Abstract
The use of hormonal contraception (HC) may affect salivary cortisol levels at rest and in response to a pharmacological or stress challenge. Therefore, the current study used a secondary data analysis to investigate the effect of HC on salivary cortisol levels in response to the mu-opioid receptor antagonist naltrexone and a psychosocial stressor, and also across the diurnal curve. Two hundred and nine women (n = 72 using hormonal contraception; HC+) completed a two-session stress response study that consisted of a stress day, in which they were exposed to public speaking and mental arithmetic, and a rest day, in which unstimulated cortisol levels were measured to assess the diurnal rhythm. A subset of seventy women (n = 24 HC+) also completed a second study in which they were administered oral naltrexone (50 mg) or placebo in a randomized, placebo-controlled, double blind fashion. Women who were HC+ had a significantly reduced salivary cortisol response to both the psychosocial stressor (p < 0.001) and naltrexone (p < 0.05) compared to HC− women. Additionally, HC+ women had a significantly altered morning diurnal cortisol rhythm (p < 0.01), with a delayed peak and higher overall levels. The results of the current study confirm that HC attenuates salivary cortisol response to a psychosocial stressor and mu-opioid receptor antagonism, and also alters the morning diurnal cortisol curve.
Keywords: Hormonal Contraception, Cortisol, Stress, Diurnal Rhythm, Naltrexone, HPA axis
1. Introduction
The present study examined the effect of hormonal contraception (HC) on diurnal salivary cortisol secretion and acute cortisol responses to the mu-opioid receptor antagonist naltrexone and a psychosocial stressor. Psychosocial stressors and mu-opioid receptor antagonists reliability activate the hypothalamic-pituitary-adrenal axis (HPA) and increase circulating cortisol levels, but do so through separate mechanisms. For example, mu-opioid receptor antagonists, such as naltrexone and naloxone, are thought to disinhibit tonic endogenous opioid-mediated suppression of CRF neurons of the paraventricular nucleus of the hypothalamus (Baker and Herkenham 1995; Mendelson and Mello 2009). In contrast, psychosocial stressors, such as public speaking and mental arithmetic, activate diffuse corticolimbic circuitry that can relieve GABAergic inhibition or provide catecholaminergic stimulation of paraventricular CRF neurons (Herman and Cullinan 1997; Radley and Sawchenko 2011; Radley 2012). Paraventricular CRF neurons also receive excitatory and inhibitory signals from the suprachiasmatic nucleus in order to regulate diurnal cortisol secretion (Kalsbeek and Buijs 2002; Buijs et al., 2003; Dickmeis 2009).
Mu-opioid receptor antagonism, psychosocial stressors, and measurement of diurnal cortisol levels are commonly used as probes of HPA axis function in laboratory paradigms and each has unique clinical implications in the identification and treatment of disease (al’Absi 2006; Kiefer et al., 2006; Heim et al., 2008; Thomson and Craighead 2008). For example, blunted cortisol response to a psychosocial stressor and attenuated diurnal levels during early abstinence are predictive of relapse in smokers (al’Absi et al., 2005; al’Absi 2006), while naltrexone’s ability to increase basal cortisol levels during treatment is associated with a reduced risk of relapse in an alcohol dependent population (Kiefer et al., 2006). Furthermore, an attenuated cortisol response to a stressor may be associated with autoimmune and inflammatory diseases (Chikanza et al., 1992; Rupprecht et al., 1995, 1997; Buske-Kirschbaum et al., 1997, 2001; Lahita 1999). Thus, for both methodological and clinical reasons, it is important to characterize intrinsic and extrinsic factors that may impact salivary cortisol response to psychosocial stress and mu-opioid receptor antagonism.
Among women, one factor that may impact salivary cortisol levels is the use of HC. Women using HC have consistently demonstrated blunted salivary or free cortisol response to a psychosocial stressor (Kirschbaum et al., 1999; Rohleder et al., 2003; Bouma et al., 2009), but shown heightened serum total cortisol levels both diurnally and in response to a psychosocial stressor or ACTH administration (Meulenberg et al., 1987; Meulenberg and Hofman 1990; Kuhl et al., 1993; Aden et al., 1998; Klose et al., 2007; Kumsta et al., 2007; Simunkova et al., 2008; Winkler and Sudik 2009). However, HC’s effect on diurnal salivary cortisol levels is less clear. Studies have reported HC dampening (Pruessner et al., 1997; Pruessner et al., 1999; Bouma et al., 2009), delaying and increasing (Meulenberg and Hofman 1990), or having no effect (Wust et al., 2000) on the cortisol awakening response, as well as increasing (Meulenberg et al., 1987; Meulenberg and Hofman 1990) or decreasing (Reinberg et al., 1996) diurnal salivary cortisol levels.
It has been speculated that the primary factor underlying HC-mediated changes in cortisol levels is increases in circulating corticosteroid-binding globulin (CBG; Kirschbaum et al., 1999; Kumsta et al., 2007; Hellhammer et al., 2009; Kudielka et al., 2009). CBG is a glycoprotein that transports cortisol to target tissues and regulates its clearance rates, with ~95% of circulating cortisol being bound to CBG or serum albumin under normal conditions (Lewis et al., 2005). Hormonal contraception that contains either an estrogen or progesterone increases circulating CBG levels (Durber et al., 1976; Wiegratz et al., 2003), which subsequently increases the ratio of total to free cortisol by both increasing CBG-bound cortisol and decreasing free cortisol levels (Meulenberg et al., 1987; Meulenberg and Hofman 1990; Wiegratz et al., 1995; Wiegratz et al., 2003; Klose et al., 2007). However, estradiol and progesterone have been shown to directly alter endogenous opioid (Foradori et al., 2002; Foradori et al., 2005; Smith et al., 2006), CRF neuron (Chen et al., 2008; Lalmansingh and Uht 2008; Zhu and Zhou 2008) and HPA axis activity (Kirschbaum et al., 1996; Kudielka et al., 1998; Thammacharoen et al., 2009), all of which could feasibly contribute to differences in diurnal cortisol secretion and stress-induced salivary cortisol response.
To date, no studies have examined the effects of HC on salivary cortisol response to mu-opioid receptor antagonism. Therefore, the primary goal of the current study, which was a secondary data analysis, was to replicate prior findings that HC use impacts salivary cortisol response to a psychosocial stressor and to extend these findings by examining naltrexone responsivity. Based on the results of previous stressor studies, we hypothesized that women using HC (HC+) would demonstrate a blunted salivary cortisol response to both a psychosocial stressor and naltrexone in comparison to women not using HC (HC−). Since blood sampling was not included in the original study design, CBG levels could not be ascertained. Instead, subjective response to both stimuli and heart rate response to the stressor were examined as secondary measures to help elucidate whether HC is exerting its effects through peripheral or central mechanisms. For example, heart rate is under the control of the autonomic nervous system, which, like the HPA axis, is regulated by the hypothalamus (Gunnar and Quevedo 2007). Therefore, if HC was directly affecting hypothalamic reactivity we would expect both cortisol and heart rate response to a stressor to be altered. However, we expected that a blunted cortisol response to a stressor or naltrexone would be primarily due to HC’s effects on peripherally circulating CBG levels rather than changes in HPA axis or central opioidergic function. Thus, we hypothesized that subjective and heart rate response to the stimuli would not differ between HC+ and HC− women. Finally, given the inconsistent results of previous studies examining unstimulated, basal cortisol levels, we explored whether HC affects the diurnal cortisol rhythm.
2. Methods
2.1 Participants
Participants were women who were taking part in the Oklahoma Family Health Patterns Project (OFHP), previously described elsewhere (Lovallo et al., 2010; Lovallo et al., 2012a; Lovallo et al., 2012b). Subjects signed a consent form approved by the Institutional Review Board of the University of Oklahoma Health Sciences Center and the Veterans Affairs Medical Center, Oklahoma City, OK, USA, and received financial compensation for participating.
Two hundred and nine women (n = 72 using hormonal contraception; HC+) participated in the stressor study and seventy of those women (n = 24 HC+) also completed the naltrexone study (Table 1). Inclusion and exclusion criteria for both studies were previously described in detail (Lovallo et al. 2012a; Lovallo et al. 2012b). In brief, all participants were in good physical health, between the ages of 18 and 30 years, had BMI between 18.5 – 29 kg/m2, were not using prescription medications other than hormonal contraceptives, had daytime job or school schedules with a normal nighttime sleep pattern, and had no reported history of serious medical or psychiatric disorder. Exclusion criteria were: diagnosis of a current or past Axis I disorder [other than past depression (> 60 days prior)], history of alcohol or drug dependence, met any criteria for substance abuse within the previous 2 months, or a positive urine drug screen, pregnancy test, or breath-alcohol test on days of testing. Smoking and smokeless tobacco use were not exclusionary. Thirty subjects (14%) reported using tobacco (Table 1). Smokers were allowed a cigarette immediately prior to the start of the protocol to reduce confounds of tobacco withdrawal symptoms on study assessments; no smoking was allowed during the sessions.
Table 1.
Subject Demographic and Background Characteristics
| Stressor Study | Naltrexone Study | |||||
|---|---|---|---|---|---|---|
| HC− | HC+ | p value | HC− | HC+ | p value | |
| N | 137 | 72 | 46 | 24 | ||
| Age (yrs) | 23.3 (3.1) | 24.0 (2.8) | .14 | 23.4 (2.6) | 24.8 (2.4) | .03 |
| Education (yrs) | 15.2 (1.9) | 16.0 (1.8) | .001 | 15.5 (1.9) | 16.6 (1.5) | .02 |
| SES | 43.6 (14.1) | 47.4 (11.8) | .05 | 47.2 (12.6) | 47.5 (11.2) | .91 |
| Race: n (% White) | 112 (82) | 65 (90) | .11 | 42 (91) | 23 (96) | .65 |
| BECK | 4.9 (5.2) | 3.9 (4.0) | .16 | 3.6 (3.9) | 3.0 (3.2) | .52 |
| BMI (kg/m2) | 23.5 (3.3) | 23.1 (3.5) | .52 | 22.8 (2.7) | 22.9 (3.2) | .96 |
| AUDIT | 3.2 (2.8) | 3.6 (2.5) | .29 | 4.1 (2.7) | 3.5 (1.8) | .31 |
| Smokers: n (%) | 20 (15) | 5 (7) | .12 | 5 (11) | 0 (0) | .16 |
Note: Data are mean (SD) unless otherwise noted. SES – Hollingshead socioeconomic status; BECK – Beck Depression Inventory; BMI – Body Mass Index; AUDIT – Alcohol Use Disorders Identification Test
Hormonal contraceptive use was determined based on a health history and current medications questionnaire taken during screening and reconfirmed on days of testing. Based on this self-report, women were divided into 2 groups, those who reported current use of HC (HC+, including birth control pills, patch, hormonal IUD, or ring) and those who reported no current use (HC−).
2.2 Study design and procedure
2.2.1 Stressor Study
The procedure for the stressor study was previously described in detail (Lovallo et al. 2012a). Subjects participated in two sessions that consisted of either stress or rest protocols, in a fixed order. To maximize stress response, the first session always consisted of the stress protocol and the rest day was the second session. Prior to the start of the stress session, subjects self-reported the start date of their most recent menstrual cycle. The sessions began at either 0900 h (n = 99) or 1300 h (n = 110), and subjects were tested at the same time for both sessions. These scheduling block options were offered to facilitate enrollment and was chosen because they would not confound within-subject change score analyses of cortisol response. Subjects received a standardized snack upon arrival at the laboratory account for the effects of blood glucose levels on cortisol secretion (Dallman 2003).
The stress protocol was 105 min in total, and consisted of a 30 min baseline period, a 45 min stress test, and a 30 min recovery period. During the baseline period, the subject relaxed and read magazines. The stress test included public speaking (30 min) followed by a mental arithmetic (15 min) task. The speech task consisted of three prepared speeches on randomly generated topics, given consecutively in front of a video camera and a white-coated experimenter holding a clipboard. The mental arithmetic task consisted of three consecutive 5 min periods, in each of which the subject was given a three-digit number (e.g., 137), told to sum the three digits (11), then add aloud that total to the original number (148), and to proceed in that fashion until told to stop.
The subject provided five saliva samples during the stress protocol: at 10 and 20 min of the baseline period (Baseline 1 and Baseline 2), at 15 and 30 min of the stress test (Stress 1 and Stress 2), and at the end of the 30 min recovery period (Recovery). To assess subjective response to the stress protocol, subjects rated their moods at each saliva sample using ten-point visual-analogue scales ranging from “Least ever felt” to “Most ever felt” (Lundberg 1980), which contained Distress (sum of scores for impatience, irritability, distress, pleasantness, and control) and Activation (sum of scores for effort, tension, concentration, interest, and stimulation) subscales. Heart rate was continuously measured with an oscillometric monitor (Dinamap, V100, General Electric, Waukesha, Wisconsin) during the entire period of the protocol. The mean heart rate during the baseline period, the stress test, and the recovery period were each examined as dependent variables in analyses of stress-responsivity.
In order to allow comparisons between sessions, the rest day was identical to the stress testing day in terms of time of day (morning or afternoon), duration (105 min), and measures (saliva samples, subjective scales, and heart rate recordings) but differed in that the subject relaxed and read general interest magazines or watched nature programs on television throughout the session with no task to complete. In order to study diurnal variation in cortisol levels, four additional saliva samples were collected on the rest day (i.e., nine total samples): by the subject upon awakening at home (Wake), immediately at arrival to the lab (Pre-Baseline), minute 45 corresponding to the stress protocol (Stress 3), and by the subject at home before bedtime (Bed).
2.2.2 Naltrexone Study
The procedure for the naltrexone study was previously described in Lovallo et al. (2012b). Subjects participated in a randomized, placebo-controlled, double-blind study that consisted of 2 counterbalanced sessions separated by at least 72 hrs, in which they received placebo or naltrexone (50 mg, Malinkrodt, St. Louis, MO, USA). The naltrexone study was always performed after completion of the stress study. Subjects arrived at the General Clinical Research Center at the University of Oklahoma Health Sciences Center at 0800 h, provided a urine sample to check for the presence of pregnancy and drugs, and were served a light breakfast. At 0900 h subjects provided a baseline saliva sample and immediately consumed the naltrexone or placebo capsule. Saliva was then collected every 30 min for the next 180 min. Additionally, every 60 min, the subject rated their moods using the same visual-analogue scales as described in the stressor study and also completed a naltrexone-specific adverse effects questionnaire (King et al., 2002) assessing nausea, vomiting, headache, distress, warm or flushed feelings, anxiety, libido, hives or rash, insomnia, diarrhea, pain, sleepiness and agitation on a 3-point scale (scored from 0 – 2), “none (0),” “mild (1),” or “severe (2).” At each timepoint, the scores for the 13 variables were summed to create one adverse side effect composite score. The subject remained seated in a recliner chair through the entire protocol and read general interest magazines or watched videos of nature or history programs.
2.3 Salivary cortisol assay
Saliva samples were collected using the Salivette device (Sarstedt, Newton, NC, USA). Salivettes were centrifuged at 4200 RPM for 20 min. The saliva was transferred to cryogenic storage tubes and placed into a −20° C freezer until shipping. Salivary cortisol assays were conducted by Salimetrics (State College, PA, USA) using a competitive enzymatic immunoassay (Salimetrics, 2011) with a sensitivity of < .003 μg/dL, an intraassay coefficient of variation of < 3.6%, and an interassay coefficient of variation of < 4.0%.
2.4 Statistical Analysis
Demographic data were compared between HC groups by t-tests and chi-squares, where appropriate. Variables that significantly differed between groups were examined as covariates in the main analyses. Since prior evidence suggests smoking may impact salivary cortisol response (al’Absi et al., 2003; al’Absi et al., 2008), smoking status was also examined as a covariate in all analyses. As previously described, the stress study sessions were conducted during either morning or afternoon blocks and, therefore, time of day (morning or afternoon) was examined as a covariate to account for potential diurnal effects on the HPA axis.
Salivary cortisol and heart rate response were analyzed as a difference score, calculated as the value obtained during the stress or naltrexone sessions minus the comparable timepoint from the rest or placebo day, respectively. As we have previously shown, difference scores detect stress-induced changes in salivary cortisol levels independent of diurnal effects on stress responsivity (Lovallo et al., 2010). Both variables were analyzed using repeated measures ANOVAs with time as a within subject factor and HC group as a between subject factor. In contrast, raw data was used for the analysis of diurnal cortisol levels over the rest day and sum scores were used for the analysis of subjective response (visual analog and adverse effect scales) in both the naltrexone and stressor studies. Diurnal data was analyzed using a repeated measures ANOVA with time as a within subject factor and HC group and time of day of the session as between subject factors. Subjective data analyses included time and session/medication (rest or stress/naltrexone or placebo, depending on whether the stress or naltrexone study) as within subject factors and HC group as a between subjects factor. Significant interactions involving cortisol response were explored with planned comparison post hoc testing at the 90, 120, and 150 min timepoints in the naltrexone study and at the Stress 1, Stress 2, and Recovery timepoints in the stressor study. These particular timepoints were chosen as they were previously shown to be the peak times of salivary cortisol response (Lovallo et al. 2012a; Lovallo et al. 2012b).
3. Results
3.1 Stressor Study
The HC− and HC+ groups were similar on all demographic measures, with the exception that HC+ women had significantly more years of education (p < 0.05; Table 1). Figure 1 illustrates salivary cortisol response to the psychological stressor in HC− and HC+ women. All reported results remained significant when the time of day of the stress session was entered into the model. The stressor increased salivary cortisol levels in HC−, but not HC+ women [Group x Time, F(4,828) = 3.60, p < 0.001, Cohen’s f = 0.13; Post Hoc: HC− > HC+ at Stress 1, Stress 2, and Recovery, p’s < 0.05]. Within HC− women, salivary cortisol levels significantly increased from baseline at Stress 1, Stress 2, and Recovery timepoints (Post Hoc: Stress 1 = Stress 2 = Recovery > Baseline 2, p < 0.05). However, there was no significant change across timepoints in HC+ women (p’s > 0.45). As expected, the stressor increased heart rate [Time, F(3, 435) = 102.1, p < 0.0001, Cohen’s f = 0.84], ratings of Activation (Session x Time, F(4, 596) = 27.8, p < 0.0001, Cohen’s f = 0.43), and ratings of Distress (Session x Time, F(4, 596) = 11.0, p < 0.0001, Cohen’s f = 0.29), but none of the three variables differed between HC groups (p’s > 0.62). All significant interactions remained after controlling for menstrual cycle, smoking status, and years of education and none of these variables were significantly related to salivary cortisol response to the stressor (p > 0.30).
Figure 1. Salivary Cortisol Response to a Psychosocial Stressor.
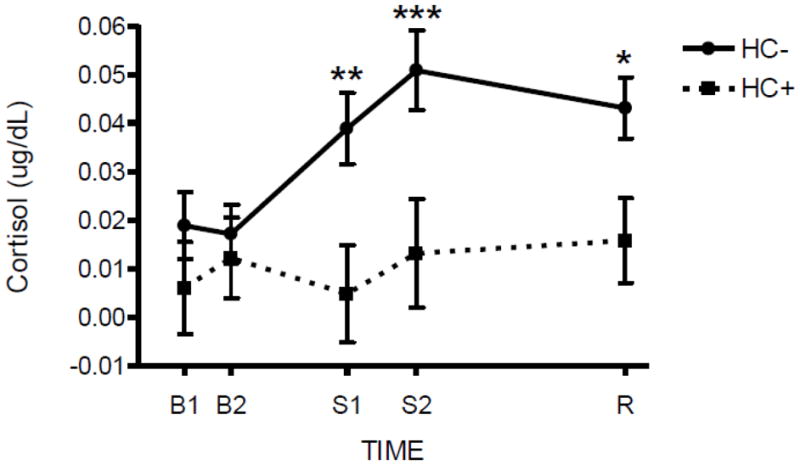
HC+ women demonstrated a significantly attenuated salivary cortisol response to a psychosocial stressor compared to HC− women at Stress 1 (S1), Stress 2 (S2), and Recovery (R) timepoints (Group x Time, p<0.001). Hormone levels are reported as mean difference scores (stress minus rest session) ± SEM. Asterisks indicate a significant difference between stress and rest sessions at that particular timepoint *p < 0.05, **p < 0.01, ***p < 0.001
Figure 2 demonstrates diurnal cortisol levels over the course of the rest day in HC− and HC+ women based upon the time of day the session was commenced (Morning: 9 AM or Afternoon: 1 PM). Among morning session participants, HC+ women had significantly reduced salivary cortisol levels upon awakening, but greater concentrations across the remainder of the rest day session compared to HC− women (Group x AM/PM x Time, F(8,1176) = 2.73, p < 0.01, Cohen’s f = 0.14; Post Hoc: Morning, HC+ < HC− at Wake, p < 0.05; HC+ > HC− at Baseline 1 through Recovery, p < 0.001). However, in subjects who participated in afternoon sessions, there were no differences between HC+ and HC− women on salivary cortisol levels at any timepoint (p’s > 0.17). As expected, there were also differences in salivary cortisol level within each HC group based on the time of day the session was completed. In HC+ women, cortisol levels in the morning session were significantly greater than those in the afternoon session from PreBaseline through Recovery (Post Hoc: Morning > Afternoon, p < 0.001). In HC− women, cortisol levels in the morning session significantly differed at PreBaseline, Baseline 1, and Stress 1 from those in the afternoon session (Post Hoc: Morning > Afternoon, p < 0.05). There were no within group differences based on time of session for Wake and Bed saliva samples (Morning = Afternoon, p’s > 0.17). Despite the differences in basal cortisol levels between HC+ women in the morning and afternoon sessions, salivary cortisol response to stress remained significantly blunted at both times of day.
Figure 2. Salivary Cortisol Diurnal Levels.
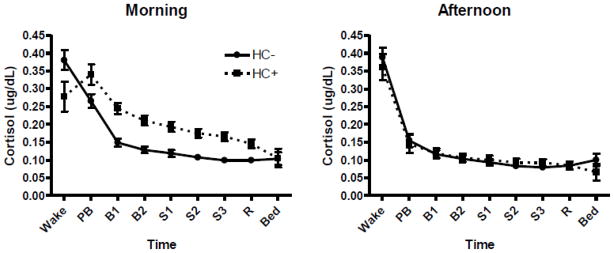
Salivary cortisol at nine time points on the rest day taken upon awakening at home (Wake), immediately at arrival to the lab (Pre-Baseline; PB), min 10 and 20 of baseline (Baseline 1 and Baseline 2; B1 and B2), min 15, 30, and 45 corresponding to the stress protocol (Stress 1, Stress 2, and Stress 3; S1, S2, S3), 30 min poststress (Recovery; R), and at home before bedtime (Bed). HC+ women had significantly lower salivary cortisol levels at Wake (p < 0.05), but significantly higher levels from Baseline 1 through Recovery (p < 0.001) than HC− women during the morning session (Group x AM/PM x Time, F(8/1176) = 2.73, p<0.01). There was no difference between HC groups in the afternoon session. Hormone levels are reported as mean ± SEM.
3.2 Naltrexone Study
Demographic comparisons in the naltrexone subsample are presented in Table 1. The data showed that HC+ women were slightly older and had more years of education than HC− women (p’s < 0.05), but the groups were otherwise similar on background characteristics. Figure 3 depicts salivary cortisol response to naltrexone in HC− and HC+ women. Naltrexone significantly increased salivary cortisol levels to a greater extent in HC− than HC+ women [Group x Time, F(6, 408) = 2.37, p < 0.05, Cohen’s f = 0.19; Post Hoc: HC− > HC+ at 90 min, p < 0.01]. Naltrexone increased salivary cortisol levels in HC− starting at 90 minutes and levels remained elevated through the final timepoint (Post Hoc: 90 min = 120 min = 150 min > 0 min, p < 0.01). However, in HC+, salivary cortisol increased to a lesser extent than in HC−, with an increase evident starting at 120 minutes after baseline (Post Hoc: 120 min = 150 min > 0 min, p < 0.05). Salivary cortisol levels did not differ between contraceptive groups during the placebo session. Naltrexone did not alter self-reports of Activation or Distress, but significantly increased the severity of reported side effects (Medication, F(1,68) = 26.1, p < 0.0001, Cohen’s f = 0.62). The HC groups did not differ in reported adverse side effects or mood status in response to naltrexone (p’s > 0.54). All reported significant interactions remained after controlling for age, years of education, and smoking status. None of these three covariates were significantly related to cortisol response to naltrexone (p’s > 0.21).
Figure 3. Salivary Cortisol Response to 50 mg Naltrexone.
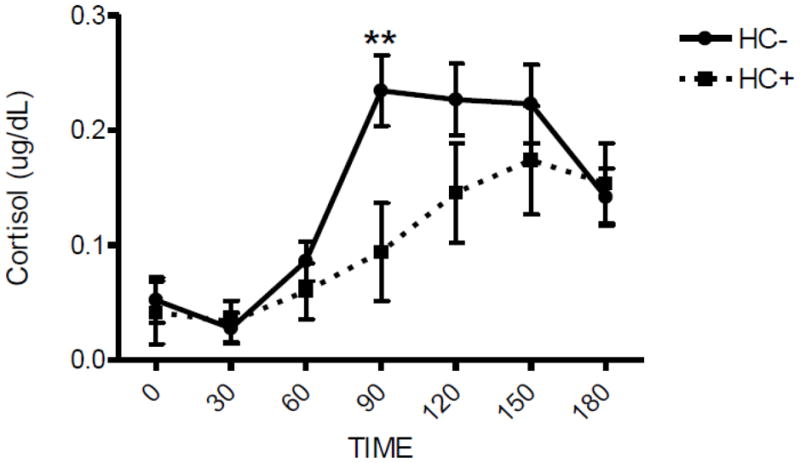
HC+ women demonstrated a significantly attenuated salivary cortisol response to naltrexone compared to HC− women 90 min after pill administration (Group x Time, p<0.05). Hormone levels are reported as mean difference scores (naltrexone minus placebo session) ± SEM. Asterisks indicate a significant difference between naltrexone and placebo sessions at that particular timepoint **p < 0.01
4. Discussion
The current study demonstrates that HC+ women have a significantly blunted salivary cortisol response to both a psychosocial stressor and 50 mg oral naltrexone compared to HC− women. In response to the psychosocial stressor, HC+ women did not display a change in salivary cortisol secretion from baseline or when compared to the rest session, while HC− women demonstrated a significant and sustained increase. HC+ women did have an increase in salivary cortisol in response to naltrexone, but their peak levels remained significantly lower and occurred later in the session than in HC− women. Additionally, in subjects who completed the morning session, HC+ women exhibited significantly reduced cortisol levels upon awakening, but greater basal cortisol levels during the rest day than HC− women. As there was no difference in cortisol secretion between HC groups in the afternoon rest session, this effect may be due to a delayed and increased morning cortisol rhythm. The results indicate that HC significantly alters salivary cortisol response to a stressor and mu-opioid receptor antagonism, as well as the morning diurnal cortisol rhythm.
Naltrexone and stress activate the HPA axis through different mechanisms, yet HC affected salivary cortisol response to both challenges. In contrast, naltrexone significantly increased opioid-specific adverse effects to a similar degree in HC− and HC+ women. This may indicate that HC is not substantially affecting central endogenous opioid activity, though it should be noted that some effects, such as nausea, may be related to opioid activity in the gastrointestinal tract. Furthermore, while the stressor significantly increased heart rate, HC− and HC+ women did not differ in this response, which may indicate that HC is not affecting hypothalamic control of the autonomic nervous system. In combination, these results suggest that HC is exerting influence on free cortisol concentrations through a peripheral mechanism rather than hypothalamic reactivity or central opioid function. It has been consistently shown that a major factor underlying HC-mediated changes in free and total cortisol levels is increases in circulating CBG (Kirschbaum et al., 1999; Kumsta et al., 2007; Hellhammer et al., 2009; Kudielka et al., 2009). Hormonal contraception-induced augmentations in CBG levels are associated with an increase in basal and stress-induced total cortisol secretion (Wiegratz et al., 1995; Dhillo et al., 2002; Klose et al., 2007), as well as a reduction in salivary cortisol in response to a stressor (Kirschbaum et al. 1999; Kumsta et al. 2007). Thus, these prior findings suggest that HC increases total cortisol secretion basally and during stress by enhancing levels of peripherally circulating CBG, which decreases the amount of available free cortisol, by proxy. This effect could explain the reduction in salivary cortisol response observed in each of the current stressor and naltrexone studies.
For example, whereas salivary cortisol response to the psychosocial stressor was completely blunted in HC+, naltrexone administration significantly increased cortisol levels. This finding may be related to naltrexone being a more potent disinhibitor of the HPA axis compared to a psychosocial stressor. In HC− women, the peak stressor-induced change from rest averaged 0.05 μg/dL, while naltrexone induced an average peak increase of 0.23 μg/dL from placebo levels. This difference in net response between the two methods is consistent with the hypothesis that elevated CBG levels in HC+ women can fully saturate low levels of free cortisol released over a short period of time (~1 hr), as observed in the stressor study. Yet, in response to the substantially larger increases in free cortisol levels observed over a relatively prolonged period of time (> 3 hrs) in the naltrexone study, available CBG may eventually become fully saturated, subsequently allowing free cortisol levels to rise in a manner observed under normal conditions. Our naltrexone study results appear to support this possibility, with HC+ showing a longer latency to exhibit measureable increases in free cortisol levels compared to HC− women (120 min in HC+ vs. 90 min in HC−), potentially due to the HC+ having to overcome increased CBG levels. However, in order to know whether CBG saturation was reached, serum CBG and total cortisol levels would need to have been measured (Hellhammer et al., 2009). We note that the foregoing argument about the role of CBG is an extrapolation from existing data. However, this set of considerations suggests the potential importance of examining CBG along with total and free cortisol in relation to HC use in future work.
While it is likely that the findings of the present study are due to HC affecting CBG levels, it is also possible that HC may also be directly and indirectly affecting the responsivity of the HPA axis itself. It has been theorized that the initial increase in CBG after HC use results in decreased fraction of unbound, free cortisol, which subsequently decreases negative feedback to hypothalamic glucocorticoid receptors, thereby increasing tonic CRF neuron activity in order to reestablish “normal” free cortisol levels (Hellhammer et al., 2009). This initial increase in CRF activity would eventually result in numerous downstream adaptations to CRF, ACTH, and glucocorticoid receptor number and sensitivity. In support of this notion, HC users have demonstrated decreased ACTH release in response to CRF administration and a stressor (Jacobs et al., 1989; Kumsta et al., 2007), increased total cortisol release in response to ACTH administration and a stressor (Henderson and Shively 2004; Klose et al., 2007; Kumsta et al., 2007; Simunkova et al., 2008; Winkler and Sudik 2009), and altered glucocorticoid sensitivity (Rohleder et al., 2003; Kuhlmann and Wolf 2005). The altered function of the HPA axis, particularly an increase in ACTH receptor sensitivity coupled with a desensitization of central glucocorticoid receptors, may be related to the delayed and heightened morning diurnal cortisol rhythm observed in HC users both in the current study and by others (Meulenberg et al., 1987; Meulenberg and Hofman 1990).
An additional possibility is that chronic changes in estrogen or progesterone levels due to HC use may have directly altered the endogenous opioid system (Foradori et al., 2002; Foradori et al., 2005; Smith et al., 2006), CRF neuron reactivity (Kirschbaum et al., 1996; Kudielka et al., 1998; Thammacharoen et al., 2009), and luteinizing hormone pulsatility (Elstein et al., 1974; Brenner et al., 1977; Mishell et al., 1977; Snowden et al., 1986), each of which are involved in the regulation of the HPA axis and stress response at the level of the hypothalamus, pituitary, and amygdala (Smith et al., 1998; Drolet et al., 2001; Bilkei-Gorzo et al., 2008). Furthermore, there is evidence that estrogens mediate the diurnal rhythm of the HPA axis (Morin et al., 1977; Burgess and Handa 1992; Norman et al., 1992), which may be due to estradiol’s regulation of CRF gene expression (Vamvakopoulos and Chrousos 1993; Roy et al., 1999; Chen et al., 2008; Lalmansingh and Uht 2008; Zhu and Zhou 2008). Therefore, HC could directly alter the dynamics and responsivity of the HPA axis at the level of the hypothalamus, which could underlie the observed changes in salivary cortisol levels at rest and in response to naltrexone and a stressor.
The present study has several limitations. Because this is a secondary data analysis, examining the impact of HC on salivary cortisol response was not the primary goal when designing the original study. Therefore, specific information regarding the type of HC being used, which could range from IUD to oral pill, was not obtained. Collecting this information might have allowed analyses of whether distinct types of HC differentially affect HPA axis responsivity. However, HC containing estrogen or progesterone both appear to significantly increase CBG levels, so the main analyses and data interpretations may not have been affected by the type of HC (Durber et al., 1976; Wiegratz et al., 2003). Additionally, as previously noted, serum total cortisol and CBG levels were not measured in this study. Future studies employing these measures will help discern the causal effect of HC on free cortisol levels and overall CBG/cortisol saturation rates. Finally, as noted by others, caution must be used when interpreting self-collected salivary samples due to the possibility of poor time-related compliance (Kudielka et al., 2003; Kudielka and Kirschbaum 2003). With regard to the samples that were instructed to be collected upon awakening, differences between HC groups were observed only in those participating in the early morning session. It is plausible that this result could have been due of poorer compliance with those subjects who did not have to start their session until later in the day and, therefore, may have been less motivated to immediately collect a saliva sample upon awakening.
The main strength of the study is the large sample size, which increases our confidence to conclude that HC significantly blunts salivary cortisol response to a psychosocial stressor and naltrexone while increasing morning secretion during rest. These findings confirm that contraceptive use should be routinely used as exclusionary criteria in human studies of HPA axis responsivity to stress and opioid receptor antagonism, and should be either excluded or minimally accounted for in studies assessing the diurnal cortisol rhythm. While it is becoming more common for studies of HPA axis function to include only non-HC using women, several recent studies have included both HC+ and HC− women without including contraceptive use as a variable in their analysis. Future research is warranted to elucidate the mechanisms underlying the effect of HC on salivary cortisol levels and to determine whether different types of HC have varying effects on the HPA axis. Finally, a decrease in cortisol response to a stressor has been associated with the presence of autoimmune and inflammatory diseases (Chikanza et al., 1992; Rupprecht et al., 1995, 1997; Buske-Kirschbaum et al., 1997, 2001; Lahita 1999). While glucocorticoid receptor sensitivity may increase in HC users to accommodate decreased free cortisol levels (Rohleder et al., 2003), more research is needed to confirm whether HC use is associated with altered glucocorticoid receptor function and the prevalence of disorders related to attenuated stress responsivity.
Highlights.
The effects of hormonal contraception (HC) on response to naltrexone and a stressor were examined
Women using HC had a blunted cortisol response to naltrexone and the stressor compared to non-users
HC did not affect subjective and cardiovascular to either stimuli
HC users also had a delayed and heightened diurnal cortisol rhythm compared to non-users
Acknowledgments
Role of the funding source
This work was supported by the Department of Veterans Affairs, the National Institutes of Health, NIAAA (R01 AA12207), NIRR (M01 RR14467, UL1 RR025767, KL2 RR025766, and RR025766), and NHLBI (F32 HL083689). The funding sources had no role in the design of the study or in the analysis and interpretation of the data. The content is solely the view of the authors and does not necessarily represent the official view of the National Institutes of Health or the VA.
Footnotes
Contributors
WRL designed the study and details of the protocol. DJOR analyzed the data. All authors contributed to writing the article and approve of its content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aden U, Jung-Hoffmann C, Kuhl H. A randomized cross-over study on various hormonal parameters of two triphasic oral contraceptives. Contraception. 1998;58:75–81. doi: 10.1016/s0010-7824(98)00071-7. [DOI] [PubMed] [Google Scholar]
- al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59:218–227. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74:401–410. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Hatsukami D, Westra R. Blunted opiate modulation of hypothalamic-pituitary-adrenocortical activity in men and women who smoke. Psychosom Med. 2008;70:928–935. doi: 10.1097/PSY.0b013e31818434ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RA, Herkenham M. Arcuate nucleus neurons that project to the hypothalamic paraventricular nucleus: neuropeptidergic identity and consequences of adrenalectomy on mRNA levels in the rat. The Journal of comparative neurology. 1995;358:518–530. doi: 10.1002/cne.903580405. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Michel K, Mauer D, Zimmer A, Klingmuller D. Control of hormonal stress reactivity by the endogenous opioid system. Psychoneuroendocrinology. 2008;33:425–436. doi: 10.1016/j.psyneuen.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Bouma EM, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. Adolescents’ cortisol responses to awakening and social stress; effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology. 2009;34:884–893. doi: 10.1016/j.psyneuen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Brenner PF, Mishell DR, Jr, Stanczyk FZ, Goebelsmann U. Serum levels of d-norgestrel, luteinizing hormone, follicle-stimulating hormone, estradiol, and progesterone in women during and following ingestion of combination oral contraceptives containing dl-norgestrel. Am J Obstet Gynecol. 1977;129:133–140. doi: 10.1016/0002-9378(77)90733-5. [DOI] [PubMed] [Google Scholar]
- Buijs RM, laFleur SE, Wortel J, vanHeyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. The Journal of comparative neurology. 2003;464:36–48. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- Burgess L, Handa R. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Geiben A, Hellhammer D. Psychobiological aspects of atopic dermatitis: an overview. Psychother Psychosom. 2001;70:6–16. doi: 10.1159/000056219. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Chen XN, Zhu H, Meng QY, Zhou JN. Estrogen receptor-α and-β regulate the human corticotropin-releasing hormone gene through similar pathways. Brain research. 2008;1223:1–10. doi: 10.1016/j.brainres.2008.05.043. [DOI] [PubMed] [Google Scholar]
- Chikanza IC, Petrou P, Kingsley G, Chrousos G, Panayi GS. Defective hypothalamic response to immune and inflammatory stimuli in patients with rheumatoid arthritis. Arthritis Rheum. 1992;35:1281–1288. doi: 10.1002/art.1780351107. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Fast glucocorticoid feedback favors ‘the munchies’. Trends Endocrinol Metab. 2003;14:394–396. doi: 10.1016/j.tem.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Kong WM, Le Roux CW, Alaghband-Zadeh J, Jones J, Carter G, Mendoza N, Meeran K, O’Shea D. Cortisol-binding globulin is important in the interpretation of dynamic tests of the hypothalamic--pituitary--adrenal axis. Eur J Endocrinol. 2002;146:231–235. doi: 10.1530/eje.0.1460231. [DOI] [PubMed] [Google Scholar]
- Dickmeis T. Glucocorticoids and the circadian clock. Journal of Endocrinology. 2009;200:3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Durber SM, Lawson J, Daly JR. The effect of oral contraceptives on plasma cortisol and cortisol binding capacity throughout the menstrual cycle in normal women. Br J Obstet Gynaecol. 1976;83:814–818. doi: 10.1111/j.1471-0528.1976.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Elstein M, Briston PG, Jenkins M, Kirk D, Miller H. Effects of a low-oestrogen oral contraceptive on urinary excretion of luteinizing hormone and ovarian steroids. Br Med J. 1974;1:11–13. doi: 10.1136/bmj.1.5896.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143:4366–4374. doi: 10.1210/en.2002-220586. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic Acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146:1835–1842. doi: 10.1210/en.2004-1326. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–73. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Wust S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Henderson JA, Shively CA. Triphasic oral contraceptive treatment alters the behavior and neurobiology of female cynomolgus monkeys. Psychoneuroendocrinology. 2004;29:21–34. doi: 10.1016/s0306-4530(02)00132-4. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Jacobs AJ, Odom MJ, Word RA, Carr BR. Effect of oral contraceptives on adrenocorticotropin and growth hormone secretion following CRH and GHRH administration. Contraception. 1989;40:691–699. doi: 10.1016/0010-7824(89)90072-3. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell and tissue research. 2002;309:109–118. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Otte C, Naber D, Wiedemann K. Hypothalamic-pituitary-adrenocortical axis activity: a target of pharmacological anticraving treatment? Biol Psychiatry. 2006;60:74–76. doi: 10.1016/j.biopsych.2005.11.023. [DOI] [PubMed] [Google Scholar]
- King AC, Schluger J, Gunduz M, Borg L, Perret G, Ho A, Kreek MJ. Hypothalamic-pituitary-adrenocortical (HPA) axis response and biotransformation of oral naltrexone: preliminary examination of relationship to family history of alcoholism. Neuropsychopharmacology. 2002;26:778–788. doi: 10.1016/S0893-133X(01)00416-X. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Schommer N, Federenko I, Gaab J, Neumann O, Oellers M, Rohleder N, Untiedt A, Hanker J, Pirke KM, Hellhammer DH. Short-term estradiol treatment enhances pituitary-adrenal axis and sympathetic responses to psychosocial stress in healthy young men. J Clin Endocrinol Metab. 1996;81:3639–3643. doi: 10.1210/jcem.81.10.8855815. [DOI] [PubMed] [Google Scholar]
- Klose M, Lange M, Rasmussen AK, Skakkebaek NE, Hilsted L, Haug E, Andersen M, Feldt-Rasmussen U. Factors influencing the adrenocorticotropin test: role of contemporary cortisol assays, body composition, and oral contraceptive agents. J Clin Endocrinol Metab. 2007;92:1326–1333. doi: 10.1210/jc.2006-1791. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med. 2003;65:313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer J, Hellhammer DH, Wolf OT, Pirke KM, Varadi E, Pilz J, Kirschbaum C. Sex differences in endocrine and psychological responses to psychosocial stress in healthy elderly subjects and the impact of a 2-week dehydroepiandrosterone treatment. J Clin Endocrinol Metab. 1998;83:1756–1761. doi: 10.1210/jcem.83.5.4758. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Kuhl H, Jung-Hoffmann C, Weber J, Boehm BO. The effect of a biphasic desogestrel-containing oral contraceptive on carbohydrate metabolism and various hormonal parameters. Contraception. 1993;47:55–68. doi: 10.1016/0010-7824(93)90109-k. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Wolf OT. Cortisol and memory retrieval in women: influence of menstrual cycle and oral contraceptives. Psychopharmacology (Berl) 2005;183:65–71. doi: 10.1007/s00213-005-0143-z. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Entringer S, Hellhammer DH, Wust S. Cortisol and ACTH responses to psychosocial stress are modulated by corticosteroid binding globulin levels. Psychoneuroendocrinology. 2007;32:1153–1157. doi: 10.1016/j.psyneuen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352–356. doi: 10.1097/00002281-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Lalmansingh AS, Uht RM. Estradiol regulates corticotropin-releasing hormone gene (crh) expression in a rapid and phasic manner that parallels estrogen receptor-α and-β recruitment to a 3′, 5′-cyclic adenosine 5′-monophosphate regulatory region of the proximal crh promoter. Endocrinology. 2008;149:346–357. doi: 10.1210/en.2007-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JG, Bagley CJ, Elder PA, Bachmann AW, Torpy DJ. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin Chim Acta. 2005;359:189–194. doi: 10.1016/j.cccn.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma Family Health Patterns Project. Biol Psychiatry. 2012a;71:344–349. doi: 10.1016/j.biopsych.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Vincent AS. Use of a resting control day in measuring the cortisol response to mental stress: diurnal patterns, time of day, and gender effects. Psychoneuroendocrinology. 2010;35:1253–1258. doi: 10.1016/j.psyneuen.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, King AC, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Naltrexone effects on cortisol secretion in women and men in relation to a family history of alcoholism: Studies from the Oklahoma Family Health Patterns Project. Psychoneuroendocrinology. 2012b doi: 10.1016/j.psyneuen.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg PK. Assessment of drugs’ side effects: Visual Analogue Scale versus check-list format. Percept Mot Skills. 1980;50:1067–1073. doi: 10.2466/pms.1980.50.3c.1067. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Endocrine effects of opioid antagonists. Opiate Receptors and Antagonists. 2009:581–604. [Google Scholar]
- Meulenberg PM, Hofman JA. The effect of oral contraceptive use and pregnancy on the daily rhythm of cortisol and cortisone. Clin Chim Acta. 1990;190:211–221. doi: 10.1016/0009-8981(90)90175-r. [DOI] [PubMed] [Google Scholar]
- Meulenberg PM, Ross HA, Swinkels LM, Benraad TJ. The effect of oral contraceptives on plasma-free and salivary cortisol and cortisone. Clin Chim Acta. 1987;165:379–385. doi: 10.1016/0009-8981(87)90183-5. [DOI] [PubMed] [Google Scholar]
- Mishell DR, Jr, Kletzky OA, Brenner PF, Roy S, Nicoloff J. The effect of contraceptive steroids on hypothalamic-pituitary function. Am J Obstet Gynecol. 1977;128:60–74. doi: 10.1016/0002-9378(77)90295-2. [DOI] [PubMed] [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science (New York, NY) 1977;196:305. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- Norman RL, Smith CJ, Pappas JD, Hall J. Exposure to ovarian steroids elicits a female pattern of plasma cortisol levels in castrated male macaques. Steroids. 1992;57:37–43. doi: 10.1016/0039-128x(92)90094-p. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, vonAuer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Radley JJ. Toward a limbic cortical inhibitory network: implications for hypothalamic-pituitary-adrenal responses following chronic stress. Front Behav Neurosci. 2012;6:7. doi: 10.3389/fnbeh.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci. 2011;31:9683–9695. doi: 10.1523/JNEUROSCI.6040-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinberg AE, Touitou Y, Soudant E, Bernard D, Bazin R, Mechkouri M. Oral contraceptives alter circadian rhythm parameters of cortisol, melatonin, blood pressure, heart rate, skin blood flow, transepidermal water loss, and skin amino acids of healthy young women. Chronobiol Int. 1996;13:199–211. doi: 10.3109/07420529609012653. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Wolf JM, Piel M, Kirschbaum C. Impact of oral contraceptive use on glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Psychoneuroendocrinology. 2003;28:261–273. doi: 10.1016/s0306-4530(02)00019-7. [DOI] [PubMed] [Google Scholar]
- Roy BN, Reid RL, Van Vugt DA. The Effects of Estrogen and Progesterone on Corticotropin-Releasing Hormone and Arginine Vasopressin Messenger Ribonucleic Acid Levels in the Paraventricular Nucleus and Supraoptic Nucleus of the Rhesus Monkey. Endocrinology. 1999;140:2191–2198. doi: 10.1210/endo.140.5.6684. [DOI] [PubMed] [Google Scholar]
- Rupprecht M, Hornstein OP, Schluter D, Schafers HJ, Koch HU, Beck G, Rupprecht R. Cortisol, corticotropin, and beta-endorphin responses to corticotropin-releasing hormone in patients with atopic eczema. Psychoneuroendocrinology. 1995;20:543–551. doi: 10.1016/0306-4530(94)00082-l. [DOI] [PubMed] [Google Scholar]
- Rupprecht M, Salzer B, Raum B, Hornstein OP, Koch HU, Riederer P, Sofic E, Rupprecht R. Physical stress-induced secretion of adrenal and pituitary hormones in patients with atopic eczema compared with normal controls. Exp Clin Endocrinol Diabetes. 1997;105:39–45. doi: 10.1055/s-0029-1211725. [DOI] [PubMed] [Google Scholar]
- Simunkova K, Starka L, Hill M, Kriz L, Hampl R, Vondra K. Comparison of total and salivary cortisol in a low-dose ACTH (Synacthen) test: influence of three-month oral contraceptives administration to healthy women. Physiol Res. 2008;57(Suppl 1):S193–199. doi: 10.33549/physiolres.931505. [DOI] [PubMed] [Google Scholar]
- Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci. 2006;26:5777–5785. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YR, Zubieta JK, del Carmen MG, Dannals RF, Ravert HT, Zacur HA, Frost JJ. Brain opioid receptor measurements by positron emission tomography in normal cycling women: relationship to luteinizing hormone pulsatility and gonadal steroid hormones. J Clin Endocrinol Metab. 1998;83:4498–4505. doi: 10.1210/jcem.83.12.5351. [DOI] [PubMed] [Google Scholar]
- Snowden EU, Khan-Dawood FS, Dawood MY. Opioid regulation of pituitary gonadotropins and prolactin in women using oral contraceptives. Am J Obstet Gynecol. 1986;154:440–444. doi: 10.1016/0002-9378(86)90687-3. [DOI] [PubMed] [Google Scholar]
- Thammacharoen S, Geary N, Lutz TA, Ogawa S, Asarian L. Divergent effects of estradiol and the estrogen receptor-α agonist PPT on eating and activation of PVN CRH neurons in ovariectomized rats and mice. Brain research. 2009;1268:88–96. doi: 10.1016/j.brainres.2009.02.067. [DOI] [PubMed] [Google Scholar]
- Thomson F, Craighead M. Innovative approaches for the treatment of depression: targeting the HPA axis. Neurochem Res. 2008;33:691–707. doi: 10.1007/s11064-007-9518-3. [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. The Journal of Clinical Investigation. 1993;92:1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegratz I, Jung-Hoffmann C, Kuhl H. Effect of two oral contraceptives containing ethinylestradiol and gestodene or norgestimate upon androgen parameters and serum binding proteins. Contraception. 1995;51:341–346. doi: 10.1016/0010-7824(95)00098-u. [DOI] [PubMed] [Google Scholar]
- Wiegratz I, Kutschera E, Lee JH, Moore C, Mellinger U, Winkler UH, Kuhl H. Effect of four different oral contraceptives on various sex hormones and serum-binding globulins. Contraception. 2003;67:25–32. doi: 10.1016/s0010-7824(02)00436-5. [DOI] [PubMed] [Google Scholar]
- Winkler UH, Sudik R. The effects of two monophasic oral contraceptives containing 30 mcg of ethinyl estradiol and either 2 mg of chlormadinone acetate or 0.15 mg of desogestrel on lipid, hormone and metabolic parameters. Contraception. 2009;79:15–23. doi: 10.1016/j.contraception.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response - normal values and confounds. Noise Health. 2000;2:79–88. [PubMed] [Google Scholar]
- Zhu H, Zhou JN. SUMO1 enhances 17-β estradiol’s effect on CRH promoter activation through estrogen receptors. Neuroendocrinology Letters. 2008:29. [PubMed] [Google Scholar]


