Abstract
Background
Limited epidemiologic data are available concerning the cardiovascular effects of cadmium exposure, although recent studies suggest associations with myocardial infarction and peripheral arterial disease. We examined the associations of cadmium exposure with cardiovascular disease in nationally representative general Korean adults.
Methods
We used cross-sectional data on blood cadmium and self-reported diagnoses of ischemic heart disease (IHD), stroke, and hypertension in a sub-sample of 1908 adults, aged 20 years and older, who participated in the 2005 Korea National Health and Nutrition Examination Survey (KNHANES). We used survey logistic regression models accounting for the complex sampling design to estimate the odds ratios (OR), adjusting for age, education, income, alcohol, smoking, body mass index, waist circumference, family history of hypertension, blood pressure, and blood lead.
Results
The geometric mean of blood cadmium was 1.53 μg/L. After adjusting for potential confounders, an interquartile range (IQR) increase in blood cadmium (0.91 μg/L) was found to be associated with an increased risk for IHD (OR 2.1, 95% confidence interval (CI) 1.3–3.4). An IQR increase in blood cadmium was found to be associated with an elevated risk for hypertension only among men (OR 1.4, 95% CI 1.1–1.8) but not among women. No association was observed with stroke in both genders.
Conclusions
These findings suggest that cadmium in blood may be associated with an increased risk for IHD and hypertension in the general Korean adult population.
Keywords: Blood cadmium, Cardiovascular disease, Ischemic heart disease, Hypertension, KNHANES
1. Introduction
Cadmium is a ubiquitous environmental pollutant and carcinogenic metal (ATSDR, 2008; IARC, 2004). Animal and human epidemiologic studies have suggested cadmium as a potential novel risk factor for cardiovascular disease (CVD), the leading cause of death worldwide (Menke et al., 2009; Perry et al., 1979). Possible biological mechanisms include reduction in endothelial barrier function, endothelial cell death, oxidative stress and lipid peroxidation in the vessel wall, vascular inflammation and secretion of cytokines, accumulation of smooth muscle cells, and reduced nitric oxide levels (Messner and Bernhard, 2010).
In recent cross-sectional studies using data from the National Health and Nutrition Examination Survey (NHANES), cadmium exposure measured in urine and/or blood has been associated with cardiovascular outcomes: stroke and heart failure (Peters et al., 2010), myocardial infarction (Everett and Frithsen, 2008), peripheral arterial disease (Navas-Acien et al., 2004, 2005), and elevation in blood pressure (Tellez-Plaza et al., 2008). Another population-based cross-sectional study conducted in Korea reported an association between blood cadmium and hypertension (Eum et al., 2008). However, some studies did not find associations (Staessen et al., 1991, 1984; Tellez-Plaza et al., 2008) and the observed associations differed by gender (Everett and Frithsen, 2008) and smoking status (Tellez-Plaza et al., 2008). Furthermore, CVD endpoints other than hypertension, such as ischemic heart disease (IHD), have not been well characterized in the general population.
The Korea NHANES (KNHANES) is a cross-sectional nationwide representative survey based on a complex, stratified, multistage probability cluster sampling design. The analysis of complex sample survey data must be performed with appropriate statistical procedures – to accurately compute estimates of population statistics – which take the complex nature of the sample strata and weights into account. We assessed the relationship between cadmium exposure and cardiovascular events, such as IHD, stroke, and hypertension, in a representative sample of the Korean general population accounting for such a complex sampling design. We also examined whether the associations were modified by gender and smoking status.
2. Materials and methods
2.1. Study population
We obtained the study data sets from the Third KNHANES, conducted by the Korea Ministry of Health and Welfare in 2005. Detailed information on the KNHANES design and protocol was provided previously (Eum et al., 2008). Briefly, the survey consisted of three sections: the Health and Behavior Interview, the Health Examination, and the Nutrition Survey. For the present analysis, we used data from 1998 adult ≥20 years of age who participated in KNHANES 2005 with detailed in-person interviews, standardized physical examinations, anthropometric measures, nutritional data, and blood sample collection for biochemistry and laboratory analysis of heavy metals (cadmium and lead) in blood.
2.2. Cardiovascular outcomes
The status of IHD [angina pectoris or myocardial infarction (Jain et al., 2007)] and stroke was collected by the self-reported questionnaire. We counted subjects as having IHD or stroke only if subjects answered “yes” to the question whether it was self-reported physician-diagnosed. Blood pressure was measured by standard methods, using a sphygmomanometer (Baumanometer, WA Baum Co. Inc., New York, NY) with the subject in a sitting position after 5 min rest. Three measures with 30 s intervals were made on all the participants. The averages of the second and third measurements were used in the analysis (Eum et al., 2008). Hypertension was defined as a systolic blood pressure (SBP) ≥ 140 mmHg, a diastolic blood pressure (DBP) ≥ 90 mmHg, a self-reported physician diagnosis, or use of anti-hypertensive medications.
2.3. Determination of cadmium in blood
As a part of the environmental heavy metal exposure study, 1998 participants aged ≥ 20 years were randomly and proportionally selected from the sampling units to measure heavy metals including cadmium in blood by the Korean Ministry of Environment (MOE). Blood samples were collected into trace-metal-free tubes after confirmation of no background contamination in all collection and storage materials. The determination of blood cadmium concentrations was done at the Seoul Medical Science Institute using an atomic adsorption spectrometer with Zeeman background correction (SpectrAA-800, Varian, Australia). The limit of detection for blood cadmium was 0.0029 μg/L. Standard reference materials were obtained from BIO-RAD (Lyphochek® Whole Blood Metals Control) for internal quality assurance and control. The coefficient of variation (CV) for blood cadmium was 8.2%.
2.4. Other covariates
Socio-demographic factors (age, gender, education, household income, smoking, and alcohol consumption) were collected using a self-reported questionnaire. Anthropometric measurements such as height, weight, and waist circumference were taken during the health examination survey. Height was determined to the nearest 0.1 cm with individuals standing barefoot using a Seriter stadiometer (850–2060 mm; Holtain Ltd., Crymych, UK). Body weight was assessed to the nearest 0.1 kg with a calibrated balance beam scale (Giant 150 N; HANA Co. Ltd., Seoul, Korea). Body mass index (BMI) was calculated as body weight in kilograms divided by height in meters squared. Waist circumference was taken in centimeters at a midway between the bottom of the rib cage and the top of the iliac crest at the end of a gentle expiration.
2.5. Statistical analysis
We conducted statistical analyses using SAS (version 9.2; SAS Institute Inc., Carry, NC, USA) and the survey package in R (version 2.11.0; The R Foundation for Statistical Computing 2010) to account for the complex sampling design. Strata, sampling units, and sample weights were used to obtain unbiased point estimates and robust linearized standard errors.
The difference in blood cadmium levels between genders was tested using a survey t-test. Demographic and clinical differences between men and women were tested using the Rao–Scott Chi-square test. We used the survey logistic regression model to estimate adjusted odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) for the prevalence of each outcome (IHD, stroke, and hypertension) associated with an interquartile range (IQR) increase in blood cadmium. All models were adjusted for age, age squared, education level (less than high school, high school, and above college or more), household income (<$9000, $9000–$16,000, $16,000–$25,000, and >$25,000), smoking status (current, former, and never smoker), alcohol (heavy, mild, and none), BMI (kg/m2), waist circumference (cm), family history of hypertension (yes/no), and blood lead (μg/dL).
To check for nonlinear relationships, we fitted blood cadmium using a natural cubic spline with 5 degrees of freedom. To assess effect modification by gender and smoking status, multiplicative interaction terms along with the main effects were included in logistic regression models and compared the ORs among the groups. For effect modification by smoking status, we examined only hypertension because of the small numbers of IHD (n=36) and stroke (n=44) and thus very small numbers in each category of smoking status. The P-value of significance was <0.05.
3. Results
The geometric mean (GM) for blood cadmium levels by demographic and clinical characteristics stratified by gender is given in Table 1. The study population consisted of 948 men and 960 women. Overall, 481 (weighted prevalence=24%) were hypertensive, and 36 (2%) and 44 (2%) had IHD and stroke, respectively. Statistical differences between men and women were found in age, education, income, smoking status, alcohol consumption, BMI, and waist circumference. Blood pressure parameters (SBP and DBP) were significantly higher in men than in women (weighted means of SBP and DBP: 120.4 versus 114.8 mmHg and 80.6 versus 74.4 mmHg, respectively, P<0.001). The GM of blood cadmium in the entire population was 1.53 μg/L. Men had statistically significantly higher levels of blood cadmium than women (1.57 versus 1.49 μg/L, P=0.01). Trends toward increased blood cadmium were seen for increasing years of age (P for trend=0.002), education (P for trend=0.0007), and household annual income (P for trend=0.075), but not for BMI and alcohol. The subjects who had IHD (1.94 versus 1.52 μg/L, P=0.006), stroke (1.69 versus 1.53 μg/L, P=0.039), or hypertension (1.67 versus 1.49 μg/L, P<0.0001) had higher cadmium levels than those without the corresponding diseases.
Table 1.
Geometric mean (GM) of blood cadmium by general and clinical characteristics of the study population, KNHANES, 2005.
| Variables | Total (N=1908)
|
Male (N=948)
|
Female (N=960)
|
P-valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Weighted percentage | Cadmium (SE)b | N | Weighted percentage | Cadmium (SE)b | N | Weighted percentage | Cadmium (SE)b | ||
| Blood cadmium, μg/L | 1908 | 100.0 | 1.53 (0.02) | 948 | 49.2 | 1.57 (0.02) | 960 | 50.8 | 1.49 (0.02) | 0.01 |
| Age, years | ||||||||||
| 20–29 | 246 | 20.8 | 1.50 (0.04) | 104 | 21.3 | 1.63 (0.05) | 142 | 20.3 | 1.38 (0.04) | <0.001 |
| 30–39 | 466 | 24.3 | 1.42 (0.03) | 234 | 25.1 | 1.52 (0.04) | 232 | 24.5 | 1.33 (0.03) | |
| 40–49 | 476 | 23.0 | 1.54 (0.02) | 263 | 23.6 | 1.51 (0.03) | 213 | 22.5 | 1.58 (0.03) | |
| 50–59 | 385 | 14.3 | 1.61 (0.03) | 188 | 14.9 | 1.59 (0.04) | 197 | 13.7 | 1.64 (0.04) | |
| 60 ≥ | 335 | 17.6 | 1.64 (0.03) | 159 | 15.1 | 1.68 (0.03) | 176 | 20.0 | 1.61 (0.04) | |
| P for trend | 0.002 | 0.508 | 0.0001 | |||||||
| Education | ||||||||||
| Less than high school diploma | 597 | 27.5 | 1.63 (0.02) | 221 | 19.4 | 1.63 (0.03) | 376 | 35.5 | 1.63 (0.03) | <0.001 |
| High school diploma | 714 | 37.8 | 1.52 (0.02) | 366 | 38.9 | 1.60 (0.03) | 348 | 36.7 | 1.45 (0.02) | |
| Some college education | 597 | 34.7 | 1.46 (0.03) | 361 | 41.7 | 1.53 (0.03) | 236 | 27.8 | 1.37 (0.03) | |
| P for trend | 0.0007 | 0.097 | <0.0001 | |||||||
| Household annual income, quartile | ||||||||||
| <$9000 | 389 | 18.9 | 1.56 (0.03) | 170 | 15.7 | 1.56 (0.04) | 219 | 22.0 | 1.55 (0.03) | 0.003 |
| $9000–$16,000 | 486 | 26.8 | 1.56 (0.02) | 238 | 27.5 | 1.65 (0.03) | 248 | 26.0 | 1.47 (0.03) | |
| $16,000–$25,000 | 502 | 27.3 | 1.55 (0.03) | 251 | 27.2 | 1.63 (0.04) | 251 | 27.5 | 1.47 (0.03) | |
| $25,000> | 531 | 27.0 | 1.46 (0.02) | 289 | 29.6 | 1.46 (0.03) | 242 | 24.5 | 1.47 (0.04) | |
| P for trend | 0.075 | 0.051 | 0.333 | |||||||
| BMI, kg/cm2 | ||||||||||
| <18.5 | 79 | 5.2 | 1.52 (0.05) | 30 | 4.1 | 1.71 (0.07) | 49 | 6.3 | 1.40 (0.05) | <0.001 |
| 18.5–25 | 1155 | 60.8 | 1.53 (0.02) | 541 | 57.7 | 1.61 (0.02) | 614 | 63.8 | 1.46 (0.02) | |
| ≥25 | 674 | 34.0 | 1.53 (0.02) | 377 | 38.2 | 1.51 (0.03) | 297 | 29.9 | 1.56 (0.03) | |
| P for trend | 0.815 | 0.065 | 0.037 | |||||||
| Alcohol | ||||||||||
| None | 555 | 30.1 | 1.51 (0.02) | 190 | 21.3 | 1.56 (0.03) | 365 | 37.5 | 1.48 (0.03) | <0.001 |
| Moderate | 468 | 25.7 | 1.50 (0.03) | 241 | 27.5 | 1.59 (0.04) | 227 | 24.0 | 1.42 (0.04) | |
| Heavy | 885 | 44.2 | 1.56 (0.02) | 517 | 51.2 | 1.57 (0.03) | 368 | 38.5 | 1.54 (0.03) | |
| P for trend | 0.201 | 0.884 | 0.341 | |||||||
| Smoking status | ||||||||||
| Never | 1007 | 53.4 | 1.49 (0.02) | 140 | 16.7 | 1.63 (0.04) | 867 | 88.9 | 1.46 (0.02) | <0.001 |
| Former | 381 | 19.2 | 1.50 (0.03) | 339 | 33.5 | 1.49 (0.03) | 42 | 5.4 | 1.57 (0.06) | |
| Current | 520 | 27.4 | 1.63 (0.02)** | 469 | 49.8 | 1.61 (0.03) | 51 | 5.7 | 1.79 (0.06)* | |
| Waist circumference, cm | ||||||||||
| <80 (female) or <90 (male) | 1254 | 66.9 | 1.52 (0.02) | 692 | 74.5 | 1.58 (0.02) | 562 | 59.6 | 1.44 (0.02) | <0.001 |
| ≥80 (female) or ≥90 (male) | 654 | 33.1 | 1.56 (0.02) | 256 | 25.5 | 1.55 (0.03) | 398 | 40.4 | 1.56 (0.03)* | |
| Family hypertension history | ||||||||||
| Yes | 592 | 31.4 | 1.49 (0.03) | 302 | 32.8 | 1.54 (0.04) | 290 | 30.1 | 1.44 (0.03) | 0.25 |
| No | 1316 | 68.6 | 1.55 (0.02) | 646 | 67.2 | 1.59 (0.02) | 670 | 69.9 | 1.51 (0.02) | |
| Ischemic heart disease | ||||||||||
| Yes | 36 | 1.8 | 1.94 (0.08)* | 19 | 1.8 | 1.81 (0.07) | 17 | 1.8 | 2.08 (0.04)* | 1.00 |
| No | 1872 | 98.2 | 1.52 (0.02) | 929 | 98.2 | 1.57 (0.02) | 943 | 98.2 | 1.48 (0.02) | |
| Stroke | ||||||||||
| Yes | 44 | 2.1 | 1.69 (0.03)* | 29 | 2.7 | 1.73 (0.05) | 15 | 1.5 | 1.63 (0.08) | 0.04 |
| No | 1864 | 97.9 | 1.53 (0.02) | 919 | 97.3 | 1.57 (0.02) | 945 | 98.5 | 1.49 (0.02) | |
| Hypertension | ||||||||||
| Yes | 481 | 23.5 | 1.67 (0.03)** | 281 | 27.2 | 1.68 (0.03)* | 200 | 19.8 | 1.65 (0.03)** | <0.001 |
| No | 1427 | 76.5 | 1.49 (0.02) | 667 | 72.8 | 1.54 (0.02) | 760 | 90.2 | 1.45 (0.02) | |
P<0.05,
P<0.001 for the comparison with the reference group in each variable (never in smoking status; <80 (female) or <90 (male) in waist circumference; no in ischemic heart disease, stroke, and hypertension).
P-value for the differences in frequency of each variable between genders from the Rao–Scott Chi-square test and for the difference in blood cadmium between genders from survey t-test.
SE is standard error of the mean.
Table 2 shows the associations of blood cadmium with IHD, stroke, and hypertension in the whole population and stratified by gender. After adjusting for cardiovascular risk and potential confounding factors, an IQR (0.91 μg/L) increase in blood cadmium was associated with a two-fold increase in the odds of IHD (OR=2.1, 95% CI, 1.3–3.4). There was no gender difference in the association between blood cadmium and IHD. The observed association among women did not change after further adjustment for menopausal status. The adjusted OR for hypertension associated with an IQR increase in blood cadmium was 1.3 (95% CI, 1.1–1.5). Such an association was statistically significant only among men (OR=1.4, 95% CI, 1.1–1.8), but the difference between men and women was not statistically significant (P for interaction=0.39). We confirm the previous findings by Eum et al. (2008) that blood cadmium was significantly associated with both systolic and diastolic blood pressure after accounting for the complex sampling design (data not shown). No associations were observed between blood cadmium and stroke in both genders.
Table 2.
Odds ratios (95% CIs) of cardiovascular diseases associated with an IQR (0.91 μg/L) increase of blood cadmium by gender.
| Total
|
Male
|
Female
|
||||
|---|---|---|---|---|---|---|
| Cases/non-cases | OR (95% CIs) | Cases/non-cases | OR (95% CIs) | Cases/non-cases | OR (95% CIs) | |
| IHD | 36/1870 | 2.10 (1.29–3.43)** | 19/928 | 1.88 (0.96–3.69) | 17/942 | 2.28 (1.26 –4.15)** |
| Stroke | 44/1862 | 1.10 (0.79–1.54) | 29/918 | 1.26 (0.79–1.98) | 15/944 | 0.94 (0.50–1.75) |
| Hypertension | 481/1427 | 1.29 (1.09–1.52)** | 281/667 | 1.40 (1.09–1.79)** | 200/760 | 1.16 (0.92–1.46) |
Adjusted for age, age2, education level (less than high school, high school, and above college or more), income (<$9000, $9000–$16,000, $16,000–$25,000, and >$25,000), family hypertension history (yes/no), systolic blood pressure, alcohol (none, moderate, and heavy), smoking status (current, former, and never), BMI, waist circumference, and blood lead; systolic blood pressure was not adjusted for in hypertension.
P<0.01.
We examined the assumption of the nonlinear associations (Fig. 1). The odds of hypertension increased with elevating blood cadmium through 3 μg/L, but it plateaued with wide 95% CIs after 3 μg/L. There seemed to be a threshold up to 2 μg/L of blood cadmium in relation to IHD, and then the positive association was evident.
Fig. 1.
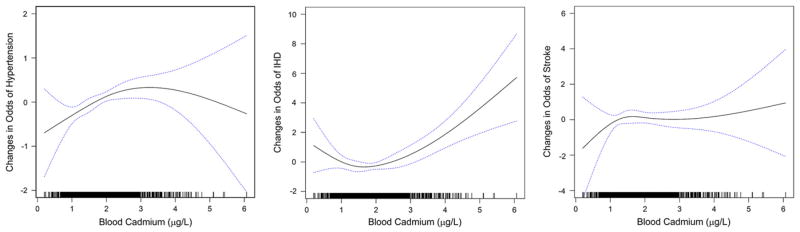
Nonlinear association between cadmium levels and the changes in odds of CVD outcomes adjusting for age, age2, gender, education level (less than high school, high school, and above college or more), income (<$9000, $9000–$16,000, $16,000–$25,000, and $25,000>), family hypertension history (yes/no), alcohol (none, moderate, and heavy), BMI, waist circumference, and blood lead. The predicted values are indicated by the solid line and their 95% confidence intervals by the blue dashed lines. Blood cadmium levels of all individual subjects are indicated by short vertical lines on the abscissa. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
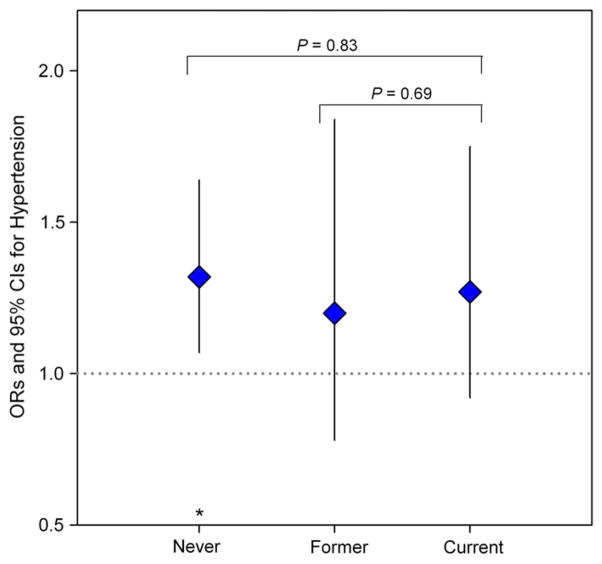
We also assessed effect modification by smoking status in the association between blood cadmium and hypertension, but no significant effect modification was observed (Fig. 2).
Fig. 2.
Odds ratios (95% CIs) of hypertension associated with an IQR (0.91 μg/L) increase in blood cadmium stratified by smoking status, adjusting for age, age2, gender, education level (less than high school, high school, and above college or more), income (<$9000, $9000–$16,000, $16,000–$25,000, and > $25,000), family hypertension history (yes/no), alcohol (none, moderate, and heavy), BMI, waist circumference, and blood lead. *P<0.05.
4. Discussion
In a representative sample of Korean adults from the 2005 KNHANES, blood cadmium at current levels was significantly associated with an increased risk of self-reported IHD and hypertension. The significant association between blood cadmium and hypertension was observed only among men, but not among women. The observed associations did not differ by smoking status. There was no significant association between blood cadmium and reported stroke.
The mechanisms associated with the potential toxic effects of cadmium exposure on the cardiovascular system are unclear. Cadmium is transferred into the bloodstream after absorption through the respiratory and digestive tracts and then transported in either as a free ion or as protein-bound mainly to metallothio-neins, which plays a role in cadmium metabolism and toxicoki-netics (Messner et al., 2009; Nordberg et al., 1992). Cadmium can deplete glutathione (GSH) and disrupt sulfhydryl homeostasis (Valko et al., 2005), which promotes oxidative stress and lipid peroxidation (Yiin et al., 1999). Cadmium-induced oxidative stress can inactivate kidney proteins, and thus lead to elevation in blood pressure in rat models (Lall et al., 1997). Epidemiologic evidence suggests that cadmium may be linked to the increased risk of hypertension via renal tubular and glomerular damages and dysfunction (Satarug et al., 2005). In vivo and in vitro studies have shown that cadmium inhibits endothelial proliferation and cell death (Messner et al., 2009) and angiogenesis (Woods et al., 2008). These cellular phenomena play an important role in hypertension and IHD.
Limited epidemiologic data are available on the cardiovascular effects of cadmium exposure. Positive associations were found with stroke and heart failure in the US general population (NHANES 1999–2006) (Peters et al., 2010). A 50% increase in blood cadmium was associated with 35% and 48% increases in prevalent stroke and heart failure, respectively (Peters et al., 2010). In the NHANES 1999–2000, both blood cadmium and urinary cadmium were associated with an increased risk of peripheral arterial disease (Navas-Acien et al., 2004, 2005). A significant association of urinary cadmium with myocardial infarction was found among women, but not among men in the NHANES-III (Everett and Frithsen, 2008). In the present study, blood cadmium was associated with IHD in both genders, but not with stroke. The aforementioned atherosclerotic effect of cadmium may be an underlying mechanism for IHD. It is not clear why cadmium was not associated with stroke in this population despite a significant association found in the US population (Peters et al., 2010). Stroke has two subtypes, ischemic and hemorrhagic, and it is known that hemorrhagic stroke is more common in Asian than in Caucasian (Ng et al., 1998). We speculate that cadmium exposure is more likely to be linked with ischemic stroke through pro-ischemic effects rather than with hemorrhagic stroke, but no subtype information in this national survey precluded us from examining stroke subtype-specific associations with cadmium.
The associations of cadmium exposure with hypertension and blood pressure remain controversial. Cadmium levels in blood were associated with an elevation in blood pressure parameters, particularly DBP, in the 1999–2004 NHANES (Tellez-Plaza et al., 2008), but no or inverse associations were also reported for urinary cadmium with blood pressure levels or the prevalence of hypertension (Staessen et al., 1991, 1984; Tellez-Plaza et al., 2008).
In our data, blood cadmium was positively associated with an elevated risk of hypertension in men but not in women, although the difference in effect was not statistically significant. Menke et al. (2009) reported that the association between urinary cadmium and increased risk of CVD mortality was reported only among men, but not among women in NHANES-III. It is unclear why men would be more susceptible to cadmium-related cardiovascular responses than women. Females are considered to accumulate more cadmium than males probably because of iron deficiency and accordingly higher absorption of other divalent metals including cadmium (Vahter et al., 2007). Indeed, many population-based studies have shown a higher body burden of cadmium in women than in men (Menke et al., 2009; Nishijo et al., 2004; Peters et al., 2010; Satarug et al., 2002). However, in this Korean population, men had higher cadmium levels than women (1.57 versus 1.49 μg/L, P=0.01). This might be because of the extremely higher prevalence of current and former smokers in men than that in women (83.3% versus 11.1%). Cigarette smoking is the primary source of cadmium exposure among smokers (Jarup et al., 1998; Satarug and Moore, 2004). On the other hand, several animal and human studies have shown that females have higher levels of metallothionein, a metal-binding protein to provide protection against oxidative stress and metal toxicity (Kumari et al., 1998), than males (Blazka and Shaikh, 1991; Kwon et al., 2007). Female hormones, especially estradiol, may be linked to increased metallothionein induction (Blazka and Shaikh, 1991). Given the higher cadmium exposure levels in men compared with women, lower metallothinein levels may confer susceptibility to cadmium-induced hypertension in men. However, there was no difference in the effect of cadmium on IHD between genders in the current study and it is unclear whether female hormones and metallothionein play a different role in different CVD outcomes. Further studies are warranted to confirm and elucidate the gender-specific association between cadmium and CVD.
We evaluated whether cigarette smoking modified the association between blood cadmium and hypertension, but there was no evidence of such effect modification. A stronger cadmium–hypertension association was seen among never smokers compared with current smokers in the NHANES 1999–2004 (Tellez-Plaza et al., 2008) and Satarug et al. (2010, 2005) suggested that this finding may indicate “pressor” effects, in that, the cadmium–hypertension association is evident in low-dose cadmium, but not in the high-dose one. In the present study, cadmium levels did not differ by smoking status, probably because of the gender effect described above (both cigarette smoking and female gender are predictors of blood cadmium, and the majority of ever smokers is men whereas the majority of never smokers is women), which appears to eliminate possible effect modification by cigarette smoking.
The findings of our study need to be interpreted with caution. First, our observed associations were found in a cross-sectional analysis using a single blood cadmium measurement at a single time point. Although urinary cadmium is considered a better cumulative marker of cadmium exposure, blood cadmium is also a good proxy for cadmium body burden in populations with low-level environmental exposures (Jarup et al., 1998). However, we cannot completely exclude a possibility of reverse causality, i.e., hypertension or IHD caused increased blood cadmium. Reverse causality may be unlikely if associations persist with cadmium biomarkers in a different matrix (Bernard, 2008), but unavailability of urinary cadmium did not allow us to resolve this issue. Second, because this study was based on national survey data which was not designed to examine specific exposures and outcomes, our regression models may not fully adjust for potential confounders. However, our results were obtained after adjusting for most well known confounders including age, smoking, BMI, socioeconomic status, family history of hypertension, and even blood lead levels. Therefore, it is unlikely that the observed findings are due to unmeasured confounding.
In summary, cadmium exposure as measured in blood was associated with an increased risk of IHD and hypertension in data representative of the Korean adult population. Men seem to be more susceptible to hypertension in association with cadmium exposure than women. Further prospective studies are needed to better understand the role of cadmium in cardiovascular toxicity, and thus ultimately to have important implications for health policy and disease prevention.
Abbreviations
- CI
95% confidence interval
- CVD
cardiovascular disease
- GM
geometric mean
- GSD
geometric standard deviation
- IHD
ischemic heart disease
- IQR
interquartile range (75–25%)
- KNHANES
Korea National Health and Nutrition Examination Survey
- MI
myocardial infarction
- SD
standard deviation
- SE
standard error
Footnotes
Funding support/Acknowledgment: SKP was supported by the National Institute of Environmental Health Sciences (NIEHS) K01-ES016587.
Disclaimer of competing interest: The authors declared no potential competing financial interests.
References
- ATSDR. Toxicological Profile for Cadmium. Agency for Toxic Substances and Disease Registry; Atlanta, GA: 2008. [PubMed] [Google Scholar]
- Bernard A. Biomarkers of metal toxicity in population studies: research potential and interpretation issues. J Toxicol Environ Health A. 2008;71:1259–1265. doi: 10.1080/15287390802211885. [DOI] [PubMed] [Google Scholar]
- Blazka ME, Shaikh ZA. Sex differences in hepatic and renal cadmium accumulation and metallothionein induction. Role of estradiol Biochem Pharmacol. 1991;41:775–780. doi: 10.1016/0006-2952(91)90080-o. [DOI] [PubMed] [Google Scholar]
- Eum KD, et al. Cadmium in blood and hypertension. Sci Total Environ. 2008;407:147–153. doi: 10.1016/j.scitotenv.2008.08.037. [DOI] [PubMed] [Google Scholar]
- Everett CJ, Frithsen IL. Association of urinary cadmium and myocardial infarction. Environ Res. 2008;106:284–286. doi: 10.1016/j.envres.2007.10.009. [DOI] [PubMed] [Google Scholar]
- IARC. Overall Evaluations of Carcinogenicity to Humans: As Evaluated in IARC Monographs. 1–82. International Agency for Research on Cancer; Lyon, France: 2004. (At Total of 900 Agents, Mixtures and Exposures) [Google Scholar]
- Jain NB, et al. Lead levels and ischemic heart disease in a prospective study of middle-aged and elderly men: the VA normative aging study. Environ Health Perspect. 2007;115:871–875. doi: 10.1289/ehp.9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarup L, et al. Health effects of cadmium exposure—a review of the literature and a risk estimate. Scand J Work Environ Health. 1998;24 (Suppl 1):1–51. [PubMed] [Google Scholar]
- Kumari MV, et al. Free radical scavenging actions of metallothionein isoforms I and II. Free Radic Res. 1998;29:93–101. doi: 10.1080/10715769800300111. [DOI] [PubMed] [Google Scholar]
- Kwon CS, et al. Mononuclear cell metallothionein mRNA levels in human subjects with poor zinc nutrition. Br J Nutr. 2007;97:247–254. doi: 10.1017/S0007114507328614. [DOI] [PubMed] [Google Scholar]
- Lall SB, et al. Cadmium induced nephrotoxicity in rats. Indian J Exp Biol. 1997;35:151–154. [PubMed] [Google Scholar]
- Menke A, et al. Cadmium levels in urine and mortality among U.S. adults. Environ Health Perspect. 2009;117:190–196. doi: 10.1289/ehp.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner B, Bernhard D. Cadmium and cardiovascular diseases: cell biology, pathophysiology, and epidemiological relevance. Biometals. 2010;23:811–822. doi: 10.1007/s10534-010-9314-4. [DOI] [PubMed] [Google Scholar]
- Messner B, et al. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler Thromb Vasc Biol. 2009;29:1392–1398. doi: 10.1161/ATVBAHA.109.190082. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, et al. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109:3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, et al. Metals in urine and peripheral arterial disease. Environ Health Perspect. 2005;113:164–169. doi: 10.1289/ehp.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng WK, et al. A comparative study of stroke subtype between Asian and Caucasians in two hospital based stroke registries. Neurol J Southeast Asia. 1998;3:19–26. [Google Scholar]
- Nishijo M, et al. The gender differences in health effects of environmental cadmium exposure and potential mechanisms. Mol Cell Biochem. 2004;255:87–92. doi: 10.1023/b:mcbi.0000007264.37170.39. [DOI] [PubMed] [Google Scholar]
- Nordberg M, et al. Cadmium, Metallothionein and Renal Tubular Toxicity. IARC Scientific Publications; 1992. pp. 293–297. [PubMed] [Google Scholar]
- Perry HM, et al. Increase in the systolic pressure of rats chronically fed cadmium. Environ Health Perspect. 1979;28:251–260. doi: 10.1289/ehp.7928251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JL, et al. Cadmium exposure in association with history of stroke and heart failure. Environ Res. 2010;110:199–206. doi: 10.1016/j.envres.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarug S, et al. Cadmium levels in the lung, liver, kidney cortex, and urine samples from Australians without occupational exposure to metals. Arch Environ Health. 2002;57:69–77. doi: 10.1080/00039890209602919. [DOI] [PubMed] [Google Scholar]
- Satarug S, et al. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. 2010;118:182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect. 2004;112:1099–1103. doi: 10.1289/ehp.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarug S, et al. Cadmium-induced nephropathy in the development of high blood pressure. Toxicol Lett. 2005;157:57–68. doi: 10.1016/j.toxlet.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Staessen J, et al. Blood pressure, the prevalence of cardiovascular diseases, and exposure to cadmium: a population study. Am J Epidemiol. 1991;134:257–267. doi: 10.1093/oxfordjournals.aje.a116079. [DOI] [PubMed] [Google Scholar]
- Staessen J, et al. Urinary cadmium and lead concentrations and their relation to blood pressure in a population with low exposure. Br J Ind Med. 1984;41:241–248. doi: 10.1136/oem.41.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Plaza M, et al. Cadmium exposure and hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES) Environ Health Perspect. 2008;116:51–56. doi: 10.1289/ehp.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M, et al. Gender differences in the disposition and toxicity of metals. Environ Res. 2007;104:85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Valko M, et al. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Woods JM, et al. Direct antiangiogenic actions of cadmium on human vascular endothelial cells. Toxicol In Vitro. 2008;22:643–651. doi: 10.1016/j.tiv.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiin SJ, et al. Cadmium-induced renal lipid peroxidation in rats and protection by selenium. J Toxicol Environ Health A. 1999;57:403–413. doi: 10.1080/009841099157601. [DOI] [PubMed] [Google Scholar]