Summary
The matching of blood flow to regional brain function, called functional hyperemia or neurovascular coupling, involves the coordinated activity of neurons, astrocytes and parenchymal arterioles. Under physiological conditions, localized neuronal activation leads to elevated astrocyte endfoot Ca2+ and vasodilation, resulting in an increase in cerebral blood flow. In this study, we examined the impact of subarachnoid hemorrhage (SAH) on neurovascular coupling. SAH model rats received two injections of autologous blood into the cisterna magna 24 hours apart. Cortical brain slices from SAH model animals were prepared four days after the initial blood injection. Arteriolar diameter and astrocyte endfoot Ca2+ were simultaneously measured using two-photon microscopy. As expected, neuronal activity evoked by electrical field stimulation (EFS) caused an elevation in endfoot Ca2+ and vasodilation in brain slices from control animals. However, in brain slices from SAH animals, EFS induced a similar increase in astrocyte endfoot Ca2+ that caused arteriolar constriction rather than vasodilation. Vasoconstriction was observed in approximately 90 % of brain slices from SAH animals in response to EFS, with 40 % exhibiting a sustained vasoconstriction, 30 % exhibiting a transient vasoconstriction (diameter restored within 1 min after EFS), and 20 % responded with a biphasic response (brief vasodilation followed by vasoconstriction). This inversion of neurovascular coupling may play a role in the development of neurological deficits following SAH.
Keywords: Subarachnoid hemorrhage, neurovascular coupling, cerebral artery, parenchymal arteriole, astrocyte, microcirculation, cortical brain slice, vasospasm
Introduction
Neurovascular coupling forms the basis of functional hyperemia, whereby localized neuronal activity leads to vasodilation and increased blood flow to enhance the supply of oxygen and nutrients to active neurons. This physiologically crucial mechanism is mediated by the coordinated activity of neurons, astrocytes and parenchymal arterioles. During neurovascular coupling, neurally released glutamate activates astrocytic metabotropic glutamate receptors leading to an inositol triphosphate (IP3) receptor-mediated wave of intracellular Ca2+ release in astrocytes. Astrocyte endfeet completely encase parenchymal arterioles and evoked increases in endfoot Ca2+ lead to the release of vasodilatory substances (e.g. vasodilatory arachidonic acid metabolites, K+ efflux from large-conductance Ca2+-activated K+ channels) from astrocytic endfeet onto parenchymal arterioles [1, 4, 7, 23].
For decades, angiographycally detected cerebral vasospasm was considered the major cause of delayed ischemic neurological deficits following SAH [11, 21]. However, recent studies suggest that prevention of angiographycally defined cerebral vasospasm did not improve outcome in SAH patients [15, 16]. Perfusion deficiencies within the microcirculation, which cannot be resolved using conventional angiography, may be one mechanism contributing to delayed ischemic neurological deficits following SAH [8, 9, 14, 21]. Restricted blood flow through intracerebral/parenchymal arterioles could result from microthrombi [24], vasoconstriction due to extravascular blood or blood breakdown products [19, 20], or impaired neurovascular communication [2].
In this study, we examined the impact of SAH on neurovascular coupling using a rat double-injection SAH model. Here, we report that SAH converts neurovascular coupling from vasodilation to vasoconstriction. Local vasoconstriction in response to neuronal activity would promote a decrease in local blood flow and could potentially compromise neuronal viability.
Materials and Methods
Rat SAH model
Sprague-Dawly rats (males, 10–12 weeks old) were used in accordance with the Guide for the Care and use of Laboratory Animals (NIH Pub. No. 85-23, revised 1996) following protocols approved by the Institutional Animal Use and Care Committee of the University of Vermont. Autologous and unheparinized arterial blood (0.5 mL) was injected twice into the cisterna magna of anesthetized animals at an interval of 24 hours [20]. Animals were euthanized by decapitation under the deep anesthesia (pentobarbital, 60 mg/kg, i.p.) 4 days after the initial blood injection.
Brain slice experiments
Cortical brain slices (coronal sections, 160 μm thick) were prepared from the territory of the middle cerebral artery using a vibratome (VT1000S, Leica), and then loaded with the Ca2+ indicator dye fluo-4 (10 μM, acetoxymethyl ester) with 0.05 % pluronic acid at 29 °C for 1 hr. Parenchymal arteriolar diameter and astrocytic endfoot Ca2+ were simultaneously imaged (~1 Hz) using a BioRad Radiance 2100 MP dedicated multiphoton imaging system. To mimic in vivo conditions, brain slices were superfused with artificial cerebrospinal fluid containing U46619 (100 nM) to induce a modest level of arterial tone. Electrical field stimulation (EFS, 50 Hz; 0.3 ms duration alternating square pulse for 3 seconds) with a pair of platinum wires placed parallel to the brain slice was used to stimulate neurons and induce an elevation in astrocytic endfoot Ca2+. Arteriolar diameter was determined by averaging the internal diameter obtained from three points along the arteriole on a given image, and expressed as percent change from the diameter recorded prior to EFS (first image of the recording). Astrocyte endfoot Ca2+ was quantified using the maximal fluorescent method [5]. All experiments were conducted at 37 °C.
Results
In the vast majority (52 of 53) of brain slices from un-operated control animals, neuronal activation by EFS elicited the predicted elevation in astrocyte endfoot Ca2+, followed by parenchymal arteriole vasodilation. In about half of these control brain slices (28/53 brain slices), the vasodilation to EFS was transient in nature, with arterioles returning to their pre-stimulation diameter within 1 minute after EFS. In an additional 19 brain slices from control animals, parenchymal arterioles showed sustained (> 1 minute) vasodilation to EFS. The remainder of brain slices from control animals (5/53 brain slices, 9.4 %) exhibited “biphasic” vascular responses; brief/transient dilation followed by constriction. In one brain slice from a control animal, EFS evoked only vasoconstriction.
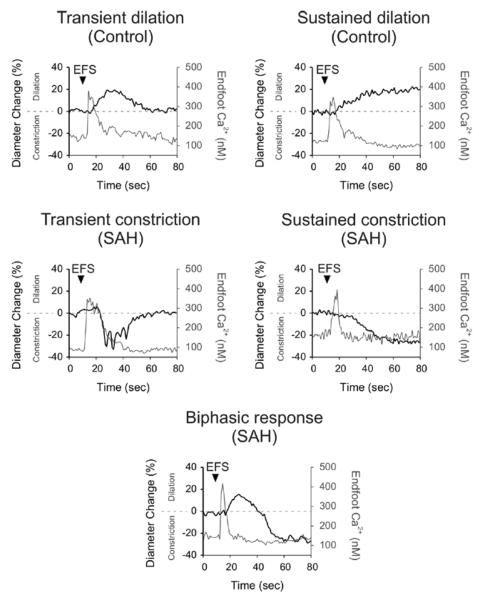
In marked contrast, EFS caused parenchymal arteriolar vasoconstriction in over 90 % of brain slices (54/59) from SAH animals. This EFS-induced vasoconstriction after SAH was transient in 17 out of 59 brain slices, and sustained in 25 out of 59 brain slices. In 12 out of 59 brain slices, parenchymal arteries showed a “biphasic” vascular response (brief vasodilation followed by vasoconstriction). The remainder of brain slices (5 out of 59) from SAH animals responded with vasodilation after EFS (transient: 3 brain slices, sustained: 2 brain slices). These observations demonstrate an inversion in EFS-induced arteriolar response from dilation to constriction in brain slices from SAH model animals (table). Interestingly, despite the dramatic SAH-induced change in the polarity of the neurovascular response, EFS-induced increases in astrocyte endfoot Ca2+ that preceded vasodilation (control animals) and vasoconstriction (SAH model animals) were remarkably similar (figure 1).
Table.
Summary of EFS-induced arteriolar responses.
| cont |
SAH |
|||
|---|---|---|---|---|
| Dilation-transient | 52.8% | ( 28 /53 ) | 5.1% | ( 3 /59 ) |
| Dilation-sustained | 35.8% | ( 19 /53 ) | 3.4% | ( 2 /59 ) |
| Biphasic response | 9.4% | ( 5 /53 ) | 20.3% | ( 12 /59 ) |
| Constriction-transient | 1.9% | ( 1/53) | 28.8% | ( 17 /59 ) |
| Constriction-sustained | 0.0% | ( 0/53) | 42.4% | ( 25 /59 ) |
| slices | ||||
Figure 1. EFS-induced parenchymal arteriolar responses in rat cortical brain slices.
Parenchymal arteriolar diameter and astrocyte endfoot Ca2+ concentration were simultaneously measured using two-photon microscopy. Increased neuronal activity evoked by electrical field stimulation (EFS) caused an elevation in astrocyte endfoot Ca2+, leading to several patterns of arteriolar responses. Representative traces of changes in arteriolar diameter and astrocyte endfoot Ca2+ concentration are shown in brain slices obtained from control and SAH animals. Responses were defined as “transient” if arteriolar diameter returned to baseline within 1 minute after EFS. Arteriolar responses that were maintained for 1 minute after EFS were counted as “sustained” responses. Recordings exhibiting both dilation and constriction were classified as “biphasic” responses. All “biphasic” responses showed an initial brief dilation followed by a sustained constriction. Transient dilations and sustained dilations were observed in brain slices from control animals. Traces of transient constriction, sustained constriction and biphasic response were recorded from brain slices from SAH model animals. All patterns of arteriolar responses were observed after similar increases of astrocytic endfoot Ca2+ evoked by EFS.
Discussion
Our observations demonstrate a fundamental change in neurovascular coupling following SAH. In over 90 % of cortical brain slices from SAH model animals, evoked neuronal activity caused an elevation in astrocytic endfoot Ca2+ followed by vasoconstriction. This vasoconstriction observed after SAH is in marked contrast to neurally evoked vasodilation typically observed in brain slices from control animals. Vasodilation is the predicted physiological response to increased neuronal activity and is the basis of functional hyperemia. On the other hand, vasoconstriction to neuronal activity represents a pathological response that could severely limit blood flow to active neurons. Our work is consistent with recent in vivo and clinical studies that have reported decreased cerebral blood flow in response to global neuronal hyperactivity under certain pathological conditions such as ischemic depolarizations in mice [22] and cortical spreading depression in human aneurismal SAH patients [3]. Our studies suggest that SAH can also induce more subtle changes in brain function that have a profound impact on neurovascular coupling.
The majority of neurovascular coupling studies, obtained using healthy animals, have reported neurally evoked vasodilation, consistent with functional hyperemia. However, a few reports have demonstrated that neuronal activation and elevated astrocytic endfoot Ca2+ can, under certain conditions, lead to parenchymal arteriolar constriction in healthy “control” animals [5, 6, 17]. Several mechanisms have been found to underlie neurally evoked vasoconstriction in brain slices from control animals, including production of the vasoconstrictor, 20-hydroxyeicosatetraenoic acid [17], excessive release of K+ from large conductance Ca2+-activated K+ channels located on astrocyte endfeet caused by abnormally high levels of endfoot Ca2+ [5] and hyperoxia-induced decrease of vasodilators, prostagrandin E2 and adenosine [6]. Our unpublished observations suggest the above mechanisms are not involved in neurally evoked vasoconstriction in brain slices from SAH animals.
We have previously reported that SAH can induce reactive astrogliosis, leading to altered astrocyte structure and function [18]. The cellular mechanisms linking SAH to altered astrocyte function and inversion of neurovascular coupling are currently under investigation and may involve an elevation in perivascular potassium (Koide and Wellman, unpublished observations). Inversion of neurovascular coupling may act in concert with SAH-induced enhanced constriction of pial arteries [10, 12, 13, 25] and parenchymal arterioles [20] to induce ischemic neuronal damage. In summary, we report the conversion of neurovascular coupling from vasodilation to vasoconstriction in brain slices from SAH models. This phenomenon may play an important role in the development of cortical infarcts following cerebral aneurysm rupture.
Acknowledgement
The authors acknowledge the University of Vermont Neuroscience COBRE molecular biology and imaging core facilities. This work was supported by the Totman Trust for Medical Research, the Peter Martin Brain Aneurysm Endowment and the NIH (P01 HL095488, R01 HL078983, R01 HL078983-05S1 and R01 HL044455).
Footnotes
Conflict of interest statement: None.
References
- [1].Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17:439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- [3].Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, Tolias C, Oliveira-Ferreira AI, Fabricius M, Hartings JA, Vajkoczy P, Lauritzen M, Dirnagl U, Bohner G, Strong AJ. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132:7–81. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- [5].Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, Macvicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gordon GR, Mulligan SJ, Macvicar BA. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- [8].Hansen-Schwartz J, Vajkoczy P, Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm: looking beyond vasoconstriction. Trends Pharmacol Sci. 2007;28:252–256. doi: 10.1016/j.tips.2007.04.002. [DOI] [PubMed] [Google Scholar]
- [9].Iadecola C. Bleeding in the brain: Killer waves of depolarization in subarachnoid bleed. Nat Med. 2009;15:1131–1132. doi: 10.1038/nm1009-1131. [DOI] [PubMed] [Google Scholar]
- [10].Ishiguro M, Puryear CB, Bisson E, Saundry CM, Nathan DJ, Russell SR, Tranmer BI, Wellman GC. Enhanced myogenic tone in cerebral arteries from a rabbit model of subarachnoid hemorrhage. Am J Physiol Heart Circ Physiol. 2002;283:H2217–H2225. doi: 10.1152/ajpheart.00629.2002. [DOI] [PubMed] [Google Scholar]
- [11].Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–572. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- [12].Koide M, Nystoriak MA, Krishnamoorthy G, O'Connor KP, Bonev AD, Nelson MT, Wellman GC. Reduced Ca2+ spark activity after subarachnoid hemorrhage disables BK channel control of cerebral artery tone. J Cereb Blood Flow Metab. 2011;31:3–16. doi: 10.1038/jcbfm.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Koide M, Penar PL, Tranmer BI, Wellman GC. Heparin-binding EGF-like growth factor mediates oxyhemoglobin-induced suppression of voltage-dependent potassium channels in rabbit cerebral artery myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H1750–H1759. doi: 10.1152/ajpheart.00443.2007. [DOI] [PubMed] [Google Scholar]
- [14].Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31:17–35. doi: 10.1038/jcbfm.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, Vajkoczy P, Wanke I, Bach D, Frey A, Marr A, Roux S, Kassell N. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2) Lancet Neurol. 2011;10:618–625. doi: 10.1016/S1474-4422(11)70108-9. [DOI] [PubMed] [Google Scholar]
- [16].Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, Frey A, Roux S, Pasqualin A. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–3021. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- [17].Mulligan SJ, Macvicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- [18].Murakami K, Koide M, Dumont TM, Russell SR, Tranmer BI, Wellman GC. Subarachnoid Hemorrhage Induces Gliosis and Increased Expression of the Pro-inflammatory Cytokine High Mobility Group Box 1 Protein. Transl Stroke Res. 2011;2:72–79. doi: 10.1007/s12975-010-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nishizawa S, Laher I. Signaling mechanisms in cerebral vasospasm. Trends Cardiovasc Med. 2005;15:24–34. doi: 10.1016/j.tcm.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [20].Nystoriak MA, O'Connor KP, Sonkusare SK, Brayden JE, Nelson MT, Wellman GC. Fundamental increase in pressure-dependent constriction of brain parenchymal arterioles from subarachnoid hemorrhage model rats due to membrane depolarization. Am J Physiol Heart Circ Physiol. 2011;300:H803–H812. doi: 10.1152/ajpheart.00760.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pluta RM, Hansen-Schwartz J, Dreier J, Vajkoczy P, Macdonald RL, Nishizawa S, Kasuya H, Wellman G, Keller E, Zauner A, Dorsch N, Clark J, Ono S, Kiris T, Leroux P, Zhang JH. Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res. 2009;31:151–158. doi: 10.1179/174313209X393564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26:1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- [23].Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- [24].Vergouwen MD, Vermeulen M, Coert BA, Stroes ES, Roos YB. Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1761–1770. doi: 10.1038/jcbfm.2008.74. [DOI] [PubMed] [Google Scholar]
- [25].Wellman GC. Ion channels and calcium signaling in cerebral arteries following subarachnoid hemorrhage. Neurol Res. 2006;28:690–702. doi: 10.1179/016164106X151972. [DOI] [PubMed] [Google Scholar]