Abstract
We studied the role of bacterial secondary metabolites in the context of grazing protection against protozoans. A model system was used to examine the impact of violacein-producing bacteria on feeding rates, growth, and survival of three common bacterivorous nanoflagellates. Freshwater isolates of Janthinobacterium lividum and Chromobacterium violaceum produced the purple pigment violacein and exhibited acute toxicity to the nanoflagellates tested. High-resolution video microscopy revealed that these bacteria were ingested by the flagellates at high rates. The uptake of less than three bacteria resulted in rapid flagellate cell death after about 20 min and cell lysis within 1 to 2 h. In selectivity experiments with nontoxic Pseudomonas putida MM1, flagellates did not discriminate against pigmented strains. Purified violacein from cell extracts of C. violaceum showed high toxicity to nanoflagellates. In addition, antiprotozoal activity was found to positively correlate with the violacein content of the bacterial strains. Pigment synthesis in C. violaceum is regulated by an N-acylhomoserine lactone (AHL)-dependent quorum-sensing system. An AHL-deficient, nonpigmented mutant provided high flagellate growth rates, while the addition of the natural C. violaceum AHL could restore toxicity. Moreover, it was shown that the presence of violacein-producing bacteria in an otherwise nontoxic bacterial diet considerably inhibited flagellate population growth. Our results suggest that violacein-producing bacteria possess a highly effective survival mechanism which may exemplify the potential of some bacterial secondary metabolites to undermine protozoan grazing pressure and population dynamics.
In most aquatic and terrestrial ecosystems, bacterial communities are exposed to grazing by bacterivorous protozoans. Predation by protozoans is viewed as a major factor in loss of bacterial biomass and production and an important selective force for bacterial community structure (for a review, see reference 25). In response to protozoan grazing, bacterial communities have been shown to develop inedible morphotypes, leading to changes in the structural and taxonomic composition (17, 26, 42, 43). Inedible morphologies, like cell filaments and aggregates or microcolonies, are an effective defense mechanism against a certain size class of predators. In addition to such size-related mechanisms, evidence has been recently provided for a number of size-unrelated, nonmorphological resistance mechanisms. Motility and swimming speed were identified as bacterial traits that successfully inhibit the process of prey capture in interception-feeding flagellates (34). Chemostat studies with mixed bacterial communities demonstrated that highly motile bacteria are able to establish a stable subpopulation in the presence of bacterivorous flagellates (36). Furthermore, the influence of the biochemical cell surface composition on the uptake of bacteria became evident (34). It has also been shown that indigestible prey is prematurely egested by nanoflagellates (2) and that some pathogenic bacteria are capable of surviving in food vacuoles (27).
Given the pronounced metabolic and physiological capabilities of bacteria, the potential for the production of secondary metabolites can be considered immense. Many of these secondary metabolites exhibit antibiotic and cytotoxic activity, so that aquatic microorganisms have become major targets in natural product research (14). The increasing number of biologically active compounds isolated from aquatic bacteria raises the question of their environmental functions. While microbial competition for limiting natural resources is widely thought to be the selective force that promotes biosynthesis of antimicrobial compounds (29, 30, 46), the role of microbial predation has been given hardly any credit so far. In plant-herbivore interactions, however, the production of secondary metabolites is generally regarded as a chemical defense strategy against grazing (19). As bacterial populations are ubiquitously exposed to protozoan grazing, it can be presumed that the production and accumulation of secondary metabolites in bacteria may play a major role in protection against protozoan predators.
The production of biologically active compounds is sometimes correlated with the presence of pigments in environmental isolates (13, 20, 21). In many experiments with enhanced protozoan grazing pressure, we observed an accumulation of pigmented strains in the bacterial community (C. Matz and K. Jürgens, unpublished data). It has been suggested that pigment formation by heterotrophic bacteria may be a protective mechanism not only against solar radiation (32) but also against protozoan grazing (45). Singh (44) reported that bacterial lawns with red, green, or violet pigmentation were not eaten by soil amoebae or even inhibited amoebal growth. Bacteria of the genus Janthinobacterium and Chromobacterium, which produce the purple pigment violacein, have repeatedly been isolated from lake bacterioplankton (12, 40; H. Güde, personal communication), rivers (7, 18), activated sludge (8; L. Eberl, unpublished data), marine environments (15), and soil (6). We isolated a violacein-producing bacterium from a low-nutrient chemostat that contained lake bacterioplankton growing in the presence of intense grazing activity.
In this study, we wanted to examine the mechanism underlying the successful persistence of violacein-producing bacteria in times of protozoan grazing and its consequences for protozoan population growth and survival. Moreover, this study was designed to give a first insight into the role of bacterial secondary metabolites in chemical defense against protozoan predation.
MATERIALS AND METHODS
Organisms and cultivation.
In our study we used two violacein-producing bacterial isolates. Janthinobacterium lividum CM37 was isolated from a mesotrophic lake. Chromobacterium violaceum ATCC 31532 (synonym CV0) was originally isolated from freshwater (47). Two mutants of C. violaceum CV0, which are affected in quorum-sensing regulation, were used in this study: CV017 (Smr mini-Tn5 Hgr), like the parental strain, produces the N-acylhomoserine lactone (AHL) N-hexanoyl-homoserine lactone (C6-HSL) but carries a genetically uncharacterized mutation causing derepression of AHL-inducible violacein production and thus produces much more pigment relative to the parental strain (3); CV026 (Smr mini-Tn5 Hgr cviI::Tn5xylE Kmr) is an AHL-negative mutant which was derived from CV0 as a result of a mini-Tn5 insertion in the cviI gene, encoding the C6-HSL synthase of this organism. This mutant is nonpigmented unless provided with exogenous AHL. For this reason CV026 is used in many laboratories as a sensitive biosensor for the detection of AHLs (37). All bacterial strains were routinely grown on yeast extract (5 g liter−1) at 20°C.
As model protozoan predators we used the three bacterivorous nanoflagellates Ochromonas sp. (Chrysomonadida), Spumella sp. (Chrysomonadida), and Bodo saltans (Kinetoplastida), which are among the most commonly reported heterotrophic flagellates (39). Ochromonas sp. was isolated by D. Springmann from mesotrophic Lake Constance, Germany. B. saltans and Spumella sp. were isolated from a mesotrophic lake (Schöhsee) in northern Germany. The flagellates were maintained on Pseudomonas putida MM1 (5) in WC medium (16) with a glucose concentration of 100 mg liter−1 and were kept in the dark at 16 ± 0.5°C. For all experiments, flagellates were taken from 5-day-old stock cultures when bacteria were reduced below 104 cells ml−1 and flagellate abundances reached approximately 106 cells ml−1.
Violacein extraction, quantification, and purification.
In order to determine the amount of pigment produced by each bacterial strain, the pigment was extracted and quantified photometrically by a method described by Blosser and Gray (1). Two-milliliter samples of the bacterial cultures were harvested by centrifugation (16,000 × g for 15 min), and the pellet was resuspended in WC medium. Four hundred microliters of the bacterial suspension was mixed with 400 μl of 10% sodium dodecyl sulfate and then incubated for 5 min at room temperature. Violacein was quantitatively removed from the cell fragments by vortexing with 900 μl of water-saturated butanol. The upper phase, containing violacein, was separated from the aqueous phase by centrifugation (16,000 × g for 10 min). The violacein content of the butanol phase was measured as absorbance at 585 nm with a spectrophotometer.
For examination of the antiprotozoal activity of violacein, the butanol phase was purified by high-performance liquid chromatography (HPLC) using a silica gel column (250 by 10 mm). Separation was achieved by applying a linear gradient solvent system of hexane-ethyl acetate (from 20:80 to 15:85 in 35 min). Violacein was detected at its absorbance maximum (585 nm).
Determination of cell numbers and cell size.
Bacterial and flagellate cell numbers were determined from formaldehyde (2%)-fixed samples. They were stained with 4′,6-diamidino-2-phenylindole (DAPI) and counted by epifluorescence microscopy (41). For the enumeration of flagellate cell numbers only cells with intact cell boundaries were taken into account as dead cells show diffuse structures due to lysis. Bacterial cell sizes were measured from DAPI preparations for 200 to 300 cells by an automated image analysis system (SIS GmbH, Münster, Germany) and an image processing procedure modified from Massana et al. (33).
Video microscopy.
In order to examine the behavior of flagellates feeding on violacein-producing bacteria, live observations were performed on Spumella sp. and Ochromonas sp. by means of high-resolution video microscopy employing an inverted microscope, a standard video camera, and a VCR. The experimental design largely follows one previously described (2, 34). Fifteen starved and 15 satiated cells of each flagellate species were monitored individually for at least 30 min after the addition of J. lividum CM37 and C. violaceum CV017 at a concentration of 107 cells ml−1. Starved and satiated flagellates were precultured at a prey density of 5 × 105 and 1 × 107 bacteria ml−1, respectively.
Feeding selectivity experiment.
Selective feeding of Ochromonas sp. on the three strains of C. violaceum (CV017, CV0, and CV026) was tested on a 1:1 mixture with the nontoxic reference strain P. putida MM1, which has been repeatedly proven to be highly edible for bacterivorous nanoflagellates (34, 35). Three-day-old bacterial cultures were harvested by centrifugation and resuspended in WC medium. From these suspensions cell numbers, sizes, and violacein content were determined (see above) before the following combinations were prepared: 50% CV0 plus 50% MM1, 50% CV017 plus 50% MM1, and 50% CV026 plus 50% MM1. Bacteria were added to the flagellate culture at a final concentration of 107 cells ml−1. After 10 min the experiment was terminated by the addition of ice-cold glutaraldehyde (2% final concentration). For the determination of ingestion rates, bacteria were counted in flagellate food vacuoles by using immunofluorescence microscopy (35). Sixty flagellate cells per replicate were inspected. Polyclonal antibodies against C. violaceum and P. putida MM1 were developed by immunizing rabbits (Eurogentec, Herstal, Belgium).
Survival experiments.
A series of batch culture experiments was performed to examine the population response of the flagellates to the presence of J. lividum CM37, the wild-type C. violaceum CV0, the violacein-overproducing mutant CV017, the nonpigmented strain CV026, and nontoxic P. putida MM1. Bacterial cultures were harvested by centrifugation (17,000 × g for 15 min) and washed with WC medium. All experiments were run in triplicate at 20°C in the dark. (i) The first experiment compared the response of Ochromonas sp. to suspensions of the two violacein-producing isolates C. violaceum CV0 and J. lividum CM37 at a concentration of 107 bacteria ml−1. Over a period of 6 h samples were fixed every hour for the enumeration of flagellate cells. (ii) The antiprotozoal activity of violacein was examined by adding the purified pigment (dissolved in methanol) to cultures of Spumella sp. and Ochromonas sp. at final concentrations of 200, 100, 50, 10, and 1 μM. Flagellates were counted after 72 h and compared with flagellate numbers in the methanol control. (iii) The toxic effects of the three C. violaceum strains (CV0, CV026, and CV017) were tested on cultures of Ochromonas sp. The three strains were applied separately in 1:1 mixtures with nontoxic P. putida MM1. The final bacterial concentration was 107 cells ml−1, and a flagellate culture without the addition of any bacteria served as nonfood control. Flagellate numbers were monitored every 3 h for a period of 12 h. (iv) In a fourth experiment, growth and survival of Ochromonas sp. and B. saltans were examined on different concentrations of the violacein-producing strain C. violaceum CV017 in an otherwise nontoxic bacterial diet. This was tested on an increasing proportion of CV017 (0, 10, 25, and 50%) mixed with the nontoxic P. putida MM1. Bacterial mixtures were added at a final concentration of 107 cells ml−1; no bacteria were added in the nonfood control. Flagellate numbers were followed over 55 h as described above.
AHL experiment.
The regulation of antiprotozoal activity in C. violaceum was investigated in a complementation experiment with the AHL-negative mutant CV026 and the signal molecule C6-HSL. CV026 was grown on yeast extract (5 g liter−1) in the presence and absence of 200 nM C6-HSL (dissolved in ethyl acetate) and then compared with strain CV017. Bacterial cultures were harvested by centrifugation (17,000 × g for 15 min) and washed with WC medium. The production of violacein was measured photometrically (see above) before the three strains were added to Ochromonas sp. cultures at a concentration of 107 bacteria ml−1. Samples for the determination of flagellate numbers were taken every hour.
Data analysis.
In the survival experiments, LT50 values were used to describe the time needed to kill 50% of the initial flagellate population. In the feeding selectivity experiment, the flagellate clearance rate, F, was determined from the ingestion rate I (bacteria flagellate−1 hour−1) by using the following formula: F = I/B = nanoliters flagellate−1 hour−1. B refers to the bacterial concentration (cells milliliter−1) used. Feeding selectivity was then calculated by using the Jacobs index D (23). The selectivity D with regard to particle 1 (here C. violaceum) is calculated as follows: D1 = (F1 − F2)/(F1 + F2). D1 can have values from +1 (exclusive ingestion of particle 1) to −1 (exclusive ingestion of particle 2). If D1 = 0, feeding is unselective.
One-way analysis of variance was used to test for significant differences between the bacterial strains and between flagellate abundances, feeding, and growth rates. Changes in flagellate numbers over time were tested for significance with repeated measures analysis of variance. Tukey tests provided post-hoc comparisons of means (STATISTICA Version 5.1; StatSoft).
Nucleotide sequence accession number.
The 16S rRNA gene sequence of J. lividum has been deposited in the GenBank database under accession number AY24741.
RESULTS
Acute toxicity of two violacein-producing bacterial isolates to Ochromonas sp.
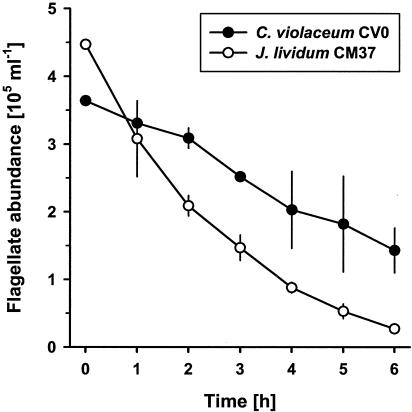
The toxicities of the two violacein-producing freshwater isolates J. lividum CM37 and C. violaceum CV0 to a bacterivorous nanoflagellate were compared. Both bacterial isolates revealed an acute toxic effect on Ochromonas sp., resulting in a 50% drop in flagellate cell numbers within the first 6 h of the experiment (Fig. 1). Specific LT50 values determined from the flagellate survivor curves indicated a significantly higher toxicity of J. lividum CM37 (P < 0.05): while the mean LT50 value for C. violaceum CV0 exceeded 5 h, the mean LT50 value for J. lividum CM37 was 1.8 h.
FIG. 1.
Cell numbers of the flagellate Ochromonas sp. feeding on suspensions of the two violacein-producing isolates, C. violaceum CV0 and J. lividum CM37. Flagellate numbers include only structurally intact cells and are given as means ± standard deviations (n = 3).
Flagellate cell death and lysis after ingestion of violacein-containing bacteria.
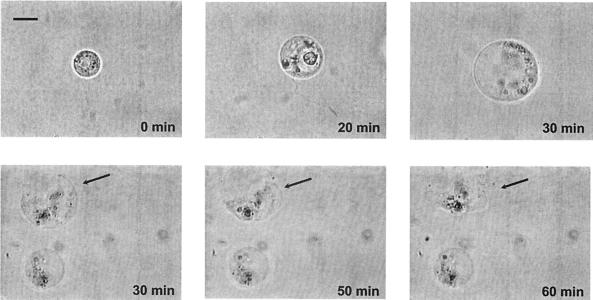
By means of high-resolution video microscopy we documented the ingestion process and the cellular response of two flagellates, Ochromonas sp. and Spumella sp., feeding on C. violaceum CV0 and J. lividum CM37. We consistently observed for both bacteria and both flagellates that flagellar beating ceased and the protozoan cell was swollen about 20 min after ingestion began (Fig. 2). Further swelling and pronounced vacuolization of the flagellate caused the cell to burst after 1 to 2 h. Table 1 gives feeding parameters of starved and satiated Ochromonas sp. and Spumella sp. on J. lividum CM37 as directly measured for individual cells. Satiated flagellate cells were killed by the uptake of one to three bacteria (Spumella sp.) and one to seven bacteria (Ochromonas sp.). After internalizing J. lividum CM37 cells, satiated flagellates showed an early egestion of all food vacuoles. Ingestion rates of starved flagellates were two to three times higher, but times of flagellate cell death did not differ significantly from those of satiated flagellates.
FIG. 2.
Lysis of Ochromonas sp. cells feeding on C. violaceum CV017. After the ingestion of bacteria, flagellar beating by the flagellate becomes irregular until it ceases after about 20 min. Note the swelling of the flagellate cell and the final burst after 60 min. Light video microscopy (magnification, ×1200; oil immersion). Scale bar = 5 μm.
TABLE 1.
Feeding of the two nanoflagellates Spumella sp. and Ochromonas sp. on J. lividum CM37a
| Nanoflagellate and treatment | No. of ingested bacteria (cells flagellate−1) | Time of egestion (min) | Time of cell death (min) |
|---|---|---|---|
| Spumella sp. | |||
| Starved | 5.0 ± 1.8 | 18.5 ± 5.8 | |
| Satiated | 1.7 ± 0.7 | 13.2 ± 4.9 | 21.3 ± 5.1 |
| Ochromonas sp. | |||
| Starved | 6.7 ± 2.4 | 18.8 ± 6.9 | |
| Satiated | 3.8 ± 2.7 | 18.7 ± 4.8 | 23.0 ± 7.2 |
The number of ingested bacteria is the total number ingested from the start of the experiment until the death of the flagellates. Time of egestion and time of flagellate cell death were calculated as the interval between ingestion of the first bacterium and either egestion or cessation of flagellar movement, respectively. The data (presented as means ± standard deviations) are based on 15 individual flagellate cells.
Antiprotozoal activity of violacein.
HPLC-purified violacein from C. violaceum exhibited high toxicity to cultures of Spumella sp. and Ochromonas sp. The concentration causing 50% reduction of flagellate numbers was approximately 10 μM. The methanol concentration used for dissolving violacein had no effect on flagellate numbers. At a violacein concentration of 10 μM, cell numbers were reduced by 51.2% ± 4.6% for Spumella sp. and by 48.7% ± 3.9% for Ochromonas sp. Higher concentrations of violacein resulted in the complete reduction of flagellate numbers.
Variability in violacein production between three C. violaceum strains.
The three C. violaceum strains CV0, CV017, and CV026 were analyzed with respect to the production of violacein during growth in batch cultures. At the same time we also measured cell numbers and cell length. In agreement with a previous study (3), marked differences in the violacein content were observed between the three strains. While cells of the wild-type CV0 and the hyperpigmented mutant CV017 showed a steady increase in violacein content over a 4-day period, no pigment production was detected in the case of the AHL-deficient strain CV026 (data not shown). CV017 cells permanently contained more than twice as much violacein relative to CV0 cells (Table 2). Differences for the calculated violacein content per cell between the three strains were significant (P < 0.05).
TABLE 2.
Feeding selectivity of Ochromonas sp. on a 1:1 mixture of the nontoxic P. putida MM1 with each of three strains of C. violaceum, CV017, CV0, and CV026a
| Strain | Violacein content (A cell−1) | Cell length (μm) | C. violaceum clearance rate (nanoliters flagellate−1 h−1) | MM1 clearance rate (nanoliters flagellate−1 h−1) | Selectivity index D |
|---|---|---|---|---|---|
| CV017 | 0.32 ± 0.01 | 2.0 ± 0.29 | 1.9 ± 0.09 | 1.5 ± 0.17 | 0.13 ± 0.07 |
| CV0 | 0.12 ± 0.01 | 1.7 ± 0.10 | 1.8 ± 0.18 | 1.5 ± 0.21 | 0.08 ± 0.07 |
| CV026 | 0.00 ± 0.00 | 1.9 ± 0.02 | 2.1 ± 0.03 | 1.3 ± 0.24 | 0.24 ± 0.09 |
The violacein content per cell was calculated from the cell number in suspension and the absorbance (A) (at 585 nm) of the respective cell extract. Clearance rates were determined from the numbers of bacteria in the food vacuoles of 60 flagellates per replicate. Feeding selectivity was calculated by using the Jacobs index D. All values are given as means ± standard deviations (n = 3).
Unselective feeding of Ochromonas sp. on violacein-producing bacteria.
Feeding selectivity of Ochromonas sp. was examined on 1:1 mixtures of nontoxic P. putida MM1 with each of the three C. violaceum strains (CV017, CV0, and CV026). The three strains were similar in cell size but differed significantly in their violacein content per cell (P < 0.002; Table 2): Pigmentation was highest for strain CV017, while strain CV026 remained nonpigmented. The clearance rates calculated from flagellate ingestion rates were not significantly different between the C. violaceum strains. Selectivity indices (D values) were positive, indicating that Ochromonas sp. did not select in favor of the nontoxic P. putida MM1. Rather, a value of D greater than 0 revealed a slight but insignificant preference for the C. violaceum strains (P > 0.1).
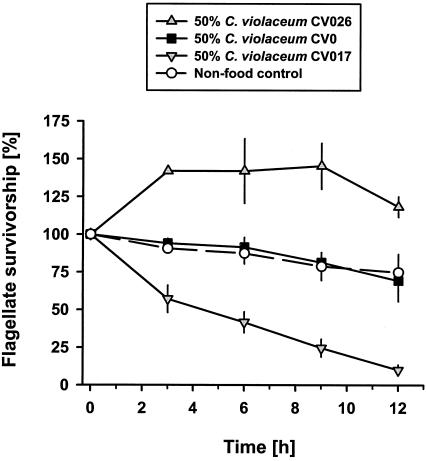
Violacein-content-dependent mortality of Ochromonas sp.
The influence of bacterial violacein content on flagellate survival was tested on 1:1 mixtures of the nontoxic P. putida MM1 and the wild-type C. violaceum CV0, the hyperpigmented strain CV017, and the nonpigmented strain CV026. When grazing on the nonpigmented CV026, Ochromonas sp. exhibited a distinct increase in cell numbers by 45% to 13.8 × 104 flagellates ml−1 within the first 3 h of the experiment (Fig. 3). After 9 h flagellate numbers decreased slowly due to food shortage. The toxicity of the wild-type CV0 compensated for flagellate growth on the nontoxic P. putida MM1, leading to a 30% reduction of flagellate survival within 12 h. In the presence of CV0, the flagellate population was reduced at the same rate as in the nonfood control, where Ochromonas sp. numbers slightly decreased due to starvation. The strongest decrease in flagellate abundance was observed on the hyperpigmented CV017. Despite the presence of the nontoxic P. putida MM1, flagellate numbers were reduced by 50% within less than 4.5 h and only 10% were left after 12 h at the end of the experiment.
FIG. 3.
Growth and survival of Ochromonas sp. on three strains of C. violaceum containing different amounts of violacein. The wild-type C. violaceum CV0, the hyperpigmented strain CV017, and the nonpigmented strain CV026 were offered in 1:1 mixtures with nontoxic P. putida MM1. No bacteria were added to the nonfood control. Survivorship values are given as means ± standard deviations (n = 3).
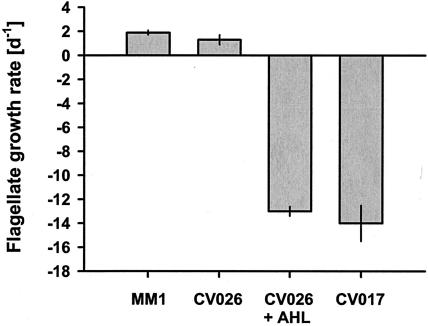
Antiprotozoal activity is quorum sensing regulated.
The role of quorum sensing in the production of the antiprotozoal compound was examined with C. violaceum CV026, which carries a mutation in the cviI gene, encoding the C6-HSL synthase. We tested whether the addition of the natural C. violaceum AHL could restore toxicity of the AHL-deficient strain CV026. As described above, no pigmentation was found for CV026 when grown without C6-HSL. With C6-HSL, pigment production in CV026 was nearly as high as in CV017 (0.30 ± 0.01 absorbance unit cell−1 versus 0.33 ± 0.01 absorbance unit cell−1). While the growth rate of Ochromonas sp. on CV026 grown without C6-HSL (1.3 ± 0.4 day−1) was comparable to that on the nontoxic P. putida MM1 (1.9 ± 0.2 day−1), flagellates feeding on CV026 grown in the presence of C6-HSL had a mortality rate as high as on CV017 (Fig. 4).
FIG. 4.
Influence of quorum sensing on population growth rates of Ochromonas sp. The AHL-deficient strain CV026 was grown in the presence and absence of the AHL C6-HSL before being fed to Ochromonas sp. The violacein-overproducing strain CV017 and nontoxic P. putida MM1 were used as reference strains. Flagellate growth rates are given as means ± standard deviations (n = 3).
Flagellate growth is reduced by the occurrence of violacein-producing bacteria in an otherwise nontoxic bacterial diet.
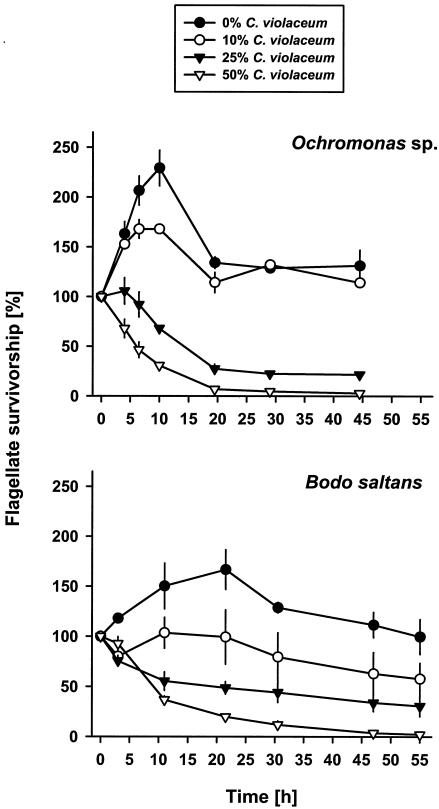
We tested the impact of violacein-producing cells on growth and survival of two bacterivorous nanoflagellates in an otherwise nontoxic prey assortment by offering four different food ratios of the pigmented strain CV017 and nontoxic P. putida MM1. Figure 5 (upper graph) shows that survival of Ochromonas sp. decreased with an increasing proportion of CV017 in the bacterial prey offered. In the 0% CV017 (100% P. putida MM1) treatment, flagellate numbers more than doubled in the first 10 h followed by a decrease to densities only slightly greater than initial values. Growth on 10% CV017 (90% P. putida MM1) was less pronounced, leading to a 42% lower flagellate peak abundance compared to the 0% CV017 treatment. Higher concentrations of CV017 (25 and 50%) resulted in an overall reduction of flagellate cell numbers (P < 0.001). In the 50% CV017 treatment half of the flagellate population was killed after 6 ± 1 h (LT50 value). Growth experiments with B. saltans provided comparable results (Fig. 5, lower graph). While the 0% CV017 treatment provided a more than 50% increase of flagellate numbers, all other treatments showed a slight-to-dramatic drop in flagellate survival. Other than for Ochromonas sp., growth of B. saltans was completely inhibited on 10% CV017. Again, the greatest decrease was observed in the 50% CV017 treatment, where flagellate numbers decreased by 50% (LT50 value) after about 9 ± 0.5 h.
FIG. 5.
Growth and survival of Ochromonas sp. (upper graph) and B. saltans (lower graph) at different concentrations of C. violaceum. Strain CV017 was added to nontoxic P. putida MM1 in four ratios (0, 10, 25, and 50% of CV017). Flagellate survivorship is given as means ± standard deviations (n = 3).
DISCUSSION
Toxic effect on flagellate predators.
Our data confirm reports from the 1940s and 1960s that C. violaceum has an inhibitory effect on protozoans (8, 44, 45). Most of these studies lacked detailed information on the bacterial concentrations applied and allowed only very limited conclusions on the ecological implications. Here we provide evidence that the uptake of a single bacterial cell can have a fatal effect on the consuming protozoan cell. This illustrates the high toxicity of violacein-producing bacteria to their protozoan predators. In fact, our data showed that the occurrence of 10% violacein-producing bacteria in an otherwise nontoxic food assemblage lowers the peak abundance of the flagellate population by more than 40%. A less pronounced impact on Ochromonas sp. in the 10% CV017 treatment may have resulted from an uncontrolled light regime in that treatment. Responses of the three flagellate species tested were generally consistent. All of them were killed rapidly, so that for instance, the time of cell death did not differ between Spumella sp. and Ochromonas sp. Higher LT50 values of Ochromonas sp. compared to B. saltans in the cell-concentration-dependent mortality experiment can be explained by higher feeding rates of Ochromonas sp (35). The ability of interception-feeding nanoflagellates to chemically discriminate between different food items has previously been demonstrated (24, 34, 49). However, selectivity indices from our selection experiment clearly indicate that the ingestion of the violacein-producing strains could not be avoided by the flagellates. Neither discriminative food uptake nor discriminative egestion from food vacuoles was observed. Taken together, the combination of acute toxicity and the inability to avoid or discriminate against violacein-producing bacteria has deleterious consequences for the flagellate cell.
We have indications that ingested bacteria need to be slightly digested for the toxins to be released. Hence, it is probable that ingested bacteria do not survive. Even though some individuals are ingested and do not survive to reproduce, it seems that the remaining clonal prey population benefits from reduced grazing pressure (see reference 51 for a review). Moreover, it seems to be economically beneficial to store the toxin rather than secrete it. Antiprotozoal activity in cell-free supernatants was found only in late-stationary-phase cultures, which was possibly due to cell lysis. Compared to the cells, the supernatant still showed considerably lower activity (data not shown).
Antiprotozoal compound and its regulation.
Our results from the toxicity assays with purified violacein provided evidence for the direct role of the pigment in the antiprotozoal activity of violacein-producing bacteria. This is in accordance with studies reporting that violacein exhibits biological activity against a number of organisms, including bacteria (28), mammalian cell lines (38), and trypanosomes (11). The pronounced biological activity of violacein, together with our finding that the violacein content of the strains investigated correlated well with their protozoan killing efficiency, strongly supports the idea that violacein is the major antiprotozoal compound produced by C. violaceum and J. lividum. However, violacein is not the only biologically active compound produced by C. violaceum (11). Therefore, further work will be required to elucidate whether other factors also contribute to the toxicity of the organism.
The production of violacein in C. violaceum is controlled by the cvi quorum-sensing system, which ensures that some phenotypic traits are expressed only when the bacterial population has reached a certain density. The cvi system consists of the AHL synthase CviI, which directs the synthesis of C6-HSL, and CviR, which after binding of C6-HSL, is thought to activate or repress transcription of target genes. Previous work has shown that, in addition to pigment synthesis, the cvi system positively regulates production of extracellular proteases, chitinases, hydrogen cyanide, and antibiotics (3). The coordinated expression of these factors by the quorum-sensing system is thought to contribute to the competitive fitness of the organism in its natural habitat. In full agreement with this hypothesis, we showed that C6-HSL-regulated production of the pigment violacein is toxic for protozoa and thus appears to be an essential factor for the survival of the bacterium in the environment. Interestingly, in Serratia sp. strain ATCC 39006 the production of the red pigment prodigiosin is also quorum sensing regulated (48). Like violacein, prodigiosins have a broad range of biological properties, including antibacterial, antimalarial, antifungal, and antiprotozoal activities (50). Although the true function of this pigment remains unclear, it has been suggested that it plays an active role in the competitive survival of the organism (9).
Ecological significance and implications for microbial food webs.
Despite the growing interest in the study of secondary metabolites from aquatic bacteria insights into their ecological roles are still limited. Bacteria producing biologically active compounds seem to be ubiquitous in both seawater and freshwater. Some studies have focused on the role of algicidal bacteria in the termination and decomposition of algal blooms and raised the possibility that they act as natural regulators of harmful algal blooms (10). There are several examples of bacterial interactions with algal species, and evidence accumulates that destructive effects of bacteria might be temporarily an important factor regulating primary production in marine ecosystems (e.g., see reference 22). Lovejoy et al. (31) isolated a yellow-pigmented Pseudoalteromonas strain from an algal bloom which caused rapid cell lysis and death (within 3 h) of autotrophic flagellates. Yoshinaga et al. (52) monitored a bloom of the dinoflagellate Gymnodinium mikimotoi in Tanabe Bay, Japan, and isolated a total of 28 bacterial strains that killed the dinoflagellate in laboratory experiments.
All of these findings strongly suggest that toxic bacteria may also be an important factor in the population dynamics of bacterivorous protists. A reduction of protozoan feeding activity due to the occurrence of toxic bacteria would undermine the grazing pressure on the entire bacterial community. In addition, the killing of flagellates may not serve solely as a protective mechanism for the bacteria; the lysis of the flagellates and the released organic matter may also serve as a nutrient source and may support bacterial growth (Matz and Jürgens, unpublished). Finally, the high sensitivity of heterotrophic nanoflagellates to bacterial toxins is of potential ecological significance because changes in the abundance, size, and species distribution within the nanoplankton communities will also affect higher trophic levels of the zooplankton community (4). Bacterivorous metazooplankton may even be directly affected in fitness by toxin-producing bacteria (P. Deines, C. Matz, and K. Jürgens, unpublished data). We suggest that bacterial antipredator compounds are important for the understanding of microbial food webs and the coupling of biogeochemical processes.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (grants Ju 367/2-1 and /2-2, Ar 288/3-1 and /3-2, and Ma 2491/1-1).
We thank Paul Williams for providing C. violaceum strains and Martin W. Hahn for providing Ochromonas sp.
REFERENCES
- 1.Blosser, R. S., and K. M. Gray. 2000. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J. Microbiol. Methods 40:47-55. [DOI] [PubMed] [Google Scholar]
- 2.Boenigk, J., C. Matz, K. Jürgens, and H. Arndt. 2001. The influence of preculture conditions and food quality on the ingestion and digestion process of three species of heterotrophic nanoflagellates. Microb. Ecol. 42:168-176. [DOI] [PubMed] [Google Scholar]
- 3.Chernin, L. S., M. K. Winson, J. M. Thompson, S. Haran, B. W. Bycroft, I. Chet, P. Williams, and G. S. Stewart. 1998. Chitinolytic activity in Chromobacterium violaceum: substrate analysis and regulation by quorum sensing. J. Bacteriol. 180:4435-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christoffersen, K. 1996. Ecological implications of cyanobacterial toxins in aquatic food webs. Phycologia 35:42-50. [Google Scholar]
- 5.Christoffersen, K., O. Nybroe, K. Jürgens, and M. Hansen. 1997. Measurement of bacterivory by heterotrophic nanoflagellates using immunofluorescence labelling of ingested cells. Aquat. Microb. Ecol. 13:127-134. [Google Scholar]
- 6.Corpe, W. 1953. Variation in pigmentation and morphology of colonies of gelatinous strains of Chromobacterium species from soil. J. Bacteriol. 66:470-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covert, J. S., and M. A. Moran. 2001. Molecular characterization of estuarine bacterial communities that use high- and low-molecular weight fractions of dissolved organic carbon. Aquat. Microb. Ecol. 25:127-139. [Google Scholar]
- 8.Curds, C. R., and J. M. Vandyke. 1966. The feeding habits and growth rates of some fresh-water ciliates found in activated-sludge plants. J. Appl. Ecol. 3:127-137. [Google Scholar]
- 9.Demain, A. L. 1995. Why do microorganisms produce antimicrobials?, p. 205-228. In P. A. Hunter, G. K. Darby, and N. J. Russell (ed.), Fifty years of antimicrobials: past perspectives and future trends. Symposium 53. Society for General Microbiology, Cambridge, United Kingdom.
- 10.Doucette, G. J. 1995. Interactions between bacteria and harmful algae: a review. Nat. Toxins 3:65-74. [DOI] [PubMed] [Google Scholar]
- 11.Duran, N., and C. F. Menck. 2001. Chromobacterium violaceum: a review of pharmacological and industiral perspectives. Crit. Rev. Microbiol. 27:201-222. [DOI] [PubMed] [Google Scholar]
- 12.Edwards, M. L., A. K. Lilley, T. H. Timms-Wilson, I. P. Thompson, and I. Cooper. 2001. Characterisation of the culturable heterotrophic bacterial community in a small eutrophic lake (Priest Pot). FEMS Microbiol. Ecol. 35:295-304. [DOI] [PubMed] [Google Scholar]
- 13.Egan, S., S. James, C. Holmström, and S. Kjelleberg. 2002. Correlation between pigmentation and antifouling compounds produced by Pseudoalteromonas tunicata. Environ. Microbiol. 4:433-442. [DOI] [PubMed] [Google Scholar]
- 14.Fenical, W. 1993. Chemical studies of marine bacteria: developing a new resource. Chem. Rev. 93:1673-1683. [Google Scholar]
- 15.Gauthier, M. J., J. M. Shewan, D. M. Gibson, and J. V. Lee. 1975. Taxonomic position and seasonal variations in marine neritic environment of some gram-negative antibiotic-producing bacteria. J. Gen. Microbiol. 87:211-218. [DOI] [PubMed] [Google Scholar]
- 16.Guillard, R. R. L., and C. J. Lorenzen. 1972. Yellow-green algae with chlorophyllide c. J. Phycol. 8:10-14. [Google Scholar]
- 17.Hahn, M. W., and M. G. Höfle. 2001. Grazing of protozoa and its effect on populations of aquatic bacteria. FEMS Microbiol. Ecol. 35:113-121. [DOI] [PubMed] [Google Scholar]
- 18.Halda-Alija, L., and T. C. Johnston. 1999. Diversity of culturable heterotrophic aerobic bacteria in pristine stream bed sediments. Can. J. Microbiol. 45:879-884. [PubMed] [Google Scholar]
- 19.Hay, M. E., and W. Fenical. 1988. Marine plant-herbivore interactions: the ecology of chemical defense. Annu. Rev. Ecol. Syst. 19:111-145. [Google Scholar]
- 20.Holmström, C., S. Egan, A. Franks, S. McCloy, and S. Kjelleberg. 2002. Antifouling activities expressed by marine surface associated Pseudoalteromonas species. FEMS Microbiol. Ecol. 41:47-58. [DOI] [PubMed] [Google Scholar]
- 21.Holmström, C., S. James, S. Egan, and S. Kjelleberg. 1996. Inhibition of common fouling organisms by marine bacterial isolates with special reference to the role of pigmented bacteria. Biofouling 10:251-259. [DOI] [PubMed] [Google Scholar]
- 22.Imai, I., Y. Ishida, and Y. Hata. 1993. Killing of marine phytoplankton by a gliding bacterium Cytophaga sp., isolated from the coastal sea of Japan. Mar. Biol. 116:527-532. [Google Scholar]
- 23.Jacobs, J. 1974. Quantitative measurement of food selection—modification of the forage ratio and Ivlev's electivity index. Oecologia 14:413-417. [DOI] [PubMed] [Google Scholar]
- 24.Jürgens, K., and W. R. De Mott. 1995. Behavioral flexibility in prey selection by bacterivorous nanoflagellates. Limnol. Oceanogr. 40:1503-1507. [Google Scholar]
- 25.Jürgens, K., and C. Matz. 2002. Predation as a shaping force for the phenotypic and genotypic composition of planktonic bacteria. Antonie Leeuwenhoek 81:413-434. [DOI] [PubMed] [Google Scholar]
- 26.Jürgens, K., J. Pernthaler, S. Schalla, and R. Amann. 1999. Morphological and compositional changes in a planktonic bacterial community in response to enhanced protozoan grazing. Appl. Environ. Microbiol. 65:1241-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King, C. H., E. B. Shotts, Jr., R. E. Wooley, and K. G. Porter. 1988. Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl. Environ. Microbiol. 54:3023-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichstein, H. C., and V. F. Van de Sand. 1945. Violacein, an antibiotic pigment produced by Chromobacterium violaceum. J. Infect. Dis. 76:47-51. [Google Scholar]
- 29.Long, R. A., and F. Azam. 2001. Antagonistic interactions among marine pelagic bacteria. Appl. Environ. Microbiol. 67:4975-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long, R. A., A. Qureshi, D. J. Faulkner, and F. Azam. 2003. 2-n-Pentyl-4-quinolinol produced by a marine Alteromonas sp. and its potential ecological and biogeochemical roles. Appl. Environ. Microbiol. 69:568-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovejoy, C., J. P. Bowman, and G. M. Hallegraeff. 1998. Algicidal effects of a novel marine Pseudoalteromonas isolate (class Proteobacteria, gamma subdivision) on harmful algal bloom species of the genera Chattonella, Gymnodinium, and Heterosigma. Appl. Environ. Microbiol. 64:2806-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margalith, P. 1992. Pigment microbiology. Chapman & Hall, London, United Kingdom.
- 33.Massana, R., J. M. Gasol, P. K. Bjornsen, N. Blackburn, A. Hagström, S. Hietanen, B. H. Hygum, J. Kuparinen, and C. Pedros-Alio. 1997. Measurement of bacterial size via image analysis of epifluorescence preparations—description of an inexpensive system and solutions to some of the most common problems. Sci. Mar. 61:397-407. [Google Scholar]
- 34.Matz, C., J. Boenigk, H. Arndt, and K. Jürgens. 2002. Role of bacterial phenotypic traits in selective feeding of the heterotrophic nanoflagellate Spumella sp. Aquat. Microb. Ecol. 27:137-148. [Google Scholar]
- 35.Matz, C., and K. Jürgens. 2001. Effects of hydrophobic and electrostatic cell surface properties of bacteria on feeding rates of heterotrophic nanoflagellates. Appl. Environ. Microbiol. 67:814-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matz, C., and K. Jürgens. 2003. Interaction of nutrient limitation and protozoan grazing determines the phenotypic structure of a bacterial community. Microb. Ecol. 45:384-398. [DOI] [PubMed] [Google Scholar]
- 37.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 38.Melo, P. S., G. Z. Justo, M. B. de Azevedo, N. Duran, and M. Haun. 2003. Violacein and its beta-cyclodextrin complexes induce apoptosis and differentiation in HL60 cells. Toxicology 186:217-225. [DOI] [PubMed] [Google Scholar]
- 39.Patterson, D. J., and W. Y. Lee. 2000. Geographic distribution and diversity of free-living heterotrophic flagellates, p. 269-287. In B. S. C. Leadbeater and J. C. Green (ed.), The flagellates—unity, diversity and evolution. Taylor & Francis, London, United Kingdom.
- 40.Pearce, D. A., C. J. van der Gast, B. Lawley, and J. C. Ellis-Evans. 2003. Bacterioplankton community diversity in a maritime Antarctic lake, determined by culture-dependent and culture-independent techniques. FEMS Microbiol. Ecol. 45:59-70. [DOI] [PubMed] [Google Scholar]
- 41.Porter, K. G., and Y. S. Feig. 1980. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25:943-948. [Google Scholar]
- 42.Rønn, R., A. E. McCaig, B. S. Griffiths, and J. I. Prosser. 2002. Impact of protozoan grazing on bacterial community structure in soil microcosms. Appl. Environ. Microbiol. 68:6094-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Šimek, K., J. Pernthaler, M. G. Weinbauer, K. Hornak, J. R. Dolan, J. Nedoma, M. Masin, and R. Amann. 2001. Changes in bacterial community composition and dynamics and viral mortality rates associated with enhanced flagellate grazing in a mesoeutrophic reservoir. Appl. Environ. Microbiol. 67:2723-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh, B. N. 1945. The selection of bacterial food by soil amoebae, and the toxic effects of bacterial pigments and other products on soil protozoa. Br. J. Exp. Pathol. 26:316-325. [Google Scholar]
- 45.Singh, B. N. 1942. Toxic effects of certain bacterial metabolic products on soil protozoa. Nature 149:168. [Google Scholar]
- 46.Slattery, M., I. Rajbhandari, and K. Wesson. 2001. Competition-mediated antibiotic induction in the marine bacterium Streptomyces tenjimariensis. Microb. Ecol. 41:90-96. [DOI] [PubMed] [Google Scholar]
- 47.Sneath, P. H. A. 1956. Cultural and biochemical characteristics of the genus Chromobacterium. J. Gen. Microbiol. 15:70-98. [DOI] [PubMed] [Google Scholar]
- 48.Thomson, N. R., M. A. Crow, S. J. McGowan, A. Cox, and G. P. Salmond. 2000. Biosynthesis of carbapenem antibiotic and prodigiosin pigment in Serratia is under quorum sensing control. Mol. Microbiol. 36:539-556. [DOI] [PubMed] [Google Scholar]
- 49.Verity, P. G. 1991. Feeding in planktonic protozoans: evidence for non-random acquisition of prey. J. Protozool. 38:69-76. [Google Scholar]
- 50.Williams, R. P., and S. M. Quadri. 1980. The pigments of Serratia, p. 31-75. In A. Von Graevenitz and S. J. Rubin (ed.), The genus Serratia. CRC Press Inc, Boca Raton, Fla.
- 51.Wolfe, G. V. 2000. The chemical defense ecology of marine unicellular plankton: constraints, mechanisms, and impacts. Biol. Bull. 198:225-244. [DOI] [PubMed] [Google Scholar]
- 52.Yoshinaga, I., T. Kawai, and Y. Ishida. 1997. Analysis of algicidal ranges of the bacteria killing the marine dinoflagellate Gymnodinium mikimotoi isolated from Tanabe Bay, Wakayama Pref., Japan. Fish Sci. 63:94-98. [Google Scholar]