Abstract
Background
Tibolone has estrogenic, progestogenic, and androgenic effects. Although tibolone prevents bone loss, its effects on fractures, breast cancer, and cardiovascular disease are uncertain.
Methods
In this randomized study, we assigned 4538 women, who were between the ages of 60 and 85 years and had a bone mineral density T score of −2.5 or less at the hip or spine or a T score of −2.0 or less and radiologic evidence of a vertebral fracture, to receive once-daily tibolone (at a dose of 1.25 mg) or placebo. Annual spine radiographs were used to assess for vertebral fracture. Rates of cardiovascular events and breast cancer were adjudicated by expert panels.
Results
During a median of 34 months of treatment, the tibolone group, as compared wit h the placebo group, had a decreased risk of vertebral fracture, with 70 cases versus 126 cases per 1000 person-years (relative hazard, 0.55; 95% confidence interval [CI], 0.41 to 0.74; P<0.001), and a decreased risk of nonvertebral fracture, with 122 cases versus 166 cases per 1000 person-years (relative hazard, 0.74; 95% CI, 0.58 to 0.93; P = 0.01). The tibolone group also had a decreased risk of invasive breast cancer (relative hazard, 0.32; 95% CI, 0.13 to 0.80; P = 0.02) and colon cancer (relative hazard, 0.31; 95% CI, 0.10 to 0.96; P=0.04). However, the tibolone group had an increased risk of stroke (relative hazard, 2.19; 95% CI, 1.14 to 4.23; P = 0.02), for which the study was stopped in February 2006 at the recommendation of the data and safety monitoring board. There were no significant differences in the risk of either coronary heart disease or venous thromboembolism between the two groups.
Conclusions
Tibolone reduced the risk of fracture and breast cancer and possibly colon cancer but increased the risk of stroke in older women with osteoporosis. (ClinicalTrials. gov number, NCT00519857.)
Tibolone is approved in 90 countries to treat menopausal symptoms and in 45 countries to prevent osteoporosis. Tibolone metabolites have estrogenic, progestogenic, and androgenic activities.1-3 Tibolone preserves bone mineral density,4-6 reduce shot flushes,7-9 and may increase libido and vaginal lubrication.10,11 Tibolone treatment has little effect on levels of low-density-lipoprotein cholesterol but decreases levels of high-density-lipoprotein (HDL) cholesterol and triglycerides.5,12 Our study, called the Long-Term Intervention on Fractures with Tibolone (LIFT), tested the primary hypothesis that treatment with tibolone reduces the risk of vertebral fracture and, secondarily, modifies the risks of nonvertebral fracture, breast cancer, deep-vein thrombosis, and cardiovascular disease in older women with osteoporosis.
Methods
Study Patients
In this randomized, double-blind, placebo-controlled clinical trial, we examined the effect of 1.25 mg of tibolone daily on the risk of vertebral and clinical fractures after 3 years and planned to assess the risks of breast cancer, cardiovascular disease, and endometrial cancer after 5 years.
From July 2001 to June 2003, we recruited women between the ages of 60 and 85 years who had a bone mineral density T score of −2.5 or less at the hip or lumbar spine or a T score of −2.0 or less with radiologic evidence of a vertebral fracture. Women with more than two vertebral fractures, a T score of less than −4.0 at the hip or spine, or a clinical diagnosis of vertebral fracture in the past year were excluded. Other criteria for exclusion were cancer (other than nonmelanoma skin cancer) within the past 5 years, previous thromboembolic disease, mammographic results with a suspicion of cancer, the use of estrogen during the previous 3 months, current use of raloxifene or tamoxifen, the use of a bisphosphonate for at least 1 month during the previous year, or a body-mass index (the weight in kilograms divided by the square of the height in meters) of more than 34. Women with a uterus underwent transvaginal ultrasonography, and the finding of a double-layer endometrial thickness of more than 4 mm was a reason for exclusion.
Patients were randomly assigned to receive either 1.25 mg of tibolone or an identical placebo daily. All patients received two to four tablets of calcium with vitamin D (315 mg of calcium citrate plus 200 IU of vitamin D3) daily. Patients who discontinued a study drug were allowed to continue with follow-up assessments and were included in the intention-to-treat analyses.
Assessment of Baseline Vertebral Fractures
A radiologist at the reading center (Synarc) graded vertebrae semiquantitatively on the severity of deformity as none (grade 0), mild (grade 1, a 20 to 25% reduction in vertebral height), moderate (grade 2, >25 to 40% reduction), or severe (grade 3, >40% reduction).13 A second radiologist qualitatively assessed whether a fracture was present (binary semiquantitative grading) and assessed vertebral dimensions (quantitative morphometry). A vertebral fracture was diagnosed if there was agreement in two assessments.14
Outcome Assessment
Incident fractures were defined as a change from grade 0 to at least grade 1, as confirmed either by the presence of a fracture as seen clearly by the radiologist (binary semiquantitative grading) or by a decrease in vertebral height of 20% or more and 4 mm or more. Patients' reports of nonvertebral fractures were confirmed by written reports from a radiologist or an orthopedic surgeon. Pathologic fractures, those attributed by the investigator to excessive trauma, and those unrelated to low bone mineral density (i.e., fractures of the face, skull, toe, or finger15) were excluded.
Dual-energy x-ray absorptiometry scans of the lumbar spine and proximal femur, which were performed by Lunar or Hologic densitometers, were analyzed centrally (Synarc). If bone mineral density at the hip or spine decreased by 7% or more from baseline or between visits, as confirmed by a repeat scan, study treatment was stopped, and the patient was advised about other approved therapies for osteoporosis.
Mammograms were repeated either annually at sites in which local guidelines recommended annual mammography or after 3 years at sites in which local guidelines did not recommend periodic mammography. Patients in each group underwent an average of 3.5 mammograms, including the baseline imaging. The local study physician managed follow-up of abnormal results; breast cancer was confirmed from pathological reports by consensus of the Breast and Gynecological Cancer Adjudication Committee.
Women with a uterus underwent annual transvaginal ultrasonography, and it was recommended that patients in whom a double-layer endometrial thickness of more than 4 mm developed should undergo endometrial biopsy (Pipelle, Prodimed) if feasible. Women with persistent, severe vaginal bleeding during the first 3 months, persistent bleeding during the first 6 months, or frequent bleeding at any other time were also advised to undergo endometrial biopsy; slides were read by two gynecologic pathologists, and a third pathologist resolved disagreements.16 Cervical cytologic smears were performed annually in patients with a cervix.
A committee of cardiologists and a neurologist defined terms from the Medical Dictionary for Regulatory Activities that might be cardiovascular outcomes of the trial, and such potential events and causes of death were adjudicated by prospectively defined criteria. Strokes were diagnosed and classified on the basis of computed tomography or magnetic resonance imaging or on the basis of typical neurologic findings lasting longer than 24 hours.
Weight was measured every 6 months. Blood tests that were performed at either 36 months or the end of the study included measurements of liver function.
Study Design
The study was approved by institutional review boards at 80 sites in 22 countries. Ten sites in the United States discontinued the trial in January 2003 after assigning 41 women to receive tibolone and 36 women to receive placebo because the central review board at each site changed its rules regarding placebo-controlled osteoporosis trials; those patients are included in the intention-to-treat analyses.
The data and safety monitoring committee reviewed unblinded data at least every 6 months. The committee prospectively established stopping boundaries for the primary end point (vertebral fracture) according to the Lan–DeMets procedure with a symmetric 0.05-level, two-sided O'Brien– Fleming spending function.17 The committee considered continuation of the study on the basis of a balance of risks and benefits.
A steering committee whose members were not employed by the sponsor, Organon, oversaw the design and conduct of the trial and unanimously approved the decision to publish the results. The study sponsor held the data, and all analyses were performed by a statistician employed by t he sponsor. The authors had access to the data and the analyses. Results for primary and secondary outcomes were independently confirmed by an analyst at the San Francisco Coordinating Center. All authors vouch for the accuracy and completeness of the acquired data and reported results.
Statistical Analysis
In determining that 4000 patients were needed for the study, we assumed that 20% of the patients would already have had a vertebral fracture at baseline and that the annual rate of vertebral fracture in the placebo group would be 6% in those with a vertebral fracture and 1.4% in those without a vertebral fracture. The study had a power of 90% to detect a 40% reduction in the risk of new vertebral fracture at 3 years.
Analyses generally included all women who had undergone randomization and had received at least one dose of a study drug (2249 in the tibolone group and 2257 in the placebo group), except as noted. Analyses of fractures included patients with at least one follow-up radiograph (2059 in the tibolone group and 2087 in the placebo group). Outcomes were analyzed with the use of Cox regression models, and results are reported as relative hazards with 95% confidence intervals. We used Poisson regression models to analyze differences in absolute rates. Analyses of potential interactions were prespecified and limited to the effect on fractures in subgroups, according to the presence of a vertebral fracture at baseline, and the effects on stroke, according to age group and the duration of treatment. The significance was tested according to treatment-by-stratum interactions in Cox models.
Results
Patients
A total of 4538 patients were randomly assigned to receive either 125 mg of tibolone or an indistinguishable placebo once daily. Of these patients, 4534 underwent randomization with use of an interactive voice-response system. Another four patients underwent randomization without the sequence specified by the voice-response system; these patients were included in the as-treated group, since they each received a study drug. About 40% of the patients were 70 years of age or older, and 26% had already had a vertebral fracture (Table 1).
Table 1.
Characteristics of the Patients.*
| Characteristic | Tibolone (N = 2267) | Placebo (N = 2267) |
|---|---|---|
| Age | ||
| Mean — yr | 68.3±5.2 | 68.2±5.2 |
| ≤69 yr — no. (%) | 1352 (60) | 1349 (60) |
| ≥70 yr — no. (%) | 915 (40) | 918 (40) |
| Body-mass index | 25.7±3.4 | 25.7±3.4 |
| Previous hormone therapy — no. (%) | 482 (21) | 461 (20) |
| Family history of breast cancer — no. (%)† | 208 (9) | 223 (10) |
| Current smoker — no. (%) | 289 (13) | 254 (11) |
| Hypertension — no. (%) | 807 (36) | 806 (36) |
| Diabetes — no. (%) | 106 (5) | 115 (5) |
| Prevalent vertebral fracture — no. (%) | 607 (27) | 584 (26) |
| Previous nonvertebral fracture — no. (%) | 528 (23) | 479 (21) |
| Bone mineral density T score‡ | ||
| Total hip | −1.8±0.78 | −1.8±0.79 |
| Lumbar spine | −2.9±0.61 | −2.9±0.55 |
| Intact uterus — no. (%) | 1746 (77) | 1773 (78) |
Plus–minus values are means ±SD. The total numbers of patients in each group include all those who underwent randomization with the use of an interactive voice-response system, although 18 patients in the tibolone group and 10 in the placebo group did not receive a study drug. The body-mass index is the weight in kilograms divided by the square of the height in meters.
Patients had a history of breast cancer in a first-degree female relative.
Measurements of bone mineral density that were obtained with the use of Lunar and Hologic densitometers were standardized for comparability.
In October 2005, the dat a and safety monitoring board notified the sponsor about a potential increased risk of stroke in the tibolone group; the sponsor notified the patients, and 496 women discontinued a study drug. In February 2006, the committee recommended stopping the trial because of an increased risk of stroke and because the effect of treatment on the risk of vertebral fracture met the formal criteria for stopping the trial for efficacy. During a median of 34 months of treatment, 91% of the patients had received at least 80% of the scheduled doses of a prescribed study drug.
Fractures and Bone Density
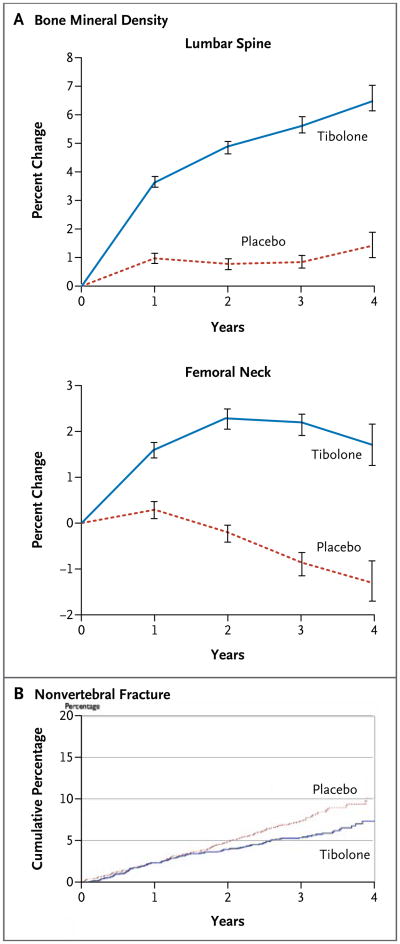
As compared with the placebo group, the tibolone group had an increase in bone mineral density of 4.8 percentage points (95% confidence interval [CI], 4.5 to 5.2) in the spine and of 3.1 percentage points (95% CI, 2.7 to 3.4) in the femoral neck (Fig. 1A). In the tibolone group, there was a reduction in the absolute risk of vertebral fracture of 8.6 (95% CI, 4.4 to 12.9) per 1000 person-years and a reduction in the relative hazard of 45% (95% CI, 26 to 59) (Table 2). There was also a reduction in the absolute risk of nonvertebral fracture of 6.9 (95% CI, 1.6 to 12.2) per 1000 person-years and a reduction in the relative hazard by 26% (95% CI, 7 to 42) (Table 2 and Fig. 1B). In the tibolone group, there was a reduction in the absolute risk of wrist fracture of 4.4 per 1000 person-years (95% CI, 1.5 to 7.4) and a reduction in the relative hazard by 46% (95% CI, 18 to 65). Hip fracture occurred in 10 patients in the tibolone group and in 14 in the placebo group (relative hazard, 0.72; 95% CI, 0.32 to 1.63).
Figure 1. Percent Changes in Bone Mineral Density and Cumulative Proportions of Patients with Nonvertebral Fractures.
Panel A shows the percent changes in bone mineral density at the lumbar spine and femoral neck in the two study groups after 4 years. The differences between the tibolone group and the placebo group were significant (P<0.001) at each year. The I bars denote 95% confidence intervals. Panel B shows the cumulative proportions of patients in the two groups with nonvertebral fractures (relative hazard in the tibolone group, 0.75; 95% CI, 0.58 to 0.93).
Table 2.
Major Outcomes.*
| Outcome | Tibolone (N=2249) | Placebo (N=2257) | Relative Hazard (95% CI) | P Value | Difference in Tibolone Group (95% CI)† | ||
|---|---|---|---|---|---|---|---|
| no. of events | no. of cases per 1000 person-years | no. of events | no. of cases per 1000 person-years | no. of cases per 1000 person-years | |||
| New vertebral fracture | 70 | 10.9 | 126 | 19.6 | 0.55 (0.41 to 0.74) | <0.001 | −8.6 (− 12.9 to −4.4) |
| Nonvertebral fracture‡ | 122 | 19.5 | 166 | 26.3 | 0.74 (0.58 to 0.93) | 0.01 | −6.9 (− 12.2 to −1.6) |
| Breast cancer | 6 | 0.9 | 19 | 2.8 | 0.32 (0.13 to 0.80) | 0.02 | −1.9 (− 3.4 to −0.5) |
| Colon cancer | 4 | 0.6 | 13 | 1.9 | 0.31 (0.10 to 0.96) | 0.04 | −1.3 (− 2.6 to −0.1) |
| Stroke (ischemic or hemorrhagic) | 28 | 4.3 | 13 | 1.9 | 2.19 (1.14 to 4.23) | 0.02 | 2.3 (0.4 to 4.2) |
| Coronary heart disease | 27 | 4.1 | 20 | 3.0 | 1.37 (0.77 to 2.45) | 0.28 | 1.1 (− 0.9 to 3.2) |
| Venous thromboembolism | 5 | 0.8 | 9 | 1.3 | 0.57 (0.19 to 1.69) | 0.31 | −0.6 (− 1.7 to 0.5) |
The analysis includes all patients who received at least one dose of a study drug. Patients could have more than one event.
Values may not equal numerical differences because of rounding.
The analysis of nonvertebral fracture included all patients in the intention-to-treat analysis (2059 patients in the tibolone group and 2087 in the placebo group).
Women who had already had a vertebral fracture at baseline had higher rates of vertebral and nonvertebral fractures; therefore, tibolone appeared to reduce the absolute risk of these fractures more for women who had already had a vertebral fracture than in those who had not had such a fracture (Table 3). Among women who had already had a vertebral fracture at baseline, tibolone was associated with a decrease in the absolute risk of vertebral fracture of 20.8 per 1000 person-years and with a decrease in the relative hazard of 61%. In these women, tibolone reduced the absolute risk of nonvertebral fracture by 17.7 per 1000 person-years and the relative hazard by 47% (Table 3). In contrast, among women who had not had a vertebral fracture at baseline, tibolone was associated with a reduction in the absolute risk of vertebral fracture of 4.6 per 1000 person-years and a reduction in the relative hazard of 31%; it was associated with a reduction in the absolute risk of nonvertebral fracture of 3.2 per 1000 person-years and a reduction in the relative hazard of 14% (P = 0.07 for both interactions).
Table 3.
Effects of Tibolone on New Factures, According to the Presence or Absence of a Vertebral Fracture at Baseline.*
| Variable | Tibolone (N = 2059) | Placebo (N = 2087) | Relative Hazard (95% CI) | P Value | Risk Difference in Tibolone Group (95% CI)† | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Treatment | Interaction | |||||||
|
| ||||||||
| no. of events | no. of cases per 1000 person-years | no. of events | no. of cases per 1000 person-years | no. of cases per 1000 person-years | ||||
|
| ||||||||
| New vertebral fracture | ||||||||
|
| ||||||||
| No prevalent vertebral fracture | 47 | 10.0 | 70 | 14.6 | 0.69 (0.48 to 1.00) | 0.05 | 0.07 | −4.6 (− 9.0 to −0.1) |
|
| ||||||||
| Prevalent vertebral fracture | 23 | 13.6 | 56 | 34.3 | 0.39 (0.24 to 0.63) | <0.001 | −20.8 (− 31.3 to −10.2) | |
| Nonvertebral fracture | ||||||||
|
| ||||||||
| No prevalent vertebral fracture | 89 | 19.3 | 106 | 22.5 | 0.86 (0.65 to 1.14) | 0.28 | 0.07 | −3.2 (− 9.1 to 2.7) |
|
| ||||||||
| Prevalent vertebral fracture | 33 | 20.0 | 60 | 37.7 | 0.53 (0.35 to 0.81) | 0.004 | −17.7 (− 29.4 to −6.0) | |
The analysis includes all patients who had undergone radiography at baseline and during at least one follow-up visit.
Values may not equal numerical differences because of rounding.
Breast and Colon Cancer
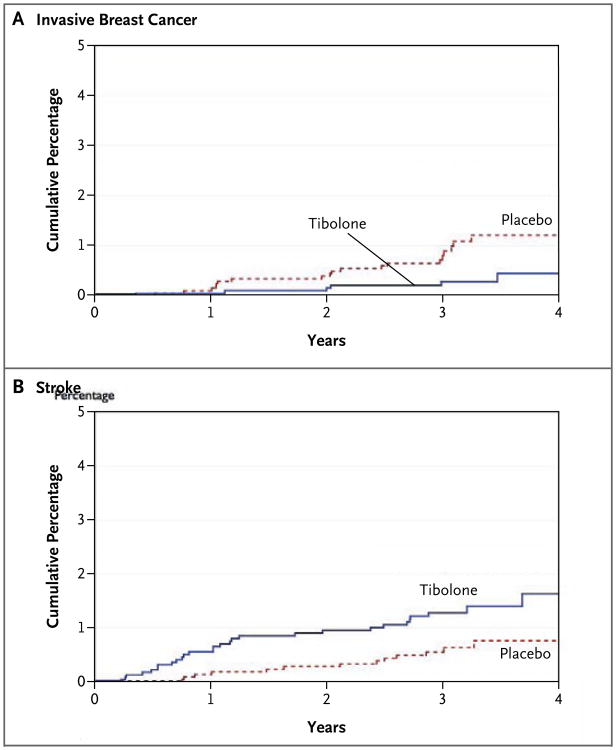
In the tibolone group, there was a decrease in the absolute incidence of invasive breast cancer of 1.9 (95% CI, 0.5 to 3.4) per 1000 person-years and a decrease in the relative risk of 68% (95% CI, 20 to 87), as compared with the placebo group (Table 2 and Fig. 2A). One case of ductal carcinoma in situ was also diagnosed in each group. In the tibolone group, t here was a decrease in the absolute incidence of colon cancer of 1.3 (95% CI, 0.1 to 2.6) per 1000 person-years and a decrease in the relative hazard of 69% (95% CI, 4 to 90), as compared with the placebo group (Table 2). There were no significant differences in the risk of other types of cancers.
Figure 2. Cumulative Percentages of Patients with Breast Cancer and Stroke.
At 4 years, therapy with tibolone was associated with a decrease in the risk of invasive breast cancer (relative hazard, 0.32; 95% CI, 0.13 to 0.80; P=0.02) (Panel A) but with an increase in the risk of stroke (relative hazard, 2.19; 95% CI, 1.14 to 4.23; P=0.02) (Panel B).
Cardiovascular and Cerebrovascular Disease
Women in the tibolone group had an increase in the absolute risk of stroke of 2.3 (95% CI, 0.4 to 4.2) per 1000 person-years and an increase in the relative hazard of 2.19 (95% CI, 1.14 to 4.23) (Table 2 and Fig. 2B). Among patients 70 years of age or older, the risk of stroke was 6.6 per 1000 person-years in the tibolone group and 3.4 per 1000 person-years in the placebo group (difference in absolute risk, 3.1 per 1000 person-years); among those between the ages of 60 and 69 years, the risks were 2.8 and 1.0 per 1000 person-years, respectively (difference in absolute risk, 1.8 per 1000 person-years). The increased risk of stroke appeared to be greater in the first year (relative hazard, 4.1) than in later years (relative hazard, 1.6), but the treatment-by-time interaction was not significant (P = 0.23). Transient ischemic attacks were rare in both the tibolone group (0.3%) and the placebo group (0.2%). The two groups did not differ significantly in rates of venous thromboembolism or coronary heart disease events (Table 2).
Gynecologic Outcomes
Among study patients who had a uterus, endometrial cancer was diagnosed in 4 of 1746 women in the tibolone group (0.8 per 100 0 per son-years) and in none of 1773 patients in the placebo group (P = 0.06). Vaginal bleeding was reported as an adverse event by 9.5% of women in the tibolone group and 2.5% in the placebo group. At some point in the trial, 533 women in the tibolone group and 168 in the placebo group were found to have an endometrial thickness of more than 4 mm, as seen on ultrasonography For bleeding or increased endometrial thickness, 499 women in the tibolone group and 136 in the placebo group underwent endometrial biopsy. Endometrial hyperplasia was diagnosed in two women in the tibolone group and one woman in the placebo group.
Of women who had a cervix and underwent at least one cervical cytologic smear, 132 of 1746 patients (7.6%) in the tibolone group and 56 of 1773 patients (3.2%) in the placebo group had mild dysplasia or atypical cells of unknown significance on cytologic analysis (P=0.009); there was no significant difference in the incidence of moderate or severe dysplasia (0.4% vs. 0.3%), and only one case of cervical cancer occurred in each group. Hysterectomies were performed in 18 women (1.0%) in the tibolone group and in 15 (0.8%) in the placebo group.
Other Effects
More patients in the tibolone group than in the placebo group discontinued treatment because of an adverse event, most commonly vaginal discharge, vaginal bleeding, or breast discomfort (Table 4). As compared with placebo, tibolone was associated with a mean increase in weight of 0.6 kg; 109 of 2050 women (5.3%) in the tibolone group and 81 of 2121 (3.8%) in the placebo group gained at least 10% of their baseline weight. Liver-function tests at baseline and at 36 months of follow-up (in 1312 patients in the tibolone group and 1368 in the placebo group) revealed that serum γ-glutamyltransferase levels increased by a mean of 9.9 IU per liter (median, 4.5) in women receiving tibolone and by 0.9 IU per liter (median, 1.0) in those receiving placebo (P<0.001); 60 women (4.6%) in the tibolone group and 20 (1.5%) in the placebo group had levels of at least 96 IU per liter (≥3 times the upper limit of the normal range [ ULN ], P<0.001). Increases in alanine aminotransferase levels of more than 90 IU per liter (≥3 times the ULN) occurred in 12 women (0.9%) in the tibolone group and 3 (0.2%) in the placebo group (P=0.02). There was no significant difference in serum bilirubin levels between the two groups. Only one woman in the tibolone group and 14 in the placebo group stopped treatment because of bone loss at t he hip or spine exceeding 7%, as compared with baseline.
Table 4.
Adverse Events.*
| Event | Tibolone (N = 2249) | Placebo (N = 2257) | P Value |
|---|---|---|---|
| no. of events (%) | |||
| Death | 26 (1.2) | 28 (1.2) | 0.89 |
| Serious event† | 548 (24.4) | 517 (22.9) | 0.26 |
| Any event | 2098 (93.3) | 2067 (91.6) | 0.03 |
| Gynecologic event‡ | |||
| Vaginal discharge | 221 (9.8) | 40 (1.8) | <0.001 |
| Breast discomfort | 203 (9.0) | 65 (2.9) | <0.001 |
| Vaginal bleeding§ | 165 (9.5) | 45 (2.5) | <0.001 |
| Vaginal infection | 186 (8.3) | 56 (2.5) | <0.001 |
| Pelvic pain | 53 (2.4) | 29 (1.3) | 0.007 |
| Other event‡ | |||
| Falling | 154 (6.8) | 205 (9.1) | 0.006 |
| Gastroenteritis | 57 (2.5) | 87 (3.9) | 0.01 |
| Sinus bradycardia | 33 (1.5) | 52 (2.3) | 0.008 |
| Discontinued treatment owing to adverse event | 422 (18.8) | 296 (13.1) | <0.001 |
| Reason for discontinuation | |||
| Vaginal bleeding§ | 31 (1.8) | 7 (0.4) | <0.001 |
| Increased endometrial thickness | 26 (1.2) | 6 (0.3) | <0.001 |
| Breast discomfort | 25 (1.1) | 4 (0.2) | <0.001 |
| Vaginal discharge | 24 (1.1) | 2 (0.1) | <0.001 |
The analysis includes all patients who received at least one dose of a study drug. Patients could have more than one adverse event.
Serious adverse events were all deaths, life-threatening events, hospitalizations, and other events that caused disability or prompted intervention to prevent permanent impairment. Such events included cardiovascular events, breast cancer, endometrial cancer, and other events that were adjudicated in a blinded fashion and are reported in the text and in Figure 2.
Other adverse events were included if they occurred in at least 2% of either group (P≤0.01).
The percentages are based on the number of patients who had a uterus (1746 in the tibolone group and 1773 in the placebo group).
The number of women who reported having fallen was 25% less in the tibolone group than in the placebo group. There were also fewer reports of gastroenteritis and sinus bradycardia in the tibolone group.
Discussion
Tibolone was associated with a reduction in the risk of vertebral fracture in older women with osteoporosis. The absolute reduction was greater (20.8 per 1000 person-years) among women who had already had a vertebral fracture than among those who had not had such a fracture (4.6 per 1000 person-years). The magnitude of the reduction in relative risk was similar to those observed for therapy with estrogen, bisphosphonates, and raloxifene.18 Tibolone was also associated with a reduction in the risk of nonvertebral fracture, an improvement that was also greater in women who had already had a vertebral fracture. A similarly decreased risk of nonvertebral fracture has been demonstrated for estrogen therapy19,20 but not for the selective estrogen-receptor modulators (SERMs) raloxifene and tamoxifen.21-23
Tibolone was associated with a reduction in the risk of invasive breast cancer to a degree that was similar to that observed for treatment with tamoxifen or raloxifene.24-26 Estrogen-receptor status was not available for the study patients, but tibolone was probably associated with a decreased risk of estrogen-receptor–positive disease because it is by far the most common type in this age group.27
The observational Million Women Study reported that the use of tibolone for up to 5 years was associated with an increased risk of breast cancer, as compared with nonuse of hormone therapy (relative risk, 1.21; 95% CI, 1.07 to 1.37).28 In contrast, a large case–control study, also conducted in the United Kingdom, showed no increased risk of breast cancer (relative risk, 0.86; 95% CI, 0.65 to 1.13) with an average of 6.5 years of use of tibolone.29 It is not clear why the results of these observational studies differ from the reduced risk that we report. If tibolone were prescribed instead of estrogen therapy for women at increased risk of breast cancer, this would spuriously increase the risk of breast cancer associated with tibolone. However, risk factors for breast cancer were similar in women taking tibolone and estrogen therapies in the Million Women Study.28
It is not clear how tibolone would decrease the risk of breast cancer. Tibolone appears to stimulate proliferation of human MCF-7 cells,30 albeit less than estradiol does.31,32 Other studies have shown that tibolone decreases the proliferation of breast epithelial cells and causes apoptosis of breast-cancer cells.33-35 Tibolone might decrease the intracellular production of estradiol by inhibiting aromatase,36 and it might inhibit sulfatase and stimulate sulfotransferase activities, thereby increasing circulating levels of the weak or inactive sulfated forms of estrogen that might competitively inhibit estradiol.37,38
The increased risk of stroke with tibolone has also been reported with estrogen therapy,20,39 but the biologic mechanism is not certain. A randomized, placebo-controlled trial showed that 2.5 mg of tibolone and conjugated equine estrogen plus medroxyprogesterone slightly increased the intima–media thickness by 0.004 mm per year.40 Tibolone increases levels of C-reactive protein, a risk factor for stroke, to a degree similar to that of conjugated equine estrogen.41 In randomized trials, tibolone decreased HDL cholesterol levels but improved lipoprotein(a) levels, did not significantly change homocysteine levels, and increased plasminogen levels.41,42 Treatment with tibolone had no effect on blood pressure or fasting blood glucose levels.40
Since the risk of stroke rises exponentially with age, tibolone should generally not be used in elderly women. Tibolone has been used by women between the ages of 50 and 60 years for menopausal symptoms and the prevention of osteoporosis when the risk of stroke was low, but it should be avoided in women who have strong risk factors for stroke, such as hypertension, smoking, diabetes, and atrial fibrillation.43 Although the overall number of adverse events was small, there was no increased risk of venous thromboembolism, as has been seen with hormone therapy and SERMs, or an increased risk of coronary events, as has been seen with conjugated estrogen combined with medroxyprogestrone.21,23,39
With respect to the four women in the tibolone group in whom endometrial cancer developed, the Million Women Study reported an association between the use of tibolone and an increased risk of endometrial cancer.44 In a 2-year randomized trial of 2.5 mg of tibolone daily involving 1598 women who were younger (mean age, 54 years) than the patients in our study (mean age, 68 years), no cases of endometrial cancer occurred.45 We found that women in the tibolone group were three times as likely to report vaginal bleeding and have an endometrial thickness of more than 4 mm (leading to an increase in the rate of endometrial biopsy by a factor of 3) as were those in t he placebo group.46 The increased surveillance might account, at least in part, for the increased diagnosis of endometrial cancer.46 There was no difference between the two groups in the low rate of endometrial hyperplasia. Although tibolone might increase the risk of endometrial cancer, the absolute risk was small during 3 years of therapy. There was a slight increase in reports of pelvic pain, as was seen in a previous trial of tibolone, but the cause was not clear.7
Treatment with tibolone was associated with several gynecologic symptoms that have also been observed with postmenopausal hormone therapy, including vaginal bleeding, vaginal discharge, and breast discomfort. The increased rate of minimally abnormal cervical cytologic results in the tibolone group was also seen with conjugated equine estrogens plus medroxyprogesterone in the Heart and Estrogen/Progestin Replacement Study (HERS)47 and the Women's Health Initiative (WHI) study48; the cause of this effect is not known.
The incidence of colon cancer was not a pre-specified trial outcome, and the number of cases was small. Nevertheless, the decreased rate of colon cancer in the tibolone group, as compared with the placebo group, resembles the reduction in the risk of colon cancer associated with the combination of estrogen and progestin49 but not estrogen alone20 in the WHI study. Potential mechanisms for an effect of estrogen include decreased production of potentially carcinogenic bile acids, decreased proliferation of intestinal epithelial cells, and reduced levels of insulin and insulin-like growth factor 1.49
Treatment with tibolone occasionally increased γ-glutamyltransferase levels, typical of intrahepatic nonalcoholic steatohepatitis, an uncommon effect of estrogen therapy that generally resolves when treatment stops.50 This effect has not previously been reported with tibolone. Because follow-up liver-function tests were obtained only at 36 months, the time course of this effect is not known.
Previous trials reported that tibolone increased weight by increasing lean body mass or water.51 A small trial suggested that tibolone might increase grip and leg-extensor strength.8 Thus, the lower rate of falling in the tibolone group, reported as an adverse event, might reflect an androgenic effect on muscular function. It has been observed that among older men, higher levels of testosterone have been associated with lower rates of falling.52 In contrast, estrogen therapy does not reduce the risk of falling.53
Our study adjudicated incident fractures, breast cancer, cardiovascular events, and the endometrial effects of tibolone. However, the study had limited power to analyze other important outcomes, such as coronary heart disease and endometrial cancer. Since the study lasted for a mean of 3 years, the profile of risks and benefits might change with longer treatment. For example, although not significant, the risk of stroke appeared to decrease with time. On the other hand, the influence of tibolone on the risk of endometrial cancer might increase with time.
Racial or ethnic background and dose are factors to consider when interpreting these data. The study was an international trial, but few patients were black or Asian. We examined the effects of 1.25 mg of tibolone daily, but 2.5 mg is used for vasomotor symptoms. Previous studies have found that 2.5 mg of tibolone had only a slightly greater effect than 1.25 mg on bone density, markers of bone resorption, and lipid levels.4,5,12
We conclude that among older women with osteoporosis, tibolone decreased the risk of vertebral and nonvertebral fractures, particularly in patients who had already had a vertebral fracture. It was associated with a decreased risk of breast cancer and perhaps colon cancer. However, it was associated with an increased risk of stroke and therefore should not be used in elderly women and women with risk factors for stroke. Tibolone had other effects resembling those of therapy combining estrogen and progestin. In instances in which tibolone is approved for use, these potential risks and benefits and other effects should be weighed when considering the use of tibolone for the treatment of menopausal symptoms or fracture prevention.
Acknowledgments
Supported by Organon.
Dr. Cummings reports receiving consulting fees from or serving on a paid advisory board for Pfizer, Amgen, Eli Lilly, Procter & Gamble, GlaxoSmithKline, and Organon; Dr. Ettinger, receiving consulting fees from or serving on a paid advisory board for Eli Lilly, Procter & Gamble, Duramed-Bart, Roche, GlaxoSmithKline, Novartis, and Organon and receiving lecture fees from Eli Lilly; Dr. Delmas, receiving consulting and lecture fees from and serving on a paid advisory board for Organon; Dr. Kenemans, receiving consulting fees from and serving on a paid advisory board for Organon and Procter & Gamble and receiving lecture fees from Solvay, Theramex, and Organon; Drs. Stathopoulos, Verweij, Mol-Arts, Kloosterboer, and Seifert, being employees of Organon; Dr. Mosca, receiving consulting fees from and serving on a paid advisory board for Organon; Dr. Christiansen, receiving consulting fees from and serving on a paid advisory board for Servier Laboratories and Synarc and having an equity interest in Nordic Bioscience and Sybarc; Dr. Bilezekian, receiving consulting fees from or serving on a paid advisory board for Eli Lilly, Novartis, Procter & Gamble, Sanofi-Aventis, GlaxoSmithKline, and Merck and receiving lecture fees from Procter & Gamble, Sanofi-Aventis, and Novartis and grant support from the Alliance for Better Bone Health and Radius Pharmaceutical; Dr. Kerzberg, receiving consulting fees from Organon; Dr. Johnson, receiving consulting fees from and serving on a paid advisory board for Pfizer and receiving grant support from Organon; Dr. Zanchetta, receiving consulting fees from or serving on a paid advisory board for Pfizer, Servier, Eli Lilly, and Organon; Dr. Grobbee, receiving consulting and lecture fees from and serving on a paid advisory board for Organon and receiving grant support from Organon, Pfizer, and AstraZeneca; and Dr. Eastell, receiving consulting fees from or serving on a paid advisory board for Nastech, Kyphon, Unipath, Osteologix, Maxygen, Transpharma, Procter & Gamble, Ono Pharma, Unilever, Fonterra Brands, Roche, and Novartis, receiving lecture fees from Sanofi Aventis, Procter & Gamble, Amgen, Eli Lilly, Novartis, Servier, and GlaxoSmithKline, and receiving grant support from Organon, Pfizer, Eli Lilly, Novartis, Procter & Gamble, and Osteologix. No other potential conflict of interest relevant to this article was reported.
We thank Stephanie Litwack Harrison for performing the confirmatory analyses and Liezl Concepcion and Jamie Low of the San Francisco Coordinating Center for their assistance with the preparation of the manuscript.
Appendix.
Investigators in the LIFT trial were as follows: Clinical Sites — Argentina: J. Zanchetta, E. Kerzberg, Buenos Aires; Australia: J. Eden, Randwick; J. Eisman, Sydney; J. Howarda, Herston; A. MacLennan, Adelaide; R. Norman, Dulwich; Belgium: J. Devogelaer, Brussels; Y. Reginster, Liege; Brazil: S. Ragi Eis, Espirito Santo; L. Griz, Recife; C. Zerbini, São Paulo; Costa Rica: L. Jiménez, San Jose; Czech Republic: J. Stepan, Prague; Denmark: C. Christiansen, Aalborg, Vejle, and Ballerup; Estonia: I. Valter, Tallinn; K. Maasalu, Tartu; K. Vahula, Pärnu; France: P. Orcel, Paris; P. Delmas, Lyon; L. Benhamou, Orleans; C. Ribot, Toulouse; Germany: D. Felsenberg, Berlin; W. Spieler, Zerbst; H. Dammann, Hamburg; Hungary: P. Lakatos, I. Szombati, Budapest; L. Korányi, Balatonfüred; B. Spengler, Zalaegerszeg; Z. Szabo, Miskolc; L. Nafradi, Szombathely; K. Takacs, Kiskunhalas; Lithuania: V. Alekna, Vilnius; A. Krasauskiene, Kaunas; Mexico: R. Jurgutis, Guanajuato; S. Jasqui, Huixquilucan; P. de la Pena, Guadalajara; P. Garcia, Monterrey; the Netherlands: A. Baka, Utrecht; J. Jonker, Zoetermeer, Groningen, and Rotterdam; Norway: E. Øfjord, Paradis; T. Lunde, Larvik; A. Skag, Hamar; Poland: A. Sawicki, A. Bochenek, Warsaw; J. Badurski, Bialystok; E. Czerwinski, K. Lipinski, Krakow; Slovak Republic: J. Payer, Bratislava; Spain: A. Díez, Barcelona; R. Gabriel, Madrid; Turkey: M. Erenus, Istanbul; F. Saracoglu, Ankara; F. Sendag, Izmir; United Kingdom: D. Reid, Inverness; P. Albertazzia, Hull; R. Eastell, Sheffield; I. Pavela, Liverpool; R. Pawaa, Wigan; J. Robinson, Manchester; United States: E. Barrett-Connor, La Jolla, CA; E. Boling, Rancho Cucamonga, CA; J. Caldwell, Daytona, FL; P. Bowen, Atlanta; R. Wasnich, Honolulu; S. Johnson, Iowa City; J. Simon, Laurel, MD; E. Lewiecki, Albuquerque, NM; D. McCluskey, Mogadore, OH; G. Sultany, Portland, OR; A. Kivitz, Duncansville, PA; W. Henry, Greer, SC; R. Downs, Richmond,VA; Venezuela: G. Riera, Valencia; A. Pérez Monteverde, Caracas. Data and Safety Monitoring Board — D. Grady, University of California, San Francisco; M. McClung, Oregon Osteoporosis Center, Portland; H. Pols, Erasmus University Rotterdam, Rotterdam, the Netherlands; K. Davis, Seattle; D. Herrington, Winston-Salem, NC; S. Hendrix, Wayne State University–Hutzel Hospital, Detroit; P. Neven, Universitair Ziekenhuis Gasthuisberg, Leuven, Belgium. Gynecology End-Points Committee — P. van Diest (chair), Department of Pathology, University Medical Center Utrecht, Utrecht, the Netherlands; N. Harbeck, Frauenklinik der Technischen Universität München, Munich; H. Senn, Facharzt FMH für Innere Medizin und Onkologie–Hematologie, St. Gallen, Switzerland; G. Svane, Department of Diagnostic Radiology, Karolinska Hospital, Stockholm. Cardiovascular Adjudication Committee — M. Longo and B. Davidson, Virginia Mason Medical Center, Seattle; J. Kappelle, University Hospital Utrecht, Utrecht, the Netherlands; R. Peters, Academic Medical Center, Amsterdam; M. Prins, Acedemisch Ziekenhuis Maastricht, Maastricht, the Netherlands.
Footnotes
Investigators in the Long-Term Intervention on Fractures with Tibolone (LIFT) Trial are listed in the Appendix.
Contributor Information
Steven R. Cummings, San Francisco Coordinating Center and the California Pacific Medical Center Research Institute, San Francisco; The University of California, San Francisco —all in San Francisco
Bruce Ettinger, The University of California, San Francisco —all in San Francisco
Pierre D. Delmas, Université de Lyon and INSERM Research Unit 831, Lyon, France
Peter Kenemans, Amsterdam Free University Hospital, Amsterdam
Victoria Stathopoulos, Organon, Roseland, NJ
Pierre Verweij, Oss, the Netherlands
Mirjam Mol-Arts, Oss, the Netherlands
Lenus Kloosterboer, Oss, the Netherlands
Lori Mosca, The College of Physicians and Surgeons, Columbia University, New York
Claus Christiansen, The Center for Clinical and Basic Research, Ballerup, Denmark
John Bilezikian, New York–Presbyterian Hospital, Columbia University, and Cornell University, New York
Eduardo Mario Kerzberg, The National Reference Center on Osteoporosis, Hospital J.M. Ramos Me-jía, Buenos Aires
Susan Johnson, Carver College of Medicine, University of Iowa, Iowa City
Jose Zanchetta, Instituto de Investigationes Me-tabólicas, Buenos Aires
Diederich E. Grobbee, The Julius Center for Health Sciences and Primary Care, Utrecht University Hospital, Utrecht, the Netherlands
Wilfried Seifert, Oss, the Netherlands
Richard Eastell, The University of Sheffield, Sheffield, United Kingdom
References
- 1.de Gooyer ME, Deckers GH, Schoonen WG, Verheul HA, Kloosterboer HJ. Receptor profiling and endocrine interactions of tibolone. Steroids. 2003;68:21–30. doi: 10.1016/s0039-128x(02)00112-5. [DOI] [PubMed] [Google Scholar]
- 2.Jelinek J, Kappen A, Schönbaum E, Lomax P. A primate model of human postmenopausal hot flushes. J Clin Endocrinol Metab. 1984;59:1224–8. doi: 10.1210/jcem-59-6-1224. [DOI] [PubMed] [Google Scholar]
- 3.Landgren MB, Helmond FA, Engelen S. Tibolone relieves climacteric symptoms in highly symptomatic women with at least seven hot flushes and sweats per day. Maturitas. 2005;50:222–30. doi: 10.1016/j.maturitas.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Bjarnason NH, Bjarnason K, Haarbo J, Rosenquist C, Christiansen C. Tibolone: prevention of bone loss in late postmenopausal women. J Clin Endocrinol Metab. 1996;81:2419–22. doi: 10.1210/jcem.81.7.8675554. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher JC, Baylink DJ, Freeman R, McClung M. Prevention of bone loss with tibolone in postmenopausal women: results of two randomized, double-blind, placebo-controlled, dose-finding studies. J Clin Endocrinol Metab. 2001;86:4717–26. doi: 10.1210/jcem.86.10.7937. [DOI] [PubMed] [Google Scholar]
- 6.Ederveen AG, Kloosterboer HJ. Tibolone exerts its protective effect on trabecular bone loss through the estrogen receptor. J Bone Miner Res. 2001;16:1651–7. doi: 10.1359/jbmr.2001.16.9.1651. [DOI] [PubMed] [Google Scholar]
- 7.Swanson SG, Drosman S, Helmond FA, Stathopoulos VM. Tibolone for the treatment of moderate to severe vasomotor symptoms and genital atrophy in post-menopausal women: a multicenter, randomized, double-blind, placebo-controlled study. Menopause. 2006;13:917–25. doi: 10.1097/01.gme.0000247016.41007.c9. [DOI] [PubMed] [Google Scholar]
- 8.Meeuwsen IB, Samson MM, Duursma SA, Verhaar HJ. The influence of tibolone on quality of life in postmenopausal women. Maturitas. 2002;41:35–43. doi: 10.1016/s0378-5122(01)00251-1. [DOI] [PubMed] [Google Scholar]
- 9.Modelska K, Cummings S. Tibolone for postmenopausal women: systematic review of randomized trials. J Clin Endocrinol Metab. 2002;87:16–23. doi: 10.1210/jcem.87.1.8141. [DOI] [PubMed] [Google Scholar]
- 10.Laan E, van Lunsen RH, Everaerd W. The effects of tibolone on vaginal blood flow, sexual desire and arousability in postmenopausal women. Climacteric. 2001;4:28–41. [PubMed] [Google Scholar]
- 11.Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286–93. doi: 10.1067/mob.2003.117. [DOI] [PubMed] [Google Scholar]
- 12.Bjarnason NH, Bjarnason K, Haarbo J, Bennink HJ, Christiansen C. Tibolone: influence on markers of cardiovascular disease. J Clin Endocrinol Metab. 1997;82:1752–6. doi: 10.1210/jcem.82.6.3995. [DOI] [PubMed] [Google Scholar]
- 13.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–48. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 14.Genant HK, Jergas M, Palermo L, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. J Bone Miner Res. 1996;11:984–96. doi: 10.1002/jbmr.5650110716. [DOI] [PubMed] [Google Scholar]
- 15.Stone KL, Seeley DG, Lui LY, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947–54. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 16.Kurman R, Norris H. Endometrial hyperplasia and related cellular changes. In: Kurman R, editor. Blaustein's pathology of the female genital tract. 4th. New York: Springer-Verlag; 1994. pp. 411–86. [Google Scholar]
- 17.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–63. [Google Scholar]
- 18.Cummings SR. A 55-year-old woman with osteopenia. JAMA. 2006;296:2601–10. doi: 10.1001/jama.296.21.2601. [DOI] [PubMed] [Google Scholar]
- 19.Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. JAMA. 2003;290:1729–38. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 20.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 21.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637–45. doi: 10.1001/jama.282.7.637. Erratum, JAMA 1999; 282:2124. [DOI] [PubMed] [Google Scholar]
- 22.Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial: Multiple Outcomes of Raloxifene Evaluation. Breast Cancer Res Treat. 2001;65:125–34. doi: 10.1023/a:1006478317173. Erratum, Breast Cancer Res Treat 2001;67:191. [DOI] [PubMed] [Google Scholar]
- 23.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 24.Cuzick J, Powles T, Veronesi U, et al. Over view of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 25.Martino S, Cauley JA, Barrett-Connor E, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–61. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 26.Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–37. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 27.Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21:28–34. doi: 10.1200/JCO.2003.03.088. [DOI] [PubMed] [Google Scholar]
- 28.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–27. doi: 10.1016/s0140-6736(03)14065-2. Erratum, Lancet 2003;362:1160. [DOI] [PubMed] [Google Scholar]
- 29.Opatrny L, Dell'Aniello S, Assouline S, Suissa S. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG. 2008;115:169–75. doi: 10.1111/j.1471-0528.2007.01520.x. [DOI] [PubMed] [Google Scholar]
- 30.Mueck AO, Lippert C, Seeger H, Wall-wiener D. Effects of tibolone on human breast cancer cells and human vascular coronary cells. Arch Gynecol Obstet. 2003;267:139–44. doi: 10.1007/s00404-002-0291-x. [DOI] [PubMed] [Google Scholar]
- 31.Kloosterboer HJ, Schoonen WG, Deckers GH, Klijn JG. Effects of progestagens and Org OD14 in in vitro and in vivo tumor models. J Steroid Biochem Mol Biol. 1994;49:311–8. doi: 10.1016/0960-0760(94)90273-9. [DOI] [PubMed] [Google Scholar]
- 32.Lippert C, Seeger H, Wallwiener D, Mueck AO. Tibolone versus 17beta-estradiol/norethisterone: effects on the proliferation of human breast cancer cells. Eur J Gynaecol Oncol. 2002;23:127–30. [PubMed] [Google Scholar]
- 33.Gompel A, Chaouat M, Jacob D, Perrot JY, Kloosterboer HJ, Rostene W. In vitro studies of tibolone in breast cells. Fertil Steril. 2002;78:351–9. doi: 10.1016/s0015-0282(02)03203-x. [DOI] [PubMed] [Google Scholar]
- 34.Werner HM, Franke HR, Vermes I. Apoptosis and proliferation in breast cancer cells, cultured in vitro: effects of SERMs. Climacteric. 2005;8:294–9. doi: 10.1080/13697130500197526. [DOI] [PubMed] [Google Scholar]
- 35.Conner P, Christow A, Kersemaekers W, et al. A comparative study of breast cell proliferation during hormone replacement therapy: effects of tibolone and continuous combined estrogen-progestogen treatment. Climacteric. 2004;7:50–8. doi: 10.1080/13697130310001651472. [DOI] [PubMed] [Google Scholar]
- 36.Raobaikady B, Parsons MF, Reed MJ, Purohit A. Tibolone and its delta-4, 7alpha-methyl norethisterone metabolite are reversible inhibitors of human aromatase. J Steroid Biochem Mol Biol. 2007;104:154–60. doi: 10.1016/j.jsbmb.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 37.Chetrite G, Kloosterboer HJ, Pasqualini JR. Effect of tibolone (Org OD14) and its metabolites on estrone sulphatase activity in MCF-7 and T-47D mammary cancer cells. Anticancer Res. 1997;17:135–40. [PubMed] [Google Scholar]
- 38.Chetrite GS, Kloosterboer HJ, Philippe JC, Pasqualini JR. Effect of Org OD14 (LIVIAL) and its metabolites on human estrogen sulphotransferase activity in the hormone-dependent MCF-7 and T-47D, and the hormone-independent MDA-MB-231, breast cancer cell lines. Anticancer Res. 1999;19:269–75. [PubMed] [Google Scholar]
- 39.Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 40.Bots ML, Evans GW, Riley W, et al. The effect of tibolone and continuous combined conjugated equine oestrogens plus medroxyprogesterone acetate on progression of carotid intima-media thickness: the Osteoporosis Prevention and Arterial effects of tiboLone (OPAL) study. Eur Heart J. 2006;27:746–55. doi: 10.1093/eurheartj/ehi695. [DOI] [PubMed] [Google Scholar]
- 41.Barnes JF, Farish E, Rankin M, Hart DM. Effects of two continuous hormone therapy regimens on C-reactive protein and homocysteine. Menopause. 2005;12:92–8. doi: 10.1097/00042192-200512010-00016. [DOI] [PubMed] [Google Scholar]
- 42.Davison S, Davis SR. New markers for cardiovascular disease risk in women: impact of endogenous estrogen status and exogenous postmenopausal hormone therapy. J Clin Endocrinol Metab. 2003;88:2470–8. doi: 10.1210/jc.2002-021929. [DOI] [PubMed] [Google Scholar]
- 43.Tegos TJ, Kalodiki E, Daskalopoulou SS, Nicolaides AN. Stroke: epidemiology, clinical picture, and risk factors. Angiology. 2000;51:793–808. doi: 10.1177/000331970005101001. [DOI] [PubMed] [Google Scholar]
- 44.Beral V, Bull D, Reeves G. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365:1543–51. doi: 10.1016/S0140-6736(05)66455-0. [DOI] [PubMed] [Google Scholar]
- 45.Archer DF, Hendrix S, Gallagher JC, et al. Endometrial effects of tibolone. J Clin Endocrinol Metab. 2007;92:911–8. doi: 10.1210/jc.2006-2207. [DOI] [PubMed] [Google Scholar]
- 46.Ettinger B, Kenemans P, Johnson S, et al. Endometrial effects of tibolone in elderly osteoporotic women. Obstet Gynecol. doi: 10.1097/AOG.0b013e3181809e25. in press. [DOI] [PubMed] [Google Scholar]
- 47.Sawaya GF, Grady D, Kerlikowske K, et al. The positive predictive value of cervical smears in previously screened post-menopausal women: the Heart and Estrogen/progestin Replacement Study (HERS) Ann Intern Med. 2000;133:942–50. doi: 10.7326/0003-4819-133-12-200012190-00009. [DOI] [PubMed] [Google Scholar]
- 48.Anderson GL, Judd HL, Kaunitz AM, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women's Health Initiative randomized trial. JAMA. 2003;290:1739–48. doi: 10.1001/jama.290.13.1739. [DOI] [PubMed] [Google Scholar]
- 49.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 50.Stravitz RT, Sanyal AJ. Drug-induced steatohepatitis. Clin Liver Dis. 2003;7:435–51. doi: 10.1016/s1089-3261(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 51.Jacobsen DE, Samson MM, Kezic S, Verhaar HJ. Postmenopausal HRT and tibolone in relation to muscle strength and body composition. Maturitas. 2007;58:7–18. doi: 10.1016/j.maturitas.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Orwoll E, Lambert LC, Marshall LM, et al. Endogenous testosterone levels, physical performance, and fall risk in older men. Arch Intern Med. 2006;166:2124–31. doi: 10.1001/archinte.166.19.2124. [DOI] [PubMed] [Google Scholar]
- 53.Greenspan SL, Resnick NM, Parker RA. The effect of hormone replacement on physical performance in community-dwelling elderly women. Am J Med. 2005;118:1232–9. doi: 10.1016/j.amjmed.2005.03.004. [DOI] [PubMed] [Google Scholar]