Abstract
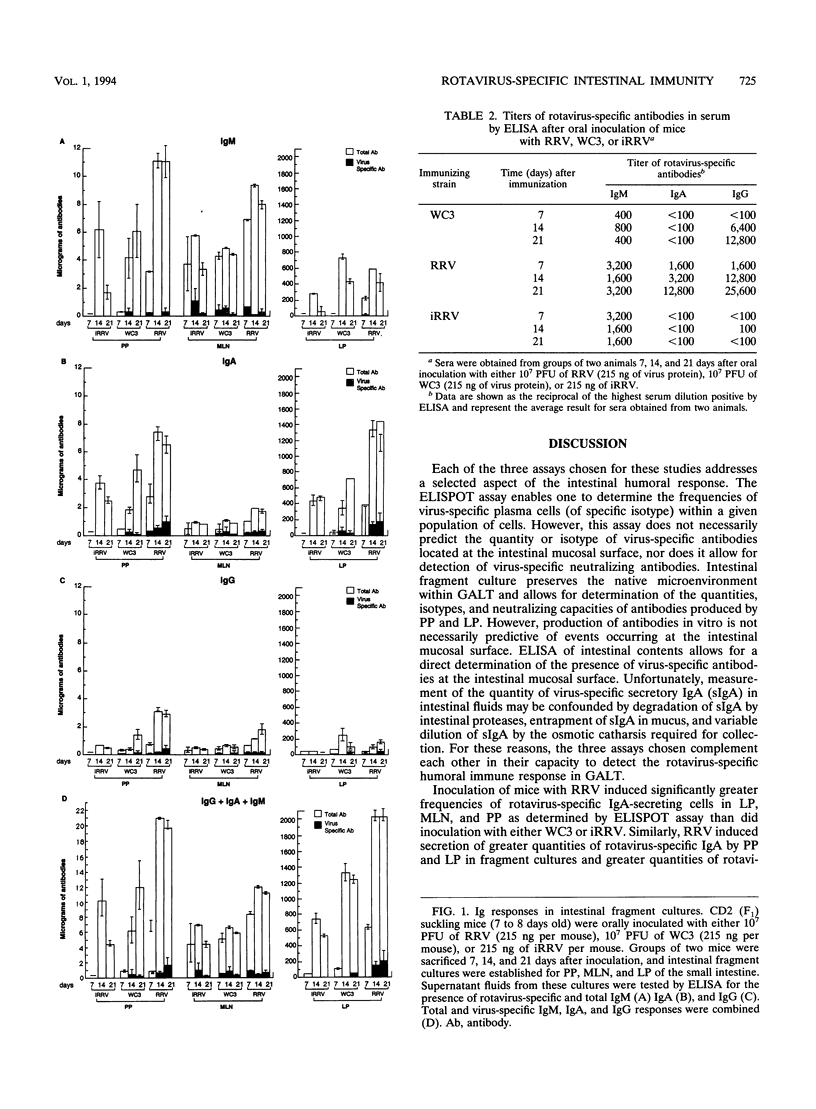
Primate rotavirus strain RRV and bovine strain WC3 or reassortants made between these animal viruses and human rotaviruses have been administered to infants as candidate vaccines. We compared RRV and WC3 in a murine model of oral infection. We determined the relative capacities of these viruses to induce a virus-specific humoral immune response by intestinal lymphocytes as tested by enzyme-linked immunospot assay, intestinal fragment culture, and enzyme-linked immunosorbent assay of intestinal contents. We found that inoculation of mice with RRV induced higher frequencies of virus-specific immunoglobulin A (IgA)-secreting cells in the lamina propria, greater quantities of virus-specific IgA in intestinal fragment cultures, and greater quantities of virus-specific IgA in intestinal secretions than did inoculation with WC3 or inactivated RRV (iRRV). The induction of an IgA response in serum was predictive of an IgA response among intestinal lymphocytes after inoculation with RRV but not WC3. In addition, large quantities of IgG, IgA, and IgM not specific for rotavirus were produced in fragment cultures from mice inoculated with RRV but not in cultures from mice inoculated with WC3 or iRRV. Possible mechanisms of RRV-induced polyclonal stimulation of intestinal B cells are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beagley K. W., Eldridge J. H., Kiyono H., Everson M. P., Koopman W. J., Honjo T., McGhee J. R. Recombinant murine IL-5 induces high rate IgA synthesis in cycling IgA-positive Peyer's patch B cells. J Immunol. 1988 Sep 15;141(6):2035–2042. [PubMed] [Google Scholar]
- Beagley K. W., Eldridge J. H., Lee F., Kiyono H., Everson M. P., Koopman W. J., Hirano T., Kishimoto T., McGhee J. R. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989 Jun 1;169(6):2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell L. M., Clark H. F., O'Brien E. A., Kornstein M. J., Plotkin S. A., Offit P. A. Gastroenteritis caused by human rotaviruses (serotype three) in a suckling mouse model. Proc Soc Exp Biol Med. 1987 Jan;184(1):127–132. doi: 10.3181/00379727-184-rc2. [DOI] [PubMed] [Google Scholar]
- Bernstein D. I., Kacica M. A., McNeal M. M., Schiff G. M., Ward R. L. Local and systemic antibody response to rotavirus WC3 vaccine in adult volunteers. Antiviral Res. 1989 Dec;12(5-6):293–300. doi: 10.1016/0166-3542(89)90056-9. [DOI] [PubMed] [Google Scholar]
- Bernstein D. I., Smith V. E., Sander D. S., Pax K. A., Schiff G. M., Ward R. L. Evaluation of WC3 rotavirus vaccine and correlates of protection in healthy infants. J Infect Dis. 1990 Nov;162(5):1055–1062. doi: 10.1093/infdis/162.5.1055. [DOI] [PubMed] [Google Scholar]
- Bland P. W., Warren L. G. Antigen presentation by epithelial cells of the rat small intestine. I. Kinetics, antigen specificity and blocking by anti-Ia antisera. Immunology. 1986 May;58(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Ceyhan M., Kanra G., Seçmeer G., Midthun K., Davidson B. L., Zito E. T., Vesikari T. Take of rhesus-human reassortant tetravalent rotavirus vaccine in breast-fed infants. Acta Paediatr. 1993 Mar;82(3):223–227. doi: 10.1111/j.1651-2227.1993.tb12646.x. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Borian F. E., Bell L. M., Modesto K., Gouvea V., Plotkin S. A. Protective effect of WC3 vaccine against rotavirus diarrhea in infants during a predominantly serotype 1 rotavirus season. J Infect Dis. 1988 Sep;158(3):570–587. doi: 10.1093/infdis/158.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F., Borian F. E., Plotkin S. A. Immune protection of infants against rotavirus gastroenteritis by a serotype 1 reassortant of bovine rotavirus WC3. J Infect Dis. 1990 Jun;161(6):1099–1104. doi: 10.1093/infdis/161.6.1099. [DOI] [PubMed] [Google Scholar]
- Coulson B. S., Grimwood K., Masendycz P. J., Lund J. S., Mermelstein N., Bishop R. F., Barnes G. L. Comparison of rotavirus immunoglobulin A coproconversion with other indices of rotavirus infection in a longitudinal study in childhood. J Clin Microbiol. 1990 Jun;28(6):1367–1374. doi: 10.1128/jcm.28.6.1367-1374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C., Prince S. J., Michalek S. M., Jackson S., Russell M. W., Moldoveanu Z., McGhee J. R., Mestecky J. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2449–2453. doi: 10.1073/pnas.84.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagan R., Kassis I., Sarov B., Midthun K., Davidson B. L., Vesikari T., Sarov I. Safety and immunogenicity of oral tetravalent human-rhesus reassortant rotavirus vaccine in neonates. Pediatr Infect Dis J. 1992 Dec;11(12):991–996. doi: 10.1097/00006454-199211120-00001. [DOI] [PubMed] [Google Scholar]
- Dharakul T., Riepenhoff-Talty M., Albini B., Ogra P. L. Distribution of rotavirus antigen in intestinal lymphoid tissues: potential role in development of the mucosal immune response to rotavirus. Clin Exp Immunol. 1988 Oct;74(1):14–19. [PMC free article] [PubMed] [Google Scholar]
- Flores J., Daoud G., Daoud N., Puig M., Martinez M., Perez-Schael I., Shaw R., Greenberg H. B., Midthun K., Kapikian A. Z. Reactogenicity and antigenicity of rhesus rotavirus vaccine (MMU-18006) in newborn infants in Venezuela. Pediatr Infect Dis J. 1988 Nov;7(11):776–780. doi: 10.1097/00006454-198811000-00006. [DOI] [PubMed] [Google Scholar]
- Groene W. S., Shaw R. D. Psoralen preparation of antigenically intact noninfectious rotavirus particles. J Virol Methods. 1992 Jul;38(1):93–102. doi: 10.1016/0166-0934(92)90172-a. [DOI] [PubMed] [Google Scholar]
- Ho M. S., Floyd R. L., Glass R. I., Pallansch M. A., Jones B., Hamby B., Woods P., Penaranda M. E., Kapikian A. Z., Bohan G. Simultaneous administration of rhesus rotavirus vaccine and oral poliovirus vaccine: immunogenicity and reactogenicity. Pediatr Infect Dis J. 1989 Oct;8(10):692–696. doi: 10.1097/00006454-198910000-00006. [DOI] [PubMed] [Google Scholar]
- Ho M. S., Glass R. I., Pinsky P. F., Anderson L. J. Rotavirus as a cause of diarrheal morbidity and mortality in the United States. J Infect Dis. 1988 Nov;158(5):1112–1116. doi: 10.1093/infdis/158.5.1112. [DOI] [PubMed] [Google Scholar]
- Ibbotson J. P., Lowes J. R., Chahal H., Gaston J. S., Life P., Kumararatne D. S., Sharif H., Alexander-Williams J., Allan R. N. Mucosal cell-mediated immunity to mycobacterial, enterobacterial and other microbial antigens in inflammatory bowel disease. Clin Exp Immunol. 1992 Feb;87(2):224–230. doi: 10.1111/j.1365-2249.1992.tb02979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantele A., Arvilommi H., Jokinen I. Specific immunoglobulin-secreting human blood cells after peroral vaccination against Salmonella typhi. J Infect Dis. 1986 Jun;153(6):1126–1131. doi: 10.1093/infdis/153.6.1126. [DOI] [PubMed] [Google Scholar]
- Kataaha P. K., Mortazavi-Milani S. M., Russell G., Holborow E. J. Anti-intermediate filament antibodies, antikeratin antibody, and antiperinuclear factor in rheumatoid arthritis and infectious mononucleosis. Ann Rheum Dis. 1985 Jul;44(7):446–449. doi: 10.1136/ard.44.7.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keljo D. J., Butler D. G., Hamilton J. R. Altered jejunal permeability to macromolecules during viral enteritis in the piglet. Gastroenterology. 1985 Apr;88(4):998–1004. doi: 10.1016/S0016-5085(85)80020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. M., MacPherson G. G. Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J Exp Med. 1993 May 1;177(5):1299–1307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. M., MacPherson G. G. Lymph-borne (veiled) dendritic cells can acquire and present intestinally administered antigens. Immunology. 1991 Jul;73(3):281–286. [PMC free article] [PubMed] [Google Scholar]
- Logan A. C., Chow K. P., George A., Weinstein P. D., Cebra J. J. Use of Peyer's patch and lymph node fragment cultures to compare local immune responses to Morganella morganii. Infect Immun. 1991 Mar;59(3):1024–1031. doi: 10.1128/iai.59.3.1024-1031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonsky G. A., Rennels M. B., Lim Y., Krall G., Kapikian A. Z., Levine M. M. Systemic and mucosal immune responses to rhesus rotavirus vaccine MMU 18006. Pediatr Infect Dis J. 1988 Jun;7(6):388–393. doi: 10.1097/00006454-198806000-00004. [DOI] [PubMed] [Google Scholar]
- Madore H. P., Christy C., Pichichero M., Long C., Pincus P., Vosefsky D., Kapikian A. Z., Dolin R. Field trial of rhesus rotavirus or human-rhesus rotavirus reassortant vaccine of VP7 serotype 3 or 1 specificity in infants. The Elmwood, Panorama, and Westfall Pediatric Groups. J Infect Dis. 1992 Aug;166(2):235–243. doi: 10.1093/infdis/166.2.235. [DOI] [PubMed] [Google Scholar]
- Matson D. O., O'Ryan M. L., Herrera I., Pickering L. K., Estes M. K. Fecal antibody responses to symptomatic and asymptomatic rotavirus infections. J Infect Dis. 1993 Mar;167(3):577–583. doi: 10.1093/infdis/167.3.577. [DOI] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Dertzbaugh M. T., Eldridge J. H., Hirasawa M., Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10(2):75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- Mebus C. A. Reovirus-like calf enteritis. Am J Dig Dis. 1976 Jul;21(7):592–598. doi: 10.1007/BF01464768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant A. A., Groene W. S., Cheng E. H., Shaw R. D. Murine intestinal antibody response to heterologous rotavirus infection. J Clin Microbiol. 1991 Aug;29(8):1693–1701. doi: 10.1128/jcm.29.8.1693-1701.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Pang L. Z., Flores J., Kapikian A. Z. Comparison of immunoglobulin A (IgA), IgG, and IgM enzyme-linked immunosorbent assays, plaque reduction neutralization assay, and complement fixation in detecting seroresponses to rotavirus vaccine candidates. J Clin Microbiol. 1989 Dec;27(12):2799–2804. doi: 10.1128/jcm.27.12.2799-2804.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Plotkin S. A. Response of mice to rotaviruses of bovine or primate origin assessed by radioimmunoassay, radioimmunoprecipitation, and plaque reduction neutralization. Infect Immun. 1983 Oct;42(1):293–300. doi: 10.1128/iai.42.1.293-300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Stroop W. G., Twist E. M., Plotkin S. A. The cultivation of human rotavirus, strain 'Wa', to high titer in cell culture and characterization of the viral structural polypeptides. J Virol Methods. 1983 Jul;7(1):29–40. doi: 10.1016/0166-0934(83)90020-4. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Cunningham S. L., Dudzik K. I. Memory and distribution of virus-specific cytotoxic T lymphocytes (CTLs) and CTL precursors after rotavirus infection. J Virol. 1991 Mar;65(3):1318–1324. doi: 10.1128/jvi.65.3.1318-1324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Schael I., Blanco M., Vilar M., Garcia D., White L., Gonzalez R., Kapikian A. Z., Flores J. Clinical studies of a quadrivalent rotavirus vaccine in Venezuelan infants. J Clin Microbiol. 1990 Mar;28(3):553–558. doi: 10.1128/jcm.28.3.553-558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Schael I., Garcia D., Gonzalez M., Gonzalez R., Daoud N., Perez M., Cunto W., Kapikian A. Z., Flores J. Prospective study of diarrheal diseases in Venezuelan children to evaluate the efficacy of rhesus rotavirus vaccine. J Med Virol. 1990 Mar;30(3):219–229. doi: 10.1002/jmv.1890300315. [DOI] [PubMed] [Google Scholar]
- Quiding M., Nordström I., Kilander A., Andersson G., Hanson L. A., Holmgren J., Czerkinsky C. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-gamma production and evokes local immunological memory. J Clin Invest. 1991 Jul;88(1):143–148. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. E., Stevens K. C. Production of autoantibodies to cellular antigens by human B cells transformed by Epstein-Barr virus. Clin Immunol Immunopathol. 1984 Dec;33(3):339–350. doi: 10.1016/0090-1229(84)90305-2. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Merchant A. A., Groene W. S., Cheng E. H. Persistence of intestinal antibody response to heterologous rotavirus infection in a murine model beyond 1 year. J Clin Microbiol. 1993 Feb;31(2):188–191. doi: 10.1128/jcm.31.2.188-191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A., Cosentino M., Leitman-Klinman S. F., Klinman D. M. Human immunodeficiency virus infection induces both polyclonal and virus-specific B cell activation. J Clin Invest. 1992 Feb;89(2):561–566. doi: 10.1172/JCI115621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey W. G., Collins J., Wallis T. S., Clarke G. J., Spencer A. J., Haddon S. J., Osborne M. P., Candy D. C., Stephen J. Kinetics, tissue specificity and pathological changes in murine rotavirus infection of mice. J Gen Virol. 1986 Dec;67(Pt 12):2625–2634. doi: 10.1099/0022-1317-67-12-2625. [DOI] [PubMed] [Google Scholar]
- Van der Heijden P. J., Bianchi A. T., Stok W., Bokhout B. A. Background (spontaneous) immunoglobulin production in the murine small intestine as a function of age. Immunology. 1988 Oct;65(2):243–248. [PMC free article] [PubMed] [Google Scholar]
- VanCott J. L., Brim T. A., Simkins R. A., Saif L. J. Isotype-specific antibody-secreting cells to transmissible gastroenteritis virus and porcine respiratory coronavirus in gut- and bronchus-associated lymphoid tissues of suckling pigs. J Immunol. 1993 May 1;150(9):3990–4000. [PubMed] [Google Scholar]
- Vesikari T., Kapikian A. Z., Delem A., Zissis G. A comparative trial of rhesus monkey (RRV-1) and bovine (RIT 4237) oral rotavirus vaccines in young children. J Infect Dis. 1986 May;153(5):832–839. doi: 10.1093/infdis/153.5.832. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Ruuska T., Delem A., André F. E. Oral rotavirus vaccination in breast- and bottle-fed infants aged 6 to 12 months. Acta Paediatr Scand. 1986 Jul;75(4):573–578. doi: 10.1111/j.1651-2227.1986.tb10253.x. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Ruuska T., Green K. Y., Flores J., Kapikian A. Z. Protective efficacy against serotype 1 rotavirus diarrhea by live oral rhesus-human reassortant rotavirus vaccines with human rotavirus VP7 serotype 1 or 2 specificity. Pediatr Infect Dis J. 1992 Jul;11(7):535–542. doi: 10.1097/00006454-199207000-00006. [DOI] [PubMed] [Google Scholar]
- Walsh J. A., Warren K. S. Selective primary health care: an interim strategy for disease control in developing countries. N Engl J Med. 1979 Nov 1;301(18):967–974. doi: 10.1056/NEJM197911013011804. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Bernstein D. I., Shukla R., Young E. C., Sherwood J. R., McNeal M. M., Walker M. C., Schiff G. M. Effects of antibody to rotavirus on protection of adults challenged with a human rotavirus. J Infect Dis. 1989 Jan;159(1):79–88. doi: 10.1093/infdis/159.1.79. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Greenberg H. B., Schiff G. M., Bernstein D. I. Serum-neutralizing antibody to VP4 and VP7 proteins in infants following vaccination with WC3 bovine rotavirus. J Virol. 1990 Jun;64(6):2687–2691. doi: 10.1128/jvi.64.6.2687-2691.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K. C., Mahida Y. R., Priddle J. D., Jewell D. P. Effect of human intestinal macrophages on immunoglobulin production by human intestinal mononuclear cells isolated from patients with inflammatory bowel disease. Clin Exp Immunol. 1990 Jan;79(1):35–40. doi: 10.1111/j.1365-2249.1990.tb05123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



