Abstract
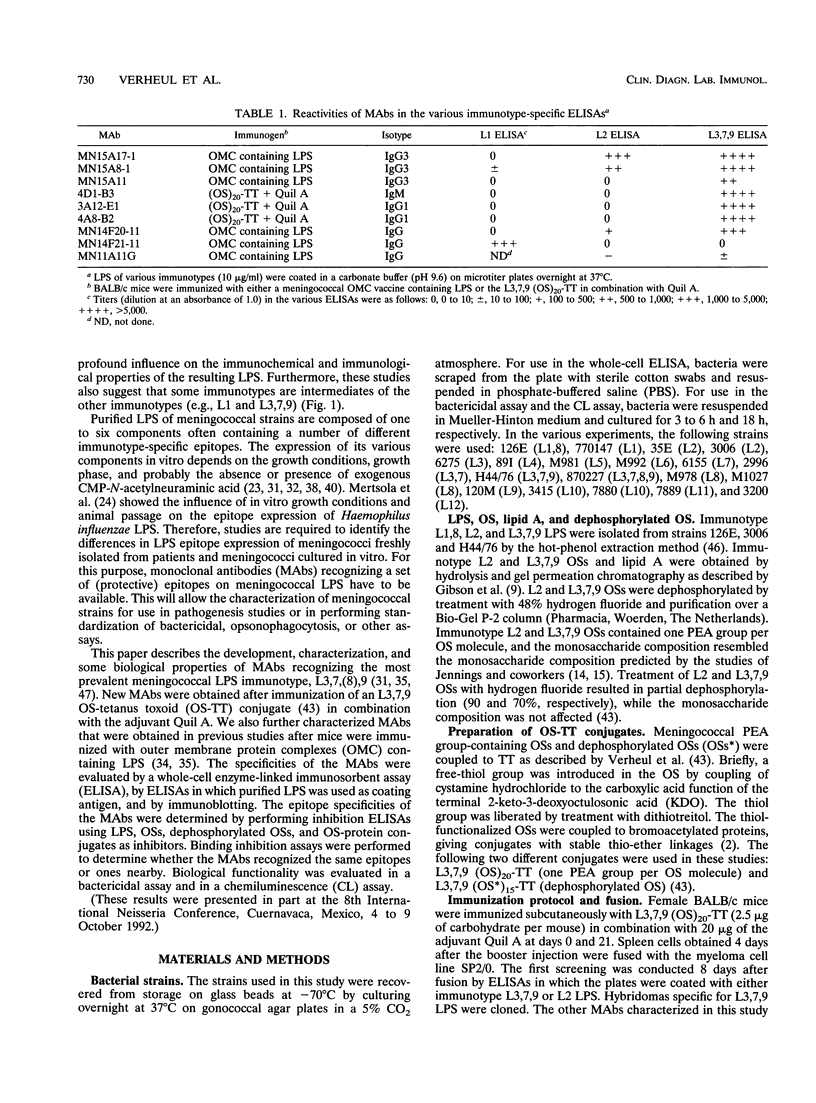
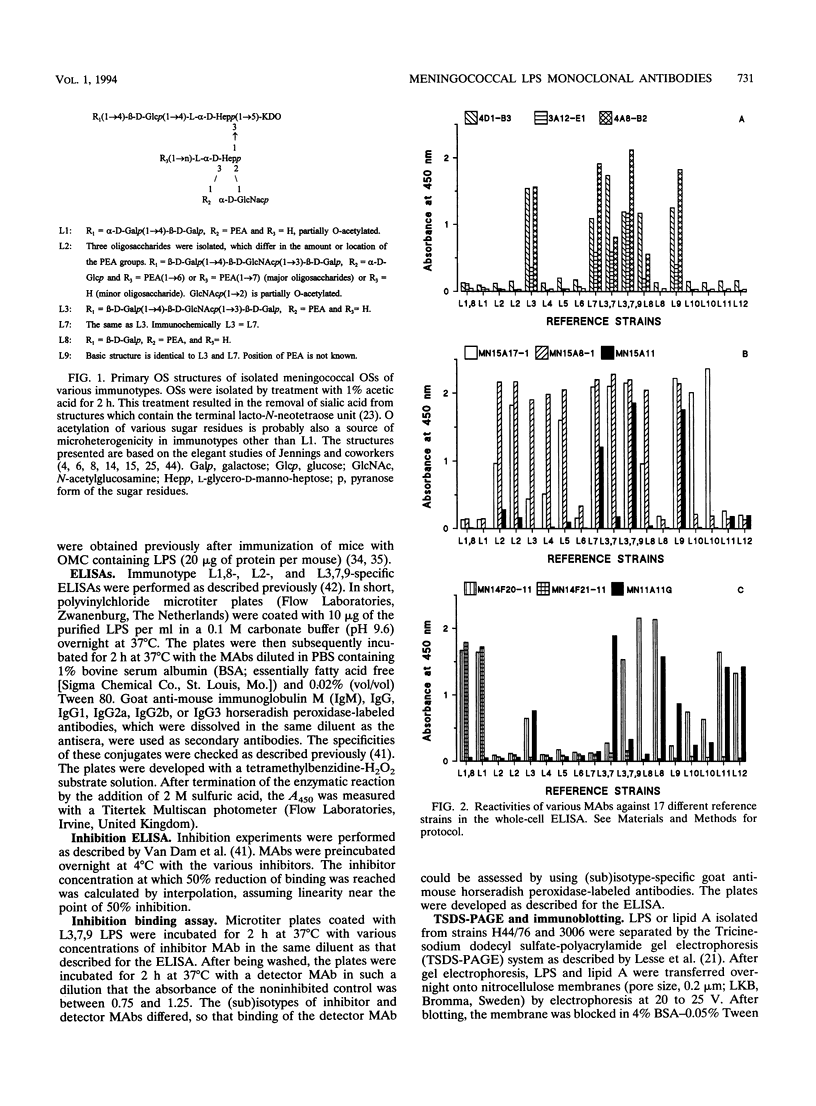
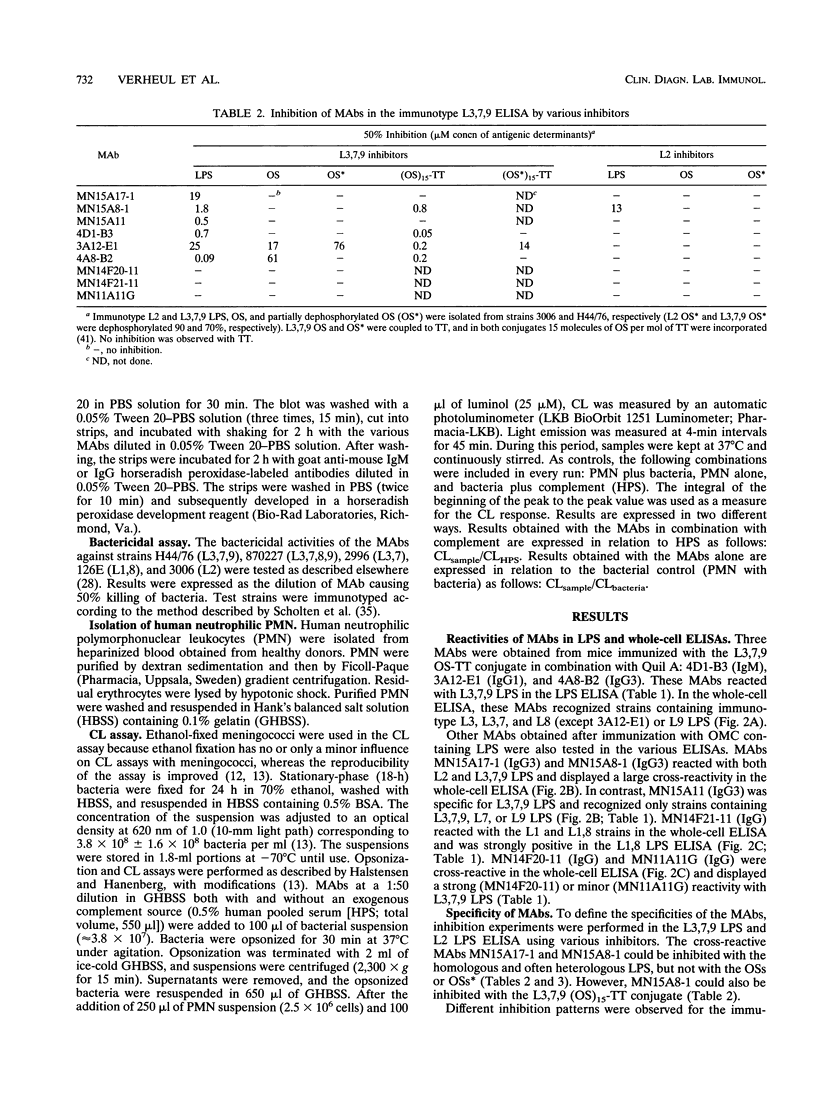
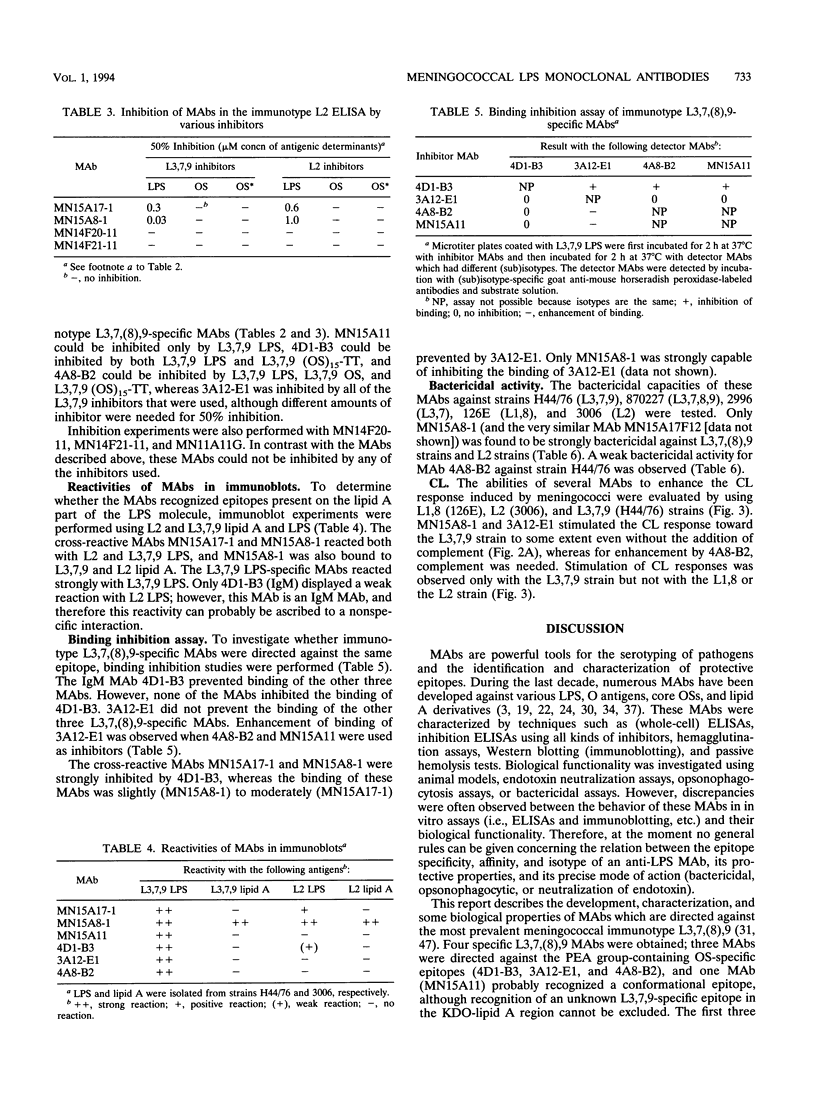
In this study, we characterize the properties of nine monoclonal antibodies (MAbs) that recognize meningococcal lipopolysaccharides (LPS). The following three specific MAbs that had not been described previously were elicited in BALB/c mice by using an immunotype L3,7,9 oligosaccharide-tetanus toxoid conjugate in combination with Quil A: 4D1-B3, 3A12-E1, and 4A8-B2. These MAbs reacted with L3,7,9 LPS on immunoblots and in the LPS enzyme-linked immunosorbent assay (ELISA) and recognised strains containing L3, L3,7, L8 (except 3A12-E1), or L9 LPS in the whole-cell ELISA. The six other MAbs have been described in previous studies (K. Saukkonen, M. Leinonen, H. Abdillahi, and J.T. Poolman, Vaccine 7:325-328, 1989; R.J.P.M. Scholten, B. Kuipers, H.A. Valkenburg, J. Danjert, W.D. Zollinger, and J.T. Poolman, J. Med. Microbiol., in press) and were obtained after immunization with outer membrane protein complexes containing LPS: MN15A11, MN15A8-1, MN15A17-1, MN11A11G, MN14F20-11, and MN14F21-11. MN15A11 was specific for L3,7,9 LPS and displayed properties similar to those of 3A12-E1. MN15A17-1, MN14F20-1, and MN11A11G were cross-reactive, and MN14F21-11 was specific for the L1,8 immunotype. Epitope specificities of MAbs reacting with L3,7,(8),9 strains were analyzed. MAbs 4D1-B3, 3A12-E1, and 4A8-B2 recognized phosphoethanolamine group-containing oligosaccharide-specific epitopes. MN15A11 and MN15A17-1 were probably directed against a conformational epitope, although for MN5A11 recognition of an unknown L3,7,9-specific epitope in the 2-keto-3-deoxyoctulosonic acid (KDO)-lipid A region cannot be excluded. MN15A8-1, a strongly cross-reactive MAb, recognized a determinant which included the KDO-lipid A region and the more terminal saccharides.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernatowicz M. S., Matsueda G. R. Preparation of peptide-protein immunogens using N-succinimidyl bromoacetate as a heterobifunctional crosslinking reagent. Anal Biochem. 1986 May 15;155(1):95–102. doi: 10.1016/0003-2697(86)90231-9. [DOI] [PubMed] [Google Scholar]
- Dell A., Azadi P., Tiller P., Thomas-Oates J., Jennings H. J., Beurret M., Michon F. Analysis of oligosaccharide epitopes of meningococcal lipopolysaccharides by fast-atom-bombardment mass spectrometry. Carbohydr Res. 1990 Apr 25;200:59–76. doi: 10.1016/0008-6215(90)84182-t. [DOI] [PubMed] [Google Scholar]
- Estabrook M. M., Mandrell R. E., Apicella M. A., Griffiss J. M. Measurement of the human immune response to meningococcal lipooligosaccharide antigens by using serum to inhibit monoclonal antibody binding to purified lipooligosaccharide. Infect Immun. 1990 Jul;58(7):2204–2213. doi: 10.1128/iai.58.7.2204-2213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamian A., Beurret M., Michon F., Brisson J. R., Jennings H. J. Structure of the L2 lipopolysaccharide core oligosaccharides of Neisseria meningitidis. J Biol Chem. 1992 Jan 15;267(2):922–925. [PubMed] [Google Scholar]
- Gibson B. W., Webb J. W., Yamasaki R., Fisher S. J., Burlingame A. L., Mandrell R. E., Schneider H., Griffiss J. M. Structure and heterogeneity of the oligosaccharides from the lipopolysaccharides of a pyocin-resistant Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1989 Jan;86(1):17–21. doi: 10.1073/pnas.86.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M., Brandt B. L., Broud D. D., Goroff D. K., Baker C. J. Immune response of infants and children to disseminated infections with Neisseria meningitidis. J Infect Dis. 1984 Jul;150(1):71–79. doi: 10.1093/infdis/150.1.71. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., O'Brien J. P., Yamasaki R., Williams G. D., Rice P. A., Schneider H. Physical heterogeneity of neisserial lipooligosaccharides reflects oligosaccharides that differ in apparent molecular weight, chemical composition, and antigenic expression. Infect Immun. 1987 Aug;55(8):1792–1800. doi: 10.1128/iai.55.8.1792-1800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttormsen H. K., Bjerknes R., Naess A., Lehmann V., Halstensen A., Sørnes S., Solberg C. O. Cross-reacting serum opsonins in patients with meningococcal disease. Infect Immun. 1992 Jul;60(7):2777–2783. doi: 10.1128/iai.60.7.2777-2783.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstensen A., Haneberg B. Standardization of a chemiluminescence method for the measurement of meningococcal opsonins using ethanol fixed meningococci. Acta Pathol Microbiol Immunol Scand C. 1987 Aug;95(4):155–160. doi: 10.1111/j.1699-0463.1987.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Beurret M., Gamian A., Michon F. Structure and immunochemistry of meningococcal lipopolysaccharides. Antonie Van Leeuwenhoek. 1987;53(6):519–522. doi: 10.1007/BF00415511. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Johnson K. G., Kenne L. The structure of an R-type oligosaccharide core obtained from some lipopolysaccharides of Neisseria meningitidis. Carbohydr Res. 1983 Sep 16;121:233–241. doi: 10.1016/0008-6215(83)84020-8. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Ashton F. E. Conjugation of meningococcal lipopolysaccharide R-type oligosaccharides to tetanus toxoid as route to a potential vaccine against group B Neisseria meningitidis. Infect Immun. 1984 Jan;43(1):407–412. doi: 10.1128/iai.43.1.407-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Griffiss J. M. Neisseria lactamica and Neisseria meningitidis share lipooligosaccharide epitopes but lack common capsular and class 1, 2, and 3 protein epitopes. Infect Immun. 1989 Feb;57(2):602–608. doi: 10.1128/iai.57.2.602-608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Hu Z., Westerink M. A., Poolman J. T., Griffiss J. M. Electromorphic characterization and description of conserved epitopes of the lipooligosaccharides of group A Neisseria meningitidis. Infect Immun. 1988 Oct;56(10):2631–2638. doi: 10.1128/iai.56.10.2631-2638.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H. M., Brade L., Appelmelk B. J., Kusumoto S., Rietschel E. T., Brade H. Characterization of the epitope specificity of murine monoclonal antibodies directed against lipid A. Infect Immun. 1992 Jun;60(6):2201–2210. doi: 10.1128/iai.60.6.2201-2210.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancios L., Lyles D. S. The interactionof antiody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology. 1982 Aug;121(1):157–167. [PubMed] [Google Scholar]
- Lesse A. J., Campagnari A. A., Bittner W. E., Apicella M. A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990 Jan 24;126(1):109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- Mandine E., Salles M. F., Zalisz R., Guenounou M., Smets P. Murine monoclonal antibodies to Klebsiella pneumoniae protect against lethal endotoxemia and experimental infection with capsulated K. pneumoniae. Infect Immun. 1990 Sep;58(9):2828–2833. doi: 10.1128/iai.58.9.2828-2833.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Kim J. J., John C. M., Gibson B. W., Sugai J. V., Apicella M. A., Griffiss J. M., Yamasaki R. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J Bacteriol. 1991 May;173(9):2823–2832. doi: 10.1128/jb.173.9.2823-2832.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michon F., Beurret M., Gamian A., Brisson J. R., Jennings H. J. Structure of the L5 lipopolysaccharide core oligosaccharides of Neisseria meningitidis. J Biol Chem. 1990 May 5;265(13):7243–7247. [PubMed] [Google Scholar]
- Morrison D. C. Bacterial endotoxins and pathogenesis. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S733–S747. doi: 10.1093/clinids/5.supplement_4.s733. [DOI] [PubMed] [Google Scholar]
- Peltola H. Meningococcal disease: still with us. Rev Infect Dis. 1983 Jan-Feb;5(1):71–91. doi: 10.1093/clinids/5.1.71. [DOI] [PubMed] [Google Scholar]
- Pollack M., Chia J. K., Koles N. L., Miller M., Guelde G. Specificity and cross-reactivity of monoclonal antibodies reactive with the core and lipid A regions of bacterial lipopolysaccharide. J Infect Dis. 1989 Feb;159(2):168–188. doi: 10.1093/infdis/159.2.168. [DOI] [PubMed] [Google Scholar]
- Saukkonen K., Leinonen M., Abdillahi H., Poolman J. T. Comparative evaluation of potential components for group B meningococcal vaccine by passive protection in the infant rat and in vitro bactericidal assay. Vaccine. 1989 Aug;7(4):325–328. doi: 10.1016/0264-410x(89)90194-1. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., Farley M. M. Pathogenic events during infection of the human nasopharynx with Neisseria meningitidis and Haemophilus influenzae. Rev Infect Dis. 1991 Jan-Feb;13(1):22–33. doi: 10.1093/clinids/13.1.22. [DOI] [PubMed] [Google Scholar]
- Terashima M., Uezumi I., Tomio T., Kato M., Irie K., Okuda T., Yokota S., Noguchi H. A protective human monoclonal antibody directed to the outer core region of Pseudomonas aeruginosa lipopolysaccharide. Infect Immun. 1991 Jan;59(1):1–6. doi: 10.1128/iai.59.1.1-6.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Civin C. I. Eight lipooligosaccharides of Neisseria meningitidis react with a monoclonal antibody which binds lacto-N-neotetraose (Gal beta 1-4GlcNAc beta 1-3Gal beta 1-4Glc). Infect Immun. 1991 Oct;59(10):3604–3609. doi: 10.1128/iai.59.10.3604-3609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Mocca L. F., Frasch C. E. Immunotype epitopes of Neisseria meningitidis lipooligosaccharide types 1 through 8. Infect Immun. 1987 Jul;55(7):1652–1656. doi: 10.1128/iai.55.7.1652-1656.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam G. J., Verheul A. F., Zigterman G. J., De Reuver M. J., Snippe H. Nonionic block polymer surfactants enhance the avidity of antibodies in polyclonal antisera against Streptococcus pneumoniae type 3 in normal and Xid mice. J Immunol. 1989 Nov 1;143(9):3049–3053. [PubMed] [Google Scholar]
- Verheul A. F., Boons G. J., Van der Marel G. A., Van Boom J. H., Jennings H. J., Snippe H., Verhoef J., Hoogerhout P., Poolman J. T. Minimal oligosaccharide structures required for induction of immune responses against meningococcal immunotype L1, L2, and L3,7,9 lipopolysaccharides determined by using synthetic oligosaccharide-protein conjugates. Infect Immun. 1991 Oct;59(10):3566–3573. doi: 10.1128/iai.59.10.3566-3573.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheul A. F., Braat A. K., Leenhouts J. M., Hoogerhout P., Poolman J. T., Snippe H., Verhoef J. Preparation, characterization, and immunogenicity of meningococcal immunotype L2 and L3,7,9 phosphoethanolamine group-containing oligosaccharide-protein conjugates. Infect Immun. 1991 Mar;59(3):843–851. doi: 10.1128/iai.59.3.843-851.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheul A. F., Poolman J. T., Snippe H., Verhoef J. The influence of the adjuvant Quil A on the epitope specificity of meningococcal lipopolysaccharide anti-carbohydrate antibodies. Mol Immunol. 1991 Nov;28(11):1193–1200. doi: 10.1016/0161-5890(91)90005-5. [DOI] [PubMed] [Google Scholar]
- Verheul A. F., Van Gaans J. A., Wiertz E. J., Snippe H., Verhoef J., Poolman J. T. Meningococcal lipopolysaccharide (LPS)-derived oligosaccharide-protein conjugates evoke outer membrane protein- but not LPS-specific bactericidal antibodies in mice: influence of adjuvants. Infect Immun. 1993 Jan;61(1):187–196. doi: 10.1128/iai.61.1.187-196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Outer-membrane protein and lipopolysaccharide serotyping of Neisseria meningitidis by inhibition of a solid-phase radioimmunoassay. Infect Immun. 1977 Nov;18(2):424–433. doi: 10.1128/iai.18.2.424-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jongh-Leuvenink J., Schellekens J., Verhoef J. Characterization of anti-core glycolipid monoclonal antibodies with chemically defined lipopolysaccharides. Infect Immun. 1990 Feb;58(2):421–426. doi: 10.1128/iai.58.2.421-426.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


