Abstract
We examined the role of the hippocampus in list-memory processing. Three rhesus monkeys that had extensive experience in this task and had demonstrated full abstract-concept learning and excellent list memory performance (Katz et al., 2002; Wright et al., 2003) received bilateral neurotoxic hippocampal lesions and were re-tested in the serial list memory task. Effects of delays on memory performance were measured in all monkeys, whereas the effects of proactive interference were assessed in only one. Despite a slight change in performance of one of the three animals during re-learning of the same/different task, selective hippocampal damage had little or no effects on list memory accuracy. In addition, the hippocampal damage did not impact serial list position functions (SPFs) but slightly altered the dynamic of the SPF curves. Finally, even more remarkable was that accurate memory performance of one animal remained intact despite the use of small set size of 8 items that created high proactive interference across lists thereby eliminating any use of familiarity judgments to support performance. Together the findings indicate that, with short list items and extensive training in the task (i.e., reference memory), monkeys with selective hippocampal lesions may be able to use alternative memory processes (i.e., working memory) that are mediated by structures other than the hippocampus.
Keywords: Recognition memory, Primacy, Recency, Serial list memory, Proactive interference
1. Introduction
Over the past decade, the contributions of the hippocampus and medial temporal lobe cortex to recognition have generated a host of studies in many species, but at the current time the specific role of each of these brain structures remains heavily debated. An example is provided by the recent issue of the journal “Hippocampus” (2010, vol. 20) exposing the different views fueling this debate. One of the reasons this controversy has lasted so long is the disagreement over how to measure hippocampal and cortical contributions to recognition memory. Recognition memory in humans is commonly assessed with list learning tasks in which participants study a set of stimuli (pictures of objects, visual patterns, faces, or words), and after a delay, judge whether the stimuli are familiar (included in the list) or new. Studies on human amnesic patients with damage to the hippocampus or adjacent cortical areas (Aggleton and Shaw, 1996; Bowles et al., 2007; Holdstock et al., 2002; Mayes et al., 2003; Mishkin et al., 1998; Reed et al., 1997; Stark et al., 2002; Vargha-Khadem et al., 1997) and functional imaging studies (Yonelinas and Parks, 2007; for reviews, see Eichenbaum et al., 2007; Skinner and Fernandes, 2007; Wais, 2008) have suggested that the hippocampus is involved in recognition memory only when participants fully recollect the items (i.e., the items and all other information associated with the items, such as whether the words were shown in red or green or the pictures were emotionally positive or negative), but not when they simply used familiarity judgments (was the item in the list or not?), which are supported by the medial temporal cortical areas. Another view, however, proposed that the strength of the memory traces is the critical attribute such that memory traces with strong or weak memory load may require the hippocampus and medial temporal cortex, respectively (Squire et al., 2007; Wixted et al., 2010).
Animal studies have attempted to resolve this disagreement but without convincing success so far. For example in monkeys, recognition memory has generally been investigated using delayed matching-to-sample (DMTS) or delayed nonmatching-to-sample (DNMTS) tasks in which the animal has to indicate which of two stimuli has been seen earlier by choosing either the familiar (match) or the novel (nonmatch) stimuli presented together during a choice test. Generally, these tasks employed a large pool of stimuli (500 to thousands). Memory is then further assessed by increasing the delays between the sample presentation and the choice or by increasing the list of items to be remembered. Using these tasks, lesion studies have provided conflicting results. Thus, whereas some studies have reported recognition deficits at the long delays or long lists following selective hippocampal lesions (Beason-Held et al., 1999; Zola et al., 2000), others found no impairment (Baxter and Murray, 2001; Murray and Mishkin, 1998; Nemanic et al., 2004). One potential limitation with the nonhuman primate studies is that the DMTS and DNMTS paradigms may rely on memory processes different from those that support the list memory tasks in humans (see Nemanic et al., 2004). The memory processes supporting DNMTS performance could include familiarity judgment, working memory, or retrospective processing, which could recruit brain areas other than the hippocampus, such as the medial temporal and prefrontal cortices known to be critical for normal performance on DNMTS tasks (Bachevalier and Mishkin, 1986; Brown and Aggleton, 2001; Ennaceur et al., 1996; Fahy et al., 1993; Gaffan and Murray, 1992; Kolb et al., 1994; Meunier et al., 1993; Miller et al., 1996; Murray and Bussey, 1999; Nemanic et al., 2004; Pihlajamaki et al., 2004; Simons and Spiers, 2003; Suzuki et al., 1993; Xiang and Brown, 2004).
In an attempt to investigate further the reasons for this disagreement and enable better comparisons with results from the human literature, the present study employed a serial list memory task similar to that used in humans (Wright et al., 1985) to re-assess the effects of selective hippocampal lesions on recognition memory in monkeys. In this task, animals are presented with a short list of items on a computer monitor followed by a probe test. The probe test presents either an item seen in the list or a new item together with a white rectangle. To receive a reward, the animal has to touch the item on the screen if it was an item of the list or touch the white rectangle if the item was new. The serial list memory task offers several advantages relative to the previous matching tasks. First, as compared to the DNMTS task in which both the familiar and new items are present together on the screen during the animal’s selection (familiar versus novel), the serial list memory task presents only one item necessitating a “yes/no” or “same/different” response. Thus, the forced-choice response in the DNMTS task may favor the use of familiarity/novelty judgment that are more dependent upon the medial temporal cortex to the detriment of same/different relational representations and retrieval strategies, which depend more heavily upon the hippocampus (Damasio et al., 1985; Eichenbaum et al., 1989, 2007; O’Keefe and Nadel, 1978; Rudy and Sutherland, 1989, 1992; Shapiro and Olton, 1994; Sutherland and Rudy, 1989).
Another important advantage of the serial list memory task over the DNMTS in the investigation of the participation of the hippocampus in recognition memory is that the list memory task can better dissociate different memory processes. In a previous study comparing serial list memory abilities in pigeons, monkeys and humans, Wright et al. (1985) demonstrated that the typical serial U-shaped position function with good (long-term) memory of the first list items (primacy effect) and a good (short-term) memory of the last list items (recency effect) normally found in human studies was also present for pigeons and monkeys. Furthermore, the authors demonstrated that in those three species, the shape of the serial-position function changed with varying the retention intervals between the end of the list and the probe test. That is, at short retention delays, recognition memory increased monotonically with better memory for the last items of the list; for intermediate delays, the serial list curve had U-shape functions with better memory for the first and last items than for the middle ones; lastly, for long retention delays, recognition memory decreased monotonically with better memory for the first items of the list. The authors suggested that these dynamic changes in serial-position functions reflect the participation of two or more memory processes. This conclusion is strengthened by the numerous demonstrations showing that the primacy and recency effects can be independently altered. Variables that selectively affect the recency effect include: moderate to long retention delays (e.g., Gardiner, 1974; Glanzer and Cunitz, 1966; Postman and Phillips, 1965; Roediger and Crowder, 1976; Wright et al., 1985); auditory vs. visual modality of stimulus presentation (e.g., Crowder, 1986; Crowder and Morton, 1969; Murdock, 1966; Wright, 2007); and knowledge about the end of the list (Watkins and Watkins, 1974). Variables that selectively affect the primacy effect include: fast presentation rates (Glanzer and Cunitz, 1966), long list lengths (Murdock, 1962), very short retention delays in single-item recognition tasks (Wright et al., 1985), alcohol intoxication (Jones, 1973), and mental retardation (Belmont and Butterfield, 1971).
Interestingly, there exists also neuropsychological evidence to support this functional dissociation of memory processes in serial list learning task. Thus, different brain areas seem to independently support the primacy and recency effects. The prefrontal cortex known to be critical for working memory processes and perirhinal cortex known to mediate short term memory have been associated with the recency effect (Barker and Warburton, 2011; Goldman-Rakic, 1987; Kesner, 1985; Saffran and Marin, 1975; Warrington et al., 1971; Warrington and Shallice, 1984; Weiskrantz, 1987), whereas the hippocampus has been associated with long-term (primacy) memory (e.g., Baddeley and Warrington, 1970; Hermann et al., 1996; Hopkins and Kesner, 1995; Hopkins et al., 1995; Kesner, 1998; Kesner and Novak, 1982).
The advantages provided by the serial list learning task over the DMTS and DNMTS offers an improved method with which to assess the role of hippocampus in recognition memory. More importantly, task manipulations, such as length of the delays and magnitude of the proactive interference across list items, may inform recent theories concerning the precise role of the hippocampus in recognition memory (see reviews of the current neural models in the review Hippocampus, 2010, vol. 20). Therefore, in this study, three rhesus monkeys with extensive experience in a serial list memory task were used (Katz et al., 2002; Wright et al., 2003). All monkeys had demonstrated full abstract-concept learning and excellent list memory performance before receiving bilateral neurotoxic lesions of the hippocampal formation. After recovery from surgical procedures the monkeys were then re-tested in the serial list learning task.
2. General methods
All procedures were approved by the Animal Care and Use Committee of the University of Texas Health Science Center at Houston in Houston, TX and carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used, as well as any pain and suffering.
2.1. Subjects
Subjects were three, 6–12 year-old, rhesus monkeys (Macaca mulatta) of both sexes, weighing 5–12 kg (Cuba, Gracie, and Slim). They were housed individually and maintained on a 12:12h light–dark cycle. Multi-vitamins were given daily and fresh fruit weekly. All three monkeys received presurgical training on a two-item same/different task and then list memory (Katz et al., 2002; Wright et al., 2003). Experimental training sessions were conducted 5–7 days a week. On testing days, access to food (Purina Monkey Chow) and water in their home cages was restricted about 15 h before testing. On non-testing days, they had accessed to food and water ad libitum. Additionally, vegetable and fruit supplements were provided at the end of each week. All monkeys were first trained on the same/different task and then received a transfer test to assess same/different concept learning, followed by a list memory task to measure serial position functions. After this training, all animals received bilateral neurotoxic (ibotenic acid) lesions of the hippocampal formation. After recovery from the surgical procedure, they were re-trained on the list memory task, as described below.
2.2. Apparatus
2.2.1. Chamber
Monkeys were tested unrestrained in one of two identical (47.5 cm wide × 53.13 cm deep × 66.25 cm high) custom aluminum test chambers (see Bhatt and Wright, 1992 for further details). A fan (Dayton 4C440, Niles, IL) located in the ceiling of the chamber, provided ventilation and white noise. Bio-Serv banana pellets (300 mg) were dispensed (model # ENV-203–300 MED Associates, Inc., Georgia, VT) into a pellet cup (5.6 cm diameter and 2.5 cm deep) that was 10 cm from the left edge and 52.5 cm from the top edge of the front panel. Tang orange drink was delivered (model #71215 Honeywell, Inc., New Britain, CT) through a juice spout that was 10 cm from the right edge and 42.5 cm from the top edge of the front panel. Touches to a computer monitor were detected by an infrared touch screen bezel (model # 81009703-01 Carroll Touch, Round Rock, TX). The bezel fitted snugly within a 40 cm × 33.75 cm cut-out in the front panel that was centered 9.38 cm from the top of the operant chamber. Touch responses were directed by a Plexiglas template (32.5 cm × 40 cm) with cut-outs matching the size and location of the stimuli.
2.2.2. Stimuli
Travel-slide color pictures were digitized via a Howtek Photomaster (87RU) camera and a Truevision TARGA-16 processing card in a 256 × 256 resolution. Stimuli were presented on a 39 cm color monitor (Eizo FX-C6; Ishikawa, Japan, 600 × 480-pixel resolution). Stimulus displays consisted of two travel-slide color pictures (each 13.75 cm × 9.7 cm) and a white rectangle (6.25 cm × 5.6 cm) on a black background. The pictures were vertically aligned with a 3.4 cm gap between them. The top picture was centered 20.63 cm from the left edge and 18.75 cm from the top of the front panel. The bottom of the white rectangle was horizontally aligned with the bottom of the lower picture with a 3.7 cm gap between them. A set of 432 travel slides was used in these experiments (for the complete set of stimuli see Wright and Katz, 2006).
2.2.3. Experimental control
Experimental events were controlled and recorded via custom software written in Visual Basic on a Pentium personal computer. A video card (Appian Jeronimo J2 Advanced Graphics Accelerator) controlled the monitor. A computer-controlled relay interface (model # PI0-12, Metrabyte, Taunton, MA) operated the juice valve and pellet dispenser.
2.3. Pre-surgical training procedures
Detailed description of data obtained on presurgical training procedures has been published (Katz et al., 2002; Wright et al., 2003). Table 1 summarizes the presurgical training history for each monkey as well as the number of sessions they received for each phase.
Table 1.
Number of sessions received for each training phase prior to surgery. Session consisted of 100 trials and Cuba started training with 10 touches to the sample.
| Monkey | Gracie | Slim | Cuba |
|---|---|---|---|
| Training | |||
| 0-Response | 250 | 250 | NA |
| Fixed-time | 54 | 30 | NA |
| 10-Responses | 0 | 26 | 35 |
| Transfer training | 41 | 46 | 33 |
| List-length testing | 31 | 37 | 45 |
| Delays testing | 205 | 995 | 497 |
Briefly, monkeys were first trained to retrieve banana pellets (Noyes 300 mg) from the food cup and drink Tang orange juice from the juice spout. Responses to the video monitor and touch screen were shaped by successive approximations to a white rectangle positioned at the right bottom corner of the screen (later to become the different response area) and another white rectangle (13.75 cm × 9.7 cm) placed in the position where the lower picture will appear on each trial. These two rectangles appeared on separate trials and were randomly and equally presented over a 100-trial session. A single touch to the white rectangle was followed by a 1.1 s, 660 Hz tone.The rewards were either food (1 pellet, delivered simultaneously with the tone) or juice (3–5 cm3, delivered 1 s after the tone) and a 15 s intertrial interval (ITI) followed reinforcement. Once a monkey was consistently responding (1–5 sessions), same/different (S/D) training began.
2.3.1. Same/different training
Eight pictures were used for S/D training. They were arranged in 64 pairs (8 “same” and 56 “different”) and each session consisted of 100 trials (50 “same”/50 “different”). For Gracie and Slim, a trial began with presentation of the two pictures (one above the other) and the white rectangle. If the two pictures were the same, a touch response to the lower picture was rewarded. If the two pictures were different, a touch response to the white rectangle was rewarded. For Cuba, a trial began with presentation of the upper picture (sample stimulus) only. Initially, the monkey was required to touch the picture once, before the two pictures appeared on the screen. Then, the number of touches to the sample picture was progressively increased to a maximum of 10 touches. Only correct choices were rewarded and starting on the fifth training session, incorrect choices were followed by a repeat of the incorrect trial (correction procedure). Training continued until performance was 80% or better on three consecutive sessions. The correction procedure was then removed and training continued until the same criterion was met. Whereas Cuba learned the task in 35 sessions (100 trials/session), neither Gracie nor Slim learned the same/different task in the limit of testing, i.e., 250 sessions (Katz et al, 2002). Slight modifications were done on the task to allow Gracie and Slim to learn the same/different task. Both monkeys received additional sessions (54 and 30 sessions, respectively) in which the sample stimulus remained on the screen for a fixed-time period before the choice test was presented. The length of time that the upper picture remained on the screen was made equivalent to the average time required by Cuba and two other monkeys (see Katz et al., 2002) to touch 10 times the sample picture. Gracie learned the S/D with the fixed-time procedure, but Slim did not. Slim received an additional 26 sessions during which he was required to touch 10 times the sample stimulus before the choice test was presented (as did Cuba).
2.3.2. Transfer testing
Following S/D training, all three monkeys received six consecutive transfer test sessions to assess abstract-concept learning. Like training, each transfer session had 100 trials. They received 10 transfer trials (5 “same” and 5 “different”) consisting of novel stimuli pseudo-randomly intermixed with 90 baseline training trials (45 “same” and 45 “different”) consisting of the same 8 pictures used in the S/D training. The set size was then progressively increased from 8 to 16, 32, 64, and 128 pictures. Transfer tests were conducted after reaching the performance criterion following the 32, 64, and 128 set sizes.
2.3.3. List-length and retention delay training
List-length training began with one list item. The upper image was presented and after 10 touches, the list item disappeared for 1 s, following which the lower picture and the white rectangle appeared on the screen. Monkeys received 100 trials per session until reaching the criterion of 85% correct or better. The list was then extended progressively to 2, 3, 4, 6, 8 and 10 items. The inter-stimulus interval (ISI) and the delay between the last item of the list and the probe item were 1 s. The training set size was increased from 128 items to as many as 432 items during this training.
Following list-length expansion training, monkeys were returned to training and testing with a list length of 4 items. Again list items were presented for 1 s, 1 s ISI, and a 1 s retention delay was interposed between the last list item and the probe item. After performance stabilized with this procedure, retention delays of 0, 1, 2, 10, 20 and 30 s were tested in a block design. Each block consisted of six sessions of 32 trials (16 same/16 different) each with the retention delay fixed. The serial position of the matching picture on same trials was counterbalanced and randomized within each session. Two sessions, with different retention delays, were tested daily. One delay was short (0, 1 and 2 s) and one was long (10, 20 and 30 s). The order (short, long) was counterbalanced over successive six session blocks. Each delay was selected randomly with the constraint that each delay occurred once within a block. Training items were unique in a session. Training continued until performance was accurate and the serial position functions were stable over several days of testing.
3. Experiment 1
To investigate the role of the hippocampal formation on the retention of S/D abstract-concept learning and on the shape and time-course of serial position functions, the three animals received MRI-guided neurotoxic lesions of the hippocampal formation bilaterally, and, following recovery, were re-tested on the “S/D” and list memory tasks.
3.1. Methods
3.1.1. Neuroimaging procedures
Briefly, one to three weeks prior to surgery, each subject was placed in a non-ferromagnetic stereotaxic device (Crist Instruments, Co., Inc., Damascus, MD), and received a T1-weighted, structural MRI scan through the entire brain at 1 mm slice intervals in the coronal plane (see Nemanic et al., 2002 for details). These high-resolution images were used to create an individual atlas for each monkey from which the coordinate values for each neurotoxin injection site for the hippocampal lesion were calculated.
The hippocampal lesions were produced by injections of ibotenic acid and were intended to include all ammonic fields, the dentate gyrus, and subicular complex. For each monkey, MRI coordinates (i.e., anterior–posterior, medial–lateral, dorsal–ventral) of the injection targets were taken through the entire rostral–caudal extent of the structure. For the posterior two-thirds of the hippocampal formation, one coordinate was selected per MR image (every millimeter) to target the center of the hippocampus body. For the most anterior portion of the hippocampus, where the uncus was clearly visible, two coordinates were taken per image, one to target the body of the hippocampal formation (lateral) and one to target the uncus (medial). The MRI coordinates for the selected injection sites were then converted into stereotaxic coordinates relative to the stereotaxic point zero. A total of 11–12 injection sites were selected per hippocampus, 9–10 sites were spaced 1.5 mm apart, through the body of the hippocampal formation, and 2 additional sites were spaced 1.5 mm apart, at the uncus.
3.1.2. Surgery
All surgical procedures were carried out under deep anesthesia (7:3 mixture of ketamine hydrochloride, 100 mg/ml, and robinul 0.2 mg/ml, 0.1 ml/kg i.m.), followed by isoflurane inhalation (2% to effect) using aseptic conditions. The anesthetized animal was repositioned in the stereotaxic apparatus for the hippocampal lesions. Heart and respiration rates, blood pressure, expired CO2, and body temperature were monitored throughout the procedure.
Following disinfection (Nolvasan solution) of the scalp and application of local anesthetic (Marcaine 25%, 1.5 ml) along the incision line, the skin was cut from the orbit to the occiput, and the connective tissue and temporal muscles were gently retracted and the dura cut.
To access the injection sites, a bone flap was made on the top of the skull and small slits were cut in the dura over the location of the injection sites. Injections were performed simultaneously in the left and right hemispheres. The needle of the 10 µl Hamilton syringe, held in a Kopf electrode manipulator (David Kopf Instruments, Tujunga, CA), was slowly lowered to each injection site and a total of 1.5–2.4 µl of ibotenic acid (Regis Chemical, Morton Grove, IL, 15 mg/ml in PBS, pH 7.4) was injected at each site at a rate of 0.2 µl/min. A 5 min delay was imposed before retraction of the needle to permit diffusion of the neurotoxin and minimize its spread along the needle track. The needle was then swabbed to remove any residual neurotoxin, and repositioned and lowered to the next injection coordinate.
When the injections were completed, the dura openings were sewn, and all tissues were closed in anatomical layers. To minimize brain swelling, all operated animals received an intravenous drip of 30 ml of mannitol (20%, delivered at a rate of 1 ml/min) before beginning the final injection.
Beginning 12 h prior to and continuing for one week after surgery, all operated monkeys were treated with dexamethazone sodium phosphate (0.4 mg/kg, i.m.) and Cephazolin (Bristol-Myers Squib, 25 mg/kg, i.m.) to reduce inflammation and protect against infection, respectively. For 3 days after surgery, the monkeys also received an analgesic (acetaminophen 10 mg/kg, p.o.).
3.1.3. Lesion assessment
Seven to ten days after surgery, all monkeys received a second MRI procedure, including a high resolution T1 and a Fluid Attenuated Inversion Recovery (FLAIR) scans to visualize areas of increased water density induced by cell death at the injection sites. The post-surgical scanning procedures have been shown to provide a rapid and accurate way to quantify lesion location and extent after neurotoxin infusion (Málková et al., 2001; Nemanic et al., 2002). In one case (Gracie), the extent of damage was minimal on the left side and so a second surgery was performed 1 month later during which ibotenic acid was injected unilaterally at 8 sites along the spared hippocampus. A second FLAIR, one week later, confirmed the additional damage by the ibotenic acid to the left hippocampus.
All three monkeys died during Tropical Storm Allison, thus, hypersignals observed onto post-surgical FLAIR images were the only means to examine the location and extent of damaged areas. However, estimation of the extent of hypersignals was shown to provide a good estimate of the extent of cell loss observed histologically and to correlate with the amount of volume reduction observed months after surgery (r = 0.893, p< 0.005; Málková et al., 2001; Nemanic et al., 2002). For each animal, FLAIR images were matched with digitized drawings of coronal sections at 1 mm intervals through a normal monkey brain. The extent of hypersignals on each image was drawn on each coronal section of the normal monkey. Using Scion Image® software, the extent of hypersignals seen within the hippocampal formation as well as in adjacent structures was measured on each section and percent damage for each structure (as compared to the normal brain) was calculated. For Gracie, which received ibotenic acid injections in two stages in the left hemisphere, the extent of hypersignals seen on the 2 post-surgical scans were combined and drawn onto the coronal sections of the normal brain.
3.1.4. Postsurgical training
Following recovery from surgery, which lasted 40, 49 and 52 days for Cuba, Gracie and Slim, respectively, all monkeys were re-trained on the list learning task, using the same procedures described above for the pre-surgical training. The animals were first given training S/D sessions followed by three transfer sessions to assess retention of abstract-concept learning. Training sessions consisted of 32 trials (16 same, 16 different) with stimuli randomly selected for each trial from a set size of 432 images. Upon completing an FR 1 to the upper image, it disappeared, followed by a 1 s retention delay and the lower image. Transfer sessions were identical to training sessions except 16 transfer trials (8 same, 8 different) replaced baseline trial. Slim was not given the transfer sessions at this time because of poor performance in the S/D task, but he was tested later, after completing list memory testing. Next, two monkeys (Cuba and Gracie) were returned to list memory testing using a list of 4 items in the block design previously described. Testing continued in this design until the serial position functions were stable. Slim also returned to list memory testing but with short delays (0, 1 and 2 s) because of his difficulty in the S/D task. After 24 sessions (8 of each short delay) his performance improved and he began training in the block design with all six retention delays.
3.2. Results
3.2.1. Lesion extent
Examples of post-surgical FLAIR images matched with corresponding coronal sections of the normal monkey are shown for the three cases in Figs. 1–3. The percent damage to each field of the hippocampal formation for each hemisphere in each case is presented in Table 2.
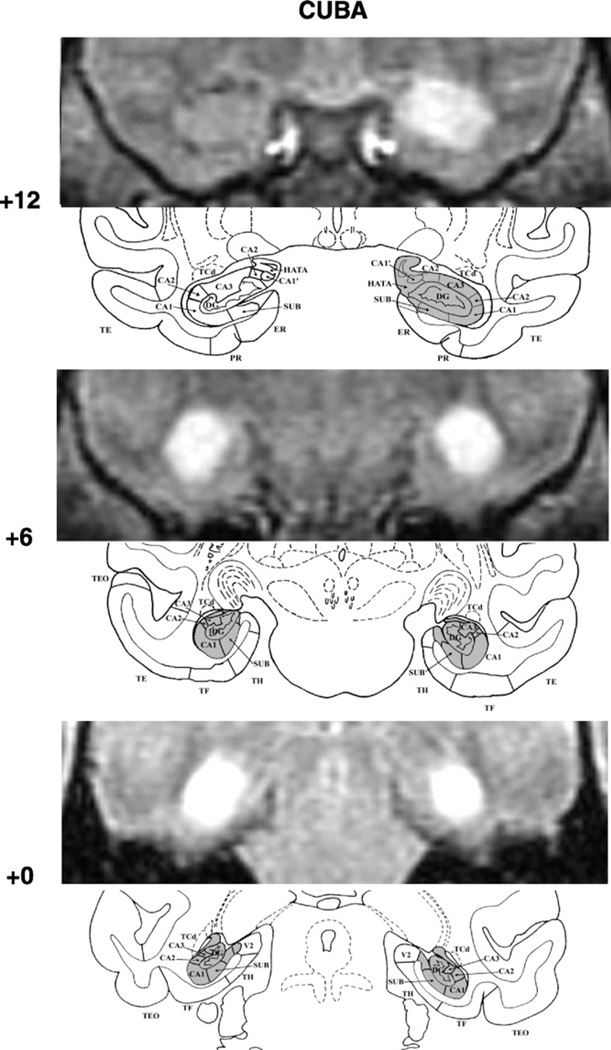
Fig. 1.
FLAIR MR coronal images (top) matched with drawing sections of a normal monkey brain (bottom) for three A–P levels through the hippocampal formation in CUBA. White areas on the FLAIR images depict areas of hypersignals that were reconstructed onto the corresponding section (gray area) of the normal monkey brain. MR acquisition parameters for the three scans were: slice thickness: 3.0 mm (with 1 mm offset between scans), repetition time: 10, echo time: 1.47, inversion time: 2.2, number of averages: 2.0, echo numbers(s): 1, magnetic field strength: 1.5 T, spacing between slices: 3 mm echo train length: 24. The numeral on the left indicates the distance in millimeters from the interaural plane. Abbreviations: CA1, CA2, and CA3, cornu ammonis fields of the hippocampus; DG, dentate gyrus; ERh, entorhinal area 28; HATA, amygdala–hippocampus transition area; PRh, perirhinal areas 35 and 36, SUB, subicular complex; TE, TEO, TH, and TF: cytoarchitectonic fields described by von Bonin and Bailey (1947), and V2, visual extrastriate cortical area.
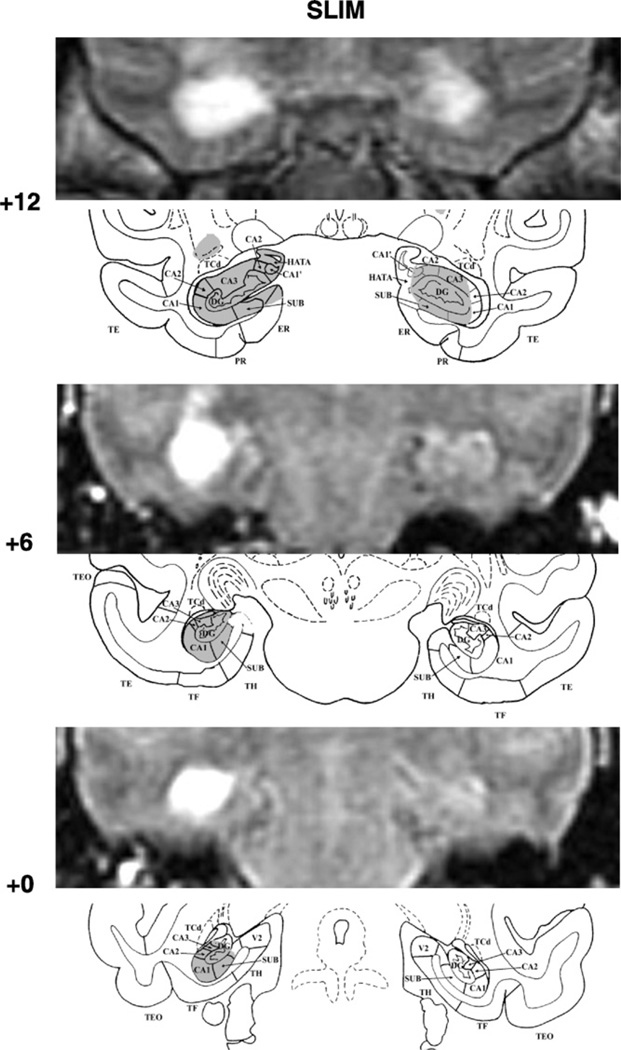
Fig. 3.
FLAIR MR coronal images (top) matched with drawing sections of a normal monkey brain (bottom) for three A–P levels through the hippocampal formation in SLIM. Conventions as in Fig. 1.
Table 2.
Percent of intended damage to the hippocampal formation.a
| Subjects | CA1 |
CA2 |
CA3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | |
| Cuba | 80.9 | 100 | 90.5 | 80.9 | 61.7 | 100 | 80.9 | 61.7 | 58.9 | 97.9 | 78.4 | 57.7 |
| Gracie | 71.2 | 100 | 85.6 | 71.2 | 91.4 | 92.2 | 91.8 | 84.2 | 34.6 | 89.9 | 62.2 | 31.1 |
| Slim | 94.1 | 5.7 | 49.9 | 5.4 | 90.1 | 7.7 | 48.9 | 6.9 | 95.2 | 32.3 | 63.7 | 30.7 |
| X | 82.1 | 68.6 | 75.3 | 52.5 | 81.1 | 66.6 | 73.9 | 50.9 | 62.9 | 73.4 | 68.1 | 39.8 |
| Subjects | Dentate gyrus |
Subicular complex |
Total |
|||||||||
| L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | |
| Cuba | 66.6 | 96.6 | 81.6 | 64.4 | 66.5 | 94.0 | 80.2 | 62.5 | 66.2 | 97.4 | 81.8 | 64.5 |
| Gracie | 34.0 | 84.3 | 59.2 | 28.7 | 16.9 | 84.4 | 50.6 | 14.2 | 43.3 | 85.7 | 64.5 | 37.1 |
| Slim | 78.6 | 35.7 | 57.1 | 28.0 | 89.8 | 23.5 | 56.7 | 21.1 | 88.2 | 25.9 | 57.1 | 22.9 |
| X | 59.7 | 72.2 | 65.9 | 40.4 | 57.7 | 67.3 | 62.5 | 32.6 | 65.9 | 69.7 | 67.8 | 41.5 |
Data are estimated intended damage (in percent of normal) to the ammon fields (CA1, CA2, and CA3), the dentate gyrus and subicular complex. Total refers to average of all five hippocampal regions. Note that for Gracie, percent of damage included extent of hypersignals found after both the first and second surgical procedures. Abbreviations: L, percent damage to the left hemisphere; R, percent damage to the right hemisphere; Avg, average of L and R, W = (L× R)/100 (weighted index as defined by Hodos and Bobko, 1984).
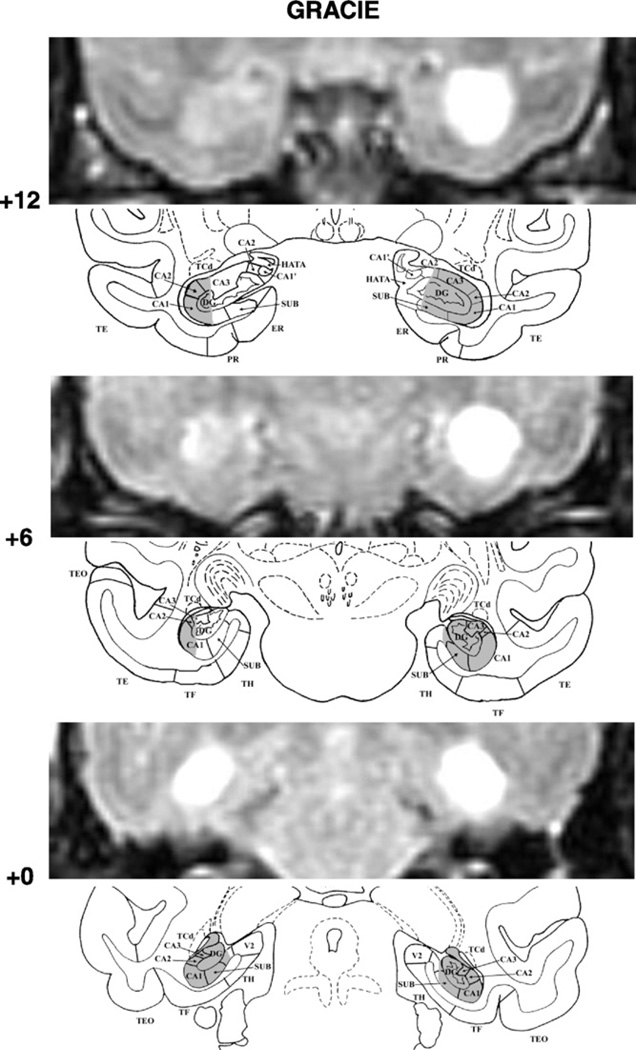
Damage to the hippocampal formation was incomplete, but substantial, in all cases, ranging from 57% to 97%. In one case, Cuba, cell loss was extensive, encompassing all but the rostral most 2 mm of the hippocampal formations on the right (see Fig. 1, level +12), whereas in the others, Gracie (Fig. 2) and Slim (Fig. 3), cell loss was extensive (>85%) on one side, but only moderate on the other (<43%). Nevertheless, despite an incomplete lesion on the left hemisphere, Gracie had 71% and 91% damage to the CA1 and CA2 fields, respectively. Importantly, none of the three monkeys had unintended damage to the cortical areas surrounding the hippocampal formation. Slim’s lesions, however, encroached on the posterior amygdala bilaterally (12% and 9% on the right and left, respectively). In addition, Slim incurred damage to the head of the caudate and tail of the putamen (more left than right hemisphere), resulting most likely from the injection needles.
Fig. 2.
FLAIR MR coronal images (top) matched with drawing sections of a normal monkey brain (bottom) for three A–P levels through the hippocampal formation in GRACIE. Note that the MR images shown were those obtained after the first surgical procedure; note the extensive sparing of the hippocampal formation on the left hemisphere as reproduced on the drawing below. Conventions as in Fig. 1.
3.2.2. Re-training and transfer on the same/different task
Two monkeys (Gracie and Cuba) showed excellent retention of the S/D task they had learned pre-surgically and were retrained for only two sessions (64 trials) averaging 93.75% and 81.3%, respectively. By contrast, Slim performed more poorly averaging only 55% over 11 sessions (352 trials). Therefore, Gracie and Cuba immediately proceeded to transfer testing and showed full abstract-concept learning again (Gracie: baseline = 98%, transfer = 98%; Cuba: baseline = 98%, transfer =95.8%). For Slim, after completing list memory testing, he also showed full abstract-concept learning again (baseline = 89.6% and transfer = 91.7%).
3.2.3. List memory
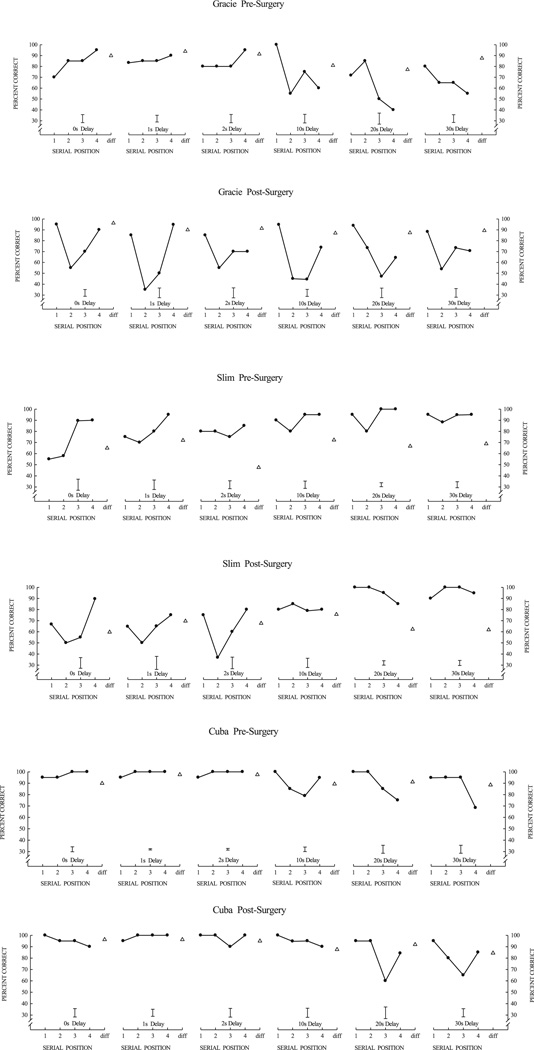
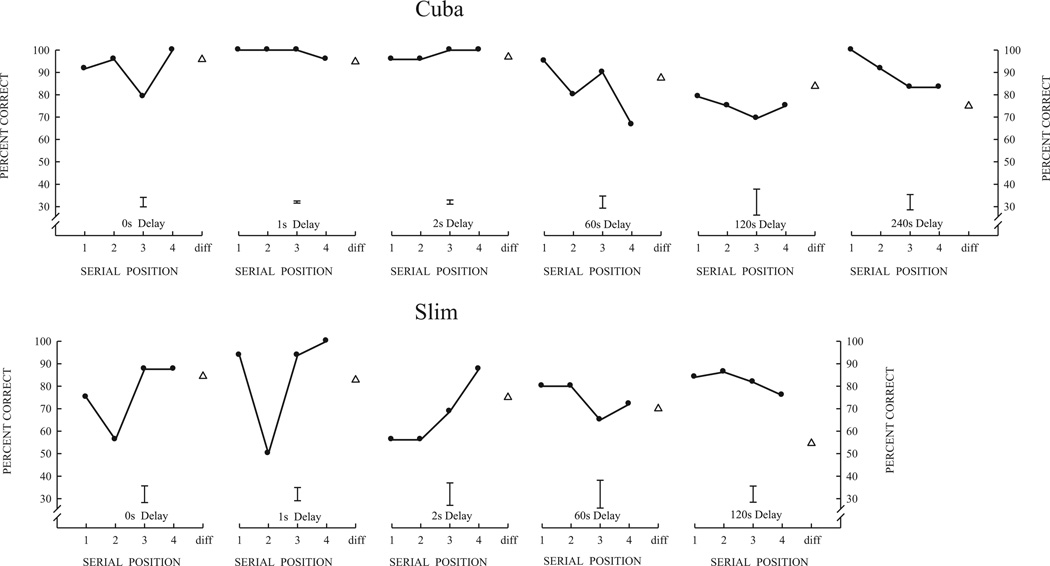
Fig. 4 illustrates serial position functions (SPFs) obtained during the last 5 blocks of pre-surgical and post-surgical list-memory testing for each monkey. At the end of post-surgical list-memory training, all animals showed high accuracy on the list memory task with some changes in the shape of the SPFs compared to pre-surgical SPFs. For Gracie (Fig. 4, top), the primacy effect during the pre-surgery training only emerged after a 10 s delay, whereas the primacy effect during post-surgery was present at all delays, including those shorter than 10 s. In addition, the recency effect dissipated at a slightly shorter delay (2 s) after surgery as compared to pre-surgery (10 s). Slim, like Gracie, also showed an earlier primacy effect (0 s delay) post-surgery than pre-surgery (Fig. 4, center), but the primacy effect grew more slowly with increasing delays than for Gracie. Slim did not show dissipation of the recency effect (pre-surgery or post-surgery) at longer delays, but his good accuracy was somewhat confounded by a bias to respond “same” at long delays. Monkey Cuba was clearly the most exceptional performer both pre-and post-surgically (Fig. 4, bottom). Cuba’s primacy effect remained very strong across all delays and interestingly Cuba’s post-surgery recency effect did not dissipate at long delays (even at the longest delay of 30 s) as it had pre-surgically.
Fig. 4.
Serial position functions curves at the end of list-learning training for Gracie, Slim, and Cuba. For each animal, top graphs illustrate pre-surgery performance and bottom graphs illustrate post-surgery performance. Data points are mean performance for four-item serial lists at different probe delays (retention intervals), the interval between the last list item and the probe test item. The bar shown for each serial-position function is the average standard error of the mean for the four serial positions (“same” trials). Open triangles show performance on “different” trials where the probe item matched none of the four list items.
3.2.4. Extended delay training
Given the unexpected failure to identify important changes in memory performance at delays up to 30 s with compromised or absent hippocampi, we extended the delays of Cuba and Slim further as shown in Fig. 5. Cuba was tested with longer delays of 60, 120, and 240 s and Slim with longer delays of 60 and 120 s (along with the 0, 1 and 2 s short delays) in the block design previously described. Short-delay performance was basically unaltered for these two monkeys, and remarkable was that their longer delay performance was basically unchanged from what it had been with the 30 s delay. Cuba’s performance averaged 84%, 82%, and 93% for long delays of 60, 120, and 240 s, and Slim’s performance averaged 78% and 89% for long delays of 60 and 120 s. In general, the longer delays had very little effect on their overall list memory performance and only slightly affected the form of some of their serial position functions.
Fig. 5.
Cuba and Slim post-surgical serial position functions with delays increased to 60 s, 120 s, and 240 s. Error bars are the average standard error of the mean for the four serial positions of each function.
4. Experiment 2
The accurate performance and regular serial position functions from Experiment 1, even when the delays between the end of the list and the probe test were increased, suggest that this good list-memory performance was not dependent upon the hippocampus. The majority of the serial position functions for each animal remained similar to those they obtained prior to the hippocampal lesions, with the only changes being improved primacy performance for Gracie and Slim at short delays, and improved recency performance for Cuba at long delays.
Indeed, the complete lack of decrements in memory performance following hippocampal lesions on a S/D recognition task in fact parallels many of the findings reported in humans with temporal lobe amnesia using yes/no recognition tasks (Bastin et al., 2004; Freed et al., 1987; Holdstock et al., 2005; Mayes et al., 2002). One reason for this lack of effects might be that these tasks may be solved using familiarity judgments “Have I seen this item before or not?” mediated by the medial temporal cortex rather than by accurate recollection about “when” or “where” (contexts) that this item (“what”) occurred. Such context learning is the basis for episodic memory and has been concluded to depend on the hippocampus (see for review Brown and Aggleton, 2001; Eichenbaum et al., 2007; Norman and O’Reilly, 2003; Rugg and Yonelinas, 2003). According to these theorists, in the absence of a functional hippocampus, serial list memory of the three monkeys could only have been maintained at a high level of proficiency by the use of familiarity judgments possibly mediated by the medial temporal cortical areas.
One way to test this possibility of familiarity processing would be to reduce the stimulus set size from the original large pool of items (used only once in a daily session) to a set size of 8 items that are repeated many times in different lists within the daily session. Thus, simple familiarity “Have I seen this item today” could not support good list memory performance. With this manipulation, after just 1 or 2 list presentations, all items become very familiar to the monkeys. If they are simply using familiarity judgments, they should respond “same” more often on “different” trials. Thus, under this condition, memory performance should become very poor, unless as in humans, these monkeys could remember that the test on a “different” trial does not match the current sample/list-item even though they may have seen it on the previous trial. Said otherwise, they would need to maintain memory of which items they have seen that day and in which list (i.e., prior list) they have seen them. Given that such strategies have been thought to depend on the integrity of the hippocampus, we would predict that removal of the hippocampus would render the subjects more susceptible to increases in proactive interference from previous list items and impair memory performance.
4.1. Method
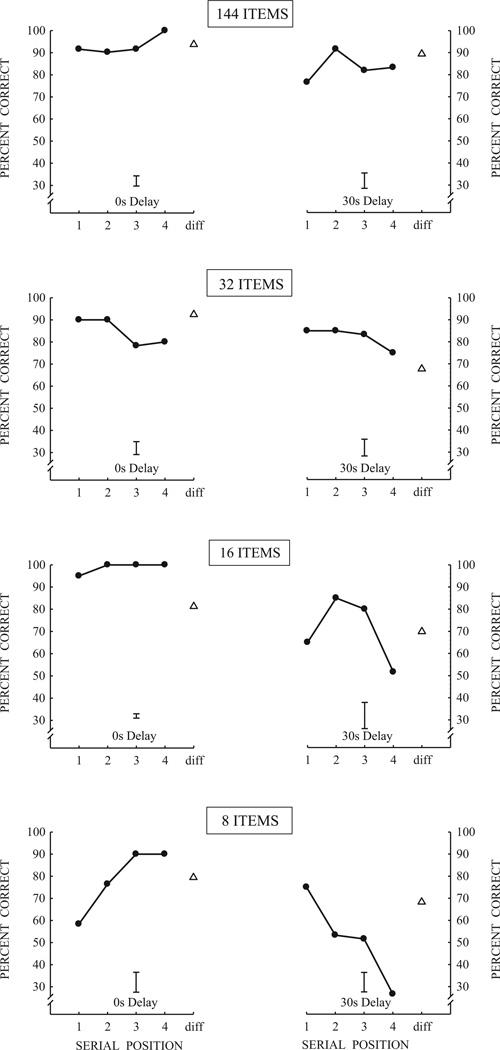
Cuba was the only monkey that was available to be tested for an across list interference effect on list memory performance. For this testing, the stimulus set size was progressively reduced from 144, 32, 16 and then 8 stimuli with tests at the shortest delay of 0 s and the longest delay of 30 s and different sets of stimuli at the two delays. Finally, the effect of interference on serial position functions was assessed using a stimulus set of 8 items with all 6 probe delays (0 s, 1 s, 2 s, 10 s, 20 s and 30 s) in the block design previously described. In this phase of testing, one set of 8 stimuli was used for delays of 0 s, 1 s, and 2 s, and a different set of 8 stimuli was used for delays of 10 s, 20 s, and 30 s, so that tests in the first block of a daily test session would not adversely affect (buildup of proactive interference) and confound tests in the second block.
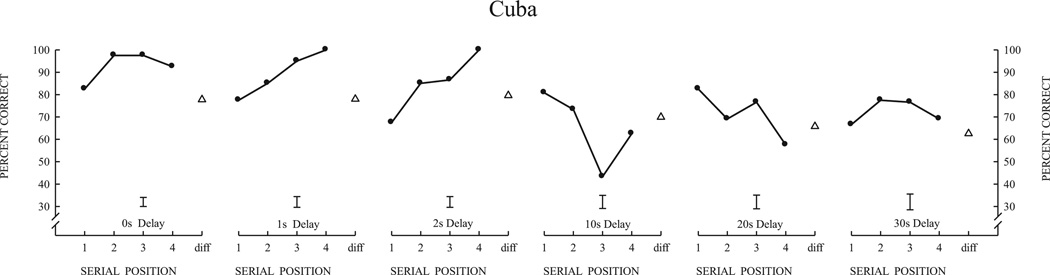
4.2. Results
As shown in Fig. 6, it is remarkable that Cuba maintained excellent performance even when the set size was decreased from 144 items to 8 items. Thus, despite the high level of proactive interference and overall high level of familiarity created by the use of a small set of 8 items, this animal nevertheless maintained a strong recency effect at the short delay of 0 s and a strong primacy effect at the long delay of 30 s. In addition, memory performance remained high in this animal when the six delays were blocked. As shown in Fig. 7, Cuba’s performance shows a strong recency effect at the 1 s delay, and, as the delays increased, memory for the first items of the list became stronger (primacy effect) and progressively inhibited memory for the last items.
Fig. 6.
Serial position functions for Cuba with the set size of items progressively reduced from 144 to 32, 16 and finally 8 items. Delays used were 0 s and 30 s. Error bars are the average standard error of the mean for the four serial positions of each function.
Fig. 7.
Serial position functions across retention delays with a set size of 8-items for Cuba. Error bars are the average standard error of the mean for the four serial positions of each function.
5. Discussion
The main findings of this study indicate that contrary to most memory models of the role of the hippocampus, selective damage to the hippocampus had only minor effects on list memory accuracy and dynamic time changes of the serial position function. Most memory models would predict: loss of primacy (first item) memory, loss of accuracy with delay, and loss in the ability to combat proactive interference. Contrary to those predictions, primacy memory actually increased for two monkeys (Gracie and Slim) at shorter delays and recency memory increased for a third monkey (Cuba) at longer delays, accuracy was maintained at long delays including delays up to 240 s (8 times longer than any pre-surgery testing), and accuracy was maintained despite high levels of proactive interference. Implication of these findings is discussed in the following sections.
5.1. Hippocampus and serial list memory performance
Two monkeys, Gracie and Cuba, were shown to perform the S/D task to high levels of proficiency in only 64 trials (i.e., no loss of learning) following hippocampal lesions. These monkeys also showed full abstract-concept learning where transfer was equivalent to baseline performance. A third monkey, Slim required some re-learning (1636 trials), but there was a huge savings relative to the original acquisition (30,600 trials) and he also eventually achieved accurate performance and full abstract-concept learning. All three monkeys also demonstrated good performance on the serial list memory task at 0–30 s delays, with strong recency and primacy effects. The lack of effects of hippocampal lesions on the serial position functions differs from the absence of primacy effect that has been previously reported in patients with medial temporal lobe damage (e.g., Baddeley and Warrington, 1970; Hermann et al., 1996; Hopkins et al., 1995; Hopkins and Kesner, 1995) as well as rodents with selective hippocampal damage (Kesner, 1998; Kesner and Novak, 1982). The difference in results most likely stems from several differences between these previous studies and the one reported here.
First, differences between the human and monkey data could have resulted from varying extent of medial temporal lobe tissue included in the hippocampal lesions. In the earlier human studies, damage to the hippocampus was not selective and extended to the surrounding cortical areas that may have had greater impact on memory performance. Alternatively, one could argue that in two cases of the present study the hippocampal damage was not complete and the spared hippocampal tissue may have allowed for good performance. But this latter possibility seems unlikely given that in one case (Cuba) the hippocampal damage was selective, bilateral, and complete and nevertheless Cuba’s recognition memory accuracy remained virtually unchanged from his pre-surgery levels. Another factor that could be used to explain the different outcomes between the human and monkey results was a difference in list lengths. Longer lists have been used in many human studies than the list of 4 items used here with the monkeys. Longer lists do emphasize the primacy effect more than shorter lists (Murdock, 1962). Nevertheless, there are a number of mitigating factors that bear upon this issue. For example, none of the human studies with longer lists systematically varied the retention interval. And varying the retention interval does emphasize the visual memory primacy effect. Moreover, by varying the retention interval the return-to-primacy or Law of Primacy becomes apparent (see Tulving, 2008; Wright, 2013, this volume). Moreover, primacy effects have also been shown for humans with short 4-item lists of kaleidoscope patterns along with similar dynamic SPF changes with delay (e.g., Wright et al., 1985).
Second, differences between the rodent and monkey data cannot be accounted by differences in the extent of hippocampal lesion. The rodents, however, were trained in a radial-arm maze which tends to favor memory for spatial locations instead of memory for items. Given the well-known role of the hippocampus in spatial memory (for review see O’Keefe and Nadel, 1978; Kessels et al., 2001; King et al., 2004), differences between the outcomes of rodent studies and the monkey study of this article could be due to the difference between spatial memory (“where” memory) and item memory (“what” memory) of the present study.
Additionally, the intact performance of the three monkeys on the list memory task actually is supported by other findings of unimpaired recognition memory performance by monkeys, rodents, and amnesic patients. Monkeys with selective hippocampal lesions have demonstrated intact recognition memory in the delayed nonmatching-to-sample (DNMTS) task, even with substantial memory delays and long lists of sample stimuli. Monkeys with selective hippocampal lesions have demonstrated intact recognition memory in memory span tasks (Heuer and Bachevalier, 2011; Murray and Mishkin, 1998; Nemanic et al., 2004; but see also Beason-Held et al., 1999; Zola et al., 2000). Similarly, DNMTS recognition performance in rodents is also intact after selective hippocampal damage, although other studies report partial impairment at long delays or long lists (Clark et al., 2000, 2001; Mumby, 2001; Steckler et al., 1998). Intact recognition memory was also found in several cases of amnesic patients with damage to the hippocampal formation, specifically in those tested with a yes/no recognition task, a task similar to the S/D task used in the current study (Adlam et al., 2009; Mayes et al., 2002; Vargha-Khadem et al., 1997; but see also Cipolotti et al., 2001; Manns et al., 2003).
5.2. Hippocampus and within-list interference
Given that one well-accepted role of the hippocampus in memory processes is to combat interference among items of a list (Eichenbaum and Buckingham, 1990; Shapiro and Olton, 1994), we predicted two possible outcomes following hippocampal lesions. First, retroactive interference of the last item upon the first item of the list would likely increase such that the primacy effect would be delayed or eliminated, as had been shown in previous studies with humans (Baddeley and Warrington, 1970; Hermann et al., 1996; Hopkins et al., 1995; Hopkins and Kesner, 1995) and rodents (Kesner, 1998; Kesner and Novak, 1982). Alternatively, under trial-unique conditions where the first list items exert proactive interference on memory for the last list items, removal of the hippocampus may increase this within-list proactive interference and actually delay or eliminate the recency effect. The data showed that, after selective hippocampal lesions, the shapes of some serial position curves were slightly altered for all 3 animals, but contrary to our expectations neither the primacy effect nor the recency effect was delayed or abolished (see Fig. 4).
Extensive pre-surgical training may have allowed the animals to rely on their well-learned reference memory of the task, which in turn may have provided the necessary basis for good working memory (remembering items of the current list) and their robust retrieval memory.
5.3. Hippocampus and across-list interference
Several studies have provided evidence that not all recognition memory tasks are sensitive to hippocampal lesions (Holdstock et al., 2002; Nemanic et al., 2004; Reed et al., 1997) and more particularly that memory performance on the DNMTS and yes/no recognition tasks may be supported by familiarity judgments that do not require the contribution of the hippocampus (for review see Aggleton and Brown, 2006; Diana et al., 2007; Eichenbaum et al., 2007). This proposal was specifically tested in the second experiment that minimized the effectiveness with which animals could use a familiarity strategy by increasing item repetition across trials and proactive interference. We did this by reducing the stimulus set size from 432 items (trial unique per daily session) to 8 items so that items would be repeated every few trials and proactive interference would accumulate over the block of 32 trials tested at a particular delay. Hence, if monkeys were simply using familiarity judgments, then they should respond “same” more often and increase their “same” bias as the trial block wore on. But as shown in Figs. 6 and 7, there is no evidence supporting a familiarity judgment strategy for the animal with hippocampal damage tested in this experiment. Contrary to the familiary strategy, this animal maintained high levels of memory performance even at the longest delays tested. This result suggests that this animal was using a strategy (or strategies) other than familiarity judgments to solve the task and recollect whether a memory item was in the current list or in some previous list. Whether they shifted their strategy from familiarity with session-unique stimuli to a recollective memory processes with repeated stimuli or were using a more recollective process all along is an open question. Other studies have shown that monkeys trained and tested with repeated stimuli and high proactive interference do gradually improve their performance (see for review Wright, 2007). Either way, the extensive training this monkey received prior to surgery was likely instrumental in its post-surgery accurate performance.
Lastly, the good memory performance of Cuba that had nearly complete, bilateral, damage to the hippocampus is intriguing and needs further investigation because it contradicts several theories that have been advanced to support the role the hippocampus in episodic memory. For example, two proposals have mainly driven research on the role of the hippocampus in recognition memory. The first is the one-process theory (Squire, 1994; Squire et al., 2007) that suggests that familiarity and recollection can both be supported by the hippocampus when memory strength of signals is high. The second is the two-process theory proposing that the hippocampus is important for recognition memory for events of episodes (what, where and when) but not for familiarity judgments (Eichenbaum et al., 2007). However, neither theory would predict that Cuba without a functional hippocampus (bilaterally), could accurately perform the list-memory task under high proactive interference with only a familiarity strategy. The findings presented in this article will need to be followed up and replicated in a larger number of animals, perhaps with differing amounts of pre-operative training in the serial list memory task. Nevertheless, if confirmed these findings may change current conceptions of the role of the hippocampus in recognition memory and further our understanding underlying hippocampal-dependent processes of relational memory, familiarity, episodic memory.
Acknowledgments
This research was supported by National Institutes of Health Grant MH-58846 to J.B., and MH072616 and MH091038 to A.A.W. The authors would like to thank Jacquelyne J. Rivera for her unwavering dedication in conducting the research. The authors wish to express their appreciation to Christopher S. Smith, Terry L. Blasdel, Peggy Bek, and Tammy L. Humbird for their expertise and valuable help in the pre- and post-surgical care of the monkeys, Roger E. Price and Belinda Rivera for the care and handling of the animals during the MR imaging procedures, Edward F.Jackson for his expert assistance in neuroimaging scanning techniques, and Maria C. Alvarado for help in the neuroimaging and surgical procedures. Writing of this manuscript was also supported in part by the Yerkes Base Grant NIH RR00165, and the Center for Behavioral Neuroscience grant NSF IBN-9876754.
Footnotes
Jonathon Crystal was the acting editor for this paper.
References
- Adlam AL, Malloy M, Mishkin M, Vargha-Khadem F. Dissociation between recognition and recall in developmental amnesia. Neuropsychologia. 2009;47(11):2207–2210. doi: 10.1016/j.neuropsychologia.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends Cogn. Sci. 2006;10:455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Shaw C. Amnesia and recognition memory: a re-analysis of psychometric data. Neuropsychologia. 1996;34(1):51–62. doi: 10.1016/0028-3932(95)00150-6. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. Visual recognition memory impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav. Brain Res. 1986;20:249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Warrington EK. Amnesia and the distinction between short and long-term memory. J. Verbal Learn. Verbal Behav. 1970;9:176–189. [Google Scholar]
- Barker GRI, Warburton EC. When is the hippocampus involved in recognition memory? J. Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin C, Linden M, Charnallet A, Denby C, Montaldi D, Roberts N, Andrew M. Dissociation between recall and recognition memory performance in an amnesic patient with hippocampal damage following carbon monoxide poisoning. Neurocase. 2004;10:330–344. doi: 10.1080/13554790490507650. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching-to-sample deficits in monkeys. Hippocampus. 2001;11(1):61–71. doi: 10.1002/1098-1063(2001)11:1<61::AID-HIPO1021>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9(5):562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Belmont JM, Butterfield EC. What the development of short-term memory is? Hum. Dev. 1971;14(4):236–248. doi: 10.1159/000271218. [DOI] [PubMed] [Google Scholar]
- Bhatt RS, Wright AA. Concept leaning by monkeys with video picture images and a touch screen. J. Exp. Anal. Behav. 1992;57:219–225. doi: 10.1901/jeab.1992.57-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles B, Crupi C, Mirsattari SM, Pigott S, Parrent AG, Pruessner JC, Yonelinas AP, Kohler S. Impaired familiarity with preserved recollection after anterior temporal lobe resection that spares hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16382–16387. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Prog. Neurobiol. 2001;55:149–189. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Shallice T, Chan D, Fox N, Scahill R, Harrison G, Stevens J, Rudge P. Long-term retrograde amnesia.. the crucial role of the hippocampus. Neuropsychologia. 2001;39(2):151–172. doi: 10.1016/s0028-3932(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Clark RE, West AN, Zola SM, Squire LR. Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus. 2001;11:176–186. doi: 10.1002/hipo.1035. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J. Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder RG. Auditory and temporal factors in the modality effect. J. Exp. Psychol. Learn. Mem. Cogn. 1986;12(2):268–278. doi: 10.1037//0278-7393.12.2.268. [DOI] [PubMed] [Google Scholar]
- Crowder RG, Morton J. Precategorical acoustic storage (PAS) Percept. Psychophys. 1969;5(6):365–373. [Google Scholar]
- Damasio AR, Eslinger PJ, Damasio H, Van Hoesen GW, Cornell S. Multimodal amnesic syndrome following bilateral temporal and basal forebrain damage. Arch. Neurol. 1985;42(3):252–259. doi: 10.1001/archneur.1985.04060030070012. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn. Sci. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Buckingham J. Studies on hippocampal processing: experiment, theory and model. In: Gabriel M, Moore J, editors. Learning and Computational Neuroscience: Foundation of Adaptive Networks. Cambridge, MA: MIT; 1990. pp. 171–231. [Google Scholar]
- Eichenbaum H, Mathews P, Cohen NJ. Further studies of hippocampal representation during odor discrimination learning. Behav. Neurosci. 1989;103(6):1207–1216. doi: 10.1037//0735-7044.103.6.1207. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum and the fornix. Exp. Brain Res. 1996;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Fahy F, Riches I, Brown M. Neuronal activity related to visual recognition memory, long-term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Exp. Brain Res. 1993;96:457–472. doi: 10.1007/BF00234113. [DOI] [PubMed] [Google Scholar]
- Freed DM, Corkin S, Cohen NJ. Forgetting in H.M.: a second look. Neuropsychologia. 1987;25:461–471. doi: 10.1016/0028-3932(87)90071-6. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Murray EA. Monkeys (Macaca fascicularis) with rhinal cortex ablations succeed in object discrimination learning despite 24-hr intertribal intervals and fail matching to sample despite double sample presentations. Behav. Neurosci. 1992;106:30–38. doi: 10.1037//0735-7044.106.1.30. [DOI] [PubMed] [Google Scholar]
- Gardiner JM. Levels of processing in word recognition and subsequent free recall. J. Exp. Psychol. 1974;102(1):101–105. doi: 10.1037/h0035694. [DOI] [PubMed] [Google Scholar]
- Glanzer M, Cunitz AR. Two storage mechanisms in free recall. J. Verbal Learn. Verbal Behav. 1966;5:351–360. [Google Scholar]
- Goldman-Rakic PS. Circuitry of the frontal association cortex and its relevance to dementia. Arch. Gerontol. Geriatr. 1987;6(3):299–309. doi: 10.1016/0167-4943(87)90029-x. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Wyler A, Davies K, Christeson J, Moran M, Stroup E. The effects of human hippocampal resection on the serial position curve. Cortex. 1996;32(2):323–334. doi: 10.1016/s0010-9452(96)80054-2. [DOI] [PubMed] [Google Scholar]
- Heuer E, Bachevalier J. Effects of selective neonatal hippocampal lesions on tests of object and spatial recognition memory in monkeys. Behav. Neurosci. 2011;125:137–149. doi: 10.1037/a0022539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W, Bobko PA. A weighted index of bilateral brain lesions. J. Neurosci. Methods. 1984;12:43–47. doi: 10.1016/0165-0270(84)90046-3. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Gong QY, Roberts N, Kapur N. Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus. 2005;15:203–215. doi: 10.1002/hipo.20046. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Roberts N, Cezayirli E, Isaac CL, O’Reilly RC, Norman KA. Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus. 2002;12:341–351. doi: 10.1002/hipo.10011. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Kesner RP. Item and order recognition memory in subjects with hypoxic brain injury. Brain Cogn. 1995;27:180–201. doi: 10.1006/brcg.1995.1016. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Kesner RP, Golsdtein M. Memory for novel and familiar spatial and linguistic temporal distance information in hypoxic subjects. J. Int. Neuropsychol. Soc. 1995;1(5):454–468. doi: 10.1017/s1355617700000552. [DOI] [PubMed] [Google Scholar]
- Jones BM. Memory impairment on the ascending and descending limbs of the blood alcohol curve. J. Abnorm. Psychol. 1973;82(1):24–32. doi: 10.1037/h0034872. [DOI] [PubMed] [Google Scholar]
- Katz JS, Wright AA, Bachevalier J. Mechanisms of same/different abstract-consept learning by rhesus monkeys (Macaca mulatta) J. Exp. Psychol. Anim. Behav. Process. 2002;28(4):358–368. [PubMed] [Google Scholar]
- Kesner RP. Correspondence between humans and animals in coding of temporal attributes: role of hippocampus and prefrontal cortex. Ann. N.Y. Acad. Sci. 1985;444:122–36. doi: 10.1111/j.1749-6632.1985.tb37584.x. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Neural mediation of memory for time: role of the hippocampus and medial prefrontal cortex. Psychon. Bull. Rev. 1998;5:585–596. [Google Scholar]
- Kesner RP, Novak JM. Serial position curve in rats: role of the dorsal Hippocampus. Science. 1982;218:173–174. doi: 10.1126/science.7123228. [DOI] [PubMed] [Google Scholar]
- Kessels RPC, de Haan EHF, Kappelle LJ, Postma A. Varieties of human spatial memory: a meta-analysis on the effects of hippocampal lesions. Brain Res. Rev. 2001;35:295–303. doi: 10.1016/s0165-0173(01)00058-3. [DOI] [PubMed] [Google Scholar]
- King JA, Trinkler I, Hartley T, Vargha-Khadem F, Burgess N. The hippocampal role in spatial memory and the familiarity-recollection distinction: a case study. Neuropsychology. 2004;18(3):405–417. doi: 10.1037/0894-4105.18.3.405. [DOI] [PubMed] [Google Scholar]
- Kolb B, Buhrmann K, McDonald R, Sutherland RJ. Dissociation of the medial prefrontal, posterior parietal, and posterior temporal cortex for spatial navigation and recognition memory. Cereb. Cortex. 1994;4:664–680. doi: 10.1093/cercor/4.6.664. [DOI] [PubMed] [Google Scholar]
- Málková L, Lex CK, Mishkin M, Saunders RC. MRI-based evaluation of locus and extent of neurotoxic lesions in monkeys. Hippocampus. 2001;11:361–370. doi: 10.1002/hipo.1050. [DOI] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37(1):171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Hunkin NM, Roberts N. Relative sparing of item recognition memory in a patient with adult-onset damage limited to the hippocampus. Hippocampus. 2003;12:325–340. doi: 10.1002/hipo.1111. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Isaac CL, Holdstock JS, Cariga P, Gummer A, Roberts N. Relative sparing of item recognition memory in a patient with adult-onset damage limited to the hippocampus. Hippocampus. 2002;12:325–340. doi: 10.1002/hipo.1111. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray E. Effects on visual recognition of combined and separate abaltions of the entorhinal andperirhinal cortex in rhesus monkeys. J. Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J. Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Vargha-Khadem F, Gadian DG. Amnesia and the organization of the hippocampal system. Hippocampus. 1998;8:212–216. doi: 10.1002/(SICI)1098-1063(1998)8:3<212::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behav. Brain Res. 2001;127(1/2):159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Murdock BB., Jr. The serial position effect in free recall. J. Exp. Psychol. 1962;64:482–488. [Google Scholar]
- Murdock BB., Jr Visual and auditory stores in short-term memory. Q. J. Exp. Psychol. 1966;18(3):206–211. doi: 10.1080/14640746608400031. [DOI] [PubMed] [Google Scholar]
- Murray E, Bussey T. Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn. Sci. 1999;3:142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J. Neurosci. 1998;18:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J. Neurosci. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Price RE, Jackson EF, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: a replication. J. Neurosci. Methods. 2002;121:199–209. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol. Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford, UK: Oxford University Press; 1978. [Google Scholar]
- Pihlajamaki M, Tanila H, Kononen M, Hanninen T, Hamalainen A, Soininen H, Aronen HJ. Visual representation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe sub-areas in humans. Eur. J. Neurosci. 2004;19:1939–1949. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Postman L, Phillips L. Short-term temporal changes in free-recall. Q. J. Exp. Psychol. 1965;17:132–138. [Google Scholar]
- Reed JM, Hamann SB, Stefanacci L, Squire LR. When amnesic patients perform well on recognition memory tests? Behav. Neurosci. 1997;111:1163–1170. doi: 10.1037//0735-7044.111.6.1163. [DOI] [PubMed] [Google Scholar]
- Roediger HL, III, Crowder RG. A serial position effect in recall of United States presidents. Bull. Psychon. Soc. 1976;8:275–278. [Google Scholar]
- Rudy JW, Sutherland KJ. The hippocampal formation is necessary for rats to learn and remember configural discriminations. Behav. Brain Res. 1989;34:97–109. doi: 10.1016/s0166-4328(89)80093-2. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Sutherland KJ. Configural and elemental associations and the memory coherence problem. J. Cogn. Neurosci. 1992;4(3):208–216. doi: 10.1162/jocn.1992.4.3.208. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Yonelinas AP. Human recognition memory: a cognitive neuro-science perspective. Trends Cogn. Sci. 2003;7:313–319. doi: 10.1016/s1364-6613(03)00131-1. [DOI] [PubMed] [Google Scholar]
- Saffran EM, Marin OS. Immediate memory for word lists and sentences in a patient with deficient auditory short-term memory. Brain Lang. 1975;2(4):420–433. doi: 10.1016/s0093-934x(75)80081-2. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, Olton DS. Hippocampal function and interference. In: Schacter DL, Tulving E, editors. Memory Systems. Cambridge, MA: The MIT Press; 1994. pp. 87–117. [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat. Rev. Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Skinner EI, Fernandes MA. Neural correlates of recollection and familiarity: a review of neuroimaging and patients data. Neuropsychologia. 2007;45:2163–2179. doi: 10.1016/j.neuropsychologia.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Squire LR. Declarative and nondeclarative memory: multiple brain systems supporting learning and memory. In: Schacter DL, Tulving E, editors. Memory Systems. Cambridge, MA: The MIT Press; 1994. pp. 203–231. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat. Rev. Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Bayley PJ, Squire LR. Recognition memory for single items and for associations is similarly impaired following damage limited to the hippocampal region. Learn. Mem. 2002;9:238–242. doi: 10.1101/lm.51802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, Drinkenburg WH, Saghal A, Aggleton JP. Recognition memory in rats. II. Neuroanatomical substrates. Prog. Neurobiol. 1998;54:313–332. doi: 10.1016/s0301-0082(97)00061-0. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Rudy JW. Configural association theory: the role of the hippocampal formation in learning, memory, and amnesia. Psychobiology. 1989;17(2):129–144. [Google Scholar]
- Suzuki W, Zola-Morgan S, Squire L, Amaral D. Lesions of the perirhinal and parahippocampal cortices in monkeys produce long-lasting memory impairment in visual and tactual modalities. J. Neurosci. 1993;13:2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. On the law of primacy. In: Gluck MA, Anderson JR, Kosslyn SM, editors. Memory and Mind. New York, NY: Erlbaum; 2008. pp. 31–48. [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277(53234):376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- von Bonin G, Bailey P. The Neocortex of Macaca mulatta. Urbana, IL: University of Illinois Press; 1947. [Google Scholar]
- Wais PE. fMRI signals associated with memory strength in the medial temporal lobes: a meta-analysis. Neuropsychologia. 2008;46:3185–3196. doi: 10.1016/j.neuropsychologia.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Logue V, Pratt RT. The anatomical localisation of selective impairment of auditory verbal short-term memory. Neuropsychologia. 1971;9(4):377–387. doi: 10.1016/0028-3932(71)90002-9. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107(3):829–854. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Watkins MJ, Watkins OC. Processing of recency items for free recall. J. Exp. Psychol. 1974;102(3):488–493. [Google Scholar]
- Weiskrantz L. Neuroanatomy of memory and amnesia: a case for multiple memory systems. Hum. Neurobiol. 1987;6(2):93–105. [PubMed] [Google Scholar]
- Wixted JT, Mickes L, Squire LR. Measuring recollection and familiarity in the medial temporal lobe. Hippocampus. 2010;20(11):1195–1205. doi: 10.1002/hipo.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AA. An experimental analysis of memory processing. J. Exp. Anal. Behav. 2007;88:405–433. doi: 10.1901/jeab.2007.88-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AA, Katz JS. Mechanisms of same/different concept learning in primates and avians. Behav. Process. 2006;72:234–254. doi: 10.1016/j.beproc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Wright AA, Rivera JJ, Katz JS, Bachevalier J. Abstract-concept learning and list-memory processing by capuchin and rhesus monkeys. J. Exp. Psychol. Anim. Behav. Process. 2003;29:184–198. doi: 10.1037/0097-7403.29.3.184. [DOI] [PubMed] [Google Scholar]
- Wright AA, Santiago HC, Sands SF, Kendrick DF, Cook RG. Memory processing of serial lists by pigeons, monkeys, and people. Science. 1985;229(4710):287–289. doi: 10.1126/science.9304205. [DOI] [PubMed] [Google Scholar]
- Wright AA. Functional Relationships for Investigating Cognitive Processes. Behav. Process. 2013 doi: 10.1016/j.beproc.2012.11.003. http://dx.doi.org/10.1016/j.beproc.2012.11.003, this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang JZ, Brown MW. Neuronal responses related to long-term recognition memory processes in prefrontal cortex. Neuron. 2004;42:817–829. doi: 10.1016/j.neuron.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Parks CM. Receiver operating characteristics (ROCs) in recognition memory: a review. Psychol. Bull. 2007;133(5):800–832. doi: 10.1037/0033-2909.133.5.800. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark SK. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J. Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]