Abstract
Background
Hypothalamic-pituitary-adrenal (HPA) axis abnormalities have been reported in bipolar disorder and also in suicidal behavior, but few studies have examined the relationship between suicidal behaviors and the HPA axis function in bipolar disorder, attending to and minimizing confounding factors. We compare HPA axis activity in bipolar individuals with and without suicidal behavior and unaffected healthy controls through measurement of salivary cortisol.
Method
Salivary cortisol was collected for three consecutive days in 29 controls, 80 bipolar individuals without a history of suicide and 56 bipolar individuals with a past history of suicide. Clinical factors that affect salivary cortisol were also examined.
Results
A past history of suicide was associated with a 7.4% higher bedtime salivary cortisol level in bipolar individuals. There was no statistical difference between non-suicidal bipolar individuals and controls in bedtime salivary cortisol and awakening salivary cortisol was not different between the three groups.
Limitations
The measure of salivary cortisol was a home based collection by the study subjects and the retrospective clinical data was primarily based on their historical account.
Conclusions
Bipolar individuals with a past history of suicidal behavior exhibit hyperactivity in the HPA axis. This biological marker remains significant regardless of demographic factors, mood state, severity and course of illness. This finding in bipolar disorder is consistent with the evidence for altered HPA axis functioning in suicide and mood disorders and is associated with a clinical subgroup of bipolar patients at elevated risk for suicide based on their history, and in need of further attention and study.
Keywords: Bipolar, Suicide, Cortisol, HPA axis, Biomarker
1. Introduction
Altered functioning of the HPA axis has been reported in suicidal behavior and in bipolar disorder (Daban et al., 2005; Mann, 2003). However, many studies of HPA axis function in bipolar disorder have not examined the potential effects of suicidal behavior (Cassidy et al., 1998; Cervantes et al., 2001; Cookson et al., 1985; Godwin, 1984; Linkowski et al., 1994; Rybakowski and Twardowska, 1999; Schmider et al., 1995) and studies of the association between HPA axis activity and suicidal behavior in varied diagnostic groups have had mixed results (Black et al., 2002; Coryell and Schlesser, 2001; Dahl et al., 1991; Duval et al., 2001; Jokinen and Nordström, 2008, 2009; Lindqvist et al., 2008; Pfennig et al., 2005; Pitchot et al., 2008; Tripodianakis et al., 2000; Yerevanian et al., 2004).
The HPA axis has been examined using a number of methods. Basal cortisol secretion has been measured with 24 hour urinary cortisol secretion and serum or salivary cortisol levels. The feedback and suppression mechanisms of the HPA axis have been investigated with the dexamethasone suppression test (DST) (Carroll et al., 1981) or the dexamethasone/corticotropin-releasing hormone (DEX/CRH) challenge test (Heuser et al., 1994).
HPA axis hyperactivity has been demonstrated in bipolar disorder during depression and less consistently during manic and mixed episodes. The findings include elevated basal plasma cortisol levels during depression and mania (Cervantes et al., 2001; Cookson et al., 1985; Linkowski et al., 1994) and non-suppression of cortisol secretion in the DST or DEX/CRH tests during depressed (Rybakowski and Twardowska, 1999) and manic or mixed (Cassidy et al., 1998; Godwin, 1984; Schmider et al., 1995) phases of illness. HPA axis hyperactivity has been considered a marker of mood state and an indicator of response to treatment and outcome, with research in depression showing worse outcome and more recurrence in individuals that do not show normalization of HPA axis hyperactivity after treatment (Ribeiro et al., 1993). During euthymia, most measurements of baseline salivary cortisol in bipolar disorder have not shown any significant difference compared to unaffected controls (Deshauer et al., 2006; Havermans et al., 2011; Thompson et al., 2005; Watson et al., 2004). One small pilot study found an enhanced salivary cortisol response to awakening in euthymic bipolar disorder (Deshauer et al., 2003).
However, some remitted bipolar individuals still show DST non-suppression when euthymic (Deshauer et al., 1999; Hwu and Lin, 1990) and other methods of examining the HPA axis such as the DEX/CRH challenge test have also demonstrated evidence of hyperactivity even after remission of mood states (Rybakowski and Twardowska, 1999; Schmider et al., 1995; Watson et al., 2004). There is also evidence of HPA axis hyper-activity in unaffected relatives of bipolar probands (Ellenbogen et al., 2004; Holsboer et al., 1995), further strengthening the argument that HPA axis hyperactivity is not solely related to the acute mood state and may be a trait marker.
HPA axis hyperactivity, as shown by DST non-suppression has been associated with future completed suicide (Coryell and Schlesser, 2001; Jokinen and Nordström, 2008) and to a lesser extent, future suicide attempts (Jokinen and Nordström, 2009). Elevated cortisol levels are found in individuals with depression or adjustment disorder and a history of suicide attempts, compared to healthy controls (Dahl et al., 1991; Tripodianakis et al., 2000). Association between DST non-suppression and future suicide attempts and completions was reported in a meta-analysis of eleven studies (Lester, 1992) and also in several subsequent studies (Coryell and Schlesser, 2001; Jokinen and Nordström, 2008, 2009; Jokinen et al., 2009; Yerevanian et al., 2004). Though the meta-analysis by Lester (1992) did not find consistent association with prior attempts and DST non-suppression, Jokinen et al. (Jokinen and Nordström, 2009) recently reported an association between past suicide attempts and DST non-suppression. The method of attempt may play a role; higher 24 hour urinary cortisol has been detected in violent suicide attempters compared to non-suicidal psychiatric controls (van Heeringen et al., 2000), and Roy et al. (Roy, 1992) found significantly increased DST non-suppression rates in patients who had made a violent suicide attempt at baseline compared to those who had made a non-violent attempt.
In contrast, several studies have had findings indicating no difference in rates of DST non-suppression based on a history of suicide attempts, prospectively observed attempts or completed suicides (Black et al., 2002; Duval et al., 2001; Pitchot et al., 2008). Other studies have even demonstrated evidence of lower HPA axis activity in individuals with suicide attempts using the DEX/CRH challenge test (Pfennig et al., 2005) or by measuring basal salivary cortisol (Lindqvist et al., 2008). Pfennig et al. examined the HPA axis activity in a large sample of 310 individuals with unipolar, bipolar and other depressive disorders with the DEX/CRH challenge test. Both suicidal and non-suicidal depressed individuals had higher post dexamethasone cortisol levels but the suicidal group had a trend towards lower cortisol compared to the non-suicidal depressed group. All participants had significant mood symptoms at time of testing (mean HAM-D = 26) (Pfennig et al., 2005). Inconsistencies in results may be due to the effects of factors such as demographic differences, mood state, diagnosis, illness severity and diagnostic comorbidities.
The present study examines HPA axis activity as a trait marker for bipolar disorder and suicide by measuring salivary cortisol in a bipolar cohort with a history of suicide and compares it with non-suicidal bipolar individuals and unaffected controls. To address the discrepancies discussed above, we examined the role of potential confounding factors on salivary cortisol including age and sex (Seeman et al., 2001), menopausal status, BMI (Kumari et al., 2010), smoking status (Badrick et al., 2007), childhood sexual abuse (Heim et al., 2010; Nicolson et al., 2010; Stein et al., 1997), medications, mood state at time of sampling, substance use disorders, chronicity of illness, history of rapid cycling, history of mixed states, years of illness, age of onset, co-morbid anxiety disorders and history of psychosis. Our hypothesis was that those with bipolar disorder would have elevated basal salivary cortisol compared to unaffected controls, and that the suicidal bipolar individuals (defined by a lifetime history of attempted suicide) would have higher levels of salivary cortisol compared to those with no history of suicidal behavior and unaffected controls.
2. Methods
2.1. Participants
Individuals were enrolled between April of 2004 and July of 2008 as part of two studies at the University of Michigan; the Prechter Longitudinal Study of Bipolar Disorder which ascertains and evaluates the individuals for at least 5 years, and a cross sectional genetic study of bipolar disorder which utilized many of the same evaluations as the Prechter project. Both studies were approved by the Institutional Review Board of the University of Michigan Medical School (IRBMED). Participants were recruited from the University of Michigan outpatient clinic and Depression Center, inpatient psychiatric unit, and through advertisements in the local community.
After obtaining informed consent, all participants were evaluated utilizing the Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger et al., 1994). Interviews were performed by experienced clinicians and a best estimate process involving two independent physicians, psychologists or advanced graduate students was used to reach a consensus DSM IV diagnosis, based on the DIGS interview as well as records of past treatment, when requested for diagnostic or treatment clarification.
At the time of analysis, a total of 185 individuals participated in the study and provided at least one salivary cortisol sample. The majority (152 individuals) were enrolled in the Prechter Longitudinal Study. One subject subsequently retracted their consent, the salivary samples from one subject were missing at time of analysis, the saliva volume from two individuals was insufficient for analysis and three individuals did not complete enough of the diagnostic interview to reach a diagnosis. Of the remaining 178, 118 (66.3%) had a diagnosis of bipolar I, 14 (7.9%) had bipolar II with recurrent depression, 7 (3.9%) had schizoaffective disorder—bipolar type, 8 (4.5%) had other affective diagnosis (depressive disorder NOS, MDD, Bipolar II with single depressive episode), 2 (1.1%) had only non-affective diagnoses (alcohol abuse and dependence) and 29 (16.3%) were unaffected controls. For the purpose of this study, those with bipolar I, bipolar II with recurrent depression and schizoaffective disorder bipolar type were grouped together as the bipolar group (N = 139) and were categorized based on reported suicide history obtained during the DIGS. If the subject reported a history of attempted suicide, the DIGS interviewer followed up with more detailed questions regarding the attempt. Two (1.4%) of the participants were missing information on suicide history in their DIGS interviews despite several efforts to contact them for follow-up, and their suicide history status could not be determined.
102 (73%) of the bipolar individuals completed the Childhood Trauma Questionnaire (CTQ). Most of the participants missing the CTQ were from those ascertained in the earlier phases of the study. Based on the scores on the sexual abuse section of the CTQ, we divided them into three groups, those with a positive (score>5), a negative (score = 5) and an unclear sexual abuse history.
Clinical data including DSM-IV diagnoses and information on the course of the disorder such as presence of lifetime history of psychosis, comorbid anxiety disorder, substance use disorders, rapid cycling or mixed states were gathered from the DIGS and documented during the diagnostic best estimate process. For a number of participants, the history of clinical factors such as mixed episodes, rapid cycling and psychosis could not be determined with certainty. Rather than discarding the data related to these individuals, they were categorized as unclear and the statistical analysis was performed with and without their data included. Per our inclusion criteria, none of the controls (N = 29) had any formofmood, anxiety, psychosis or substance use diagnosis, presently or historically.
Years of illness were determined using the best estimated age of onset of the disorder (mean 22.01 years, S.D. 12.36). Smoking status was determined using the data from the DIGS nicotine use section or the subject's response to the Fagerstrom test for Nicotine Dependence (Heatherton et al., 1991). For the majority of individuals, height and weight were measured at baseline and body mass index (BMI) was calculated (mean 27.99, S.D. 6.18). However, this data was not collected at the time of salivary sampling on 38 individuals enrolled in the early years of the study. We did not believe this to represent a systematic selection bias, so as not to lose power and be able to use the data collected on these participants, we imputed the BMI for them using the series mean value.
2.2. Procedures
Participants collected specimens at home over 3 consecutive days. Salivary cortisol was collected using the Salivette synthetic swab (Sarstedt, Nümbrecht, Germany). Detailed written and verbal instructions were given for the collection process, and to avoid eating, drinking anything other than water, smoking or brushing their teeth at least 30 min prior to collecting the samples. They were to collect samples 10–15 min after waking up in the morning and 10–15 min before going to bed at night, keep the samples in the refrigerator until completion of the collection, and send them back via the provided mailing envelope. Samples were centrifuged at a relative centrifugal force of approximately 2500×g for 10 min and the collected specimen was kept frozen at −20 °F until ready to analyze.
Analysis was performed at the University of Michigan CLASS Laboratory, Ann Arbor, Michigan. The competitive immunoassay was on a Siemen Centaur automated analyzer, using chemiluminescent technology. The inter- and intra-assay coefficients of variation at 0.7 µg/dl were 12.4% and 3.6% respectively.
Overall, 158 individuals had three bedtime cortisol measures, 14 had two and 4 had one valid bedtime cortisol measure. 157 individuals had three morning cortisol measures, 13 had two and 6 had one valid morning cortisol measure.
2.3. Statistical analysis
Average awakening and bedtime cortisol levels were not normally distributed and several individuals had extreme values. Even after log transformation, values of bedtime cortisol remained significantly different from the normal distribution. Due to the non-normal distribution, the presence of extreme outliers and the difference of sample sizes we tested the overall differences between groups using the Kruskal–Wallis H test and report the median and interquartile range. Where the Kruskal–Wallis test was found to be significant, pairwise comparisons were performed using the Mann–Whitney U test. Statistical significance was set at 0.05 and Bonferroni corrections were made for multiple testing.
We used t tests to compare mean differences and the χ2 test for frequency data. To control for the effects of any clinical variable that might be different among the suicidal and non-suicidal bipolar groups on salivary cortisol, variables that showed significant difference among the two groups were entered into a linear regression model with bedtime cortisol as the dependent variable. We tested R2 changes between models for statistical significance. Statistical analysis was performed using the statistical program, PASW version 18.
3. Results
3.1. Clinical description of participants
The characteristics of the bipolar and control group are summarized in Table 1. Bipolar individuals were more likely to smoke (χ2 = 5.527, p = 0.019) and had elevated BMIs compared to unaffected controls (t = 16.003, p<0.001). Participants who had made a serious suicide attempt or an impulsive less serious attempt with ambivalence or minimal intent were all categorized as attempters (N = 56, 40.3%). Individuals who had thoughts of suicide but never attempted along with those who had never had thoughts of suicide were categorized as non-attempters (N = 81, 58.3%).
Table 1.
Characteristics of bipolar and control individuals.
| Unaffected controls N = 29 | Bipolar individuals N = 139 | Statistical test (df) | p value | |
|---|---|---|---|---|
| Age, mean (SD) | 37.52 (16.83) | 39.89 (12.66) | t = 0.747 (1, 166) | 0.389 |
| BMI, mean (SD) | 24.75 (4.03) | 29.08 (5.52) | t = 16.003 (1, 166) | <0.001a |
| Female-nonmenopausal Number (%) | 14 (48.3%) | 63 (45.3%) | χ2 = 0.246 (3) | 0.970 |
| Female-menopausal Number (%) | 4 (13.8%) | 24 (17.3%) | ||
| Female-unknown-menopausal status Number (%) | 1 (3.4%) | 4 (2.9%) | ||
| Male, Number (%) | 10 (34.5%) | 48 (34.5%) | ||
| Number currently smoking (%) | 2 (6.9%) | 38 (27.3%) | χ2 = 5.527 (1) | 0.019a |
Statistically significant difference (p<0.05).
Table 2 summarized the clinical characteristics of the bipolar individuals categorized by suicide history. Bipolar individuals who presented with illness duration of less than two years or whose periods of remission were longer than mood episodes were categorized as “not chronic” (N = 67, 48.2%). If they had some form of mood symptom for most of the duration of illness, they were categorized as “chronic” (N = 66, 47.5%). The chronicity status of 6 (4.3%) individuals could not be determined based on the DIGS and records.
Table 2.
Clinical characteristics of bipolar individuals with and without a history of suicide attempt
| Total bipolar individuals (N = 139) |
Bipolar without a history of suicide attempts (N = 80) |
Bipolar with a history of suicide attempts (N = 55) |
Statistical test of the difference between bipolar with and without a history of suicide attempt (df) |
Significance (2-sided) |
|
|---|---|---|---|---|---|
| History of substance use disorder N (%) | 78 (56.1%) | 41 (51.3%) | 36 (65.5%) | χ2 = 2.684 (1) | 0.101 |
| Comorbid anxiety diagnosis N (%) | 58 (41.7%) | 31 (38.8%) | 27 (49.1%) | χ2 = 1.422 (1) | 0.233 |
| Male N (%) | 48 (34.5%) | 34 (42.5%) | 11 (20.0%) | χ2 = 8.562 (3) | 0.036a |
| Female non-menopausal N (%) | 63 (45.3%) | 30 (37.5%) | 33 (60.0%) | ||
| Female menopausal N (%) | 24 (17.3%) | 14 (17.5%) | 10 (18.2%) | ||
| Female unknown status N (%) | 4 (2.9%) | 2 (2.5%) | 1 (1.2%) | ||
| Euthymic mood state N (%) | 55 (39.6%) | 35 (43.8%) | 19 (34.5%) | χ2 = 1.769 (3) | 0.622 |
| Depressed mood state N (%) | 44 (31.7%) | 26 (32.5%) | 18 (32.7%) | ||
| Hypomanic/mixed mood state N (%) | 9 (6.5%) | 4 (5%) | 3 (5.5%) | ||
| Unknown mood state N (%) | 31 (22.3%) | 15 (18.8%) | 15 (27.3%) | ||
| Current smoker N (%) | 38 (27.3%) | 15 (18.8%) | 22 (40.0%) | χ2 = 7.397 (1) | 0.007a |
| Positive history of mixed episodes N (%) | 41 (29.5%) | 20 (25.0%) | 21 (38.2%) | χ2 = 8.583 (2) | 0.014a |
| Unclear history of mixed episodes N (%) | 7 (5.0%) | 1 (1.3%) | 5 (9.1%) | ||
| No history of mixed episodes N (%) | 91 (65.5%) | 59 (73.8%) | 29 (52.7%) | ||
| Positive history of psychosis N (%) | 88 (63.3%) | 53 (66.3%) | 32 (58.2%) | χ2 = 0.943 (2) | 0.624 |
| Unclear history of psychosis N (%) | 8 (5.8%) | 4 (5.0%) | 3 (2.9%) | ||
| No history of psychosis N (%) | 43 (30.9%) | 23 (28.8%) | 20 (36.4%) | ||
| Positive history of rapid cycling N (%) | 48 (34.5%) | 23 (28.8%) | 23 (41.8%) | χ2 = 9.294 (2) | 0.010a |
| Unclear history of rapid cycling N (%) | 25 (18.0%) | 10 (12.5%) | 14 (25.5%) | ||
| No history of rapid cycling N (%) | 66 (47.5%) | 47 (58.8%) | 18 (32.7%) | ||
| Chronically ill N (%) | 66 (47.5%) | 26 (32.5%) | 37 (67.3%) | χ2 = 17.255 (2) | <0.001a |
| Unclear course N (%) | 6 (4.3%) | 5 (6.3%) | 0 (0%) | ||
| Not chronically ill N (%) | 67 (48.2%) | 49 (61.3%) | 18 (32.7%) | ||
| BMI mean (SD) | 29.08 (5.52) | 28.03 (4.54) | 30.89 (6.37) | t = 9.308 (1) | 0.003a |
| Age mean (SD) | 39.89 (12.66) | 41.04 (13.18) | 38.31 (11.23) | t = 1.571 (1) | 0.212 |
| Years of illness mean (SD) | 22.01 (12.36) | 20.61 (13.20) | 24.33 (10.72) | t = 2.993 | 0.086 |
| Positive history of sexual abuse N (%) | 40 (28.8%) | 21 (26.3%) | 19 (34.5%) | χ2 = 3.165 (2) | 0.206 |
| Unclear history of sexual abuse N (%) | 37 (26.6%) | 19 (23.8%) | 17 (30.9%) | ||
| Negative history of sexual abuse N (%) | 62 (44.6%) | 40 (50%) | 19 (34.5%) |
Statistically significant difference (p <0.05).
Almost 40% of the bipolar sample was considered euthymic (HAMD-17≤9 and YMRS≤7) at entry to the study (N = 55, 39.6%, Mean Hamilton = 4.1 S.D. = 2.6, Mean YMRS = 1.1 S.D. = 1.8) and one-third of the sample was depressed (HAMD-17>9) (N = 44, 31.7%: Mean Hamilton = 14.3, S.D. = 2.9). Less than 7% of the sample was either manic, hypomanic or in a mixed state (YMRS>7) (N = 9, 6.5%: Mean Hamilton = 10.9 S.D. = 5.8; Mean YMRS = 11.4, S.D. = 3.5). Thirty one of the participants enrolled in the earlier phases in the project did not have HAMD-17 or YMRS scores and their mood state was categorized as unknown (N = 31, 22.3%). All 29 unaffected controls were euthymic at the time of evaluation.
Overall the bipolar individuals with a history of suicide attempt were significantly more likely to be female and non-menopausal (p = 0.036), currently smoke cigarettes (p = 0.007), have a history of mixed mood episodes (p = 0.014) or rapid cycling (p = 0.010), an elevated BMI (p = 0.003) and more chronic and persistent course of illness (p< 0.001). Bipolar individuals with a history of suicide also had more years of illness (p = 0.086) and an elevated rate of substance use disorders (p = 0.10) although the differences did not reach statistical significance.
There was no statistical difference in the types of medications used by the two bipolar groups (Table 3). None was on oral corticosteroids.
Table 3.
Difference in medications groups between bipolar individuals with and without a history of suicide attempts.
| Medication group: number taking it (%) |
Bipolar without a history of suicide attempts N = 80 |
Bipolar with a history of suicide attempts N = 55 |
Pearson Chi-square (df) |
Significance (2-sided) |
|---|---|---|---|---|
| Sex Hormones: 16 (11.9%) | 6 (7.5%) | 10 (18.2%) | 3.560 (1) | 0.059 |
| Anti-diabetics: 8 (5.9%) | 4 (5%) | 4 (7.3%) | 0.302 (1) | 0.583 |
| Inhalers: 6 (4.4%) | 3 (3.8%) | 3 (5.5%) | 0.223 (1) | 0.637 |
| Simulants: 6 (4.4%) | 3 (3.8%) | 3 (5.5%) | 0.223 (1) | 0.637 |
| Benzodiazepines: 40 (29.6%) | 24 (30.0%) | 16 (29.1%) | 0.013 (1) | 0.910 |
| Mood stabilizers: 109 (80.7%) | 68 (85.0%) | 41 (74.5%) | 2.291 (1) | 0.130 |
| Antipsychotics: 66 (48.9%) | 36 (45.0%) | 30 (54.5%) | 1.188 (1) | 0.276 |
| Antidepressants: 69 (51.1%) | 38 (47.5%) | 31 (56.4%) | 1.025 (1) | 0.311 |
| Other: 87 (64.4%) | 50 (62.5%) | 37 (67.3%) | 0.324 (1) | 0.569 |
3.2. Cortisol
There was no statistically significant difference in average awakening cortisol between bipolar or control participants (N = 165, Kruskal-Wallis χ2 = 2.117, df = 2, p = 0.347) (Table 4). For bedtime salivary cortisol, the overall difference between the three groups was statistically significant (N = 164, Kruskal–Wallis χ2 = 7.375, df = 2, p = 0.025, significant for Bonferroni adjusted alpha level of 0.05/2 = 0.025). Comparison of the three groups using the Mann–Whitney U test showed only a statistical difference between the bipolar individuals with and without a history of suicide attempt. (N = 135, Mann–Whitney U = 1629.5, p = 0.011, significant for Bonferroni adjusted alpha level of 0.05/3 = 0.016). Although the bipolar individuals with a history of suicide had a higher mean rank on their bedtime cortisol compared to unaffected controls, the difference did not maintain statistical significance after correction for multiple testing. (N = 84, Mann–Whitney U = 589, p = 0.050, not significant for Bonferroni adjusted alpha level of 0.05/3 = 0.016).
Table 4.
Awakening and bedtime cortisol levels (µg/dl) in bipolar individuals with and without a history of suicide attempt and controls.
| Controls (N = 29) | Bipolar without a history of suicide attempt (80) |
Bipolar with a history of suicide attempt (N = 56 awakening, 55 bedtime) |
|
|---|---|---|---|
| Awakening cortisol | |||
| Median, (IQR) | 0.93 (0.32) | 0.90, (0.29) | 0.90, (0.25) |
| Mean rank | 94.33 | 81.86 | 78.76 |
| Bedtime cortisol | |||
| Median, (IQR) | 0.45, (0.09) | 0.45, (0.08) | 0.50, (0.11)a |
| Mean rank | 74.59 | 75.63 | 96.66 |
Statistically higher mean rank compared to non-suicidal bipolar participants at p<0.05 level after Bonferroni correction.
The bipolar group with suicide attempts had more individuals who were on sex hormones (oral or injectable contraceptives) with a significance of 0.059. We examined the difference in bedtime cortisol levels among the two bipolar groups with the 16 participants on sex hormones removed. We found similar results (N = 119, Mann–Whitney U = 1146.00, p = 0.004) and they were therefore included in the analyses.
To better understand the effects of the observed clinical differences on bedtime cortisol we performed a linear regression analysis. When average bedtime cortisol was used as the dependent variable, the distribution of the studentized deleted residuals had high skewness (7.96 with standard error = 0.209) and kurtosis (79.14 with standard error = 0.414) and was significantly different from normal (Kolmogorov–Smirnov statistic <0.001). This was mainly due to three bipolar participants, one with a history of suicide attempt and two without that had average bedtime cortisol levels that were extreme outliers. Each had one very elevated bedtime cortisol level (2.05, 2.59 and 5.28 µg/dl). After removal of these three extreme values, recalculating the average cortisol level and log transformation of the data, the residuals displayed much closer to a normal distribution (skewness of 0.741 with standard error = 0.209 and Kurtosis of 0.869 with standard error = 0.414) and the Kolmogorov–Smirnov test of deviation from normality was no longer significant (0.200). We used this log transformed bedtime cortisol levels as the dependent variable and the clinical factors that were different between the two groups as independent variables. Considering the effect that mood state could have on cortisol levels, we also added mood state to the model, even though there was no difference in the proportion of bipolar individuals in a given mood state by history of suicide attempts. There was no evidence of multicollinearity in our data. All independent variables displayed a tolerance of at least 0.586 and a maximum variance-inflation factor (VIF) of 1.705
In the first step we created a model with the significant clinical variables (BMI at baseline, years ill, smoking status, history of substance abuse, mixed state history, rapid cycling history, chronicity of course of illness, mood state and sex) and checked for significance of the individual variables and the model as a whole. We used dummy variables for the categorical variables that were not binary. In the following step we added suicide attempt history to the model and looked at the significance of R2 change, the significance of the whole model and the significance of each standardized coefficient (β). The results are reported in Table 5.
Table 5.
Result of linear regression with log transformed bedtime cortisol levels as dependent variable and clinical factors as independent variables.
| Model 1. |
Model 2. |
|||
|---|---|---|---|---|
| β | p value | β | p value | |
| Positive substance abuse history | 0.014 | 0.888 | 0.002 | 0.980 |
| Years of illness | 0.065 | 0.534 | 0.044 | 0.671 |
| Positive current smoking status | 0.032 | 0.750 | −0.022 | 0.831 |
| BMI at baseline | 0.082 | 0.363 | 0.043 | 0.636 |
| Positive history of mixed episodes | −0.145 | 0.137 | −0.161 | 0.096 |
| Unclear history of mixed episodes | −0.206 | 0.023a | −0.233 | 0.010a |
| Positive history of rapid cycling | 0.166 | 0.103 | 0.133 | 0.189 |
| Unclear history of rapid cycling | −0.039 | 0.690 | −0.074 | 0.446 |
| Illness pattern is chronic | 0.014 | 0.897 | −0.014 | 0.895 |
| Illness pattern is unclear | −0.095 | 0.276 | −0.074 | 0.392 |
| Depressed at baseline | −0.100 | 0.334 | −0.069 | 0.502 |
| Hypomanic/manic/mixed at baseline | −0.062 | 0.489 | −0.049 | 0.580 |
| Mood state at baseline is unknown | 0.264 | 0.009a | 0.258 | 0.010a |
| Male | −0.032 | 0.738 | 0.014 | 0.885 |
| Female and menopausal | −0.154 | 0.158 | −0.137 | 0.204 |
| Female and menopausal status is unknown | −0.062 | 0.499 | −0.055 | 0.543 |
| Suicide attempt history | 0.208 | 0.041a | ||
| Intercept (B) | −.814 | <0.001 | −0.791 | <0.001 |
| R2 | 0.180 | 0.209 | ||
| Adjusted R2 | 0.069 | 0.094 | ||
| R2 change | 0.029 | |||
| F value of R2 change (df) | 4.289 (1, 117) | |||
| p value of R2 change | 0.041a | |||
| p value for full model | 0.074 | 0.033a | ||
| Sample Size | 135 | 135 | ||
Statistically significant difference (p<0.05).
Only model 2 showed statistical significance (p = 0.033). The addition of suicide attempt history to the model also made a significant increase in R2 (p = 0.041) and suicide attempt history retained its statistical significance in the full model (p = 0.041). Two other clinical variables showed statistical significance in this model. An unclear history of mixed episodes reduced bedtime cortisol compared to bipolar individuals who had no past mixed episodes (p = 0.010) and bipolar individuals with unclear mood status at baseline had a higher bedtime cortisol compared to the euthymic bipolar group (p = 0.010).
We also repeated the analysis removing those missing any clinical data (related to baseline mood status, history of mixed episodes, rapid cycling, chronicity of illness and meno-pausal status in women). 82 bipolar individuals remained. The statistical results were similar, with the bipolar individuals with suicide history exhibiting higher bedtime cortisol levels (Mann–Whitney U = 506.5, p = 0.005). Although the full linear regression model was not significant (p = 0.169) the change in R2 from adding the history of suicide was highly significant (β = 0.354, R2 change = 0.085, F change = 7.312, df = 1, 69, p = 0.009). In this model no other clinical variable had a statistically significant effect on bedtime cortisol.
We examined bedtime salivary cortisol levels in euthymic bipolar individuals separately (N = 35 with no suicide attempt and N = 19 with a past history of suicide attempts). Again the mean rank was statistically higher for bipolar individuals with a past history of suicide attempts (Mann–Whitney U = 209, p = 0.025).
As our inter-assay coefficient of variance of the cortisol test was 12.4%, a repeated measures ANOVA was performed on participants who had three days of bedtime cortisol results (controls = 28, non-suicidal bipolar = 67 and suicidal bipolar = 48) to examine the effects of day of sampling on cortisol levels, along with bipolar and suicide status. Though there was a statistically significant difference (Wilks Lambda F = 4.217 df(2,139) p = 0.017) in the estimated marginal means between sampling days two and three (Mean difference = 0.045 µg/dl, 95% Confidence Interval 0.007–0.082), the difference in estimated marginal means between bipolar participants with and without suicide attempts (Mean Difference = 0.042 µg/dl, 95% Confidence interval 0.004–0.079) remained statistically significant (F = 3.665 df(2,140) p = 0.028)
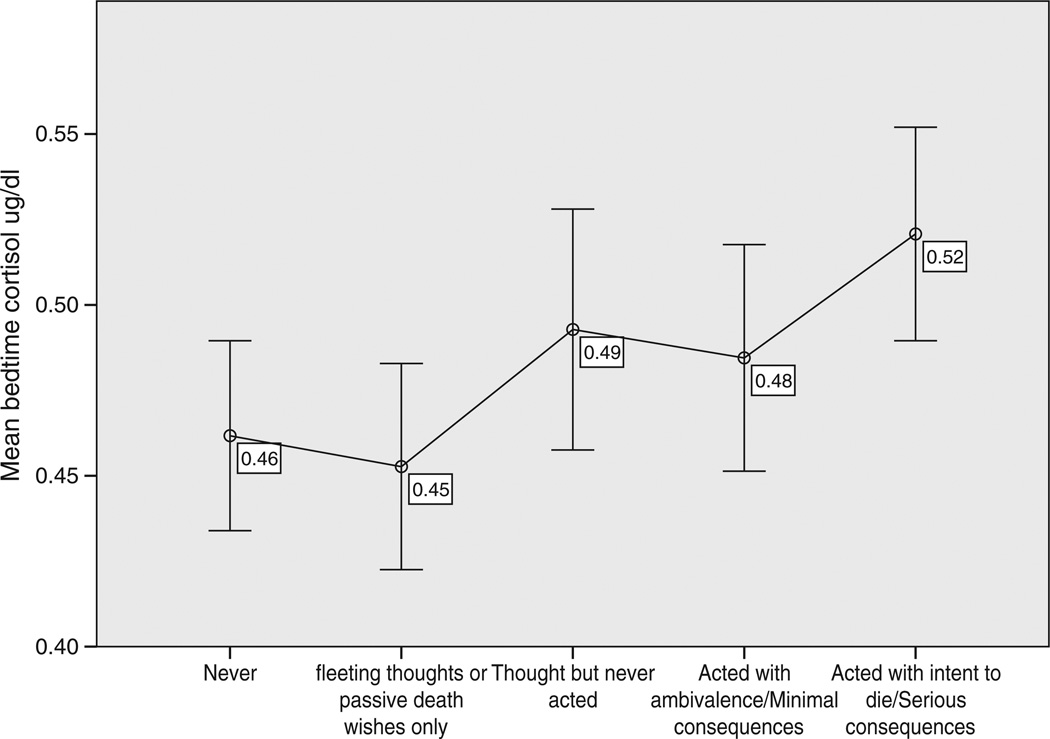
In the full model, the estimated coefficient (b) for suicide was 0.072. We calculated that a history of suicidal attempts increases salivary cortisol by 7.46% [100(e0.072−1)] (Allison, 1999). In post hoc exploratory analysis, we also looked at severity of suicide attempts and bedtime salivary cortisol. Based on information collected in the DIGS, we categorized the severity of suicidal thoughts and behaviors on a five-point scale. There was a significant positive correlation between severity of suicidal behavior or thoughts and bedtime salivary cortisol (N = 135, Spearman's rho = 0.255, p = 0.003). Bipolar individuals with more serious suicide attempts had the highest bedtime cortisol levels (Fig. 1).
Fig. 1.
Levels of bedtime salivary cortisol in bipolar individuals based on severity of suicidal thoughts and behaviors. Error Bars: 95% CI.
4. Discussion
We found elevated bedtime salivary cortisol in bipolar individuals with a history of suicide attempts compared to non-suicidal bipolar individuals. Further supporting our findings, secondary analysis of the intensity of suicidal behavior and level of bedtime cortisol indicated a positive correlation, with the highest cortisol levels reported in individuals that had made a past serious suicide attempt. The difference in bedtime salivary cortisol between suicidal and non-suicidal bipolar individuals remained significant even after controlling for age and sex, BMI, smoking status, childhood sexual abuse, medications, mood state at time of sampling and several clinical factors related to course and severity of illness (substance use disorders, chronicity of illness, rapid cycling, mixed states, years of illness, age of onset, anxiety and psychosis). This is a strong indicator that our finding is related to the presence of a past history of suicidality and not related to severity of illness, mood state, or demographic confounders. The presence of this finding during different mood states and also in the euthymic state indicates that hyperactivity of the HPA axis is a biological marker related to suicidality in bipolar disorder and warrants more detailed investigation.
The difference between bipolar participants with and without suicidal behavior was only 0.05 µg/dl. Currently, this test has low sensitivity and specificity in detecting individuals with suicidal history in practical clinical applications. However, the observation of a sustained correlation between increasing suicidality and cortisol levels while controlling for confounding clinical and biological factors clearly indicates the relevance of HPA axis abnormalities in this potentially lethal clinical condition.
Compared to other studies of salivary cortisol in bipolar disorder (Deshauer et al., 2003, 2006; Havermans et al., 2011; Thompson et al., 2005; Watson et al., 2004) our study sampled the largest number of bipolar individuals. Despite this and contrary to our initial predictions, non-suicidal bipolar individuals did not have a statistically detectable elevated salivary cortisol compared to unaffected controls on awakening or at bedtime. This may reflect the limited sensitivity of salivary cortisol to detect smaller differences in HPA axis activity. In euthymic bipolar individuals, Watson et al. (2004) were unable to detect HPA axis hyperactivity measuring basal salivary cortisol secretion, but identified hyperactivity in the same individuals utilizing the more sensitive DEX/CRH challenge test. We also found no statistically significant difference in awakening cortisol levels in any of the three groups.
In direct contrast to our study, a smaller study by Lindqvist et al. (2008) reported lower salivary cortisol in the evening in the individuals with a history of suicide. A possible explanation for the difference in our findings could be that their study had a more heterogeneous diagnostic population compared to our study, which is limited to bipolar individuals.
Mood state has a significant effect on the level of HPA axis activity and may also explain some of the reported inconsistencies. Individuals have DST non-suppression during acute mood episodes and in many instances, the DST non-suppression returns to normal with resolution of mood symptoms (Ribeiro et al., 1993). In most studies described above, the DST or DEX/CRH tests were done at the time of an acute mood episode. The level of HPA axis hyperactivity during symptomatic mood states may be much larger than the levels of hyperactivity seen in suicidal individuals during euthymic mood states. Our finding suggests that at least part of the HPA axis hyperactivity during acute mood episodes in individuals with a history of suicide does not return to baseline levels and this hyperactivity does not appear to be a marker of other aspects of disease severity or chronicity.
Clearly, measures of HPA axis activity alone cannot completely explain the causes for such a complex behavior as suicide. Coryell et al. (2006) did a follow up study on the causes of death in 334 individuals who had a baseline DST on average 18 years earlier. There were 32 completed suicides. They found an association between completed suicide and baseline DST non-suppression, but only in individuals who were recruited while on an inpatient unit or had reported suicidal thoughts at the index episode, suggesting that the HPA axis hyperactivity may be more of an additive risk for suicide, which factors itself in when other predisposing elements are present.
Our study has a number of limitations. The use of salivary cortisol as a marker of HPA axis activity has both strengths and weaknesses. The collection process is straightforward and simple and relatively free of stress. The specimens can be processed without need for deep freezing and the samples are relatively stable at room temperature. Salivary cortisol has also been closely associated with unbound plasma cortisol (Hellhammer et al., 2009). However, salivary cortisol levels can be affected by many factors, including smoking cigarettes (Badrick et al., 2007), food intake (Rosmond et al., 2000) or brushing teeth prior to sampling. Every attempt was made to mitigate these confounding factors with detailed written instructions and verbal explanations. Cortisol secretion also exhibits a diurnal variation with the highest levels in the morning shortly after awakening and low levels in the evening (Levine et al., 2007). This highlights the importance of consistency in timing of the samples and we cannot eliminate variability due to non-adherence to instructions.
Although we found no difference in the mood states of bipolar individuals with and without a history of suicide, due to the cross sectional design of the study, we are unable to compare the HPA activity of the same individuals in specific mood states and cannot definitely rule out the role of mood state in the final analysis. In the linear regression model, we only found that participants that had missing mood state had higher bedtime salivary cortisol compared to euthymic bipolar individuals. The significance of this finding is unclear to us and it could be a spurious finding, related to the number of statistical tests that were performed. Limiting our analysis to euthymic individuals provided the same results.
Our participants were not medication free at the time of evaluation. Given the effect of medications on cortisol levels, this could have affected the results. Only sex hormones showed a difference bordering on statistical significance between the suicidal and non-suicidal groups. This difference most likely reflects our finding that more females were in the bipolar group with a history of suicide attempts. Repeat analysis with these individuals removed showed similar results.
Another limitation is that information on several clinical variables was missing in some of our participants. However, we found the same results in the analysis of data with these individuals excluded. Finally, since we have no psychiatric comparison group, the findings may not be generalizable to other diagnostic categories.
In this study, elevated bedtime salivary cortisol was associated with a history of suicidal behavior in bipolar individuals. While HPA axis hyperactivity has been closely related to acute mood episodes, based on our findings, it also may be a trait marker of suicidal behavior during euthymic periods in bipolar individuals.
Acknowledgments
Funding for this study was provided by the Prechter Bipolar Research Fund at the University of Michigan (MK, EFHS, ARP, CBB, GJH, SAL, MGM) and the University of Michigan Depression Center, Rachel Upjohn Clinical Scholars Award (EFHS). The authors wish to thank all the individuals who volunteered to participate in this study.
Role of funding source
Funding for this study was provided by the Prechter Bipolar Research Fund at the University of Michigan and the University of Michigan Depression Center, Rachel Upjohn Clinical Scholars Award. The funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest
In the past 5 years Dr. McInnis has received honoraria for service on speaker's bureaus or consultative boards with Pfizer, BMS, GSK, Astra-Zeneca, Eli Lilly, Janssen, and Merck Pharmaceuticals. All other authors declare that they have no conflicts of interest.
References
- Allison PD. Multiple Regression: A Primer. USA: Pine Forge Press; 1999. [Google Scholar]
- Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. Journal of Clinical Endocrinology and Metabolism. 2007;92:819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- Black D, Monahan P, Winokur G. The relationship between DST results and suicidal behavior. Annals of Clinical Psychiatry. 2002;14:83–88. doi: 10.1023/a:1016839404032. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Feinberg M, Greden JF, Tarika J, Albala AA, Haskett RF, James NM, Kronfol Z, Lohr N, Steiner M, de Vigne JP, Young E. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Archives of General Psychiatry. 1981;38:15–22. doi: 10.1001/archpsyc.1981.01780260017001. [DOI] [PubMed] [Google Scholar]
- Cassidy F, Ritchie JC, Carroll BJ. Plasma dexamethasone concentration and cortisol response during manic episodes. Biological Psychiatry. 1998;43:747–754. doi: 10.1016/s0006-3223(97)00274-6. [DOI] [PubMed] [Google Scholar]
- Cervantes P, Gelber S, Kin FN, Nair VN, Schwartz G. Circadian secretion of cortisol in bipolar disorder. Journal of Psychiatry Neuroscience: JPN. 2001;26:411–416. [PMC free article] [PubMed] [Google Scholar]
- Cookson JC, Silverstone T, Williams S, Besser GM. Plasma cortisol levels in mania: associated clinical ratings and changes during treatment with haloperidol. The British Journal of Psychiatry. 1985;146:498–502. doi: 10.1192/bjp.146.5.498. [DOI] [PubMed] [Google Scholar]
- Coryell W, Schlesser M. The dexamethasone suppression test and suicide prediction. The American Journal of Psychiatry. 2001;158:748. doi: 10.1176/appi.ajp.158.5.748. [DOI] [PubMed] [Google Scholar]
- Coryell W, Young E, Carroll B. Hyperactivity of the hypothalamicpituitary-adrenal axis and mortality in major depressive disorder. Psychiatry Research. 2006;142:99. doi: 10.1016/j.psychres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Daban C, Vieta E, Mackin P, Young AH. Hypothalamic-pituitary-adrenal axis and bipolar disorder. The Psychiatric Clinics of North America. 2005;28:469–480. doi: 10.1016/j.psc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Ryan ND, Puig-Antich J, Nguyen NA, al-Shabbout M, Meyer VA, Perel J. 24-hour cortisol measures in adolescents with major depression: a controlled study. Biological Psychiatry. 1991;30:25–36. doi: 10.1016/0006-3223(91)90067-v. [DOI] [PubMed] [Google Scholar]
- Deshauer D, Grof E, Alda M, Grof P. Patterns of DST positivity in remitted affective disorders. Biological Psychiatry. 1999;45:1023–1029. doi: 10.1016/s0006-3223(98)00334-5. [DOI] [PubMed] [Google Scholar]
- Deshauer D, Duffy A, Alda M, Grof E, Albuquerque J, Grof P. The cortisol awakening response in bipolar illness: a pilot study. Canadian Journal of Psychiatry. 2003;48:462–466. doi: 10.1177/070674370304800706. [DOI] [PubMed] [Google Scholar]
- Deshauer D, Duffy A, Meaney M, Sharma S, Grof P. Salivary cortisol secretion in remitted bipolar patients and offspring of bipolar parents. Bipolar Disorders. 2006;8:345. doi: 10.1111/j.1399-5618.2006.00338.x. [DOI] [PubMed] [Google Scholar]
- Duval F, Mokrani MC, Correa H, Bailey P, Valdebenito M, Monreal J, Crocq MA, Macher JP. Lack of effect of HPA axis hyperactivity on hormonal responses to d-fenfluramine in major depressed patients: implications for pathogenesis of suicidal behaviour. Psychoneuroendo-crinology. 2001;26:521–537. doi: 10.1016/s0306-4530(01)00011-7. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Hodgins S, Walker C. High levels of cortisol among adolescent offspring of parents with bipolar disorder: a pilot study. Psychoneuroendocrinology. 2004;29:99–106. doi: 10.1016/s0306-4530(02)00135-x. [DOI] [PubMed] [Google Scholar]
- Godwin CD. The dexamethasone suppression test in acute mania. Journal of Affective Disorders. 1984;7:281–286. doi: 10.1016/0165-0327(84)90049-1. [DOI] [PubMed] [Google Scholar]
- Havermans R, Nicolson N, Berkhof J, Devries M. Patterns of salivary cortisol secretion and responses to daily events in patients with remitted bipolar disorder. Psychoneuroendocrinology. 2011;36:258–265. doi: 10.1016/j.psyneuen.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrm KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, Nemeroff C. Neurobiological and psychiatric consequences of child abuse and neglect. Developmental Psychobiology. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- Hellhammer D, Wst S, Kudielka B. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/ CRH test: a refined laboratory test for psychiatric disorders. Journal of Psychiatric Research. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Lauer CJ, Schreiber W, Krieg JC. Altered hypothalamic-pituitary-adrenocortical regulation in healthy subjects at high familial risk for affective disorders. Neuroendocrinology. 1995;62:340–347. doi: 10.1159/000127023. [DOI] [PubMed] [Google Scholar]
- Hwu HG, Lin HN. Serial dexamethasone suppression test in psychiatric inpatients. Biological Psychiatry. 1990;27:609–616. doi: 10.1016/0006-3223(90)90528-a. [DOI] [PubMed] [Google Scholar]
- Jokinen J, Nordström P. HPA axis hyperactivity as suicide predictor in elderly mood disorder inpatients. Psychoneuroendocrinology. 2008;33:1387. doi: 10.1016/j.psyneuen.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Jokinen J, Nordström P. HPA axis hyperactivity and attempted suicide in young adult mood disorder inpatients. Journal of Affective Disorders. 2009;116:117. doi: 10.1016/j.jad.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Jokinen J, Nordström A, Nordström P. CSF 5-HIAA and DST non-suppression-orthogonal biologic risk factors for suicide in male mood disorder inpatients. Psychiatry Research. 2009;165:96. doi: 10.1016/j.psychres.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Kumari M, Chandola T, Brunner E, Kivimaki M. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II study. Journal of Clinical Endocrinology and Metabolism. 2010;95:4415–4423. doi: 10.1210/jc.2009-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester D. The dexamethasone suppression test as an indicator of suicide: a meta-analysis. Pharmacopsychiatry. 1992;25:265. doi: 10.1055/s-2007-1014419. [DOI] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Lewis J, Weller A. Measuring cortisol in human psychobiological studies. Physiology & Behavior. 2007;90:43. doi: 10.1016/j.physbeh.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Isaksson A, Trskman-Bendz L, Brundin L. Salivary cortisol and suicidal behavior—a follow-up study. Psychoneuroendocrinology. 2008;33:1061. doi: 10.1016/j.psyneuen.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Linkowski P, Kerkhofs M, Van Onderbergen A, Hubain P, Copinschi G, L'Hermite-Balriaux M, Leclercq R, Brasseur M, Mendlewicz J, Van Cauter E. The 24-hour profiles of cortisol, prolactin, and growth hormone secretion in mania. Archives of General Psychiatry. 1994;51:616–624. doi: 10.1001/archpsyc.1994.03950080028004. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Neurobiology of suicidal behaviour. Nature Reviews. Neuro-science. 2003;4:819. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- Nicolson N, Davis M, Kruszewski D, Zautra A. Childhood maltreatment and diurnal cortisol patterns in women with chronic pain. Psychosomatic Medicine. 2010;72:471–480. doi: 10.1097/PSY.0b013e3181d9a104. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Pfennig A, Kunzel H, Kern N, Ising M, Majer M, Fuchs B, Ernst G, Holsboer F, Binder E. Hypothalamus-pituitary-adrenal system regulation and suicidal behavior in depression. Biological Psychiatry. 2005;57:336–342. doi: 10.1016/j.biopsych.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Pitchot W, Scantamburlo G, Pinto E, Hansenne M, Reggers J, Ansseau M, Legros J. Vasopressin-neurophysin and DST in major depression: relationship with suicidal behavior. Journal of Psychiatric Research. 2008;42:684–688. doi: 10.1016/j.jpsychires.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ribeiro SC, Tandon R, Grunhaus L, Greden JF. The DST as a predictor of outcome in depression: a meta-analysis. The American Journal of Psychiatry. 1993;150:1618–1629. doi: 10.1176/ajp.150.11.1618. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Holm G, Bjrntorp P. Food-induced cortisol secretion in relation to anthropometric, metabolic and haemodynamic variables in men. International Journal of Obesity. 2000;24:416–422. doi: 10.1038/sj.ijo.0801173. [DOI] [PubMed] [Google Scholar]
- Roy A. Hypothalamic-pituitary-adrenal axis function and suicidal behavior in depression. Biological Psychiatry. 1992;32:812–816. doi: 10.1016/0006-3223(92)90084-d. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Twardowska K. The dexamethasone/corticotropin-releasing hormone test in depression in bipolar and unipolar affective illness. Journal of Psychiatric Research. 1999;33:363–370. doi: 10.1016/s0022-3956(99)00014-x. [DOI] [PubMed] [Google Scholar]
- Schmider J, Lammers CH, Gotthardt U, Dettling M, Holsboer F, Heuser IJ. Combined dexamethasone/corticotropin-releasing hormone test in acute and remitted manic patients, in acute depression, and in normal controls: I. Biological Psychiatry. 1995;38:797–802. doi: 10.1016/0006-3223(95)00064-X. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Singer B, Wilkinson CW, McEwen B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendo-crinology. 2001;26:225–240. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Stein MB, Yehuda R, Koverola C, Hanna C. Enhanced dexametha-sone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biological Psychiatry. 1997;42:680–686. doi: 10.1016/s0006-3223(96)00489-1. [DOI] [PubMed] [Google Scholar]
- Thompson J, Gallagher P, Hughes J, Watson S, Gray J, Ferrier IN, Young A. Neurocognitive impairment in euthymic patients with bipolar affective disorder. The British Journal of Psychiatry. 2005;186:32–40. doi: 10.1192/bjp.186.1.32. [DOI] [PubMed] [Google Scholar]
- Tripodianakis J, Markianos M, Sarantidis D, Leotsakou C. Neuro-chemical variables in subjects with adjustment disorder after suicide attempts. European Psychiatry. 2000;15:190–195. doi: 10.1016/s0924-9338(00)00226-1. [DOI] [PubMed] [Google Scholar]
- van Heeringen K, Audenaert K, Van de Wiele L, Verstraete A. Cortisol in violent suicidal behaviour: association with personality and monoaminergic activity. Journal of Affective Disorders. 2000;60:181–189. doi: 10.1016/s0165-0327(99)00180-9. [DOI] [PubMed] [Google Scholar]
- Watson S, Gallagher P, Ritchie J, Ferrier IN, Young A. Hypotha-lamic-pituitary-adrenal axis function in patients with bipolar disorder. The British Journal of Psychiatry. 2004;184:496. doi: 10.1192/bjp.184.6.496. [DOI] [PubMed] [Google Scholar]
- Yerevanian B, Feusner J, Koek R, Mintz J. The dexamethasone suppression test as a predictor of suicidal behavior in unipolar depression. Journal of Affective Disorders. 2004;83:103. doi: 10.1016/j.jad.2004.08.009. [DOI] [PubMed] [Google Scholar]