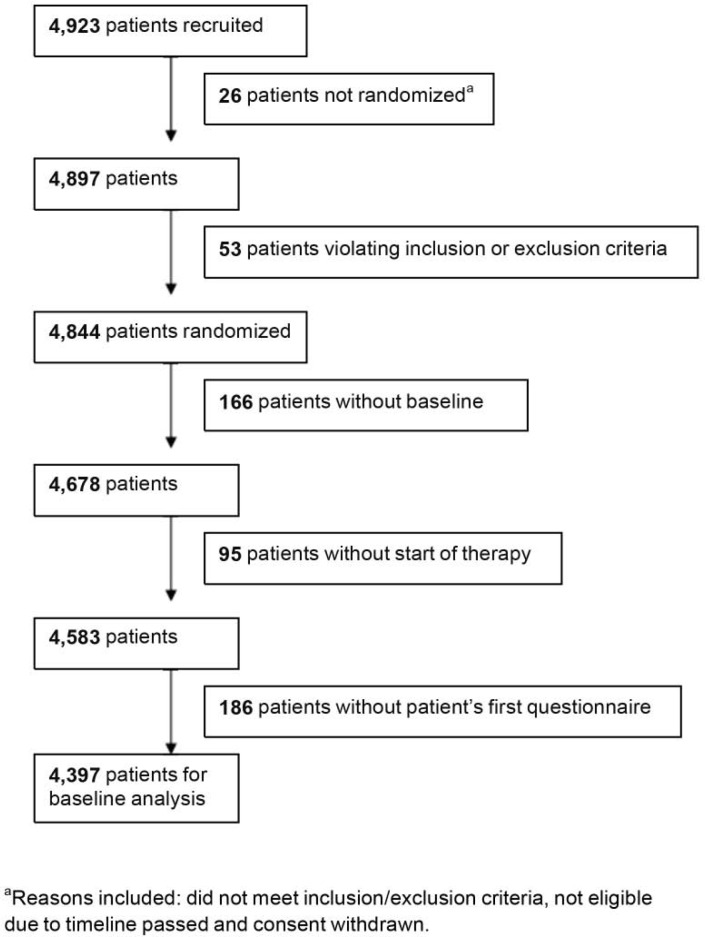
Between October 2006 and November 2008, 4,923 postmenopausal women starting adjuvant treatment with anastrozole were recruited to the PACT program at 109 breast cancer centers/clinics in cooperation with 1,361 office-based gynecologists and oncologists (fig. 2). A total of 4,844 women were then randomized 1:1 to standard therapy (n = 2,402) or standard therapy plus EM (n = 2,442). Of these, 4,397 patients were evaluable for baseline characteristics (fig. 3). The treatment groups were well balanced at baseline with regard to patient demographics, tumor characteristics, prior therapies, and attitudes to therapy.
Fig. 3.
Patient flow in PACT study.
The patient demographics are shown in table 1. The median patient age in the study was 65.5 years and the majority of the patients had a body mass index (BMI) of either < 25 (36.5%) or ≥ 25 to < 30 (37.8%). Almost all (95%) patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. The majority of the patients were native to Germany (78%) and were either retired or considered themselves as housewives (66%). Most patients reported being married and living with their partner (59%); however, 19% were widowed and 8% were divorced. The majority of the patients had children (79%), with 2 children being most frequent (35%) and 1 child being the next most frequent (25%). The highest school qualifications reported by most patients were Mittlere Reife (awarded after 10 years of schooling; 48%) and Hauptschulabschluss (awarded after 9 years of schooling; 28%).
Table 1.
Baseline demographics of the study population and the German epidemiological patient population
| Characteristic | Study population, n(%) | Epidemiological population, n (%) |
|---|---|---|
| Total number of patients | 4,397 | 89,317 |
| Age, years | ||
| Median | 65.5 | 62 |
| ≤65 | 2,099 (47.7) | 41,373 (46.3) |
| >65 | 2,298 (52.3) | 47,944 (53.7) |
| BMI, kg/m2 | ||
| <25 | 1,605 (36.5) | 12,657 (14.2) |
| ≥ 25 to < 30 | 1,662 (37.8) | 9,819 (11.0) |
| ≥30 | 1,102 (25.1) | 6,770 (7.6) |
| Not disclosed | 28 (0.6) | 60,071 (67.3) |
| ECOG PS (Karnofsky scale) | ||
| 0 (100%) | 2,466 (56.1) | 36,276 (40.6) |
| 1 (80–90%) | 1,721 (39.1) | 31,361 (35.1) |
| ≥ 2 (10–70%) | 182 (4.1) | 6,044 (6.8) |
| Not disclosed | 28 (0.6) | 15,636(17.5) |
| Native country | – | |
| Germany | 3,414 (77.6) | |
| Eastern Europe (excluding Russia) | 235 (5.3) | |
| Western Europe (excluding Germany) | 45 (1.0) | |
| Other | 703 (16.0) | |
| Employment status | – | |
| Employed (full- or part-time) | 416 (9.5) | |
| Retired/homemaker | 2,887 (65.7) | |
| Unemployed | 107 (2.4) | |
| Certified unfit for work | 557 (12.7) | |
| Other/not disclosed | 430 (9.8) | |
| Marital status | – | |
| Divorced | 368 (8.4) | |
| Married and living with partner | 2,585 (58.8) | |
| Married and not living with partner | 69 (1.6) | |
| Single | 197 (4.5) | |
| Widowed | 827 (18.8) | |
| Not disclosed | 351 (8.0) | |
| Number of children | – | |
| 0 | 915 (20.8) | |
| 1 | 1,114(25.3) | |
| 2 | 1,547 (35.2) | |
| 3 | 543 (12.4) | |
| ≥ 4 | 273 (6.2) | |
| Not disclosed | 5 (0.1) | |
| Highest school qualification | ||
| – | ||
| Mittlere Reife (awarded after 10 years of schooling) | 2,095 (47.7) | |
| Hauptschulabschluss (awarded after 9 years of schooling) | 1,209 (27.5) | |
| Abitur (awarded after 13 years of schooling) | 492 (11.2) | |
| No qualification | 75 (1.7) | |
| Other | 132 (3.0) | |
| Not disclosed | 394 (9.0) | |
| Additional number of tablets per day | ||
| – | ||
| 0 | 101 (2.3) | |
| 1–2 | 1,243 (28.3) | |
| 3–5 | 836 (19.0) | |
| 6–10 | 376 (8.6) | |
| >10 | 79 (1.8) | |
| Not disclosed | 1,762 (40.1) | |
| Concomitant conditions > 3%a | ||
| – | ||
| Cardiovascular system | 2,029 (24.7) | |
| Thyroid disorder | 904 (11.0) | |
| Other (undisclosed) | 755 (9.2) | |
| Joint pain | 533 (6.5) | |
| Back pain | 508 (6.2) | |
| Other types of angiopathy | 459 (5.6) | |
| Diabetes | 435 (5.3) | |
| Hot flushes | 380 (4.6) | |
| Gastrointestinal symptoms | 336 (4.1) | |
| Other metabolic diseases | 309 (3.8) | |
| Thromboembolism | 306 (3.7) | |
| Osteopenia/osteoporosis | 276 (3.4) |
Total number of conditions reported = 8,226. Multiple responses permitted per patient.
BMI = Body mass index, ECOG PS = Eastern Cooperative Oncology Group performance status.
At baseline, patients were asked about the number of other tablets they were taking (not including anastrozole). Data are available for 60% of all patients. Only 2% of all patients reported taking no concomitant medication. Most patients reported taking 1–2 (28% of patients) or 3–5 additional tablets per day (19% of patients), while approximately 10% of the patients reported taking more than 5 tablets per day. Concomitant conditions were widely reported within this population (n = 8,226, multiple answers permitted per patient). The most frequently reported conditions included: cardiovascular disease (25%), thyroid disorders (11%), joint pain (7%), back pain (6%), angiopathies (‘other conditions of the vascular system’ excluding thromboembolism; 6%), and diabetes (5%).
The baseline tumor characteristics are presented in table 2; not all patients received all possible tests to determine the tumor characteristics. The majority of the tumors were grade 2 (n = 3,011, 66%); 42% were diagnosed as stage IA (T1 N0; n = 1,910). Bilateral tumors were reported in 136 patients (3%), whereas histology revealed invasive ductal carcinoma in 77% of all patients (n = 3,485) and invasive lobular tumors in approximately 16%. Axillary dissection had been conducted in 1,886 patients, and 2,881 sentinel node biopsies had been performed, the majority (77.9%) of which were found to be tumor free. In total, 98% of the tested tumors were ER positive. Immunohistochemistry testing showed moderate (2+) overexpression of HER2 in 364 samples (8% of those tested) and high (3+) overexpression in 372 samples (8% of those tested). Fluorescent or chromogen in-situ hybridization (FISH/CISH) testing was performed on 873 tumors, of which 111 (12.7%) were assessed as positive. FISH/CISH tests were performed on 19.8% of the total patient population, of which 2.5% were assessed as positive.
Table 2.
Baseline tumor characteristics of the study population and the German epidemiological patient population
| Characteristic | Study population, n (%) | Epidemiological population, n (%) | |||
|---|---|---|---|---|---|
| Tumor localization (n = 4,397) | |||||
| Unilateral | 4,261 (96.9) | 83,046 (93.0) | |||
| Bilateral | 136 (3.1) | 6,271 (7.0) | |||
| Histology (n = 4,533) | |||||
| Invasive ductal carcinoma | 3,485 (76.9) | 58,078 (65.0) | |||
| Invasive lobular carcinoma | 735 (16.2) | 12,525 (14.0) | |||
| Other invasive carcinoma | 313 (6.9) | 5,167 (5.7) | |||
| Axillary staging (n = 2,435) | |||||
| Axillary dissection | 1,886 (77.5) | 40,393 (45.2) | |||
| SLN biopsy (n = 2,881) | (n = 55,791) | ||||
| SLN affected | 637 (22.1) | 11,817 (21.2) | |||
| SLN not affected | 2,244 (77.9) | 43,974 (78.8)a | |||
| Tumor grade (n = 4,533) | |||||
| 1 | 625 (13.8) | 14,653 (16.4) | |||
| 2 | 3,011 (66.4) | 56,558 (63.3) | |||
| 3 | 856 (18.9) | 16,206 (18.1) | |||
| Not determined | 41 (0.9) | 1,863 (2.1) | |||
| Tumor stage (n = 4,533)b | – | ||||
| Stage 0 | Tis N0 | 17 (0.38) | |||
| Stage IA | T1 N0 | 1,910 (42.14) | |||
| Stage IB | T0 or T1 N1 mi | 71 (1.57) | |||
| Stage IIA | T0 or T1 N1 | 407 (8.98) | |||
| T2 N0 | 845 (18.64) | ||||
| Stage IIB | T2 N1 | 490 (10.81) 46 (1.01) | |||
| Stage IIIA | T0 or T1 or T2 N2 T3 N1 or N2 | 278 (6.13) 88 (1.94) | |||
| Stage IIIB | T4 N0 or N1 or N2 | 72 (1.59) | |||
| Stage IIIC | any T N3 | 183 (4.04) | |||
| Stage unknown | Tx or Nx or T0 N0 | 126 (2.78) | |||
| ER status (n = 4,530) | |||||
| ER+ | 4,440 (98.0) | 89,317 (100.0) | |||
| PgR status (n = 4,524) | |||||
| PgR+ | 4,024 (88.8) | 77,122 (86.3) | |||
| HER2 status by IHC (n = 4,420) | |||||
| 0/1+ | 3,515 (79.5) | 69,179 (77.5) | |||
| 2+ | 364 (8.2) | 8,015 (9.0) | |||
| 3+ | 372 (8.4) | 7,365 (8.2) | |||
| Not donec | 169 (3.8) | 4,758 (5.3) | |||
| HER2 status by FISH/CISH (n = 873) | ISH positive | ISH negative | ISH positive | ISH negative | |
| 0/1+ | 0 | 453 (51.8) | – | – | |
| 2+ | 46 (5.2) | 244 (27.9) | 1,129 (1.2) | 4,618d(5.2) | |
| 3+ | 65 (7.4) | 6 (0.69) | – | – | |
| Not donec | 0 | 59 (6.76) | – | – |
SLN negative or not documented.
Tumor staging according to TNM UICC (Union for International Cancer Control) ed 7.
Patients for whom the HER2 status was determined but the investigator did not document the result.
FISH/CISH tumor status was unknown for 2,268 patients.
SLN = Sentinel lymph node, pT = pathological assessment of the primary tumor, pN = pathological assessment of regional lymph nodes, ER = estrogen receptor, PgR = progesterone receptor, HER2 = human epidermal growth factor receptor 2, IHC = immunohistochemistry, FISH = fluorescent in-situ hybridization, CISH = chromogen in-situ hybridization, ISH = in-situ hybridization.
Prior Surgery, Radiotherapy and Chemotherapy
Approximately three-quarters of the total 4,397 patients had received breast-conserving surgery (BCS) and 24% a mastectomy. Although defined as being mutually exclusive within the primary surgery classification, the physician responses showed that 57 patients received both BCS and mastectomy. BCS had been received by 79% of the patients aged ≤ 65 years and by 75% of the patients aged > 65 years. Mastectomy had been performed on 22% of the patients ≤ 65 years and on 26% of the patients > 65 years (p = 0.0006).
Radiotherapy was planned for 85% of the patients (n = 3,751). At study start, 19% of the patients had completed radiotherapy and, for 57%, treatment was in progress or had not yet started; there were missing data for 9% of the patients. Analysis of the use of radiotherapy according to the type of surgery received showed that post-operative radiotherapy was scheduled for 95% of the patients who had undergone BCS, compared with 53% of the patients who had received mastectomy. Reflecting the postmenopausal, hormone-sensitive nature of this patient population, only 285 patients (7%) had received neoadjuvant chemotherapy, the majority (79%, n = 225) of whom had received combination therapy including an anthracycline and a taxane. Adjuvant chemotherapy was known to be scheduled for 38% (n = 1,682) of the patients, and 86% (n = 1,440) of these patients had completed their planned adjuvant chemotherapy at baseline (table 3). As in the neoadjuvant setting, the most frequently scheduled adjuvant chemotherapy regimen was a combination of an anthracycline and a taxane (56.1%).
Table 3.
Prior surgery, radiotherapy and chemotherapy of the patients
| Characteristic | n (%) | |
|---|---|---|
| Primary breast surgerya | ||
| Axillary lymphnodectomy | 1,809 (20.3) | |
| BCS | 3,377 (38.0) | |
| Mastectomy | 1,062(11.9) | |
| Oncoplastic surgery | 191 (2.2) | |
| No surgery | 2 (< 0.1) | |
| Other | 65 (0.8) | |
| Sentinel lymph node biopsy | 2,393 (26.9) | |
| Neoadjuvant chemotherapy (n = 4,397) | ||
| Anthracycline-containing therapy | 42 (1.0) | |
| Anthracycline- and taxane-containing therapy | 225 (5.1) | |
| No chemotherapy scheduled | 4,112 (93.5) | |
| Other | 18 (0.4) | |
| Post-operative chemotherapy (n = 4,397) | ||
| Not scheduled | 2,715 (61.8) | |
| Scheduled | 1,682 (38.3) | |
| Completed | 1,440 (32.8) | |
| In progress/not started | 99 (2.3) | |
| Not disclosed | 143 (3.3) | |
| Scheduled post-operative chemotherapy regimens (n = 1,682) | ||
| Anthracycline-containing therapy | 610 (36.3) | |
| Anthracycline- and taxane-containing therapy | 943 (56.1) | |
| CMF | 20 (1.2) | |
| Other therapy | 109 (6.5) | |
| Post-operative radiotherapy (n = 4,397) | ||
| Not scheduled | 646 (14.7) | |
| Scheduled | 3,751 (85.3) | |
| Completed | 837 (19.0) | |
| In progress/not started | 2,519 (57.3) | |
| Not disclosed | 395 (9.0) | |
| Post-operative radiotherapy according to surgery (n = 4,333) | BCS (n = 3,301)b | Mastectomy (n = 1,032)b |
| Not scheduled | 163 (4.9) | 484 (46.9) |
| Scheduled | 3,138 (95.1) | 548 (53.1) |
Total number of procedures reported = 8,899. Multiple responses permitted per patient, 57 patients received both BCS and mastectomy.
Number of patients receiving BCS or mastectomy (not number of procedures performed). CMF = Cyclophosphamide, methotrexate and 5-fluorouracil; BCS = breast-conserving surgery.
Patient-Reported Breast Cancer Therapy and Information
The majority of the patients (82%) considered themselves to be compliant with the medication they were prescribed prior to participation in the PACT trial. Only 8% reported that they did not consistently take their medication; 10% of the patients did not respond to this question. At study start, the patients received a questionnaire concerning patient satisfaction (EORTC IN-PATSAT32 plus questions specific to the antihormonal treatment). They were asked if they had had a say in their treatment decision, and more than half (n = 2,429) reported ‘good’ or ‘satisfactory’ levels of involvement. Approximately 80% of the patients (n = 3,588) said they received ‘good’ or ‘satisfactory’ levels of information about their therapy and drug effects; approximately 75% (n = 3,321) reported being content with the level of information they had received regarding side effects (table 4).
Table 4.
Patients’ self-assessment about medication, involvement in treatment decision, understanding of their therapies and the side effects (n = 4,397)
| Level of response | Reported patient behavior concerning intake of medicine, n(%) | Level of response | How much say did you have in the selection of your breast cancer treatment?, n (%) | Did you receive a good explanation about the antihormonal therapy and the prescribed drug?, n (%) | Did you receive a good explanation about the side effects of the antihormonal therapy and the prescribed drug?, n (%) |
|---|---|---|---|---|---|
| Always | 3,592 (81.7) | good | 1,908 (43.4) | 2,971 (67.6) | 2,648 (60.2) |
| At start, but later less | 219 (5.0) | satisfactory | 521 (11.9) | 617 (14.0) | 673 (15.3) |
| Do not like it; only if I remember it | 27 (0.6) | limited | 632 (14.4) | 246 (5.6) | 342 (7.8) |
| Regularly forget | 84 (1.9) | none | 801 (18.2) | 120 (2.7) | 264 (6.0) |
| Never, although the doctor prescribed it | 9 (0.2) | ||||
| Not disclosed | 466 (10.6) | not disclosed | 535 (12.2) | 443 (10.1) | 470 (10.7) |
Patient-Reported Global Health Status and Quality of Life
Results from the EORTC QLQ-C30 and -BR23 functional scales showed that, at study start, the most positive score was for cognitive functioning (mean value 77.58), followed by physical functioning (72.89) and body image (72.15). The patients scored sexual functioning particularly negatively (18.41), with 1,981 (54.5%) reporting no interest in or participation in sexual activity at all (table 5). Results from the symptom scales showed that hair loss was the most significant symptom reported (mean value 57.98) at study start, perhaps related to prior chemotherapy treatment. This was followed by fatigue (45.41) and sleep disturbance (44.24). Nausea/vomiting (10.05) and diarrhea (11.23) were considered to be the mildest symptoms (table 6).
Table 5.
Patients’ quality of life at start of treatment assessed via the EORTC QLQ-C30 and the EORTC QLQ-BR23
| Endpoint | Mean score |
|---|---|
| Physical functioning (n = 4,029) | 72.89 |
| Role functioning (n = 3,996) | 57.70 |
| Emotional functioning (n = 4,019) | 58.93 |
| Cognitive functioning (n = 4,025) | 77.58 |
| Social functioning (n = 4,010) | 67.61 |
| Global health status/QoL (n = 3,983) | 56.70 |
| Body image (n = 3,977) | 72.15 |
| Sexual functioning (n = 3,633) | 18.41a |
| Sexual enjoyment (n = 1,010) | 49.60 |
| Future perspective (n = 3,984) | 35.58 |
1,981 patients reported no interest in or participation in sexual activity at all.
0 = ‘Very poor’ refers to a score of 1 in the EORTC QLQ-C30;
100 = ‘excellent’ refers to a score of 7 in the Global health status/QoL and to a score of 4 for all other questions.
SD = Standard deviation, QoL = quality of life, EORTC = European Organization for Research and Treatment of Cancer.
Table 6.
Symptom reporting by the patients at the start of treatment, assessed via the EORTC QLQ-C30 and the EORTC QLQ-BR23
| Symptoms | Mean score |
|---|---|
| Breast symptoms (n = 3,948) | 28.81 |
| Arm symptoms (n = 3,942) | 29.53 |
| Hair loss (n = 1,780) | 57.98 |
| Fatigue (n = 4,013) | 45.41 |
| Nausea and vomiting (n = 4,007) | 10.05 |
| Pain (n = 4,023) | 32.71 |
| Dyspnea (n = 3,962) | 28.57 |
| Sleep disturbances (n = 3,994) | 44.24 |
| Loss of appetite (n = 4,017) | 20.92a |
| Constipation (n = 3,983) | 18.08a |
| Diarrhea (n = 3,979) | 11.23a |
The majority of patients were not symptomatic in this category.
0 = ‘No symptoms’ refers to a score of 1 in this questionnaire; 100 = ‘very strong symptoms’ refers to a score of 4 for all symptoms in the EORTC QLQ-30.
EORTC = European Organization for Research and Treatment of Cancer.
Discussion
The PACT study was designed to achieve enrollment of a wide range of patients from across Germany. It is the first prospectively designed, randomized controlled study evaluating therapy compliance in patients with primary breast cancer. PACT was designed to analyze compliance with adjuvant AI therapy, and factors considered to potentially influence compliance such as concurrent medications and attitudes towards treatment were also collected. The number of patients enrolled in PACT generated the largest database compiled to date in a single country to comprise a homogeneous cohort of postmenopausal patients with ER-positive early breast cancer. The database was interrogated to analyze patient demographic and baseline disease characteristics as well as patterns of prior treatment selection and quality-of-life endpoints.
The patients in the PACT study met clear inclusion/exclusion criteria and were recruited throughout Germany, from both research units and centers that do not frequently participate in clinical studies, providing a much more heterogeneous population than if patients had been recruited from a single center or region. As they were preselected for AI therapy before study enrollment (non-interventional design) and had received or were scheduled for prior and concomitant treatments before enrollment, we believe these patients are truly representative of current treatment practices in Germany.
Patient demographics were similar between PACT and the phase III international ATAC trial with anastrozole [20] in terms of median patient age (65.5 vs. 64.3 years, respectively), mean height (164 vs. 161 cm), mean weight (73 vs. 71 kg) and mean BMI (27.2 vs. 27.7 kg/m2). Therefore, this suggests that patients in the PACT study are representative of a general early breast cancer population and that its findings may be applicable beyond Germany.
The majority of the patients in the PACT study were married, of retirement age and spent much of their time at home. These factors have been associated with good compliance levels [12, 17, 18], suggesting that the patients in PACT may have been more predisposed to compliance with taking medication than other populations.
Significantly more patients had undergone mastectomy in the more elderly age group than in the younger patient group. This finding has been reported previously [19] despite similar proportions of older (≥ 80 years) and younger (67–79 years) women reporting concerns about physical appearance: 27% and 32%, respectively (p = 0.25). Furthermore, data have shown that a greater number of elderly patients (aged ≤ 70 years) given the choice select BCS over mastectomy [20]. It is possible that clinicians are selecting mastectomy over BCS in more elderly patients to minimize the need for radiotherapy, which is less well tolerated by these patients. This option may appear preferable for patients who have concerns about the safety or efficacy of radiotherapy versus surgery [21, 22], and such practices have been reported previously in the Tamoxifen Exemestane Adjuvant Multinational (TEAM) and Intergroup Exemestane Study (IES) trials [23, 24]. In the overall PACT population, the majority of patients received BCS. However, data collected from other European studies indicate differences in surgical treatment patterns across the continent. In a Netherlands-specific sub-analysis of the TEAM study, mastectomy was the most frequently utilized surgical option for patients with early invasive breast cancer, ahead of BCS [25]. In Italy, while the Italian Association of Medical Oncology (AIOM) guidelines recommend BCS for patients with stage I–II breast cancer, patient and tumor characteristics, patient preference and technical reasons all limit adherence to this guidance [26]. It is likely that these external pressures vary across Europe, restricting the ability of physicians to strictly follow treatment recommendations.
A previous study found that patients of older age (> 60 years), those who had not attended further education and/or those without internet access were less likely to have been involved in the decision regarding endocrine treatment for breast cancer [27]. This puts them at increased risk for poor compliance and makes them particularly suitable for the PACT program. Indeed, over 70% (n = 3,107) of the patients randomized in the PACT study were > 60 years old.
The baseline data gathered in PACT provided the opportunity to understand patient perceptions about medication taking and compliance prior to participation in the trial. Approximately 80% of the patients reported that they ‘always’ took their medicine. Prospective data suggest that, at 24 months, 82% of patients are still compliant with their AI treatment [28]. In comparison, retrospective data suggest that 19–28% of patients may be non-compliant with their AI medication at 12 months (not taking at least 80%), with this figure rising to 32–50% of patients at 3 years [29]. It will be interesting to see whether the compliance results from PACT concur with the patients’ self-reported compliance, and with previously reported data on this topic. While the majority of the patients reported that their involvement in the treatment decision and information about side effects had been ‘good’ or ‘satisfactory’, 20–25% of the patients were unsatisfied. If this level of dissatisfaction is translated into compliance behavior in the study, a substantial proportion of patients may not be taking their medication correctly. The PACT study is investigating whether EM could improve patients’ understanding about their treatment and ultimately could improve compliance.
While all attempts were made to enroll patients throughout Germany, we do not know how representative our sample is compared with the entire German breast cancer population. Additionally, patients who are able to and do consent to participate in clinical trials are a self-selected population. It has been previously reported that patients who join clinical trials are well motivated and may therefore be more likely to be compliant with their medication [30]. To answer this question, we consulted the epidemiological patient population from the West German Breast Center (WBC). This center compiles data on the primary treatment of breast cancer from 220 German breast centers, which collectively represent approximately two-thirds of the nationwide breast cancer cases. This information is used for benchmarking purposes, and the data on women with postmenopausal ER-positive breast cancer give a useful reference point to assess whether the PACT population was representative of the overall German breast cancer population. While comparable data are not available for all parameters, the patient disease characteristics were similar across both populations (written communication, Harbeck and West German Breast Cancer Center 2012). Unilateral disease was most frequently observed and the majority of patients had a grade 2 tumor. Invasive ductal carcinoma was the most frequently observed histology in both groups, and the majority of patients had no sentinel lymph node involvement (77.9% vs. 78.8%). FISH/CISH analysis of patients with moderate overexpression of HER2 was more frequently used by the PACT investigators than by the collection of German breast centers (33% vs. 6.4%). Overall, this indicates that, despite the implementation of patient inclusion criteria, the PACT cohort appears to provide a fair representation of the overall German breast cancer population.
There are several limitations to this baseline analysis that can be attributed to the nature of its non-interventional design. Over 4,900 patients were enrolled across 109 breast cancer centers and clinics. As such, inconsistencies in the classification and documentation of patient treatment by physicians were expected and indeed observed. In particular, despite being mutually exclusive within the primary surgery classification, 57 patients received both BCS and mastectomy. These 57 patients may have undergone BCS and subsequent mastectomy, as multiple procedures were permitted per patient. However, this could also have been the result of differences in the interpretation of the CRF where physicians recorded partial mastectomies as both BCS and mastectomy, or due to irregularities in patient notes. A further limitation was the use of patient questionnaires to collect information on certain parameters (e.g. concomitant medication and conditions). This led to a large amount of missing data, and the results derived using this methodology may underestimate the actual patient demographics.
Following the initiation of PACT, a similar international trial (Compliance of ARomatase Inhibitors AssessmenT In Daily practice through Educational approach (CARIATIDE)) was developed, which is ongoing in Europe, Australia and South America [28]. The data from this study will provide a more international perspective on the PACT data and provide a broader database of the treatment of postmenopausal patients with early breast cancer.
In summary, PACT is the first clinical trial of its kind, designed to prospectively evaluate compliance with an AI (anastrozole) in breast cancer. This study has generated the largest homogeneous database to date of prospectively collected information on patients with early breast cancer, including treatment practices and patient attitudes, in a single country. We consider our population to be representative of patients throughout Germany and, therefore, the results of the PACT study will raise relevant concerns for physicians.
Disclosure Statement
N.H. has received honoraria and served as consultant for AstraZeneca. M.B. has served as consultant for Astellas and AstraZeneca. P.H. has served as consultant for AstraZeneca, Pfizer, and Novartis. H.-J.L. has served as consultant for Roche, Sanofi, Novartis, and Celgene. D.S. and R.K. have served as consultants for AstraZeneca. S.Z. is an employee of AstraZeneca. C.J., C.W.-K., R.H., H.S., and U.N. have no conflicts of interest to declare.
Acknowledgements
The authors would like to thank their co-investigators, the patients and their families for their participation in the PACT trial. The authors are grateful to the patient advocates who supported the PACT trial with their advice. They would also like to acknowledge AstraZeneca for funding and supporting the trial, as well as the assistance of Regina Eickhoff and Ina Santjer-Schnabel from Alcedis GmbH for data management. This study was sponsored by AstraZeneca. We thank Kerry Acheson and Lucy Hurst from iMed Comms who provided medical writing support, funded by AstraZeneca. Final approval of the manuscript rested solely with the authors.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Kaatsch P, Spix C, Katalinic A, Hentschel S, Baras N, Barnes B, Bertz J, Dahm S, Haberland J, Kraywinkel K, Laudi A, Wolf U. Berlin: Robert Koch-Institut; 2012. Krebs in Deutschland 2007/2008. Gesundheitsberichterstattung des Bundes 2012. [Google Scholar]
- 3.Carlson RW, Allred DC, Anderson BO, Burstein HJ, Edge S, Farrar WB, Forero A, Hermes Giordano S, Goldstein LJ, Gradishar W, Hayes D, Hudis CA, Isakoff SJ, Ljung BM, Marcom PK, Mayer IA, McCormick B, Pierce L. Fort Washington: National Comprehensive Cancer Network; 2012. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer (Version 3.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes – dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aebi S, Davidson T, Gruber G, Cardoso F. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22((suppl 6)):vi12–vi24. doi: 10.1093/annonc/mdr371. [DOI] [PubMed] [Google Scholar]
- 8.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, Forbes JF. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–1141. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 10.Ward JH. Duration of adjuvant endocrine therapy of breast cancer: how much is enough? Curr Opin Obstet Gynecol. 2010;22:51–55. doi: 10.1097/GCO.0b013e328334ff40. [DOI] [PubMed] [Google Scholar]
- 11.Ma AM, Barone J, Wallis AE, Wu NJ, Garcia LB, Estabrook A, Rosenbaum-Smith SM, Tartter PI. Noncompliance with adjuvant radiation, chemotherapy, or hormonal therapy in breast cancer patients. Am J Surg. 2008;196:500–504. doi: 10.1016/j.amjsurg.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Hadji P. Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol. 2010;73:156–166. doi: 10.1016/j.critrevonc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 14.Ziller V, Kalder M, Albert US, Holzhauer W, Ziller M, Wagner U, Hadji P. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20:431–436. doi: 10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 15.Engel J, Nagel G, Breuer E, Meisner C, Albert US, Strelocke K, Sauer H, Katenkamp D, Mittermayer C, Heidemann E, Schulz KD, Kunath H, Lorenz W, Holzel D. Primary breast cancer therapy in six regions of Germany. Eur J Cancer. 2002;38:578–585. doi: 10.1016/s0959-8049(01)00407-5. [DOI] [PubMed] [Google Scholar]
- 16.Luftner D, Henschke P, Pollmann D, Schildhauer S, Possinger K. Prescription pattern of aromatase inhibitors in the adjuvant setting in Germany – final results of a survey among German breast cancer specialists. Onkologie. 2005;28:639–644. doi: 10.1159/000089339. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett JA. Addressing the challenges of adherence. J Acquir Immune Defic Syndr. 2002;29((suppl 1)):S2–S10. doi: 10.1097/00126334-200202011-00002. [DOI] [PubMed] [Google Scholar]
- 18.Valldeoriola F, Coronell C, Pont C, Buongiorno MT, Camara A, Gaig C, Compta Y. Socio-demographic and clinical factors influencing the adherence to treatment in Parkinson's disease: the ADHESON study. Eur J Neurol. 2011;18:980–987. doi: 10.1111/j.1468-1331.2010.03320.x. [DOI] [PubMed] [Google Scholar]
- 19.Mandelblatt JS, Hadley J, Kerner JF, Schulman KA, Gold K, Dunmore-Griffith J, Edge S, Guadagnoli E, Lynch JJ, Meropol NJ, Weeks JC, Winn R. Patterns of breast carcinoma treatment in older women: patient preference and clinical and physical influences. Cancer. 2000;89:561–573. [PubMed] [Google Scholar]
- 20.Sandison AJ, Gold DM, Wright P, Jones PA. Breast conservation or mastectomy: treatment choice of women aged 70 years and older. Br J Surg. 1996;83:994–996. doi: 10.1002/bjs.1800830736. [DOI] [PubMed] [Google Scholar]
- 21.Nattinger AB. Variation in the choice of breast-conserving surgery or mastectomy: patient or physician decision making? J Clin Oncol. 2005;23:5429–5431. doi: 10.1200/JCO.2005.04.913. [DOI] [PubMed] [Google Scholar]
- 22.Nold RJ, Beamer RL, Helmer SD, McBoyle MF. Factors influencing a woman's choice to undergo breast-conserving surgery versus modified radical mastectomy. Am J Surg. 2000;180:413–418. doi: 10.1016/s0002-9610(00)00501-8. [DOI] [PubMed] [Google Scholar]
- 23.Jassem J, Coombes RC, Ireland B, Hall E, Snowdon CF, Bliss JM. Approaches to post-mastectomy radiotherapy (PMRT) in the inter-group exemestane study (IES) European Society for Radiotherapy and Oncology (ESTRO) 2006 Poster 680. [Google Scholar]
- 24.van de Water W. Markopoulos C. van de Velde CJ. Seynaeve C. Hasenburg A. Rea D. Putter H. Nortier JW. de Craen AJ. Hille ET. Bastiaannet E. Hadji P. Westendorp RG. Liefers GJ. Jones SE. Association between age at diagnosis and disease-specific mortality among postmenopausal women with hormone receptor-positive breast cancer. JAMA. 2012;307:590–597. doi: 10.1001/jama.2012.84. [DOI] [PubMed] [Google Scholar]
- 25.van Nes JG. Seynaeve C. Jones S. Markopoulos C. Putter H. van de Velde CJ. Hasenburg A. Rea DW. Vannetzel JM. Dirix L. Hozumi Y. Kerin MJ. Kieback DG. Meershoek-Klein Kranenbarg WM. Hille ET. Nortier JW. Variations in locoregional therapy in postmenopausal patients with early breast cancer treated in different countries. Br J Surg. 2010;97:671–679. doi: 10.1002/bjs.6962. [DOI] [PubMed] [Google Scholar]
- 26.Barni S, Venturini M, Molino A, Donadio M, Rizzoli S, Maiello E, Gori S. Importance of adherence to guidelines in breast cancer clinical practice. The Italian experience (AIOM) Tumori. 2011;97:559–563. doi: 10.1177/030089161109700503. [DOI] [PubMed] [Google Scholar]
- 27.Wengstrom Y, Aapro M, Leto di Priolo S, Cannon H, Georgiou V. Patients’ knowledge and experience of adjuvant endocrine therapy for early breast cancer: a European study. Breast. 2007;16:462–468. doi: 10.1016/j.breast.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Neven P, Markopoulos C, Tanner M, Marty M, Kreienberg R, Atkins L, Franquet A, Serin D, Gulcelik M, Deschamp V. The impact of educational materials on compliance and persistence with adjuvant aromatase inhibitors: 2 year follow-up and final results from the CARIATIDE study. Cancer Res. 2011;71((suppl 24)):5–16. –02. [Google Scholar]
- 29.Partridge AH, Avorn J, Wang PS, Winer EP. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652–661. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 30.Caro JJ, Speckman JL. Existing treatment strategies: does noncompliance make a difference? J Hypertens Suppl. 1998;16:S31–S34. [PubMed] [Google Scholar]