Abstract
Introduction
Stress and stress hormone are known to play important roles in modulating different stages of memory including reconsolidation. In a previous study, we found that treatment with stress or corticosterone after a single memory reactivation disrupted reconsolidation of a drug-related memory in rats. Here we presumed that stress after memory reactivation can effectively inhibit drug-related memory by disrupting its reconsolidation in abstinent heroin addicts.
Materials and methods
In the present study, 21 abstinent heroin addicts learned a word list (containing ten neutral, ten heroin-related negative, and ten heroin-related positive words) on day 1; retrieval of a word list (learned 24 h earlier) was made on day 2; and immediately after retrieval, they were exposed to either a standardized psychosocial laboratory stressor (Trier Social Stress Test) or a control condition in a crossover manner. On day 3, free recall of the word list and other psychological and physical responses were assessed.
Results
The stressor induced a significant increase in salivary free cortisol and a decrease in mood. Memory recall was significantly impaired after the stress condition. Follow-up analysis revealed that heroin-related negative and positive words (i.e., heroin-related words) were affected, whereas no effect was observed for neutral words. No changes were detected for cued recall, working memory, or attention. Stress after drug-related memory retrieval significantly decreased its subsequent recall, likely through impaired drug-related memory reconsolidation process.
Conclusion
Reconsolidation blockade may thus provide a potential therapeutic strategy for the prevention of relapse in drug addiction.
Keywords: Stress, Glucocorticoids, Heroin-related memory, Reconsolidation
Introduction
A pathological usurpation of neural processes that normally serve reward-related learning and memory has been thought to underlie the progress of drug addiction (Berger et al. 1989; Hyman and Malenka 2001; Robbins and Everitt 1999, 2002; White 1996). Maladaptive memories associated with the effects of drugs of abuse is possible to result in relapse to drug-seeking and -taking behaviors that are often found in drug-dependent patients. Nonetheless, previously formed memories are susceptible to disruption immediately after recall due to a necessity for reconsolidation. According to current reconsolidation theories, reactivation of a consolidated memory renders it once again vulnerable to amnestic treatment (Campbell et al. 1968), and the so-called reconsolidation of this old memory requires de novo protein synthesis (Amorapanth et al. 2000; Aoki et al. 2003). Notably, repeated relapse induced by drug-related cues is likely to be influenced by memory reconsolidation in which a consolidated memory could again return to a labile state after retrieval (Lewis et al. 1968; Misanin et al. 1968; Nader 2003; Nader et al. 2000b; Przybyslawski and Sara 1997; Sara 2000a, b).
Ample evidence of animal studies has demonstrated that the reconsolidation of memory is subjective to various behavioral and pharmacological manipulations. The process of reconsolidation can be profoundly affected by amnesic effects induced by the administration of blockers, such as the protein synthesis inhibitors or β-blockers (Nader et al. 2000a; Przybyslawski et al. 1999), or also by the learning of a new memory (Boccia et al. 2005; Walker et al. 2003), after the presentation of a reminder. Using the task of conditioned place preference, various substrates in the brain, including Zif268 antisense oligodeoxynucleotides, β-adrenoceptor, and glutamate receptors have been demonstrated to play an integrated role in modulating the reconsolidation of drug-conditioned memory (Bernardi et al. 2006; Kreek and Koob 1998; Lee et al. 2006; Popik et al. 2006). Miller and Marshall (2005) showed that reconsolidation of cocaine-conditioned place preference (CPP) can be disrupted by interfering in the molecular processes within the nucleus accumbens core.
The importance of stress and stress hormone in the different stages of memory processes including reconsolidation has been implicated in the literature (Diamond et al. 1996; Loscertales et al. 1998; Newcomer et al. 1994, 1999; Roozendaal 2002). Stress and glucocorticoids enhance (Loscertales et al. 1998; Roozendaal 2002) as well as impair (Diamond et al. 1996; Newcomer et al. 1994, 1999) memory consolidation, and memory retrieval is usually impaired (de Quervain et al. 1998; Kuhlmann et al. 2005b). Moreover, in our recent study, we found that treatment with stress or corticosterone after a single memory reactivation blocks reconsolidation of a drug-related memory in rats. However, little is known regarding the effects of stress on reconsolidation of drug-related memories in human. So, we presumed that stress after memory reactivation can effectively inhibit drug-related memory by disrupting its reconsolidation in abstinent heroin addicts.
The current research was designed to investigate the amnesic effect on heroin-related memory by psychosocial stress immediately after heroin-related memory retrieval in abstinent heroin addicts. A wide range of psychological and physical responses were assessed to examine the effects of psychological stress on drug-related memory reconsolidation.
Materials and methods
Participants
The study was conducted according to human research guidelines and was approved by the Peking University Research Ethics Board. Informed consent was obtained from all subjects before testing, who were compensated for taking part in the study. Prior to enrollment, participants underwent thorough physical and psychiatric evaluations, including medical-history interview, physical examination, Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorder-IV (DSM-IV), and clinical laboratory tests (including electrocardiogram, blood chemistry, and urinalysis). Participants met DSM-IV criteria for heroin dependence. Exclusion criteria were: current DSM-IV diagnosis of any affective, anxiety, or psychotic disorder and current dependence on any substance besides heroin and nicotine (assessed by urine test and self-report). Twenty-one male inpatient abstinent heroin addicts between 24 and 45 years of age (mean±SE; 31.4±1.3) were recruited from Addiction Treatment Center of Beijing Ankang Hospital, Beijing, China. They had a mean lifetime history of regular heroin use of 7.1±0.8 (mean±SE) years and average heroin use 0.6±0.1 (mean±SE) g/day. Upon enrollment, they reported having been abstinent 3.9±0.3 (mean±SE) months. Urine drug screens were conducted three times per week.
Demographic characteristics of the participants are summarized in Table 1.
Table 1.
Demographic characteristics of participants
| Characteristic | Mean±SEM/N (%) |
|---|---|
| Age | 31.4±1.3 |
| Years of education | 9.2±0.5 |
| Dose used (g/day) | 0.6±0.1 |
| Duration of heroin use (years) | 7.1±0.8 |
| Months since last heroin use | 3.9±0.3 |
| Marital status/N (%) | |
| Married or cohabiting | 9/42.9 |
| Separated or divorced | 2/9.5 |
| Never married | 10/47.6 |
Procedure
This was a crossover study, consisting of a total of six sessions per participant, with each session occurring on a separate day. An interval of 4 weeks separated the first three sessions from the second three. On day 1, participants arrived in the morning (9:30–10:30 A.M.) and learned a list of 30 words (ten heroin-related positive words, ten heroin-related negative words, and ten neutral words; for details, see below). Participants were initially given 2 min to learn the words and subsequently asked to write down the words they were able to recall. The same learning trial was immediately repeated once (Kuhlmann et al. 2005b).
On day 2, participants filled out a mood questionnaire upon arrival in the morning. Thirty minutes later, participants were asked to write down the words they had learned on the previous day. Immediately, they were challenged with the Trier Social Stress Test (TSST; Kirschbaum et al. 1993) or underwent a control condition for 15 min. After that, participants filled out the same mood questionnaire again. Cortisol, heart rate, and blood pressure were measured before TSST or control condition (0 min), immediately afterward (+20 min), and 10 min later (+30 min).
On day 3, participants arrived at the laboratory in the morning at the same time, and participants were asked to write down the words they had learned on day 1. Cued recall was assessed immediately after free recall by randomly presenting part of the word on a piece of paper, with instructions to complete the word with the previously learned words. Finally, attention and working memory were assessed.
After 4 weeks, participants returned for days 4, 5, and 6, during which the same procedures were repeated with the alternate condition (TSST or control) counterbalanced among participants.
Task battery
TSST task
The TSST is a well-established paradigm to produce a significant hypothalamic-pituitary-adrenal response to stressful events in a clinical setting (Dickerson and Kemeny 2004; Kirschbaum et al. 1993; Kuhlmann et al. 2005b). The TSST began with a 2-min preparation period followed by 5 min of public speaking (a simulated job interview focusing on personal strengths and weaknesses) in front of a group of staff (containing one man and one woman wearing formal coats). Immediately following the 5 min of speaking, the participants were asked to do mental arithmetic (i.e., counting backward from 2,308 by 13) aloud in front of staff for 5 min (Dickerson and Kemeny 2004; Kuhlmann et al. 2005b; Mason 1968). In addition, the subjects are videotaped. The control condition consisted of a 5-min chat with a tester (about a movie or a book) and 5 min of rest. The challenges were given immediately after retrieval of words, but the subjects were not informed in advance.
Memory for words
Two parallel Chinese word lists were used in the current study. They were presented to participants on paper. The word list contained ten heroin-related positive words (e.g., heroin, syringe), ten heroin-related negative words (e.g., withdrawal, diarrhea), and ten neutral words (table, refrigerator). In a pilot study, 100 abstinent heroin patients (who did not participate in the present study) rated the emotional valence of the words on a seven-point scale ranging from negative (1) over neutral (4) to positive (7) (Kuhlmann et al. 2005a). Positive heroin words received an average rating of 6.48±0.42 (mean±SD), range 5.0–7.0; negative heroin words received an average rating of 1.23±0.28 (mean±SD), range 1.0–1.9, while neutral nondrug words received an average rating of 4.39±0.38 (mean±SD), range 3.6–5.2. This difference was significant (F(2,198)=5,635.55, p<0.001; post hoc t test, all p<0.01). This word list was used to test the effect of psychosocial stress on the reconsolidation of heroin-related words in abstinent heroin addicts.
Working memory (digit-span test)
This task was used to assess working memory in all participants. Several series of digits of increasing length were read to the participants, who were required to repeat each series. Each set length was tested twice. A forward and a backward condition were used. Participants earned one point for each correctly repeated set (Wechsler 1987).
d2 test of attention/psychomotor speed
From a series of the letters d and p, with one or two lines above and/or below each letter, the participants were asked to mark the d's with two lines as quickly and accurately as possible. A summary test score was calculated using the number of correctly marked d's minus the number of errors (Brickenkamp 1994).
Mood assessment
Participants completed an adjective checklist containing 32 words to assess bad versus good mood (16 items), alertness versus fatigue (eight items), and calmness versus restlessness (eight items). On a scale of 0 to 5, participants had to rate how much each word matched their current state (Kuhlmann et al. 2005a; Steyer et al. 1994).
Cortisol, blood pressure, and heart rate assessment
Saliva was collected using Salivette collection devices (Sarstedt, Nümbrecht, Germany). Free cortisol levels were measured using a commercially available immunoassay (Immuno-Biological Laboratories, Furui Company, Beijing, China). Interassay and intra-assay variations were <15%. Blood pressure was measured with a 9062D monitor (Baozhong Biotechnology Company, China). Heart rate was measured continuously with a sensor attached to the participant's finger (Shi et al. 2007).
Statistical analysis
Repeated measures analyses of variance (ANOVAs) with the within-subjects factors Challenge (stress and control) and Valence (positive, negative, and neutral) were used to compare word free recall on days 3 and 6. Repeated measures ANOVAs with the within-subjects factors Challenge (stress and control) and Time (0, +20, +30 min) were used to analyze cortisol levels, systolic pressure, diastolic pressure, and heart rate. Repeated measures ANOVAs with the within-subjects factors Challenge (stress and control) and Time (pre-challenge and post-challenge) were used to analyze subjective ratings. Post hoc pairwise comparisons were done with Fisher's least significant difference. Other cognitive measures (cued recall, working memory, and attention) were analyzed by paired t tests. p values less than or equal to 0.05 were considered statistically significant. The analyses were performed with SPSS 13.0.
Results
The effect of psychosocial stress on heroin-related word memory reconsolidation
The stress challenge significantly affected reconsolidation of the words in abstinent heroin users (the main effect of Challenge, F(2,40)=6.29; p<0.05, interaction of challenge by valence, F(2,40)=5.27; p<0.05). Post hoc analysis revealed that the stress condition reduced recall of heroin-related positive words (t(20)=3.52, p<0.05) and heroin-related negative words (t(20)=5.06, p<0.01) but not neutral words (t(20)=0.45, p>0.10; Fig. 1).
Fig. 1.
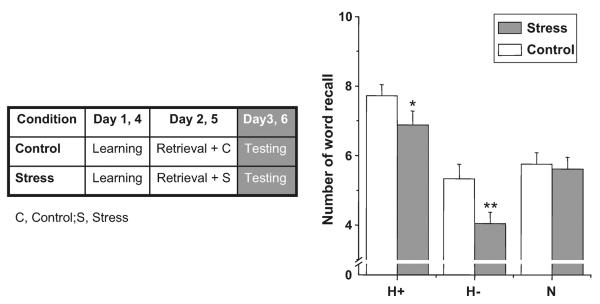
Recall of words on day 3 or 6. Results are expressed as number of word recalls. Error bars represent SE. H+ heroin-related positive words, H− heroin-related negative words, N neutral words. *p<0.05, **p<0.01 differences in post hoc t tests. For additional statistical analysis, see “Results”
Salivary cortisol levels
As expected, the stress challenge significantly increased salivary cortisol concentrations (main effect of Challenge, F(2,40)=10.06, p<0.01; interaction of Challenge by Time, F(2,40)=8.86, p<0.05). Post hoc t tests between the stress and control conditions showed significantly higher cortisol concentrations at +30 min (t(20)=−5.263, p<0.01; Fig. 2).
Fig. 2.
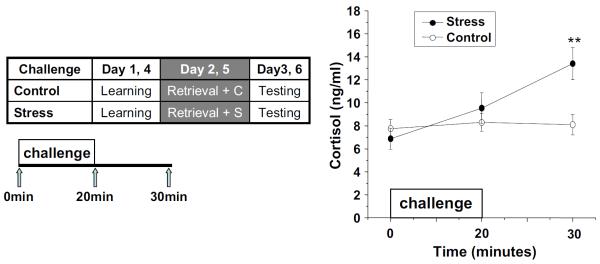
Salivary cortisol concentrations. “Challenge” refers to stress versus control condition (for details, see “Materials and methods.” **p<0.01 in post hoc t tests. Error bars represent SE
Blood pressure and heart rate measures
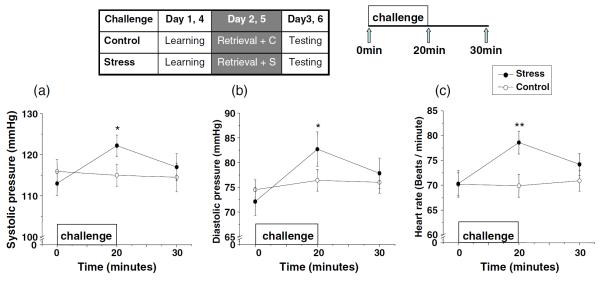
The stress condition (compared to the control condition) significantly increased systolic pressure (main effect of Challenge, F(2,40)=6.81, p<0.01; interaction of Challenge by Time, F(2,40)=5.03, p<0.05), diastolic pressure (main effect of Challenge, F(2,40)=3.14, p<0.05) and heart rate (main effect of Challenge, F(2,40)=25.4, p<0.01); interaction of Challenge by Time, F(2,40)=18.83, p<0.01). Post hoc t tests showed that significant differences were at +20 min (all p values <0.05, Fig. 3a–c).
Fig. 3.
Systolic pressure (a), diastolic pressure (b), and heart rate (c) in response to the stress or the control condition in participants with heroin dependence. “Challenge” refers to stress versus control condition (for details, see “Materials and methods”). *p<0.05 and **p<0.01 versus control in post hoc t tests. Error bars represent SE
Other cognitive measures
The stress challenge had no effect on measures of cued recall, working memory, or attention (all p values >0.10; Table 2).
Table 2.
Results of the working memory, attention test, and the mood scale
| Control condition | Stress condition | |
|---|---|---|
| Digital-span forward | 8.62±0.27 | 8.95±0.23 |
| Digital-span backward | 5.81±0.31 | 6.09±0.27 |
| Attention test | 209.28±7.48 | 194.71±10.25 |
| Calmness versus restlessness mood (before challenge) | 3.29±0.29 | 3.58±0.33 |
| Calmness versus restlessness mood (after challenge) | 3.42±0.27 | 2.73±0.28* |
All results are mean±SE. For statistical analysis, see “Results”
p<0.05 differences in paired t tests
Subjective ratings
A significant Challenge by Time interaction was observed only for calm versus restlessness (F(1,20)=8.72, p<0.05), reflecting a decrease in calm after the TSST (confirmed by a paired t test, p<0.05). For the other two scales, there was no Challenge by Time interaction (all p values >0.10; data not shown; Table 2).
Discussion
We found that acute stress immediately after the word list retrieval impairs recall of drug-related positive and negative but not neutral words in abstinent heroin addicts (Table 3 and Fig. 1). The impaired recall of drug-related words is consistent with the hypothesis that the process of reconsolidation of drug-related memory can be profoundly affected by amnesic effects induced by stress after the presentation of a reminder in abstinent heroin addicts.
Table 3.
Numbers of word recall for the three categories separately
| Control condition |
Stress condition |
|||||
|---|---|---|---|---|---|---|
| H+ | H− | N | H+ | H− | N | |
| First learning trail on day 1 | 5.26±0.39 | 3.57±0.33 | 4.01±0.34 | 5.43±0.36 | 3.85±0.44 | 4.10±0.37 |
| Second learning trial on day 1 | 7.91±0.31 | 6.09±0.44 | 6.40±0.38 | 7.95±0.39 | 5.96±0.43 | 6.37±0.42 |
| Recall of words on day 2 | 7.50±0.36 | 5.23±0.49 | 5.58±0.49 | 7.43±0.39 | 5.02±0.36 | 5.01±0.49 |
| Cued recall on day 3 | 9.13±0.39 | 7.50±0.41 | 7.67±0.35 | 9.04±0.27 | 7.24±0.42 | 7.43±0.28 |
All results are mean±SE. For statistical analysis, see “Results.” There are no significant differences between control and stress condition for all variances.
Numerous studies have demonstrated that stress plays an important but complex role in learning and memory. Stress or glucocorticoids have shown an inverse U-shaped dose–response relationship with consolidation (Lupien and McEwen 1997; Sandi et al. 1997; Roozendaal et al. 1999) and an inhibitory effect on retrieval (de Quervain et al. 1998; Roozendaal et al. 2002). In humans, acute stress or glucocorticoid administration has beneficial or detrimental effects, depending on several modulatory variables (Lupien and Lepage 2001; Wolf 2003). The previous studies have found a significant negative effect of acute stress or glucocorticoids on delayed memory retrieval in humans (Buss et al. 2004; de Quervain et al. 2000, 2003; Kuhlmann et al. 2005a; Payne et al. 2002; Wolf et al. 2001). Emotionally arousing words were more affected by stress than neutral words (Kuhlmann et al. 2005b). Moreover, moderate cortisol elevations in response to stress, most likely in combination with activation of the autonomous nervous system, can lead to negative effects on retrieval that are similar to those seen with oral cortisol treatment. Only a few groups have studied the effects of stress or glucocorticoids on reconsolidation of memory. Maroun and Akirav (2008) provided the first evidence that stress might have an inhibitory effect on the reconsolidation of memory. They found that, in habituated (low arousal level) and nonhabituated (high arousal level) rats, exposure to an out-of-context stressor impaired long-term reconsolidation of objective recognition memory (Maroun and Akirav 2008). In our recent study, morphine CPP was blocked in rats that received a cold-water stress or corticosterone after a single-trial reactivation by disrupting reconsolidation of morphine reward memory (Wang et al. 2008). In this study, we found that stress after drug-related memory retrieval significantly decreased its subsequent recall through impaired drug-related memory reconsolidation process, a result consistent with the previous studies that stress impairs reconsolidation of recognition memory (Maroun and Akirav 2008; Wang et al. 2008).
Two previous studies have observed enhanced consolidation of emotionally arousing material when compared with neutral material after cortisol or stress treatment (Buchanan and Lovallo 2001; Cahill et al. 2003). However, our present study found stress impaired heroin-related emotional word reconsolidation when compared with neutral words. Thus, the beneficial and detrimental effects of stress might be depend on whether stress was given after drug-related memory acquisition or after reactivation. The knowledge accumulated so far indicates that reconsolidation of a reactivated memory and consolidation of an initial learning are characterized by distinctive features (Alberini 2005). First, they involve different brain areas and circuits. Consolidation appears to require several areas that are not essential for reconsolidation, and reconsolidation might involve mostly modulatory systems. Second, consolidation and reconsolidation also differ in their temporal dynamics.
The previous finding documented that the interference following reactivation specifically disrupted subsequent reconsolidation rather than immediately reversing prior learning (Walker et al. 2003). In that study, the subjects were required to rehearse the materials learned on day 1 immediately before giving the interference on day 2. However, when retested again on the material on day 3, the material recall was decreased significantly. To confirm that inference following reactivation specifically disrupted subsequent reconsolidation rather than immediately reversing prior learning, another group of subjects was retested again directly after the interference on day 2 rather than on day 3 and showed no decrease compared to the earlier test (Walker et al. 2003). Taken together with previous findings, our results indicated that brief periods of recall of drug-related material return the memory to labile state, rendering it once again vulnerable to interference from stress and in need of reconsolidation.
A large body of evidences has demonstrated that drug-related memory can be prevented by inhibition of its reconsolidation (Bernardi et al. 2006; Kreek and Koob 1998; Lee et al. 2006; Miller and Marshall 2005; Popik et al. 2006). Memory consolidation and reactivation-induced memory “lability” appear to be dependent on some overlapping molecular mechanisms such as N-methyl-d-aspartate (NMDA) receptor activation, β-adrenergic receptor activation, and cyclic adenosine monophosphate response element-binding protein activation, whereas some manipulations can affect one process and not the other (Bourtchuladze et al. 1994; Cahill et al. 2000; Debiec and Ledoux 2004; Kida et al. 2002; Lee et al. 2004; McGaugh 2002; McGaugh and Roozendaal 2002; Przybyslawski et al. 1999; Przybyslawski and Sara 1997; Sara 2000a). Systemic NMDA antagonist MK-801 after memory reactivation can reduce the expression of amphetamine-CPP (Sadler et al. 2007). Zif268 ASO can induce blockade of drug memory reconsolidation and impair the maintenance of cocaine-seeking behavior (Baran et al. 2005; Bellani et al. 2006). In our current study as well as in previous studies (Wang et al. 2008), we found that stress or glucocorticoids also can block the drug-related memory reconsolidation.
An important conceptual advance in the past decade has been the process of drug addiction that is a pathological usurpation of neural processes that normally serve reward-related learning and memory (Hyman and Malenka 2001; Robbins and Everitt 1999). Maladaptive memories associated with the effects of drugs of abuse are possible to result in priming motivation to drug seeking. Primed motivation to engage in addictive behavior has been linked with increased “incentive salience” of target reinforcers (Robinson and Berridge 1993, 2001). From a cognitive science perspective, salience involves selective activation of concepts related to the target reinforcer in memory (Volkow et al. 2002). Such activation should be measurable in terms of recall of drug-relevant versus drug-irrelevant concepts (Zack and Poulos 2004). Therefore, in the present study, we used memory recall for previously learned words including drug-relevant and -irrelevant ones to investigate the effect of stress on heroin-related material memory in abstinent heroin addicts.
The finding of our study is that heroin-related words appear to be more affected by stress than neutral words. A probable mechanism of our finding is that post-reactivation stress inhibiting reconsolidation of the reactivated drug-related memory may act by cortisol, but neutral material is not involved in the impairment effect of cortisol. Stress and stress hormones have important roles in susceptibility to and maintenance of drug dependence. Patients with heroin dependence have been shown to have higher circulating levels of cortisol and altered hypothalamic–pituitary axis activity (Day et al. 1997; Kreek and Koob 1998) which might actually lead to persistent, pathologically strong, drug-related memories in addicts. Another probable interpretation of our findings is that the recall of emotionally arousing words appears to be more affected by stress than recall of neutral words if both word categories are presented within one word list. In this study, we found the arousing rating of these heroin-related words were significantly higher than that of neutral words. Previous studies have observed that there was significant enhancement or impairment effect of cortisol or stress on emotionally arousing material when compared with neutral material (Buchanan and Lovallo 2001; Cahill 2003; Kuhlmann et al. 2005a, b). In our study, the effects of cortisol were similar for positive as well as negative heroin-related memory, which suggests that emotional arousal rather than valence is the crucial aspect of the observed interactions. Pharmacological functional magnetic resonance imaging studies have shown that this effect of glucocorticoids on emotional arousal materials is dependent on β-adrenergic activation in the amygdala (Strange and Dolan 2004; van Stegeren et al. 2005). Amygdala is a critical brain site that mediates memory consolidation and reconsolidation (Debiec and LeDoux 2006; Doyere et al. 2007; Hellemans et al. 2006; Jin et al. 2007; Lee et al. 2005; Maroun and Akirav 2008; McGaugh 2002, 2004; McIntyre et al. 2003; Milekic and Alberini 2002; Nathan et al. 2004; Pare 2003). However, the role of the amygdala in heroin-related memory reconsolidation is not as well understood. In our previous studies, infusion of microinjection of RU28362 (a glucocorticoid receptor (GR) agonist) into the basolateral amygdala (BLA), disrupted reconsolidation of morphine reward memory (Wang et al. 2008). BLA may be a critical brain region involved in integrating the influences of stress or glucocorticoids on drug-related memory.
In addicted individuals, heroin “highs” are inexorably followed by a severe opiate withdrawal syndrome composed of somatic signs and negative affective states, such as dysphoria and depressed mood (O'Brien 1996). The negative affective states of opiate withdrawal dramatically motivate compulsive heroin-seeking behavior and opiate abuse (Baker et al. 2004; Koob and Le Moal 2001). The heroin-related negative words used in this study were almost withdrawal syndrome- and negative affective states-related ones, so the inhibition effect of stress on heroin-related negative word reconsolidation was also prospectively useful for therapy.
Interestingly, the inhibited effect of stress or stress hormone on subsequent drug-related memory recall is inconsistent with its effect on relapse (Shaham 1996; Shaham et al. 2000). The stress effects are modulated by whether the stress is administered before or after drug-related memory reactivation. In our study, administration of stress after retrieval test inhibits reconsolidation of drug-related memory.
The limitation of our study is whether the effect of stress on reconsolidation is specific for heroin-related words or would have also occurred for other emotional words as well. In the present study, the main purpose is to examine the effect of stress on reconsolidation of heroin-related emotional memory. Another limitation is that we did not assess heroin craving, so we do not know whether the mnemonic and physiological effects of the stressor were correlated with a decrease in craving (as we suspect they were). We can state, however, that the effects of the stressor were not so generalized as to change overall cognitive function (e.g., cued recall, attention, and working memory).
In conclusion, stress after drug-related memory retrieval significantly decreased its subsequent recall, likely through impaired the drug-related memory reconsolidation process. These studies provide a model for a therapeutic approach in the treatment of pathological drug-related memories in human research and suggest future experiments designed to explore the specific neurobiological mechanisms of this effect.
Acknowledgments
The authors wish to thank the National Basic Research Program of China (973 Program, 2007CB512302 and 2009CB522004), the National High Technology Research and Development Program of China (863 Program, 2006AA02Z4D1), and the Natural Science Foundation of China (nos. 30570576 and 30670713).
Footnotes
Disclosure/Conflict of Interest All of the authors declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci. 2000;3:74–79. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- Aoki C, Fujisawa S, Mahadomrongkul V, Shah PJ, Nader K, Erisir A. NMDA receptor blockade in intact adult cortex increases trafficking of NR2A subunits into spines, postsynaptic densities, and axon terminals. Brain Res. 2003;963:139–149. doi: 10.1016/s0006-8993(02)03962-8. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baran SE, Campbell AM, Kleen JK, Foltz CH, Wright RL, Diamond DM, Conrad CD. Combination of high fat diet and chronic stress retracts hippocampal dendrites. Neuroreport. 2005;16:39–43. doi: 10.1097/00001756-200501190-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellani R, Luecken LJ, Conrad CD. Peripubertal anxiety profile can predict predisposition to spatial memory impairments following chronic stress. Behav Brain Res. 2006;166:263–270. doi: 10.1016/j.bbr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Berger P, Gawin F, Kosten TR. Treatment of cocaine abuse with mazindol. Lancet. 1989;1:283. doi: 10.1016/s0140-6736(89)91299-3. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport. 2006;17:1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- Boccia MM, Blake MG, Acosta GB, Baratti CM. Memory consolidation and reconsolidation of an inhibitory avoidance task in mice: effects of a new different learning task. Neuroscience. 2005;135:19–29. doi: 10.1016/j.neuroscience.2005.04.068. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Brickenkamp R. Test d2: Aufmerksamkeits-Belastungs-Test; Handanweisungen. Hogrefe; Germany: 1994. [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Buss C, Wolf OT, Witt J, Hellhammer DH. Autobiographic memory impairment following acute cortisol administration. Psychoneuroendocrinology. 2004;29:1093–1096. doi: 10.1016/j.psyneuen.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Cahill L. Sex-related influences on the neurobiology of emotionally influenced memory. Ann N Y Acad Sci. 2003;985:163–173. doi: 10.1111/j.1749-6632.2003.tb07080.x. [DOI] [PubMed] [Google Scholar]
- Cahill L, Pham CA, Setlow B. Impaired memory consolidation in rats produced with beta-adrenergic blockade. Neurobiol Learn Mem. 2000;74:259–266. doi: 10.1006/nlme.1999.3950. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learn Mem. 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BA, Jaynes J, Misanin JR. Retention of a light-dark discrimination in rats of different ages. J Comp Physiol Psychol. 1968;66:467–472. doi: 10.1037/h0026360. [DOI] [PubMed] [Google Scholar]
- Day JC, Piazza PV, Le Moal M, Maccari S. Cocaine-induced increase in cortical acetylcholine release: interaction with the hypothalamo-pituitary-adrenal axis. Eur J Neurosci. 1997;9:1130–1136. doi: 10.1111/j.1460-9568.1997.tb01466.x. [DOI] [PubMed] [Google Scholar]
- Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: treatment implications for PTSD. Ann N Y Acad Sci. 2006;1071:521–524. doi: 10.1196/annals.1364.056. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Henke K, Aerni A, Treyer V, McGaugh JL, Berthold T, Nitsch RM, Buck A, Roozendaal B, Hock C. Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci. 2003;17:1296–1302. doi: 10.1046/j.1460-9568.2003.02542.x. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Fleshner M, Ingersoll N, Rose GM. Psychological stress impairs spatial working memory: relevance to electrophysiological studies of hippocampal function. Behav Neurosci. 1996;110:661–672. doi: 10.1037//0735-7044.110.4.661. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Doyere V, Debiec J, Monfils MH, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007;10:414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Everitt BJ, Lee JL. Disrupting reconsolidation of conditioned withdrawal memories in the basolateral amygdala reduces suppression of heroin seeking in rats. J Neurosci. 2006;26:12694–12699. doi: 10.1523/JNEUROSCI.3101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Jin XC, Lu YF, Yang XF, Ma L, Li BM. Glucocorticoid receptors in the basolateral nucleus of amygdala are required for postreactivation reconsolidation of auditory fear memory. Eur J Neurosci. 2007;25:3702–3712. doi: 10.1111/j.1460-9568.2007.05621.x. [DOI] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, Silva AJ. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The `Trier Social Stress Test'—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Kirschbaum C, Wolf OT. Effects of oral cortisol treatment in healthy young women on memory retrieval of negative and neutral words. Neurobiol Learn Mem. 2005a;83:158–162. doi: 10.1016/j.nlm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. J Neurosci. 2005b;25:2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DJ, Misanin JR, Miller RR. Recovery of memory following amnesia. Nature. 1968;220:704–705. doi: 10.1038/220704a0. [DOI] [PubMed] [Google Scholar]
- Loscertales M, Rose SP, Daisley JN, Sandi C. Piracetam facilitates long-term memory for a passive avoidance task in chicks through a mechanism that requires a brain corticosteroid action. Eur J Neurosci. 1998;10:2238–2243. doi: 10.1046/j.1460-9568.1998.00234.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: can't live with it, can't live without it. Behav Brain Res. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Maroun M, Akirav I. Arousal and stress effects on consolidation and reconsolidation of recognition memory. Neuropsychopharmacology. 2008;33:394–405. doi: 10.1038/sj.npp.1301401. [DOI] [PubMed] [Google Scholar]
- Mason JW. A review of psychoendocrine research on the pituitary-adrenal cortical system. Psychosom Med. 1968;30(Suppl):576–607. [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends Neurosci. 2002;25:456. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Power AE, Roozendaal B, McGaugh JL. Role of the basolateral amygdala in memory consolidation. Ann N Y Acad Sci. 2003;985:273–293. doi: 10.1111/j.1749-6632.2003.tb07088.x. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000a;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nat Rev Neurosci. 2000b;1:216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- Nathan SV, Griffith QK, McReynolds JR, Hahn EL, Roozendaal B. Basolateral amygdala interacts with other brain regions in regulating glucocorticoid effects on different memory functions. Ann N Y Acad Sci. 2004;1032:179–182. doi: 10.1196/annals.1314.015. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME. Glucocorticoid-induced impairment in declarative memory performance in adult humans. J Neurosci. 1994;14:2047–2053. doi: 10.1523/JNEUROSCI.14-04-02047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer JW, Craft S, Fucetola R, Moldin SO, Selke G, Paras L, Miller R. Glucose-induced increase in memory performance in patients with schizophrenia. Schizophr Bull. 1999;25:321–335. doi: 10.1093/oxfordjournals.schbul.a033381. [DOI] [PubMed] [Google Scholar]
- O'Brien CP. Drug addiction and drug abuse. In: Hardman JG, et al., editors. The Pharmacological Basis of Therapeutics. 9th ed. Mc Graw-Hill; New York: 1996. pp. 557–577. [Google Scholar]
- Pare D. Role of the basolateral amygdala in memory consolidation. Prog Neurobiol. 2003;70:409–420. doi: 10.1016/s0301-0082(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Payne JD, Nadel L, Allen JJ, Thomas KG, Jacobs WJ. The effects of experimentally induced stress on false recognition. Memory. 2002;10:1–6. doi: 10.1080/09658210143000119. [DOI] [PubMed] [Google Scholar]
- Popik P, Wrobel M, Bisaga A. Reinstatement of morphine-conditioned reward is blocked by memantine. Neuropsychopharmacology. 2006;31:160–170. doi: 10.1038/sj.npp.1300760. [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Sara SJ. Reconsolidation of memory after its reactivation. Behav Brain Res. 1997;84:241–246. doi: 10.1016/s0166-4328(96)00153-2. [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 1999;19:6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Williams CL, McGaugh JL. Glucocorticoid receptor activation in the rat nucleus of the solitary tract facilitates memory consolidation: involvement of the basolateral amygdala. Eur J Neurosci. 1999;11:1317–1323. doi: 10.1046/j.1460-9568.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor–cAMP/cAMP/PKA system in influencing memory consolidation. Eur J Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- Sadler R, Herzig V, Schmidt WJ. Repeated treatment with the NMDA antagonist MK-801 disrupts reconsolidation of memory for amphetamine-conditioned place preference. Behav Pharmacol. 2007;18:699–703. doi: 10.1097/FBP.0b013e3282effb81. [DOI] [PubMed] [Google Scholar]
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000a;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Strengthening the shaky trace through retrieval. Nat Rev Neurosci. 2000b;1:212–213. doi: 10.1038/35044575. [DOI] [PubMed] [Google Scholar]
- Shaham Y. Effect of stress on opioid-seeking behavior: evidence from studies with rats. Ann Behav Med. 1996;18:255–263. doi: 10.1007/BF02895287. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao LY, Epstein DH, Zhang XL, Lu L. Long-term methadone maintenance reduces protracted symptoms of heroin abstinence and cue-induced craving in Chinese heroin abusers. Pharmacol Biochem Behav. 2007;87:141–145. doi: 10.1016/j.pbb.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Steyer R, Schwenkmezger P, Notz P, Eid M. Testtheoretische Analysen des Mehrdimensionalen Befindlichkeitsfragebogens (MDBF) Diagnostica. 1994;40:320–328. [Google Scholar]
- Strange BA, Dolan RJ. Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proc Natl Acad Sci USA. 2004;101:11454–11458. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stegeren AH, Goekoop R, Everaerd W, Scheltens P, Barkhof F, Kuijer JP, Rombouts SA. Noradrenaline mediates amygdala activation in men and women during encoding of emotional material. Neuroimage. 2005;24:898–909. doi: 10.1016/j.neuroimage.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Wang XY, Zhao M, Ghitza UE, Li YQ, Lu L. Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci. 2008;28:5602–5610. doi: 10.1523/JNEUROSCI.0750-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale-revised. Harcourt Brace Jovanovich; TX: 1987. [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction. 1996;91:921–949. (discussion 951–65) [PubMed] [Google Scholar]
- Wolf OT. HPA axis and memory. Best Pract Res Clin Endocrinol Metab. 2003;17:287–299. doi: 10.1016/s1521-690x(02)00101-x. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Convit A, McHugh PF, Kandil E, Thorn EL, De Santi S, McEwen BS, de Leon MJ. Cortisol differentially affects memory in young and elderly men. Behav Neurosci. 2001;115:1002–1011. doi: 10.1037//0735-7044.115.5.1002. [DOI] [PubMed] [Google Scholar]
- Zack M, Poulos CX. Amphetamine primes motivation to gamble and gambling-related semantic networks in problem gamblers. Neuropsychopharmacology. 2004;29:195–207. doi: 10.1038/sj.npp.1300333. [DOI] [PubMed] [Google Scholar]