Abstract
The Szabolcs Lab is focused on understanding the biology of donor-derived cellular immunity in recipients of allogeneic hematopoietic cell transplantation (HCT) that can be translated into new immunotherapy strategies. To this end, we are focused on developing novel laboratory approaches to analyze and augment immune recovery for high risk patient cohorts without increasing graft versus host disease (GVHD). Much of our work has focused on unrelated cord blood transplantation (UCBT) as the dominant clinical scenario and laboratory model. Our overarching goal is to minimize transplant related mortality and morbidity and render HLA-mismatched unrelated cord blood transplant a widely accepted safe cellular therapy. Donor leukocyte infusions (DLI) in the allogeneic hematopoietic transplant setting can provide a clinically relevant boost of immunity to reduce opportunistic infections and to increase graft-versus-leukemia activity. Our Lab has a major focus towards ex-vivo expansion of cord blood T cells with anti-apoptotic cytokines and CD3/CD28 co-stimulatory beads. Expanded lymphocytes lack alloreactivity against recipient and other allogeneic cells indicating a favorable safety profile from graft-versus-host disease. Nevertheless, expanded T cells can be primed subsequently against lymphoid and myeloid leukemia cells to generate tumor-specific cytotoxic T cells. These findings offer a major step in fulfilling critical biological requirements to quickly generate a cellular product ex vivo, using a negligible fraction of a cord blood graft that provides a flexible adoptive immunotherapy platform for both for children and adults.
Keywords: cord blood, transplantation, lymphopenia, plasmacytoid dendritic cells, CD4+ T lymphocytes, ex vivo expansion, donor leukocyte infusion (DLI), graft-versus-host disease (GVHD), graft-versus-leukemia (GVL)
INTRODUCTION
Cord blood as a transplantable hematopoietic graft was first used in 1989 for a child with severe Fanconi anemia (1). Since 1993, when the first ever unrelated cord blood transplant (UCBT) was performed at Duke University (2), >20,000 cord blood (CB) transplants are estimated to have been performed worldwide (3). The increasing use of cord blood in the unrelated donor setting is partially explained by the following significant findings. UCBT apparently offers a similar (4) or reduced risk (5) of severe graft-versus-host-disease (GVHD) permitting the use of 1–2 MHC/HLA allele mismatched grafts, reviewed in (6). Despite the increased experience with this unique hematopoietic graft transplant related mortality is typically between 20–40% in the first 6 months. Of the possible causes, infection-related mortality (IRM) is the primary or secondary cause of death (with or without another major cause such as GvHD) in ≥ 50% of deaths after UCBT with the majority of them occurring in the first 100 days (7–10). Needless to say, inadequate numerical and/of functional immunity is the cause of these deaths.
The Szabolcs Lab has been interested in understanding the biology of immune reconstitutions in this unique MHC-mismatched setting where tolerance is eventually achieved in all survivors, typically ~12–24 months after UCBT permitting discontinuation of all immunosuppressive (IS) medications. In the cord blood graft itself, significant numbers of immune cells are also transferred including 1–10 million/kg CD3+ T cells along with long term repopulating stem cells. One of our major hypothesis is that the fate of these adoptively transferred lymphocytes in an inflamed pancytopenic host (after radio/chemotherapy) will have great impact on infectious morbidity, alloreactivity, and overall survival in the immediate and coincidentally most vulnerable post-transplant period. Typically, thymopoiesis does not recover until 3–6 months after transplantation regardless of the graft source.
PREVIOUSLY DESCRIBED FEATURES OF IMMUNITY AFTER CORD BLOOD TRANPLANTATION
The tempo of cellular recovery is quite variable after UCBT. Although mitogenic responses may reach normal range in children 6–9 months after UCBT, numerical T cell reconstitution is gradual and typically does not reach age appropriate numbers before 9–12 months. This is still much earlier than what is experienced with adult patients, where T cell reconstitution typically extends beyond the first year, presumably as a result from age-dependent decline in pre-transplant thymic function (11). While the incidence of life threatening viral infections is high in the first 6 months after UCBT suggesting deficits in T cell numbers and/or function, when monitored beyond 9 months post-transplant the speed of T cell recovery seems to be at least comparable (12) to or even better than that seen after unrelated bone marrow transplant (BMT), (13–15). Likely, the smaller inoculum of lymphocytes, dendritic cells, etc, per kg body weight will contribute to the numerical deficits. The impact of damage suffered by secondary lymphoid organs after chemo and radiation therapy remains unknown. Extremely severe T cell lymphopenia may extend throughout the first year (16). Notably, NK cell recovery is prompt both in numbers and function in both adults and children by the first 2 months, comparable to recipients of bone marrow grafts (13, 17). Significant B lymphocyte recovery and immunoglobulin production starts ~3–4 months after transplant that may reach normal numbers by 6–9 months (18).
It has been convincingly demonstrated that attaining a vigorous cellular immune response in particular against herpes viruses, the most common viral pathogens isolated in the first 3 months, favorably impacts survival after UCBT. Investigators from the Cord Blood Transplantation Study (COBLT) analyzed antigen-specific proliferation after UCBT (19). Children with malignancies were longitudinally tested over the first 3 years post transplant for herpes virus specific responses (HSV, VZV, CMV). Approximately 43% of the patients studied eventually developed a positive T-lymphocyte proliferative response to at least one herpes virus at some point over the 3 year observational period. In a few, proliferative responses developed as early as within the first 30–50 days, indicating that naïve, antigen-inexperienced T lymphocytes transferred in the graft can give rise to antigen-specific T-lymphocyte immunity before thymic recovery(19). Significantly, patients with a proliferative response at any time in the first 3 years to any of the herpesviruses had a lower probability of leukemia relapse and a higher overall survival (19). One may speculate that the superior proliferative T cell response represents a powerful surrogate marker for functional immune reconstitution lading to more effective graft-versus-leukemia (GVL) activity. However, the development and kinetics of protective antigen-specific function was not evaluable (20). These data underscore the high clinical relevance to design novel immunotherapy approaches aimed at alleviating post-transplant lymphopenia and the Th1/Tc1 functional deficits displayed by cord blood T cells infused in the graft.
Functional deficits in lymphocytes and dendritic cells present in cord blood grafts
CB contains significantly higher absolute numbers of T, NK, and B lymphocytes than adult peripheral blood (PB) or even marrow (14, 21–23). However, most UCB T cells infused with the graft are CD45RA+/CD62L+ ‘Recent Thymic Emigrants’ (RTE) with smaller TCRγδ and CD25 + subsets (23) than in adult blood or bone marrow. CB T cells have been even called ‘immature’ compared to bulk adult T cells due to their impaired capacity for cytokine production (24) and diminished lytic activity (25). While RTE predominate in cord blood it contains some antigen-experienced T cells, most notably those specific for maternal minor histocompatibility antigens (26). The reduced capacity of bulk cord blood mononuclear cells to secrete cytokines and lymphokines has been demonstrated to affect GM-CSF, M-CSF, IL-4, IL-8, IL-12, IL-15, and IL-18, reviewed in (27, 28). There is reduced expression of NFATc2 (nuclear factor of activated T cells c2, a critical transcription factor necessary for up-regulation of these and other cytokines known to amplify T-cell responses. The relative cytolytic deficiency of CB T cells is associated with absent expression of granzymes and perforin (29), essential for the control of viral and other pathogens. CD25+ T cells in cord blood are naturally occurring regulatory T cells (30) with potent suppressor function as opposed to peripherally activated CD25+ cells (31). Fewer UCB T cells display HLA-DR and CCR-5 activation markers, while the CD8+/CD57+/CD28− and CD8+/CD45RA+/CD27− ‘cytotoxic’, along with the ‘skin homing’ CLA+ T cell subsets are absent altogether (23). Compared with adult blood more cord blood T cells progress through cell cycle and enter apoptosis. However, unlike in adult PB the majority of proliferating Ki-67+ T cells in UCB retain a CD45RA+/RO−, CD69−, CD25−, HLA-DR− ‘resting’ phenotype (23, 32). Unlike in adult blood there is also significant expression of telomerase in CB T cells (32). In contrast with T cells, CB NK cells are functionally “mature” with comparable or better lytic activity than their bone marrow derived counterparts (33). Not surprisingly, ‘naïve’ B lymphocytes are in excess in CB with an abundance of CD5+ B1 cells and CD23− immature B cells (23, 34).
Placental factors shape the functional profile of fetal T cells and dendritic cells
Despite an overlapping CD45RA+/CD28+/CD27+ phenotype CB T cells are fundamentally different from ‘naïve’ adult T cells mostly because they demonstrate a relative Th2/Tc2 bias. Multiple tiers of Th2/Tc2 biasing factors exist at the maternal-fetal interface; IL-10 secreted by cytotrophoblasts (35), reduced local Tryptophan levels (36), increased progesterone levels (37) and other placental factors (IL-4, PGE2), reviewed in (38 ). This immunoregulatory network could be viewed as part of an evolutionary adaptation to permit survival and avoid rejection of the fetus. Fas-ligand expression at the maternal-fetal interface may eliminate T cells activated despite these factors above (39). The exuberant production of IL-13 primarily by CD8+ T cells (40) is in sharp contrst with lower IFNγ production that persists even after stimulation via CD3/CD28 signaling and exogenous IL-2 (40). This is a consequence of differential patterns of methylation of the IFNγ promoter (41). Independently, impaired APC function of neonatal/cord blood dendritic cells (DC) restricts the potential for optimal Th1 cell responses in neonates due to their low IL-12 expression (42–44). Despite these limits there is evidence that intrauterine viral infections e.g. CMV could generate partial Th1 immune responses (38), though persistent and selective deficiency of antiviral Th1 CD4+ T cell is documented into early childhood (45).
IMMUNE RECONSTITUTION STUDIES BY THE SZABOLCS LAB AFTER HLA-MISMATCHED CORD BLOOD TRANSPLANTATION
Patient and graft specific factors predict the risk of death from opportunistic infections (OI) in the first 6 months after UCBT
Over the past several years Szabolcs lab has studied the reconstitution of immunity in the immediate post-UCBT period (prior to thymic recovery) in >150 pediatric recipients of single unit UCB at Duke University to identify surrogate immune markers for those at risk for OI.
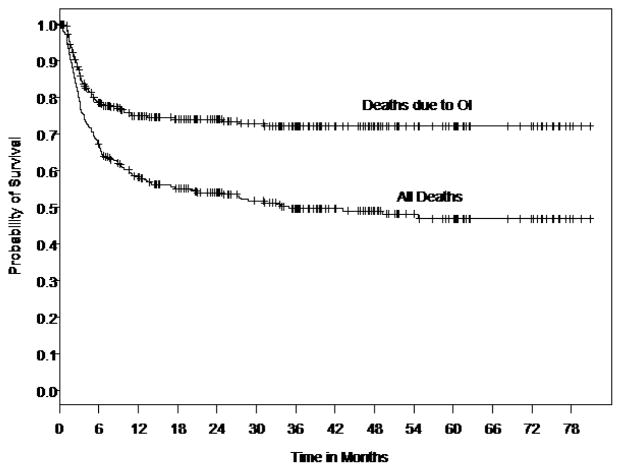
Several graft and patient-specific variables were also identified as significant factors when the laboratory measurements of DC and T cell reconstitution were analyzed. To determine the impact of patient and graft-specific factors on 6-month post-UCBT OI-related mortality we reviewed all consecutive pediatric UCB recipients transplanted at Duke University Medical Center between June 1999 and Oct. 2005 to overlap with the immune monitoring studies (46). Three hundred thirty (330) pediatric recipients of single UCB grafts were identified. Those receiving a second transplant for primary graft failure were excluded. Two hundred twenty (220) of the 330 patients (67%) were alive at 6 months, Figure 1. Of those who died by 6 months, (58%) were identified with OI (viral, fungal, protozoal infections) implicated as a cause of death (Fig. 1). Those who died prior to 6 months and for whom OI was not implicated as a cause of death were omitted from the study dataset, resulting in 284 patients.
Figure 1. Kaplan-Meier curve of survival (months) after UCBT in 330 consecutive pts.
Death related to opportunistic infections (OI )is the major cause of failure, most occurring by 6 months. Reproduced with permission from Cytotherapy c2007, Informa Healthcare Journals
Multivariate modeling revealed that a significantly greater probability of 6-month OI-related death was associated with CMV positive serology, greater HLA mismatch, and older age. Higher total graft cell dose, including CD34+ progenitor cell dose and CD3+ cell dose were each associated with lower probability of death due to OI at 6 months (47). Here we demonstrated for the first time the protective immunity afforded by expansion and functional contribution of post-thymic T cells infused with the graft prior to the recovery of the “central” de novo thymic pathway. We are in the midst of analysis of longitudinal monitoring of dendritic cell and lymphocyte recovery during the first 2 years after UCBT with emerging protecting influence of Tregs in the first 6 months coupled with the protective effects of thymus regeneration at the 6 months time point and CD123+ plasmacytoid dendritic cell recovery, data not shown.
Dendritic and T cell subsets at Day +50 after UCBT serve as surrogate markers of protection from OI
To identify patients who were at increased risk for developing OI in the first 100 days a prospective cross-sectional study has been conducted at ~day + 50 post-UCBT, with the latest analysis extended to 111 patients (47). Utilizing Trucount™ methodology (23, 48, 49) 4-color surface and intracellular (ic) FACS was employed to accurately enumerate and characterize lymphocyte along with myeloid and plasmacytoid DC subsets.
All patients received myeloablative conditioning regimes, (TBI/CY, Bu/CY, Bu/MEL, TBI/MEL) and equine ATG at 30mg/kg/day between day-3 to day-1. All received identical GvHD prophylaxis consisting of Cyclosporine A plus steroids, slowly tapered after day+21 in the absence of ≥grade II aGvHD). Varriable degree of cellular reconstitution was noted for most immune cells except for the striking absence of B lymphocytes despite millions infused/kg during transplant.
Table I. lists those immune parameters that remain significant predictors for the presence of de novo developed OI. Figure 2. shows that individuals that develop OI by day +100 have a significantly reduced probability of overall survival (Fig. 2A) and that death due to OI is related to Grade III/IV GvHD (Fig. 2B). Based on these data (50), and also on data not shown, we hypothesize that the increased prevalence of CD8+ T cells expressing/secreting HLA-DR, IFNγ, Granzymes A, B, Perforin represent an effort by the emerging immune system to control the infectious agent. These changes accompany down regulation of CD28 and CD27 expression along with CD57 upregulation thus represent an evolution towards effector phenotype and cytotoxic function. Along with the skewing of the T cell profile, significantly fewer CD123+ plasmacytoid/lymphoid DC circulate in those with infection (p=0.007) demonstrating that antigen presenting cell deficiency occurs along with lymphocyte alterations (47).
Table I.
Continuous variables of immunity associated with OI incidence in the first 100 days. Measurements in the “Day + 50 study” group
| Variable | Median Value For Patients with OI | Median Value For Patients without OI | Logistic Regression (P-value) |
|---|---|---|---|
| Abs # CD4+ T Cells (cell #/μl) | 44 | 137 | 0.02 without age in model |
| % CD8+ T Cells | 44 | 14 | <0.0001 |
| % CD57+/CD28−/CD8+ T cells | 9 | 3 | <0.02 |
| % CD25+/CD3+T Cells | 22 | 40 | <0.016 |
| % TCRγδ T cell subset | 2.3 | 0.97 | <0.017 |
| % ‘activated’ HLA-DR+ T cells | 53 | 38 | <0.009 |
| % ‘NKT’ CD3+/CD56+ T cells | 8 | 4 | <0.01 |
| % IFNγ Secreting T cells | 18 | 4 | <0.006 |
Confounders tested: Race, age, gender, weight, CMV status, HLA mismatch, malignancy, TBI, GvHD, High Dose steroid pulse (yes, no), Anti-CD25/Daclizumab pulse (yes, no), infused total cell dose/kg, CD34+ cell/kg, CD3+ T cell dose/kg.
Figure 2.
Fig. 2A.) Time to death from all causes in the “Day 50” cohort by Opportunistic Infection status. Fig. 2B.) Time to death from OI by presence or absence of severe GvHD. Reproduced with permission from Cytotherapy c2007, Informa Healthcare Journals
Circulating effector T cells ~day+20 after UCBT, can predict those at risk for OI
In a technically more challenging study (47), we aimed to gain insight into the fate and maturational biology of adoptively transferred naive T cells in the lymphopenic hosts even prior to the onset of OI to develop predictive models for OI incidence in the first 100 days. Blood was obtained at a median 18 days post-UCBT if the WBC exceeded 400 cells/mm3. Circulating T-cell subsets and DC counts were monitored. We have analyzed seventy six (76) patients at a median age of 62 months with at least 12 months follow-up. Forty four patients (58%) presented de novo with OI (>90% viral) at a median of 35 days. Both the OI+ and OI− patient cohorts had low but equivalent absolute WBC, CD3+, CD4+ T cells, and NK lymphocytes. DC subsets were largely undetectable. Strikingly, ~40% of circulating T cells were proliferating (Ki-67+), regardless of OI status, reflecting vigorous peripheral expansion reducing the CD45RA+/CD62L+ RTE pool to <20% from >90% infused in the graft only 2–3 weeks earlier (23). While most cells (67% ± 27%) expressed a ‘memory like’ CD45RA−/CD45RO+ phenotype, a significant population (14±28%) co-expressed CD45RA and RO. The robust T cell expansion was accompanied by upregulation of markers of activation with a median of 66% of T cells HLA-DR+. Interestingly, ~10% of the circulating T cells were entering apoptosis (ic activated Caspase-3+), regardless of OI status. In those who developed OI, significantly higher proportion of the circulating T cells were CD8+ (40% vs. 28%, p=0.04), expressed CCR-5 (82% vs. 55%, p=0.009), were secreting IFNγ (35% vs. 12%, p=0.01), and acquired a CD57+/CD28− ‘effector CTL’ phenotype. In patients developing OI significantly more Perforin+/CD8+ T cells circulated (48% vs. 26%, p=0.02).
In conclusion, in the immediate post-transplant lymphopenic period extensive T cell proliferation via peripheral expansion leads to major immunophenotypic alterations accompanied by a gradual loss of the original naïve phenotype. In parallel, new T cell subsets emerge displaying a phenotype associated with antigenic stimulation (51). We hypothesize that in patients who will develop OI, even clinically undetectable levels of virus could induce phenotypic acquisition of Th1/Tc1 cytotoxic effector profile.
CORD BLOOD T CELL EXPANSION IN VITRO WITH TH1 TC1 MATURATION; PRECLINICAL RESEARCH TOWARDS ADOPTIVE T CELL IMMUNOTHERAPY
Recently, we and others have demonstrated the feasibility of ex vivo CB T cell expansion (52, 53), drawing from the pioneering work by June et al. (54) utilizing paramagnetic Dynal beads coated with anti-CD3 and anti-CD28 stimulatory antibodies. These artificial antigen presenting cells (APC) simultaneously provide agonistic TCR and co-stimulatory signals triggering sufficient T cell proliferation in vitro to generate clinically relevant DLI products from living donors (55–57). Although in our previous work (52), robust T cell expansion and even partial Th1/Tc1 maturation was evident starting with frozen/thawed CB specimens, significant apoptosis (~16%) resulted in an inverted CD4/CD8 ratio and diminished yield despite relatively low concentrations of IL-2 in the medium. The high degree of apoptosis was likely the result of activation induced cell death (AICD) following strong TCR signaling on CB T cells as previously described (58). Infusion of overstimulated, apoptosis prone DLI product would likely lead to a narrow T cell repertoire and shortened T cell survival in vivo. Moreover, it could falsely suggest futility of ex vivo expanded DLI strategies.
In the current study (59), we tested and confirmed our hypotheses, that interleukin-7 (IL-7) acting in concert with a new, clinical grade CD3/CD28 costimulatory bead and IL-2, would not only enhance ex vivo CB T cell proliferation while retaining a broad TCR repertoire as predicted (60), but it would also reduce activation induced cell death.
Favorable impact of IL-7 on CB T cell survival, proliferation, and TCR Vβ repertoire during CD3/28 mediated expansion
Purified T cells obtained from frozen/thawed cord blood specimens were split and cultured in parallel with and without IL-7. Matched pair analysis demonstrated significantly more viable T cells when IL-7 was added to IL-2 in the medium leading to an average of 165 fold T cell expansion (Figure 3A) Following 14 days of expansion, striking dilution of TCR excision circles was noted as the sjTREC content in CD3+ T cells was depleted by ~ 2log in both culture conditions as compared to the starting population of pre-expansion cord blood T cells (Figure 3B), irrespective of IL-7 exposure. Significantly more viable CD45 bright T lymphocytes were identified in cultures supplanted with IL-7 (71% ±10) compared to cultures with IL-2 alone (mean 46% ±15) As determined by ic activated Caspase-3 expression and 7-AAD staining, there were significantly fewer T cells undergoing active apoptosis in the presence of IL-7 (median 4% versus 8%) (Fig. 4B. The anti-apoptotic effect if IL-7 was evident in both CD4+ and CD8+ subsets. To test T cell survival promoting effects of IL-7 beyond the in vitro expansion period, expanded cells were frozen on Day 14 and subsequently thawed and rested for 24h in culture medium devoid of cytokines. Although the rest period in vitro can not mimic the in vivo post-infusion conditions exactly, these experiments demonstrate that T cells post expansion retain the potential to up-regulate IL-7 receptor/CD127 (Fig. 34A), and that the majority of IL-7 + IL-2 expanded T cells are still alive after freeze, thaw, and 24 hour culture in medium (Fig. 4B). Independent of the described anti-apoptotic effects, IL-7 promoted significantly greater T cell proliferation. About 2/3rd of all T cells were still actively cycling at the termination of the expansion period, as detected by intracellular Ki-67 expression, data not shown. Since naïve T cells with recent thymic emigrant phenotype (CD28+/CD27+CD45RA+/CD62L+) represent the vast majority of unmanipulated CB T cells, these findings corroborate earlier studies demonstrating the proliferative and anti-apoptotic effects of IL-7 to be operational predominantly in the naïve/CD45RA+ T cell compartment (60, 61). In addition to superior T cell proliferation and reduced apoptosis in IL-7 supplanted conditions, we also found higher TCRVβ diversity per family (p=0.04, n=3) displaying a broad polyclon al spectrum (59).
Figure 3.
(A) CD3+ T cell expansion is superior in the presence of IL-7. Frozen/thawed cord blood T cells were enriched by negative selection then split equally into two under identical culture conditions except for the presence of IL-7 as indicated. cell were cultured for 12—14 days with ClinExVivo™ Dynabeads® while medium and cytokines were replenished x 3/week. A 50ul aliquot was removed from the bags at indicated time points and absolute T cells number was enumerated in Trucount® tubes. (B) Irrespective of IL-7 in the culture medium, expansion leads to dilution and near complete loss of sjTREC in day 14 progeny. The signal joint TCR excision circles (sjTREC) were measured before and after expansion, n=4.. For each sample total nucleated cell count and absolute T cells content was enumerated by Trucount FACS method. TREC content was expressed after adjustment for 105 T cells/sample. Reproduced with permission from Canceer Research ©2010,
Figure 4.

(A) Kinetic analysis of surface CD25 and CD127 expression. Simultaneous monitoring of IL-2Rα (CD25) and IL-7Rα (CD127) was performed after FACS surface staining and acquisition as described (23, 49) on serial aliquots obtained before (Day 0) and during expansion onn the indicated days. (B) Cell death after 24h of rest in cytokine free medium was assayed and scored by positive staining for Annexin and 7-AAD in parallel after freeze and thaw of expanded day 14 T cells, representative of four experiments. Reproduced with permission from Canceer Research ©2010,
Limited Th1/Tc1 ‘maturation’ during expansion and low expression levels of 4-1BB/CD137, CD40L, and perforin correlate with absent alloreactivity
Once we have demonstrated the salutary effects of IL-7 on T cell viability, expansion, and overall T cell receptor diversity, we sought to determine its impact on surface and intracellular phenotype and overall T cell function as measured by cytokine secretion profile and cytotoxicity. Despite undergoing several cycles of cell division triggered by IL-2 + IL-7 in concert with TCR and CD28 co-stimulation, significantly more CB T cells retained the naïve starting phenotype, CD45RA+/CD62L+ in the IL-7-containing condition (90% +/− 5%) compared to cells cultured in IL-2 alone (73% +/− 14%, p=0.03) (Fig. 2D). Surface expression of L-selectin (CD62L) is essential for effective T cell homing to secondary lymphoid organs, a desired destination for antigen inexperienced, unprimed adoptive T cell infusions. CCR-7, a chemokine receptor implicated in both the entry and also in the retaining of T cells in lymph nodes, was also expressed on the majority of expanded T cells, data not shown. Interestingly, while the surface of post-expansion T cells appeared identical to unmanipulated fresh cord blood T cells in terms of CD28+/CD27+/CD45RA+/CD62L co-expression, expanded T cells displayed several upregulated surface molecules commonly seen after activation, including CD25, HLA-DR, OX40. However, <10% of cells expressed CD40L. Despite the preservation of resting, naïve, ‘RTE-mimicking’ surface phenotype, as indicated by CD28+/CD27+/CD45RA+/CD62L co-expression, CD3/CD28 co-stimulation led to rapid down-regulation of membrane CD127 (IL7Rα) in parallel with surface CD25 (IL2Rα) up-regulation on the very same T cells (Fig. 3A). This “receptor switching process’ is not dependent on the presence of IL-7 in our cultures as CD25 and CD127 expression levels were superimposable in the presence and absence of IL-7 (Fig. 3A). Moreover, since a near complete reversal between CD25 and CD127 expression has occurred by ~24–48h of culture (Fig. 3A) this phenomenon appears independent of cell division. Numerical T cell expansion does not begin in earnest in the cultures until day-3–4, (Fig. 1, and data not shown). Interestingly, when expanded T cells were frozen on Day 14 and subsequently thawed and rested for 24h in culture medium devoid of cytokines, CD127 was re-expressed on nearly half of the viable T cells (Fig. 4A). IL-7 receptor re-expression could permit delivery of pro-survival signals to expanded T cells administered by clinical DLI infusions in the lymphopenic post-transplant state where endogenous IL-7 level has been demonstrated to be elevated up to 10–30 pg/ml weeks after transplant (62). Although it is possible that high levels of IL-7 in vivo could induce down-regulation of CD127 in the responding T cells, nevertheless our results suggest that the expanded T cells retain the capacity to re-express CD127 even when rested post-thaw with IL-7 at 15–30 pg/ml, data not shown.
CD3/CD28 co-stimulation with ClinExVivo™ Dynabeads® in this series of experiments enhanced in a larger fraction of post-expansion T cells the capacity for intracellular expression of IFNγ, TNFα, and Granzyme B (Table 1) than we previously reported using different artificial-APC beads (52). Nevertheless, despite the potential for an increase in alloreactivity (63) after the more robust expansion in the presence of IL-7, the expanded progeny lacked cytotoxicity against a highly immunogenic (CD40+, CD80+, CD86+) EBV+ allogeneic lymphoblastoid cell line (IM9) (n=7), or recipient fibroblasts (n=2), despite a week long pre-sensitization prior to performing the CTL assay (Fig. 5). Interestingly, absent cytotoxicity coincided with low expression of 4-1BB/CD137, CD40L, and perforin (Table I). Together, these features support a favorable safety profile of ‘day 14” ClinExVivo™ expanded T cells with reduced likelihood for inducing GVHD in vivo upon adoptive transfer.
Figure 5. Leukemia-specific CTL can be in vitro primed starting with the CD3/28-expanded CB T cells.
T cells were first CD3/28-expanded in the presence of IL2+ IL-7 over 14 days as described and thereafter were primed/sensitized against 2 killed leukemia cell lines in parallel cultures for 7–9 days at 10:1 responder: stimulator ratio in the presence of IL-12, IL-7, and IL-15. A, CTL primed in vitro with Mitomycin C-treated IM9 cells. B, CTL primed in vitro with IFNγ-treated and Mitomycin C-treated U937 cells. Each CTL culture was re-stimulated 2 more times (1st IL7+ IL-15, thereafter 2nd in IL15 alone) for a total of 3 weeks with their respective killed leukemia cells. Cytotoxicity of washed effectors after 3 weeks in CTL culture was tested against fresh, unmodified, BATDA®-loaded IM9, U937 cells, and recipient PHA blasts at the indicated E:T ratios for 3h, as indicated. Europium release was measured by the Delfia® EuTDA cytotoxicity assay and the calculated percent specific cytotoxicity is presented on the Y-axis. Reproduced with permission from Canceer Research ©2010
Ex vivo expanded, CD3/CD28-costimulated cord blood T cells can be primed in vitro against lymphoid and myeloid leukemia
Donor leukocyte infusion with ‘day 14’ ClinExVivo™ +IL-2 +IL-7 expanded T cells generated from the originally infused cord blood graft could alleviate post-transplant lymphopenia and qualitative T cell defects until thymic regeneration could contribute new T cells. However, such DLI would be antigen non-specific and will require microbial and/or tumor antigens to in vivo prime infused T cells in the transplant recipients. In a series of experiments we evaluated the potential of ‘day 14’ CD3/CD28-costimulated/expanded T cells to undergo in vitro priming against specific leukemic targets. In vitro generated tumor-specific CTL responses could be adoptively infused to treat leukemia patients with minimal residual disease and/or relapse. CD3/CD28-expanded ‘day 14’ T cells were stimulated vitro for 3 weeks in parallel cultures with killed, Mitomycin C treated lymphoid leukemia cells (IM9) and IFNγ-treated myeloid leukemia cells (U937). U937 cells by themselves can not induce allogeneic T cell response unless a stimulating anti-CD3 antibody is added to cultures (64). In addition, they do not provide co-stimulation via the CD80/CD86-CD28 pathway, but likely via the ubiquitously expressed CD147, CD98 molecules (64). CTL priming was performed in the decreasing presence of IL-12, IL7, and IL-15 drawing from our published experimental strategy to in vitro prime anti-viral responses from cord blood (65). Robust T cell expansion (195X, ±115, n=4) ensued over the course of ~ 3 weeks when killed leukemia cells rather than ClinExVivo™ beads served as APC. After the course of two to three repeated stimulations, strong leukemia-specific cytotoxicity was detected in CTL assays, killing the stimulating leukemia cells but not the other leukemia or most importantly cord blood transplant recipient PHA blasts, n=4, p=<0.01, (Fig. 5A and Fig. 5B). Failure to recognize and kill CB transplant recipient PHA blasts indicates future clinical safety from the potential toxicity of GVHD.
Acknowledgments
The author thanks Joanne Kurtzberg and Nelson Chao for their continued encouragement in these studies, Donna Niedzwiecki and Adam Mendizabal for expert statistical support, Melissa Reese, Luciana Marti, Antony Jeyaraj, Susan Buntz and Richard Vinesett for expert technical help. This work was supported in part by The National Marrow Donor Program-Marrow Transplant Research Grant (P. Sz.), 1PO1-HL-67314-01A1 (PI N. Chao), 1R01CA132110, and 1R01HL091749 (Multiple PI: P. Sz. & K. Komanduri)
References
- 1.Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321(17):1174–8. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 2.Kurtzberg J, Graham M, Casey J, Olson J, Stevens CE, Rubinstein P. The use of umbilical cord blood in mismatched related and unrelated hemopoietic stem cell transplantation. Blood Cells. 1994;20(2–3):275–83. [PubMed] [Google Scholar]
- 3.Gluckman E, Rocha V. Cord blood transplantation: state of the art. Haematologica. 2009;94(4):451–4. doi: 10.3324/haematol.2009.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker JN, Davies SM, DeFor T, Ramsay NK, Weisdorf DJ, Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001;97(10):2957–61. doi: 10.1182/blood.v97.10.2957. [DOI] [PubMed] [Google Scholar]
- 5.Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97(10):2962–71. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 6.Rocha V, Gluckman E. Improving outcomes of cord blood transplantation: HLA matching, cell dose and other graft- and transplantation-related factors. Br J Haematol. 2009;147(2):262–74. doi: 10.1111/j.1365-2141.2009.07883.x. [DOI] [PubMed] [Google Scholar]
- 7.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339(22):1565–77. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 8.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351(22):2276–85. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 9.Rocha V, Gluckman E. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006;12(1 Suppl 1):34–41. doi: 10.1016/j.bbmt.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Kurtzberg J, Carter SL, Baxter-Lowe LA, Feig SA, Guinan EC, Kamani NR, Kapoor N, Delaney C, Haut PR, Wall D, Kernan NA. Results of the cord blood transplantation study (COBLT): Clinical outcomes of 193 unrelated donor umbilical cord blood transplantation in pediatric patients with malignant conditions. [abstract] Biol Blood Marrow Transplant; 2005. 2005 Feb;2005:2. ( abst 6) [Google Scholar]
- 11.Klein AK, Patel DD, Gooding ME, et al. T-Cell recovery in adults and children following umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2001;7(8):454–66. doi: 10.1016/s1083-8791(01)80013-6. [DOI] [PubMed] [Google Scholar]
- 12.Thomson BG, Robertson KA, Gowan D, et al. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000;96(8):2703–11. [PubMed] [Google Scholar]
- 13.Moretta A, Maccario R, Fagioli F, et al. Analysis of immune reconstitution in children undergoing cord blood transplantation. Exp Hematol. 2001;29(3):371–9. doi: 10.1016/s0301-472x(00)00667-6. [DOI] [PubMed] [Google Scholar]
- 14.Broxmeyer HE American Association of Blood Banks. Cord blood: biology, immunology, and clinical transplantation. Bethesda, Md: AABB Press; 2004. [Google Scholar]
- 15.Koh LP, Chao NJ. Umbilical cord blood transplantation in adults using myeloablative and nonmyeloablative preparative regimens. Biol Blood Marrow Transplant. 2004;10(1):1–22. doi: 10.1016/j.bbmt.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Komanduri KV, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110(13):4543–51. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmi Z, Hommel-Berrey G, Smith F, Thomson B. NK cells recover early and mediate cytotoxicity via perforin/granzyme and Fas/FasL pathways in umbilical cord blood recipients. Hum Immunol. 2001;62(8):782–90. doi: 10.1016/s0198-8859(01)00275-0. [DOI] [PubMed] [Google Scholar]
- 18.Niehues T, Rocha V, Filipovich AH, et al. Factors affecting lymphocyte subset reconstitution after either related or unrelated cord blood transplantation in children -- a Eurocord analysis. Br J Haematol. 2001;114(1):42–8. doi: 10.1046/j.1365-2141.2001.02900.x. [DOI] [PubMed] [Google Scholar]
- 19.Cohen G, Carter SL, Weinberg KI, et al. Antigen-specific T-lymphocyte function after cord blood transplantation. Biol Blood Marrow Transplant. 2006;12(12):1335–42. doi: 10.1016/j.bbmt.2006.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parkman R, Cohen G, Carter SL, et al. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2006;12(9):919–27. doi: 10.1016/j.bbmt.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Han P, Hodge G, Story C, Xu X. Phenotypic analysis of functional T-lymphocyte subtypes and natural killer cells in human cord blood: relevance to umbilical cord blood transplantation. Br J Haematol. 1995;89(4):733–40. doi: 10.1111/j.1365-2141.1995.tb08409.x. [DOI] [PubMed] [Google Scholar]
- 22.D’Arena G, Musto P, Cascavilla N, et al. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica. 1998;83(3):197–203. [PubMed] [Google Scholar]
- 23.Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Coexistent naive phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Exp Hematol. 2003;31(8):708–14. doi: 10.1016/s0301-472x(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 24.Chalmers IM, Janossy G, Contreras M, Navarrete C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. 1998;92(1):11–8. [PubMed] [Google Scholar]
- 25.Risdon G, Gaddy J, Stehman FB, Broxmeyer HE. Proliferative and cytotoxic responses of human cord blood T lymphocytes following allogeneic stimulation. Cell Immunol. 1994;154(1):14–24. doi: 10.1006/cimm.1994.1053. [DOI] [PubMed] [Google Scholar]
- 26.Mommaas B, Stegehuis-Kamp JA, van Halteren AG, et al. Cord blood comprises antigen-experienced T cells specific for maternal minor histocompatibility antigen HA-1. Blood. 2005;105(4):1823–7. doi: 10.1182/blood-2004-07-2832. [DOI] [PubMed] [Google Scholar]
- 27.Suen Y, Lee SM, Qian J, van de Ven C, Cairo MS. Dysregulation of lymphokine production in the neonate and its impact on neonatal cell mediated immunity. Vaccine. 1998;16(14–15):1369–77. doi: 10.1016/s0264-410x(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 28.Bradley MB, Cairo MS. Cord blood immunology and stem cell transplantation. Hum Immunol. 2005;66(5):431–46. doi: 10.1016/j.humimm.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Berthou C, Legros-Maida S, Soulie A, et al. Cord blood T lymphocytes lack constitutive perforin expression in contrast to adult peripheral blood T lymphocytes. Blood. 1995;85(6):1540–6. [PubMed] [Google Scholar]
- 30.Takahata Y, Nomura A, Takada H, et al. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. 2004;32(7):622–9. doi: 10.1016/j.exphem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Godfrey WR, Spoden DJ, Ge YG, et al. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105(2):750–8. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- 32.Schonland SO, Zimmer JK, Lopez-Benitez CM, et al. Homeostatic control of T-cell generation in neonates. Blood. 2003;102(4):1428–34. doi: 10.1182/blood-2002-11-3591. [DOI] [PubMed] [Google Scholar]
- 33.Gardiner CM, Meara AO, Reen DJ. Differential cytotoxicity of cord blood and bone marrow-derived natural killer cells. Blood. 1998;91(1):207–13. [PubMed] [Google Scholar]
- 34.Harris DT, Schumacher MJ, Locascio J, et al. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci U S A. 1992;89(21):10006–10. doi: 10.1073/pnas.89.21.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth I, Corry DB, Locksley RM, Abrams JS, Litton MJ, Fisher SJ. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184(2):539–48. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 37.Szekeres-Bartho J, Faust Z, Varga P, Szereday L, Kelemen K. The immunological pregnancy protective effect of progesterone is manifested via controlling cytokine production. Am J Reprod Immunol. 1996;35(4):348–51. doi: 10.1111/j.1600-0897.1996.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 38.Marchant A, Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clin Exp Immunol. 2005;141(1):10–8. doi: 10.1111/j.1365-2249.2005.02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guller S, LaChapelle L. The role of placental Fas ligand in maintaining immune privilege at maternal-fetal interfaces. Semin Reprod Endocrinol. 1999;17(1):39–44. doi: 10.1055/s-2007-1016210. [DOI] [PubMed] [Google Scholar]
- 40.Ribeiro-do-Couto LM, Boeije LC, Kroon JS, et al. High IL-13 production by human neonatal T cells: neonate immune system regulator? Eur J Immunol. 2001;31(11):3394–402. doi: 10.1002/1521-4141(200111)31:11<3394::aid-immu3394>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 41.White GP, Watt PM, Holt BJ, Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO− T cells. J Immunol. 2002;168(6):2820–7. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- 42.Goriely S, Van Lint C, Dadkhah R, et al. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J Exp Med. 2004;199(7):1011–6. doi: 10.1084/jem.20031272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goriely S, Vincart B, Stordeur P, et al. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol. 2001;166(3):2141–6. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- 44.Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002;128(1):118–23. doi: 10.1046/j.1365-2249.2002.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tu W, Chen S, Sharp M, et al. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol. 2004;172(5):3260–7. doi: 10.4049/jimmunol.172.5.3260. [DOI] [PubMed] [Google Scholar]
- 46.Szabolcs PP, BL, Niedzwiecki D, Chao N, Kurtzberg J. Multivariate Analysis of Patient and Graft Specific Factors among 330 Recipients of Unrelated Cord Blood Transplant (UCBT) To Predict Risk of Death from Opportunistic Infections in the First 6 Months after UCBT. [abstract] Blood. 2006;108(11):2860a. [Google Scholar]
- 47.Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007;9(2):111–22. doi: 10.1016/j.bbmt.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Absolute values of dendritic cell subsets in bone marrow, cord blood, and peripheral blood enumerated by a novel method. Stem Cells. 2003;21(3):296–303. doi: 10.1634/stemcells.21-3-296. [DOI] [PubMed] [Google Scholar]
- 49.Szabolcs P, Park KD, Marti L, et al. Superior depletion of alloreactive T cells from peripheral blood stem cell and umbilical cord blood grafts by the combined use of trimetrexate and interleukin-2 immunotoxin. Biol Blood Marrow Transplant. 2004;10(11):772–83. doi: 10.1016/j.bbmt.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Szabolcs P, Park KD, Marti L, Reese M, Lee M, DeOliveira, Sanders L, Niedzwiecki D, Kurtzberg J. The impact of immune reconstitution in the early post grafting period on the development of opportunistic infections after unrelated cord blood transplantation: a multivariate analysis of host, graft, and day +50 immune profile. [abstract] Biology of Blood and Marrow Transplantation. 2004;10(2):24, 48a. [Google Scholar]
- 51.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186(9):1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazur MA, Davis CC, Szabolcs P. Ex vivo expansion and Th1/Tc1 maturation of umbilical cord blood T cells by CD3/CD28 costimulation. Biol Blood Marrow Transplant. 2008;14(10):1190–6. doi: 10.1016/j.bbmt.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parmar S, Robinson SN, Komanduri K, et al. Ex vivo expanded umbilical cord blood T cells maintain naive phenotype and TCR diversity. Cytotherapy. 2006;8(2):149–57. doi: 10.1080/14653240600620812. [DOI] [PubMed] [Google Scholar]
- 54.June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990;11(6):211–6. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 55.Levine BL, Bernstein WB, Aronson NE, et al. Adoptive transfer of costimulated CD4+ T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection. Nat Med. 2002;8(1):47–53. doi: 10.1038/nm0102-47. [DOI] [PubMed] [Google Scholar]
- 56.Laport GG, Levine BL, Stadtmauer EA, et al. Adoptive transfer of costimulated T cells induces lymphocytosis in patients with relapsed/refractory non-Hodgkin lymphoma following CD34+-selected hematopoietic cell transplantation. Blood. 2003;102(6):2004–13. doi: 10.1182/blood-2003-01-0095. [DOI] [PubMed] [Google Scholar]
- 57.Porter DL, Levine BL, Bunin N, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006;107(4):1325–31. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 58.Hagihara M, Chargui J, Gansuvd B, et al. Umbilical cord blood T lymphocytes are induced to apoptosis after being allo-primed in vitro. Bone Marrow Transplant. 1999;24(11):1229–33. doi: 10.1038/sj.bmt.1702050. [DOI] [PubMed] [Google Scholar]
- 59.Davis CCML, Sempowski G, Jeyaraj DA, Szabolcs P. IL-7 Permits Th1/Tc1 Maturation And Promotes Ex-Vivo Expansion of Cord Blood T Cells: A Critical Step Toward Adoptive Immunotherapy After Cord Blood Transplantation. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-09-2860. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snyder KM, Mackall CL, Fry TJ. IL-7 in allogeneic transplant: clinical promise and potential pitfalls. Leuk Lymphoma. 2006;47(7):1222–8. doi: 10.1080/10428190600555876. [DOI] [PubMed] [Google Scholar]
- 61.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–63. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 62.Bolotin E, Annett G, Parkman R, Weinberg K. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 1999;23(8):783–8. doi: 10.1038/sj.bmt.1701655. [DOI] [PubMed] [Google Scholar]
- 63.Chung B, Dudl E, Toyama A, Barsky L, Weinberg KI. Importance of interleukin-7 in the development of experimental graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14(1):16–27. doi: 10.1016/j.bbmt.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 64.Stonehouse TJ, Woodhead VE, Herridge PS, et al. Molecular characterization of U937-dependent T-cell co-stimulation. Immunology. 1999;96(1):35–47. doi: 10.1046/j.1365-2567.1999.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park KD, Marti L, Kurtzberg J, Szabolcs P. In vitro priming and expansion of cytomegalovirus-specific Th1 and Tc1 T cells from naive cord blood lymphocytes. Blood. 2006;108(5):1770–3. doi: 10.1182/blood-2005-10-006536. [DOI] [PMC free article] [PubMed] [Google Scholar]