Abstract
Despite improvements in cancer therapies in the past 50 years, neuroblastoma remains a devastating clinical problem and a leading cause of childhood cancer deaths. Advances in treatments for children with high-risk neuroblastoma have, until recently, involved addition of cytotoxic therapy to dose-intensive regimens. In this era of targeted therapies, substantial efforts have been made to identify optimal targets for different types of cancer. The discovery of hereditary and somatic activating mutations in the oncogene ALK has now placed neuroblastoma among other cancers, such as melanoma and non-small-cell lung cancer (NSCLC), which benefit from therapies with oncogene-specific small-molecule tyrosine kinase inhibitors. Crizotinib, a small-molecule inhibitor of ALK, has transformed the landscape for the treatment of NSCLC harbouring ALK translocations and has demonstrated activity in preclinical models of ALK-driven neuroblastomas. However, inhibition of mutated ALK is complex when compared with translocated ALK and remains a therapeutic challenge. This Review discusses the biology of ALK in the development of neuroblastoma, preclinical and clinical progress with the use of ALK inhibitors and immunotherapy, challenges associated with resistance to such therapies and the steps being taken to overcome some of these hurdles.
Introduction
Neuroblastoma is an embryonal tumour of the autonomic nervous system that is most commonly diagnosed in early childhood and accounts for 10% of paediatric cancer mortality.1 It is the most frequent form of malignancy diagnosed within the first year of age, and represents a spectrum of diseases with diverse and often dramatic clinical behaviour, as well as distinct biological features in different subsets of patients.2,3 Neuroblastoma constitutes the highest proportion of human cancer cases that undergo spontaneous regression even when metastasis forms,4–6 but it also accounts for a disproportionate amount of childhood cancer morbidity and mortality.
High-risk neuroblastomas have a near-diploid or near-tetraploid karyotype and are characterized by complex chromosomal aberrations. A subset of tumours are characterized by deletions in chromosomes 1p and 11q,7 but to date, no tumour suppressor genes have been identified in these regions. Another major subgroup of high-risk neuroblastomas have a high level of amplification of the MYCN oncogene, a biomarker of poor prognosis8,9 that when aberrantly expressed in neuroblastomas, is challenging to target pharmacologically.
Neuroblastoma is one of the few solid cancers in which a randomized clinical trial has shown that myeloablative consolidation therapy with autologous stem-cell rescue results in substantial improvement in event-free survival (EFS).10 In addition, findings from the 1980s show that neuroblastoma cell lines can be induced to terminally differentiate when exposed to retinoid compounds.11,12 This observation prompted a randomized clinical trial in which isotretinoin (a retinoid compound and derivative of vitamin A) was used after myeloablative therapy and reduced the risk of relapse among children with high-risk neuroblastoma.10 Efficacy of stem cell transplant and isotretinoin together improved survival by ~20% compared to patients who received chemotherapy alone. These findings have motivated studies with increased dose-intensity in both induction and consolidation therapies during the past 15 years; one such study is the ongoing phase III trial testing whether tandem myelo-ablative chemotherapy improves EFS for children with high-risk neuroblastoma.13
Survivors of neuroblastoma are often left with considerable long-term adverse effects, many of which can be life-threatening.1 While increasing the intensity of therapies could improve outcomes, it can be contended that no substantial changes in survival rates of children with neuroblastoma will be observed until new treatment strategies can be developed targeting fundamental molecular alterations in the tumour cells.
Until recently, survival of high-risk patients has been around 35%, with only modest improvements in the past few years.10 The Children’s Oncology Group recently reported the results of a randomized clinical trial of a new dose-intensive immunotherapeutic regimen using ch14.18, a monoclonal antibody against disialoganglioside GD2, in combination with alternating cycles of cytokines GM-CSF or IL-2 added to a regimen of isotretinoin.14 The 2-year EFS was dramatically improved from 46% to 66% in immunotherapy-treated patients compared with those who received isotretinoin alone. Unfortunately, no other innovative treatment approaches have been used in frontline therapy. For several years, multiple tractable molecular targets have been investigated in neuroblastoma, including the neurotrophic tyrosine kinase receptor pathways,15–17 c-Kit and PDGFR,18,19 angiogenic factors such as VEGF,20–22 histone deacetylases,23,24 and programmed cell death pathways;25 however, there is limited biological rationale and evidence of preclinical efficacy to help prioritize drug development targeting these molecules. To improve the overall survival in patients with neuroblastoma demands additional novel treatment approaches targeting validated molecular and genetic abnormalities underlying tumorigenesis and/or disease progression.
After decades of research into the neuroblastoma predisposition genes using positional cloning approaches, the discovery of activating mutations in the tyrosine kinase domain of the ALK oncogene as the leading cause for most cases of hereditary neuroblastoma, and the finding that these mutations are also somatically acquired in 7–10% of sporadic cases have provided the first tractable molecular target in this disease. ALK is a receptor tyrosine kinase (RTK) involved in the formation of several other human cancers, most notably anaplastic large-cell lymphoma (ALCL)—a cancer typically of childhood—and non-small-cell lung cancer (NSCLC), and could have a role in the pathogenesis of other unknown types of cancers. Constitutive ALK signalling induces cell transformation in vitro and in vivo by controlling key cellular processes, such as cell-cycle progression, survival, cell migration and cell shaping.26–28 ALK, therefore, represents an attractive target for innovative therapies that are based on selective small-molecule inhibitors and target the tyrosine kinase activity of ALK. In this Review, we describe the scientific basis for targeting ALK aberrations in neuroblastoma, the emerging challenges and how research has influenced strategies to study ALK inhibition in the clinic.
ALK structure and signalling
ALK is an orphan RTK normally expressed only in the developing embryonic and neonatal brain,29,30 minimizing the potential risk of ALK inhibition in young children. ALK is a member of the insulin receptor superfamily and was first discovered in an anaplastic large-cell non- Hodgkin’s lymphoma in 1994 in a translocated form fused to the N-terminal of nucleophosmin resulting in constitutive activation of ALK tyrosine kinase domain.31 Over a dozen ALK fusion partners have since been identified including RANBP2 in inflammatory myofibroblastic tumour, EML4 in 3–7% of NSCLC and renal cancer,31,32 and TPM3 in renal cancer,33 which make ALK one of the few RTKs implicated as an oncogene in both haematopoietic and non-haematopoietic malignancies.34 We have shown that full-length ALK protein and mRNA are expressed in the majority of primary neuroblastoma tumours,29 and others have suggested an oncogenic role for full-length ALK in lung cancer,35 thyroid cancer,36 glioblastoma37 and rhabdomyosarcoma.38
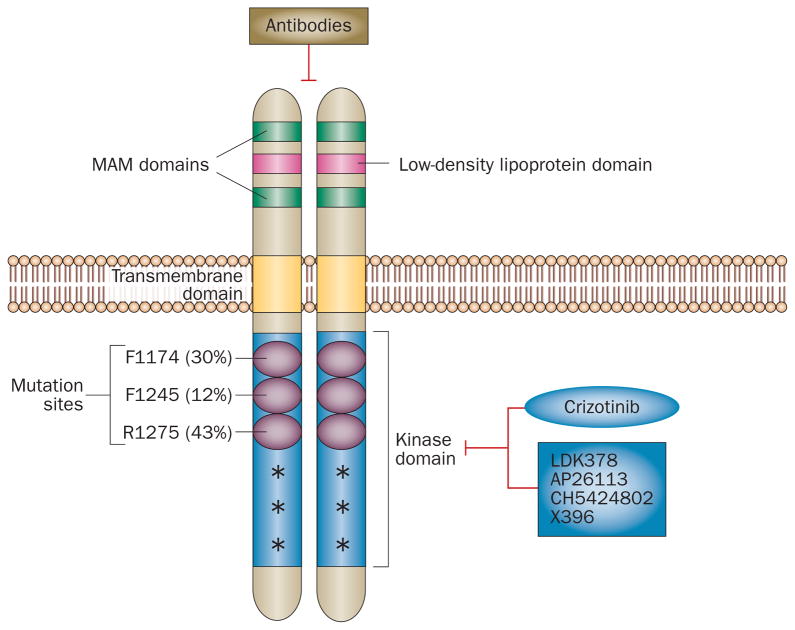
ALK is a 220 kD, 1,620 amino acid single-chain receptor with an extracellular domain containing two MAM domains, as well as transmembrane and intracellular domains (Figure 1). Jelly belly has been identified as the ligand of Drosophila ALK and activates ALK through the MAPK pathway.39 Although the extracellular low-density lipoprotein domain could have a role in ligand binding of human ALK, whether the proposed ligands midkine and pleiotrophin actually bind and activate ALK is unclear.40–42 Constitutive ALK activation has been linked to cellular processes related to oncogenesis including cell-cycle progression, survival, and cell migration in cells expressing translocated ALK. Downstream signalling of ALK is also best characterized in these cells and involves STAT3, PI3K/AKT, and MAPK,43–46 although signalling through these molecules has not been demonstrated for full-length ALK in neuroblastoma. Signal transduction pathways vary among different ALK aberrations; however, both translocated and full-length ALK are sensitive to crizotinib, a small-molecule inhibitor of ALK, suggesting that ALK inhibition is a relevant therapeutic strategy.47–50
Figure 1.
Schematic representation of ALK protein structure and mutations found in neuroblastoma. The low-density lipoprotein domain, two MAM domains, and the transmembrane and kinase domains of ALK are shown. R1275, F1174 and F1245 are three most common ALK mutations in neuroblastoma; the frequency of these mutations are provided in parenthesis. Other low frequency mutations (>20) are denoted with an asterisk. The tyrosine kinase inhibitor crizotinib is in clinical trials and other second-generation ALK inhibitors are in clinical development. ALK-targeting antibodies are also being developed.
The biology of ALK in neuroblastoma
Hereditary neuroblastoma
In 1972, Knudson and Strong predicted that neuroblastoma, similar to the analogous embryonal cancer retinoblastoma, follows a two-hit model of tumorigenesis explaining hereditary and sporadic cases.51 This model is correct for the majority of childhood and adult cancers, and the susceptibility genes are generally tumour suppressors, in which two genomic events constitute sequential inactivation of both alleles. The discovery of germline mutations in the oncogenes RET, MET and KIT in the aetiology of multiple endocrine neoplasia type 2 cancer, papillary renal carcinoma and gastrointestinal stromal tumours, respectively, challenged this paradigm, but it is now clear that somatically acquired duplication or amplification of the mutant allele provides the second genetic event.52 The discovery of activating mutations in the tyrosine kinase domain of ALK as the major cause of hereditary neuroblastoma53 provides the first example of a paediatric cancer arising from germline mutations in an oncogene. We and others have shown that ALK activation can be acquired somatically,53–57 providing the first evidence for oncogenic activation of ALK via mutation of the kinase domain. This finding is in contrast to previous reports describing ALK rearrangements, including translocations and gene amplification, as the genetic basis for the initial observations of sensitivity to ALK inhibition in NSCLC, ALCL and neuroblastoma.58
The discovery of highly penetrant, heritable ALK mutations as the cause of hereditary neuroblastoma is of immediate relevance to patients with a family history of this disease and opens a new field that will likely become intrinsic to the cancer risk assessment for individuals and families with neuroblastoma. Although hereditary cases of neuroblastoma are rare relative to sporadic cases, efforts to sequence matched germline DNA for each tumour with a somatic mutation will help to understand the frequency of hereditary predisposition to neuroblastoma even in the absence of a family history. The ability to identify individuals at high risk for developing this disease brings with it the responsibility to devise effective surveillance strategies, and some fundamental questions need to be considered: what are the processes that control the genetic penetrance of mutations in ALK? Can understanding the genetic basis help predict clinical severity and biological grade of neuroblastoma? What is the risk of developing the disease given that there is incomplete penetrance? Answers to these questions will have a major affect on the future management of patients with neuroblastoma. This work will hopefully lead to effective early detection strategies resulting in favourable clinical outcomes, much like those achieved in Li-Fraumeni syndrome.59
Functional expression of ALK in neuroblastoma
Full-length ALK expression was first linked to neuroblastoma in 2000,60 followed by a report showing consistent expression of activated ALK in tumours of neural origin,61 but no correlation was evident between the levels of ALK expression and prognostic factors for neuroblastoma, such as age, MYCN amplification, tumour stage, tumour histology and DNA content. Rare cases of ALK amplification have also been described,62 but the definitive role of this oncogene has only recently been reported.
Reports of crystal structures of the unphosphorylated human ALK tyrosine kinase domain of human ALK63,64 revealed unexpected similarities to the EGFR tyrosine kinase domain, which could be useful for predicting the consequences of ALK mutations in neuroblastoma. Activating mutations in ALK occur as single-base mis-sense alterations in the tyrosine kinase domain;53 no disease-causing mutations have been reported within the extracellular domain. ALK mutations are heterozygous and, to date, no direct evidence has pointed to acquisition of a second genetic event, such as amplification of the mutant allele.
The R1275 mutation is the most frequent mutation in ALK in neuroblastoma, as it occurs in the germline of patients with hereditary predisposition,53 and is detected in almost 50% of tumours with ALK mutation. The most common ALK mutations in sporadic cases of neuro-blastoma are found at positions R1275, F1174, and F1245, all of which activate ALK, are located in key regulatory regions of the RTK domain, and have transformation capabilities in vitro and in vivo (Figure 1).55,56 Biochemical data further showed that the R1275Q mutation in ALK stabilizes the autoinhibited conformation of the tyrosine kinase domain and coincides closely with Lys837 in EGFR, where a mutation to Gln activates EGFR in NSCLC.47 Once R1275 is replaced with Gln in ALK, the autoinhibitory interactions are disrupted, ALK is activated and, in the presence of a tyrosine kinase inhibitor (TKI), results in preferential binding of crizotinib versus ATP. F1174 in ALK contributes to a well-packed hydrophobic core near the activation loop and when mutated, the packing of this hydrophobic core is disrupted, weakening the autoinhibitory interactions and enables the tyrosine kinase domain to adopt its active configuration.47 F1174L mutation in ALK combines the characteristics of an activating mutation and de novo resistance mutation as it resembles the drug-resistant variant of EGFR (harbouring Y858R and T766M double mutation), and has increased ATP-binding affinity—a feature which, in contrast to R1275Q mutation, reduces the potency of ATP-competitive inhibitors.47 Interestingly, the F1174L mutation has emerged as an escape mechanism in adults with neuroblastoma who have an ALK translocation treated with crizotinib,65 highlighting the importance of findings in neuroblastoma for other types of ALK-positive cancers.
Targeted therapy with ALK inhibitors
Through systematic resequencing of 1,600 sporadic neuroblastoma tumour samples obtained at diagnosis that are representative of the natural spectrum of the disease, we confirmed66 that ALK is mutated in 8% of diagnostic tumour samples, and mutations are distributed across the range of phenotypes, as shown in a previous meta-analysis.67 All three mutations at amino acid locations R1275, F1174 and F1245 account for 86% of mutations found in neuroblastoma and correlate with the constitutively active form of ALK.47,68 Whether rare mutations occurring at other locations within the tyrosine kinase domain, such as the germline mutation at I1250T (a kinase-dead mutation),69 are disease causing and respond to pharmacological ALK inhibition is under investigation, signifying the importance of tumour sequencing in the clinic. The prognostic importance of ALK alterations needs to be evaluated in a wide variety of diagnostic tumour samples taken from a large number of patients representative of the whole spectrum of neuroblastoma. This characterization is paramount for the identification of high-risk patients who are most likely to benefit from therapeutic stratification. Elucidation of the target population is crucial to the success and clinical efficacy of treatment, as was shown for RAF inhibition in BRAF-mutant melanoma,70 as well as ALK inhibition in ALK-translocated NSCLC.49
Notably, ALK mRNA expression71 and native ALK protein expression measured by immunohistochemistry,72,73 have been proposed as indicators of ALK inhibition in neuroblastoma, suggesting these measurements as biomarkers of mutation-independent ALK activation. Although overall ALK mRNA expression levels in neuroblastoma are associated with a poor prognosis (independent of genomic ALK status67) and are significantly higher in tumour cells expressing mutated ALK, no direct correlation was found between endogenous ALK mRNA levels and constitutive activation of ALK protein. Interestingly, one study demonstrated that while ALK protein levels do not always correlate with ALK genetic alterations or mRNA abundance, both mutated and wild-type ALK can exert oncogenic activity in neuroblastoma cells above a threshold expression level, suggesting that wild-type ALK is a potential therapeutic target when overexpressed.72
For the clinical application of ALK-targeted therapy in neuroblastoma, a diagnostic test that can be performed using small amounts of tumour tissue is essential to screen patients and identify potential responders. ALK mRNA and native protein expression could be necessary, but insufficient, for predicting sensitivity to pharmacological ALK inhibition. While constitutive ALK activation could be a suitable biomarker, immunohistochemistry is associated with both technical and interpretive challenges, highlighting the need to develop a sensitive and reliable clinical assay for detection of constitutively active ALK. Kinases that are intimately involved in processes leading to tumour proliferation and survival become oncogenic by genetic mutation, amplification or translocation, leading to constitutive activation and transforming capacity. The success of ALK kinase inhibition therapy in neuroblastoma, therefore, relies on the identification of appropriate predictive bio-markers, and genetic alterations (mutation and amplification) remain the hallmark that render the cancer cells susceptible to kinase inhibition.
Preclinical and early phase clinical trials
The integration of cancer genomics and chemical compound screening is important for the development of patient-based cancer therapeutics.74 One study investigated sensitivity to TAE684, a tool compound inhibitor of ALK, in a panel of 602 established cancer cell lines derived from a wide variety of tumour types.58,75 Whereas the majority of cell lines were refractory to the treatment, a small subset of cells displayed substantial growth inhibition. Notably, the drug-sensitive cells were derived from NSCLC, ALCL and neuroblastoma. While no correlation was found between genotypic alterations and phenotypes in the neuroblastoma cells, these findings serve as further proof-of-concept that selective kinase inhibitors can elicit dramatic responses in tumour cells harbouring specific genetic lesions.
The development of ALK-targeted therapy has benefitted from previous studies of agents targeting other druggable kinases, such as BCR–ABL in chronic myeloid leukaemia and EGFR in NSCLC. Rapid clinical development of ALK-targeted therapy in neuroblastoma and other tumours has been greatly accelerated, in part, because crizotinib (originally developed as a MET inhibitor) was developed before the discovery of ALK translocations in NSCLC and activating ALK mutations in neuroblastoma.76 In 2010, one study showed that crizotinib yielded dramatic response rates in pre-treated patients with advanced NSCLC containing ALK-rearrangements.49 In addition, mechanisms of resistance associated with mutations in the ALK tyrosine kinase domain were elucidated, further implicating ALK as a valuable target (Figure 2).65
Figure 2.
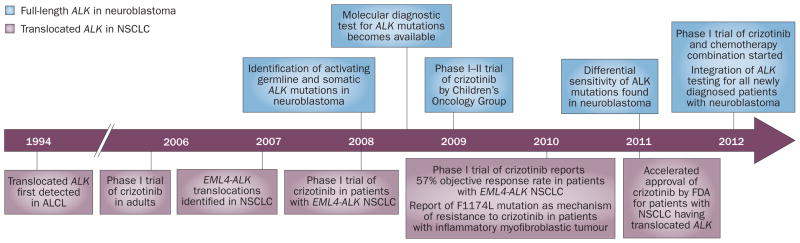
Timeline depicting the milestones leading to the clinical testing of the ALK inhibitor crizotinib targeting the full-length ALK in neuroblastoma (above) and translocated ALK in non-small-cell lung cancer (below).
ALK genomics, biochemical studies and preclinical drug development have firmly established ALK as a tractable molecular target in neuroblastoma. We have established that neuroblastoma cells and xenografts harbouring an ALK aberration (mutation or amplification) are significantly more sensitive to growth inhibition when treated with crizotinib than cells with wild-type ALK,47 although each mutation confers different levels of sensitivity to crizotinib. Nevertheless, these data provide the preclinical rationale for ALK inhibition as a useful therapeutic strategy in neuroblastoma.
In 2009, within 18 months of the initial discovery of a mutation in a neuroblastoma pedigree, the ADVL0912 trial,77 a phase I–II clinical trial of crizotinib, was initiated by the Children’s Oncology Group for paediatric patients with relapsed solid tumours and ALCL, and demonstrated a rapid translation of preclinical molecular findings into the clinic (Figure 2). As the focus of anticancer drug discovery has shifted to molecularly targeted drugs, this trial was designed, at least in part, to define the optimal dose of crizotinib by emphasizing therapeutic and pharmacokinetic end points. Pharmacokinetic studies, often considered to be optional when used in paediatric phase I trials, are mandatory and critical to guide selection of the appropriate dose in future trials. In parallel with the dose-escalation phase that uses a rolling-six design,78 patients with ALK-positive tumours are allowed to enroll in the study at any time and have access to the drug that has prospect for direct benefit. In addition, the trial enriches for patients with neuroblastoma, who are typically more heavily pretreated than other patients and often do not meet the eligibility criteria for phase I trials. Such trial enrichment enables patients to enroll at one dose lower than that of the highest dose used in the study, followed by intrapatient dose escalation once the next higher dose level has been deemed safe. Correlative biology studies are an essential component of this type of trial to understand tumour heterogeneity, as well as mechanisms of response and resistance to the drug. Greaves and Maley have elegantly shown that whereas therapeutic intervention could eliminate cancer clones, it can also inadvertently lead to the expansion of resistant clones and treatment failure.79 An important goal, therefore, is to develop a reliable method for monitoring the presence of residual cancer cells at regular stages during the treatment course to obtain a good understanding of how to approach the problem of drug resistance, either acquired or caused by selection of rare resistant clones from a heterogeneous tumour environment.
Preclinical and early phase clinical development of ALK-targeted therapy in neuroblastoma has advanced rapidly, in a manner that has enabled real-time integration of discoveries to define an optimal dose of crizotinib for phase II II trials. Our current understanding of neuroblastoma biology suggests that near-complete inhibition of constitutively active ALK is necessary to achieve a clinical response, similar to that achieved in BRAF-mutant melanoma following >80% inhibition of ERK activation.80
Resistance to therapy
The development of resistance to targeted cancer therapy is now considered largely inevitable.81 While TKIs targeting ALK, including crizotinib, are effective treatments in preclinical models, their clinical efficacy will ultimately be hindered by drug resistance. One study showed that crizotinib resistance in neuroblastoma cells expressing F1174L-mutated ALK arises from increased ATP-binding affinity for this mutant, thereby reducing the potency of ATP-competitive inhibitors.47 These data also suggest that crizotinib resistance is surmountable by using higher doses of the drug or new high-affinity inhibitors.47 This work has directly affected the clinical trial of crizotinib that is in progress by the Children’s Oncology Group in an effort to circumvent the de novo resistance caused by the F1174L mutation (Figure 2), with ongoing dose-escalation beyond the traditional four doses to define a true recommended phase II dose. This approach is important for proper design of front-line therapy and for guiding the development of next-generation ALK inhibitors, several of which are in early phase clinical trials48 in neuroblastoma and other cancers driven by ALK translocations.
Mechanisms of acquired resistance have been identified for BCR–ABL inhibition in acute myeloid leukaemia, EGFR inhibition in lung cancer, HER2 inhibition in breast cancer, and Smoothened (a G-protein-coupled receptor encoded by SMO) inhibition in medulloblastoma.82 In light of these challenges, we and others are working to develop a model system for predicting crizotinib resistance mechanisms in the laboratory. To achieve this, neuroblastoma cell lines of known crizotinib sensitivity are treated with sustained and increasing doses of the drug for several months and resistance evaluated by the reduced sensitivity of these cells to treatment as compared to an untreated cell line. Interestingly, Sanger sequencing of resistant cell lines revealed no additional mutations in ALK tyrosine kinase domain, even after addition of the chemical mutagen N-ethyl-nitrosourea, a potent inducer of point mutations. In addition, genomic profiling also failed to detect any changes in ALK copy number.83 Probing of a phospho-array to assess changes in the activation level of a panel of RTKs revealed significant upregulation of phosphorylated EGFR; this result is corroborated by in situ analysis of ALK mRNA showing a significant increase in EGFR expression exclusively in resistant cells.83 These data suggest the importance of elucidating the upstream and downstream signalling pathways in tumours with activated oncoproteins to design effective therapies that are likely to require combining inhibitors of specific signalling pathways. Cautious extrapolation from these preclinical models will enable us to define biomarkers predictive of response and resistance to ALK-targeted therapy.
ALK-targeted immunotherapy
Although small-molecule inhibition of ALK remains a promising strategy, development of parallel approaches is necessary. As mentioned above, preclinical studies have shown that ALK copy number and genotype strongly predict responsiveness to ALK inhibition by TKIs, with de novo resistant mutations, such as F1174L, rendering the tumour largely refractory to crizotinib.47 Neuroblastoma cells almost uniformly express GD2 on their surface, which provides a tractable target for immunotherapeutic approaches and sets the stage for immunotherapy in neuroblastoma, as recently shown in the study of the Children’s Oncology Group.14
ALK is an ideal tumour antigen for targeting treatment given that it is detectable by immunohistochemistry on the majority of neuroblastoma cells, and yet its expression is restricted to the tumour, thus minimizing the risk of cytotoxicity in non-malignant tissue; these attributes may simplify patient selection.29,30 Although clinically relevant ALK antibodies are being developed, so far pre-clinical analysis has been limited.84 Our work has shown not only that such an ALK antibody can lead to growth inhibition of neuroblastoma cell lines in the absence of immune effector cells, but also that ALK antibodies are able to mediate antibody-dependent cell-mediated cytotoxicity (ADCC), suggesting two possible mechanisms of action.29 Moreover, these effects were shown in a neuroblastoma cell line that harbours the crizotinib-refractory mutation F1174L,29 further suggesting that ALK-targeted immunotherapy would provide a therapeutic option for patients who are least likely to respond to crizotinib treatment. Unlike GD2 antibodies, ALK antibodies bind to and inhibit an oncogenic receptor, providing an additional inhibitory mechanism independent of the immune system.
Therapeutic antibodies targeting the RTKs HER2 and EGFR were first developed in late 1990s and have since demonstrated therapeutic efficacy.85,86 Several studies have highlighted the potential for dual targeting of oncogenic RTKs in cancer,87,88 and in one preclinical study in lung cancer, dual targeting of EGFR and erlotinib-resistant EGFR was the only therapeutic approach that induced tumour regression.87 TKI-induced cell surface accumulation of the targeted tumour antigen has been suggested as one mechanism for the enhanced efficacy of dual targeting with a TKI and an RTK antibody.88 Other studies have suggested that the combination of small-molecule inhibitors with antibodies results in enhanced apoptosis of cancer cells.89 Indeed, our results are consistent with these studies and suggest that small-molecule inhibition of ALK would sensitize neuroblastoma cells to treatment with an ALK antibody, and that the combination leads to enhanced programmed cell death.29 Whether the direct cytotoxic effects of ALK-targeted immunotherapy are due to ligand blockade, inhibition of dimerization, or enhanced endocytosis and proteosomal degradation is under investigation. Preclinical optimization will reduce immunogenicity and antibody–drug conjugate technology will improve therapeutic index to maximize success in the clinic.
Future perspectives
An important obstacle in the development and assessment of anticancer therapies is the failure of preclinical mouse tumour models to reliably predict how a drug will perform in humans. There has been a gradual movement to use transgenic oncomouse models for anticancer drug testing. The most characteristic transgenic model of neuroblastoma is the TH-MYCN model,90 in which the animals develop an aggressive malignancy similar to the human MYCN-amplified neuroblastomas. This model has been used widely for a multitude of basic biology and therapeutic sensitivity studies.91–99 Recent data suggest that inhibition of PI3K and mTOR signalling pathways affect angiogenic blockade, in part, by degradation of MYCN in vivo, and should be tested in children with high-risk MYCN-amplified neuroblastoma.100 Besides the TH-MYCN model, generating genetically engineered mouse models (GEMM) that overexpress various ALK mutants will require sophisticated approaches,101 but such approaches are vital to assess the efficacy of novel ALK-targeted therapeutics. GEMM will also enable small interfering RNA screens of the druggable genome in combination with ALK inhibitors to evaluate synergy and antagonism with existing chemotherapy backbones. Transgenic zebra fish models have also emerged as an invaluable model system of human cancer to analyze underlying cellular processes. Zebra fish also present an exciting in vivo model for high-throughput drug screening as they enable visual assessment of both drug efficacy and toxicity,102 and are likely to emerge as an essential tool for the study of ALK-driven neuroblastoma, complementing in vitro cell culture-based drug screens. The advances in GEMM and zebra fish studies will help to address fundamental questions about the regulation of ALK signalling and mechanisms of de novo and acquired drug resistance.
Another obstacle in the development of effective cancer therapies is patient selection and trial design. When first-generation anti-EGFR therapies for the treatment of NSCLC entered clinical trials in the 1990s, this approach was directed against the wild-type receptor that was shown to be overexpressed in many epithelial cancer types.103 Mutations in the EGFR kinase domain in NSCLC were discovered in 2004, showing that this subset of tumours was linked to increased sensitivity of lung tumours to gefitinib and erlotinib.104–106 A small proportion of patients with no detectable EGFR-activating mutations show a radiographic response when treated with TKIs targeting EGFR, suggesting that there are probably other types of genetic alterations that activate EGFR signalling in the absence of mutations.107,108 Today, we know that clinical and biological selection of these patients is important for providing appropriate treatment, and stratification based on EGFR mutation status is mandatory prior to the initiation of frontline therapy. Rapid development of ALK inhibition in patients with NSCLC stems from this experience, and the same theory will hold true for neuroblastoma, in which determining the appropriate target population for treatment, the frequency of this target aberration in patients and how to combine therapeutic interventions with molecular diagnostics will all need to be considered. In addition, combining targeted therapies in both early phase and late-phase clinical drug development will raise practical issues at the bench and the bedside, including tumour resistance mechanisms, existence of redundant signalling pathways, tumour heterogeneity and the signalling feedback loops that may attenuate antitumour activity. Future phase III trials of ALK inhibition in neuroblastoma will benefit from previous studies of targeted agents, but several substantial challenges remain that will require adopting new paradigms for drug development and clinical trial design.
Conclusions
Neuroblastoma remains a debilitating disease that provokes both exasperation and excitement among clinical and laboratory investigators. For decades, dramatic intensification of cytotoxic therapy was only moderately effective at improving outcome, but substantial advances have been made that incorporate targeted immunotherapy. The discovery of ALK as a mutated oncogenic receptor in a subset of neuroblastoma tumours provides the basis for the development of innovative targeted treatment approaches. Preclinical data suggest that targeting mutated ALK in neuroblastoma is complex and that the ultimate design of a phase III clinical trial will require identification of an appropriate biomarker for therapeutic stratification. Equally important is the collaboration between the laboratory and clinical settings to facilitate the development of ALK-targeted therapy, to develop dual-targeting strategies aimed at preventing or delaying drug resistance, and to ensure that appropriate measures are in place for collecting adequate tissue samples to study drug sensitivity and resistance, thereby enabling translational research to improve the outcomes of patients.
Key points.
The discovery of germline and somatic mutations in ALK provides the first tractable oncogenic target in neuroblastoma, prompting the initiation of a phase I–II trial of the ALK inhibitor crizotinib
ALK is mutated in 8% of all neuroblastoma cases; mutations are distributed across the range of phenotypes and predict for an inferior outcome
Full-length ALK is expressed on the surface of neuroblastoma tumours in both the presence and absence of a genetic alteration, suggesting that ALK antibody therapy is relevant in neuroblastoma
The F1174L mutation in ALK results in de novo resistance in neuroblastoma and has emerged as a resistance mechanism in ALK-translocated tumours treated with crizotinib
Structural and biochemical data suggest that the increase in ATP-binding affinity for the F1174L-mutated ALK can be overcome with higher doses of crizotinib
Stratification of patients based on ALK alteration status will likely become an integral part of frontline therapy for patients with high-risk neuroblastoma
Acknowledgments
This work is supported in part by grants from the National Institute of Health (R01-CA140198), the Children’s Oncology Group, the Carly Hillman Fund and the US Army Peer-Reviewed Medical Research Program (W81XWH-10-1-0212/3) granted to Y. P. Mossé.
Footnotes
Author contributions
Both authors made a substantial contribution to researching data for the article, discussion of content, and to writing, revising and editing the manuscript before submission.
Competing interests
Y. P. Mossé declares an association with the following company: Pfizer. See the article online for full details of the relationship. E. L. Carpenter declares no competing interests.
References
- 1.Smith MA, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 3.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsen NL. How frequent is spontaneous remission of neuroblastomas? Implications for screening. Br J Cancer. 1990;61:441–446. doi: 10.1038/bjc.1990.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everson TC, Cole WH. Spontaneous regression of neuroblastoma. W. B. Saunders Co; Philadelphia: 1966. pp. 88–163. [Google Scholar]
- 6.Yamamoto K, et al. Spontaneous regression of localized neuroblastoma detected by mass screening. J Clin Oncol. 1998;16:1265–1269. doi: 10.1200/JCO.1998.16.4.1265. [DOI] [PubMed] [Google Scholar]
- 7.Attiyeh EF, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med. 2005;353:2243–2253. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- 8.Brodeur G, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 9.Schwab M, et al. Enhanced expression of the human gene N-myc consequent to amplification of DNA may contribute to malignant progression of neuroblastoma. Proc Natl Acad Sci USA. 1984;81:4940–4944. doi: 10.1073/pnas.81.15.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthay KK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 11.Sidell N. Retinoic acid-induced growth inhibition and morphologic differentiation of human neuroblastoma cells in vitro. J Natl Cancer Inst. 1982;68:589–596. [PubMed] [Google Scholar]
- 12.Thiele CJ, Reynolds CP, Israel M. Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. Nature. 1986;313:404–406. doi: 10.1038/313404a0. [DOI] [PubMed] [Google Scholar]
- 13.US National Library of Medicine. ClinicalTrials gov. 2012 [online], http://clinicaltrials.gov/ct/show/NCT00567567.
- 14.Yu AL, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodeur GM, et al. Expression of TrkA, TrkB and TrkC in human neuroblastomas. J Neurooncol. 1997;31:49–55. doi: 10.1023/a:1005729329526. [DOI] [PubMed] [Google Scholar]
- 16.Minturn JE, et al. Phase I trial of lestaurtinib for children with refractory neuroblastoma: a new approaches to neuroblastoma therapy consortium study. Cancer Chemother Pharmacol. 2011;68:1057–1065. doi: 10.1007/s00280-011-1581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norris RE, Minturn JE, Brodeur GM, Maris JM, Adamson PC. Preclinical evaluation of lestaurtinib (CEP-701) in combination with retinoids for neuroblastoma. Cancer Chemother Pharmacol. 2011;68:1469–1475. doi: 10.1007/s00280-011-1623-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beppu K, Jaboine J, Merchant MS, Mackall CL, Thiele CJ. Effect of imatinib mesylate on neuroblastoma tumorigenesis and vascular endothelial growth factor expression. J Natl Cancer Inst. 2004;96:46–55. doi: 10.1093/jnci/djh004. [DOI] [PubMed] [Google Scholar]
- 19.Vitali R, et al. c-Kit is preferentially expressed in MYCN-amplified neuroblastoma and its effect on cell proliferation is inhibited in vitro by STI-571. Int J Cancer. 2003;106:147–152. doi: 10.1002/ijc.11187. [DOI] [PubMed] [Google Scholar]
- 20.Eggert A, et al. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res. 2000;6:1900–1908. [PubMed] [Google Scholar]
- 21.Fotsis T, et al. Down-regulation of endothelial cell growth inhibitors by enhanced MYCN oncogene expression in human neuroblastoma cells. Eur J Biochem. 1999;263:757–764. doi: 10.1046/j.1432-1327.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 22.Maris JM, et al. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:581–587. doi: 10.1002/pbc.21232. [DOI] [PubMed] [Google Scholar]
- 23.Coffey DC, et al. The histone deacetylase inhibitor, CBHA, inhibits growth of human neuroblastoma xenografts in vivo, alone and synergistically with all-trans retinoic acid. Cancer Res. 2001;61:3591–3594. [PubMed] [Google Scholar]
- 24.Jaboin J, et al. MS-27–275, an inhibitor of histone deacetylase, has marked in vitro and in vivo antitumor activity against pediatric solid tumors. Cancer Res. 2002;62:6108–6115. [PubMed] [Google Scholar]
- 25.Goldsmith KC, Hogarty MD. Targeting programmed cell death pathways with experimental therapeutics: opportunities in high-risk neuroblastoma. Cancer Lett. 2005;228:133–141. doi: 10.1016/j.canlet.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 26.Osajima-Hakomori Y, et al. Biological role of anaplastic lymphoma kinase in neuroblastoma. Am J Pathol. 2005;167:213–222. doi: 10.1016/S0002-9440(10)62966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim MS, et al. The proteomic signature of NPM/ALK reveals deregulation of multiple cellular pathways. Blood. 2009;114:1585–1595. doi: 10.1182/blood-2009-02-204735. [DOI] [PubMed] [Google Scholar]
- 28.Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009;420:345–361. doi: 10.1042/BJ20090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenter EL, et al. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene. doi: 10.1038/onc.2011.647. http://dx.doi.org/10.1038/onc.2011.647. [DOI] [PMC free article] [PubMed]
- 30.Iwahara T, et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439–449. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- 31.Morris SW, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 32.Cheng M, Ott GR. Anaplastic lymphoma kinase as a therapeutic target in anaplastic large cell lymphoma, non-small cell lung cancer and neuroblastoma. Anticancer Agents Med Chem. 2010;10:236–249. doi: 10.2174/1871520611009030236. [DOI] [PubMed] [Google Scholar]
- 33.Sugawara E, et al. Identification of anaplastic lymphoma kinase fusions in renal cancer: Large-scale immunohistochemical screening by the intercalated antibody-enhanced polymer method. Cancer. doi: 10.1002/cncr.27391. http://dx.doi.org/10.1002/cncr.27391. [DOI] [PubMed]
- 34.Mosse YP, Wood A, Maris JM. Inhibition of ALK signaling for cancer therapy. Clin Cancer Res. 2009;15:5609–5614. doi: 10.1158/1078-0432.CCR-08-2762. [DOI] [PubMed] [Google Scholar]
- 35.Wang YW, et al. Identification of oncogenic point mutations and hyperphosphorylation of anaplastic lymphoma kinase in lung cancer. Neoplasia. 2011;13:704–715. doi: 10.1593/neo.11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murugan AK, Xing M. Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene. Cancer Res. 2011;71:4403–4411. doi: 10.1158/0008-5472.CAN-10-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grzelinski M, et al. Enhanced antitumorigenic effects in glioblastoma on double targeting of pleiotrophin and its receptor ALK. Neoplasia. 2009;11:145–156. doi: 10.1593/neo.81040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Gaal JC, et al. Anaplastic lymphoma kinase aberrations in rhabdomyosarcoma: clinical and prognostic implications. J Clin Oncol. 2012;30:308–315. doi: 10.1200/JCO.2011.37.8588. [DOI] [PubMed] [Google Scholar]
- 39.Englund C, et al. Jeb signals through the Alk receptor tyrosine kinase to drive visceral muscle fusion. Nature. 2003;425:512–516. doi: 10.1038/nature01950. [DOI] [PubMed] [Google Scholar]
- 40.Mathivet T, Mazot P, Vigny M. In contrast to agonist monoclonal antibodies, both C-terminal truncated form and full length form of Pleiotrophin failed to activate vertebrate ALK (anaplastic lymphoma kinase)? Cell Signal. 2007;19:2434–2443. doi: 10.1016/j.cellsig.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Stoica GE, et al. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J Biol Chem. 2001;276:16772–16779. doi: 10.1074/jbc.M010660200. [DOI] [PubMed] [Google Scholar]
- 42.Stoica GE, et al. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J Biol Chem. 2002;277:35990–35998. doi: 10.1074/jbc.M205749200. [DOI] [PubMed] [Google Scholar]
- 43.Bai RY, et al. Nucleophosmin-anaplastic lymphoma kinase associated with anaplastic large-cell lymphoma activates the phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway. Blood. 2000;96:4319–4327. [PubMed] [Google Scholar]
- 44.Chiarle R, et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med. 2005;11:623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- 45.Kasprzycka M, Marzec M, Liu X, Zhang Q, Wasik MA. Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein induces the T regulatory cell phenotype by activating STAT3. Proc Natl Acad Sci USA. 2006;103:9964–9969. doi: 10.1073/pnas.0603507103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou HY, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408–4417. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 47.Bresler SC, et al. Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Sci Transl Med. 2011;3:108ra114. doi: 10.1126/scitranslmed.3002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hallberg B, Palmer RH. ALK and NSCLC: Targeted therapy with ALK inhibitors F1000. Med Rep. 2011;3:21. doi: 10.3410/M3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwak EL, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasaki T, Rodig SJ, Chirieac LR, Janne PA. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer. 2010;46:1773–1780. doi: 10.1016/j.ejca.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knudson AG, Jr, Strong LC. Mutation and cancer: neuroblastoma and pheochromocytoma. Am J Hum Genet. 1972;24:514–532. [PMC free article] [PubMed] [Google Scholar]
- 52.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 53.Mosse YP, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–935. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caren H, Abel F, Kogner P, Martinsson T. High incidence of DNA mutations and gene amplifications of the ALK gene in advanced sporadic neuroblastoma tumours. Biochem J. 2008;416:153–159. doi: 10.1042/bj20081834. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–974. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 56.George RE, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–978. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janoueix-Lerosey I, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455:967–970. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 58.McDermott U, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008;68:3389–3395. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]
- 59.Tabori U, Malkin D. Risk stratification in cancer predisposition syndromes: lessons learned from novel molecular developments in Li-Fraumeni syndrome. Cancer Res. 2008;68:2053–2057. doi: 10.1158/0008-5472.CAN-07-2091. [DOI] [PubMed] [Google Scholar]
- 60.Lamant L, et al. Expression of the ALK tyrosine kinase gene in neuroblastoma. Am J Pathol. 2000;156:1711–1721. doi: 10.1016/S0002-9440(10)65042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dirks WG, et al. Expression and functional analysis of the anaplastic lymphoma kinase (ALK) gene in tumor cell lines. Int J Cancer. 2002;100:49–56. doi: 10.1002/ijc.10435. [DOI] [PubMed] [Google Scholar]
- 62.George R, et al. Genome-wide analysis of neuroblastomas using high-density single nucleotide polymorphism arrays. PLoS ONE. 2007;2:e255. doi: 10.1371/journal.pone.0000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bossi RT, et al. Crystal structures of anaplastic lymphoma kinase in complex with ATP competitive inhibitors. Biochemistry. 2010;49:6813–6825. doi: 10.1021/bi1005514. [DOI] [PubMed] [Google Scholar]
- 64.Lee CC, et al. Crystal structure of the ALK (anaplastic lymphoma kinase) catalytic domain. Biochem J. 2010;430:425–437. doi: 10.1042/BJ20100609. [DOI] [PubMed] [Google Scholar]
- 65.Sasaki T, et al. The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res. 2010;70:10038–10043. doi: 10.1158/0008-5472.CAN-10-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiser D, et al. Stratification of patients with neuroblastoma for targeted ALK inhibitor therapy. J Clin Oncol. 2011;29 (Suppl 15):9514. [Google Scholar]
- 67.De Brouwer S, et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin Cancer Res. 2010;16:4353–4362. doi: 10.1158/1078-0432.CCR-09-2660. [DOI] [PubMed] [Google Scholar]
- 68.Schonherr C, et al. Activating ALK mutations found in neuroblastoma are inhibited by crizotinib and NVP-TAE684. Biochem J. 2011;440:405–413. doi: 10.1042/BJ20101796. [DOI] [PubMed] [Google Scholar]
- 69.Schonherr C, et al. The neuroblastoma ALK(I1250T) mutation is a kinase-dead RTK in vitro and in vivo. Transl Oncol. 2011;4:258–265. doi: 10.1593/tlo.11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chapman PB, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schulte JH, et al. High ALK receptor tyrosine kinase expression supersedes ALK mutation as a determining factor of an unfavorable phenotype in primary neuroblastoma. Clin Cancer Res. 2011;17:5082–5092. doi: 10.1158/1078-0432.CCR-10-2809. [DOI] [PubMed] [Google Scholar]
- 72.Passoni L, et al. Mutation-independent anaplastic lymphoma kinase overexpression in poor prognosis neuroblastoma patients. Cancer Res. 2009;69:7338–7346. doi: 10.1158/0008-5472.CAN-08-4419. [DOI] [PubMed] [Google Scholar]
- 73.Duijkers FA, et al. Anaplastic lymphoma kinase (ALK) inhibitor response in neuroblastoma is highly correlated with ALK mutation status, ALK mRNA and protein levels. Cell Oncol. 2011;34:409–417. doi: 10.1007/s13402-011-0048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cancer Target Discovery and Development Network et al. Towards patient-based cancer therapeutics. Nat Biotechnol. 2010;28:904–906. doi: 10.1038/nbt0910-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galkin AV, et al. Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK. Proc Natl Acad Sci USA. 2007;104:270–275. doi: 10.1073/pnas.0609412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Christensen JG, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 2007;6:3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 77.US National Library of Medicine. ClinicalTrials gov. 2012 [online], http://clinicaltrials.gov/ct2/show/NCT00939770.
- 78.Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26:190–195. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 79.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bollag G, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr Opin Genet Dev. 2008;18:73–79. doi: 10.1016/j.gde.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 82.Buonamici S, et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2:51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carpenter EL, et al. Mechanisms of resistance to small molecule inhibition of anaplastic lymphoma kinase in human neuroblastoma. Presented at the 102nd American Association for Cancer Research Conference. [Google Scholar]
- 84.Moog-Lutz C, et al. Activation and inhibition of anaplastic lymphoma kinase receptor tyrosine kinase by monoclonal antibodies and absence of agonist activity of pleiotrophin. J Biol Chem. 2005;280:26039–26048. doi: 10.1074/jbc.M501972200. [DOI] [PubMed] [Google Scholar]
- 85.Baselga J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 86.Robert F, et al. Phase I study of anti--epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:3234–3243. doi: 10.1200/JCO.2001.19.13.3234. [DOI] [PubMed] [Google Scholar]
- 87.Regales L, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119:3000–3010. doi: 10.1172/JCI38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scaltriti M, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–814. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 89.Xia W, et al. Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene. 2005;24:6213–6221. doi: 10.1038/sj.onc.1208774. [DOI] [PubMed] [Google Scholar]
- 90.Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997;16:2985–2995. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Faisal A, et al. The aurora kinase inhibitor CCT137690 downregulates MYCN and sensitizes MYCN-amplified neuroblastoma in vivo. Mol Cancer Ther. 2011;10:2115–2123. doi: 10.1158/1535-7163.MCT-11-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Terrile M, et al. miRNA expression profiling of the murine TH-MYCN neuroblastoma model reveals similarities with human tumors and identifies novel candidate miRNAs. PLoS ONE. 2011;6:e28356. doi: 10.1371/journal.pone.0028356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chayka O, et al. Clusterin, a haploinsufficient tumor suppressor gene in neuroblastomas. J Natl Cancer Inst. 2009;101:663–677. doi: 10.1093/jnci/djp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Teitz T, et al. Preclinical models for neuroblastoma: establishing a baseline for treatment. PLoS ONE. 2011;6:e19133. doi: 10.1371/journal.pone.0019133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chesler L, et al. Chemotherapy-induced apoptosis in a transgenic model of neuroblastoma proceeds through p53 induction. Neoplasia. 2008;10:1268–74. doi: 10.1593/neo.08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hogarty MD, et al. ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Res. 2008;68:9735–9745. doi: 10.1158/0008-5472.CAN-07-6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chesler L, et al. Malignant progression and blockade of angiogenesis in a murine transgenic model of neuroblastoma. Cancer Res. 2007;67:9435–9442. doi: 10.1158/0008-5472.CAN-07-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng AJ, et al. Cell lines from MYCN transgenic murine tumours reflect the molecular and biological characteristics of human neuroblastoma. Eur J Cancer. 2007;43:1467–1475. doi: 10.1016/j.ejca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burkhart CA, et al. Effects of MYCN antisense oligonucleotide administration on tumorigenesis in a murine model of neuroblastoma. J Natl Cancer Inst. 2003;95:1394–1403. doi: 10.1093/jnci/djg045. [DOI] [PubMed] [Google Scholar]
- 100.Chanthery YH, et al. Paracrine signaling through MYCN enhances tumor-vascular interactions in neuroblastoma. Sci Transl Med. 2012;4:115ra3. doi: 10.1126/scitranslmed.3002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chesler L, Weiss WA. Genetically engineered murine models--contribution to our understanding of the genetics, molecular pathology and therapeutic targeting of neuroblastoma. Semin Cancer Biol. 2011;21:245–255. doi: 10.1016/j.semcancer.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Etchin J, Kanki JP, Look AT. Zebrafish as a model for the study of human cancer. Methods Cell Biol. 2011;105:309–337. doi: 10.1016/B978-0-12-381320-6.00013-8. [DOI] [PubMed] [Google Scholar]
- 103.Ozanne B, Richards CS, Hendler F, Burns D, Gusterson B. Over-expression of the EGF receptor is a hallmark of squamous cell carcinomas. J Pathol. 1986;149:9–14. doi: 10.1002/path.1711490104. [DOI] [PubMed] [Google Scholar]
- 104.Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 105.Paez JG, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 106.Pao W, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mok TS, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 108.Han SW, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005;23:2493–2501. doi: 10.1200/JCO.2005.01.388. [DOI] [PubMed] [Google Scholar]