Abstract
Background
Chronic hepatitis C (HCV) infected patients with coexisting mental health and/or substance abuse issues face significant barriers to treatment and are often deferred. This paper sought to highlight critical pre-treatment strategies and provide tangible resources for HCV clinicians to facilitate preparation and successful treatment of these patients.
Methods
Guided by the clinical experience of our liver center, a large, tertiary academic medical center, and informed by the extant literature, we summarize pre-treatment strategies and specific resources and recommendations for HCV providers.
Results
Four key pre-treatment strategies include: 1) screening for mental health/substance abuse issues using brief, reliable and validated instruments; 2) locating and establishing collaborative care with mental health and substance abuse specialists; 3) using a motivational interviewing communication style; and 4) addressing adherence-related issues.
Conclusions
HCV clinicians are in a unique position to prepare patients with coexisting mental health and/or substance abuse issues for antiviral therapy.
Keywords: Psychiatric, Depression, Substance abuse, Multidisciplinary
Introduction
The first generation of direct acting antivirals recently approved by the U.S. Food and Drug Administration has instilled new hope in improving treatment outcomes in patients with genotype 1 chronic hepatitis C (HCV) infection [1, 2]. Despite recent advances in treatment, many obstacles continue to plague HCV management and treatment. There are critical junctures along the path from initial HCV screening and diagnosis, to referral to specialty care, to approval and successful completion of HCV treatment, that tremendously limit the overall impact of antiviral therapy (Fig. 1) [3–8]. Even in an ideal situation where 80 % of patients are appropriately screened, 80 % are appropriately referred, etc., less than one third of patients will ultimately be cured due to the number of steps in this complex process. This funneling-down process will dampen the impact of new antiviral therapies on public health outcomes unless current management strategies along this continuum are modified.
Fig. 1.
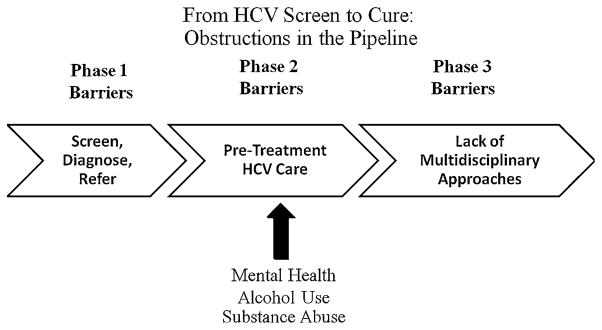
Multiple barriers obstruct the pipeline at different phases along the continuum from HCV screening to HCV cure, which ultimately diminish the overall impact antiviral therapy will have on public health outcomes. Barriers related to MH/SA issues can be addressed by HCV clinicians in the time leading up to antiviral treatment
Patients with mental health and/or substance abuse (MH/SA) issues are especially vulnerable to this funneling down process as their rates of referral and treatment are lower than the “ideal,” in spite of documented evidence that efficacy rates are the same [9–26]. Gastroenterologists and hepatologists are in a unique position to impact Phase 2 of this pipeline by keeping referred patients in the pipeline and preparing them for antiviral therapy. Screening for MH/SA, using a motivational communication style, discussing nonadherence issues, and establishing collaborative care with mental health, addictions, and other support systems need to become key elements of the pre-treatment phase. The purpose of the present paper is twofold: to highlight critical pre-treatment clinical procedures and to provide tangible resources which HCV clinicians can utilize to assist patients with coexisting MH/SA issues to prepare for antiviral treatment.
Screen Patients to Identify MH/SA Care Needs
Patients with chronic HCV and coexisting MH/SA problems have a number of psychosocial issues that can pose challenges to initiating and completing antiviral treatment. While most clinicians are diligent about screening for the “usual suspects” (i.e., depression, suicidality, ongoing alcohol and/or illicit drug use), there are a number of other significant psychosocial issues that can complicate treatment, reduce adherence, and create management challenges. Among these other issues include bona fide psychiatric diagnoses, as well as subclinical mood, personality or behavioral symptoms (e.g., Bipolar disorder, anxiety disorders, irritability, impulsivity, etc.). Additionally, general life instability and inadequate resources are present in a large number of patients who do not meet criteria for a diagnosable psychiatric or addiction disorder, but nonetheless these psychosocial factors can sabotage treatment success. Examples of these problems include no access to healthcare and insurance, transportation, housing, limited financial resources, and chaotic interpersonal or living environments. Recently, some of these factors have been linked to medication nonadherence [27]. This instability, paired with limited adaptive coping skills to handle daily stressors and side effects of treatment, can wreak havoc during antiviral therapy. The impact of these issues can be limited and treatment success optimized by screening for and collaborating with specialists to manage these issues during the preparation phase.
During the pre-treatment phase, proper screening for MH/SA issues is critical, as they can interfere with safety, adherence, and efficacy if not properly identified and managed. Importantly, psychiatric symptoms can be easily overlooked during routine clinical exams, as evidenced by a recent study which found that only 32 % of patients who developed major depression during HCV treatment were correctly identified as “depressed” by clinicians [28]. Thus, the use of brief, validated screening tools can help clinicians before and during HCV treatment to identify patients at risk for emotional disturbances. Use of such instruments can then prompt hepatology clinicians to address these issues, prescribe prophylactic or on-treatment antidepressants, or refer to specialized MH/SA services, thereby lessening the chance of further complications during treatment.
With respect to mental health issues, common reliable and validated screening measures for depression include the: (1) Center for Epidemiologic Studies—Depression Scale [29, 30], the Beck Depression Inventory-II [31], and the Patient Health Questionnaire-9 [32]. A reliable and validated measure of bipolar-spectrum symptoms (e.g., hypomania and mania, respectively) is the Bipolar Spectrum Diagnostic Scale and Mood Disorder Questionnaire [33, 34]. Given the demands placed on clinicians during a standard medical visit, screening for these issues can be daunting and viewed as one more burden-some task. However, these instruments can be administered very quickly by clinic staff (e.g., nursing) or self-administered by patients as they wait in the waiting or exam room.
With respect to substance abuse, a reliable and validated measure of alcohol use is the Alcohol Use Disorders Identification Test [35] while illicit drug abuse can be measured using the validated Drug Abuse Screening Test [36]. Additionally, clinicians should also consider systematic urine toxicology screens on patients suspected of potential substance abuse to validate self-report instruments. HCV clinicians are in a unique position to assist patients with ongoing substance abuse by referral to appropriate services, such as methadone or buprenorphine treatments for opioid users, or licensed certified addiction counselors. Once under the care of an addiction specialist, the current body of evidence suggests that these patients can effectively and safely be treated for HCV, as long as multidisciplinary care continues [17, 20, 23–25, 37].
Locating Community Mental Health/Substance Abuse Services and Other Resources
With broader access to the Internet, clinicians can quickly locate services and clinics in the patients’ community (see Table 1). At the UNC Liver Center, we rely heavily upon the online treatment locator provided by the Substance Abuse and Mental Health Services Administration (SAMHSA) website to locate services for both MH/SA issues. We also utilize nationwide and statewide Internet databases to locate psychiatrists, psychologists, or therapists in our patients’ local communities who specialize in evidence-based treatments for psychological and addiction problems. For some patients who are not interested in formal SA programs but would be willing to participate in peer groups, clinicians can locate Alcoholics Anonymous or Narcotics Anonymous meetings in local areas by searching by city or zip codes on their websites.
Table 1.
Internet resources for mental health, substance abuse, and other assistance
| Organization | Type of support | Website |
|---|---|---|
| The Substance Abuse and Mental Health Services Administration (SAMHSA) | MH/SA | http://www.samhsa.gov |
| Psychology Today | MH/SA | http://therapists.psychologytoday.com/rms |
| National Association of Social Workers | MH/SA | http://www.naswdc.org |
| Motivational Interviewing (MI) | MH/SA | http://motivationalinterview.org |
| MI for Alcohol Use/Abuse | SA | http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/clinicians_guide.htm |
| Alcoholics Anonymous | SA | http://www.aa.org |
| Narcotics Anonymous | SA | http://www.na.org |
| Genetech/Roche Patient Assistance | Financial | http://www.gene.com/gene/products/access |
| Merck Patient Assistance | Financial | http://www.merck.com/merckhelps/patientassistance/home.html |
| Vertex Patient Assistance | Financial | http://www.incivek.com/ |
| U.S. Dept. of Housing and Urban Development | Housing | http://portal.hud.gov/hudportal/HUD |
| Center for Medicare and Medicaid Services | Medicare Medicaid Assistance | http://www.cms.gov |
For non-MH/SA issues (i.e., life instability, lack of resources), there are a variety of resources available. Each State has a Department of Health and Human Services, where patients can access county resources, such as case managers who can assist patients with navigating a daunting system. Patients with Medicare/Medicaid can be referred to the Center for Medicare and Medicaid Services website to determine if they may be eligible for public assistance or transportation to medical visits by county transportation. Patients seeking support in housing could be referred to national agencies such as the U.S. Department of Housing and Urban Development. For patient financial assistance with antiviral therapy medications, Roche/Genentech, Merck, and Vertex have established pharmaceutical patient assistance programs that can be accessed via the Internet (Table 1). Many HCV patients come from very impoverished and resource-limited homes, and may not have easy access to computers. We cannot stress the value of providing patients with tangible print-outs of resources, and how this simple gesture may increase the likelihood of following through with treatment recommendations, as well as strengthens rapport between clinicians and patients, which is a core ingredient in getting patients through antiviral treatment safely and effectively.
Utilize a Motivational-Enhancing Communication Style
Patients may be ambivalent or resistant to engaging in pre-treatment recommendations, such as stopping alcohol use or participating in MH/SA services. The manner in which the clinical staff addresses patient ambivalence is critical to build rapport, decrease resistance, and increase motivation to follow through with treatment recommendations. A communication style known as “motivational interviewing” helps enhance patient motivation to make positive behavioral changes (e.g., stop alcohol use) through the use of brief, nonjudgmental questioning or conversations that increase the patient’s awareness of their issues without making them too defensive, as well as bolsters internal motivation and confidence to make desirable behavior changes [38–40]. Motivational interviewing has been effective in enhancing motivation to quit drug or alcohol use, engage in MH/SA treatment, and adhere to medical recommendations in a number of clinical settings [41–46]. More importantly, these interventions can be delivered by medical clinicians from non-counseling backgrounds [38, 47].
Within the hepatology setting, motivational interviewing has been understudied but a few studies demonstrate the promise of this technique. One study suggested that a recent diagnosis of HCV was the strongest motivator and predictor of completing a substance abuse program, among patients enrolled in residential drug treatment [48]. Another study found that hepatologists trained in motivational interviewing techniques could deliver these brief interventions to their patients, resulting in significant alcohol reduction (>50 %) or abstinence from alcohol among 62 % of study participants [49]. Clinicians interested in learning more about motivational interviewing techniques can access resources via the Internet.
Communicate About Adherence
With the advent of protease inhibitors, thrice daily dosing, and concerns about viral resistance, nonadherence with treatment (i.e., missing doses or lack of persisting with regimen) may become a prominent issue that clinicians need to address before and during treatment. Borrowing from the HIV and broader adherence literature, several patient, treatment, and provider-patient relationship factors have been associated with medication nonadherence, and we might expect these same factors to affect nonadherence to HCV treatment [50–53]. Patient factors related to nonadherence include: mental health and emotional problems (e.g., depression); substance abuse; self-efficacy/confidence; health beliefs about illness severity and benefits of treatment; cognitive impairment; and socioeconomic factors. Treatment factors related to nonadherence include: complexity and duration of therapy, number of pills and frequency of dosing, and side effect profile. Finally, a poor working relationship with the provider, patient dissatisfaction, poor communication, and lack of sufficient and detailed information are also related to nonadherence.
Very few studies have identified patient characteristics associated with missed doses and lack of persistence to HCV treatment [21, 54, 55]. In a recent analysis of the Virahep-C database, we found that age, race, and marital, employment, and insurance status, as well as baseline depression and headaches were risk factors for missing doses of medications or failing to persist for the full course of therapy [27]. These characteristics identify patients at risk for missing doses or failing to persist with HCV therapy, who may need additional information, support, or interventions [56].
Clinicians may need to be more cognizant of how they discuss adherence with their patients. Interested readers are referred to recent articles on assessing and discussing adherence. Although the patient population may be different, the broad themes are directly applicable to HCV treatment [57]. The author describes seven common mistakes in assessing adherence, such as using improper tone, attitudes about discovering nonadherence, and assignment of blame. Recommendations for assessing and discussing adherence are then described, including accepting nonadherence, turning the focus away from obedience, respecting reasons for nonadherence, and prevention of nonadherence. With the complexity of new antiviral regimens, coupled with the high prevalence of risk factors for nonadherence present in the HCV population, better adherence assessment and interventions will need to be integrated into clinical practice. As such, we have recommended several readings on adherence from the broader adherence literature [52, 57–63].
Conclusion
Recent approval of the first generation of direct acting antiviral agents has generated hope and enthusiasm for improved SVR rates and shorter treatment durations for many patients with genotype 1 HCV. However, interferon-based therapy is expected to remain a key component of antiviral treatment for at least the next 5 years for all genotypes [64]. During this time, neuropsychiatric side effects, which can exacerbate pre-morbid MH/SA issues, will continue to instill trepidation in HCV providers due to concerns over safety, nonadherence, and efficacy in patients with such issues. While patients with these MH/SA and other psychosocial issues can pose management problems during treatment, the empirical evidence and our clinical experience has demonstrated that these patients, with proper pre-treatment preparation and an integrated, multidisciplinary setting, can effectively and safely undergo antiviral treatment for HCV [13–23]. Moreover, as the HCV population ages and liver disease complications proliferate, failure to extend treatment to these traditionally “difficult to treat” patients will stymie potential gains that are promised by advances in antiviral drug agents [5, 65]. Clinicians are in a unique position to increase the number of patients who exist in the pipeline from HCV diagnosis to treatment, by establishing multidisciplinary, collaborative relationships and employing the recommendations and resources described in this article.
Acknowledgments
Donna Evon receives research grant support from Roche, and served on an advisory board for Vertex Pharmaceuticals in the past 12 months. Michael Fried receives research grants from Genentech, Vertex, Tibotec, Janssen, Gilead, Abbott, Bristol-Myers Squibb, Anadys. Dr. Fried also serves as an ad hoc consultant to Genentech, Vertex, Merck, Tibotec, Janssen, Bristol-Myers Squibb, Novartis and Gilead. Sid Barritt is on the speaker’s bureau for Salix Pharmaceuticals and receives research grant support from Tibotec. This work was supported, in part, by a UNC Clinical and Translational Science Award (UL1RR025747; Bonner); the National Institutes of Health Awards (1KL2-RR025746-03; Barritt; and K23DK089004-02; Evon) and a mentoring grant (K24 DK066144-01; Fried).
Abbreviations
- MH/SA
Mental health/substance abuse
- HCV
Chronic hepatitis C virus
- SVR
Sustained virological response
Footnotes
Conflict of interest: Dr. Bonner has nothing to disclose.
References
- 1.U.S. Food and Drug Administration. [Accessed 7 June 2011.];FDA approves Victrelis for Hepatitis C [FDA News Release web site] 2011 May 13; Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm255390.htm.
- 2.U.S. Food and Drug Administration. [Accessed 20 March 2012.];FDA approves Incivek for hepatitis C [FDA News Release web site] 2011 May 23; Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm256299.htm.
- 3.Volk ML. Antiviral therapy for hepatitis C: why are so few patients being treated? J Antimicrob Chemother. 2010;65:1327– 1329. doi: 10.1093/jac/dkq157. [DOI] [PubMed] [Google Scholar]
- 4.Volk ML, Tocco R, Saini S, et al. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009;50:1750–1755. doi: 10.1002/hep.23220. [DOI] [PubMed] [Google Scholar]
- 5.Davis GL, Alter MJ, El-Serag H, et al. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 6.Kramer JR, Kanwal F, Richardson P, et al. Importance of patient, provider, and facility predictors of hepatitis C virus treatment in veterans: a national study. Am J Gastroenterol. 2011;106:483– 491. doi: 10.1038/ajg.2010.430. [DOI] [PubMed] [Google Scholar]
- 7.Kanwal F, Hoang T, Spiegel BM, et al. Predictors of treatment in patients with chronic hepatitis C infection—role of patient versus nonpatient factors. Hepatology. 2007;46:1741–1749. doi: 10.1002/hep.21927. [DOI] [PubMed] [Google Scholar]
- 8.Stepanova M, Kanwal F, El-Serag HB, et al. Insurance status and treatment candidacy of hepatitis C patients: analysis of population-based data from the United States. Hepatology. 2011;53:737–745. doi: 10.1002/hep.24131. [DOI] [PubMed] [Google Scholar]
- 9.Evon DM, Verma A, Dougherty KA, et al. High deferral rates and poorer treatment outcomes for HCV patients with psychiatric and substance use comorbidities. Dig Dis Sci. 2007;52:3251–3258. doi: 10.1007/s10620-006-9669-0. [DOI] [PubMed] [Google Scholar]
- 10.Muir AJ, Provenzale D. A descriptive evaluation of eligibility for therapy among veterans with chronic hepatitis C virus infection. J Clin Gastroenterol. 2002;34:268–271. doi: 10.1097/00004836-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Falck-Ytter Y, Kale H, Mullen KD, et al. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med. 2002;136:288–292. doi: 10.7326/0003-4819-136-4-200202190-00008. [DOI] [PubMed] [Google Scholar]
- 12.Butt AA, Wagener M, Shakil AO, et al. Reasons for non-treatment of hepatitis C in veterans in care. J Viral Hepat. 2005;12:81–85. doi: 10.1111/j.1365-2893.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer M, Schmidt F, Folwaczny C, et al. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37:443–451. doi: 10.1053/jhep.2003.50031. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer M, Heinz A, Backmund M. Treatment of chronic hepatitis C in patients with drug dependence: time to change the rules? Addiction. 2004;99:1167–1175. doi: 10.1111/j.1360-0443.2004.00821.x. [DOI] [PubMed] [Google Scholar]
- 15.Schaefer M, Schwaiger M, Garkisch AS, et al. Prevention of interferon-alpha associated depression in psychiatric risk patients with chronic hepatitis C. J Hepatol. 2005;42:793–798. doi: 10.1016/j.jhep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer M, Hinzpeter A, Mohmand A, et al. Hepatitis C treatment in “difficult-to-treat” psychiatric patients with pegylated interferon-alpha and ribavirin: response and psychiatric side effects. Hepatology. 2007;46:991–998. doi: 10.1002/hep.21791. [DOI] [PubMed] [Google Scholar]
- 17.Grebely J, Genoway K, Khara M, et al. Treatment uptake and outcomes among current and former injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment of hepatitis C virus infection. Int J Drug Policy. 2007;18:437–443. doi: 10.1016/j.drugpo.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Knott A, Dieperink E, Willenbring ML, et al. Integrated psychiatric/medical care in a chronic hepatitis C clinic: effect on antiviral treatment evaluation and outcomes. Am J Gastroenterol. 2006;101:2254–2262. doi: 10.1111/j.1572-0241.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- 19.Sylvestre DL, Loftis JM, Hauser P, et al. Co-occurring Hepatitis C, substance use, and psychiatric illness: treatment issues and developing integrated-models of care. J Urban Health. 2004;81:719–734. doi: 10.1093/jurban/jth153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sylvestre DL. Treating hepatitis C virus infection in active substance users. Clin Infect Dis. 2005;40:S321–S324. doi: 10.1086/427447. [DOI] [PubMed] [Google Scholar]
- 21.Sylvestre DL, Clements BJ. Adherence to hepatitis C treatment in recovering heroin users maintained on methadone. Eur J Gastroenterol Hepatol. 2007;19:741–747. doi: 10.1097/MEG.0b013e3281bcb8d8. [DOI] [PubMed] [Google Scholar]
- 22.Matthews G, Kronborg IJ, Dore GJ. Treatment for hepatitis C virus infection among current injection drug users in Australia. Clin Infect Dis. 2005;40:S325–S329. doi: 10.1086/427448. [DOI] [PubMed] [Google Scholar]
- 23.Freedman K, Nathanson J. Interferon-based hepatitis C treatment in patients with pre-existing severe mental illness and substance use disorders. Expert Rev Anti Infect Ther. 2009;7:363–376. doi: 10.1586/eri.09.1. [DOI] [PubMed] [Google Scholar]
- 24.Zanini B, Covolo L, Donato F, et al. Effectiveness and tolerability of combination treatment of chronic hepatitis C in illicit drug users: meta-analysis of prospective studies. Clin Ther. 2010;32:2139–2159. doi: 10.1016/S0149-2918(11)00021-X. [DOI] [PubMed] [Google Scholar]
- 25.Bruggmann P, Falcato L, Dober S, et al. Active intravenous drug use during chronic hepatitis C therapy does not reduce sustained virological response rates in adherent patients. J Viral Hepat. 2008;15:747–752. doi: 10.1111/j.1365-2893.2008.01010.x. [DOI] [PubMed] [Google Scholar]
- 26.Bruggmann P, Dampz M, Gerlach T, et al. Treatment outcome in relation to alcohol consumption during hepatitis C therapy: an analysis of the Swiss Hepatitis C Cohort study. Drug Alcohol Depend. 2010;110:167–171. doi: 10.1016/j.drugalcdep.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Evon DM, Esserman DA, Rao T, et al. Patient missed doses and treatment non-persistence during PEG/Ribavirin therapy for chronic hepatitis C: implications for triple therapy and beyond. Hepatology. 2011;54:869A. [Google Scholar]
- 28.Leutscher PD, Lagging M, Buhl MR, et al. Evaluation of depression as a risk factor for treatment failure in chronic hepatitis C. Hepatology. 2010;52:430–435. doi: 10.1002/hep.23699. [DOI] [PubMed] [Google Scholar]
- 29.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 30.Evon DM, Ramcharran D, Belle SH, et al. Prospective analysis of depression during peginterferon and ribavirin therapy of chronic hepatitis C: results of the VIRAHEP-C study. American Journal of Gastroenterology. 2009;104:2949–2958. doi: 10.1038/ajg.2009.528. [DOI] [PubMed] [Google Scholar]
- 31.Beck AT, Steer RA, Ball R, et al. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 32.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873–1875. doi: 10.1176/appi.ajp.157.11.1873. [DOI] [PubMed] [Google Scholar]
- 34.Nassir GS, Miller CJ, Berv DA, et al. Sensitivity and specificity of a new bipolar spectrum diagnostic scale. J Affect Disord. 2005;84:273–277. doi: 10.1016/S0165-0327(03)00196-4. [DOI] [PubMed] [Google Scholar]
- 35.Saunders JB, Aasland OG, Babor TF, et al. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 36.Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J Subst Abuse Treat. 2007;32:189–198. doi: 10.1016/j.jsat.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Sylvestre D. Hepatitis C treatment in drug users: perception versus evidence. Eur J Gastroenterol Hepatol. 2006;18:129–130. doi: 10.1097/00042737-200602000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Rollnick S, Miller W, Butler C. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York: The Guilford Press; 2007. [Google Scholar]
- 39.Emmons KM, Rollnick S. Motivational interviewing in health care settings. Opportunities and limitations. Am J Prev Med. 2001;20:68–74. doi: 10.1016/s0749-3797(00)00254-3. [DOI] [PubMed] [Google Scholar]
- 40.Miller WR, Rollnick S. Motivational interviewing: preparing people for change. New York: Guilford Press; 2002. [Google Scholar]
- 41.Martins RK, McNeil DW. Review of motivational interviewing in promoting health behaviors. Clin Psychol Rev. 2009;29:283–293. doi: 10.1016/j.cpr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Knight KM, McGowan L, Dickens C, et al. A systematic review of motivational interviewing in physical health care settings. Br J Health Psychol. 2006;11:319–332. doi: 10.1348/135910705X52516. [DOI] [PubMed] [Google Scholar]
- 43.Searight HR. Efficient counseling techniques for the primary care physician. Prim Care. 2007;34:551–570. doi: 10.1016/j.pop.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 44.Lev-Ran S, Nitzan U. Motivational interviewing in health care. Harefuah. 2011;150:749. [PubMed] [Google Scholar]
- 45.Callahan EJ, Flynn NM, Kuenneth CA, et al. Strategies to reduce HIV risk behavior in HIV primary care clinics: brief provider messages and specialist intervention. AIDS Behav. 2007;11:S48–S57. doi: 10.1007/s10461-006-9200-9. [DOI] [PubMed] [Google Scholar]
- 46.Rubak S, Sandbaek A, Lauritzen T, et al. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55:305–312. [PMC free article] [PubMed] [Google Scholar]
- 47.Miller WR. DHHD Publication No (SMA) 99-3354. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Substance Abuse Treatment; Rockville, MD: 1999. Enhancing motivation for change in substance abuse treatment. Treatment improvement protocol (TIP) series 35. [PubMed] [Google Scholar]
- 48.Rifai MA, Moles JK, Lehman LP, et al. Hepatitis C screening and treatment outcomes in patients with substance use/dependence disorders. Psychosomatics. 2006;47:112–121. doi: 10.1176/appi.psy.47.2.112. [DOI] [PubMed] [Google Scholar]
- 49.Dieperink E, Ho SB, Heit S, et al. Significant reductions in drinking following brief alcohol treatment provided in a hepatitis C clinic. Psychosomatics. 2010;51:149–156. doi: 10.1176/appi.psy.51.2.149. [DOI] [PubMed] [Google Scholar]
- 50.Berg CJ, Michelson SE, Safren SA. Behavioral aspects of HIV care: adherence, depression, substance use, and HIV-transmission behaviors. Infect Dis Clin North Am. 2007;21:181–200. doi: 10.1016/j.idc.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Golin C, Isasi F, Bontempi JB, et al. Secret pills: HIV-positive patients’ experiences taking antiretroviral therapy in North Carolina. AIDS Educ Prev. 2002;14:318–329. doi: 10.1521/aeap.14.5.318.23870. [DOI] [PubMed] [Google Scholar]
- 52.Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 53.Hendershot CS, Stoner SA, Pantalone DW, et al. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52:180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 55.Martin-Santos R, Diez-Quevedo C, Castellvi P, et al. De novo depression and anxiety disorders and influence on adherence during peginterferon-alpha-2a and ribavirin treatment in patients with hepatitis C. Aliment Pharmacol Ther. 2008;27:257–265. doi: 10.1111/j.1365-2036.2007.03568.x. [DOI] [PubMed] [Google Scholar]
- 56.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 57.Weiden PJ. The adherence interview: Better information, better alliance. [Accessed 15 June 2011.];Psychiatric Annals Online. 2011 :41. (online only). Available at: http://www.psychiatricannalsonline.com/view.asp?rID=84071.
- 58.Hill S, Kavookjian J. Motivational interviewing as a behavioral intervention to increase HAART adherence in patients who are HIV-positive: a systematic review of the literature. AIDS Care. doi: 10.1080/09540121.2011.630354. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 59.Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826–834. doi: 10.1097/MLR.0b013e31819a5acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin LR, Williams SL, Haskard KB, et al. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1:189–199. [PMC free article] [PubMed] [Google Scholar]
- 61.Tanioka D, Iwasaki Y, Araki Y, et al. Factors associated with adherence to combination therapy of interferon and ribavirin for patients with chronic hepatitis C: importance of patient’s motivation and physician’s treatment experience. Liver Int. 2009;29:721–729. doi: 10.1111/j.1478-3231.2008.01964.x. [DOI] [PubMed] [Google Scholar]
- 62.Simoni JM, Amico KR, Pearson CR, et al. Strategies for promoting adherence to antiretroviral therapy: a review of the literature. Curr Infect Dis Rep. 2008;10:515–521. doi: 10.1007/s11908-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Golin CE, Liu H, Hays RD, et al. A prospective study of predictors of adherence to combination antiretroviral medication. J Gen Intern Med. 2002;17:756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vezali E, Aghemo A, Colombo M. Interferon in the treatment of chronic hepatitis C: a drug caught between past and future. Expert Opin Biol Ther. 2011;11:301–313. doi: 10.1517/14712598.2011.552906. [DOI] [PubMed] [Google Scholar]
- 65.Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182–1188. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]


