Abstract
A terminal alkyne is immobilized rapidly into a full monolayer by squishing a small volume of a solution of the alkyne between an azide-modified surface and a copper plate. The monolayer is covalently attached to the surface through a copper-catalyzed alkyne-azide cycloaddition (CuAAC) reaction, and the coverages of the immobilized electroactive alkyne species are quantified by cyclic voltammetry. A reaction time of less than twenty seconds is possible with no other reagents required. The procedure is effective in aerobic conditions using either an aqueous or aprotic organic solution of the alkyne (1–100 mM).
Introduction
The ability to reproducibly immobilize small molecules to planar surfaces through covalent bonds is critical for a diverse range of applications and fundamental studies. Covalent surface immobilization enables mechanistic investigation of catalytic systems,1–3 biosensor development,4 protein function5 and enzyme activity6 studies, and advanced diagnostic tools5,7. A variety of coupling methods are available for small-molecule covalent surface immobilization.6,8–10 Among these choices, the copper-catalyzed alkyne-azide cycloaddition (CuAAC) reaction is distinguished by the orthogonality of the azide and alkyne reagents to other functional groups, the reaction's high yield and rate, its regioselectivity for the 1,4-triazole product, the oxidative and hydrolytic stability of the resulting triazole linker and the electronic conjugation of the linker11. For these reasons, the CuAAC reaction has been adopted for a wide variety of applications including surface immobilization.12–14 CuAAC has proven to be a useful immobilization strategy on silica,15 titania,12,16 gold,17 diamond,18,19 and graphitic materials3,20,21. The CuAAC reaction is catalyzed by Cu(I) complexes, and therefore requires a reductant when carried out using Cu(II) catalyst precursors, a commonly used method. However, copper metal or Cu(I) oxides can also provide the Cu(I) cycloaddition catalyst.22–28
Here we report a remarkably simple, fast and robust procedure to covalently immobilize a monolayer of discrete molecules on a flat metal-oxide surface using the CuAAC reaction. This "squish-and-CuAAC" procedure requires only a freshly cleaned copper plate, a solution of a terminal alkyne and an azide-terminated metal oxide surface. No stoichiometric reductants or supporting ligands12,17 are needed in the solution, and the reaction may be run aerobically using either an aqueous or acetonitrile solution of the alkyne. Electrochemical quantification demonstrates that this convenient, additive-free procedure can immobilize a complete monolayer in less than twenty seconds.
Results and Discussion
Glass slides coated with fluorine-doped tin oxide (FTO) were used as electrodes. Indium-doped tin oxide (ITO) surfaces were equally effective. The electrodes were modified with organic azide groups using a version of a previously reported gas-phase silane deposition method.15 A copper plate was immersed in glacial acetic acid for 1 minute and then dried in a stream of dinitrogen gas. A 5 µL drop of the alkyne solution (typically 1 mM) was deposited on the copper plate, and a 1 cm2 azide-modified electrode was placed immediately face down over the drop, squishing the solution between the electrode and the copper plate. This volume of alkyne solution wetted the entire electrode surface, indicating an average separation between the electrode and the copper plate of about 50 µm and, in the case of a 1 mM solution, providing approximately a ten-fold excess of alkyne relative to the maximum observed coverage. After a period of 10 to 180 seconds, the electrode was removed from the copper plate, rinsed with isopropanol and analyzed electrochemically.
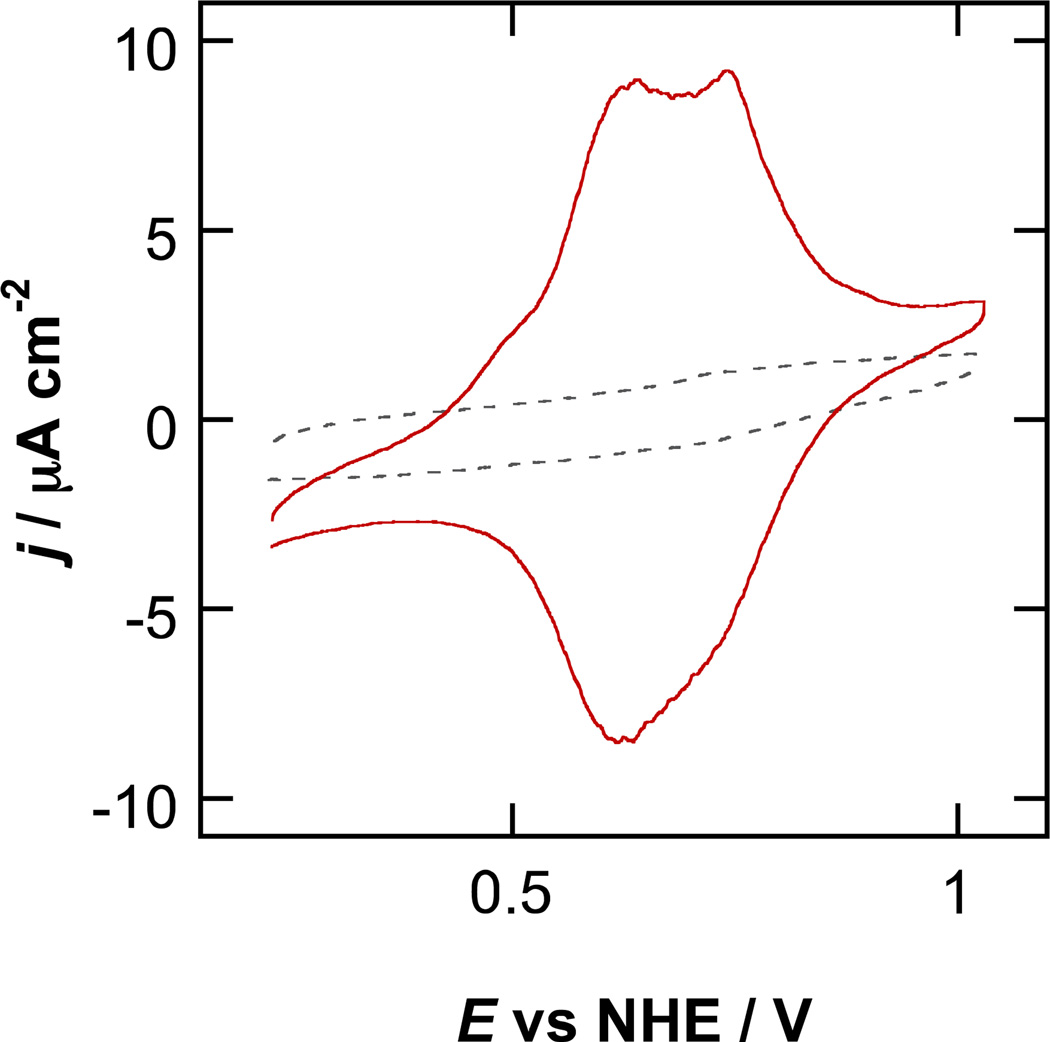
An electrode modified by this method, using a 1 mM aqueous solution of iron terpyridyl complex 1, shows a faradaic wave due to the oxidation and re-reduction of the Fe(II) center (Figure 1). The observed half-wave potential of 1.21 V vs. NHE agrees well with the potential observed in solution for the parent complex bearing no alkyne group.29 The same procedure carried out with an acetonitrile solution of 1 provided similar results (Figure S1). No faradaic wave is observed on the electrode when the procedure is carried out using the parent complex lacking an alkyne group in place of 1, or when a glass slide is used in place of the copper plate, regardless of the choice of solvent.
Figure 1.
Cyclic voltammogram of an azide-modified 1 cm2 FTO electrode after squish-and-CuAAC with 5 µL of 1 mM aqueous solution of 1 on a freshly etched copper plate (—), and after the same procedure on a glass slide (- - -) (180 second contact). (0.1 V s−1, 0.1 M aqueous HClO4.)
The anodic peak potentials vary linearly with scan rate (Figure S2), diagnostic of a redox species immobilized at the electrode.30 The nearly symmetric peak shape of immobilized 1 is characteristic of identical, independent, non-interacting redox species. The width of the faradaic wave of immobilized 1 at half its maximum height is approximately 120 mV, slightly larger than the ideal value of 90 mV.30 The splitting between the oxidative and reductive peaks is 35 mV at a scan rate of 0.1 V s−1. The highest coverage of 1 observed in our experiments is 1.6 × 1014 molecules cm−2, very close to the sterically limited coverage of 1.5 × 1014 molecules cm−2 calculated from the crystal structure dimensions31 of the parent complex.32 Based on the width of the undecyl chain linking the azide group to the oxide surface, the coverage of azide groups is estimated to be 5 × 1014 groups cm−2.33
Treatment of an azide-terminated electrode with an acetonitrile solution of ethynylferrocene (2) results in two reversible faradaic waves, originating from the oxidation and re-reduction of the Fe(II) center (Figure 2). The lower-potential couple (E1/2 = 0.62 V) is similar to previous reports of immobilized ferrocene17,20, and the splitting between the oxidative and reductive peaks for this couple is 10 mV at a scan rate of 0.1 V s−1. The second peak (E1/2 = 0.73 V) indicates the presence of a second electrochemical population of ferrocene groups in a more oxidizing environment, a phenomenon previously observed when ferrocene is immobilized onto electrodes at high coverages.34–36
Figure 2.
Cyclic voltammogram of an azide-modified 1 cm2 FTO electrode after squish-and-CuAAC with 5 µL of 1 mM acetonitrile solution of 2 on a freshly etched copper plate (—), and after the same procedure on a glass slide (- - -) (90 second contact). (0.1 V s−1, 0.1 M aqueous HClO4.)
The surface coverage of ferrocene groups approaches a maximum of approximately 4 × 1014 molecules cm−2 for alkyne concentrations of 10 mM and less. This exceeds the sterically limited closest-packing coverage of the ferrocene group by almost 50% (4 × 1014 molecules cm−2 vs. 2.7 × 1014 molecules cm−2)34, but is less than the expected coverage of the azide (5 × 1014 molecules cm−2). A reviewer offered one possible explanation of the higher than expected coverage: strong adsorption of bis(ferrrocenyl)diyne produced by oxidative alkyne homocoupling, a side reaction known to occur under some CuAAC reaction conditions.37 This product might however reasonably be expected to be extracted during the rinsing steps. Moreover, in a control procedure using rigorously anaerobic conditions, the resulting coverage of surface ferrocene groups was comparable within the variance of the measurement. Another possible explanation consistent with both the higher-than-expected coverage and the electrochemical heterogeneity is a very dense layer of covalently attached ferrocene groups at the surface, in which some groups are buried under others, with a total coverage exceeding that of a densely packed ferrocene monolayer. A higher-than-unity roughness factor may also make a contribution to the anomalously high coverage.
The squish-and-CuAAC method is very rapid. At an alkyne concentration of 10 mM, a hundredfold excess relative to the maximum observed coverage, the immobilization of ethynylferrocene is complete in less than 20 seconds with a coverage of 4 × 1014 molecules cm−2 (Figure 3a). The rate of surface coverage growth depends strongly on the alkyne concentration between 0.5 and 10 mM. The squish-and-CuAAC method is also effective for immobilizing fluorescein alkyne 3 (Figure S4).
Figure 3.
(a) Coverage of ferrocene groups on FTO electrodes, immobilized using the squish-and-CuAAC procedure, as a function of surface contact time for different concentrations of 2: 0.1 mM (⊠); 0.5 mM ( ); 1 mM (
); 1 mM ( ); 10 mM (
); 10 mM ( ); 100 mM (
); 100 mM ( ). Data points are averages of three to five individual measurements (see Figure S3 for complete data). The dotted line shows the coverage growth predicted by Equation 1 with k = 25 M−1 s−1 and [alkyne] = 1 mM. (b) Coverage growth of ferrocene groups on FTO electrodes at a freshly etched copper plate using the squish-and-CuAAC procedure with a 1 mM solution of 2 in acetonitrile, with 50 µm (
). Data points are averages of three to five individual measurements (see Figure S3 for complete data). The dotted line shows the coverage growth predicted by Equation 1 with k = 25 M−1 s−1 and [alkyne] = 1 mM. (b) Coverage growth of ferrocene groups on FTO electrodes at a freshly etched copper plate using the squish-and-CuAAC procedure with a 1 mM solution of 2 in acetonitrile, with 50 µm ( ), 250 µm (
), 250 µm ( ), and 500 µm (
), and 500 µm ( ) separating the copper plate and the electrode.
) separating the copper plate and the electrode.
The strong dependence of the coverage growth rate on the concentration of the alkyne solution (Figure 3a) is consistent with the solution-phase CuAAC reaction38,40 and its prevailing mechanistic hypothesis12,38. The growth rate observed with a 1 mM solution is described reasonably by the expression
| (1) |
with k = 25 M−1 s−1 and Γ∞ = 4.3 × 1014 cm−2 (Figure 3a). This expression describes a surface CuAAC reaction that is first-order in both alkyne and the surface azide. However, at very low alkyne concentrations (0.1 mM), where the alkyne is depleted,39 and at very high alkyne concentrations(100 mM) other processes should become kinetically important.40
Surface immobilization was carried out using a range of distances between the copper plate and the electrode. In the squish-and-CuAAC procedure, the thin layer of solution squished between the copper plate and the modified surface is approximately 50 microns thick.41 The reaction was also carried out with inter-surface distances of 250 and 500 microns, using spacers of known thickness. When the distance was widened from 50 to 500 microns, the time required to reach a coverage of 2 × 1014 molecules cm−2 increased more than ten-fold, from twenty to three hundred seconds (Figure 3b). Presumably this is because the rate of reaction becomes limited by the concentration of the copper catalyst at greater distances from the copper source.
The squish-and-CuAAC procedure exploits the convenience of using copper metal as a catalyst source for the CuAAC reaction. Copper metal is already known as a catalyst source for the CuAAC reaction from reports using copper turnings,22 copper particles,23,25,42 and flat copper surfaces26–28. In previous reports using a copper surface to initiate CuAAC, generation of the Cu(I) catalyst was attributed to aerobic oxidation of the copper surface, which we presume occurs here.26,28 Rapid aerobic oxidation of a freshly etched Cu(0) surface, following the same acetic acid cleaning procedure employed in our experiments, has been characterized by XPS and Auger spectroscopy.43,44 Acetic acid is known to associate with copper surfaces in vacuum conditions,45 and presumably remains adsorbed on the copper plate at the beginning of the squish-and-CuAAC procedure. This is unlikely to hinder the CuAAC reaction: Cu(I)(OAc) is a known source of catalytically active Cu(I) for CuAAC,46 and the reaction may in fact be catalyzed by acetate-supported binuclear copper centers37,47. Despite some claims to the contrary,48–50 the coupling of azides and terminal alkynes at ambient temperatures is generally accepted to require a Cu(I) catalyst. In the case of the squish-and-CuAAC procedure reported here, no surface coverage results when the procedure is carried out against a glass slide instead of a copper plate (Figure 1 and Figure 2). This result is consistent with the observation of Spruell et al. concerning the interfacial CuAAC reaction that "reaction without a [copper] catalyst results in very limited conversion, even over prolonged reaction times."26
Interfacial CuAAC has been carried out with micron-scale spatial resolution by Spruell et al. using patterned stamps for microcontact printing,26 and by Devaraj et al. using interdigitated electrodes51. Paxton et al. achieved 50 nm resolution when a copper-coated AFM tip was used to catalyze the interfacial CuAAC reaction.52 For those specific systems, the spatial resolution of the resulting pattern implies that the active Cu(I) catalyst does not diffuse over micron-scale distances. In contrast, surface CuAAC activity is observed in the squish-and-CuAAC system even when the catalyst source is separated from the reaction site by hundreds of microns, indicating that the active Cu(I) catalyst can diffuse across this distance under the squish-and-CuAAC conditions. The reason for this apparent difference in diffusion of the active catalyst is currently unknown.
Electrochemical analysis allows the use of quantified surface coverages when investigating the concentration-dependent kinetics of the surface CuAAC reaction. Recent work has probed the structure-dependent kinetics of the surface CuAAC reaction using fluorescence intensity as an indirect proxy for surface density.50 The kinetics of different reaction conditions were examined by Spruell et al. using electrochemical surface quantification, but the concentration-dependent investigation of a single reaction system was not studied.26 In this work, we have applied electrochemical quantification to the systematic concentration-dependent kinetic examination of a single reaction system.
Finally, the squish-and-CuAAC procedure provides the fastest CuAAC-based monolayer formation currently known – less than 20 seconds using a 10 mM alkyne solution. Several recent studies using potentially self-quenching and therefore nonlinear fluorescence detection report alkyne immobilization in minutes.26,48,53,54 Spruell et al. reported that an electrochemically quantified full monolayer was immobilized in 60 minutes using microcontact printing, a close-contact technique similar to the squish-and-CuAAC procedure.26 Under comparable conditions of 1 mM alkyne, we observed complete monolayer formation of 2 in less than two minutes (Figure 3).
Conclusion
The squish-and-CuAAC immobilization procedure is convenient for rapid immobilization of dense monolayers on flat surfaces. The Cu(I) catalyst for the CuAAC reaction is presumably generated by spontaneous aerobic oxidation at the surface of bulk copper metal, consistent with control experiments and with previous analysis of surface copper oxidation. This procedure is effective using aqueous as well as aprotic organic solutions, extending its use to biologically important species. It tolerates ordinary aerobic conditions, and requires no accelerating ligand or chemical reductant. Electrochemical quantification of surface coverage indicates that a full monolayer is immobilized in seconds, and the rate of the reaction drops off slowly with increasing distance between the copper plate and the modified surface.
Supplementary Material
Scheme 1.
Interfacial CuAAC reaction in the presence of a copper surface, and alkynes immobilized using the additive-free squish-and-CuAAC procedure.
Acknowledgements
We thank Eric D. Stenehjem and Ali Hosseini for helpful discussions. We also thank Dr. Andrew Olson and the Stanford Neuroscience Microscopy Service, supported by NIH NS069375, for assistance with fluorescence microscopy. This work was supported by the Global Climate and Energy Project at Stanford University and by NIH #GM050730.
Footnotes
Supporting Information
Experimental details, complete surface coverage data on concentration-dependent rate of immobilization, and fluorescence intensity data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Vannucci AK, Hull JF, Chen Z, Binstead RA, Concepcion JJ, Meyer TJ. Water Oxidation Intermediates Applied to Catalysis: Benzyl Alcohol Oxidation. J. Am. Chem. Soc. 2012;134:3972. doi: 10.1021/ja210718u. [DOI] [PubMed] [Google Scholar]
- 2.Decreau RA, Collman JP, Hosseini A. Electrochemical applications. How click chemistry brought biomimetic models to the next level: electrocatalysis under controlled rate of electron transfer. Chem. Soc. Rev. 2010;39:1291. doi: 10.1039/b901972n. [DOI] [PubMed] [Google Scholar]
- 3.McCrory CCL, Devadoss A, Ottenwaelder X, Lowe RD, Stack TDP, Chidsey CED. Electrocatalytic O2 Reduction by Covalently Immobilized Mononucelar Copper(I) Complexes: Evidence for a Binuclear Cu2O2 Intermediate. J. Am. Chem. Soc. 2011;133:3696. doi: 10.1021/ja106338h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samanta D, Sarkar A. Immobilization of bio-macromolecules on self-assembled monolayers: methods and sensor applications. Chem. Soc. Rev. 2011;40:2567. doi: 10.1039/c0cs00056f. [DOI] [PubMed] [Google Scholar]
- 5.Wong LS, Khan F, Micklefield J. Selective Covalent Protein Immobilization: Strategies and Applications. Chem. Rev. 2009;109:4025. doi: 10.1021/cr8004668. [DOI] [PubMed] [Google Scholar]
- 6.Winssinger N, Pianowski Z, Debaene F. Probing biology with small molecule microarrays (SMM) Top. Curr. Chem. 2007;278:311. [Google Scholar]
- 7.Balamurugan S, Obubuafo A, Soper SA, Spivak DA. Surface immobilization methods for aptamer diagnostic applications. Anal. Bioanal. Chem. 2008;390:1009. doi: 10.1007/s00216-007-1587-2. [DOI] [PubMed] [Google Scholar]
- 8.Chi YS, Lee JK, Lee KB, Kim DJ, Choi IS. Biosurface organic chemistry: Interfacial chemical reactions for applications to nanobiotechnology and biomedical sciences. Bull. Korean Chem. Soc. 2005;26:361. [Google Scholar]
- 9.Jonkheijm P, Weinrich D, Koehn M, Engelkamp H, Christianen PCM, Kuhlmann J, Maan JC, Nuesse D, Schroeder H, Wacker R, Breinbauer R, Niemeyer CM, Waldmann H. Photochemical surface patterning by the thiol-ene reaction. Angew. Chem. Int. Ed. 2008;47:4421. doi: 10.1002/anie.200800101. [DOI] [PubMed] [Google Scholar]
- 10.Manova RK, Pujari SP, Weijers CAGM, Zuilhof H, van Beek TA. Copper-Free Click Biofunctionalization of Silicon Nitride Surfaces via Strain-Promoted Alkyne-Azide Cycloaddition Reactions. Langmuir. 2012;28:8651. doi: 10.1021/la300921e. [DOI] [PubMed] [Google Scholar]
- 11.Devaraj NK, Decreau RA, Ebina W, Collman JP, Chidsey CED. Rate of interfacial electron transfer through the 1,2,3-triazole linkage. J. Phys. Chem. B. 2006;110:15955. doi: 10.1021/jp057416p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meldal M, Tornoe CW. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008;108:2952. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 13.Nandivada H, Jiang XW, Lahann J. Click chemistry: Versatility and control in the hands of materials scientists. Adv. Mater. 2007;19:2197. [Google Scholar]
- 14.Gooding JJ, Ciampi S. The molecular level modification of surfaces: from self-assembled monolayers to complex molecular assemblies. Chem. Soc. Rev. 2011;40:2704. doi: 10.1039/c0cs00139b. [DOI] [PubMed] [Google Scholar]
- 15.Lowe RD, Pellow MA, Stack TDP, Chidsey CED. Deposition of Dense Siloxane Monolayers from Water and Trimethoxyorganosilane Vapor. Langmuir. 2011;27:9928. doi: 10.1021/la201333y. [DOI] [PubMed] [Google Scholar]
- 16.Watson MA, Lyskawa J, Zobrist C, Fournier D, Jimenez M, Traisnel M, Gengembre L, Woisel P. A "Clickable" Titanium Surface Platform. Langmuir. 2010;26:15920. doi: 10.1021/la102688m. [DOI] [PubMed] [Google Scholar]
- 17.Collman JP, Devaraj NK, Chidsey CED. "Clicking" functionality onto electrode surfaces. Langmuir. 2004;20:1051. doi: 10.1021/la0362977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruther RE, Rigsby ML, Gerken JB, Hogendoorn SR, Landis EC, Stahl SS, Hamers RJ. Highly Stable Redox-Active Molecular Layers by Covalent Grafting to Conductive Diamond. J. Am. Chem. Soc. 2011;133:5692. doi: 10.1021/ja200210t. [DOI] [PubMed] [Google Scholar]
- 19.Yao SA, Ruther RE, Zhang L, Franking RA, Hamers RJ, Berry JF. Covalent Attachment of Catalyst Molecules to Conductive Diamond: CO2 Reduction Using “Smart” Electrodes. J. Am. Chem. Soc. 2012;134:15632. doi: 10.1021/ja304783j. [DOI] [PubMed] [Google Scholar]
- 20.Devadoss A, Chidsey CED. Azide-modified graphitic surfaces for covalent attachment of alkyne-terminated molecules by "click" chemistry. J. Am. Chem. Soc. 2007;129:5370. doi: 10.1021/ja071291f. [DOI] [PubMed] [Google Scholar]
- 21.Landis EC, Hamers RJ. Covalent Grafting of Redox-Active Molecules to Vertically Aligned Carbon Nanofiber Arrays via "Click" Chemistry. Chem. Mater. 2009;21:724. [Google Scholar]
- 22.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Alonso F, Moglie Y, Radivoy G, Yus M. Multicomponent Synthesis of 1,2,3-Triazoles in Water Catalyzed by Copper Nanoparticles on Activated Carbon. Adv. Synth. Catal. 2010;352:3208. [Google Scholar]
- 24.Veerakumar P, Velayudham M, Lu K-L, Rajagopal S. Highly dispersed silica-supported nanocopper as an efficient heterogeneous catalyst: application in the synthesis of 1,2,3-triazoles and thioethers. Catal. Sci. Tech. 2011;1:1512. [Google Scholar]
- 25.Shao CW, Zhu R, Luo S, Zhang Q, Wang XY, Hu YF. Copper(I) oxide and benzoic acid 'on water': a highly practical and efficient catalytic system for copper(I)-catalyzed azide-alkyne cycloaddition. Tetrahedron Lett. 2011;52:3782. [Google Scholar]
- 26.Spruell JM, Sheriff BA, Rozkiewicz DI, Dichtel WR, Rohde RD, Reinhoudt DN, Stoddart JF, Heath JR. Heterogeneous Catalysis through Microcontact Printing. Angew. Chem. Int. Ed. 2008;47:9927. doi: 10.1002/anie.200803480. [DOI] [PubMed] [Google Scholar]
- 27.Bogdan AR, James K. Efficient Access to New Chemical Space Through Flow-Construction of Druglike Macrocycles Through Copper-Surface-Catalyzed Azide-Alkyne Cycloaddition Reactions. Chem. Eur. J. 2010;16:14506. doi: 10.1002/chem.201002215. [DOI] [PubMed] [Google Scholar]
- 28.Diaz DD, Punna S, Holzer P, Mcpherson AK, Sharpless KB, Fokin VV, Finn MG. Click chemistry in materials synthesis. 1. Adhesive polymers from coppercatalyzed azide-alkyne cycloaddition. J. Polym. Sci. Part A: Polym. Chem. 2004;42:4392. [Google Scholar]
- 29.Chow HS, Constable EC, Housecroft CE, Neuburger M, Schaffner S. Ligands and complexes with supramolecular aromatic-aromatic interactions: iron(II) and ruthenium(II) complexes of 2,2' : 6', 2''-terpyridines with pendant naphthalene groups. Dalton Trans. 2006:2881. doi: 10.1039/b515610f. [DOI] [PubMed] [Google Scholar]
- 30.Bard AJ, Faulkner LR. Electrochemical Methods: Fundamentals and Applications. 2nd ed. New York: John Wiley & Sons, Inc.; 2001. [Google Scholar]
- 31.Christiansen L, Hendrickson DN, Toftlund H, Wilson SR, Xie C-L. Fepyridyl-TACN crystal structure. Inorg. Chem. 1986;25:2813. [Google Scholar]
- 32.The surface coverage of the immobilized redox species, Γ, was calculated from the faradaic charge Q, measured as the integrated cathodic current above an interpolated baseline, according to the relation Γ = Q/enA, where e is the charge on the electron, n is the number of electrons in the redox process, and A is the area of the electrode.
- 33.Ulman A, editor. Organic Thin Films and Surfaces : Directions for the Nineties. Vol. 20. San Diego: Academic Press; 1995. [Google Scholar]
- 34.Chidsey CED, Bertozzi CR, Putvinski TM, Mujsce AM. Coadsorption of ferrocene-terminated and unsubstituted alkanethiols on gold: electroactive self-assembled monolayers. J. Am. Chem. Soc. 1990;112:4301. [Google Scholar]
- 35.Vercelli B, Zotti G, Schiavon G, Zecchin S, Berlin A. Adsorption of hexylferrocene phosphonic acid on indium-tin oxide electrodes. Evidence of strong interchain interactions in ferrocene self-assembled monolayers. Langmuir. 2003;19:9351. [Google Scholar]
- 36.Peerce PJ, Bard AJ. Polymer-Films on Electrodes .2. Film Structure and Mechanism of Electron-Transfer with Electrodeposited Poly(Vinylferrocene) J. Electroanal. Chem. 1980;112:97. [Google Scholar]
- 37.Kuang GC, Guha PM, Brotherton WS, Simmons JT, Stankee LA, Nguyen BT, Clark RJ, Zhu L. Experimental Investigation on the Mechanism of Chelation-Assisted, Copper(II) Acetate-Accelerated Azide-Alkyne Cycloaddition. J. Am. Chem. Soc. 2011;133:13984. doi: 10.1021/ja203733q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rolff M, Schottenheim J, Decker H, Tuczek F. Copper-O2 reactivity of tyrosinase models towards external monophenolic substrates: molecular mechanism and comparison with the enzyme. Chem. Soc. Rev. 2011 doi: 10.1039/c0cs00202j. [DOI] [PubMed] [Google Scholar]
- 39.Rodionov VO, Fokin VV, Finn MG. Mechanism of the ligand-free Cu-I-catalyzed azide-alkyne cycloaddition reaction. Angew. Chem. Int. Ed. 2005;44:2210. doi: 10.1002/anie.200461496. [DOI] [PubMed] [Google Scholar]
- 40.The continuous increase in coverage observed with 100 mM alkyne was interpreted as an adsorption process distinct from the cycloaddition reactions.
- 41.This estimate is based on the observation that 5 µL of solution is required to completely wet a 1 cm2 electrode when squished between the copper plate and the electrode.
- 42.Alonso F, Moglie Y, Radivoy G, Yus M. Unsupported Copper Nanoparticles in the 1,3-Dipolar Cycloaddition of Terminal Alkynes and Azides. Eur. J. Org. Chem. 2010:1875. [Google Scholar]
- 43.Chavez KL, Hess DW. A novel method of etching copper oxide using acetic acid. J. Electrochem. Soc. 2001;148:G640. [Google Scholar]
- 44.The squish-and-CuAAC procedure is effective even when the copper surface has not been etched with acetic acid. The etching step was included in the procedure to ensure reproducible reactivity.
- 45.Bowker M, Madix RJ. The Adsorption and Oxidation of Acetic-Acid and Acetaldehyde on Cu(110) Appl. Surf. Sci. 1981;8:299. [Google Scholar]
- 46.Zhang Q, Wang X, Cheng C, Zhu R, Liu N, Hu Y. Copper(I) acetate-catalyzed azide–alkyne cycloaddition for highly efficient preparation of 1-(pyridin-2-yl)-1,2,3-triazoles. Org. Biomol. Chem. 2012;10:2847. doi: 10.1039/c2ob06942c. [DOI] [PubMed] [Google Scholar]
- 47.Shao C, Cheng G, Su D, Xu J, Wang X, Hu Y. Copper(I) Acetate: a Structurally Simple but Highly Efficient Dinuclear Catalyst for Copper-Catalyzed Azide-Alkyne Cycloaddition. Adv. Synth. Catal. 2010;352:1587. [Google Scholar]
- 48.Rozkiewicz DI, Janczewski D, Verboom W, Ravoo BJ, Reinhoudt DN. "Click" chemistry by microcontact printing. Angew. Chem. Int. Ed. 2006;45:5292. doi: 10.1002/anie.200601090. [DOI] [PubMed] [Google Scholar]
- 49.Rozkiewicz DI, Gierlich J, Burley GA, Gutsmiedl K, Carell T, Ravoo BJ, Reinhoudt DN. Transfer printing of DNA by "Click" chemistry. ChemBioChem. 2007;8:1997. doi: 10.1002/cbic.200700402. [DOI] [PubMed] [Google Scholar]
- 50.Mehlich J, Ravoo BJ. Click chemistry by microcontact printing on self-assembled monolayers: A structure–reactivity study by fluorescence microscopy. Org. Biomol. Chem. 2011;9:4108. doi: 10.1039/c1ob05187c. [DOI] [PubMed] [Google Scholar]
- 51.Devaraj NK, Dinolfo PH, Chidsey CED, Collman JP. Selective Functionalization of Independently Addressed Microelectrodes by Electrochemical Activation and Deactivation of a Coupling Catalyst. J. Am. Chem. Soc. 2006;128:1794. doi: 10.1021/ja058380h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paxton WF, Spruell JM, Stoddart JF. Heterogeneous Catalysis of a Copper-Coated Atomic Force Microscopy Tip for Direct-Write Click Chemistry. J. Am. Chem. Soc. 2009;131:6692. doi: 10.1021/ja9015974. [DOI] [PubMed] [Google Scholar]
- 53.Michel O, Ravoo BJ. Carbohydrate Microarrays by Microcontact "Click" Chemistry. Langmuir. 2008;24:12116. doi: 10.1021/la802304w. [DOI] [PubMed] [Google Scholar]
- 54.Wendeln C, Ravoo BJ. Surface Patterning by Microcontact Chemistry. Langmuir. 2012;28:5527. doi: 10.1021/la204721x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.