Abstract
The chimeric gene encoding a C-terminally-truncated form of the S-layer protein SbpA from Bacillus sphaericus CCM 2177 and two copies of the Fc-binding Z-domain was constructed, cloned, and heterologously expressed in Escherichia coli HMS174(DE3). The Z-domain is a synthetic analogue of the B-domain of protein A, capable of binding the Fc part of immunoglobulin G (IgG). The S-layer fusion protein rSbpA31-1068/ZZ retained the specific properties of the S-layer protein moiety to self-assemble in suspension and to recrystallize on supports precoated with secondary cell wall polymer (SCWP), which is the natural anchoring molecule for the S-layer protein in the bacterial cell wall. Due to the construction principle of the S-layer fusion protein, the ZZ-domains remained exposed on the outermost surface of the protein lattice. The binding capacity of the native or cross-linked monolayer for human IgG was determined by surface plasmon resonance measurements. For batch adsorption experiments, 3-μm-diameter, biocompatible cellulose-based, SCWP-coated microbeads were used for recrystallization of the S-layer fusion protein. In the case of the native monolayer, the binding capacity for human IgG was 5.1 ng/mm2, whereas after cross-linking with dimethyl pimelimidate, 4.4 ng of IgG/mm2 was bound. This corresponded to 78 and 65% of the theoretical saturation capacity of a planar surface for IgGs aligned in the upright position, respectively. Compared to commercial particles used as immunoadsorbents to remove autoantibodies from sera of patients suffering from an autoimmune disease, the IgG binding capacity of the S-layer fusion protein-coated microbeads was at least 20 times higher. For that reason, this novel type of microbeads should find application in the microsphere-based detoxification system.
Crystalline bacterial cell surface layers (S-layers) are two-dimensional proteinaceous arrays that are found as the outermost cell envelope component of many bacteria and archea (27-30). S-layers completely cover the cell surface during all stages of growth and division, and they are composed of identical species of protein or glycoprotein subunits with a molecular mass ranging from 40 to 200 kDa. S-layer lattices exhibit an oblique, square, or hexagonal symmetry. In bacteria, the S-layer subunits are linked to each other and to the underlying cell envelope layer by noncovalent interactions. In the case of members of the family Bacillaceae, the N-terminal part was found to be involved in anchoring the S-layer subunits via a distinct type of secondary cell wall polymer (SCWP) to the rigid cell wall layer (4, 7, 11-14, 18, 21, 26, 27). Even after isolaton from the cell wall, many S-layer proteins maintain the ability to self-assemble in suspension or to recrystallize on solid supports, such as silicon wafers, gold chips, silanized glass, or plastic materials; on Langmuir lipid films; on liposomes;, and at the air-water interface (16, 30, 32). Together with the high density and regular arrangement of functional groups in the S-layer lattice, this specific feature has opened a broad potential for application in biotechnology, molecular nanotechnology, and biomimetics (30). In previous studies, functional groups in the S-layer lattice were exploited for covalent binding of biologically active macromolecules, such as enzymes, antibodies, or ligands, for the production of S-layer-based biosensors (30), solid-phase dipstick-style immunoassays (5, 6, 31), and affinity microparticles (36).
The nucleotide sequence encoding the S-layer protein SbpA of Bacillus sphaericus CCM 2177 was determined by a PCR-based technique (12). The protein precursor includes a 30-amino-acid-long typical gram-positive signal peptide and consists of a total of 1,268 amino acids. The N-terminal part of the S-layer protein SbpA possesses an S-layer-like homologous (SLH) domain and recognizes a distinct type of SCWP as the proper anchoring structure in the rigid cell wall layer. The structure of this SCWP has been elucidated by nuclear magnetic resonance (NMR) analysis (11). The polymer chains consist of 8 to 9 disaccharide units that are composed of N-acetylglucosamine and N-acetyl mannosamine. Every second N-acetyl mannosamine residue carries a pyruvate ketal, which endows the polymer chains with a negative net charge. Most recently, the specific interactions between SbpA and the SCWP have been exploited for an oriented binding of the S-layer subunits on solid supports to generate monomolecular protein lattices (24).
Studies of the structure-function relationship of SbpA revealed that 200 C-terminal amino acids can be deleted without interfering with the self-assembly properties or the formation of the square lattice structure (12). Furthermore, amino acid position 1,068 was found to be located on the outer S-layer surface and was therefore exploited as a fusion site for the production of chimeric S-layer proteins. So far, S-layer fusion proteins have been constructed that comprised the sequence of the major birch pollen allergen (12) or the sequence of a single variable region of a heavy chain camel antibody directed against lysozyme (24).
In the present study, an S-layer fusion protein comprising the sequence of the Z-domain, a synthetic analogue of the immunoglobulin G (IgG)-binding B-domain of protein A of Staphylococcus aureus, has been constructed. Staphylococcal protein A (SPA) consists of a cell wall binding region and five domains, termed C, B, A, D, and E, with C next to the cell wall. The molecular interaction of SPA with Igs is well understood, and the binding sites on the Fc part of IgG1, -2, and -4 have been characterized. X-ray analysis revealed that the B-domain of SPA has two contact sites that interact with the Fc part of IgG (9). Based on this knowledge, the synthetic Z-domain, which consists of 58 amino acids and is capable of binding the Fc part of IgG, has been constructed (19, 23).
To obtain an S-layer fusion protein capable of binding IgG, the 5′ end of the sequence encoding two Z-binding domains was fused via a short linker to the 3′ end of the gene encoding the C-terminally-truncated form of the S-layer protein SbpA. After heterologous expression, the S-layer fusion protein was isolated from the host cells, purified, and recrystallized on solid supports precoated with thiolated SCWP. The IgG-binding capacity was evaluated for the native and cross-linked S-layer fusion protein. To prepare biocompatible microparticles for the microsphere-based detoxification system (MDS) to remove IgG from human plasma from patients suffering from an autoimmune disease, the S-layer fusion protein was recrystallized on SCWP-coated, 3-μm-diameter cellulose-based microbeads, and the IgG binding capacity was investigated. The MDS is an alternative approach to conventional immunoadsorption systems (1-3, 10), in which the plasma does not perfuse on an adsorption column but is recirculated into the filtrate compartment of the module (Fig. 1). The addition of microparticles to the plasma circuit allows rapid removal of the pathogenic substances (34).
FIG. 1.
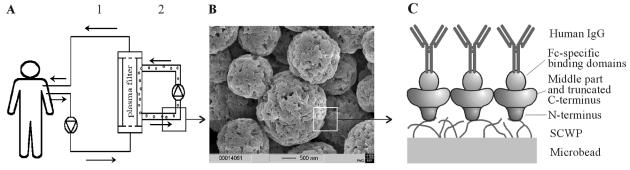
(A) Schematic drawing of the MDS, showing the primary circuit (labeled 1) containing the whole blood of the patient. The blood cells are rejected by the plasma filter. In the second circuit (labeled 2), the plasma recirculates together with the S-layer fusion protein-coated microbeads, on which IgG shall be bound. After passing the plasma filter again, the purified plasma is combined with blood cells, and the whole blood is reinfused into the patient. (B) Scanning electron micrograph of the biocompatible, cellulose-based microbeads used for recrystallization of rSbpA31-1068/ZZ. (C) Schematic drawing showing the oriented recrystallization of the S-layer fusion protein rSbpA31-1068/ZZ on microbeads precoated with SCWP and binding of IgG to the ZZ-domains.
MATERIALS AND METHODS
Cloning and expression of the chimeric gene encoding the S-layer fusion protein rSbpA31-1068/ZZ.
All PCRs and isolation of DNA were performed as described by Jarosch et al. (14). For amplification of the gene encoding rSbpA31-1068,chromosomal DNA of B. sphaericus CCM 2177 was used as a template. The oligonucleotide primers sbpA37 (5′-CGGATTCCATGGCGCAAGTAAACGACTATAACAAAATC-3′), which introduced the restriction site (boldface) NcoI at the 5′ end of the coding sequence, and the reverse primer sbpA41 (5′-CGCGGATCCTTCTGAATATGCAGTAGTTGCTGC-3′), which introduced the BamHI restriction site (boldface) at the 3′ end, were constructed. Digestion of DNA with restriction endonucleases, separation of DNA fragments by agarose gel electrophoresis, ligation of DNA fragments, and transformation procedures were performed as described in reference 25. DNA fragments were recovered from agarose gels by using the Qiaex II gel extraction kit (Qiagen). Two Z-domains were amplified from the pEZZ 18 protein A gene fusion vector (Amersham Pharmacia). For that purpose, the oligonucleotide primers ZZ3forward (5′-CGCGGATCCGACAACAAATTCAACAAAGAACAACAAAACG-3′) and ZZ2reverse (5′-GACCGCTCGAGTTATACTTTCGGCGCCTGAGCATC-3′), which introduced the restriction sites BamHI and XhoI, were used. To obtain plasmid pET28a-ZZ, the gel-purified PCR product, encoding two repeats of the synthetic Z-domain, was ligated into the corresponding restriction sites of plasmid pET28a, which was established in Escherichia coli TG1. To generate the chimeric sbpA(93-3204)/ZZ gene, the gel-purified PCR product sbpA(93-3204) was ligated into the corresponding restriction sites of plasmid pET28a-ZZ, which was used for transformation of E. coli TG1. Growth of E. coli TG1 and selection of transformants were accomplished according to reference 12. The plasmid stability test and heterologous expression of the sbpA(93-3204)/ZZ gene in E. coli HMS174(DE3) were performed as described by Jarosch et al. (14). Samples were taken 1 to 4 h after induction of sbpA(93-3204)/ZZ gene expression by addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG; GEBRU). Preparation of samples for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis was carried out as described by Laemmli (17). Electron microscopy was performed as described in reference 22.
Isolation of the S-layer fusion protein from the host cells and purification.
Isolation of the S-layer fusion protein was performed as previously described by Jarosch et al. (13), except that DNA remaining in the fraction of the S-layer fusion protein was degraded by DNase treatment. For that purpose, the insoluble pellet obtained by sonification of the cell suspension and centrifugation at 30,000 × g for 15 min at 4°C was treated with a DNase I (Roche) solution (1 mg/ml in 100 mM MgCl2 · 7H2O in MilliQ water). To remove residual DNase I, the pellet was washed twice with 1% Triton X-100 in 50 mM Tris-HCl buffer (pH 7.2) and 50 mM Tris-HCl buffer (pH 7.2). Extraction of the S-layer fusion protein was with 5 M guanidine hydrochloride (GHCl) in 50 mM Tris-HCl buffer (pH 7.2) and purification by gel permeation chromatography (GPC) were performed as described in reference 24. Immunoblotting with a polyclonal rabbit antiserum raised against the S-layer protein of B. sphaericus CCM 2177 was carried out as described by Egelseer et al. (8). The presence of the ZZ moiety in the S-layer fusion protein was checked by detection of bound human IgG (Sigma I-4506) via immunoreactivity with an antihuman-alkaline phosphatase (ALP) conjugate (Sigma A-3187).
Investigation of the self-assembly properties of the S-layer fusion protein and recrystallization on peptidoglycan-containing sacculi.
Preparation of self-assembly products and recrystallization of the S-layer fusion protein on peptidoglycan-containing sacculi of B. sphaericus were performed as described in references 11 and 12. Peptidoglycan-containing sacculi carrying a monolayer of recrystallized S-layer fusion protein are referred to as “recrystallization products” in all further experiments. To obtain water-soluble S-layer fusion protein, 1 mg of the GPC-purified and lyophilized samples was dissolved in 1 ml of 5 M GHCl in 50 mM Tris-HCl buffer (pH 7.2), and the solution was dialyzed against aqua bidest for 18 h at 4°C. After centrifugation at 16,000 × g for 5 min at 4°C, the clear supernatant containing nonassembled S-layer fusion protein was removed. For recrystallization on solid supports, 1 ml of the supernatant was diluted with 9 ml of 0.5 mM Tris-HCl buffer (pH 9.0, containing 10 mM CaCl2).
Immunodot assays for investigation of the accessibility of the fused ZZ-domains.
To investigate the accessibility of the fused ZZ-domains in the water-soluble and recrystallized S-layer fusion protein, 5 μl of each of the respective samples with an S-layer protein content of 1 mg/ml was dried onto a nitrocellulose membrane. After blocking with 2% Top Block (Fluka) in Tris-buffered saline (TBS) and incubation with 20 ng of human IgG (Sigma I-4506) in 20 ml of TBS containing 2% Top-Block for 1 h at 20°C, the membrane was washed three times with 0.5% Tween in TBS and subsequently incubated with an antihuman IgG-ALP conjugate (Sigma A-3187, diluted 1:5,000 in blocking solution) for 1 h at 20°C. After three further washing steps, detection was accomplished by treatment with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium chloride (BCIP/NBT; Roche).
Recrystallization of the S-layer fusion protein on gold chips and silanized glass slides precoated with thiolated SCWP and AFM analyses.
Cleaning of gold chips (silicon <100>, coated with 1-nm diameter Ti beads and 60-nm-diameter Au beads) with an area of 1 cm2 was performed as described before (24). Glass slides were silanized by treatment with a solution of 3-aminopropyl-trimethoxysilane-HCl (10% in MilliQ water, pH 3.5), which was followed by incubation at 200°C for 18 h. SCWP was isolated from peptidoglycan-containing sacculi of B. sphaericus CCM 2177 and purified under conditions described in reference 11. Chemical modification of the reducing end of the polymer chains and introduction of a terminal sulfhydryl group by modification with 2-iminothiolane were performed as described by Mader et al. (C. Mader, C. Huber, D. Moll, U. B. Sleytr, and M. Sára, submitted for publication). Binding of thiolated SCWP to gold chips was carried out according to the method of Pleschberger et al. (24). For covalent binding of thiolated SCWP to silanized glass slides, m-maleimidobenzoyl-N-hydroxysuccinimide ester (MBS; Pierce 22311) was used as a heterobifunctional cross-linker. For that purpose, a solution of 10 mM MBS in dimethyl sulfoxide (DMSO) was prepared and then diluted 1:10 with 20 mM phosphate buffer (pH 7.2), and silanized glass slides were incubated for 1 h at 20°C. After extensive washing with 20 mM phosphate buffer (pH 7.2) and aqua bidest, the glass slides were put into a solution of thiolated SCWP (1-mg/ml MilliQ water) and incubated at 20°C for 2 h. Subsequently, the glass slides were washed with crystallization buffer. Recrystallization of the S-layer fusion protein on solid supports and atomic force microscopy (AFM) analyses were performed as described in reference 24.
SPR studies for investigation of the IgG-binding capacity of rSbpA31-1068/ZZ recrystallized on gold chips precoated with thiolated SCWP.
Surface plasmon resonance (SPR) experiments were performed with a Biacore 2000 system (Biacore, Uppsala, Sweden). Gold chips were incubated with a solution of thiolated SCWP (100-ng/ml MilliQ water; pH adjusted to 3.5 with 10 mM HCl) at 20°C for 1 h and washed five times with MilliQ water. For recrystallization of the S-layer fusion protein, a solution of 100 μg of rSbpA31-1068/ZZ in crystallization buffer was conducted over the sensor surface in flow cell 1 (FC1) at a flow rate of 2 μl/min. For reference studies, rSbpA was recrystallized in flow cell 2 (FC2) under the same conditions. The amount of recrystallized S-layer fusion protein was expressed in terms of resonance units (RU; 1,000 RU corresponds to approximately 1 ng/mm2). In order to introduce covalent bonds between the S-layer subunits, 150 μl of a solution of 10 mM dimethyl pimelimidate · 2HCl (DMP, Pierce) in 0.2 M triethanolamine buffer (pH 8.5, containing 10 mM CaCl2) was passed over the sensor surface at a flow rate of 2 μl/min. After blocking potentially reactive groups with 0.1 M ethanolamine and washing with crystallization buffer, 200 μl of a solution of human IgG (Sigma I-4506; 100 μg/ml of crystallization buffer) was injected at a flow rate of 10 μl/min. The contact and dissociation time of IgG with the rSbpA31-1068/ZZ monolayer were 20 min and 800 s, respectively. IgG-binding and elution experiments were performed with the native and DMP-treated rSbpA31-1068/ZZ monolayer.
Determination of the IgG-binding capacity of recrystallization products obtained with rSbpA31-1068/ZZ.
For determination of the IgG-binding capacity of recrystallization products prepared with the S-layer fusion protein, 50-mg wet pellets obtained by centrifugation at 16,000 × g for 15 min at 4°C were incubated with 2 ml of a solution of human IgG (Sigma I-4506) at a concentration of 1mg/ml of Tris-HCl buffer (50 mM, pH 7.2) for 1 h at 20°C. Unbound IgG was removed by centrifugation of the suspensions at 16,000 × g for 15 min at 4°C and three washing steps with 50 mM Tris-HCl buffer (pH 7.2). Human IgG that had bound to the recrystallization products was recovered by treatment of the pellets with 0.1 M glycine-HCl buffer (pH 2.5). For comparative studies, recrystallization products prepared with rSbpA were used. The amount of human IgG in the supernatants was determined by the bicinchoninic acid (BCA) method (33) in Tris-HCl buffer and by the method of Lowry et al. (20) in glycine-HCl buffer.
Biocompatibility tests for investigation of LAL reactivity and cytotoxicity of recrystallization products prepared with rSbpA31-1068/ZZ.
Recrystallization products of the S-layer fusion protein were washed four times with NaCl solution (0.9% in MilliQ water). Subsequently, 10 mg of wet pellet was resuspended in 100 ml of NaCl solution. After incubation at 37°C for 20 h, the suspensions were centrifuged at 16,000 × g for 15 min at 4°C. The supernatants were passed through a 0.2-μm-pore-diameter microfilter (Nalgene) and tested for Limulus amebocyte lysate (LAL) reactivity and cytotoxicity, by using either a kinetic test system (Charles River Endosafe; CoaChrom Diagnostica, Austria), or the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) test (EZ4U; Biomedica, Vienna, Austria).
Recrystallization of the S-layer fusion protein on biocompatible microbeads precoated with thiolated SCWP.
Cellulose-based microbeads used for recrystallization of the S-layer fusion protein were produced at the Fraunhofer Institute for Applied Polymer Research (Golm, Germany). Such microbeads exhibited a positively charged surface with free amine groups and had a mean diameter of 3 μm.
For covalent binding of thiolated SCWP, 100 mg of wet pellet of the microbeads was resuspended in 3 ml of 20 mM phosphate buffer (pH 7.2), and 300 μl of 10 mM MBS in dimethyl sulfoxide (DMSO) was added. The suspension was stirred for 1 h at 20°C. Then the microbeads were sedimented by centrifugation at 6,000 × g for 5 min at 4°C and washed six times with 20 mM phosphate buffer (pH 7.2) containing 10% DMSO and three times with MilliQ water. Subsequently, MBS-modified microbeads were resuspended in 2 ml of a solution containing 2 mg of thiolated SCWP per ml of MilliQ water. The suspension was stirred at 20°C overnight. After that, the microbeads were washed with crystallization buffer. For recrystallization of the S-layer fusion protein, 50 mg of SCWP-coated microbeads was suspended in 1 ml of a solution of rSbpA31-1068/ZZ, and the suspension was stirred at 20°C for 18 h.
The amount of S-layer fusion protein that had bound to the microbeads was determined as described in references 35 and 36. Cross-linking of the S-layer fusion protein with DMP and washing and saturation of potentially reactive groups were done as described above. IgG-binding experiments were performed with native and DMP-treated microbeads. For that purpose, 50-mg wet pellets of microbeads were incubated in 1.5 ml of a solution of human IgG (Sigma I-4506) at a concentration of 1 mg/ml of 50 mM Tris-HCl buffer (pH 7.2) for 1 h at 20°C. After sedimentation of the microbeads at 6,000 × g for 5 min at 4°C, excess human IgG was removed by three washing steps with 50 mM Tris-HCl buffer (pH 7.2). IgG that remained bound to the microbeads was eluted with 0.1 M glycine-HCl buffer (pH 2.5). After washing with crystallization buffer, the incubation and elution steps with IgG were repeated once. Samples of each supernatant and of the microbeads were prepared for SDS-PAGE analysis.
RESULTS
Cloning and expression of the chimeric gene encoding the S-layer fusion protein rSbpA31-1068/ZZ.
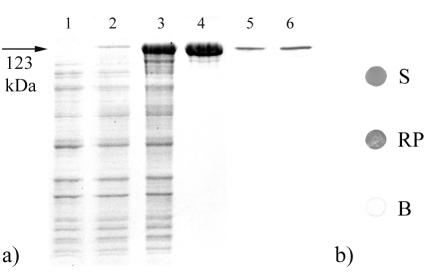
A PCR product encoding two repeats of the synthetic Z-domain based on the IgG binding B-domain of SPA was derived from PCR amplification by using the primers ZZ3forward and ZZ2reverse and ligated into the pET28a vector. After cloning in E. coli TG1, amplification, and isolation of the plasmid pET28a-ZZ, the gene encoding the truncated form of the S-layer protein SbpA, which was obtained by PCR using the primers sbpA37 and sbpA41, was ligated via the corresponding restriction sites into this plasmid. The resulting vector, pET28a-ZZ/sbpA(93-3204), was first cloned in E. coli TG1 and then established in E. coli HMS174(DE3). After induction of expression by the addition of IPTG, biomass samples were harvested at various points of time and subjected to SDS-PAGE analysis and ultrathin sectioning. In comparison to E. coli HMS174(DE3) cells harvested before the addition of IPTG (Fig. 2a, lane 1), an additional high-molecular-mass protein band was observed on SDS gels in samples from E. coli HMS174(DE3) cultures induced to express the chimeric sbpA(93-3204)/ZZ gene (Fig. 2a, lanes 2 and 3). This additional protein band had an apparent molecular mass of 123,000 Da, which corresponded to the theoretical molecular mass calculated for the S-layer fusion protein of 123,057 Da.
FIG. 2.
(a) SDS-PAGE pattern of SDS extracts of whole cells of E. coli HMS174(DE3) containing pET28a carrying the chimeric gene encoding rSbpA31-1068/ZZ before (lane 1) and 1 (lane 2) and 4 (lane 3) h after induction of expression by the addition of IPTG. Lane 4, SDS-PAGE analysis of rSbpA31-1068/ZZ after purification by GPC. Lanes 5 and 6, immunoblot analysis of SDS extracts of purified rSbpA31-1068/ZZ, using polyclonal rabbit antiserum raised against the S-layer protein of B. sphaericus CCM 2177 (lane 5) or antihuman-ALP for detection of bound human IgG (lane 6). (b) Dot blot assays indicating the accessibility of the fused ZZ-domains to human IgG of the water-soluble (S) or the recrystallized S-layer fusion protein (RP). Water-soluble rSbpA was used as a blank (B).
Isolation of the S-layer fusion protein from the host cells and purification.
As derived from SDS-PAGE analysis of samples collected during the isolation procedure, rSbpA31-1068/ZZ had accumulated in the insoluble fraction of the lysed E. coli HMS174(DE3) cells (data not shown). To isolate the S-layer fusion protein, the insoluble fraction was extracted with 5 M GHCl. After purification by GPC, only a single protein band with an apparent molecular mass of 123,000 Da was detected on SDS gels (Fig. 2a, lane 4). The presence of the SbpA-specific part in the S-layer fusion protein was confirmed by immunoblotting with the polyclonal rabbit antiserum raised against the S-layer protein of B. sphaericus CCM 2177 (Fig. 2a, lane 5). The presence and functionality of the ZZ-domains were proved via the ability to bind human IgG, which was finally detected with an antihuman-ALP conjugate (Fig. 2a, lane 6). The purified chimeric S-layer protein rSbpA31-1068/ZZ synthesized in E. coli HMS174(DE3) was subjected to N-terminal sequencing, showing that the N terminus (AQVND) was identical to that of the mature S-layer protein of B. sphaericus CCM 2177.
Investigation of the self-assembly properties of the S-layer fusion protein and recrystallization on peptidoglycan-containing sacculi.
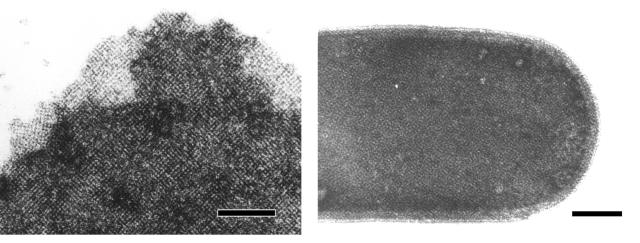
As shown by negative staining, the S-layer fusion protein reassembled into flat double or multilayer sheets, which exhibited the square lattice structure typical of the S-layer protein SbpA. The S-layer fusion protein recognized peptidoglycan-containing sacculi of B. sphaericus CCM 2177 as a binding site and was recrystallized into the square lattice (Fig. 3).
FIG. 3.
Electron micrographs of negatively stained preparations showing the square lattice formed by rSbpA31-1068/ZZ in self-assembly products (left) or on recrystallization products (right). Bars, 200 nm.
Immunodot assays for investigation of the accessibility of the fused ZZ-domains.
Assuming identical functionalities of the ZZ moiety for both samples, the intensities of the dots indicated that in comparison to the water-soluble S-layer fusion protein, the accessibility of the fused ZZ-domains for human IgG was only slightly reduced in recrystallization products (Fig. 2b).
Recrystallization of the S-layer fusion protein on gold chips and glass slides precoated with thiolated SCWP and AFM analysis.
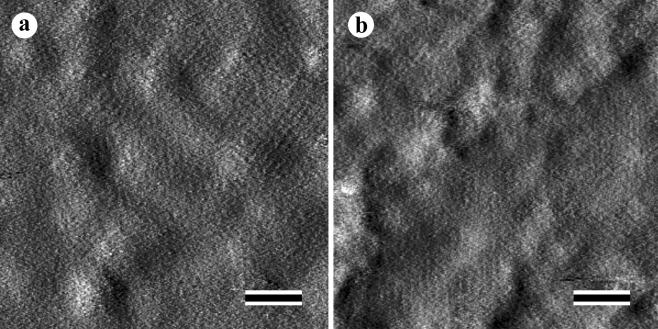
As shown by AFM analysis, the S-layer fusion protein recrystallized into a monolayer on gold chips and silanized glas slides precoated with thiolated SCWP. The monolayers consisted of numerous randomly oriented patches with an average size of 200 nm. All patches exhibited the square lattice structure with a center-to-center spacing of the morphological units of 13.1 nm (Fig. 4).
FIG. 4.
AFM images of rSbpA31-1068/ZZ recrystallized on gold chips (a) and a silanized glass slide precoated with SCWP (b). Bars, 100 nm.
SPR studies for investigation of the IgG-binding capacity of rSbpA31-1068/ZZ recrystallized on gold chips precoated with thiolated SCWP.
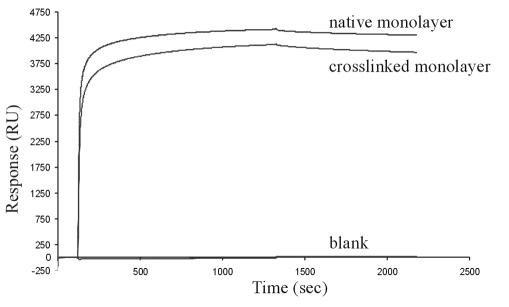
To investigate the binding capacity of the rSbpA31-1068/ZZ monolayer generated by oriented recrystallization of the S-layer fusion protein on gold chips precoated with thiolated SCWP for human IgG, SPR studies were performed. Recrystallization of the S-layer fusion protein led to an increase of 5,266 RU for the native monolayer or 5,254 RU for the cross-linked monolayer, which both corresponded to 4.3 × 10−5 nM/mm2. According to the molecular mass of the S-layer fusion protein and the area occupied by one morphological unit, the theoretical value for a monolayer lies at 3.9 × 10−5 nM/mm2, which is in good accordance with the data of 4.3 × 10−5 nM/mm2. When a solution of IgG at a concentration of 100 μg/ml was conducted over the native and the DMP-cross-linked rSbpA31-1068/ZZ monolayer, specific IgG binding was observed in both cases. On the contrary, the rSbpA monolayer used as a reference did not bind IgG at all. In the case of the native monolayer, IgG corresponding to 4,367 RU was bound, whereas an increase of 4,126 RU was observed for the DMP-cross-linked monolayer (Fig. 5). These values corresponded to either 2.9 × 10−5 nM IgG/mm2 or 2.8 × 10−5 nM IgG per mm2, respectively. Considering that 4.3 × 10−5 nM native S-layer fusion protein per mm2 sensor surface could bind 2.9 × 10−5 nM IgG per mm2, it can be calculated that, on average, 2.7 IgG molecules had attached per morphological unit of the square S-layer lattice consisting of four subunits (Table 1). In the case of the DMP-cross-linked rSbpA31-1068/ZZ monolayer, the IgG-binding capacity was slightly reduced, since on average, 2.6 IgG molecules were bound per morphological unit.
FIG. 5.
Sensorgram of SPR studies for investigation of the IgG-binding capacity of the native and DMP-cross-linked rSbpA31-1068/ZZ monolayer obtained by recrystallization of the S-layer fusion protein on gold chips precoated with thiolated SCWP. The native monolayer bound an amount of IgG corresponding to 4,367 RU, whereas a slightly reduced binding capacity of 4,126 RU was observed for the DMP-cross-linked monolayer. A monolayer of rSbpA was used as a blank. The signal obtained for each monolayer was set to zero before the injection of IgG-containing solutions.
TABLE 1.
IgG-binding capacity of native and DMP-cross-linked monolayers, obtained by oriented recrystallization of S-layer fusion protein rSbpA31-1068/ZZ on SCWP-coated SPR gold chips or SCWP-coated cellulose-based microbeadsa
| Parameter | Result for:
|
|||
|---|---|---|---|---|
| SPR
|
Microbeads
|
|||
| Native | DMP-treated | Native | DMP-treated | |
| Bound S-layer fusion protein | 4.3 × 10−5 nM/mm2b | 4.3 × 10−5 nM/mm2b | 620 μg/mg of microbead dry wt | 620 μg/mg of microbead dry wt |
| Bound IgG | 2.9 × 10−5 nM/mm2b | 2.8 × 10−5 nM/mm2b | 1,065 μg of IgG/mg of S-layer fusion protein | 870 μg of IgG/mg of S-layer fusion protein |
| nmol of IgG/nmol of S-layer fusion protein | 2.9/4.3 | 2.8/4.3 | 7.1/8.1 | 5.8/8.1 |
| Molar ratio (IgG/S-layer subunit) | 0.67 | 0.65 | 0.88 | 0.72 |
| No. of IgG molecules attached/morphological unit | 2.7 | 2.6 | 3.5 | 2.9 |
| nmol of IgG/mm2 | 2.9 × 10−5 | 2.8 × 10−5 | 3.4 × 10−5 | 2.8 × 10−5 |
| ng of IgG/mm2 | 4.35 | 4.20 | 5.10 | 4.20 |
| % of theoretical saturation capacity (6.5 ng/mm2) | 67 | 65 | 78 | 65 |
According to the center to center spacing of the morphological units of 13.1 nm, the area occupied by 1 cell unit is 171.61 nm2. Derived from this value and the molecular mass of the S-layer fusion protein of 123,000 Da, 1 mg (8.1 nmol) covers an area of 2.1 × 1017 nm2.
The binding capacity of the gold chip for the S-layer fusion protein and of the rSbpA31-1068/ZZ monolayer for human IgG was derived from RU values obtained by SPR measurements.
Determination of the IgG-binding capacity of recrystallization products prepared with rSbpA31-1068/ZZ.
The binding capacity of recrystallization products prepared with the S-layer fusion protein for human IgG was determined by batch adsorption experiments and was found to lie at 35 mg of human IgG per g of wet pellet. This value was comparable to the IgG-binding capacity of affinity microparticles prepared by linking SPA to cyanamide-activated, glutaraldehyde-treated S-layer-carrying cell wall fragments of Thermoanaerobacter thermohydrosulfuricus L111-69, which showed a binding capacity of 40 mg of human IgG per g of wet pellet (35). Bound IgG could be eluted from recrystallization products by treatment with glycine-HCl buffer (pH 2.5). When the extract was analyzed by SDS-PAGE, only IgG but no S-layer fusion protein could be detected.
Biocompatibility of recrystallization products prepared with rSbpA31-1068/ZZ.
Supernatants from recrystallization products were tested for LAL reactivity and cytotoxicity. The samples exhibited an endotoxin contamination of <1.2 EU/ml and no cytotoxicity towards eukaryotic cells.
Recrystallization of the S-layer fusion protein on cellulose-based microbeads precoated with thiolated SCWP and determination of the IgG-binding capacity.
Celloluse-based microbeads carrying free amine groups on their surface were preactivated with MBS to bind thiolated SCWP. Subsequently, the S-layer fusion protein rSbpA31-1068/ZZ was recrystallized, and the amount of bound S-layer fusion protein was calculated and compared with the theoretical value for microbeads with a diameter of 3 μm. Instead of the theoretical value of 860 μg of S-layer fusion protein per mg of microbead dry weight, 620 μg of rSbpA31-1068/ZZ was actually bound, which corresponded to 72% coverage. Microbeads coated with the native S-layer fusion protein were capable of binding 660 μg of IgG per mg of microbead dry weight, which corresponded to 1,065 μg of IgG per mg of S-layer fusion protein, or 3.5 IgG molecules per morphological unit of the square S-layer lattice (Table 1). In the case of the DMP-cross-linked S-layer fusion protein, a binding capacity of 540 μg of IgG per mg of microbead dry weight, or 870 μg of IgG per mg of S-layer fusion protein, was obtained. This value corresponded to a binding density of 2.9 IgG molecules per morphological unit (Table 1).
Incubation of the microbeads with glycine-HCl buffer (pH 2.5) revealed that 80 to 90% of the bound IgG could be eluted in a single step. SDS-PAGE analysis showed that only IgG and no S-layer fusion protein was extracted under these conditions. After a further elution step, no IgG remained bound to the microbeads. The binding capacity of the microbeads for human IgG in the second incubation step was comparable to the amount of IgG bound in the first incubation step. Furthermore, elution of IgG by treatment with glycine-HCl buffer (pH 2.5) revealed data similar to those after the first incubation step. When rSbpA was used for recrystallization on microbeads precoated with thiolated SCWP, no IgG binding was observed at all.
DISCUSSION
In the present study, an S-layer fusion protein was constructed that comprised the SCWP-binding region and the self-assembly domain of the S-layer protein SbpA and two copies of the Fc-binding Z-domain. The latter is a synthetic analogue of the B-domain of SPA, capable of binding the Fc part of IgGs (19, 23). By exploiting the specific interactions between the N-terminal part and the SCWP, the S-layer fusion protein was used for coating of biocompatible, cellulose-based microbeads to generate specific adsorbents, which should find clinical application in the treatment of various autoimmune diseases.
For the construction of the S-layer fusion protein rSbpA31-1068/ZZ, a C-terminally-truncated form of the S-layer protein SbpA of B. sphaericus CCM 2177 was selected. As shown in a previous study, the deletion of 200 C-terminal amino acids led to a significant increase in the accessibility of the C terminus, while retaining the functionality of the S-layer protein moiety (12). The S-layer fusion protein rSbpA31-1068/ZZ could self-assemble in suspension and recrystallize on peptidoglycan-containing sacculi and on solid supports precoated with thiolated SCWP. The formation of the square lattice structure was observed on self-assembly and recrystallization products and on monolayers formed on solid supports. Due to the specific interactions between the SLH domain located on the N-terminal part and the SCWP (11), the S-layer fusion protein attached with the inner surface to the SCWP-coated supports, thereby leaving the fused ZZ-domains exposed to the ambient environment. This was demonstrated by the intensities of immunodot assays, in which the water-soluble S-layer fusion protein showed only a slightly stronger reaction than the S-layer fusion protein recrystallized on peptidoglycan-containing sacculi. The binding capacity of the monolayer obtained by oriented recrystallization of the S-layer fusion protein on supports precoated with SCWP for human IgG was determined in its native state and after cross-linking with DMP. Furthermore, the IgG binding capacity was investigated in a real-time biosensor (SPR) as a dynamic system and in batch adsorption experiments using recrystallization products, which despite the different experimental conditions led to similar results (Table 1). In both systems, the IgG binding capacity of the DMP-cross-linked monolayer was slightly reduced in comparison to that of the native monolayer. Although the native monolayer was resistant to treatment with glycine-HCl buffer (pH 2.5), which is a necessary condition to elute IgG from the Z-domain, cross-linking of the S-layer lattice is considered essential for all applications, in which the S-layer fusion protein is brought in contact with human plasma, like in immunoadsorption. The potential use of the S-layer fusion protein to bind human IgG from plasma of patients suffering from autoimmune disease was also the reason why contamination with endotoxin or the cytotoxicity for eukaryotic cells was investigated. Recrystallization products prepared with the S-layer fusion protein did not show any cytotoxicity, and the endotoxin content was very low, but to guarantee the complete absence of lipopolysaccharides, expression in a gram-positive organism is strongly demanded.
Igs are spheroid lens-shaped molecules, so the theoretical saturation capacity of a planar surface depends on whether the molecules are immobilized lying down or in an upright position. If IgG is bound via SPA or the Z-domain, all molecules should be aligned in an upright position. Assuming that the Fab regions are in the condensed state, the maximum binding capacity (theoretical saturation capacity) of a planar surface lies at 6.5 ng/mm2 (15). On microbeads covered with native S-layer fusion protein, 5.1 ng of IgG/mm2 (corresponding to 78% of the theoretical saturation capacity) was bound. After cross-linking with DMP, the binding capacity decreased to 4.4 ng of IgG/mm2, which still represented 65% of the maximum packing density (Table 1).
For comparative studies with affinity microparticles (5, 35, 36), recrystallization products were prepared with the S-layer fusion protein. Affinity microparticles are obtained by cross-linking S-layer carrying cell wall fragments with glutaraldehyde, activating free carboxylic acid groups with cyanamide and covalent binding of SPA. In previous studies, it was demonstrated that immobilization of SPA led to a dense monolayer of the ligand on the S-layer surface of affinity microparticles from T. thermohydrosulfuricus L111-69 or B. sphaericus CCM 2120, which were capable of binding 40 μg of human IgG/mg of wet pellet (35, 36). This binding capacity was rather similar to that of recrystallization products prepared with the S-layer fusion protein, but significantly higher than those of commercially available immunoadsorbents, which were <10 μg/mg of wet pellet (35). The clearly higher IgG-binding capacity of affinity microparticles was attributed to the high density and regular arrangement of functional groups on the outer surface of the S-layer lattice to which SPA was covalently linked (36). The regular arrangement of functional groups in the S-layer lattice is in contrast to the structure of conventional affinity matrices, which are composed of networks of polymers, and this feature was even improved with the S-layer fusion protein. In this case, each S-layer subunit carried exactly two ZZ-domains and thus two binding sites for IgG, but due to sterical reasons and the size of the IgG molecules, only one Z-domain could be exploited for IgG binding. The Z-domain nearest to the S-layer protein should function as a spacer and guarantee an optimal accessibility for the large IgG molecules to the second Z-domain. Since DMP-treated microbeads had a dry weight of 34.4%, about 186 μg of IgG/mg of wet pellet was bound, which was at least 20 times higher than the IgG-binding capacity of commercial immunoadsorbent particles. In addition to the high binding capacity, a further advantage in comparison to commercial adsorbents can be seen in the fact that the ZZ-domains are exposed on the outermost surface of the microbeads, so that diffusion-limited binding events can be excluded.
The use of biocompatible cellulose-based microbeads as a carrier for the S-layer fusion protein rSbpA31-1068/ZZ, the possibility to cross-link the S-layer lattice with DMP, the high levels of IgG that were still bound after cross-linking, and the possibility to elute IgG and regenerate the microbeads without loss of the binding capacity are extremely promising for their application as immunoadsorbents in the MDS.
Acknowledgments
This work was supported by project P14689-MOB of the Austrian Science Foundation, by the Competence Center “Biomolecular Therapeutics,” and by the Christian Doppler Society.
We thank Jacqueline Friedmann for the AFM images.
REFERENCES
- 1.Arason, G. J., M. S. D'Ambrogio, T. Vikingsdottir, A. Sigfusson, and H. Valdimarsson. 1999. Enzyme immunoassays for measuring complement-dependent prevention of immune precipitation (PIP) and solubilization of preformed antigen-antibody complexes (SOL). J. Immunol. Methods 223:37-46. [DOI] [PubMed] [Google Scholar]
- 2.Batocchi, A. P., A. Evoli, C. Di Schino, and P. Tonali. 2000. Therapeutic apheresis in myasthenia gravis. Ther. Apher. 4:275-279. [DOI] [PubMed] [Google Scholar]
- 3.Braun, N., M. Junger, R. Klein, S. Gutenberger, M. Guagnin, and T. Risler. 2002. Dextran sulfate (Selesorb) plasma apheresis improves vascular changes in systemic lupus erythematosus. Ther. Apher. 6:471-477. [DOI] [PubMed] [Google Scholar]
- 4.Brechtel, E., and H. Bahl. 1999. In Thermoanaerobacterium thermosulfurigenes EM1 S-layer homology domains do not attach to peptidoglycan. J. Bacteriol. 181:5017-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breitwieser, A., S. Küpcü, S. Howorka, S. Weigert, C. Langer, K. Hoffmann-Sommergruber, O. Scheiner, U. B. Sleytr, and M. Sára. 1996. 2-D protein crystals as an immobilization matrix for producing reaction zones in dipstick-style immunoassays. BioTechniques 21:918-925. [DOI] [PubMed] [Google Scholar]
- 6.Breitwieser, A., C. Mader, I. Schocher, K. Hoffmann-Sommergruber, W. Aberer, O. Scheiner, U. B. Sleytr, and M. Sára. 1998. A novel dipstick developed for rapid Bet v 1-specific IgE detection: recombinant allergen immobilized via a monoclonal antibody to crystalline bacterial cell-surface layers. Allergy 53:786-793. [DOI] [PubMed] [Google Scholar]
- 7.Chauvaux, S., M. Matuschek, and P. Beguin. 1999. Distinct affinity of binding sites for S-layer homologous domains in Clostridium thermocellum and Bacillus anthracis cell envelopes. J. Bacteriol. 181:2455-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egelseer, E. M., I. Schocher, U. B. Sleytr, and M. Sára. 1996. Evidence that an N-terminal S-layer protein fragment triggers the release of a cell-associated high-molecular-weight amylase in Bacillus stearothermophilus ATCC 12980. J. Bacteriol. 178:5602-5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliasson, M., A. Olsson, E. Palmcrantz, K. Wiberg, M. Inganas, B. Guss, M. Lindberg, and M. Uhlen. 1988. Chimeric IgG-binding receptors engineered from staphylococcal protein A and streptococcal protein G. J. Biol. Chem. 263:4323-4327. [PubMed] [Google Scholar]
- 10.Felson, D. T., M. P. LaValley, A. R. Baldassare, J. A. Block, J. R. Caldwell, G. W. Cannon, C. Deal, S. Evans, R. Fleischmann, R. M. Gendreau, E. R. Harris, E. L. Matteson, S. H. Roth, H. R. Schumacher, M. H. Weisman, and D. E. Furst. 1999. The Prosorba column for treatment of refractory rheumatoid arthritis: a randomized, double-blind, sham-controlled trial. Arthritis Rheum. 42:2153-2159. [DOI] [PubMed] [Google Scholar]
- 11.Ilk, N., P. Kosma, M. Puchberger, E. M. Egelseer, H. F. Mayer, U. B. Sleytr, and M. Sára. 1999. Structural and functional analyses of the secondary cell wall polymer of Bacillus sphaericus CCM 2177 that serves as an S-layer-specific anchor. J. Bacteriol. 181:7643-7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilk, N., C. Völlenkle, E. M. Egelseer, A. Breitwieser, U. B. Sleytr, and M. Sára. 2002. Molecular characterization of the S-layer gene, sbpA, of Bacillus sphaericus CCM 2177 and production of a functional S-layer fusion protein with the ability to recrystallize in a defined orientation while presenting the fused allergen. Appl. Environ. Microbiol. 68:3251-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarosch, M., E. M. Egelseer, C. Huber, D. Moll, D. Mattanovich, U. B. Sleytr, and M. Sára. 2001. Analysis of the structure-function relationship of the S-layer protein SbsC of Bacillus stearothermophilus ATCC 12980 by producing truncated forms. Microbiology 147:1353-1363. [DOI] [PubMed] [Google Scholar]
- 14.Jarosch, M., E. M. Egelseer, D. Mattanovich, U. B. Sleytr, and M. Sára. 2000. S-layer gene sbsC of Bacillus stearothermophilus ATCC 12980: molecular characterization and heterologous expression in Escherichia coli. Microbiology 146:273-281. [DOI] [PubMed] [Google Scholar]
- 15.Kim, M., K. Saito, S. Furusaki, T. Sugo, and I. Ishigaki. 1991. Protein adsorption capacity of porous phenylalanine-containing membrane based on a polyethylene matrix. J. Chromatogr. 586:27-33. [Google Scholar]
- 16.Küpcü, S., M. Sára, and U. B. Sleytr. 1995. Liposomes coated with crystalline bacterial cells surface protein (S-layer) as immobilization structures for macromolecules. Biochim. Biophys. Acta 1235:263-269. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 18.Lemaire, M., I. Miras, P. Gounon, and P. Beguin. 1998. Identification of a region responsible for binding to the cell wall within the S-layer protein of Clostridium thermocellum. Microbiology 144:211-217. [DOI] [PubMed] [Google Scholar]
- 19.Lowenadler, B., B. Jansson, S. Paleus, E. Holmgren, B. Nilsson, T. Moks, G. Palm, S. Josephson, L. Philipson, and M. Uhlen. 1987. A gene fusion system for generating antibodies against short peptides. Gene 58:87-97. [DOI] [PubMed] [Google Scholar]
- 20.Lowry, O., N. Rosebrough, A. Farr, and R. Randall. 1951. Protein measurements with Folin-phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 21.Mesnage, S., T. Fontaine, T. Mignot, M. Delepierre, M. Mock, and A. Fouet. 2000. Bacterial SLH domain proteins are non-covalently anchored to the cell surface via a conserved mechanism involving wall polysaccharide pyruvylation. EMBO J. 19:4473-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messner, P., F. Hollaus, and U. B. Sleytr. 1984. Paracrystalline cell wall surface layers of different Bacillus stearothermophilus strains. Int. J. Syst. Bacteriol. 34:202-210. [Google Scholar]
- 23.Nilsson, B., T. Moks, B. Jansson, L. Abrahmsen, A. Elmblad, E. Holmgren, C. Henrichson, T. A. Jones, and M. Uhlen. 1987. A synthetic IgG-binding domain based on staphylococcal protein A. Protein Eng. 1:107-113. [DOI] [PubMed] [Google Scholar]
- 24.Pleschberger, M., A. Neubauer, E. M. Egelseer, S. Weigert, B. Lindner, U. B. Sleytr, S. Muyldermans, and M. Sára. 2003. Generation of a functional monomolecular protein lattice consisting of an S-layer fusion protein comprising the variable domain of a camel heavy chain antibody. Bioconjug. Chem. 14:440-448. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Sára, M. 2001. Conserved anchoring mechanisms between crystalline cell surface S-layer proteins and secondary cell wall polymers in Gram-positive bacteria? Trends Microbiol. 9:47-49. [DOI] [PubMed] [Google Scholar]
- 27.Sára, M., and U. B. Sleytr. 2000. S-layer proteins. J. Bacteriol. 182:859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sleytr, U. B., and T. J. Beveridge. 1999. Bacterial S-layers. Trends Microbiol. 7:253-260. [DOI] [PubMed] [Google Scholar]
- 29.Sleytr, U. B., E. M. Egelseer, D. Pum, and B. Schuster. S-layers. In C. M. Niemeyer and C. A. Mirkin (ed.), Nanobiotechnology: concepts, applications, and perspectives, in press. Wiley-VCH, Weinheim, Germany.
- 30.Sleytr, U. B., P. Messner, D. Pum, and M. Sára. 1999. Crystalline bacterial cell surface layers (S-layers): from supramolecular cell structure to biomimetics and nanotechnology. Angew. Chem. Int. Ed. Engl. 38:1034-1054. [DOI] [PubMed] [Google Scholar]
- 31.Sleytr, U. B., and M. Sára. 1997. Bacterial and archaeal S-layer proteins: structure-function relationships and their biotechnological applications. Trends Biotechnol. 15:20-26. [DOI] [PubMed] [Google Scholar]
- 32.Sleytr, U. B., M. Sára, D. Pum, B. Schuster, P. Messner, and C. Schäffer. 2003. Self assembly protein systems: microbial S-layers, p. 285-338. In A. Steinbüchel and S. Fahnestock (ed.), Biopolymers, vol. 7. Wiley-VCH, Weinheim, Germany.
- 33.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 34.Weber, C., C. Rajnoch, F. Loth, H. Schima, and D. Falkenhagen. 1994. The microspheres based detoxification system (MDS). A new extracorporeal blood purification technology based on recirculated microspherical adsorbent particles. Int. J. Artif. Organs 17:595-602. [PubMed] [Google Scholar]
- 35.Weber, V., S. Weigert, M. Sára, U. B. Sleytr, and D. Falkenhagen. 2001. Development of affinity microparticles for extracorporeal blood purification based on crystalline bacterial cell surface proteins. Ther. Apher. 5:433-438. [DOI] [PubMed] [Google Scholar]
- 36.Weiner, C., M. Sára, and U. B. Sleytr. 1994. Novel protein A affinity matrix prepared from two-dimensional protein crystals. Biotechnol. Bioeng. 43:321-330. [DOI] [PubMed] [Google Scholar]