Abstract
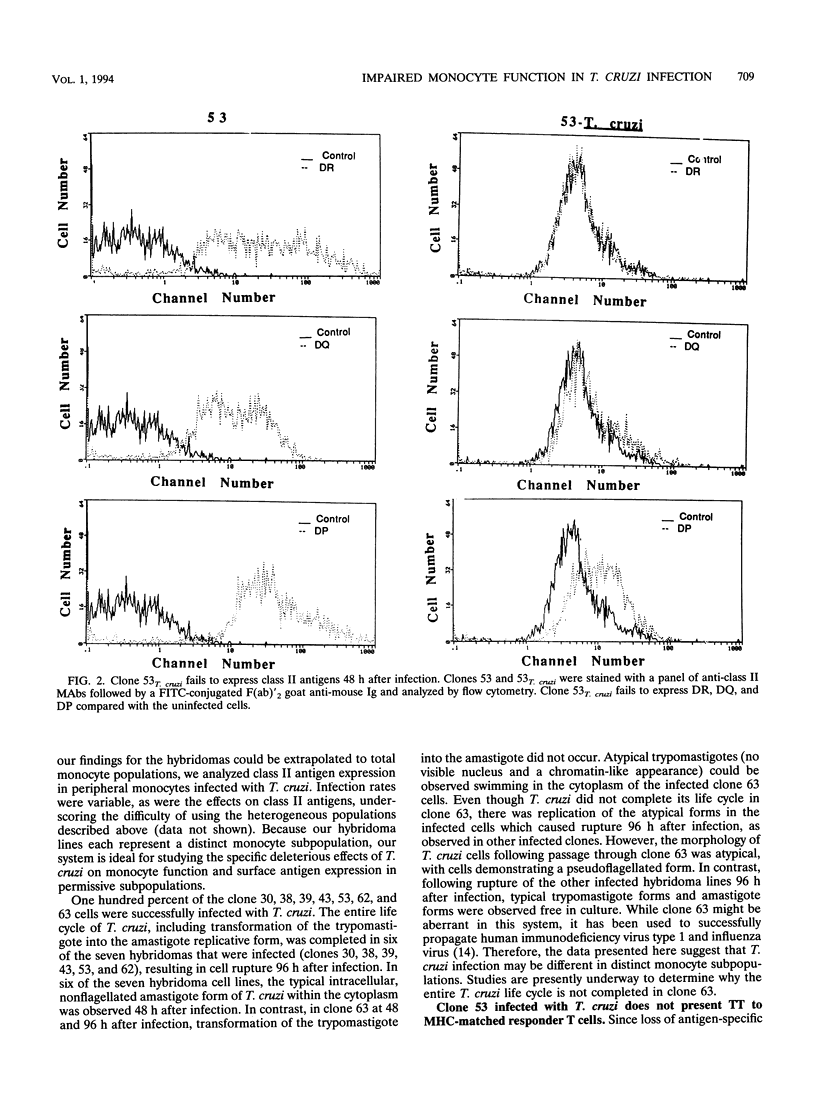
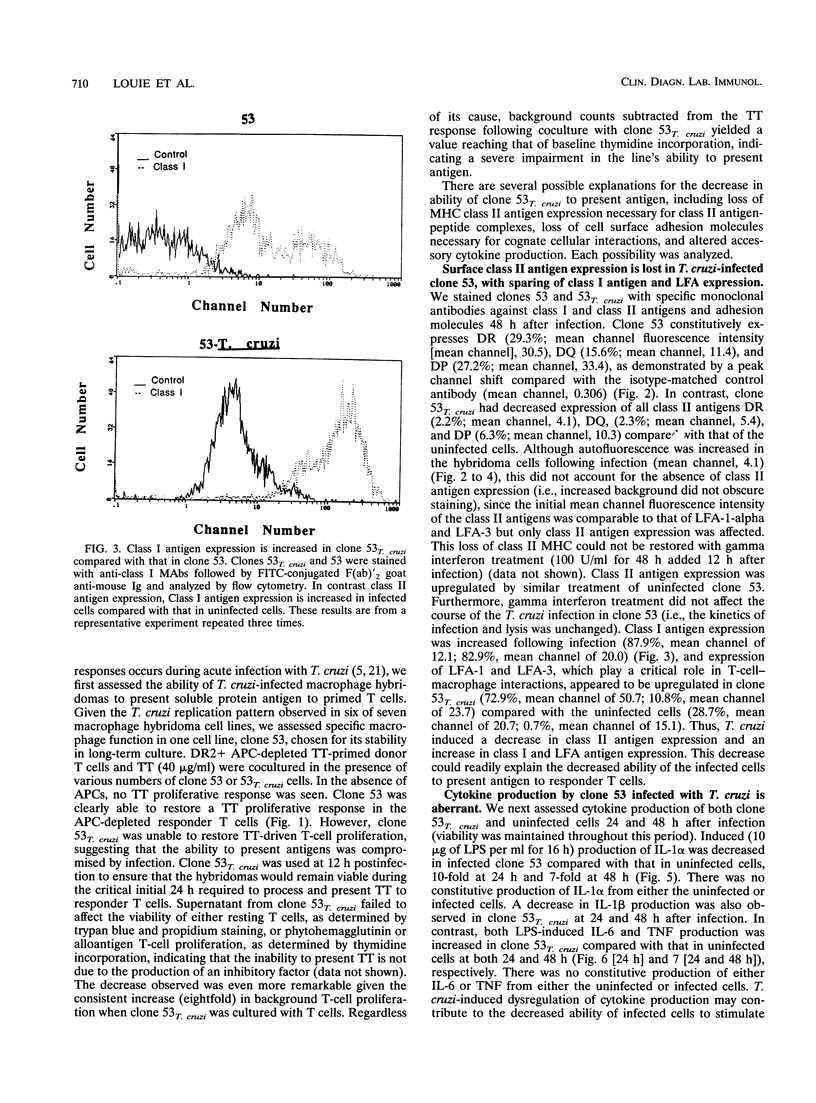
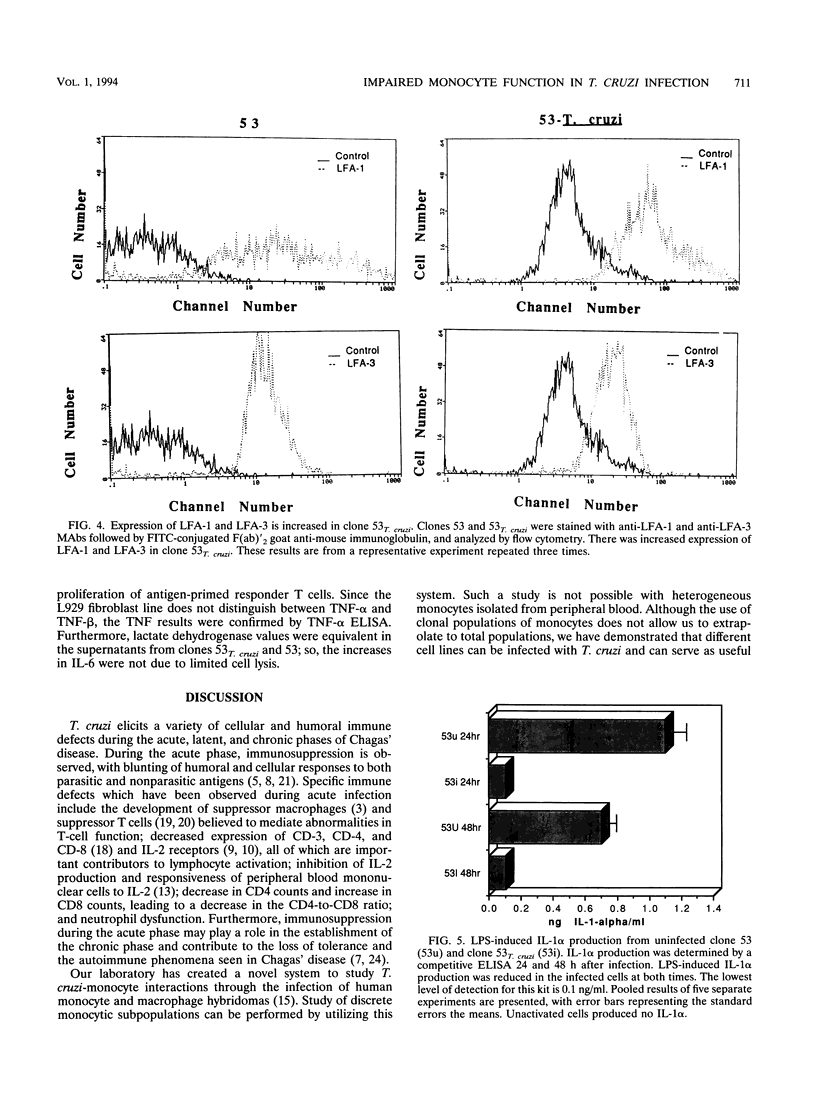
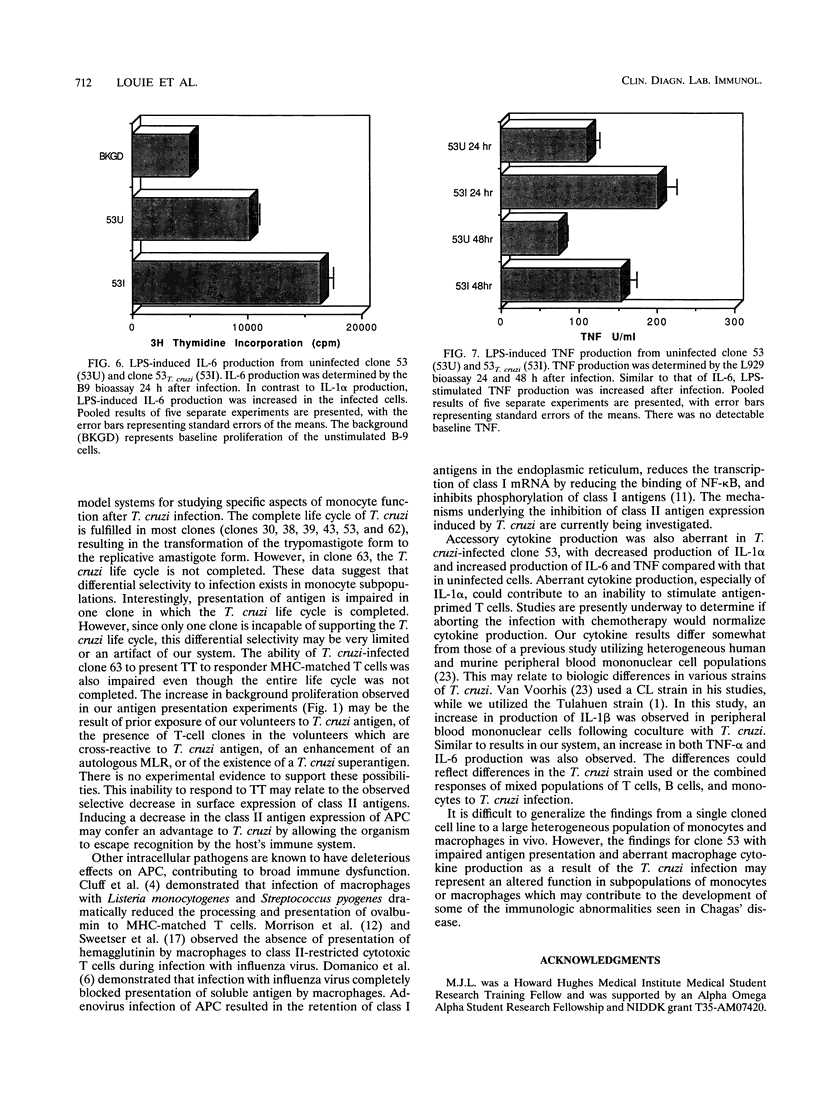
During acute infection, Trypanosoma cruzi, the etiologic agent of Chagas' disease, causes immunosuppression by mechanisms that are not fully delineated. Since mononuclear phagocytes are major target cells in trypanosomiasis, we investigated monocytic function during acute T. cruzi infection. A series of human monocyte and macrophage hybridomas, which represent clonal expansions of subpopulations of human macrophages and possess many normal monocytic functions, were successfully infected with T. cruzi. Clones 63 and 53, chosen for stability in long-term culture, were studied extensively after infection with T. cruzi. Following infection of clone 63, the trypomastigote did not transform into the amastigote multiplicative form, suggesting that clone 63 did not support the entire T. cruzi life cycle. The typical life cycle was completed in clone 53, and thus, clone 53 was used in subsequent studies. Following infection, clone 53 lost expression of class II antigens compared with uninfected cells (DR of 2.2% versus 29.3% and mean channel fluorescence intensity [mean channel] of 4.1 versus 30.5, DQ of 2.3% versus 15.6% and mean channel of 5.4 versus 11.4, and DP of 6.3% versus 27.2% and mean channel of 10.3 versus 33.4). The expression of Class I antigens (87.9% versus 82.8%; mean channel, 20 versus 120) and the adhesion molecules LFA-1 (72.9% versus 28.7%; mean channel, 50.7 versus 23.7) and LFA-3 (10.8% versus 0.7%; mean channel, 20.7 versus 15.1) was increased in infected cells compared with that in uninfected cells. Production of interleukin-1 alpha was decreased and interleukin-6 production was increased in infected clone 53 compared with those in the uninfected cells, while production of tumor necrosis factor alpha was increased.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade V., Barral-Netto M., Andrade S. G. Patterns of resistance of inbred mice to Trypanosoma cruzi are determined by parasite strain. Braz J Med Biol Res. 1985;18(4):499–506. [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Cerrone M. C., Kuhn R. E. Macrophage regulation of immune responses of spleen cells from mice infected with Trypanosoma cruzi. Cell Immunol. 1991 Dec;138(2):423–436. doi: 10.1016/0008-8749(91)90166-9. [DOI] [PubMed] [Google Scholar]
- Cluff C. W., Garcia M., Ziegler H. K. Intracellular hemolysin-producing Listeria monocytogenes strains inhibit macrophage-mediated antigen processing. Infect Immun. 1990 Nov;58(11):3601–3612. doi: 10.1128/iai.58.11.3601-3612.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D. S., Kuhn R. E. Trypanosoma cruzi-induced suppression of the primary immune response in murine cell cultures to T-cell-dependent and -independent antigens. J Parasitol. 1980 Feb;66(1):16–27. [PubMed] [Google Scholar]
- Domanico S. Z., Pierce S. K. Virus infection blocks the processing and presentation of exogenous antigen with the major histocompatibility complex class II molecules. Eur J Immunol. 1992 Aug;22(8):2055–2062. doi: 10.1002/eji.1830220815. [DOI] [PubMed] [Google Scholar]
- Harel-Bellan A., Joskowicz M., Fradelizi D., Eisen H. Modification of T-cell proliferation and interleukin 2 production in mice infected with Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3466–3469. doi: 10.1073/pnas.80.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F., Cuna W. R., Beltz L. A., Sztein M. B. Trypanosoma cruzi reduces the number of high-affinity IL-2 receptors on activated human lymphocytes by suppressing the expression of the p55 and p70 receptor components. J Immunol. 1989 Jul 1;143(1):275–279. [PubMed] [Google Scholar]
- Kierszenbaum F., Cuna W. R., Beltz L. A., Sztein M. B. Trypanosomal immunosuppressive factor: a secretion product(s) of Trypanosoma cruzi that inhibits proliferation and IL-2 receptor expression by activated human peripheral blood mononuclear cells. J Immunol. 1990 May 15;144(10):4000–4004. [PubMed] [Google Scholar]
- Kierszenbaum F. On evasion of Trypanosoma cruzi from the host immune response. Lymphoproliferative responses to trypanosomal antigens during acute and chronic experimental Chagas' disease. Immunology. 1981 Nov;44(3):641–648. [PMC free article] [PubMed] [Google Scholar]
- Meijer I., Boot A. J., Mahabir G., Zantema A., van der Eb A. J. Reduced binding activity of transcription factor NF-kappa B accounts for MHC class I repression in adenovirus type 12 E 1-transformed cells. Cell Immunol. 1992 Nov;145(1):56–65. doi: 10.1016/0008-8749(92)90312-d. [DOI] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. G., Inverso J. A., Roters S. B. Suppressed antibody responses to sheep erythrocytes in mice with chronic Trypanosoma cruzi infections are restored with interleukin 2. J Immunol. 1984 Dec;133(6):3333–3337. [PubMed] [Google Scholar]
- Sperber K., Bauer J., Pizzimenti A., Najfeld V., Mayer L. Identification of subpopulations of human macrophages through the generation of human macrophage hybridomas. J Immunol Methods. 1990 May 8;129(1):31–40. doi: 10.1016/0022-1759(90)90417-t. [DOI] [PubMed] [Google Scholar]
- Sperber K., Hamrang G., Louie M. J., Kalb T., Banerjee R., Choi H. S., Paronetto F., Mayer L. Progressive impairment of monocytic function in HIV-1-infected human macrophage hybridomas. AIDS Res Hum Retroviruses. 1993 Jul;9(7):657–667. doi: 10.1089/aid.1993.9.657. [DOI] [PubMed] [Google Scholar]
- Sperber K., Quraishi H., Kalb T. H., Panja A., Stecher V., Mayer L. Selective regulation of cytokine secretion by hydroxychloroquine: inhibition of interleukin 1 alpha (IL-1-alpha) and IL-6 in human monocytes and T cells. J Rheumatol. 1993 May;20(5):803–808. [PubMed] [Google Scholar]
- Sweetser M. T., Morrison L. A., Braciale V. L., Braciale T. J. Recognition of pre-processed endogenous antigen by class I but not class II MHC-restricted T cells. Nature. 1989 Nov 9;342(6246):180–182. doi: 10.1038/342180a0. [DOI] [PubMed] [Google Scholar]
- Sztein M. B., Cuna W. R., Kierszenbaum F. Trypanosoma cruzi inhibits the expression of CD3, CD4, CD8, and IL-2R by mitogen-activated helper and cytotoxic human lymphocytes. J Immunol. 1990 May 1;144(9):3558–3562. [PubMed] [Google Scholar]
- Tarleton R. L. Trypanosoma cruzi-induced suppression of IL-2 production. I. Evidence for the presence of IL-2-producing cells. J Immunol. 1988 Apr 15;140(8):2763–2768. [PubMed] [Google Scholar]
- Tarleton R. L. Trypanosoma cruzi-induced suppression of IL-2 production. II. Evidence for a role for suppressor cells. J Immunol. 1988 Apr 15;140(8):2769–2773. [PubMed] [Google Scholar]
- Teixeira A. R., Teixeira G., Macêdo V., Prata A. Acquired cell-mediated immunodepression in acute Chagas' disease. J Clin Invest. 1978 Dec;62(6):1132–1141. doi: 10.1172/JCI109232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulich T. R., Guo K. Z., Remick D., del Castillo J., Yin S. M. Endotoxin-induced cytokine gene expression in vivo. III. IL-6 mRNA and serum protein expression and the in vivo hematologic effects of IL-6. J Immunol. 1991 Apr 1;146(7):2316–2323. [PubMed] [Google Scholar]
- Van Voorhis W. C. Coculture of human peripheral blood mononuclear cells with Trypanosoma cruzi leads to proliferation of lymphocytes and cytokine production. J Immunol. 1992 Jan 1;148(1):239–248. [PubMed] [Google Scholar]
- Voltarelli J. C., Donadi E. A., Falcao R. P. Immunosuppression in human acute Chagas disease. Trans R Soc Trop Med Hyg. 1987;81(1):169–170. doi: 10.1016/0035-9203(87)90324-5. [DOI] [PubMed] [Google Scholar]



