Abstract
Ethanol’s effects on intracellular signaling pathways contribute to acute effects of ethanol as well as to neuroadaptive responses to repeated ethanol exposure. In this chapter we review recent discoveries that demonstrate how ethanol alters signaling pathways involving several receptor tyrosine kinases and intracellular tyrosine and serine-threonine kinases, with consequences for regulation of cell surface receptor function, gene expression, protein translation, neuronal excitability and animal behavior. We also describe recent work that demonstrates a key role for ethanol in regulating the function of scaffolding proteins that organize signaling complexes into functional units. Finally, we review recent exciting studies demonstrating ethanol modulation of DNA and histone modification and the expression of microRNAs, indicating epigenetic mechanisms by which ethanol regulates neuronal gene expression and addictive behaviors.
Keywords: Phosphorylation, Signal transduction, Growth factor, Epigenetic, Kinase
1 Introduction
Ethanol is a psychoactive substance with rewarding and sedative-hypnotic properties that stem largely from its acute effects on specific signaling proteins that lead to changes in localization and post-translational modifications, gene expression and neuronal excitability. Neurons adapt to repeated ethanol exposure through homeostatic changes in cellular signaling pathways that serve to maintain nervous system function in the presence of ethanol. Such neuroadaptations are thought to contribute to addiction partly because the absence of ethanol produces an aversive withdrawal state that negatively reinforces continued ethanol consumption. Such neuroadaptive changes include long-term changes in gene expression. This chapter reviews recent advances in the field of signal transduction in Drosophila melanogaster and rodents as it relates to alcohol use disorders, with emphasis on mechanisms that hold promise for discovery of new drug targets for treatment. Earlier findings are described in (Ron and Jurd 2005; Newton and Ron 2007; Nagy 2008; Lee and Messing 2008).
2 Serine–Threonine Kinases
Serine/threonine kinases are a large and heterogeneous group of enzymes that phosphorylate protein substrates on serine or threonine residues. Some are receptors (e.g. TGFβ receptors) but the majority are intracellular such as protein kinases A, B (also known as AKT), C, calcium/calmodulin-dependent protein kinases and mitogen-activated protein kinases.
2.1 cAMP-Dependent Protein Kinase A (PKA)
PKA plays a key role in learning and memory (Abel and Nguyen 2008) and in behavioral responses to drugs of abuse (Lee and Messing 2008). It exists as an inactive tetramer of two regulatory subunits and two catalytic subunits. Adenylyl cyclase (AC) activation catalyzes the hydrolysis of ATP to cyclic adenosine 3′, 5′-monophosphate (cAMP). cAMP activates PKA by binding to the regulatory subunits, causing their dissociation from catalytic subunits, which then become active (Brandon et al. 1997). All PKA subunits (RIα, RIβ, RIIα, RIIβ, Cα and Cβ) are expressed in distinct but overlapping patterns in the brain (Cadd and McKnight 1989). There are nine AC isoforms and all are regulated by subunits of heterotrimeric G-proteins (Cooper 2003). Gsα activates all except possibly AC8 (Wang and Storm 2003), while Golfα activates AC5, and Gβγ activates AC2, AC4 and AC7. Conversely, Gi/oα inhibits AC1, AC5, AC6 and AC8, while Gβγ inhibits AC1. Production of cAMP can also be regulated by protein kinase C (PKC) which inhibits AC6 and activates AC2, AC4 and AC7, and by calcium which inhibits AC5 and AC6, activates AC8 and together with Gsα synergistically activates AC1 (Wang and Storm 2003; Cooper 2003).
2.1.1 Ethanol Regulation of AC/PKA Signaling
Like other addictive drugs, ethanol acutely increases levels of extracellular dopamine in the nucleus accumbens (NAc) (Di Chiara and Imperato 1988), which activates D1 dopamine receptors coupled to Gs and Golf, and leads to activation of AC and PKA. Dopamine also activates D2 receptors coupled to Gi/o, which inhibits several AC isoforms. Dopamine activation of D2 receptors also releases Gβγ subunits, which stimulate G-protein-regulated inwardly rectifying K+ (GIRK) channels, and inhibit L–, N–, and P/Q-type calcium channels. The net effect of these actions on ion channel function is to depress neuronal excitability. However, in NAc neurons, Gβγ activation of AC is required for dopamine-stimulated firing, which requires co-activation of D1 and D2 receptors (Hopf et al. 2003).
An important downstream regulator of dopaminergic signaling in striatal neurons is the dopamine- and cAMP-regulated neuronal phosphoprotein of 32 kDa (DARPP-32), which acts as a bidirectional switch that is regulated by phosphorylation (Svenningsson et al. 2005). PKA phosphorylation of Thr-34 makes DARPP-32 a potent inhibitor of the protein phosphatase PP1, which in turn amplifies PKA-mediated phosphorylation of substrates. Cyclin-dependent protein kinase 5 (Cdk5) phosphorylates DARPP-32 at Thr-75, which turns DARPP-32 into an inhibitor of PKA and antagonizes several acute effects of dopamine in the striatum. DARPP-32 appears critical for ethanol reinforcement and reward since mice lacking DARRP-32 show reduced ethanol self-administration and conditioned place preference (Maldve et al. 2002; Risinger et al. 2001).
Ethanol activates AC/PKA/DARPP-32 signaling through several mechanisms. Ethanol increases levels of extracellular dopamine in the NAc (Di Chiara and Imperato 1988; Weiss et al. 1993) by increasing firing of ventral tegmental area (VTA) dopamine neurons (Gessa et al. 1985; Brodie et al. 1990). Ethanol also enhances dopamine D1 receptor-mediated activation of AC (Rex et al. 2008). In addition, ethanol indirectly activates Golf-coupled adenosine A2a receptors by inhibiting adenosine reuptake through type I equilibrative nucleoside transporters, thereby increasing extracellular concentrations of adenosine (Nagy et al. 1990; Choi et al. 2004). Low doses of ethanol and other addictive drugs such as opiates, cannabinoids and nicotine can act synergistically to stimulate ACs through combined effects at A2a receptors, which activate Golf, and dopamine D2 receptors, which cause release of Gβγ subunits (Yao et al. 2003; Yao et al. 2002). These events result in cAMP response element (CRE)-mediated gene expression not only in the NAc but also in several other limbic brain regions (Asyyed et al. 2006).
An important substrate of PKA is the cyclic AMP response element binding protein (CREB), a transcription factor activated by phosphorylation at Ser-133 by PKA and also by calcium/calmodulin-dependent protein kinase IV, or mitogen-and stress-activated protein kinases (MSK1 and 2) (Lonze and Ginty 2002; Hauge and Frodin 2006). In rats, chronic consumption of ethanol for several weeks decreases Ser-133 phosphorylated CREB (p-CREB) in the striatum (Li et al. 2003) and diminishes the ability of an acute ethanol challenge to increase p-CREB and CREB function in the striatum and cerebellum (Yang et al. 1998a, b). During acute ethanol withdrawal, p-CREB is also decreased in several regions of the cerebral cortex (Pandey et al. 2001). These decreases in p-CREB may relate to increased expression of protein kinase inhibitor α (PKIα), as demonstrated in the prefrontal cortex (PFC), NAc and amygdala by transcriptional profiling of brain tissue from Wistar rats subjected to chronic intermittent exposure for 2 weeks (Repunte-Canonigo et al. 2007). PKIα is a protein that acts as a pseudosubstrate inhibitor of PKA catalytic subunits. Since acute exposure to ethanol activates PKA signaling, up-regulation of PKIα can be considered a homeostatic compensatory response that normalizes PKA signaling during chronic ethanol exposure.
2.1.2 AC/PKA Signaling in Behavioral Responses to Ethanol
Several studies indicate a role for PKA in the intoxicating effects of ethanol. Inhibition of PKA through intracerebroventricular (ICV) administration of the selective PKA inhibitor KT5720 reduces the acute ataxic and hypnotic effects of ethanol in rats (Lai et al. 2007). Likewise, RIIβ knockout mice, which have reduced cAMP-stimulated PKA activity, show decreased sensitivity to hypnotic effects of ethanol (Thiele et al. 2000). In addition, pituitary adenylate cyclase-activating polypeptide (PACAP) knockout mice, which are predicted to have a deficit in PKA signaling (Tanaka et al. 2004), show reduced hypothermic and hypnotic responses to ethanol. These studies suggest that ethanol-induced activation of PKA contributes to acute ataxic, hypothermic and sedative-hypnotic effects of ethanol.
In addition to regulating acute behavioral responses to ethanol, PKA signaling also regulates ethanol consumption. Thus, RIIβ knockout mice show reduced cAMP-stimulated PKA activity and increased ethanol intake (Thiele et al. 2000). Mice haplodeficient for the α and Δ isoforms of CREB show increased ethanol consumption (Pandey et al. 2004), while administration of the PKA inhibitor Rp-cAMPS into the central amygdala (CeA) (Pandey et al. 2003) or NAc shell (Misra and Pandey 2006) of Sprague–Dawley rats increases ethanol consumption and preference. These studies suggest that inhibiting PKA signaling, especially in the CeA and the NAc shell, leads to increased ethanol drinking through a CREB-dependent mechanism. In addition, in rats, long-term alcohol consumption with repeated episodes of deprivation produces a strong down-regulation of PACAP gene expression in the striatum that is reversed by treatment with glycine transport inhibitors, which also reduce relapse drinking in this model (Vengeliene et al. 2010), indicating that PACAP, which lies upstream of PKA, is part of a glycine-regulated gene network that undergoes neuroadaptation during long-term ethanol self-administration to promote relapse.
Not all studies, however, agree with these findings, but instead report that inhibition of PKA signaling increases acute effects of ethanol and decreases ethanol consumption. For example, mice that are haplodeficient for Gαs, that express a dominant negative form of PKA (Wand et al. 2001), or lack the calcium-sensitive adenylyl cyclases AC1 and AC8 (Maas et al. 2005), show a more prolonged ethanol-induced loss of righting and less ethanol consumption than wild type mice. Systemic injection of the A2a receptor antagonist 3, 7-dimethylpropargylxanthine, which is expected to reduce PKA signaling throughout the striatum, reduces ethanol consumption in Long-Evans (Arolfo et al. 2004) and Wistar rats (Thorsell et al. 2007). In addition, intra-striatal administration of a peptide that inhibits Gβγ and prevents both ethanol-stimulated nuclear translocation of PKA and PKA-stimulated gene expression, decreases ethanol intake in Long-Evans rats (Yao et al. 2002).
The differences between these studies and those that find reduced sensitivity to ethanol and increased ethanol drinking upon inhibition of AC/PKA signaling may relate to differential duration or level of exposure to ethanol, to effects of global versus local pharmacological and genetic manipulations, compensatory effects of gene targeting that alter non-PKA pathways, or, perhaps, effects of genetic background in the different mouse and rat strains used in these studies. However, experiments in selected and inbred lines of rats and mice do support a role for decreased PKA signaling in the amygdala and NAc shell in promoting ethanol drinking. For example, levels of CREB and p-CREB are lower in the NAc shell of high ethanol preferring C57BL/6 mice compared with DBA/2 mice, which are a low ethanol preferring strain. In addition, alcohol-preferring (P) rats show lower levels of pCREB and CREB DNA binding activity in the CeA and medial amygdala (MeA) than alcohol non-preferring (NP) rats (Pandey et al. 1999a).
2.1.3 PKA and CREB Regulation of Anxiety and Ethanol Consumption
Studies by Pandey and colleagues have identified key roles for amygdala PKA, CREB and the CREB-regulated gene neuropeptide Y (NPY) in the co-regulation of anxiety and ethanol drinking. NPY is abundantly expressed in the brain and is anxiolytic when administered into the central nervous system (CNS) (Heilig 2004). Knockout of the NPY gene in mice increases ethanol consumption, while transgenic overexpression of NPY reduces it (Thiele et al. 1998). P rats drink excessively and have lower levels of p-CREB and NPY in the amygdala compared with NP rats; P rats also show greater anxiety-like behavior than NP rats (Pandey et al. 2005). Self-administration of increasing concentrations of ethanol (7–12% over 10 days) in a two-bottle choice paradigm, or injection of 1 g/kg ethanol, normalizes anxiety-like behavior in P rats. These findings are associated with increased p-CREB and expression of NPY in the CeA and MeA of ethanol-treated P rats (Pandey et al. 2005). Infusion of the PKA activator Sp-cAMP into the CeA of P rats increases local p-CREB and NPY levels, decreases ethanol self-administration and normalizes their heightened anxiety-like behavior (Pandey et al. 2005). Conversely, in NP rats, infusion of the PKA inhibitor Rp-cAMP into the CeA decreases local p-CREB and NPY, increases anxiety-like behavior and increases ethanol intake (Pandey et al. 2005). The importance of NPY in these behavioral changes has been demonstrated by infusing NPY into the amygdala of P rats, which mimics the effect of Sp-cAMPs by decreasing anxiety-like behavior and ethanol intake.
Increased anxiety that accompanies alcohol withdrawal is argued to be one of the negatively reinforcing factors that promotes ethanol consumption (Koob 2009). Support for this concept stems from studies of diminished amygdala PKA signaling and NPY expression that accompany ethanol withdrawal in rats. One day after withdrawal from chronic daily intake of ethanol, Sprague–Dawley rats show increased anxiety-like behavior (Pandey et al. 2003), which is associated with decreased p-CREB (Pandey et al. 2003) and NPY (Roy and Pandey 2002) in the CeA and MeA. Sp-cAMPS infused into the CeA normalizes CREB phosphorylation and NPY expression, and prevents withdrawal-induced anxiety in these rats (Pandey et al. 2003; Zhang and Pandey 2003). In ethanol na rats, infusion of the PKA inhibitor Rp-cAMPS into the CeA decreases local p-CREB and NPY and increases both anxiety and ethanol consumption (Pandey et al. 2003; Zhang and Pandey 2003). Furthermore, infusion of NPY into the CeA prevents Rp-cAMPS-induced decreases in ethanol preference (Pandey et al. 2003). Intra-amygdalar infusion of NPY also reduces ethanol intake in P rats after multiple episodes of alcohol deprivation (Gilpin et al. 2003) and reduces withdrawal-induced increases in ethanol consumption in Wistar rats (Gilpin et al. 2008). These results indicate that deficient PKA and NPY signaling in the amygdala are critical for increased anxiety and drinking that accompany alcohol withdrawal.
2.2 Protein Kinase C (PKC)
The PKC family of serine–threonine kinases mediates signals derived from lipid second messengers. The members of this family share similar catalytic domains but can be subdivided into four classes based on differences in their regulatory domains that alter structure and function (Rosse et al. 2010; Newton 2010). The classical or conventional cPKCs (α, β, γ) are activated by diacylcglycerol (DAG) and calcium. Novel nPKCs (δ, ε, η, θ) are activated by diacylglycerol but not by calcium. Atypical PKCs (ι or λ in mice, and ζ), do not require calcium or diacylglycerol for activation, but can be activated by phosphatidylinositols, phosphatidic acid, arachidonic acid and ceramide, and by interaction with the partitioning defective 6 (PAR6)-CDC42 complex (Hirai and Chida 2003; Rosse et al. 2010). Recently, a fourth group of PKC-related kinases (PKN1, PKN2, PKN3) has been included as a PKC subfamily; they are activated by the small G-proteins Rac and Rho (Rosse et al. 2010).
DAG, generated by activation of phospholipase C (PLC), is the most studied lipid activator of PKC signaling (Fukami et al. 2010). Among the PLC isoforms, activation of β and γ subtypes has been best described. PLCβ is activated by Gβγ or Gαq subunits of heterotrimeric G-proteins released upon ligand binding to G-protein coupled receptors. Activation of receptor tyrosine kinases leads instead to recruitment, tyrosine phosphorylation, and activation of PLCγ. DAG can also be generated as a result of receptor-mediated activation of phospholipase D (Nishizuka 1995). PKC activation is generally associated with translocation of PKC from one cellular compartment to another containing lipid activators and proteins that bind the activated kinase near substrates. Here we summarize recent work on ethanol and PKC, focusing on three PKC isozymes, PKCε, PKCγ and PKCδ (see also section on RACK1).
2.2.1 Ethanol Regulation of PKC Activity
Ethanol has been reported to activate, inhibit or have no effect on PKC activity in vitro, depending on experimental conditions (reviewed in (Stubbs and Slater 1999). Recently, ethanol was reported to bind to PKCε and inhibit PKCε activity when assayed in vitro in the presence of DAG plus phosphatidylcholine and phosphatidylserine (Das et al. 2009). However, using DAG and phosphatidylserine with Triton-X-100 micelles, we previously found that ethanol does not alter the activity of PKC in vitro (Messing et al. 1991), while other literature indicates that ethanol exposure activates PKCε in intact cell systems (Miyame et al. 1997; Jiang and Ye 2003; Qi et al. 2007). Ethanol regulation of PKCε and other PKC isozymes is most likely to be indirect, due to modulation of upstream signaling pathways that generate DAG or that lead to phosphorylation of sites necessary for full kinase activity, such as the C-terminal hydrophobic motifs of PKCε (Wallace et al. 2007) and the cPKCs (Wilkie et al. 2007).
2.2.2 Ethanol Regulation of PKC Localization
In NG108-15 neuroblastoma × glioma cells, ethanol causes translocation of PKCδ from the Golgi to the perinucleus and PKCε from the perinucleus to the cytoplasm (Gordon et al. 1997). Cytosolic translocation of PKCε has also been observed in rat cerebral cortex following acute exposure to ethanol (Kumar et al. 2006). Dopamine D2 receptor agonists stimulate translocation of PKCδ and PKCε to these same sites in NG108-15 and CHO cells stably transfected to express D2 receptors (Gordon et al. 2001), and in cultured rat VTA dopamine neurons (Yao et al. 2010). The effects of ethanol and D2 agonists are synergistic since concentrations of ethanol and agonist that do not cause translocation alone produce robust translocation when administered together (Gordon et al. 2001). Ethanol-translocated PKCε is active (Yao et al. 2008), as shown by its binding to monoclonal antibody 14E6, which specifically detects the active conformation of PKCε (Souroujon et al. 2004). Translocation of PKCε occurs together with translocation of β′COP (Gordon et al. 2001; Yao et al. 2008), a receptor for activated PKCε (εRACK; RACK2) (Csukai et al. 1997). Ethanol stimulates translocation of this complex through activation of adenosine A2a receptors, and both ethanol and D2 agonist stimulated translocation require activation of PLC, PKCε and PKA; PKA may act by promoting the activation of PLC and by phosphorylating and facilitating the translocation of εRACK (Yao et al. 2008). These results indicate considerable cross talk between PKA and PKCε in synergistic responses to ethanol and dopamine.
Such crosstalk is important in the VTA where drugs of abuse increase extracellular levels of dopamine and thereby activate D2 autoreceptors on dopaminergic neurons. This event leads to the activation of PKC (most likely PKCε) and PKA, which phosphorylate and up-regulate tyrosine hydrolase (TH) and increase production of dopamine (Yao et al. 2010). Up-regulation of TH activity by cocaine is essential for the ability of ALDH2 inhibitors to reduce cocaine self-administration in rodents (Yao et al. 2010). ALDH2 inhibitors impair metabolism of dopamine, resulting in the generation of tetrahydropapaveroline (THP), a potent inhibitor of TH, especially phosphorylated TH. It is likely that the ability of ALDH2 inhibitors to reduce ethanol self-administration (Arolfo et al. 2009) also requires PKCε and PKA-mediated up-regulation of dopamine production to generate THP, although this possibility remains to be tested.
2.2.3 PKCε Regulation of GABAA Receptors and Intoxication
The intoxicating effects of ethanol last much longer in PKCε knockout mice than in wild type mice due to impaired development of acute functional tolerance to ethanol in the knockout (Wallace et al. 2007). Phenotypic and biochemical studies using PKCε knockout mice and selective peptide inhibitors and activators of PKCε have demonstrated that PKCε reduces the response of GABAA receptors to several positive allosteric modulators, including neurosteroids, benzodiazepines and ethanol (Hodge et al. 1999; Hodge et al. 2002). Two mechanisms appear to account for this modulation. First, PKCε phosphorylates the γ2 subunit of GABAA receptors at Ser-327, and when this site is phosphorylated, synaptic GABAA receptors show reduced activation by benzodiazepines and by ethanol (Qi et al. 2007). This phosphorylation event is important for behavior since development of acute functional tolerance is associated with increased Ser-327 phosphorylation and reduced effects of ethanol on cerebellar GABAA receptors (Qi et al. 2007; Wallace et al. 2007). Second, PKCε phosphorylates the N-ethylmaleimide-sensitive factor (NSF) at Ser-460 and Thr-461 (Chou et al. 2010). Phosphorylation at these sites increases NSF activity and binding to PKCε and alters GABAA receptor trafficking, resulting in fewer receptors at the synapse (Chou et al. 2010). Thus, inhibiting PKCε facilitates inhibitory synaptic transmission in general by increasing the density of synaptic GABAA receptors through a reduction in NSF activity and specifically enhances the positive allosteric effects of ethanol and benzodiazepines by decreasing the phosphorylation of GABA γ2 subunits.
2.2.4 PKCε and Ethanol-Induced GABA Release
Ethanol stimulates GABA release in the CeA through a mechanism that requires activation of type 1 corticotrophin releasing factor (CRF) receptors (CRF1Rs) (Nie et al. 2004). CRF is an anxiogenic neuropeptide that is upregulated in the amygdala of ethanol-dependent rodents where it promotes excessive ethanol consumption through actions at CRF1Rs (Chu et al. 2007; Sommer et al. 2008). Furthermore, a polymorphism in the Crhr1 promoter that is accompanied by increased abundance of Crhr1 transcripts in several limbic areas has been identified in Marchigian–Sardinian Preferring (msP) rats genetically selected for high alcohol preference (Hansson et al. 2007). PKCε knockout mice, which show reduced anxiety-like behavior (Hodge et al. 2002) and low levels of ethanol self-administration (Hodge et al. 1999; Olive et al. 2000), also have an ~50% reduction in levels of CRF in the CeA (Lesscher et al. 2008). Furthermore, absence or inhibition of PKCε prevents CRF or ethanol-stimulated GABA release in the CeA (Bajo et al. 2008). Therefore, PKCε is important not only for the production of CRF but also for CRF1R signaling that controls ethanol-induced GABA release in the CeA and regulates both anxiety and ethanol consumption (Lesscher et al. 2008; Lesscher et al. 2009).
2.2.5 PKCγ and Ethanol-Mediated GABAA Receptor Trafficking
Like PKCε, PKCγ is widely expressed in the CNS (Naik et al. 2000), and also regulates GABAA receptors and behavioral responses to ethanol. In contrast to PKCε knockout mice, PKCγ knockout mice are less sensitive to acute effects of ethanol, consume more ethanol and show impaired development of chronic tolerance to ethanol compared with wild type mice (Bowers et al. 1999; Bowers et al. 2000; Bowers and Wehner 2001). Exposure to ethanol for several hours causes internalization of α1 subunits of GABAA receptors in cerebral cortex (Kumar et al. 2003) and hippocampus (Liang et al. 2007), which may play a role in the hyperexcitablity that appears during ethanol withdrawal. In a recent study it was found that PKCγ co-immunoprecipitates with α1 subunits and this association is increased after 4 h of exposure to ethanol (Kumar et al. 2010). Treatment with short-interfering RNAs targeted against PKCγ prevented ethanol-induced decreases in the abundance of α1 subunits in cultured cortical neurons (Kumar et al. 2010), suggesting that PKCγ mediates ethanol-induced decreases in α1. However, while suggestive, these results should be viewed as preliminary since although three siRNAs were used against PKCγ in this study, it appears that they were administered together, not separately. Also, an inhibitory peptide derived from the pseudosubstrate sequence of PKCβ had no effect on α1 subunit trafficking which is puzzling since this peptide appears to also inhibit PKCγ activity (Correia et al. 2003). Finally, ethanol treatment results in the inhibition of PKCβII translocation and thus prevents proper substrate phosphorylation (Ron et al. 2000).
2.2.6 PKCδ and Sensitivity to Ethanol Intoxication
PKCδ is expressed in several brain regions that regulate ethanol intake (Merchenthaler et al. 1993; Choi et al. 2008) including the CeA (Koob et al. 1998; Finn et al. 2007; Funk et al. 2006; Primeaux et al. 2006; Moller et al. 1997; Hyytiä and Koob 1995), the hippocampus (Adell and Myers 1994; Huttunen and Myers 1987; Martin-García et al. 2007), the bed nucleus of the stria terminalis (BNST) (Walker et al. 2003; Hyytia et al. 1999) and the lateral septum (Ryabinin et al. 2008). Acute ethanol exposure alters the distribution, whereas chronic exposure increases the abundance and translocation of PKCδ in neural cell lines (Messing et al. 1991; Gordon et al. 1997), suggesting that PKCδ participates in responses to ethanol. This hypothesis has been confirmed in PKCδ knockout mice(Chou et al. 2004), which are less sensitive to the acute motor-impairing effects of ethanol (Choi et al. 2008). This resistance is most obvious at a dose of ethanol (1.5 g/kg) that produces blood ethanol concentrations of 150–240 mg/dl (32–51 mM) in mice (Gentry et al. 1983); similar blood levels impair coordination in humans (Messing 2007). These findings suggest that PKCδ is involved in neuronal signaling pathways that increase acute sensitivity to ethanol at ethanol doses that produce moderate intoxication.
2.2.7 PKCδ and Tonic GABA Currents
Although GABAA receptors are considered primary targets for ethanol, demonstration of direct effects at concentrations lower than those that produce anesthesia has been historically difficult (Harris et al. 1997; Harris et al. 1995; Criswell and Breese 2005). However, recent electrophysiological studies have provided evidence of low dose ethanol effects at GABAA receptors in the hippocampus, cerebral cortex, NAc and CeA (Weiner and Valenzuela 2006). Evidence from electrophysiological recordings of recombinant receptors expressed in Xenopus oocytes (Sundstrom-Poromaa et al. 2002; Wallner et al. 2003) and of native receptors in hippocampal dentate gyrus granule cells (Wei et al. 2004; Fleming et al. 2007) suggest that GABAA receptors formed by the subunit combination of α4βxδ are sensitive to low (1–30 mM) concentrations of ethanol. Concentrations of 3–20 mM produce mild intoxication in humans and 17 mM is equivalent to a blood alcohol level of 80 mg/dl (Messing 2007). It must be noted, however, that some investigators have been unable to replicate these findings (Yamashita et al. 2006; Borghese et al. 2006). The basis for this discrepancy could be related partly to differences in phosphorylation state of the receptor, as discussed below.
GABAA receptors that contain δ subunits are extrasynaptic and modulate the inhibitory tone of neurons by responding to ambient GABA levels, as opposed to synaptic receptors, which contain γ2 subunits instead of δ subunits, and provide rapid, phasic inhibition by responding to stimulated release of GABA at synapses (Farrant and Nusser 2005; Wei et al. 2003; Glykys and Mody 2007; Glykys et al. 2007). GABAA receptors that contain δ subunits have a high affinity for GABA and a slow rate of desensitization, properties that are useful for tonic regulation of inhibition. Our recent work indicates that PKCδ regulates the ethanol sensitivity of tonic inhibitory GABA currents (Choi et al. 2008). Thus, tonic currents in thalamic and hippocampal neurons of PKCδ knockout mice show no response to 30 mM ethanol. Ethanol regulation of tonic GABA current is mediated by a direct effect of ethanol on extrasynaptic GABAA receptors rather than on mechanisms that regulate extracellular concentrations of GABA (e.g. GABA transporters) since, in mouse L(tk-) fibroblasts that express α4β3δ GABAA receptors, ethanol enhancement of GABA currents is also PKCδ-dependent (Choi et al. 2008). These findings suggest that PKCδ facilitates ethanol intoxication by enhancing ethanol’s action at extrasynaptic GABAA receptors, possibly through phosphorylation of receptor subunits.
2.3 Extracellular Signal-Regulated Kinases (ERKs)
ERKs are serine–threonine protein kinases that are members of the mitogen-activated protein kinase (MAPK) family. There are two isoforms, p44 ERK1 and p42 ERK2, with functions that partly overlap. Both are widely expressed in limbic brain regions including in the mesolimbic dopaminergic system, amygdala and prefrontal cortex (Lein et al. 2007). ERKs are activated by a Ras–Raf–MEK signaling cascade that is activated by receptor tyrosine kinases (see section below on Receptor Tyrosine Kinases) or by calcium influx through NMDA and voltage-gated calcium channels. The function of these ion channels can be enhanced by PKA-mediated phosphorylation resulting from activation of dopamine D1 receptors (Lu et al. 2006; Pascoli et al. 2011). Since ERK activity is increased by dopamine and glutamate receptor stimulation, it may function as a coincidence detector that combines information about rewards and contextual information during the development of addiction (Girault et al. 2007).
Since MEK phosphorylation activates ERK1 and ERK2 (ERKs), ERK activity can be indirectly assayed by measuring MEK phosphorylation of ERKs using phospho-specific antibodies. Using this approach, previous studies have reported that acute ethanol exposure (3.5 g/kg) in adult rats inhibits ERKs in the cerebral cortex, hippocampus and cerebellum (Chandler and Sutton 2005) and that continuous or intermittent exposure to ethanol vapor for 12 days also inhibits ERKs in the amygdala, cerebellum, dorsal striatum, hippocampus and PFC (Sanna et al. 2002). We recently found that acute systemic administration of 2 g/kg of ethanol to C57BL/6 mice did not alter ERK phosphorylation in the NAc (Neasta et al. 2011a). However, Ibba et al. (Ibba et al. 2009) detected ERK activation in the BNST, CeA and the NAc 15 min after intragastric gavage of 1 but not 2 g/kg ethanol; both basal and ethanol-stimulated ERK activity could be blocked by a dopamine D1 receptor antagonist. In addition, Neznanova and colleagues (Neznanova et al. 2009) observed that alcohol-preferring AA rats showed rapid and transient dephosphorylation of ERK1/2 upon acute ethanol challenge in the medial prefrontal cortex, and to a lesser degree in the nucleus accumbens, whereas alcohol non-preferring ANA rats did not. Therefore, it is possible that, under certain conditions, ethanol stimulation of dopaminergic signaling activates ERKs. In addition, ethanol can indirectly activate ERKs by up-regulating BDNF-mediated signaling in the dorsal striatum (see section below on BDNF). The mechanisms responsible for inhibition of ERKs by ethanol elsewhere are not known.
Few studies have examined ERK activity in ethanol-dependent rodents. An older report found that ERK activity is increased during ethanol withdrawal in the dorsal striatum, cerebellum and especially in the amygdala (Sanna et al. 2002). ERK activation can induce transcription of the immediate-early gene c-fos. Acute administration of ethanol stimulates c-fos in several brain regions, but only in the MeA is this ERK-dependent (Hansson et al. 2008). However, in ethanol-dependent rats, induction of c-fos by an ethanol challenge is inhibited in orbital frontal cortex and NAc shell through an ERK-dependent mechanism (Hansson et al. 2008), suggesting that in these brain regions ERKs are part of a homeostatic response that suppresses ethanol-induced c-fos expression mediated by other signaling pathways. Overall, ethanol’s effects on ERK signaling are heterogeneous and depend not only on the brain region studied but also on whether animals are in an ethanol-naïve or -dependent state. The net effect of ERK signaling may be to suppress ethanol intake, since recent evidence indicates that systemic administration of the MEK inhibitor SL327 increases operant self-administration of ethanol in C57BL/6J mice (Faccidomo et al. 2009), and this inhibitory mechanism of ERKs on ethanol consumption may be mediated via BDNF (see section on BDNF).
2.4 P13K, AKT and GSK3beta
Phosphatidylinositol-3-kinase (P13K) is a lipid kinase that phosphorylates phosphatidylinositides (PtdIns) at the plasma membrane leading to the recruitment of the downstream serine and threonine kinases, 3-phosphoinositide-dependent protein kinase 1 (PDK1) and AKT, to the membrane, where PDK1 phosphorylates and activates AKT. The PI3/AKT pathway contributes to diverse biological functions such as cell survival and growth (Brazil and Hemmings 2001; Engelman 2009), and in the CNS, AKT phosphorylates the β2 subunit of the GABAA receptor leading to increased membranal localization of β2 containing GABAA, thereby increasing GABAA receptor-mediated synaptic transmission (Wang et al. 2003). Interestingly, several independent investigations in flies and rodents recently indicated an important role for the P13K/AKT pathway in ethanol’s actions. Specifically, inhibition of P13K in the NAc reduced binge drinking in C57BL6 mice (Cozzoli et al. 2009) and in rats (Neasta et al. 2011a). These results suggest that ethanol treatment results in the activation of P13K in the NAc, but the mechanism underlying P13K activation is not yet clear. One possibility is that ethanol activates a small Ras family G-protein upstream of P13K. Ras proteins (H-Ras, K-Ras and N-Ras) cycle between active GTP-bound and inactive GDP-bound forms. Active GTP-bound Ras interacts with several effector proteins, and among them is P13K. Interestingly, we previously observed that acute ex vivo treatment of hippocampal slices with ethanol leads to a robust activation of H-Ras (Suvarna et al. 2005).
As menioned above, AKT is activated in response to the activation of P13K. AKT activation can be measured by phosphorylation of AKT on threonine 308 and serine 473. Systemic administration of 0.75 g/kg ethanol to young adult (3-week) mice increases phosphorylation of AKT at Thr-308 in the striatum (Bjork et al. 2010). Administration of a higher dose (1.5 g/kg) increases AKT Thr-308 phosphorylation measured 45 min later in the medial prefrontal cortex, but not in the NAc of AA rats selectively bred to drink high levels of ethanol (Neznanova et al. 2009). On the other hand, we found that systemic administration of ethanol (2 g/kg) leads to the phosphorylation of AKT at both threonine 308 and serine 473 in the NAc of adult (9-week old) mice (Neasta et al. 2011a). AKT is also phosphorylated (and thus activated) in the NAc of high ethanol drinking Long-Evans rats (Neasta et al. 2011a). The activation of AKT by ethanol is likely to be an important contributor to mechanisms that lead to ethanol-drinking behaviors as the blockade of the AKT pathway within the NAc decreases excessive voluntary consumption and self-administration of ethanol in heavy drinking rats (Neasta et al. 2011a). Finally, using Drosophila as a model system, the Heberlein group conducted an elegant set of experiments suggesting that the P13K/AKT pathway contributes to the sensitivity of flies to the sedative actions of ethanol. Specifically, neuronal overexpression of PDK1, the catalytic subunit P13K or AKT increased the duration of ethanol sedation, whereas over expression of the dominant negative form of P13K or RNAi-mediated knockdown of AKT decreased the sensitivity of flies to the acute hypnotic actions of ethanol (Eddison et al. 2011). Together, the studies described above strongly suggest that the P13K/AKT signaling pathway is a key contributor to mechanisms that underlie phenotypes such as excessive ethanol drinking.
The serine/threonine kinases glycogen synthase kinase-3 α and β (GSK-3α and GSK-3β) (Jope and Johnson 2004) are important and well-characterized substrates of AKT, and phosphorylation of GSK-3α on serine 21 and GSK-3β on serine 9 by AKT results in the inhibition of GSK-3 kinase activity (Jope and Johnson 2004). Neznanova et al. did not observe changes in the level of GSK-3β phosphorylation in the NAc of AA rats in response to systemic administration of ethanol, although an increase in GSK3β phosphorylation was detected in the prefrontal cortex (Neznanova et al. 2009). We recently observed that systemic administration of ethanol (2 g/kg) as well as recurring cycles of voluntary consumption of high amounts of ethanol followed by periods of withdrawal in rats lead to an increase in level of phosphorylated GSK-3α and GSK-3β in the NAc (Neasta et al. 2011a). The contribution of GSK-3α or GSK-3β inhibiton to ethanol’s actions in the CNS is yet to be determined.
2.5 mTOR
A very important downstream target of AKT is the serine/threonine protein kinase, mammalian target of rapamycin (mTOR) (Hay and Sonenberg 2004). mTOR signals through two multiprotein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (Hoeffer and Klann 2010). The two complexes have unique protein compositions consisting of adaptor proteins, enzymes and substrates (Hoeffer and Klann 2010). mTORC1 plays an essential role in initiating local synaptic protein translation, synaptic plasticity, learning and memory (Costa-Mattioli et al. 2009). We recently found that mTORC1 within the NAc is a novel contributor to molecular mechanisms underlying ethanol drinking (Neasta et al. 2010). Systemic administration of ethanol and high levels of ethanol intake activated mTORC1-mediated signaling in the NAc of mice and rats. In addition, levels of the AMPA receptor subunit GluR1 and the scaffolding protein Homer, two synaptic proteins whose translation is regulated by mTORC1 (Slipczuk et al. 2009), were up-regulated in these rodents (Neasta et al. 2010). Importantly, the FDA-approved inhibitor of mTORC1, rapamycin, decreased ethanol induction of Homer translation, reduced ethanol-induced locomotor sensitization and place preference, and reduced excessive ethanol intake and seeking (Neasta et al. 2010). Interestingly, the translation of postsynaptic density protein 95 (PSD-95), and the activity-regulated cytoskeleton-associated protein (Arc) have been linked to the mTORC1 pathway (Lee et al. 2005; Takei et al. 2004). Both proteins play a role in neuroadaptations underlying ethanol’s actions (Carpenter-Hyland and Chandler 2006; Pandey et al. 2008b; Moonat et al. 2011; Camp et al. 2011). Therefore, it would be interesting to determine if ethanol-induced activation of mTORC1 signaling results in translation of these and other synaptic proteins.
3 Tyrosine Kinases
Tyrosine kinases are a large and diverse family of proteins that can be subdivided into two groups: receptor tyrosine kinases (RTK), and the non-receptor tyrosine kinases (NRTK). Both groups share a highly conserved kinase domain and unique protein or lipid binding domains.
3.1 Receptor Tyrsosine Kinases
RTKs are membrane-spanning receptors composed of an extracellular N-terminal region, a membrane-spanning region, and an intracellular C-terminal region, which contains the catalytic domain. Most receptors dimerize upon ligand binding allowing auto- and trans-phosphorylation to occur. Tyrosine phosphorylation at the C-terminus of the receptor serves as a docking site for adaptor proteins, which, in turn, recruit enzymes that initiate the activation of a signaling cascade. The most well-characterized ligands that interact and activate RTKs are growth factors, and in recent years several growth factors have been associated with molecular and behavioral adaptations in response to ethanol.
3.1.1 EGF
Epidermal growth factor (EGF) and its receptor (EGFR) are expressed in adult neurons in brain regions such as the hippocampus, cerebellum and cerebral cortex (Wong and Guillaud 2004). In the hippocampus EGF enhances the activity of the N-methyl-D-aspartate (NMDA) receptor (NMDAR) and increases long-term potentiation (LTP) in CA1 pyramidal neurons (Wong and Guillaud 2004). EGF also plays a protective role in stimulating the survival of rat cortical and dopaminergic neurons (Wong and Guillaud 2004). Recent evidence suggests a contribution of EGFR-mediated signaling to ethanol’s actions in the CNS. A forward genetic screen in Drosophila identified mutants in the gene happyhour, which display a high level of resistance to ethanol intoxication (Corl et al. 2009). The protein encoded by happyhour shows strong homology to mammalian Ste20 family kinases and acts to inhibit EGFR-mediated activation of ERK (Corl et al. 2009). In addition, inhibitors of the EGFR signaling increase the sensitivity of both flies and mice to the intoxicating properties of ethanol (Corl et al. 2009). Interestingly, incubation of cultured cancer cells with ethanol (43 mM) produces a rapid and robust phosphorylation and activation of the EGFR and of ERK1/2, and an inhibitor of the EGFR blocks both phosphorylation events (Forsyth et al. 2010), providing a direct link between ethanol and EGFR-mediated signaling. In rats, systemic administration of EGFR inhibitors reduces voluntary consumption of ethanol but not sucrose (Corl et al. 2009). Together, these results indicate that the EGFR is part of an evolutionary conserved signaling pathway activated by ethanol exposure that contributes to mechanisms underlying ethanol intoxication as well as consumption.
3.1.2 Insulin
Insulin is not produced in the brain, but circulating insulin crosses the blood–brain barrier. Insulin receptors (IRs) are expressed in both astrocytes and neurons in brain regions such as the hypothalamus, hippocampus, cerebellum, amygdala and cerebral cortex. Insulin’s main roles in the CNS are the control of food intake and cognitive functions such as memory (Laron 2009). In Drosophila, activation of insulin signaling in the CNS plays a regulatory role in ethanol-mediated intoxication, as inhibition of the pathway or reduction in the function of insulin-producing cells increases the severity of fly intoxication (Corl et al. 2005). Although these results are intriguing, the role of insulin in ethanol intoxication needs to be confirmed in mammals.
3.1.3 GDNF
The glial-derived neurotrophic factor (GDNF) is a distant member of the transforming growth factor β (TGF-β) superfamily. Although GDNF was originally identified in a glial cell line (Lin et al. 1993), it is mainly expressed in neurons of the adult brain (Pochon et al. 1997; Barroso-Chinea et al. 2005). Binding of GDNF to its co-receptor, GFRα1 leads to the recruitment of the RTK, Ret, to the GFRα1-GDNF complex (Jing et al. 1996), and Ret is then activated by autophosphorylation (Durbec et al. 1996). The main signaling pathways that are downstream of Ret activation are ERK1/2, P13K and PLCγ (Airaksinen and Saarma 2002). GDNF is highly expressed in the NAc, and its receptors (Ret and GFRα1), are localized in the VTA (Trupp et al. 1997). We recently showed that dopaminergic terminals in the nucleus accumbens retrogradely transport GDNF to the VTA, where the growth factor increases the spontaneous activity of dopaminergic neurons, resulting in an increase in dopamine overflow in the NAc (Wang et al. 2010a). Accumulating evidence suggests that GDNF in the mesolimbic dopaminergic system plays an important inhibitory role in ethanol-drinking behavior. A single administration of GDNF into the VTA of Long-Evans rats results in a very rapid and sustained reduction of ethanol intake in two-bottle choice continuous access and operant self-administration paradigms (Carnicella et al. 2008, 2010; Carnicella and Ron 2009). Interestingly, GDNF’s actions are specific for ethanol and are not due to a general reduction of reward or changes in locomotor activity, as the growth factor has no effect on operant self-administration of sucrose (Carnicella et al. 2008). Importantly, intra-VTA infusion of GDNF 10 min before the beginning of an operant session blocks the reacquisition of operant responding for ethanol after a period of extinction (Carnicella et al. 2008). Activation of the GDNF pathway in the VTA results in the phosphorylation of ERKs (Carnicella et al. 2008; Wang et al. 2010a), which is required to reduce ethanol consumption, since inhibition of ERKs in the VTA blocks GDNF inhibition of ethanol self-administration (Carnicella et al. 2008). Finally, mice haploinsufficient for GDNF or its receptor, GFRα1, consume more ethanol after a period of abstinence compared with wild type littermates (Carnicella et al. 2009b). In addition, these mice exhibit increased place preference for ethanol compared with wild type mice (Carnicella et al. 2009b). These results suggest that endogenous GDNF-mediated signaling contributes to mechanisms that protect against addiction by suppressing or delaying the development of ethanol reward and limiting relapse to drinking.
3.1.4 BDNF
The brain derived neurotrophic factor (BDNF) belongs to the nerve growth factor (NGF) family of neurotrophic factors. BDNF and its receptor TrkB are widely distributed throughout the brain, and the BDNF/TrkB pathway plays an important role in neuronal proliferation, differentiation and survival, as well as synaptic plasticity (Lewin and Barde 1996; Yoshii and Constantine-Paton 2010). More recently, BDNF has been implicated in psychiatric disorders such as depression and anxiety (Martinowich et al. 2007). In addition, a growing body of literature suggests a role for BDNF in drug addiction (Ghitza et al. 2010), and we and others generated evidence that suggests a unique role for BDNF in regulating behavioral responses to ethanol. Specifically, a reduction in BDNF gene expression in BDNF heterozygous knockout mice (Hensler et al. 2003; McGough et al. 2004), or inhibition of the BDNF receptor TrkB (Jeanblanc et al. 2006), increases ethanol consumption and preference. Moreover, acute systemic administration of ethanol and voluntary intake of moderate amounts of ethanol, through two-bottle choice or operant self-administration paradigms, increases BDNF expression in the dorsal striatum of mice and rats (McGough et al. 2004; Jeanblanc et al. 2009; Logrip et al. 2009). Ethanol-mediated increases in BDNF mRNA result in increased synthesis of BDNF and activation of ERK (Logrip et al. 2008), and to increased expression of downstream genes, such as the dopamine D3 receptor and dynorphin (Jeanblanc et al. 2006; Logrip et al. 2008). Interestingly, BDNF in the dorsal striatum, in turn, acts as an endogenous inhibitor of ethanol consumption (McGough et al. 2004; Jeanblanc et al. 2009), and this action is localized to the dorsolateral striatum (Jeanblanc et al. 2009), a brain region associated with habit learning (Yin and Knowlton 2006). In contrast, long-term, daily ethanol intake in C57BL6 mice results in a breakdown of this protective homeostatic pathway in the dorsal striatum (Logrip et al. 2009). In addition, chronic, high levels of ethanol intake decrease cortical BDNF mRNA (Logrip et al. 2009). These results are in line with previous data showing that a decrease in cortical BDNF can be detected 24 h after withdrawal from chronic ethanol treatment (Pandey et al. 1999b).
Elegant studies by Pandey and colleagues suggest that BDNF in the CeA and MeA plays a protective role against anxiety and ethanol consumption during ethanol withdrawal. Reducing BDNF in the amygdala increases anxiety and ethanol consumption in rats (Pandey et al. 2006; Pandey et al. 2008b). In contrast, the anxiolytic actions of ethanol are associated with increased expression of BDNF, as well as BDNF-induced expression of Arc in the CeA and MeA, and infusion of BDNF in the CeA reverses ethanol withdrawal-induced anxiety (Pandey et al. 2008b). Interestingly, BDNF mRNA and protein levels are lower in the extended amygdala of P rats compared with NP rats, which is consistent with BDNF’s role in suppressing ethanol intake (Prakash et al. 2008).
Consistent with these results in rodents, Heberlein and colleagues (Heberlein et al. 2010) recently reported that the level of BDNF in the serum of alcohol-dependent patients is negatively correlated with the severity of withdrawal symptoms. In addition, a single nucleotide polymorphism (Val66Met) in the BDNF gene, which leads to a reduction in BDNF function (Chen et al. 2004), has been linked with an earlier onset of alcoholism (Matsushita et al. 2005), and a recent human study reported a higher risk of relapse in ethanol-dependent patients with this polymorphism (Wojnar et al. 2009).
3.2 The Src Family of Protein Tyrosine Kinases
The Src family of protein tyrosine kinases (Src PTKs) are intracellular, membrane-bound enzymes that play an important role in various cellular functions. Four members of the family are expressed in the brain (Src, Fyn, Lyn and Yes) (Kalia et al. 2004). Src and Fyn have been heavily implicated in modulation of NMDARs and synaptic plasticity (Salter and Kalia 2004). Fyn is also an important mediator of neurite outgrowth and myelination by oligodendrocytes (Beggs et al. 1994; Bodrikov et al. 2005; Brackenbury et al. 2008; Sperber et al. 2001), and has been implicated in Alzheimer’s disease (Lee et al. 2004; Chin et al. 2005). Lyn was reported to interact with the Na+/K+ ATPase and to phosphorylate its α3 subunit (Wang and Yu 2005). Lyn was also shown to negatively regulate NMDAR activity (Umemori et al. 2003). A link between Lyn and the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptor (AMPAR) in the cerebellum has also been suggested (Hayashi and Huganir 2004). Very recently, we found that Lyn negatively regulates the release of dopamine in the mesolimbic system (Gibb et al. 2011). Although Yes is highly expressed in the basal ganglia (Walaas et al. 1993), its role in the CNS has yet to be determined.
3.2.1 Src and Fyn in Ethanol Modulation of NMDA Receptors
Interestingly, ethanol exposure results in opposing actions on the activity of Src and Fyn. Acute treatment of hippocampal neurons with ethanol inhibits Src (Suvarna et al. 2005), but activates Fyn (Yaka et al. 2003b). Src inhibition, in turn, results in the internalization of the NR2A subunit of the NMDAR (Suvarna et al. 2005), and this mechanism contributes to the acute inhibitory actions of ethanol on channel function (Suvarna et al. 2005). In the hippocampus, ethanol stimulates Fyn-mediated phosphorylation of NR2B, which contributes to the development of acute tolerance to ethanol both ex vivo and in vivo (Ron 2004; Ron and Jurd 2005). Fyn is also activated in the dorsal striatum in response to ethanol (Wang et al. 2007; Wang et al. 2010b). Interestingly, ethanol-stimulated phosphorylation of NR2B is not observed in a structurally related brain region, the NAc (Wang et al. 2007). Furthermore, Fyn-mediated activation and NR2B phosphorylation results in long-term facilitation (LTF) of NR2B-containing NMDARs in the dorsal striatum of ethanol exposed rodents (Wang et al. 2007). This LTF of NMDAR activity is centered in the dorsomedial striatum (DMS) (Wang et al. 2010b), a brain region implicated in goal-directed behaviors (Yin et al. 2005). Importantly, repeated daily systemic administration of ethanol leads to prolonged activation of Fyn, increased NR2B phosphorylation and membrane localization of the receptor subunit in the DMS (Wang et al. 2010b). These events are associated with a long-lasting increase in the activity of the NR2B-NMDARs in this brain region (Wang et al. 2010b). Furthermore, inhibition of NR2B–NMDARs or Src family PTKs in the DMS but not the DLS or the NAc significantly decreases operant self-administration of ethanol and reduces ethanol-primed reinstatement of ethanol seeking (Wang et al. 2007; Wang et al. 2010b). Together, these results suggest that Fyn phosphorylation of NR2B and subsequent up-regulation of NMDAR function within the DMS contribute to the maladaptive synaptic changes that promote excessive ethanol intake and relapse.
In contrast to the above results, a recent study by Wu and colleagues (Wu et al. 2010) showed that tyrosine phosphorylation of NR2B in the hippocampus is markedly reduced in rats fed a liquid ethanol diet. The discrepancy between Wu’s results and ours could be due to differences in paradigms. It is also important to note that the basal level of NR2B phosphorylation in Wu’s study was rather high, while we did not detect a significant basal level of NR2B phosphorylation in the dorsal striatum of control rats (Wang et al. 2007; Wang et al. 2010b).
3.2.2 Lyn and Rewarding Properties of Ethanol
As mentioned above, we recently found that Lyn negatively regulates the release of dopamine in SHSY5Y human neuroblastoma cells and in the mouse NAc (Gibb et al. 2011). Acute exposure of rodents to ethanol causes a rapid increase in extracellular dopamine in the NAc (Gonzales et al. 2004), and we found that overexpression of the active form of Lyn in the VTA blocks ethanol-mediated dopamine overflow. Dopamine transmission is a contributor to ethanol reward-related behaviors (Gonzales et al. 2004), and we observed an inverse relationship between the protein level and the activity of the kinase versus the rewarding properties of ethanol (Gibb et al. 2011). Place preference for ethanol was increased in Lyn knockout mice compared with wild type littermates (Gibb et al. 2011) but was reduced in mice overexpressing an active form of Lyn in VTA neurons compared with control mice (Gibb et al. 2011). Together, these results suggest that Lyn contributes to a mechanism that controls the extracellular levels of DA, and by doing so, the kinase reduces the rewarding properties of ethanol.
4 Scaffolding Proteins
Scaffolding proteins are a diverse group of proteins that allow for the orchestration of multiple signaling events, and provide a focal point of interaction between signaling proteins such as kinases, phosphatases, their substrates, intracellular organelles, the cytoskeletal network and the plasma membrane. Scaffolding proteins also provide platforms that allow spatially and temporally segregated events to occur. Changes in the protein–protein interactions between scaffolding proteins and their associated binding partners are potentially important consequences of neuroadaptation to ethanol. Here we review the contribution of three scaffolding proteins that play important roles in the actions of ethanol on the adult brain, although other proteins such as β-arrestin, and A-kinase anchoring proteins are likely to also be involved in mediating ethanol’s effects.
4.1 RACK1
RACK1 is a scaffolding protein that is highly expressed in the CNS (Ashique et al. 2006) and was originally identified as an anchoring protein of PKCβII (Ron et al. 1994). The RACK1 amino acid sequence is characterized by seven WD40 repeats that form a seven-blade β-propeller structure (Coyle et al. 2009; Smith et al. 1999), enabling the protein to interact with a large number of binding partners including several enzymes such as Fyn kinase (Yaka et al. 2002), focal adhesion kinase (FAK) (Kiely et al. 2009), receptor protein tyrosine phosphatase µ (PTPµ) (Mourton et al. 2001), the cyclic AMP-specific phosphodiesterase isoform PDE4D5 (Yarwood et al. 1999), as well as with the intracellular tails of receptors like the insulin-like growth factor 1 receptor (IGF-1R) (Kiely et al. 2002; Hermanto et al. 2002), the inositol 1, 4, 5-triphosphate receptor (Patterson et al. 2004) and the NR2B subunit of NMDARs (Yaka et al. 2002). Interestingly, the intracellular compartmentalization of RACK1 changes in response to stimuli. For example, cellular stress such as hypoxia and heat shock results in RACK1 association with cytoplasmic stress granules (Arimoto et al. 2008). In contrast, upon activation of PKCβII, RACK1 shuttles active PKCβII to its site of action (Ron et al. 1999), whereas activation of PKA induces translocation of RACK1 to the nucleus (Yaka et al. 2003a; He et al. 2010). Exposure of cells to ethanol changes the intracellular localization of RACK1 via a mechanism that requires activation of PKA (Ron et al. 2000; He et al. 2002; Yaka et al. 2003b; Wang et al. 2007). One of the consequences of RACK1 nuclear localization is the inhibition of PKCβII translocation which was observed both in cultured cells and in vivo (Ron et al. 2000). Furthermore, under basal conditions, RACK1 interacts with and localizes Fyn kinase in close proximity to the NR2B subunit of the NMDAR, but inhibits the ability of Fyn to phosphorylate the channel until the appropriate signal occurs (Yaka et al. 2002; Yaka et al. 2003a; Thornton et al. 2004). Formation of this tri-molecular complex is not ubiquitous in the brain; it is found in hippocampus and dorsal striatum, but not in the cerebral cortex or NAc (Yaka et al. 2003a; Wang et al. 2007) (see also section on Src, Fyn and modulation of NMDAR function in response to ethanol). In the hippocampus and dorsal striatum, acute ex vivo ethanol treatment releases RACK1 from the NMDAR complex, which enables Fyn to phosphorylate NR2B (Yaka et al. 2003b; Wang et al. 2007). In addition, ethanol-stimulated translocation of RACK1 to the nucleus increases expression of BDNF in hippocampal and dorsal striatal neurons (McGough et al. 2004). In SHSY5Y cells, nuclear RACK1 localizes at the promoter IV region of the BDNF gene, resulting in chromatin modifications that lead to promoter-controlled BDNF exon IV transcription (He et al. 2010). It will be of great interest to determine if RACK1-dependent epigenetic modulation of BDNF expression contributes to ethanol’s actions in the brain (see also section on Epigenetic regulation of gene expression). Finally, in vivo evidence suggests that RACK1-mediated increases in BDNF levels in the dorsal striatum are part of a homeostatic pathway that regulates behaviors such as voluntary ethanol intake and ethanol sensitization (McGough et al. 2004; Jeanblanc et al. 2006) (see also section on Receptor Tyrosine Kinases). In summary, RACK1 provides an example of how changes in the compartmentalization of a scaffolding protein, as well as modifications in protein–protein interactions, can lead to the inhibition or activation of numerous signaling pathways in response to ethanol exposure.
4.2 PSD-95
The postsynaptic density protein of 95 kDa (PSD-95) is a core scaffolding protein that clusters NMDARs at glutamatergic synapses, connecting the receptors to the cytoskeleton and to signaling proteins that regulate channel function (Kim and Sheng 2004). PSD-95 has been implicated in synaptic plasticity underlying learning and memory (Kim and Sheng 2004). A recent study shows that PSD-95 knockout mice exhibit greater signs of ethanol intoxication and show less voluntary ethanol intake than wild type littermates (Camp et al. 2011). Although both genotypes showed similar levels of ethanol preference, wild type, but not PSD-95 knockout mice, maintained their preference for ethanol when tested 14 days later. Surprisingly, the deficits attributed to deletion of PSD-95 do not seem to involve altered NMDAR function since MK801, an NMDAR antagonist-enhanced ethanol intoxication to a similar extent in both genotypes (Camp et al. 2011). These results suggest that PSD-95 contributes to the level of ethanol intoxication, which can influence ethanol intake. In addition, these results imply that PSD-95 contributes to reward memory. However, the mechanism by which PSD-95 contributes to ethanol’s actions in vivo has yet to be unraveled.
4.3 Homer
Homer proteins are structurally related scaffolding proteins that are the products of three independent genes, Homer1, 2 and 3 (Fagni et al. 2002). These genes can give rise to constitutively expressed long isoforms (Homer1b, c, d, Homer 2a, b and Homer3) and an immediate-early gene (short) isoform (Homer1a) (Soloviev et al. 2000). The long Homer isoforms contain a coil–coil domain and leucine zipper motifs allowing them to assemble as multimers (Hayashi et al. 2006). Homer proteins contain the protein–protein interaction binding motif Enabled/vasodilator-stimulated phosphoprotein homology 1 (EVH1) that enables the direct interaction of homers with various proteins. Homer proteins connect ion channels and receptors to intracellular calcium storage, the cytoskeleton (Thomas 2002; Sala et al. 2001), and to various signaling cascades including ERK (Mao et al. 2005) and P13K (Rong et al. 2003).
Several studies by Szumlinski and colleagues indicate that the Homer2 isoform plays an important role in ethanol’s actions. Consumption of high levels of ethanol increases Homer2 expression in the NAc of mice, and this increase persists even 2 months after the last ethanol-drinking session (Klugmann and Szumlinski 2008; Cozzoli et al. 2009). Our finding that high levels of ethanol intake increase Homer proteins in the NAc via mTORC1 (Neasta et al. 2010) (see section on mTOR) may provide a mechanism for ethanol-mediated induction of Homer2 protein levels, although the antibodies we used did not differentiate between the long isoforms of Homer. Homer2 knockout mice consume less ethanol than wild type mice in a two-bottle choice continuous access paradigm, and do not develop ethanol place preference or locomotor sensitization to ethanol (Szumlinski et al. 2005). Homer2 knockout mice instead show ethanol place aversion and an increased hypnotic response to high doses of ethanol compared with wild type mice. In addition, Homer2 knockout mice do not show certain characteristic neurochemical changes associated with repeated ethanol administration (Szumlinski et al. 2005). In line with these findings, knockdown of Homer2 in the shell of the NAc reduces binge drinking in C57BL/6 mice (Cozzoli et al. 2009) and both the behavioral and neurochemical abnormalities in Homer2 knockout mice can be rescued by an administration of an adeno-associated virus (AAV) expressing Homer2 into the NAc (Klugmann and Szumlinski 2008). Interestingly, the contribution of the Homer gene to ethanol’s actions has also been observed in Drosophila; flies lacking the Homer gene show increased sensitivity to the sedative actions of ethanol and do not develop acute tolerance to ethanol (Urizar et al. 2007).
5 Epigenetic Regulation of Gene Expression
Transcription factors and other regulatory proteins regulate gene expression by binding to specific sites in the genome. Layered on top of this process are epigenetic mechanisms that control the way genomic DNA is packaged into chromatin and regulate access of transcription factors to target DNA sequences. These mechanisms regulate DNA methylation, covalent modification of histones and positioning of nucleosomes, and have the potential to produce long-lasting changes in gene expression that are self-perpetuating in the absence of the signals that initiate them. Chromatin changes may be transient or long lasting, mitotically transmissible, and in some cases inherited through meiosis to the next generation (Youngson and Whitelaw 2008; Dulac 2010). Epigenetic mechanisms have recently become topics of intense interest in the addiction field since they could produce persistent neuroadaptations that underlie drug tolerance and addiction (Tsankova et al. 2007). This field of research is still young and ethanol-induced modifications of histone and DNA are just now being identified. Here we describe a few recent examples in the nervous system.
5.1 DNA Methylation
Methylation of cytosine bases in DNA is mainly restricted to CpG dinucleotides and plays an important role in silencing of genes, inactivation of one X chromosome in females and in genomic imprinting of parental alleles. Proteins such as MECP2, which contain a methyl-CpG-binding domain (MBD) can inhibit, or in some cases facilitate, gene expression by binding to methylated CpG islands commonly located in gene promoter regions. DNA methyltransferases (DNMTs) catalyze DNA methylation and are critical for normal development. The enzymes are also expressed in post-mitotic neurons and recent evidence using DNMT inhibitors suggests that DNMTs mediate neuronal plasticity associated with memory (Miller and Sweatt 2007).
Although DNA methylation had been thought to be stable once established, recent evidence indicates that it can be dynamically regulated. For example, in response to maternal care, the glucocorticoid receptor promoter undergoes demethylation leading to increased expression of the receptor and a reduced response of the hypothalamic-pituitary axis to stress in the offspring (Szyf et al. 2005). The mechanisms responsible for DNA demethylation in adult neurons are not yet known.
In humans, chronic alcoholism is associated with increased circulating levels of homocysteine, probably due to impaired homocysteine metabolism (Bleich and Hillemacher 2009). Homocysteine is methylated to yield methionine, which can be metaboilized to S-adenosyl-l-methionine (SAM); SAM is a methyl group donor for DNMTs. This mechanism may explain why chronic exposure to alcohol can lead to hypermethylation and transcriptional silencing of some genes. For example, maternal ingestion of ethanol before or during gestation in mice leads to hypermethylation and reduced gene expression at the epigentically sensitive Agouti viable yellow (Avy) allele of the Agouti gene in the offspring (Kaminen-Ahola et al. 2010). Hypermethylation has also been found in cell cycle genes of ethanol exposed neural stem cells (Hicks et al. 2010). These findings suggest that DNA hypermethylation contributes to teratogenic effects of alcohol.
Ethanol exposure can also lead to demethylation and increased expression of certain genes, such as the NR2B subunit of NMDA receptors. Specifically, the abundance of the NR2B subunit is persistently up-regulated in C57BL/6 J mouse cortical neurons following chronic intermittent ethanol (CIE) treatment and, a recent analysis suggests that the mechanism involves DNA demethylation of CpG islands within the 5′ regulatory region of the Grin2b gene (Qiang et al. 2010). CIE treatment decreased the association of MeCP2 with chomatin and with regulatory regions of the gene. Conversely, methylation of these regions in vitro decreased binding of the CREB transcription factor to the Grin2b promoter. Treatment with SAM to promote DNA methylation prevented CIE-induced demethylation of the Grin2b promoter and CIE-induced increases in NR2B mRNA. The mechanism for this effect appeared to involve a CIE-mediated decrease in the level of mRNA for the DNA methyltransferase Dnmt1 that persists for at least 5 days after ethanol treatment; however, how this decrease occurs is not yet known.
5.2 Histone Acetylation and Up-Regulation of Neuropeptide Y
Covalent modification of histone is another mechanism for epigenetic regulation of gene expression. Modifications occur at N-terminal tail regions of histones and alter histone-DNA and histone–histone binding. Identified modifications include acetylation, methylation, phosphorylation, ubiquitination, ADP-ribosylation and SUMOylation (Kouzarides 2007). Most is known about histone acetylation in regulating neuronal gene expression. Histone acetyltransferases (HATs) add acetyl groups to specific lysine residues, which relaxes local chromatin structure and permits transcription factor binding to DNA (Tsankova et al. 2007). For example, the CREB binding protein (CBP) is a HAT and its recruitment by CREB facilitates CREB-mediated gene expression. Conversely, histone deacetylases (HDACs) remove acetyl groups, thereby promoting chromatin condensation and decreasing transcription.
Recently, Pandey and colleagues (Pandey et al. 2008a) reported that the anxiolytic effect of ethanol is associated with increased levels of CBP, histone acetylation and NPY in the rat CeA and MeA, whereas ethanol withdrawal is associated with increased anxiety-like behavior and decreased CBP, histone acetylation and NPY in these brain regions. To investigate whether changes in histone acetylation are causally related to anxiety and NPY expression, the authors treated ethanol-withdrawn rats with trichostatin A (TSA), an HDAC inhibitor. TSA restored histone acetylation and NPY expression, and reduced anxiety-like behavior in rats undergoing ethanol withdrawal. These findings suggest a link between these events, but these results should be viewed with caution given the action of HDACs on other genes, as well as the limited specificity of TSA (Dulac 2010).
5.3 MicroRNA
MicroRNAs (miRNAs) are a large family of non-coding RNAs that may control translation from as many as 60% of all protein-coding transcripts (Hicks et al. 2010). Primary miRNA transcripts show internal complementarity and thus adopt a stem-loop structure. These precursors are processed by ribonucleases to form mature 21–23 bp miRNAs that form miRNA-induced silencing complexes (miRISCs) by associating with the proteins Argonaute and GW182 [glycine-tryptophan (GW) repeat-containing protein of 182 kDa]. miRNAs regulate protein synthesis by base-pairing to target mRNAs, most commonly at their 3′-untranslated region, allowing miRISC-mediated repression of translation, or induction of deadenylation and degradation of mRNA.
Recent studies have documented ethanol-induced changes in up to 3% of miRNAs in models of alcohol-induced liver disease and teratogenesis, but less is known about ethanol regulation of miRNA in the adult nervous system (Miranda et al. 2010). A recent, in-depth study by Pietrzykowski and colleagues identified miR-9 as a key regulator of ethanol-sensitive BK channel splice variants that contributes to ethanol tolerance (Pietrzykowski et al. 2008). The BK channel is a high conductance calcium- and voltage-dependent potassium channel that is potentiated by ethanol (Treistman and Martin 2009). In the rat supraoptic nucleus and striatum these channels develop tolerance to ethanol, resulting from decreased ethanol sensitivity and reduced channel density. The decrease in BK channel density is associated with decreased mRNA encoding the main pore-forming subunit of the channel, and involving, in particular, those mRNA splice variants that recognize miR-9 and encode for subunits that are most ethanol-sensitive. Analysis of other predicted miR-9 target transcripts with a known role in ethanol’s actions revealed 8 whose expression was decreased and 2 whose expression was increased by exposure to 20 mM ethanol for 15 min. These targets encode several proteins of interest for understanding addiction, such as clock, the dopamine D2 receptor, the β2 subunit of GABAA receptors and the β1 subunit of voltage-gated calcium channels (Pietrzykowski et al. 2008). How ethanol rapidly increases levels of miR-9 is not known, but may involve increased expression or processing of its precursor.
6 Summary
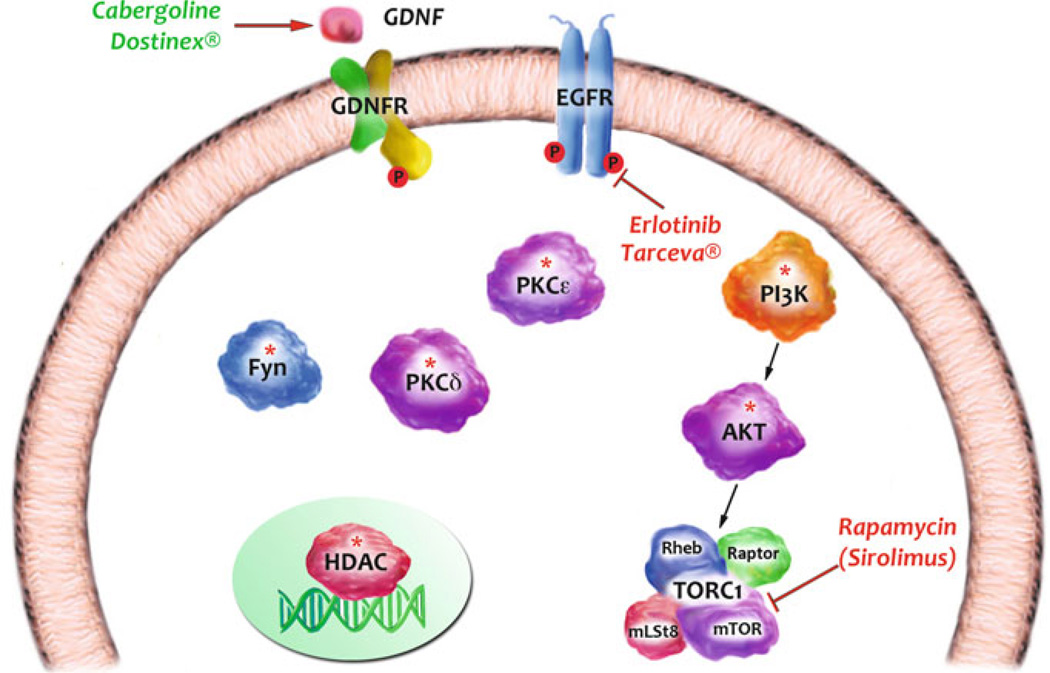
In this chapter, we covered progress that has been made on elucidating signaling pathways such as those involving PLC/PKC and P13K/AKT/mTORC1 that underlie or maintain behaviors associated with alcohol use disorders, as well as cascades initiated by growth factors such as GDNF that act in the opposite direction. We emphasized the role of signaling molecules such as protein kinases that control post-translational modifications in response to alcohol exposure in rodents, as protein kinases hold great promise as therapeutic targets for CNS diseases (Chico et al. 2009). Most research and development efforts on kinases as drug targets have been focused on oncology. However, signaling cascades are shared across cell types, and information generated in other systems can be of potential use for alcohol use disorders. For example, the EGFR inhibitor, TARCEVA® (Erlotinib), is used to treat non-small-cell lung cancer, yet was reported by Heberlein and colleagues to reduce voluntary consumption of ethanol (Corl et al. 2009) (Fig. 1). Another example is the mTORC1 inhibitor, rapamycin (sirolimus), which is used clinically to prevent rejection in organ transplantation yet was recently found in preclinical rodent models to reduce excessive ethanol intake and seeking (Neasta et al. 2010) (Fig. 1). In addition to mTORC1, its upstream kinase activators AKT and P13K, which show promise as potential drug targets in rodent models (Cozzoli et al. 2009; Neasta et al. 2011b) (Fig. 1), are currently being targeted by pharmaceutical companies for the treatment of various types of cancers (LoPiccolo et al. 2008). In addition, other signaling targets that are potentially of great interest for drug development are PKCε, PKCδ and Fyn (Hodge et al. 1999; Khasar et al. 1999; McMahon et al. 2000; Yaka et al. 2003b; Yaka et al. 2003c; Wang et al. 2007; Wang et al. 2010b), as well as HDAC (Pandey et al. 2008a) (Fig. 1). Of interest are the very recent advances that are being made in the development of small molecules that disrupt protein–protein interactions between signaling and scaffolding proteins (Arkin and Whitty 2009; Blazer and Neubig 2009) that may allow a high degree of desirable specificity in inhibitor action. Another intriguing possibility is the use of FDA-approved drugs such as cabergoline (Dostinex), which are approved for other indications but show promise in preclinical rodent models (Carnicella et al. 2009a) (Fig. 1). In summary, the examples described above put forward the possibility of developing small-molecule inhibitors or activators of specific signaling molecules as novel treatments for alcohol use disorders.
Fig. 1.
Ethanol alters the function of membrane and intracellular enzymes. Inhibitors or up-regulators of these targets can be developed as novel therapeutics for the treatment of alcohol use disorders. Depicted are examples of such targets. Asterisks denote targets for which inhibitors are in development. Red depicts FDA-approved kinase inhibitors. Green depicts an FDA-approved medication that up-regulates the expression of a protective gene
Acknowledgment
This work was supported by NIH grants AA013438, AA016848, AA014366 to D.R., AA017072 (D.R. and R.O.M), AA018316, AA013588, and U.S. Dept. of the Army contract W81XWH-07-1-0078 to R.O.M., and funds provided by the State of California for medical research on alcohol and substance abuse through the University of California (D.R. and R.O.M.).
The authors like to thank Carol Webb for editorial support.
Contributor Information
Dorit Ron, Email: dron@gallo.ucsf.edu.
Robert O. Messing, Email: romes@gallo.ucsf.edu.
References
- Abel T, Nguyen PV. Regulation of hippocampus-dependent memory by cyclic AMP-dependent protein kinase. Prog Brain Res. 2008;169:97–115. doi: 10.1016/S0079-6123(07)00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adell A, Myers RD. Increased alcohol intake in low alcohol drinking rats after chronic infusion of the [beta]-carboline harman into the hippocampus. Pharmacol Biochem Behav. 1994;49(4):949–953. doi: 10.1016/0091-3057(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3(5):383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol. 2008;10(11):1324–1332. doi: 10.1038/ncb1791. [DOI] [PubMed] [Google Scholar]
- Arkin MR, Whitty A. The road less traveled: modulating signal transduction enzymes by inhibiting their protein–protein interactions. Curr Opin Chem Biol. 2009;13(3):284–290. doi: 10.1016/j.cbpa.2009.05.125. [DOI] [PubMed] [Google Scholar]
- Arolfo MP, Yao L, Gordon AS, Diamond I, Janak PH. Ethanol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol Clin Exp Res. 2004;28(9):1308–1316. doi: 10.1097/01.alc.0000139821.38167.20. [DOI] [PubMed] [Google Scholar]
- Arolfo MP, Overstreet DH, Yao L, Fan P, Lawrence AJ, Tao G, Keung WM, Vallee BL, Olive MF, Gass JT, Rubin E, Anni H, Hodge CW, Besheer J, Zablocki J, Leung K, Blackburn BK, Lange LG, Diamond I. Suppression of heavy drinking and alcohol seeking by a selective ALDH-2 inhibitor. Alcohol Clin Exp Res. 2009;33(11):1935–1944. doi: 10.1111/j.1530-0277.2009.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashique AM, Kharazia V, Yaka R, Phamluong K, Peterson AS, Ron D. Localization of the scaffolding protein RACK1 in the developing and adult mouse brain. Brain Res. 2006;1069(1):31–38. doi: 10.1016/j.brainres.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Asyyed A, Storm D, Diamond I. Ethanol activates cAMP response element-mediated gene expression in select regions of the mouse brain. Brain Res. 2006;1106(1):63–71. doi: 10.1016/j.brainres.2006.05.107. [DOI] [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci U S A. 2008;105(24):8410–8415. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso-Chinea P, Cruz-Muros I, Aymerich MS, Rodriguez-Diaz M, Afonso-Oramas D, Lanciego JL, Gonzalez-Hernandez T. Striatal expression of GDNF and differential vulnerability of midbrain dopaminergic cells. Eur J Neurosci. 2005;21(7):1815–1827. doi: 10.1111/j.1460-9568.2005.04024.x. [DOI] [PubMed] [Google Scholar]
- Beggs HE, Soriano P, Maness PF. NCAM-dependent neurite outgrowth is inhibited in neurons from Fyn-minus mice. J Cell Biol. 1994;127(3):825–833. doi: 10.1083/jcb.127.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork K, Terasmaa A, Sun H, Thorsell A, Sommer WH, Heilig M. Ethanol-induced activation of AKT and DARPP-32 in the mouse striatum mediated by opioid receptors. Addict Biol. 2010;15(3):299–303. doi: 10.1111/j.1369-1600.2010.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer LL, Neubig RR. Small molecule protein–protein interaction inhibitors as CNS therapeutic agents: current progress and future hurdles. Neuropsychopharmacology. 2009;34(1):126–141. doi: 10.1038/npp.2008.151. [DOI] [PubMed] [Google Scholar]
- Bleich S, Hillemacher T. Homocysteine, alcoholism and its molecular networks. Pharmacopsychiatry. 2009;42(Suppl 1):S102–S109. doi: 10.1055/s-0029-1214396. [DOI] [PubMed] [Google Scholar]
- Bodrikov V, Leshchyns’ka I, Sytnyk V, Overvoorde J, den Hertog J, Schachner M. RPTPalpha is essential for NCAM-mediated p59fyn activation and neurite elongation. J Cell Biol. 2005;168(1):127–139. doi: 10.1083/jcb.200405073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Storustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. The delta subunit of gamma-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316(3):1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, Wehner JM. Ethanol consumption and behavioral impulsivity are increased in protein kinase Cγ null mutant mice. J Neurosci. 2001;21(21):RC180. doi: 10.1523/JNEUROSCI.21-21-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers BJ, Owen EH, Collins AC, Abeliovich A, Tonegawa S, Wehner JM. Decreased ethanol sensitivity and tolerance development in gamma-protein kinase C null mutant mice is dependent on genetic background. Alcohol Clin Exp Res. 1999;23(3):387–397. [PubMed] [Google Scholar]
- Bowers BJ, Collins AC, Tritto T, Wehner JM. Mice lacking PKC gamma exhibit decreased anxiety. Behav Genet. 2000;30(2):111–121. doi: 10.1023/a:1001951104208. [DOI] [PubMed] [Google Scholar]
- Brackenbury WJ, Davis TH, Chen C, Slat EA, Detrow MJ, Dickendesher TL, Ranscht B, Isom LL. Voltage-gated Na + channel beta1 subunit-mediated neurite outgrowth requires Fyn kinase and contributes to postnatal CNS development in vivo. J Neurosci. 2008;28(12):3246–3256. doi: 10.1523/JNEUROSCI.5446-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon EP, Idzerda RL, McKnight GS. PKA isoforms, neural pathways, and behaviour: making the connection. Curr Opin Neurobiol. 1997;7(3):397–403. doi: 10.1016/s0959-4388(97)80069-4. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26(11):657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508(1):65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Cadd G, McKnight GS. Distinct patterns of cAMP-dependent protein kinase gene expression in mouse brain. Neuron. 1989;3(1):71–79. doi: 10.1016/0896-6273(89)90116-5. [DOI] [PubMed] [Google Scholar]
- Camp M, Feyder M, Ihne J, Palachick B, Hurd B, Karlsson RM, Noronha B, Chen YC, Coba M, Grant S, Holmes A. A novel role for PSD-95 in mediating ethanol intoxication, drinking and place preference. Addict Biol. 2011;16(3):428–439. doi: 10.1111/j.1369-1600.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D. GDNF—a potential target to treat addiction. Pharmacol Ther. 2009;122(1):9–18. doi: 10.1016/j.pharmthera.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105(23):8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ahmadiantehrani S, He DY, Nielsen CK, Bartlett SE, Janak PH, Ron D. Cabergoline decreases alcohol drinking and seeking behaviors via glial cell line-derived neurotrophic factor. Biol Psychiatry. 2009a;66(2):146–153. doi: 10.1016/j.biopsych.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ahmadiantehrani S, Janak PH, Ron D. GDNF is an endogenous negative regulator of ethanol-mediated reward and of ethanol consumption after a period of abstinence. Alcohol Clin Exp Res. 2009b;33(6):1012–1024. doi: 10.1111/j.1530-0277.2009.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, He DY, Yowell QV, Glick SD, Ron D. Noribogaine, but not 18-MC, exhibits similar actions as ibogaine on GDNF expression and ethanol self-administration. Addict Biol. 2010;15(4):424–433. doi: 10.1111/j.1369-1600.2010.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Homeostatic plasticity during alcohol exposure promotes enlargement of dendritic spines. Eur J Neurosci. 2006;24(12):3496–3506. doi: 10.1111/j.1460-9568.2006.05247.x. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Sutton G. Acute ethanol inhibits extracellular signal-regulated kinase, protein kinase B, and adenosine 3′:5′-cyclic monophosphate response element binding protein activity in an age- and brain region-specific manner. Alcohol Clin Exp Res. 2005;294:672–682. doi: 10.1097/01.alc.0000158935.53360.5f. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24(18):4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chico LK, Van Eldik LJ, Watterson DM. Targeting protein kinases in central nervous system disorders. Nat Rev Drug Discov. 2009;8(11):892–909. doi: 10.1038/nrd2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Palop JJ, Puolivali J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2005;25(42):9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, Diamond I, Bonci A, Messing RO. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci. 2004;7(8):855–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- Choi D-S, Wei W, Deitchman JK, Kharazia VN, Lesscher HMB, McMahon T, Wang D, Qi Z-H, Sieghart W, Zhang C, Shokat KM, Mody I, Messing RO. Protein kinase C delta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J Neurosci. 2008;28:11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WH, Choi DS, Zhang H, Mu D, McMahon T, Kharazia VN, Lowell CA, Ferriero DM, Messing RO. Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J Clin Invest. 2004;114(1):49–56. doi: 10.1172/JCI21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WH, Wang D, McMahon T, Qi ZH, Song M, Zhang C, Shokat KM, Messing RO. GABAA receptor trafficking is regulated by protein kinase C(epsilon) and the N-ethylmaleimide-sensitive factor. J Neurosci. 2010;30(42):13955–13965. doi: 10.1523/JNEUROSCI.0270-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86(4):813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J. 2003;375(Pt 3):517–529. doi: 10.1042/BJ20031061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corl AB, Rodan AR, Heberlein U. Insulin signaling in the nervous system regulates ethanol intoxication in Drosophila melanogaster. Nat Neurosci. 2005;8(1):18–19. doi: 10.1038/nn1363. [DOI] [PubMed] [Google Scholar]
- Corl AB, Berger KH, Ophir-Shohat G, Gesch J, Simms JA, Bartlett SE, Heberlein U. Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanol-induced behaviors. Cell. 2009;137(5):949–960. doi: 10.1016/j.cell.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Correia SS, Duarte CB, Faro CJ, Pires EV, Carvalho AL. Protein kinase C gamma associates directly with the GluR4 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subunit. Effect on receptor phosphorylation. J Biol Chem. 2003;278(8):6307–6313. doi: 10.1074/jbc.M205587200. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61(1):10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle SM, Gilbert WV, Doudna JA. Direct link between RACK1 function and localization at the ribosome in vivo. Mol Cell Biol. 2009;29(6):1626–1634. doi: 10.1128/MCB.01718-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK. Binge drinking upregulates accumbens mGluR5-Homer2-P13K signaling: functional implications for alcoholism. J Neurosci. 2009;29(27):8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30(8):1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Csukai M, Chen C-H, Mochly-Rosen D. β′-COP: a COPI cotamer protein is also an epsilon protein kinase C specific RACK. FASEB J. 1997;11:A1187. [Google Scholar]
- Das J, Pany S, Rahman GM, Slater SJ. PKC epsilon has an alcohol-binding site in its second cysteine-rich regulatory domain. Biochem J. 2009;421(3):405–413. doi: 10.1042/BJ20082271. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C. Brain function and chromatin plasticity. Nature. 2010;465(7299):728–735. doi: 10.1038/nature09231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Costantini F, Saarma M, et al. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996;381(6585):789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- Eddison M, Guarnieri DJ, Cheng L, Liu CH, Mofatt KG, Davis G, Heberlein U. Arouser controls ethanol sensitivity through its regulation of synapse number. In revision. 2011 doi: 10.1016/j.neuron.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Engelman JA. Targeting P13K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Faccidomo S, Besheer J, Stanford PC, Hodge CW. Increased operant responding for ethanol in male C57BL/6 J mice: specific regulation by the ERK1/2, but not JNK, MAP kinase pathway. Psychopharmacology (Berl) 2009;204(1):135–147. doi: 10.1007/s00213-008-1444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagni L, Worley PF, Ango F. Homer as both a scaffold and transduction molecule. Sci STKE. 2002;2002(137):RE8. doi: 10.1126/stke.2002.137.re8. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6(3):215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31(6):939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Wilson WA, Swartzwelder HS. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiology. 2007;97(5):3806–3811. doi: 10.1152/jn.00101.2007. [DOI] [PubMed] [Google Scholar]
- Forsyth CB, Tang Y, Shaikh M, Zhang L, Keshavarzian A. Alcohol stimulates activation of Snail, epidermal growth factor receptor signaling, and biomarkers of epithelial-mesenchymal transition in colon and breast cancer cells. Alcohol Clin Exp Res. 2010;34(1):19–31. doi: 10.1111/j.1530-0277.2009.01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami K, Inanobe S, Kanemaru K, Nakamura Y. Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog Lipid Res. 2010;49(4):429–437. doi: 10.1016/j.plipres.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26(44):11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry RT, Rappaport MS, Dole VP. Serial determination of plasma ethanol concentrations in mice. Physiol Behav. 1983;31(4):529–532. doi: 10.1016/0031-9384(83)90077-x. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348(1):201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010;35(2):157–171. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb SL, Jeanblanc J, Barak S, Yowell QV, Yaka R, Ron D. Lyn kinase regulates mesolimbic dopamine release: Implication for alcohol reward. J Neurosci. 2011;31(6):2180–2187. doi: 10.1523/JNEUROSCI.5540-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Li TK, Badia-Elder NE. Neuropeptide Y reduces oral ethanol intake in alcohol-preferring (P) rats following a period of imposed ethanol abstinence. Alcohol Clin Exp Res. 2003;27(5):787–794. doi: 10.1097/01.ALC.0000065723.93234.1D. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav. 2008;90(3):475–480. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical and gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7(1):77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56(5):763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10(1):40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103(2):121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gordon AS, Yao L, Wu ZL, Coe IR, Diamond I. Ethanol alters the subcellular localization of delta- and epsilon protein kinase C in NG108–15 cells. Mol Pharmacol. 1997;52(4):554–559. doi: 10.1124/mol.52.4.554. [DOI] [PubMed] [Google Scholar]
- Gordon AS, Yao L, Jiang Z, Fishburn CS, Fuchs S, Diamond I. Ethanol acts synergistically with a D2 dopamine agonist to cause translocation of protein kinase C. Mol Pharmacol. 2001;59(1):153–160. doi: 10.1124/mol.59.1.153. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict biol. 2007;12(1):30–34. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Rimondini R, Neznanova O, Sommer WH, Heilig M. Neuroplasticity in brain reward circuitry following a history of ethanol dependence. Eur J Neurosci. 2008;27(8):1912–1922. doi: 10.1111/j.1460-9568.2008.06159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Proctor WR, McQuilkin SJ, Klein RL, Mascia MP, Whatley V, Whiting PJ, Dunwiddie TV. Ethanol increases GABAA responses in cells stably transfected with receptor subunits. Alcoholism: clinical and experimental research. 1995;19:226–232. doi: 10.1111/j.1530-0277.1995.tb01496.x. [DOI] [PubMed] [Google Scholar]
- Harris RA, Mihic SJ, Brozowski S, Hadingham K, Whiting PJ. Ethanol, flunitrazepam, and pentobarbital modulation of GABAA receptors expressed in mammalian cells and Xenopus oocytes. Alcohol Clin Exp Res. 1997;21(3):444–451. doi: 10.1111/j.1530-0277.1997.tb03789.x. [DOI] [PubMed] [Google Scholar]
- Hauge C, Frodin M. RSK and MSK in MAP kinase signalling. J Cell Sci. 2006;119(15):3021–3023. doi: 10.1242/jcs.02950. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Huganir RL. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J Neurosci. 2004;24(27):6152–6160. doi: 10.1523/JNEUROSCI.0799-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi MK, Ames HM, Hayashi Y. Tetrameric hub structure of postsynaptic scaffolding protein homer. J Neurosci. 2006;26(33):8492–8501. doi: 10.1523/JNEUROSCI.2731-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DY, Vagts AJ, Yaka R, Ron D. Ethanol induces gene expression via nuclear compartmentalization of receptor for activated C kinase 1. Mol Pharmacol. 2002;62(2):272–280. doi: 10.1124/mol.62.2.272. [DOI] [PubMed] [Google Scholar]
- He DY, Neasta J, Ron D. Epigenetic regulation of BDNF expression via the scaffolding protein RACK1. J Biol Chem. 2010;285(25):19043–19050. doi: 10.1074/jbc.M110.100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein A, Muschler M, Wilhelm J, Frieling H, Lenz B, Groschl M, Kornhuber J, Bleich S, Hillemacher T. BDNF and GDNF serum levels in alcohol-dependent patients during withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1060–1064. doi: 10.1016/j.pnpbp.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38(4):213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Ladenheim EE, Lyons WE. Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (±) mice. J Neurochem. 2003;85(5):1139–1147. doi: 10.1046/j.1471-4159.2003.01748.x. [DOI] [PubMed] [Google Scholar]
- Hermanto U, Zong CS, Li W, Wang LH. RACK1, an insulin-like growth factor I (IGF-I) receptor-interacting protein, modulates IGF-I-dependent integrin signaling and promotes cell spreading and contact with extracellular matrix. Mol Cell Biol. 2002;22(7):2345–2365. doi: 10.1128/MCB.22.7.2345-2365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks SD, Middleton FA, Miller MW. Ethanol-induced methylation of cell cycle genes in neural stem cells. J Neurochem. 2010;114(6):1767–1780. doi: 10.1111/j.1471-4159.2010.06886.x. [DOI] [PubMed] [Google Scholar]
- Hirai T, Chida K. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem. 2003;133(1):1–7. doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2(11):997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Raber J, McMahon T, Walter H, Sanchez-Perez AM, Olive MF, Mehmert K, Morrow AL, Messing RO. Decreased anxiety-like behavior, reduced stress hormones, and neurosteroid supersensitivity in mice lacking protein kinase C epsilon. J Clin Invest. 2002;110(7):1003–1010. doi: 10.1172/JCI15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33(2):67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Cascini MG, Gordon AS, Diamond I, Bonci A. Cooperative activation of dopamine D1 and D2 receptors increases spike firing of nucleus accumbens neurons via G-protein betagamma subunits. J Neurosci. 2003;23(12):5079–5087. doi: 10.1523/JNEUROSCI.23-12-05079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen P, Myers RD. Anatomical localization in hippocampus of tetrahydro-beta-carboline-induced alcohol drinking in the rat. Alcohol. 1987;4(3):181–187. doi: 10.1016/0741-8329(87)90041-3. [DOI] [PubMed] [Google Scholar]
- Hyytiä P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283(1–3):151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Ingman K, Soini SL, Laitinen JT, Korpi ER. Effects of continuous opioid receptor blockade on alcohol intake and up-regulation of opioid receptor subtype signalling in a genetic model of high alcohol drinking. Naunyn Schmiedebergs Arch Pharmacol. 1999;360(4):391–401. doi: 10.1007/s002109900070. [DOI] [PubMed] [Google Scholar]
- Ibba F, Vinci S, Spiga S, Peana AT, Assaretti AR, Spina L, Longoni R, Acquas E. Ethanol-induced extracellular signal regulated kinase: role of dopamine D1 receptors. Alcohol Clin Exp Res. 2009;33(5):858–867. doi: 10.1111/j.1530-0277.2009.00907.x. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, McGough NN, Logrip ML, Phamluong K, Janak PH, Ron D. The dopamine D3 receptor is part of a homeostatic pathway regulating ethanol consumption. J Neurosci. 2006;26(5):1457–1464. doi: 10.1523/JNEUROSCI.3786-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, Ron D. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009;29(43):13494–13502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZL, Ye J. Protein kinase C(epsilon) is involved in ethanol potentiation of glycine-gated Cl(-) current in rat neurons of ventral tegmental area. Neuropharmacology. 2003;44(4):493–502. doi: 10.1016/s0028-3908(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis JC, Hu S, Altrock BW, Fox GM. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85(7):1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Gingrich JR, Salter MW. Src in synaptic transmission and plasticity. Oncogene. 2004;23(48):8007–8016. doi: 10.1038/sj.onc.1208158. [DOI] [PubMed] [Google Scholar]
- Kaminen-Ahola N, Ahola A, Maga M, Mallitt KA, Fahey P, Cox TC, Whitelaw E, Chong S. Maternal ethanol consumption alters the epigenotype and the phenotype of offspring in a mouse model. PLoS Genet. 2010;6(1):e1000811. doi: 10.1371/journal.pgen.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24(1):253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely PA, Sant A, O’Connor R. RACK1 is an IGF-1 Receptor interacting protein that can regulate IGF-1- mediated Akt activation and protection from cell death. J Biol Chem. 2002;8(6):6. doi: 10.1074/jbc.M201758200. [DOI] [PubMed] [Google Scholar]
- Kiely PA, Baillie GS, Barrett R, Buckley DA, Adams DR, Houslay MD, O’Connor R. Phosphorylation of RACK1 on tyrosine 52 by c-Abl is required for insulin-like growth factor I-mediated regulation of focal adhesion kinase. J Biol Chem. 2009;284(30):20263–20274. doi: 10.1074/jbc.M109.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5(10):771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Klugmann M, Szumlinski KK. Targeting Homer genes using adeno-associated viral vector: lessons learned from behavioural and neurochemical studies. Behav Pharmacol. 2008;19(5–6):485–500. doi: 10.1097/FBP.0b013e32830c369f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21(3):467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O’Buckley TK, Grobin AC, Morrow AL. Chronic ethanol consumption enhances internalization of alpha1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem. 2003;86(3):700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Lane BM, Morrow AL. Differential effects of systemic ethanol administration on protein kinase cepsilon, gamma, and beta isoform expression, membrane translocation, and target phosphorylation: reversal by chronic ethanol exposure. J Pharmacol Exp Ther. 2006;319(3):1366–1375. doi: 10.1124/jpet.106.110890. [DOI] [PubMed] [Google Scholar]
- Kumar S, Suryanarayanan A, Boyd KN, Comerford CE, Lai MA, Ren Q, Morrow AL. Ethanol reduces GABAA alpha1 subunit receptor surface expression by a protein kinase Cgamma-dependent mechanism in cultured cerebral cortical neurons. Mol Pharmacol. 2010;77(5):793–803. doi: 10.1124/mol.109.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CC, Kuo TI, Lin HH. The role of protein kinase A in acute ethanol-induced neurobehavioral actions in rats. Anesth Analg. 2007;105(1):89–96. doi: 10.1213/01.ane.0000263030.13249.36. [DOI] [PubMed] [Google Scholar]
- Laron Z. Insulin and the brain. Arch Physiol Biochem. 2009;115(2):112–116. doi: 10.1080/13813450902949012. [DOI] [PubMed] [Google Scholar]
- Lee AM, Messing RO. Protein kinases and addiction. Ann N Y Acad Sci. 2008;1141:22–57. doi: 10.1196/annals.1441.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM, Do LH, Andreadis A, Van Hoesen G, Ksiezak-Reding H. Phosphorylation of tau by fyn: implications for Alzheimer’s disease. J Neurosci. 2004;24(9):2304–2312. doi: 10.1523/JNEUROSCI.4162-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Huang CC, Wu MY, Hsu KS. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J Biol Chem. 2005;280(18):18543–18550. doi: 10.1074/jbc.M414112200. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, McMahon T, Lasek AW, Chou WH, Connolly J, Kharazia V, Messing RO. Amygdala protein kinase C epsilon regulates corticotropin-releasing factor and anxiety-like behavior. Genes Brain Behav. 2008;7(3):323–333. doi: 10.1111/j.1601-183X.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, Wallace MJ, Zeng L, Wang V, Deitchman JK, McMahon T, Messing RO, Newton PM. Amygdala protein kinase C epsilon controls alcohol consumption. Genes Brain Behav Feb 11. 2009 doi: 10.1111/j.1601-183X.2009.00485.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Li J, Li YH, Yuan XR. Changes of phosphorylation of cAMP response element binding protein in rat nucleus accumbens after chronic ethanol intake: naloxone reversal. Acta Pharmacol Sin. 2003;24(9):930–936. [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27(45):12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. Faseb J. 2008;22(7):2393–2404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Escalating ethanol intake is associated with altered corticostriatal BDNF expression. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06073.x. [in Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35(4):605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the P13K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11(1–2):32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29(12):695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Maas JW, Jr, Vogt SK, Chan GC, Pineda VV, Storm DR, Muglia LJ. Calcium-stimulated adenylyl cyclases are critical modulators of neuronal ethanol sensitivity. J Neurosci. 2005;25(16):4118–4126. doi: 10.1523/JNEUROSCI.4273-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldve RE, Zhang TA, Ferrani-Kile K, Schreiber SS, Lippmann MJ, Snyder GL, Fienberg AA, Leslie SW, Gonzales RA, Morrisett RA. DARPP-32 and regulation of the ethanol sensitivity of NMDA receptors in the nucleus accumbens. Nat Neurosci. 2002;5(7):641–648. doi: 10.1038/nn877. [DOI] [PubMed] [Google Scholar]
- Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005;25(10):2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-García E, Darbra S, Pallarès M. Intrahippocampal allopregnanolone decreases voluntary chronic alcohol consumption in non-selected rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(4):823–831. doi: 10.1016/j.pnpbp.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10(9):1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Matsushita S, Arai H, Matsui T, Yuzuriha T, Urakami K, Masaki T, Higuchi S. Brain-derived neurotrophic factor gene polymorphisms and Alzheimer’s disease. J Neural Transm. 2005;112(5):703–711. doi: 10.1007/s00702-004-0210-3. [DOI] [PubMed] [Google Scholar]
- McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, Kharazia V, Janak PH, Ron D. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004;24(46):10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon T, Andersen R, Metten P, Crabbe JC, Messing RO. Protein kinase C epsilon mediates up-regulation of N-type calcium channels by ethanol. Mol Pharmacol. 2000;57(1):53–58. [PubMed] [Google Scholar]
- Merchenthaler I, Liposits Z, Reid JJ, Wetsel WC. Light and electron microscopic immunocytochemical localization of PKCδ immunoreactivity in the rat central nervous system. J Comp Neurol. 1993;336:378–399. doi: 10.1002/cne.903360306. [DOI] [PubMed] [Google Scholar]
- Messing RO. Alcohol and the nervous system. In: Aminoff MJ, editor. Neurology and General Medicine. 3rd edn. New York: Churchill Livingstone; 2007. pp. 721–734. [Google Scholar]
- Messing RO, Petersen PJ, Henrich CJ. Chronic ethanol exposure increases levels of protein kinase C δ and ε and protein kinase C-mediated phosphorylation in cultured neural cells. J Biol Chem. 1991;266:23428–23432. [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53(6):857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Miranda RC, Pietrzykowski AZ, Tang Y, Sathyan P, Mayfield D, Keshavarzian A, Sampson W, Hereld D. MicroRNAs: master regulators of ethanol abuse and toxicity? Alcohol Clin Exp Res. 2010;34(4):575–587. doi: 10.1111/j.1530-0277.2009.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra K, Pandey SC. The decreased cyclic-AMP dependent-protein kinase A function in the nucleus accumbens: a role in alcohol drinking but not in anxiety-like behaviors in rats. Neuropsychopharmacology. 2006;31(7):1406–1419. doi: 10.1038/sj.npp.1300900. [DOI] [PubMed] [Google Scholar]
- Miyame M, Diamond I, Weiner MW, Camacho SA, Figueredo VM. Regular alcohol consumption mimics cardiac preconditioning by protecting against ischemia-reperfusion injury. Proc Nat Acad Sci. 1997;94:3235–3239. doi: 10.1073/pnas.94.7.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760(1–2):94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Pandey SC. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2011;16(2):238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourton T, Hellberg CB, Burden-Gulley SM, Hinman J, Rhee A, Brady-Kalnay SM. The PTPmu protein tyrosine phosphatase binds and recruits the scaffolding protein RACK1 to cell–cell contacts. J Biol Chem. 2001;13:13. doi: 10.1074/jbc.M010823200. [DOI] [PubMed] [Google Scholar]
- Nagy J. Alcohol related changes in regulation of NMDA receptor functions. Curr Neuropharmacol. 2008;6(1):39–54. doi: 10.2174/157015908783769662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J Biol Chem. 1990;265:1946–1951. [PubMed] [Google Scholar]
- Naik MU, Benedikz E, Hernandez I, Libien J, Hrabe J, Valsamis M, Dow-Edwards D, Osman M, Sacktor TC. Distribution of protein kinase Mzeta and the complete protein kinase C isoform family in rat brain. J Comp Neurol. 2000;426(2):243–258. doi: 10.1002/1096-9861(20001016)426:2<243::aid-cne6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci U S A. 2010;107:424–433. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell QV, Carnicella S, Ron D. AKT signaling pathway in the nucleus accumbens mediates excessive alcohol drinking behaviors. Biol Psychiatry. 2011a doi: 10.1016/j.biopsych.2011.03.019. [In Revision] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Hamida SB, Yowell QV, Carnicella S, Ron D. AKT signaling pathway in the nucleus accumbens mediates excessive alcohol drinking behaviors. Biol Psychiatry. 2011b doi: 10.1016/j.biopsych.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298(3):E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton PM, Ron D. Protein kinase C and alcohol addiction. Pharmacol Res. 2007;55(6):570–577. doi: 10.1016/j.phrs.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Neznanova O, Bjork K, Rimondini R, Hansson AC, Hyytia P, Heilig M, Sommer WH. Acute ethanol challenge inhibits glycogen synthase kinase-3beta in the rat prefrontal cortex. Int J Neuropsychopharmacol. 2009;12(2):275–280. doi: 10.1017/S1461145708009620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303(5663):1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9(7):484–496. [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Messing RO, Hodge CW. Reduced operant ethanol self-administration and in vivo mesolimbic dopamine responses to ethanol in PKCε-deficient mice. Eur J Neurosci. 2000;12(11):4131–4140. doi: 10.1046/j.1460-9568.2000.00297.x. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Mittal N, Lumeng L, Li TK. Involvement of the cyclic AMP-responsive element binding protein gene transcription factor in genetic preference for alcohol drinking behavior. Alcohol Clin Exp Res. 1999a;23(9):1425–1434. [PubMed] [Google Scholar]
- Pandey SC, Zhang D, Mittal N, Nayyar D. Potential role of the gene transcription factor cyclic AMP-responsive element binding protein in ethanol withdrawal-related anxiety. J Pharmacol Exp Ther. 1999b;288(2):866–878. [PubMed] [Google Scholar]
- Pandey SC, Roy A, Mittal N. Effects of chronic ethanol intake and its withdrawal on the expression and phosphorylation of the creb gene transcription factor in rat cortex. J Pharmacol Exp Ther. 2001;296(3):857–868. [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H. The decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcohol Clin Exp Res. 2003;27(3):396–409. doi: 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H, Xu T. Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. J Neurosci. 2004;24(21):5022, 5030. doi: 10.1523/JNEUROSCI.5557-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115(10):2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26(32):8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008a;28(14):3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci. 2008b;28(10):2589–2600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Besnard A, Herve D, Pages C, Heck N, Girault JA, Caboche J, Vanhoutte P. Cyclic Adenosine Monophosphate-Independent Tyrosine Phosphorylation of NR2B Mediates Cocaine-Induced Extracellular Signal-Regulated Kinase Activation. Biol Psychiatry. 2011;69(3):218–227. doi: 10.1016/j.biopsych.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Patterson RL, van Rossum DB, Barrow RK, Snyder SH. RACK1 binds to inositol 1, 4, 5-trisphosphate receptors and mediates Ca2 + release. Proc Natl Acad Sci U S A. 2004;101(8):2328–2332. doi: 10.1073/pnas.0308567100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, Siegelmann HT, Treistman SN. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59(2):274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon NA, Menoud A, Tseng JL, Zurn AD, Aebischer P. Neuronal GDNF expression in the adult rat nervous system identified by in situ hybridization. Eur J Neurosci. 1997;9(3):463–471. doi: 10.1111/j.1460-9568.1997.tb01623.x. [DOI] [PubMed] [Google Scholar]
- Prakash A, Zhang H, Pandey SC. Innate differences in the expression of brain-derived neurotrophic factor in the regions within the extended amygdala between alcohol preferring and nonpreferring rats. Alcohol Clin Exp Res. 2008;32(6):909–920. doi: 10.1111/j.1530-0277.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, Bray GA, York DA, Wilson MA. Overexpression of neuropeptide Y in the central nucleus of the amygdala decreases ethanol self-administration in “anxious” rats. Alcohol Clin Exp Res. 2006;30(5):791–801. doi: 10.1111/j.1530-0277.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- Qi ZH, Song M, Wallace MJ, Wang D, Newton PM, McMahon T, Chou WH, Zhang C, Shokat KM, Messing RO. Protein kinase C epsilon regulates GABAA receptor sensitivity to ethanol and benzodiazepines through phosphorylation of gamma 2 subunits. J Biol Chem. 2007;282:33052–33063. doi: 10.1074/jbc.M707233200. [DOI] [PubMed] [Google Scholar]
- Qiang M, Denny A, Chen J, Ticku MK, Yan B, Henderson G. The site specific demethylation in the 5′-regulatory area of NMDA receptor 2B subunit gene associated with CIE-induced up-regulation of transcription. PLoS One. 2010;5(1):e8798. doi: 10.1371/journal.pone.0008798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Lutjens R, van der Stap LD, Sanna PP. Increased expression of protein kinase A inhibitor alpha (PKI-alpha) and decreased PKA-regulated genes in chronic intermittent alcohol exposure. Brain Res. 2007;1138:48–56. doi: 10.1016/j.brainres.2006.09.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex EB, Rankin ML, Ariano MA, Sibley DR. Ethanol regulation of D(1) dopamine receptor signaling is mediated by protein kinase C in an isozyme-specific manner. Neuropsychopharmacology. 2008;33(12):2900–2911. doi: 10.1038/npp.2008.16. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Freeman PA, Greengard P, Fienberg AA. Motivational effects of ethanol in DARPP-32 knock-out mice. J Neurosci. 2001;21(1):340–348. doi: 10.1523/JNEUROSCI.21-01-00340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D. Signaling cascades regulating NMDA receptor sensitivity to ethanol. Neuroscientist. 2004;10(4):325–336. doi: 10.1177/1073858404263516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Jurd R. The “ups and downs” of signaling cascades in addiction. Sci STKE. 2005;2005(309):re14. doi: 10.1126/stke.3092005re14. [DOI] [PubMed] [Google Scholar]
- Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci U S A. 1994;91(3):839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Jiang Z, Yao L, Vagts A, Diamond I, Gordon A. Coordinated movement of RACK1 with activated betaIIPKC. J Biol Chem. 1999;274(38):27039–27046. doi: 10.1074/jbc.274.38.27039. [DOI] [PubMed] [Google Scholar]
- Ron D, Vagts AJ, Dohrman DP, Yaka R, Jiang Z, Yao L, Crabbe J, Grisel JE, Diamond I. Uncoupling of {beta}IIPKC from its targeting protein RACK1 in response to ethanol in cultured cells and mouse brain. Faseb J. 2000;14(14):2303–2314. doi: 10.1096/fj.00-0143com. [DOI] [PubMed] [Google Scholar]
- Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. P13Kinase enhancer-Homer complex couples mGluRI to P13Kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6(11):1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11(2):103–112. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol Clin Exp Res. 2002;26(6):796–803. [PubMed] [Google Scholar]
- Ryabinin AE, Yoneyama N, Tanchuck MA, Mark GP, Finn DA. Urocortin 1 microinjection into the mouse lateral septum regulates the acquisition and expression of alcohol consumption. Neuroscience. 2008;151(3):780–790. doi: 10.1016/j.neuroscience.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31(1):115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5(4):317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Simpson C, Lutjens R, Koob G. ERK regulation in chronic ethanol exposure and withdrawal. Brain Res. 2002;948(1–2):186–191. doi: 10.1016/s0006-8993(02)03191-8. [DOI] [PubMed] [Google Scholar]
- Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One. 2009;4(6):e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TF, Gaitatzes C, Saxena K, Neer EJ. The WD repeat: a common architecture for diverse functions [In Process Citation] Trends Biochem Sci. 1999;24(5):181–185. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- Soloviev MM, Ciruela F, Chan WY, McIlhinney RA. Mouse brain and muscle tissues constitutively express high levels of Homer proteins. Eur J Biochem. 2000;267(3):634–639. doi: 10.1046/j.1432-1327.2000.01078.x. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biological psychiatry. 2008;63(2):139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Souroujon MC, Yao L, Chen H, Endemann G, Khaner H, Geeraert V, Schechtman D, Gordon AS, Diamond I, Mochly-Rosen D. State-specific monoclonal antibodies identify an intermediate state in epsilon protein kinase C activation. J Biol Chem. 2004;279(17):17617–17624. doi: 10.1074/jbc.M400962200. [DOI] [PubMed] [Google Scholar]
- Sperber BR, Boyle-Walsh EA, Engleka MJ, Gadue P, Peterson AC, Stein PL, Scherer SS, McMorris FA. A unique role for Fyn in CNS myelination. J Neurosci. 2001;21(6):2039–2047. doi: 10.1523/JNEUROSCI.21-06-02039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs CD, Slater SJ. Ethanol and protein kinase C. Alcohol Clin Exp Res. 1999;23(9):1552–1560. [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5(8):721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarna N, Borgland SL, Wang J, Phamluong K, Auberson YP, Bonci A, Ron D. Ethanol alters trafficking and functional N-methyl-D-aspartate receptor NR2 subunit ratio via H-Ras. J Biol Chem. 2005;280(36):31450–31459. doi: 10.1074/jbc.M504120200. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nairn AC, Greengard P. DARPP-32 mediates the actions of multiple drugs of abuse. AAPS J. 2005;7(2):E353–E360. doi: 10.1208/aapsj070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugman M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005;25(30):7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M, Weaver IC, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol. 2005;26(3–4):139–162. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24(44):9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Hashimoto H, Shintani N, Yamamoto A, Baba A. Reduced hypothermic and hypnotic responses to ethanol in PACAP-deficient mice. Regul Pept. 2004;123(1–3):95–98. doi: 10.1016/j.regpep.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste. Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396(6709):366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci. 2000;20(10):RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas U. Modulation of synaptic signalling complexes by Homer proteins. J Neurochem. 2002;81(3):407–413. doi: 10.1046/j.1471-4159.2002.00869.x. [DOI] [PubMed] [Google Scholar]
- Thornton C, Tang KC, Phamluong K, Luong K, Vagts A, Nikanjam D, Yaka R, Ron D. Spatial and temporal regulation of RACK1 function and NMDA receptor activity through WD40 motif-mediated dimerization. J Biol Chem. 2004 doi: 10.1074/jbc.M402316200. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Johnson J, Heilig M. Effect of the adenosine A2a receptor antagonist 3, 7-dimethyl-propargylxanthine on anxiety-like and depression-like behavior and alcohol consumption in Wistar Rats. Alcohol Clin Exp Res. 2007;31(8):1302–1307. doi: 10.1111/j.1530-0277.2007.00425.x. [DOI] [PubMed] [Google Scholar]
- Treistman SN, Martin GE. BK Channels: mediators and models for alcohol tolerance. Trends Neurosci. 2009;32(12):629–637. doi: 10.1016/j.tins.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupp M, Belluardo N, Funakoshi H, Ibanez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor- alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17(10):3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(5):355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Umemori H, Ogura H, Tozawa N, Mikoshiba K, Nishizumi H, Yamamoto T. Impairment of N-methyl-D-aspartate receptor-controlled motor activity in LYN-deficient mice. Neuroscience. 2003;118(3):709–713. doi: 10.1016/s0306-4522(03)00025-3. [DOI] [PubMed] [Google Scholar]
- Urizar NL, Yang Z, Edenberg HJ, Davis RL. Drosophila homer is required in a small set of neurons including the ellipsoid body for normal ethanol sensitivity and tolerance. J Neurosci. 2007;27(17):4541–4551. doi: 10.1523/JNEUROSCI.0305-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Leonardi-Essmann F, Sommer WH, Marston HM, Spanagel R. Glycine transporter-1 blockade leads to persistently reduced relapse-like alcohol drinking in rats. Biol Psychiatry. 2010 Oct 15;68(8):704–711. doi: 10.1016/j.biopsych.2010.05.029. [DOI] [PubMed] [Google Scholar]
- Walaas SI, Zhao YH, Sudol M. Neuronal localization of the tyrosine-specific protein kinase p62c-yes in rat basal ganglia. Neurochem Res. 1993;18(1):43–46. doi: 10.1007/BF00966921. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463(1–3):199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Newton PM, Oyasu M, McMahon T, Chou WH, Connolly J, Messing RO. Acute functional tolerance to ethanol mediated by protein kinase C epsilon. Neuropsychopharmacology. 2007;32(1):127–136. doi: 10.1038/sj.npp.1301059. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Nat Acad Sci. 2003;100(25):15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand G, Levine M, Zweifel L, Schwindinger W, Abel T. The cAMP-protein kinase A signal transduction pathway modulates ethanol consumption and sedative effects of ethanol. J Neurosci. 2001;21(14):5297–5303. doi: 10.1523/JNEUROSCI.21-14-05297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Storm DR. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Mol Pharmacol. 2003;63(3):463–468. doi: 10.1124/mol.63.3.463. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Yu SP. Novel regulation of Na, K-ATPase by Src tyrosine kinases in cortical neurons. J Neurochem. 2005;93(6):1515–1523. doi: 10.1111/j.1471-4159.2005.03147.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38(6):915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B–NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27(13):3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Ahmadiantehrani S, He DY, Barak S, Kharazia V, Ben Hamida S, Zapata A, Shippenberg TS, Ron D. Nucleus accumbens-derived glial cell line-derived neurotrophic factor is a retrograde enhancer of dopaminergic tone in the mesocorticolimbic system. J Neurosci. 2010a;30(43):14502–14512. doi: 10.1523/JNEUROSCI.3909-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010b;30(30):10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23(33):10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24(38):8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111(3):533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Wilkie MB, Besheer J, Kelley SP, Kumar S, O’Buckley TK, Morrow AL, Hodge CW. Acute ethanol administration rapidly increases phosphorylation of conventional protein kinase C in specific mammalian brain regions in vivo. Alcohol Clin Exp Res. 2007;31(7):1259–12567. doi: 10.1111/j.1530-0277.2007.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnar M, Brower KJ, Strobbe S, Ilgen M, Matsumoto H, Nowosad I, Sliwerska E, Burmeister M. Association between Val66Met brain-derived neurotrophic factor (BDNF) gene polymorphism and post-treatment relapse in alcohol dependence. Alcohol Clin Exp Res. 2009;33(4):693–702. doi: 10.1111/j.1530-0277.2008.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RW, Guillaud L. The role of epidermal growth factor and its receptors in mammalian CNS. Cytokine Growth Factor Rev. 2004;15(2–3):147–156. doi: 10.1016/j.cytogfr.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Wu PH, Coultrap S, Browning MD, Proctor WR. Correlated changes in NMDA receptor phosphorylation, functional activity, and sedation by chronic ethanol consumption. J Neurochem. 2010;115(5):1112–1122. doi: 10.1111/j.1471-4159.2010.06991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Thornton C, Vagts AJ, Phamluong K, Bonci A, Ron D. NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc Natl Acad Sci U S A. 2002;99(8):5710–5715. doi: 10.1073/pnas.062046299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, He DY, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide (PACAP(1–38)) enhances N-Methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem. 2003a;278(11):9630–9638. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]
- Yaka R, Phamluong K, Ron D. Scaffolding of Fyn kinase to the nmda receptor determines brain region sensitivity to ethanol. J Neurosci. 2003b;23(9):3623–3632. doi: 10.1523/JNEUROSCI.23-09-03623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Tang KC, Camarini R, Janak PH, Ron D. Fyn kinase and NR2B–containing NMDA receptors regulate acute ethanol sensitivity but not ethanol intake or conditioned reward. Alcohol Clin Exp Res. 2003c;27(11):1736–1742. doi: 10.1097/01.ALC.0000095924.87729.D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Marszalec W, Yeh JZ, Narahashi T. Effects of ethanol on tonic GABA currents in cerebellar granule cells and mammalian cells recombinantly expressing GABA(A) receptors. J Pharmacol Exp Ther. 2006;319(1):431–438. doi: 10.1124/jpet.106.106260. [DOI] [PubMed] [Google Scholar]
- Yang X, Horn K, Baraban JM, Wand GS. Chronic ethanol administration decreases phosphorylation of cyclic AMP response element-binding protein in granule cells of rat cerebellum. J Neurochem. 1998a;70(1):224–232. doi: 10.1046/j.1471-4159.1998.70010224.x. [DOI] [PubMed] [Google Scholar]
- Yang X, Horn K, Wand GS. Chronic ethanol exposure impairs phosphorylation of CREB and CRE-binding activity in rat striatum. Alcohol Clin Exp Res. 1998b;22(2):382–390. [PubMed] [Google Scholar]
- Yao L, Arolfo MP, Dohrman DP, Jiang Z, Fan P, Fuchs S, Janak PH, Gordon AS, Diamond I. Betagamma dimers mediate synergy of dopamine D2 and adenosine A2 receptor-stimulated PKA signaling and regulate ethanol consumption. Cell. 2002;109(6):733–743. doi: 10.1016/s0092-8674(02)00763-8. [DOI] [PubMed] [Google Scholar]
- Yao L, Fan P, Jiang Z, Mailliard WS, Gordon AS, Diamond I. Addicting drugs utilize a synergistic molecular mechanism in common requiring adenosine and Gi-beta gamma dimers. Proc Natl Acad Sci U S A. 2003;100(24):14379–14384. doi: 10.1073/pnas.2336093100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Fan P, Jiang Z, Gordon A, Mochly-Rosen D, Diamond I. Dopamine and ethanol cause translocation of epsilonPKC associated with epsilonRACK: cross-talk between cAMP-dependent protein kinase A and protein kinase C signaling pathways. Mol Pharmacol. 2008;73(4):1105–1112. doi: 10.1124/mol.107.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Fan P, Arolfo M, Jiang Z, Olive MF, Zablocki J, Sun HL, Chu N, Lee J, Kim HY, Leung K, Shryock J, Blackburn B, Diamond I. Inhibition of aldehyde dehydrogenase-2 suppresses cocaine seeking by generating THP, a cocaine use-dependent inhibitor of dopamine synthesis. Nat Med. 2010 doi: 10.1038/nm.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarwood SJ, Steele MR, Scotland G, Houslay MD, Bolger GB. The RACK1 signaling scaffold protein selectively interacts with the cAMP-specific phosphodiesterase PDE4D5 isoform. J Biol Chem. 1999;274(21):14909–14917. doi: 10.1074/jbc.274.21.14909. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22(2):513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70(5):304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngson NA, Whitelaw E. Transgenerational epigenetic effects. Annu Rev Genomics Hum Genet. 2008;9:233–257. doi: 10.1146/annurev.genom.9.081307.164445. [DOI] [PubMed] [Google Scholar]
- Zhang H, Pandey SC. Effects of PKA modulation on the expression of neuropeptide Y in rat amygdaloid structures during ethanol withdrawal. Peptides. 2003;24(9):1397–1402. doi: 10.1016/j.peptides.2003.08.008. [DOI] [PubMed] [Google Scholar]