Abstract
Objectives
Contemporary adhesives lose their bond strength to dentin regardless of the bonding system used. This loss relates to the hydrolysis of collagen matrix of the hybrid layers. The preservation of the collagen matrix integrity is a key issue in the attempts to improve the dentin bonding durability.
Methods
Dentin contains collagenolytic enzymes, matrix metalloproteinases (MMPs) and cysteine cathepsins, which are responsible for the hydrolytic degradation of collagen matrix in the bonded interface.
Results
The identities, roles and function of collagenolytic enzymes in mineralized dentin has been gathered only within last 15 years, but they have already been demonstrated to have an important role in dental hard tissue pathologies, including the degradation of the hybrid layer. Identifying responsible enzymes facilitates the development of new, more efficient methods to improve the stability of dentin-adhesive bond and durability of bond strength.
Significance
Understanding the nature and role of proteolytic degradation of dentin-adhesive interfaces has improved immensely and has practically grown to a scientific field of its own within only 10 years, holding excellent promise that stable resin-dentin bonds will be routinely available in a daily clinical setting already in a near future.
Introduction
Since the first study to demonstrate the rapid time-related loss of dentin bond strength [1], numerous studies have confirmed the finding both with etch-and-rinse (E&R) and SE (SE) adhesives [2]. The problem is specific for the resin-dentin bonds, as the resin –enamel bonds are very stable over time [3], and relates to the loss of of the hybrid layer collagen matrix [2].
Enzymatic degradation of the collagen matrix by host-derived enzymes plays a significant role in the destruction of the bonded interface [4,5]. To date, several matrix metalloproteinases (MMPs) and cysteine cathepsins have been identified in dentin, and suggested to be responsible for the digestion of collagen fibrils exposed at the adhesive interface. During the last 10 years, the understanding of the mechanisms involved in the proteolytic degradation of dentin-adhesive interfaces has gained immense attention and has practically grown to a scientific field of its own. The progression in this field has been rapid, and the clinical interest in measures to improve dentin bond durability is also increasing [6]. This has led to several tentative approaches to prevent such enzymatic activity. In general, the strategy to improve the durability of resin-dentin bonds relies on the ability to inhibit the activity of the enzymes. In this review, we will look at the presence, identity and function of the collagen-degrading proteolytic enzymes in dentin.
Dentin collagen: structural properties vs. bonding
Before going into details of dentinal enzymes, it is essential to briefly overview the dentin organic matrix to fully understand the complexity of the tissue in relation to the adhesion and function of the enzymes. Approximately 50 vol.% of dentin is composed of minerals, the rest being type I collagen and non-collagenous proteins (30 vol.%) and water (appr. 20 vol.%) [7]. During the mineralization of dentin and bone, collagen fibrils provide the aqueous compartment in which nanometer-sized apatite mineral crystals grow. In soft tissues (and in dentin and bone matrix prior mineralization), collagen intermolecular spaces contain highly ordered and tightly bound water molecules forming multi-layered cylinders around collagen molecules [8] (Figure 1). During the mineralization process, water within and between the fibrils is progressively replaced with minerals [9]. In mineralized dentin, there is no resin infiltration. To permit resin infiltration for adhesive retention, dental hard tissues are acid-etched by acids or acidic monomers to remove minerals that are replaced by rinse-water (E&R adhesives) or water used in SE primer-adhesives as a solvent (E&R and SE adhesives). Hydration of collagen prior to the monomer penetration is essential in order to avoid the matrix collapse due to the formation of interpeptide hydrogen bonds between collagen fibrils [10]. In addition, the ionizable moieties of acidic monomers in SE adhesives are also hydrophilic and promote water sorption over time.
Figure 1.

Representation of the progressive hydration of the collagen Gly-Ala peptide. Top row presents the perpendicular and the bottom row parallel view to the molecular axis at the same hydration level. (a) A view of the non-hydrated collagen, with the three peptide chains shown in different colors. (b) The first shell of water molecules (blue spheres), directly hydrogen-bonded to carbonyl, hydroxyl or amide groups on the peptide surface. (c) The second shell of water molecules, hydrogen bond to the water in the first shell, demonstrating the filling of the superhelical groove. (d) The third shell of water molecules. (Reproduced from [8] with permission.)
During application of adhesives, solvated comonomers are expected to replace the water and penetrate into and around collagen fibrils for proper hybridization [5]. While the interfibrillar spaces between collagen fibrils in hybrid layers are wide enough to allow small hydrophilic monomers (e.g. HEMA, TEGDMA) to penetrate between the fibrils, intrafibrillar penetration has been challenged. The inter-molecular distance in the lateral packing of collagen molecules within a collagen fibril (the space supposed to be occupied by monomer molecules) is within range of 1.26 nm to 1.33 nm. Since even the adhesive monomers (such as TEGDMA) units are approximately 2 nm in diameter, the complete infiltration of the adhesive material even at single monomer molecular level is restricted by the space available within a collagen fibril [11]. We will address this issue later in the article.
As adhesive comonomers diffuse into the water around collagen fibrils, their relatively high chemical concentrations (2–4 moles/L) lower the vapor pressure of water (Raoult’s law) making it more difficult to evaporate residual water using a dental air syringe [12]. The spaces between collagen fibrils are between 20–30 nm. These spaces contain proteoglycan hydrogels [13], that may restrict the free diffusion of some of the larger adhesive monomers, such as BisGMA (512 Da) compared to small monomers such as HEMA (100 Da). Figure 2 shows a schematic of collagen fibrils cut in cross-section (large circles) in a hybrid layer being infiltrated by a mixture of BisGMA/HEMA comonomers. Note that the small black dots, representing HEMA, diffuse more deeply than the large black dots representing BisGMA. This schematic was made to illustrate what might be responsible for a decreasing concentration of BisGMA that had been reported by Spencer and Wang [14] when they examined its distribution across hybrid layers by microRaman spectroscopy.
Figure 2.

Schematic of parallel collagen fibrils in demineralized dentin cut in cross-section to show the size of interfibrillar spaces. These spaces are not empty but contain proteoglycan hydrogels that may act as molecular sieves. Many dental adhesives are mixtures of comonomers. The large black dots on the right indicate dimethacrylates like BisGMA, while the smaller black dots indicate HEMA. BisGMA seems to only penetrate the top half of hybrid layers (reproduced from [170], with permission).
The schematic on the left shows that the interior of collagen fibrils is made up of hundreds of collagen molecules separated by water-filled spaces about 1.8 nm wide. Albumin, with a molecular diameter of 6 nm cannot enter “collagen water” but would be excluded as being too large (MW 68 kDa). Glucose (MW 180 Da), BGP (bone Gla protein or osteocalcin, MW 5700 Da) can equilibrate with collagen water even though the molecular diameter of osteocalcin is the same as the width of intermolecular spaces (1.8 nm in both cases) because collagen molecules are in constant rotational vibration (modified from [15]).
However, as each collagen fibril is made up of hundreds of collagen molecules that are closely packed together, Bertassoni et al. [11] argued that there is not sufficient space between packed collagen molecules (ca. 1.26–1.33 nm) to accommodate adhesive monomers (Figure 2).
Physiological experiments often provide insight into how molecules interact with collagen fibrils. Toroian et al. [15] pulverized bovine bone into small particles and packed them into 2 × 50 cm columns to permit gel filtration column chromatography of these particles before and after demineralization. They used bone collagen particles just like investigators usually use hydrated Sephadex beads. In typical gel filtration chromatography, large molecular weight molecules like blue dextran (106 Da) are too large to penetrate the Sephadex beads, so they elute with the void volume ahead of molecules that can penetrate the beads and hence, whose elution is delayed. By using phosphate ions, glucose, osteocalcin (5700 Da), cytochrome (12.3 kDa), fetuin (48 kDa), hemoglobin (64 kDa) and IgG (152 kDa), Toroian et al. [15] found that molecules less than 6 kDa easily diffuse between collagen molecules and entered “collagen water”, while molecules larger than 40 kDa were excluded from “collagen water”. However, they found that osteocalcin (5.7 kDa, molecular diameter 1.8 nm) did penetrate into “collagen water” even though x-ray diffraction studies confirmed that the Bragg spacing between hydrated collagen molecules is 1.8 nm (Figure 2). Generally, molecules whose diameters are equal to pore sizes in barriers are not permeable because the molecule would have to fit the pore perfectly. If they touched any part of the pore, they would be reflected away and not permeate.
Toroian et al. [15] conclude that hydrated collagen molecules function as if their intermolecular size is significantly larger than 1.8 nm due molecular vibration/rotation. The results support the hypothesis “that individual collagen molecules have substantial freedom to move in the lateral plane of the fibril”.
We conclude that if osteocalcin (MW 5700 Da) can equilibrate with collagen water, then BisGMA (512 Da) and HEMA (100 Da) should easily permeate into water located around and within collagen molecules. Whether water-immiscible monomers such as BisGMA can wet collagen molecules is debatable. Since BisGMA is usually solvated in ethanol or HEMA, and they are both water miscible, perhaps dimethacrylates like BisGMA can infiltrate the space between collagen molecules and polymerize to envelop them and prevent protease-induced collagen hydrolysis.
Complete coverage of the nanoscale irregularities on the collagen fibrils surface via passive monomer penetration may be difficult to achieve. These irregularities are caused by the 4 to 6 nm height difference between the fibril gap and overlap zones, and nanometer-scale microfibrils at the surface of dentin collagen fibrils [16]. Microfibrils, detected with X-ray diffraction, are discontinuous 5-mer repeats of collagen molecules with 4 to5 nm diameters [11,17,18]. Another potential hindrance may be proteoglycans (PGs), which with their glycosaminoglycan (GAG) carbohydrates envelop the collagen surface, retaining water [11]. The ability of polymeric resins to fully displace water from the crevices present on collagen fibril surfaces may require extremely low viscosity monomeric material and precise placement of the primer components to alter the surface energy of the fibril. Nanoleakage can occur in the absence of detectable gaps in the resin-dentin interface [19,20]. These “nanovoids” can be responsible for facilitating the diffusion of water molecules with diameters of about 100 pm, from the hydrated dentin [11], allowing slow but continuous degradation of the ester bonds in adhesive polymers. Degradation of the microfibrils would lead to nanoleakage, and with increasing void size, increase in dentinal fluid seepage would speed up the hydrolysis of both resins and matrix components, with eventual loss of bond strength. Others have argued that the ability of adhesive monomers to displace water from collagen is limited by their relative concentrations. Water has a concentration of 55 moles/L, while comonomers have concentrations of 3–4 moles/L [12]. Thus, although the adhesive monomer HEMA has been shown to “fit” into the groove on water-free collagen peptides in computer models [21], there are structural and chemical reasons why this may not occur.
Other components and features of dentin matrix may also have marked impacts on the durability of dentin bonds. These include e.g. collagen cross-linking and PGs in dentin. For the sake of clarity, these aspects will be described in detail in further paragraphs.
Enzymes in dentin
Matrix metalloproteinases
Matrix metalloproteinases (MMPs) are a family of Zn2+- and Ca2+-dependent enzymes, that in concert are able to degrade practically all ECM components, making them important components in many biological and pathological processes. Esterases and proteases are also classified as hydrolases. That is, they enzymatically add water across ester or peptide bonds. In humans, the MMP family contains 23 members that are frequently divided into six groups - collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs (MT-MMPs), and other MMPs - based on substrate specificity and homology [22,23]. Even though this classification is commonly used both for historical and practical reasons, it does not fully reflect the complexicity of MMP functions and biological activities, as most MMPs can degrade several substrates with variable specificity [22,23]. For example, collagenases-1 and -3 (MMP-1 and -13) can also degrade gelatin at a slow rate, and gelatinases (MMP-2 and -9) can degrade several types of collagen, especially type IV collagen (thus the old name “type IV collagenases”) [22–24]. Apart from their ECM degradation, MMPs also play important roles in the conversion of noncollagenous matrix proteins to signaling molecules that affect cell survival, proliferation, and differentiation [25].
MMPs are synthesized and mostly secreted as inactive proenzymes (zymogens), in which the so-called prodomain is present, inhibiting the functional activity of catalytic domain (Figure 3). In latent (non-activated) MMPs, the unpaired cysteine in the prodomain forms a bridge with the catalytic zinc (referred to as the “cysteine switch” mechanism [26]), preventing enzymatic activity. This conserved cysteine acts as a ligand for the catalytic zinc atom in the active site, excluding water molecules and rendering the enzyme inactive. Activation occurs when this linkage is disrupted by proteolytic cleavage of the propeptide by other MMPs, cysteine cathepsins or other proteinases, or chemically e.g. by amino phenyl mercuric acid (APMA), replacing the thiol group with water [24,25]. In addition to the pro- and catalytic domains, MMPs contain other domains responsible, for instance, for substrate specificity, recognition, and interaction [27]. Therefore, MMPs are often classified according to their molecular structures [25].
Figure 3.

A conserved cysteine residue (C) in the pro-domain coordinates with the Zn2+ ion at the functional site of the catalytic domain. The pro-domain is removed by cleavage in the pro-domain and between the pro-domain and the catalytic domain. The “propeller” is a hemopexin domain contained by most MMPs, and is attached to the catalytic domain by flexible hinge domain. The hemopexin domain e.g. mediates protein–protein interactions, contributes to substrate recognition, enzyme activation, and protease localization. Modified from [25]).
Collagen molecules contain a rigid helical center (Figure 4) with globular N- and C-terminal ends called telopeptides. In vertebrate MMP family, collagenases (MMP-1, -8, -13), MMP-2 (gelatinase A), and membrane-type 1-MMP (MT1-MMP, MMP-14) can cleave native triple-helical type I, II, and III collagens. They all cleave collagens into ¼- and ⅓-fragments at the Gly-Leu/Isoleu peptide bond where collagen peptide structure determines both specific cleavage and binding sites for MMPs [28]. The fragments denature at body temperature, and are further degraded by gelatinases and other non-specific tissue proteinases. Interstitial collagen consists of three α chains, and each chain winds around each other in a right-handed twist to form a triple helical structure with about 300 nm in length and 1.5 nm in diameter. This triple-helical structure makes interstitial collagens fibrils in their native configuration resistant to most vertebrate proteolytic enzymes. The substrate binding site of collagenases is located in a deep cleft with the entrance being only approximately 0.5 nm wide. This is not enough space to accommodate triple helical collagen with a diameter that is 3 times larger than the active site of the enzyme [27]. Currently, two mechanisms have been proposed to allow the degradation of collagen fibril by MMPs. Using a MMP-1 mutant that is unable to cleave collagen but retains the binding properties, Chung et al. [29] demonstrated that MMP-1 is capable of unwinding collagen fibril to allow the enzyme’s active site to cleave individual chains in succession [27,29] (Figure 4). The role of MMP in unwinding collagen peptide helices was further supported by the cleavage of unwound fibril by MMP-3 and neutrophil elastase, which normally do not degrade fibrillar collagen [27,29]. The similar binding-unwinding mechanisms have later been suggested for MMP-8, -2 and -14 [30]. This view was at least partially challenged by Perumal and others [28], when they demonstrated that the collagen region where cleavage begins, is fully protected by the collagen C-telopeptide (the terminal end of the molecule) restricting the access of MMPs to the cleavage site of the native collagen structure [28]. They suggest that the site must be exposed by proteolytical removal of C-terminal telopetide by telopeptidase or e.g. mechanical damage, before MMP can bind to the substrate’s “interaction domain” facilitating the triple-helix unwinding/dissociation function of the enzyme before collagenolysis. Alternatively, damage caused e.g. by physical loading may induce changes in fibril structure, such as breakage of cross-linkages at the C-terminus. This damage may expose the first collagen peptide chain to be cleaved by MMP, leading to a greater freedom of movement for other chains and their subsequent cleavage [28]. Collagen molecules distal to the original cleavage site would also become accessible to MMP cleavage, leading to further degradation [28]. This is an inviting hypothesis, as the damages caused to dentin collagenous matrix by demineralization in caries lesions, phosphoric acid or acidic monomers have been suggested to cause changes in the collagen molecular arrangement (e.g. breakage of cross-linkages) which may expose the catalytic binding site [11]. Interestingly, telopeptidase enzyme activity of MMP-9 has long been suggested to be important in bone matrix degradation [31]. MMP-9 is present in dentin [32], it is readily activated by acid pH changes [31,33–35], and telopeptidase activity of MMP-9 has been suggested to have a role in dentin matrix degradation in caries [35]. Alternatively, cysteine cathepsins may be involved. Cysteine cathepsins can activate MMPs [36], and this mechanism has been suggested to be involved in human dentinal caries lesions [37]. Cysteine cathepsins can also cleave C-terminal telopeptide of type I collagen [38], possibly exposing the collagenase cleavage site after being activated by acids.
Figure 4. Steps involved in collagenolysis by collagenase.
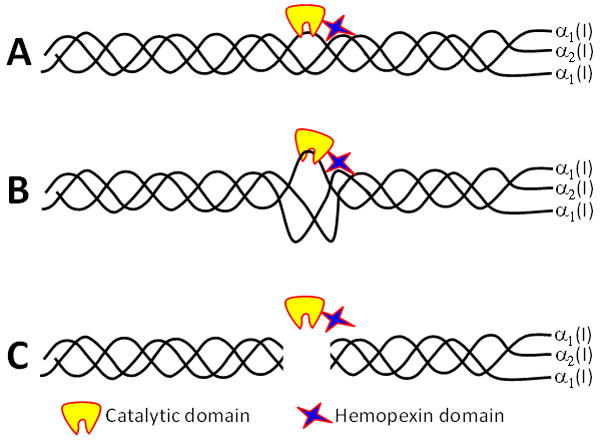
A) Collagenase bound to triple helical collagen via cooperative interaction of catalytic and hemopexin domain, but is unable to cleave in its native state due to the smallness of the cleft of the active site.
B) Unwinding the triple-helical conformation allows single α-chain to be presented to the active site of the catalytic domain for peptide hydrolysis.
C) All three α-chain are successively hydrolyzed, resulting with collagen molecule to be cut into ¼ and ⅓ fragments. (Adapted from [27]).
Although intermolecular cross-links can occur along both helical and telopeptide regions, they are especially common in the telopeptide domains. When Garnero et al. [39] incubated acid-etched bone powder with pure, soluble MMP-2, they saw increased liberation of soluble collagen peptides. When the peptides were isolated by SDS-PAGE electrophoresis, none of the bands were due to ¼ or ⅓ length collagen fragments. Using specific antibodies directed at various cross-linked telopeptides, they found that MMP-2 released relatively long cross-linked C-terminal telopeptide fragments called ICTP fragments. When they incubated bone powder with cathepsin K, the released telopeptides were much shorter. These were called CTX peptides. Both classes of peptides are now measured in plasma and urine in postmenopausal patients as indices of the degree of bone turnover.
When Osorio et al. [40] incubated exogenous soluble MMP-2 with completely demineralized beams of dentin, the dentin released ICTP telopeptide fragments. The control beams incubated in control media without MMP-2 also released ICTP peptide fragments, albeit at lower concentrations, indicating that the MMP-2 isolated from dentin [32,41,42] may function as a “telopeptidase”. If gelatinases MMP-2 and -9 can hydrolyze the bulky telopeptides off surface collagen fibrils, they may create enough space for a collagenase to bind to the proper binding site to create unwinding of the collagen molecule that promote collagen hydrolysis. It appears that MMPs may work in concert. Gelatinases may indeed hydrolyze gelatin when it forms, but may also help initiate collagenase activity by being a “telopeptidase”.
MMP activity can be regulated at multiple levels, e.g. transcription, secretion, degranulation of enzymes from intracellular granules, and by specific and non-specific inhibitors [25]. Tissue inhibitors of MMPs (TIMPs -1, to -4) are specific inhibitors that participate in controlling the local activities of MMPs in tissues. Most TIMPs inhibit active MMPs and some TIMPs can prevent pro-MMP activation. TIMPs are also involved in various cellular and tissue regulatory processes [43,44]. In body fluids, a macroglobulin protein, α2-macroglobulin, may be the primary regulator of MMP activity. α2-macroglobulin and fetuin-A (aka α-2-Heremans Schmid-glycoprotein, α-2-HS-glycoprotein, AHSG), are abundant plasma proteins that may be involved in the regulation of dentin MMPs [23]. There are also an ever-increasing number of synthetic MMP inhibitors. Most of them prevent MMP activity by chelating or replacing the active-site Zn2+, or by “coating” the substrate.
Several MMPs have been identified in the dentin–pulp complex. During tooth development, MMPs participate in the organization of the ECM components and compartments [9,23]. Mature human odontoblasts synthesize at least gelatinases MMP-2 and -9, collagenases MMP-8 and -13, and enamelysin MMP-20 [45–49]. However, the gene expression profile is much larger, covering most of the MMP family members [50].
The first time collagenase in dentin was described was in the early 1980s, when Dayan et al. [51] demonstrated collagenolytic activity in carious and intact human dentin. The finding went by pretty much unexplained and unnoticed, which is not surprising, since at that time MMP research was still in its “adolescence” phase, as described by Lapière [52]. For example, the name “matrix metalloproteinases” for the family was only suggested in 1986 [52]. Recent studies have demonstrated the presence of at least gelatinases MMP-2 and -9, collagenase MMP-8 and stromelysin MMP-3 in human dentin [32,41,42,53–57]. MMP-20 is present in dentinal fluid [47], intense gelatinolytic activity has been observed in dentinal tubules with laser confocal microscopy [58] (Figure 5), and MMP-20 [47], -2 [59] and -9 [60] have been shown to increase in dentinal tubules of carious teeth. In mineralized dentin, MMP-2 is probably the most abundant MMP [23]. TIMP-1 and -2 are also found in human dentin [49,61,62]; α2-macroglobulin and fetuin-A are also present in marked quantities in dentin [23]. The physiological roles of MMPs in dentin is not well understood, but they have been suggested to participate in peritubular and tertiary dentin formation, and in the release of dentinal growth factors [7,22,23,63–66]. The role of MMPs in collagen degradation in dentin caries progression has been well documented [35,37,67–71]. For a comprehensive overview of current knowledge of MMPs in dentin-pulp complex, the reader is referred to recent reviews [9,23].
Figure 5.

Gelatinolytic activity in dentinal tubules (green fluorescence) and in the hybrid layer at the top of the tubules of intact human tooth as seen with confocal fluorescence microscope.
Cysteine cathepsins
The lysosomal cysteine proteases belong to the clan CA of cysteine proteases; they are members of the C1 family of papain-like enzymes, the largest and the best characterized family of cysteine peptidases. There are 11 human cysteine cathepsins, namely cathepsins B, C, F, H, K, L, O, S, V, X and W [72–74]. Cathepsins B, H, L, C, X, F, O and V are ubiquitously expressed in human tissues, while cathepsins K, W and S are tissue-specific [75]. They are biosynthesized in the secretory route as inactive zymogens and are transported to endosomes via the mannose-6-phosphate receptor pathway [76]. Cysteine cathepsin zymogens are processed to the active forms either autocatalytically triggered by endosomal acidification or by other proteases [77]. The autocatalytic activation of cathepsins is substantially accelerated in the presence of anionic polysaccharides, such as GAGs and dextran sulfate [78,79]. The structure-function is best known for cathepsin B [75].
Cysteine cathepsins are active and stable in a slightly acidic pH and mostly unstable at neutral pH. They can be irreversibly inactivated at neutral pH [80], except cathepsin S which is stable at neutral or slightly alkaline pH [81]. Cathepsin B has mainly peptidyl-dipeptidase and carboxypeptidase activity [73,82,83]. On the other hand, cathepsin B endopeptidase activity has a pH optimum around 7.4 [84,85], which is expectable for an enzyme that is involved in many physiological processes related with extracellular matrix degradation. Unlike other cysteine proteases, cathepsin B has a flexible loop that partially occludes the active site aperture of mature enzyme and carries pH-sensitive arginine and histidine residues, and appears to play crucial role in modulating its endo- and exopepditase activities [86,87].
Cysteine proteases are often termed promiscuous proteases or those with broad substrate specificity [88]. Physiological substrates for cathepsin B, and others, are still a matter of speculation. However, cathepsin B degrades extracellular matrix components such as type IV collagen and fibronectin under both acidic and neutral pH [89].
Although cysteine cathepsins were initially considered as intracellular enzymes responsible for the non-specific proteolysis in the endosomal/lysosomal compartment, this view is rapidly changing [75]. In some (non-dental) cell types, the secretion processes of cathepsins are very well elucidated. Cathepsin B can be found in lysosomes [87] or secretory vesicles [76], or may follow the default pathway of secretion [90]. Furthermore, cathepsin B is consistently found associated with the plasma membrane of tumor cells [91]. Although lysosomes were for a long time considered to be dead-end organelles, recent developments show that these organelles or their contents in shuttle vesicles can move back and forth along microtubules rendering possible exocytosis or retrograde transport to endosomes [92].
Several cathepsins participate in bone resorption and remodeling. The most potent mammalian collagenase is cathepsin K which, in contrast to the cathepsins B, L and S, is highly expressed in osteoclasts [93] and odontoclasts [94], playing a special role in mineralized tissue resorption under normal and pathological conditions [95,96]. Indeed, in the absence of cathepsin K, osteoclasts are hampered in their capacity to digest bone collagen [97]. The biological relevance of the collagenolytic activity of cathepsin K was underlined by the finding that deficiency in this protease causes the bone-sclerosing dysplasia, pycnodysostosis, in man [98] and an osteopetrotic phenotype in mice [99]. When osteoclasts demineralize bone matrix by lowering the pH in the resorption lacuna, the acidic environment favors the extracellular collagenolytic activity of cysteine proteases, especially cathepsin K. Later, when the pH has increased due to an increased amount of phosphate and calcium, the MMPs digest the rest of collagen [100]. Low pH around joint implants also leads to demineralization and type I collagen degradation in peri-implant bone; the acid- and cathepsin K-driven mechanisms of periprosthetic bone resorption have been described in context with loosening of hip implants [101].
Recent data have shown that odontoblasts and pulp tissue have coding DNA sequences (CDS) of most cathepsins [102], suggesting that, in terms of protein expression, the variety of cysteine cathepsins present in dentin-pulp complex can be compared to that described for MMPs [50]. Cysteine cathepsin activity has been demonstrated both in intact [102] and carious [37] dentin, and cathepsin B has been localized in dentinal tubules [102]. Once synthesized by odontoblast and/or pulp tissue, secreted cathepsins can easily reach the dentinal tubules and enter deep into dentin [102] (Figure 6). Moreover, changes in expression levels, localization and activity have been described in carious dentin [37]. The significant increase of cysteine cathepsin activity in carious dentin with increasing depth (approaching the pulp) indicates that odontoblast- or pulp-derived cysteine cathepsins may be important in active caries lesions, especially with young patients [37]. Together these findings indicate that cysteine cathepsins may have an important role during dentinal caries development. Since clinical restorations are practically always made on carious teeth with at least some degree of pulpal inflammation, it is possible that increased levels of cysteine cathepsins are present also in dentinal tubules under the restoration.
Figure 6.
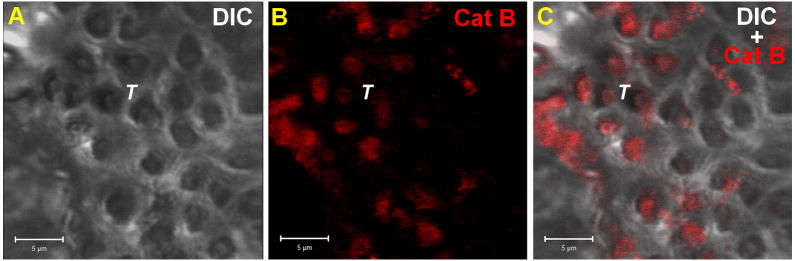
Cathepsin B in dentinal tubules. Dentin tissue cross-section visualized by Differential Interference Contrast (DIC) (A). Intratubular Cathepsin B were immunolabeled using rabbit anti-human cathepsin B as primary antibody and mouse anti-rabbit IgG conjugated with Alexa Fluor 594® as a secondary antibody at the red channel (B). Merged Images (C). T = Tubules and scale bar = 5μm.
The increased expression and/or activity of cysteine cathepsins often coincide with their presence in the extracellular environment, confirming the hypothesis that pH is not sole factor responsible for their activity. Cathepsins with high collagenolytic activities are known to be mainly responsible for remodeling the extracellular matrix [75]. The extracellular matrix consists mostly of collagen and proteoglycans, which have all been identified as the cathepsin substrates [103]. Cathepsin K is the only cysteine cathepsin capable of cleaving the collagen at the triple helical region [104,105]. However, gelatin can be cleaved by several cathepsins [106] and cathepsins B and L cleave in the non-helical telopeptide extensions of collagens [107,108]. Cathepsins L and S can release GAGs from cartilage proteins such as aggrecan [109]. PGs form interfibrillar bridges that absorb water with their negatively charged GAG side chains in dentin collagenous matrix [11,23], and GAGs play a crucial role in the formation of complexes between cysteine cathepsins and their protein substrates. This will be discussed in more detail in the next section.
The interplay of dentin proteolytic enzymes and non-collagenous proteins
Since the role of MMPs in dentin pathologies first emerged less than 15 years ago [35,45], and cysteine cathepsins were detected in dentin only two years ago [37,102], it is obvious that not much is known about their potential interactions in dentin formation, physiology, or diseases, let alone in the loss of collagen matrix in the hybrid layer. However, the exceptionally wide expression profile of MMPs [50] and cysteine cathepsins [102] in mature human odontoblasts strongly indicate that the enzymes must have various tasks in the formation and maturation of mineralized dentin. This kind of wide-scale expression of two extracellular matrix–degrading enzyme families in specific tissue is quite unique, especially the cells that in general are not considered to participate in tissue remodeling under physiologic conditions. Most of the potential interactions between MMPs and cysteine cathepsins in dentin are still based on the research with other tissues, and must be considered speculative until more work has been performed in dentin.
In cartilage collagen, cathepsin B and MMPs have been suggested to have at least partially independent pathways in cartilage proteoglycan breakdown [110]. Cysteine cathepsins are able also to activate MMPs [111]. Cathepsin B from articular chondrocytes increases MMP levels and stimulates angiogenesis by proteolytic inhibition of TIMPs [112]. Moreover, procathepsin B can be activated by active MMPs [113], and conversely, cathepsin B has been shown to be responsible for activation of MMP-1 in gingival fibroblast cultures [114]. This scenario establishes a possible and reasonable conclusion that both class of proteolytic enzymes, cysteine cathepsins and MMPs, are participating synergistically in bone resorption and make cathepsin B an attractive candidate for the potential cathepsin-MMP interplay also in dentin-pulp complex (Figure 7A,B). Identification of different cathepsins at the protein level in dentin may, in the future, better elucidate the potential cathepsin-MMP interactions also in the degradation of the hybrid layer collagen.
Figure 7.

Schematic presentation of possible mechnisms of activation and function of cysteine cathepsins and MMPs in dentin (modified from the Online-only Appendix 2 in [37]). Green blocks with MMPs (red triangles) or cathepsins (yellow arrowheads) indicate inactivity of enzyme either as a proform or in complex with specific (TIMPs, cystatins) or non-specific (e.g. α2 macroglobulin) inhibitors. Activation of protein (removal of green blocks) may represent either elimination of inhibitor, transform from latent to active form, or both. Glycosaminoglycans (GAGs) in dentin may also affect the enzyme activity either by activation or inhibition, depending on enzyme and GAGs in question [106,127].
A) pH changes caused by etching acid or acidic monomers convert dentin-bound proMMPs and/or MMP-TIMP complexes (d-MMP) into active MMP. Respectively, dentin-bound cathepsin (d-Cat) becomes active in acidic pH.
B) At least cathepsin B directly cleaves and inactivates MMP-specific tissue inhibitors TIMP-1 and TIMP-2 [36], changing the balance between MMPs and their inhibitors. Acidic pH activates cysteine cathepsins, which in turn either proteolytically activate proMMPs or degrade TIMP inhibiting MMP, or both, resulting with active MMPs and functional activity after neutralization of pH.
C) Glycosaminoglycan (GAG: black dot) activation and stabilization of cathepsin (and possibly MMPs), allowing functional activity of cathepsins even in neutral pH.
D) Odontoblast- or pulp-tissue derived enzymes may enter the hybrid layer.
Several MMPs (at least MMP-1, -2, -7, -8, -9 and -13) can bind to PGs and/or GAGs which may affect their binding properties, regulate enzyme activity but also protect them from auto- or other activation [115–118]. For example, pro-MMP-9-PG heteromers bind to type I collagen as well as gelatin in vivo, while monomeric MMP-9 has much greater affinity to gelatin [117]. Interestingly, the organomercurial compound p-aminophenylmercury acetate (APMA) does not activate pro-MMP-9-PG complex, commonly used MMP activator [117]. Instead, Ca2+, known to stabilize MMP-9 and other MMPs but not induce proenzyme activation, induced an autoactivation of the pro-MMP-9 in the complex and release of activated enzyme from the complex [115]. Since the weak response of dentinal MMPs to APMA-activation is a frequent finding [42,119–121] and calcium is known to be needed for demineralized dentin autolytic degradation [121,122], it is tempting to speculate that MMP-PG/GAG complexes would also be present in dentin matrix, where MMP-3 might participate in its activation by cleaving the heteromer PG [54]. Due to these unique characteristics, pro-MMP-9-PG heteromer (and other MMP-PG/GAG complexes) may have fundamental physiological importance in spite of their presumably low relative amounts in tissues [117], because only catalytic amounts of the enzyme are required to digest physiological targets. GAGs also participate in the regulation of cysteine cathepsin activity in a very complex manner. GAG activation (e.g. [123]) and stabilization [124] of cathepsin (and possibly MMPs [116]), allows functional activity of cathepsins even in neutral pH. GAGs are able to accelerate the conversion of zymogen forms of cysteine cathepsins into mature forms at neutral pH, activating cathepsin B [123], cathepsin L [125], cathepsin S [106,126,127] and congopain [128]. The presence of GAGs in the dentin [129] and their release during acid-demineralization and subsequent proteolytic degradation of acid-demineralized dentin matrix [130] supports the possibility that GAGs are involved in in vivo processing of cysteine cathepsins in dentin. Interestingly, procathepsin S is capable of autocatalytic activation not only at acidic pH but also at neutral pH in the presence of related GAGs [78]. Also, cathepsin B is stabilized at pH 7.4 by interaction with heparan sulfate and heparin GAGs [124]. Of the several GAGs that can complex with cysteine cathepsin K, only chondroitin sulfates and keratan sulfates (both present in dentin [129]) (Figure 8) induce its collagenolytic activity; and chondroitin sulfates can accelerate the procathepsin B processing into active form [79]. With other cysteine cathepsins, the effect is inhibitory [106,127], or they may participate on cellular trafficking of cysteine cathepsins [131]. This highly selective inhibition/activation mechanism has been suggested to be important in the control of collagen turnover [127] (Figure 7C).
Figure 8.
Collagen and chondroitin 6-sulphate distribution in dentin. Dentin tissue was visualized by confocal microscopy in longitudinal- (A, B and C) and cross-section (D, E and F). Dentin morphology was shown by Differential Interference Contrast (DIC), (A and E). Molecularly well- structured collagen was visualized by its intrinsic fluorescence at the green channel (B and F). Chondroitin 6-Sulphate were immunolabeled using rabbit anti-human chondroitin 6-sulphate as primary antibody and mouse anti-rabbit IgG conjugated with Alexa Fluor 594® as a secondary antibody at the red channel (C and G). Colocalization between collagen and chondroitin 6-sulphate can be seen as the yellow color formed by the overlapping channels green and red in merged images (D) or interacting in the tubular area (H). The three-dimensional model was constructed from the upper panel’s images and shows how collagen and chondroitin 6-sulphate molecules are anatomically distributed on dentinal tissues (I). CS = Chondroitin 6-Sulphate; scale bars = 5 μm (A, B, C and D) or 2 μm (E, F, G and H).
In vivo, the dentinal face of the adhesive interface is bathed in dentinal fluid, and the permeability of simplified polymerized adhesives has been long recognized [132]. The enzymes dissolved in dentinal fluid may thus participate in the degradation of hybrid layer collagen, especially in vivo (Figure 7D). Using the in vitro thick-slice human tooth model, Lehmann and others [133] demonstrated an increased MMP-2 protein expression in the odontoblasts when the overlying dentin was treated with SE adhesive-bonded restoration. The authors concluded that odontoblasts may participate into hybrid layer degradation by increasing enzyme synthesis [133]. Boushell and others [59] demonstrated a significant increase in MMP-2 immunoreactivity in the caries-affected tubules, regardless of the level of caries severity. MMP-13 is expressed in high levels in odontoblasts and pulp tissue both in healthy and carious teeth [48,134]. MMP-1, -2 [135] and MMP-9 [136,137] levels increase in inflamed pulp, and MMP-9 has been detected in dentinal fluid of teeth with pulpitis but not in healthy teeth [60]. Polymerized adhesive disks increase MMP-2 production in pulpal fibroblasts in culture [138]. Cysteine cathepsin B is present in dentinal tubules [102] (Figure 6), and odontoblast- or pulp -derived cysteine cathepsins, delivered via dentinal fluid, have been suggested to have an important role in dentinal caries [37]. The previous data indicates that at least MMP-20 may also be functional in dentinal caries matrix degradation [47]. Since teeth are almost always restored because of caries, usually accompanied with at least mild local pulpitis, the influx of odontoblast- or pulp tissue-derived enzymes may be significant factor in the degradation of hybrid layer in vivo. However, the presence of resin tags in dentinal tubules may prevent the outward diffusion of cysteine cathepsins or MMPs from the pulp into peripheral dentin.
Enzymatic degradation of dentin collagen
At the turn of the millenium, morphologic evidence began to emerge from in vivo studies of resin elution or degradation of collagen matrices in aged resin-dentin bonds [1,139–141]. In 2003, Hashimoto and co-workers [142] demonstrated the degradation of acid-etched dentin collagen in water storage for 500 days in vitro. While the role of MMPs in the progression of dentin matrix of caries lesion had been suggested earlier [35,47,64,67,68], the first direct evidence of this occurring also in non-carious dentin collagen came when Pashley and co-workers demonstrated that dentin demineralized by acid etching slowly degrades in the absence of bacteria [143]. Collagen matrices incubated in artificial saliva were almost completely degraded within 250 days, while those stored either in artificial saliva containing enzyme inhibitors or mineral oil were practically intact [143]. The study also confirmed the intrinsic collagenolytic activity of dentin matrix indicated in the early paper by Dayan et al. [51]. After this groundbreaking report there have been several articles using different techniques that confirm that collagenolytic enzymes are present in human mineralized dentin, they can be active after demineralization with various methods, and that they have a role in the degradation of the hybrid layer collagenous matrix.
Biochemical techniques demonstrating the degradation of dentin collagen by extrinsic or intrinsic proteases
While Pashley et al. [143] clearly demonstrated the time-related collagen destruction with TEM, quantitative techniques to measure and quantitate the actual level of degradation have been used. Quantitative methods allow to test and compare different enzyme inhibitors within reasonable time frame (e.g. four weeks) and evaluate their potential before time- and labor-consuming in vitro or in vivo bond strength testing. These techniques include measuring the release of hydroxyproline (HYP) in hydrolyzates of media or C-terminal telopeptides (ICTP, CTX) into incubation medium.
The rationale for evaluating collagen degradation by means of HYP assays relies on the fact that type I collagen contains about 10 mass % HYP, but most other proteins contain little or none of this amino acid [144]. During type I collagen degradation, the release of HYP from the incubation solution can be measured with colorimetric methods or HPLC. Urine HYP has been used as a marker of bone resorption, but due to its lack of sensitivity and specificity to bone collagen and availability of more sensitive and specific methods, there is little clinical use of HYP any more [145]. With dentin, HYP release analysis was used for the first time by Veis and Schlueter in 1964 [146] in their classical study characterizing dentin collagen. Carrilho and others [121] analyzed the effect of CHX on HYP release, along with modulus of elasticity, of demineralized dentin beams. Thirty min preincubation of demineralized dentin beams in 2% CHX resulted with significantly reduced HYP release compared to the controls, indicating decrease in collagen degradation. In addition, no changes in modulus of elasticity occurred in CHX-pretreated samples, while with controls a significant and time-dependent decrease was evident already after one week incubation in artificial saliva [121]. The study further confirmed the enzyme-inhibiting activity of CHX, but also demonstrated that HYP release can be used to quantitate collagen degradation in demineralized dentin. The method has since been used in several studies with different approaches to inhibiting demineralized dentin collagen degradation [122,147–150].
Matrix metalloproteases (MMPs)-1, -8 and -13 are true collagenases. They attack native collagen at a specific peptide bond and cleave collagen molecules into a ¼ and ⅓ segment [39]. A larger number of MMPs including -2, -9 and -13 can attack type I collagen telopeptides to release a long C-telopeptide segment called ICTP [38]. This segment includes at least two cross-linked telopeptides and the first phenylalamine of the phenylalamine-rich region. When MMPs-2, -9, -13 and -14 were added to partially demineralized human bone powder, ICTP telopeptide fragments were formed in vitro but they did not release the smaller eight amino acid C-terminal peptide, CTX [38]. However, when those authors incubated exogenous cathepsin K with demineralized bone powder, the major telopeptide that was released was CTX. No CTX was released from bone powder incubated with MMP-2, -9, -13 or -14 [38]. All MMPs (at least the above-mentioned MMPs-2, -9, -13 and 14 [38]) and cysteine cathepsins (at least cathepsins B and L [107,108,151]) that release ICTP or CTX telopeptide fragments from type I collagen would be called telopeptidases rather than collagenases. Cathepsin K is a unique protease in that it is both a telopeptidase and a collagenase. It can cleave telopeptides as well as the helical region of collagen [39].
Transmission electron microscopy
Transmission electron microscopy (TEM) is a commonly used and suitable method to examine the hybrid layers because of the small size (50–100 nm wide) of collagen fibrils and because the structural integrity can usually be evaluated by the characteristic banding of collagen. Also the interfibrillar spaces that are only approximately 20 nm wide can be seen. Numerous experiments both in vitro and in vivo have demonstrated the loss of the hybrid layer collagen structure in aged samples [2,4,5], in which collagen network and structure appear more or less disorganized or even lost, leaving empty spaces to be filled with sample embedding resin (Figure 9). Comparison of the samples prepared for TEM immediately or after aging, or comparing the control and experimental groups (as in Figure 9), offers a powerful tool to evaluate the effect of aging on the hybrid layer integrity. Similarly, TEM is frequently used to evaluate the effect of enzyme inhibition on the hybrid layer integrity. Figure 9 demonstrates the dramatic differences in the hybrid layers of the control and experimental (CHX-treated) primary teeth after six months in service in vivo. This was the first study that directly indicated the clinical efficacy of enzyme inhibition on hybrid layer preservation [152].
Figure 9.
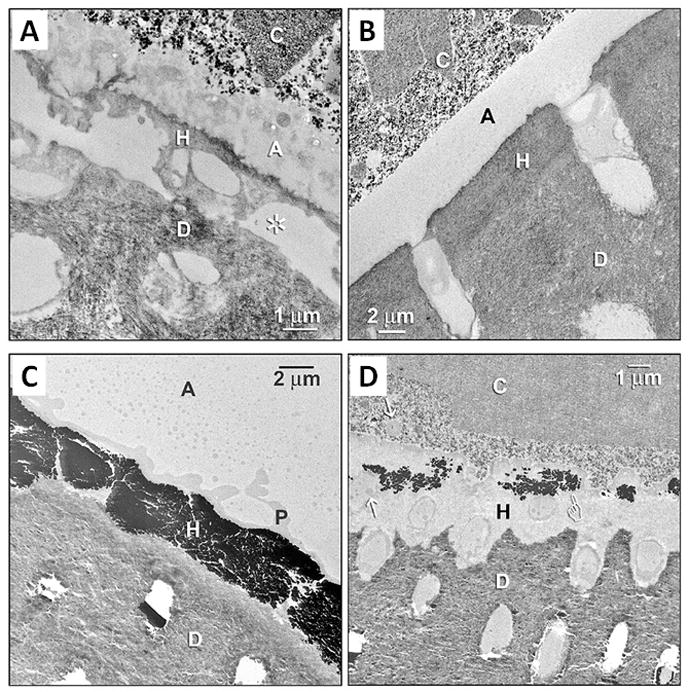
TEM analysis of hybrid layer in primary molars restored six months earlier without (controls) or with 2% CHX pretreatment (experimental group), using 2-step E&R adhesive and composite resin. A) Demineralized section of the control tooth hybrid layer stained with phosphotungstic acid and uranyl acetate. The hybrid layer (H) is almost completely degenerated and partially even missing, with empty regions occupied by epoxy resin (asterisk). C: resin composite; A: adhesive; H: hybrid layer; D: dentin. B) The section respective to A from the experimental (i.e. CHX-treatment) group. The hybrid layer (H) appears normal. C) Undemineralized, unstained, silver-impregnated section from the control tooth demonstrates a degraded hybrid layer with extensive leakage seen as black silver deposits that almost completely fill the hybrid layer. P, polyalkenoic acid copolymer. The silver filled the water-filled spaces that replaced collagen fibrils. D) An experimental tooth pretreated with CHX, with sparsely distributed silver deposits (pointer) in the hybrid layer. Arrows: polyalkenoic acid copolymer that is characteristic of 3M-ESPE adhesives. (Reproduced from [152], with permission.)
Few investigations of resin-dentin bonds use TEM because it is technically much more demanding than SEM. The ultrastructure of dentin hybrid layers was first described by Van Meerbeek et al. [153–155] and by Tay et al. [156–158]. They showed that the collagen fibrils exhibited 67 nm periodicity, took up heavy metal TEM stains and had 20 nm interfibrillar spaces between the collagen fibrils, presumably filled with infiltrated resin. Armstrong and co-workers [159] were the first to carefully examine such hybrid layers in resin-bonded dentin that had been stored in water for 5 years. Their TEMs revealed that almost 70% of the collagen fibrils had disappeared! They had a “ghost-like” appearance. The other 30% of the hybrid layers appeared to be normal in TEM appearance with 67 nm cross-striations that stained well with heavy metals. This was the first solid evidence of why resin-dentin bond strengths fell over time. The collagen fibrils in the hybrid layer that anchor the overlying adhesive resin and composites to the underlying mineralized dentin had been destroyed. Such collagen fibrils are the only continuous structural elements connecting adhesive fillings to underlying dentin. Their loss coincided with decreases in measured bond strength [160,161].
This ultrastructural observation was an important breakthrough. The presence of MMPs in dentin had been reported by Martin-De Las Heras et al. [41] in a biochemical study. They extracted MMP-2 from normal dentin. This was well-received by developmental biologist who confirmed the observation and explained the relevance of MMPs in embryological development. This observation was completely ignored by restorative dentists who had little interest in developmental biology.
Effect of adhesives on purified and dentin matrix-bound enzymes
The use of 35% phosphoric acid (pH 0.7 to -1), to acid-etch dentin in the E&R approach, was initially thought to inactivate pro-MMPs trapped within the mineralized dentin due to its low pH which was believed to denature MMPs [143]. The subsequent application of resin monomers constituting the adhesive blends of either E&R [120] or SE adhesives [119] have been shown to reactivate the quenched collagenolytic/gelatinolytic activities of the endogenous enzymes. This phenomenon occurred both in coronal [119,120] and in radicular dentin [162]. The studies were performed to detect gelatinolytic/collagenolytic enzyme activities within the dentin powder before and after the sequential application of the phosphoric acid-etchant and an E&R adhesive [120] or treating mineralized dentin with a SE adhesive (i.e. the solid phase) [119,162]. These data were obtained using powdered dentin on internally quenched fluorescent collagen/gelatin substrate.
This experimental setup clearly revealed a specific gelatinolytic activities of the dentin bonding system/dentin powder mixtures, but they did not clarify which type of endogenous enzymes (MMPs, cathepsins or others) were involved in the degradation process. Thus such activity could have not been related to a specific enzyme. For this reason, more recently, different efforts have been devoted to identify the specific MMPs activated by the bonding agents, using immunohistochemical, gel electrophoresis, and confocal laser fluorescence techniques. With field emission in-lens-scanning electron microscopy (FEI-SEM) and TEM MMP-2 and -9 [55] and MMP-3 [57] were localized in partially EDTA-mineralized dentin (Figure 10). In particular correlative biochemical and immunohistochemical approaches at TEM and high resolution-SEM with MMP-2 immunolocalization in mineralized dentin, phosphoric acid-etched dentin and adhesive-treated acid-etched dentin support the hypothesis that MMP-2 may be one of the most relevant dentin-bound MMPs involved in hybrid layer degradation [163]. MMP-2 was found to be entrapped within the hybrid layer created by a two-step E&R adhesive system and aged in artificial saliva for 12 months. The amount of MMP-2 available for detection varied as a function of dentin treatment, being minimal in 0.2% CHX-treated samples [163]. The colorimetric antibody-specific assay allowing direct measurement of specific protein levels (QuantiSir) confirmed the electron microscopy findings. Interestingly, MMP-2 expression and activity varied depending on the step of the bonding procedure: while phosphoric acid etching reduced the detection level by 43% compared to mineralized dentin, treating the acid-etched dentin with SB 1XT increased the detection by 58% compared to mineralized dentin and by 179% compared to acid-etched dentin [163]. The results support the previous finding that adhesives can reactivate the enzymes present in dentin after acid etch etching [120]. Furthermore, pretreatment of acid-etched dentin with 0.2% CHX before the adhesive application decreased detection level by 27% [163], demonstrating the binding of CHX to the enzyme in demineralized dentin matrix and its inhibition.
Figure 10.
Field emission in-lens SEM (FEI-SEM) micrographs of unfixed, partially decalcified dentin after a pre-embedding immunolabeling with antibodies for MMP-2, -9 and -3. Labeling is seen as electron-dense white spots (pointers). (a,d) Low magnification view (20 000x) of the partially decalcified dentin surface showing open tubular orifices (T) surrounded by a thick collar of organic matrix and intertubular porous dentin (ITD). MMP-2 and -9 labeling mainly localized in peritubular dentin. (b,e) A higher magnification (50 000x), showing MMP-2 (b) and -9 (e) along the collagen fibrils. (c,f) High magnification (100 000x) FEI-SEM micrographs, revealing the relationship between MMP-2 and -9 and the collagen meshwork. The specimen shows a moderate labeling for MMP-9 (e), which is uniformly weaker than MMP-2 staining (c). (g–i) FEI-SEM micrographs of partially decalcified dentin after a pre-embedding immunolabelling an antibody for MMP-3. (g) Low magnification (30 000x) showing a patent dentinal tubule (DT) and the porous intertubular dentine (ITD). MMP-3 is mainly localized within the intertubular dentine. (h) Higher magnification (50 000x) showing MMP-3 located along the collagen fibrils. (i) High magnification FEI-SEM micrograph (100 000x), revealing MMP-3 distribution within the intertubular dentine. (Figures a–f reproduced from [55] and g–i from [57], with permission).
These results were further confirmed using zymography assays performed on protein dentin extracts after different treatments of dentin powder. In gel zymography, gelatin is copolymerized in an SDS-PAGE gel as a substrate. When the protein extracts are run into the gel, proteins are separated by their molecular weight. The gel is then incubated in activation and incubation solutions, allowing gelatinolytic enzymes to degrade the gelatin in the gel, and the proteins can be detected and identified by the location of the bands in the gel [164]. While no enzymatic activity was found in proteins extracted from mineralized dentin, zymograms of phosphoric acid-demineralized dentin extracts showed bands at 66 kDa (MMP-2 active form), a fainter band at 86 kDa (active form of MMP-9) and other minor gelatinolytic bands with lower molecular weights [165]. Zymograms of adhesive-treated dentin showed an intense increases both in active (band at 66 kDa) and proform of MMP-2 (band at 72 kDa), together with a slight increase in MMP-9 (fainter band at 86 kDa) [165]. However, when SE adhesives were used, the zymographic assay was unable to reveal enzyme activation similar to what could be obtained with E&R adhesives [166,167].
Despite all these findings however, no assay has been able to evaluate the proteolytic activity in hybrid layers in situ due to the intrinsic difficulties to detect, precisely localize, and measure enzyme activities within the tissues, because zymography requires extraction of the proteolytic enzymes from the tissue prior to analysis. Recently Mazzoni et al. [58] using a correlative approach with an ELISA assay and in situ zymography, quantified and localized the MMPs activity within the hybrid layer created by an E&R adhesive. This was performed using quenched fluorescent-labeled gelatin showing intense and precise fluorescent localization indicating enzyme activity within the hybrid layer [58] (Figure 11). This may also be due to the presence of cysteine cathepsins [102] derived from the dental pulp via dentinal fluid and possibly activated by adhesive resin monomers.
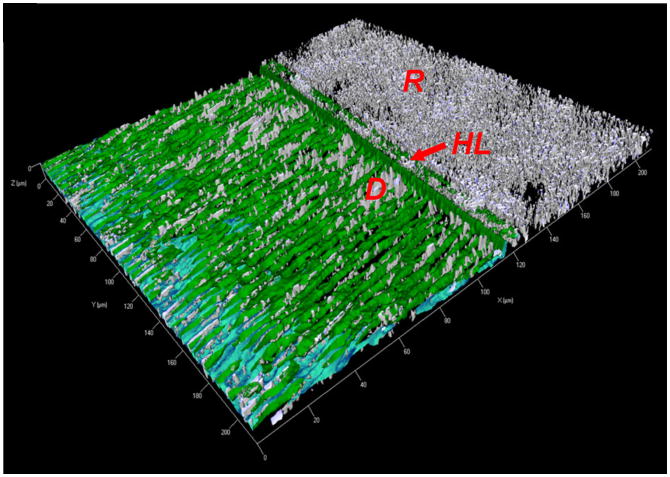
Figure 11.
Three-dimensional model of the acquired image using in situ zymography with the cross-section of composite resin (R), hybrid layer (HL: arrow) and dentin (D). In in situ gelatin zymography, samples are overlayed with quenched florescent-conjugated gelatin gel, and any gelatinolytic activity in the sample releases fluorescence (green color). Intense gelatinolytic activity is present in dentinal tubules and especially at the bottom of the hybrid layer as a 1- to 2-μm-thick, well-defined layer. (Reproduced from [58], with permission).
The intense enzymatic activity reported along the dentinal tubules [58] also supports the hypothesis shown by Lehmann et al. [133] that a SE adhesive can increase MMP-2 synthesis in human odontoblasts, possibly increasing MMP-2 penetration into the hybrid layer via dentinal fluid. Similarly Orsini et al. [138] showed that unreacted monomers eluted from polymerized adhesives are capable of inducing human pulp fibroblasts to overexpress MMP-2.
Conclusions
Identification of the presence and activity of dentinal enzymes has provided basic knowledge that allows us better understand their roles in dental hard tissue pathologies. Matrix metalloproteinases and cysteine cathepsins in mineralized dentin and dentinal fluid have been shown to contribute to dental caries [35,67,68] and erosion [168,169], but the field with most interest has been the enzymatic degradation of the composite adhesive hybrid layer collagenous matrix. This, in turn, has already led to several experimental strategies that aim to improve the durability of the resin-dentin bond strength by inhibiting the enzymatic degradation of collagen in the hybrid layer. Even though work remains to be done e.g. to identify the key enzymes and to find the optional means of inhibition that would not affect immediate bond strength but would still retain the effect for years, the currently available data already holds excellent promise that stable resin-dentin bonds will be routinely available in a daily clinical setting already in a near future.
Acknowledgments
This work was supported, in part, by grants DE 015306 from the NIDCR to DHP, P.I.; and by the Academy of Finland to LT, P.I. and AT-M, P.I.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shono Y, Terashita M, Shimada J, Kozono Y, Carvalho RM, Russell CM, Pashley DH. Durability of resin-dentin bonds. Journal of Adhesive Dentistry. 1999;1:211–8. [PubMed] [Google Scholar]
- 2.Hashimoto M. A review - micromorphological evidence of degradation in resin-dentin bonds and potential preventional solutions. Journal of Biomedical Materials Research Part B, Applied Biomaterials. 2010;92:268–80. doi: 10.1002/jbm.b.31535. [DOI] [PubMed] [Google Scholar]
- 3.Loguercio AD, Moura SK, Pellizzaro A, Dal-Bianco K, Patzlaff RT, Grande RHM, Reis A. Durability of enamel bonding using two-step self-etch systems on ground and unground enamel. Operative Dentistry. 2008;33:79–88. doi: 10.2341/07-42. [DOI] [PubMed] [Google Scholar]
- 4.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dental Materials. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A. State of the art etch-and-rinse adhesives. Dental Materials. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Botta SB. What causes durability reduction in tooth-colored resin restorations? Journal of Contemporary Dental Practice. 2012;13:i–ii. [PubMed] [Google Scholar]
- 7.Tjäderhane L, Carrilho MR, Breschi L, Tay FR, Pashley DH. Dentin basic structure and composition – an overview. Endodontic Topics. 2012;20:3–29. [Google Scholar]
- 8.Bella J, Brodsky B, Berman HM. Hydration structure of a collagen peptide. Structure. 1995;3:893–906. doi: 10.1016/S0969-2126(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 9.Tjäderhane L, Haapasalo M. Dentin-pulp border: dynamic interface between hard and soft tissues. Endodontic Topics. 2012;20:52–84. [Google Scholar]
- 10.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, Donnelly A, Garcia-Godoy F. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. American Journal of Dentistry. 2007;20:7–21. [PubMed] [Google Scholar]
- 11.Bertassoni LE, Orgel JP, Antipova O, Swain MV. The dentin organic matrix - limitations of restorative dentistry hidden on the nanometer scale. Acta Biomaterialia. 2012;8:2419–33. doi: 10.1016/j.actbio.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cadenaro M, Breschi L, Rueggeberg FA, Suchko M, Grodin E, Agee KA, De Lenarda R, Tay FR, Pashley DH. Effects of residual ethanol on the rate and degree of conversion of five experimental resins. Dental Materials. 2009;25:621–8. doi: 10.1016/j.dental.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott JE, Thomlinson AM. The structure of interfibrillar proteoglycan bridges (‘shape modules’) in extracellular matrix of fibrous connective tissues and their stability in various chemical environments. Journal of Anatomy. 1998;192:391–405. doi: 10.1046/j.1469-7580.1998.19230391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. Journal of Biomedical Materials Research. 2002;62:447–56. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 15.Toroian D, Lim JE, Price PA. The size exclusion characteristics of type I collagen: implications for the role of noncollagenous bone constituents in mineralization. Journal of Biological Chemistry. 2007;282:22437–47. doi: 10.1074/jbc.M700591200. [DOI] [PubMed] [Google Scholar]
- 16.Habelitz S, Balooch M, Marshall SJ, Balooch G, Marshall GW., Jr In situ atomic force microscopy of partially demineralized human dentin collagen fibrils. Journal of Structural Biology. 2002;138:227–36. doi: 10.1016/s1047-8477(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 17.Ottani V, Raspanti M, Ruggeri A. Collagen structure and functional implications. Micron. 2001;32:251–60. doi: 10.1016/s0968-4328(00)00042-1. [DOI] [PubMed] [Google Scholar]
- 18.Orgel JP, Irving TC, Miller A, Wess TJ. Microfibrillar structure of type I collagen in situ. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9001–5. doi: 10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrari M, Tay FR. Technique sensitivity in bonding to vital, acid-etched dentin. Operative Dentistry. 2003;28:3–8. [PubMed] [Google Scholar]
- 20.Kim J, Mai S, Carrilho MR, Yiu CK, Pashley DH, Tay FR. An all-in-one adhesive does not etch beyond hybrid layers. Journal of Dental Research. 2010;89:482–7. doi: 10.1177/0022034510363665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaidyanathan J, Vaidyanathan TK, Yadav P, Linaras CE. Collagen-ligand interaction in dentinal adhesion: computer visualization and analysis. Biomaterials. 2001;22:2911–20. doi: 10.1016/s0142-9612(01)00038-2. [DOI] [PubMed] [Google Scholar]
- 22.Hannas AR, Pereira JC, Granjeiro JM, Tjäderhane L. The role of matrix metalloproteinases in oral environment. Acta Odontologica Scandinavica. 2007;65:1–13. doi: 10.1080/00016350600963640. [DOI] [PubMed] [Google Scholar]
- 23.Mazzoni A, Breschi L, Carrilho M, Nascimento FD, Orsini G, Ruggeri A, Gobbi P, Mazzotti G, Tay FR, Pashley DH, Tjäderhane L. A review on nature, role and functions of dentin non-collagenous proteins. Part II: enzymes, serum proteins and growth factors. Endodontic Topics. 2012;21:19–40. [Google Scholar]
- 24.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circulation Research. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 25.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nature reviews Molecular Cell Biology. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:5578–82. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagase H, Fushimi K. Elucidating the function of non catalytic domains of collagenases and aggrecanases. Connective Tissue Research. 2008;49:169–74. doi: 10.1080/03008200802151698. [DOI] [PubMed] [Google Scholar]
- 28.Perumal S, Antipova O, Orgel JP. Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2824–9. doi: 10.1073/pnas.0710588105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung L, Dinakarpandian D, Yoshida N, Lauer-Fields JL, Fields GB, Visse R, Nagase H. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO Journal. 2004;23:3020–30. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gioia M, Monaco S, Fasciglione GF, Coletti A, Modesti A, Marini S, Coletta M. Characterization of the mechanisms by which gelatinase A, neutrophil collagenase, and membrane-type metalloproteinase MMP-14 recognize collagen I and enzymatically process the two alpha-chains. Journal of Molecular Biology. 2007;368:1101–13. doi: 10.1016/j.jmb.2007.02.076. [DOI] [PubMed] [Google Scholar]
- 31.Okada Y, Naka K, Kawamura K, Matsumoto T, Nakanishi I, Fujimoto N, et al. Localization of matrix metalloproteinase 9 (92-kilodalton gelatinase/type IV collagenase=gelatinase B) in osteoclasts: implications for bone resorption. Laboratory Investigations. 1995;72:311–22. [PubMed] [Google Scholar]
- 32.Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, Di Lenarda R, Pashley DH, Breschi L. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. Journal of Dental Research. 2007;86:436–40. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 33.Davis GE, Martin BM. A latent Mr 94,000 gelatin-degrading metalloprotease induced during differentiation of HL-60 promyelocytic leukemia cells: a member of the collagenase family of enzymes. Cancer Research. 1990;50:1113–20. [PubMed] [Google Scholar]
- 34.Davis GE. Identification of an abundant latent 94-kDa gelatin-degrading metalloprotease in human saliva which is activated by acid exposure: implications for a role in digestion of collagenous proteins. Archives of Biochemistry and Biophysics. 1991;286:551–4. doi: 10.1016/0003-9861(91)90078-w. [DOI] [PubMed] [Google Scholar]
- 35.Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. Journal of Dental Research. 1998;77:1622–9. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- 36.Nagase H. Activation mechanisms of matrix metalloproteinases. Biological Chemistry. 1997;378:151–60. [PubMed] [Google Scholar]
- 37.Nascimento FD, Minciotti CL, Geraldeli S, Carrilho MR, Pashley DH, Tay FR, Nader HB, Salo T, Tjäderhane L, Tersariol IL. Cysteine cathepsins in human carious dentin. Journal of Dental Research. 2011;90:506–11. doi: 10.1177/0022034510391906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garnero P, Ferreras M, Karsdal MA, Nicamhlaoibh R, Risteli J, Borel O, Qvist P, Delmas PD, Foged NT, Delaissé JM. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. Journal of Bone and Mineral Research. 2003;18:859–67. doi: 10.1359/jbmr.2003.18.5.859. [DOI] [PubMed] [Google Scholar]
- 39.Garnero P, Borel O, Byrjalsen I, Ferreras M, Drake FH, McQueney MS, Foged NT, Delmas PD, Delaissé JM. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. Journal of Biological Chemistry. 1998;273:32347–52. doi: 10.1074/jbc.273.48.32347. [DOI] [PubMed] [Google Scholar]
- 40.Osorio R, Yamauti M, Osorio E, Ruiz-Requena ME, Pashley DH, Tay FR, Toledano M. Effect of dentin etching and chlorhexidine application on metalloprotease-mediated collagen degradation. European Journal of Oral Sciences. 2011;119:79–85. doi: 10.1111/j.1600-0722.2010.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin-De Las Heras S, Valenzuela A, Overall CM. The matrix metalloproteinase gelatinase A in human dentine. Archives of Oral Biology. 2000;45:757–65. doi: 10.1016/s0003-9969(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 42.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Archives of Oral Biology. 2007;52:121–7. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochimica et Biophysica Acta. 2000;1477:267–83. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 44.Lambert E, Dassé E, Haye B, Petitfrère E. TIMPs as multifacial proteins. Critical Reviews in Oncology/Hematology. 2004;49:187–98. doi: 10.1016/j.critrevonc.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Tjäderhane L, Salo T, Larjava H, Larmas M, Overall CM. A novel organ culture method to study the function of the human odontoblasts in vitro: gelatinase expression by odontoblasts is differentially regulated by TGF-beta1. Journal of Dental Research. 1998;77:1488–98. doi: 10.1177/00220345980770070301. [DOI] [PubMed] [Google Scholar]
- 46.Palosaari H, Wahlgren J, Larmas M, Rönkä H, Sorsa T, Salo T, Tjäderhane L. The expression of MMP-8 in human odontoblasts and dental pulp cells is down-regulated by TGF-beta1. Journal of Dental Research. 2000;79:77–84. doi: 10.1177/00220345000790011401. [DOI] [PubMed] [Google Scholar]
- 47.Sulkala M, Larmas M, Sorsa T, Salo T, Tjäderhane L. The localization of matrix metalloproteinase–20 (MMP-20, Enamelysin) in mature human teeth. Journal of Dental Research. 2002;81:603–7. doi: 10.1177/154405910208100905. [DOI] [PubMed] [Google Scholar]
- 48.Sulkala M, Pääkkönen V, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-13 (MMP-13, collagenase-3) is highly expressed in human tooth pulp. Connective Tissue Research. 2004;45:231–7. doi: 10.1080/03008200490885788. [DOI] [PubMed] [Google Scholar]
- 49.Niu LN, Zhang L, Jiao K, Li F, Ding YX, Wang DY, Wang MQ, Tay FR, Chen JH. Localization of MMP-2, MMP-9, TIMP-1, and TIMP-2 in human coronal dentine. Journal of Dentistry. 2011;39:536–42. doi: 10.1016/j.jdent.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Palosaari H, Pennington CJ, Larmas M, Edwards DR, Tjäderhane L, Salo T. Expression profile of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in mature human odontoblasts and pulp tissue. European Journal of Oral Sciences. 2003;111:117–27. doi: 10.1034/j.1600-0722.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 51.Dayan D, Binderman I, Mechanic GL. A preliminary study of activation of collagenase in carious human dentine matrix. Archives of Oral Biology. 1983;28:185–7. doi: 10.1016/0003-9969(83)90126-7. [DOI] [PubMed] [Google Scholar]
- 52.Lapière ChM. Tadpole collagenase, the single parent of such a large family. Biochimie. 2005;87:243–7. doi: 10.1016/j.biochi.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 53.Boushell LW, Kaku M, Mochida Y, Bagnell R, Yamauchi M. Immunohistochemical localization of matrixmetalloproteinase-2 in human coronal dentin. Archives of Oral Biology. 2008;53:109–16. doi: 10.1016/j.archoralbio.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boukpessi T, Menashi S, Camoin L, Ten Cate JM, Goldberg M, Chaussain-Miller C. The effect of stromelysin-1 (MMP-3) on non-collagenous extracellular matrix proteins of demineralized dentin and the adhesive properties of restorative resins. Biomaterials. 2008;29:4367–73. doi: 10.1016/j.biomaterials.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 55.Mazzoni A, Pashley DH, Tay FR, Gobbi P, Orsini G, Ruggeri A, Jr, Carrilho M, Tjäderhane L, Di Lenarda R, Breschi L. Immunohistochemical identification of MMP-2 and MMP-9 in human dentin: correlative FEI-SEM/TEM analysis. Journal of Biomedical Materials Research Part A. 2009;88:697–703. doi: 10.1002/jbm.a.31920. [DOI] [PubMed] [Google Scholar]
- 56.Santos J, Carrilho MR, Tervahartiala T, Sorsa T, Breschi L, Mazzoni A, Pashley D, Tay F, Ferraz C, Tjäderhane L. Determination of matrix metalloproteinases in human radicular dentin. Journal of Endodontics. 2009;35:686–689. doi: 10.1016/j.joen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Mazzoni A, Papa V, Nato F, Carrilho M, Tjäderhane L, Ruggeri A, Jr, Gobbi P, Mazzotti G, Tay FR, Pashley DH, Breschi L. Immunohistochemical and biochemical assay of MMP-3 in human dentine. Journal of Dentistry. 2011;39:231–7. doi: 10.1016/j.jdent.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazzoni A, Nascimento FD, Carrilho M, Tersariol I, Papa V, Tjäderhane L, Di Lenarda R, Tay FR, Pashley DH, Breschi L. MMP activity in the hybrid layer detected with in situ zymography. Journal of Dental Research. 2012;91:467–72. doi: 10.1177/0022034512439210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boushell LW, Nagaoka H, Nagaoka H, Yamauchi M. Increased matrix metalloproteinase-2 and bone sialoprotein response to human coronal caries. Caries Research. 2011;45:453–9. doi: 10.1159/000330601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zehnder M, Wegehaupt FJ, Attin T. A first study on the usefulness of matrix metalloproteinase 9 from dentinal fluid to indicate pulp inflammation. Journal of Endodontics. 2011;37:17–20. doi: 10.1016/j.joen.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Ishiguro K, Yamashita K, Nakagaki H, Iwata K, Hayakawa T. Identification of tissue inhibitor of metalloproteinases-1 (TIMP-1) in human teeth and its distribution in cementum and dentine. Archives of Oral Biology. 1994;39:345–9. doi: 10.1016/0003-9969(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 62.Leonardi R, Loreto C. Immunohistochemical localization of tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) in human carious dentine. Acta Histochemica. 2010;11:298–302. doi: 10.1016/j.acthis.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Tjäderhane L, Palosaari H, Wahlgren J, Larmas M, Sorsa T, Salo T. Human odontoblast culture method: the expression of collagen and matrix metalloproteinases (MMPs) Advances in Dental Research. 2001;15:55–8. doi: 10.1177/08959374010150011401. [DOI] [PubMed] [Google Scholar]
- 64.Tjäderhane L, Palosaari H, Sulkala M, Wahlgren J, Salo T. Proceedings of the International Conference on Dentin/Pulp Complex. Tokyo: Quintessence Publishing; 2002. The expression of matrix metalloproteinases (MMPs) in human odontoblasts; pp. 45–51. [Google Scholar]
- 65.Muromachi K, Kamio N, Narita T, Annen-Kamio M, Sugiya H, Matsushima K. MMP-3 provokes CTGF/CCN2 production independently of protease activity and dependently on dynamin-related endocytosis, which contributes to human dental pulp cell migration. Journal of Cellular Biochemistry. 2012;113:1348–58. doi: 10.1002/jcb.24007. [DOI] [PubMed] [Google Scholar]
- 66.Charadram N, Farahani RM, Harty D, Rathsam C, Swain MV, Hunter N. Regulation of reactionary dentin formation by odontoblasts in response to polymicrobial invasion of dentin matrix. Bone. 2012;50:265–75. doi: 10.1016/j.bone.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tjäderhane L, Sulkala M, Sorsa T, Teronen O, Larmas M, Salo T. The effect of MMP inhibitor Metastat on fissure caries progression in rats. Annals of the New York Academy of Sciences. 1999;878:686–8. doi: 10.1111/j.1749-6632.1999.tb07762.x. [DOI] [PubMed] [Google Scholar]
- 68.Sulkala M, Wahlgren J, Larmas M, Sorsa T, Teronen O, Salo T, Tjäderhane L. The effects of MMP inhibitors on human salivary MMP activity and caries progression in rats. Journal of Dental Research. 2001;80:1545–9. doi: 10.1177/00220345010800061301. [DOI] [PubMed] [Google Scholar]
- 69.Nordbø H, Leirskar J, Ngo H, Mount GJ, Wahlgren J. The influence of a matrix metalloproteinase on the remineralization of artificially demineralized dentin. Oral Health and Preventive Dentistry. 2003;1:267–72. [PubMed] [Google Scholar]
- 70.Shimada Y, Ichinose S, Sadr A, Burrow MF, Tagami J. Localization of matrix metalloproteinases (MMPs-2, 8, 9 and 20) in normal and carious dentine. Australian Dental Journal. 2009;54:347–54. doi: 10.1111/j.1834-7819.2009.01161.x. [DOI] [PubMed] [Google Scholar]
- 71.Toledano M, Nieto-Aguilar R, Osorio R, Campos A, Osorio E, Tay FR, Alaminos M. Differential expression of matrix metalloproteinase-2 in human coronal and radicular sound and carious dentine. Journal of Dentistry. 2010;38:635–40. doi: 10.1016/j.jdent.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 72.Dickinson DP. Cysteine peptidases of mammals: their biological roles and potential effects in the oral cavity and other tissues in health and disease. Critical Reviews of Oral Biology and Medicine. 2002;13:238–75. doi: 10.1177/154411130201300304. [DOI] [PubMed] [Google Scholar]
- 73.Polgár L, Csoma C. Dissociation of ionizing groups in the binding cleft inversely controls the endo- and exopeptidase activities of cathepsin B. Journal of Biological Chemistry. 1987;262:14448–53. [PubMed] [Google Scholar]
- 74.Rossi A, Deveraux Q, Turk B, Sali A. Comprehensive search for cysteine cathepsins in the human genome. Biological Chemistry. 2004;385:363–72. doi: 10.1515/BC.2004.040. [DOI] [PubMed] [Google Scholar]
- 75.Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, Turk D. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochimica et Biophysica Acta. 2012;1824:68–88. doi: 10.1016/j.bbapap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanewinkel H, Glössl J, Kresse H. Biosynthesis of cathepsin B in cultured normal and I-cell fibroblasts. Journal of Biological Chemistry. 1987;262:12351–5. [PubMed] [Google Scholar]
- 77.Mach L, Mort JS, Glössl J. Maturation of human procathepsin B. Proenzyme activation and proteolytic processing of the precursor to the mature proteinase, in vitro, are primarily unimolecular processes. Journal of Biological Chemistry. 1994;269:13030–5. [PubMed] [Google Scholar]
- 78.Vasiljeva O, Dolinar M, Pungerčar JR, Turk V, Turk B. Recombinant human procathepsin S is capable of autocatalytic processing at neutral pH in the presence of glycosaminoglycans. FEBS Letters. 2005;579:1285–90. doi: 10.1016/j.febslet.2004.12.093. [DOI] [PubMed] [Google Scholar]
- 79.Caglic D, Pungercar JR, Pejler G, Turk V, Turk B. Glycosaminoglycans facilitate procathepsin B activation through disruption of propeptide-mature enzyme interactions. Journal of Biological Chemistry. 2007;282:33076–85. doi: 10.1074/jbc.M705761200. [DOI] [PubMed] [Google Scholar]
- 80.Turk B, Bieth JG, Björk I, Dolenc I, Turk D, Cimerman N, Kos J, Colic A, Stoka V, Turk V. Regulation of the activity of lysosomal cysteine proteinases by pH-induced inactivation and/or endogenous protein inhibitors, cystatins. Biological Chemistry Hoppe-Seyler. 1995;376:225–30. doi: 10.1515/bchm3.1995.376.4.225. [DOI] [PubMed] [Google Scholar]
- 81.Kirschke H, Wiederanders B, Brömme D, Rinne A. Cathepsin S from bovine spleen. Purification, distribution, intracellular localization and action on proteins. Biochemical Journal. 1989;264:467–73. doi: 10.1042/bj2640467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takahashi T, Dehdarani AH, Yonezawa S, Tang J. Porcine spleen cathepsin B is an exopeptidase. Journal of Biological Chemistry. 1986;261:9375–81. [PubMed] [Google Scholar]
- 83.Pohl J, Davinic S, Bláha I, Strop P, Kostka V. Chromophoric and fluorophoric peptide substrates cleaved through the dipeptidyl carboxypeptidase activity of cathepsin B. Analytical Biochemistry. 1987;165:96–101. doi: 10.1016/0003-2697(87)90205-3. [DOI] [PubMed] [Google Scholar]
- 84.Willenbrock F, Brocklehurst K. A general framework of cysteine-proteinase mechanism deduced from studies on enzymes with structurally different analogous catalytic-site residues Asp-158 and -161 (papain and actinidin), Gly-196 (cathepsin B) and Asn-165 (cathepsin H). Kinetic studies up to pH 8 of the hydrolysis of N-alpha-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide catalysed by cathepsin B and of L-arginine 2-naphthylamide catalysed by cathepsin H. Biochemical Journal. 1985;227:521–8. doi: 10.1042/bj2270521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khouri HE, Plouffe C, Hasnain S, Hirama T, Storer AC, Ménard R. A model to explain the pH-dependent specificity of cathepsin B-catalysed hydrolyses. Biochemical Journal. 1991;275 (Pt 3):751–7. doi: 10.1042/bj2750751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Musil D, Zucic D, Turk D, Engh RA, Mayr I, Huber R, Popovic T, Turk V, Towatari T, Katunuma N, Bode W. The refined 2. 15 A X-ray crystal structure of human liver cathepsin B: the structural basis for its specificity. EMBO Journal. 1991;10:2321–30. doi: 10.1002/j.1460-2075.1991.tb07771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nägler DK, Storer AC, Portaro FC, Carmona E, Juliano L, Ménard R. Major increase in endopeptidase activity of human cathepsin B upon removal of occluding loop contacts. Biochemistry. 1997;36:12608–15. doi: 10.1021/bi971264+. [DOI] [PubMed] [Google Scholar]
- 88.Barrett AJ, Kirschke H, Cathepsin B, Cathepsin H, Cathepsin L. Methods in Enzymology. 1981;80(Pt C):535–61. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- 89.Buck MR, Karustis DG, Day NA, Honn KV, Sloane BF. Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochemical Journal. 1992;282 (Pt 1):273–8. doi: 10.1042/bj2820273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keppler D, Waridel P, Abrahamson M, Bachmann D, Berdoz J, Sordat B. Latency of cathepsin B secreted by human colon carcinoma cells is not linked to secretion of cystatin C and is relieved by neutrophil elastase. Biochimica et Biophysica Acta. 1994;1226:117–25. doi: 10.1016/0925-4439(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 91.Sloane BF, Moin K, Sameni M, Tait LR, Rozhin J, Ziegler G. Membrane association of cathepsin B can be induced by transfection of human breast epithelial cells with c-Ha-ras oncogene. Journal of Cell Science. 1994;107(Pt 2):373–84. doi: 10.1242/jcs.107.2.373. [DOI] [PubMed] [Google Scholar]
- 92.Storrie B, Desjardins M. The biogenesis of lysosomes: is it a kiss and run, continuous fusion and fission process? Bioessays. 1996;18:895–903. doi: 10.1002/bies.950181108. [DOI] [PubMed] [Google Scholar]
- 93.Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S, Lee-Rykaczewski E, Coleman L, Rieman D, Barthlow R, Hastings G, Gowen M. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. Journal of Bioloical Chemistry. 1996;271:12511–6. doi: 10.1074/jbc.271.21.12511. [DOI] [PubMed] [Google Scholar]
- 94.Götz W, Quondamatteo F, Ragotzki S, Affeldt J, Jäger A. Localization of cathepsin D in human odontoclasts. a light and electron microscopical immunocytochemical study. Connective Tissue Research. 2000;41:185–94. doi: 10.3109/03008200009005289. [DOI] [PubMed] [Google Scholar]
- 95.Lecaille F, Brömme D, Lalmanach G. Biochemical properties and regulation of cathepsin K activity. Biochimie. 2008;90:208–26. doi: 10.1016/j.biochi.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 96.Wang Z, McCauley LK. Osteoclasts and odontoclasts: signaling pathways to development and disease. Oral Diseases. 2011;17:129–42. doi: 10.1111/j.1601-0825.2010.01718.x. [DOI] [PubMed] [Google Scholar]
- 97.Perez-Amodio S, Beertsen W, Everts V. (Pre-)osteoclasts induce retraction of osteoblasts before their fusion to osteoclasts. Journal of Bone and Mineral Research. 2004;19:1722–31. doi: 10.1359/JBMR.040509. [DOI] [PubMed] [Google Scholar]
- 98.Gelb BD, Shi GP, Chapman HA, Desnick RJ. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science. 1996;273:1236–8. doi: 10.1126/science.273.5279.1236. [DOI] [PubMed] [Google Scholar]
- 99.Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, Moritz JD, Schu P, von Figura K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13453–8. doi: 10.1073/pnas.95.23.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Everts V. Is cathepsin K truly involved in bone resorption? Journal of Bone and Mineral Research. 1998;13:321–2. doi: 10.1359/jbmr.1998.13.2.321.1. [DOI] [PubMed] [Google Scholar]
- 101.Konttinen YT, Takagi M, Mandelin J, Lassus J, Salo J, Ainola M, Li TF, Virtanen I, Liljestrom M, Sakai H, Kobayashi Y, Sorsa T, Lappalainen R, Demulder A, Santavirta S. Acid attack and cathepsin K in bone resorption around total hip replacement prosthesis. Journal of Bone and Mineral Research. 2001;16:1780–6. doi: 10.1359/jbmr.2001.16.10.1780. [DOI] [PubMed] [Google Scholar]
- 102.Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, Carrilho MR, Pashley DH, Tay FR, Salo T, Tjäderhane L. Cysteine cathepsins in human dentin–pulp complex. Journal of Endodontics. 2010;36:475–81. doi: 10.1016/j.joen.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 103.Lutgens SP, Cleutjens KB, Daemen MJ, Heeneman S. Cathepsin cysteine proteases in cardiovascular disease. FASEB Journal. 2007;21:3029–41. doi: 10.1096/fj.06-7924com. [DOI] [PubMed] [Google Scholar]
- 104.Brömme D, Okamoto K. Human cathepsin O2, a novel cysteine protease highly expressed in osteoclastomas and ovary molecular cloning, sequencing and tissue distribution. Biological Chemistry Hoppe-Seyler. 1995;376:379–84. doi: 10.1515/bchm3.1995.376.6.379. [DOI] [PubMed] [Google Scholar]
- 105.Hou WS, Li W, Keyszer G, Weber E, Levy R, Klein MJ, Gravallese EM, Goldring SR, Brömme D. Comparison of cathepsins K and S expression within the rheumatoid and osteoarthritic synovium. Arthritis and Rheumatism. 2002;46:663–74. doi: 10.1002/art.10114. [DOI] [PubMed] [Google Scholar]
- 106.Li Z, Hou WS, Escalante-Torres CR, Gelb BD, Bromme D. Collagenase activity of cathepsin K depends on complex formation with chondroitin sulfate. Journal of Biological Chemistry. 2002;277:28669–76. doi: 10.1074/jbc.M204004200. [DOI] [PubMed] [Google Scholar]
- 107.Burleigh MC, Barrett AJ, Lazarus GS. Cathepsin B1. A lysosomal enzyme that degrades native collagen. Biochemical Journal. 1974;137:387–98. doi: 10.1042/bj1370387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kirschke H, Kembhavi AA, Bohley P, Barrett AJ. Action of rat liver cathepsin L on collagen and other substrates. Biochemical Journal. 1982;201:367–72. doi: 10.1042/bj2010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hou WS, Li Z, Büttner FH, Bartnik E, Brömme D. Cleavage site specificity of cathepsin K toward cartilage proteoglycans and protease complex formation. Biological Chemistry. 2003;384:891–7. doi: 10.1515/BC.2003.100. [DOI] [PubMed] [Google Scholar]
- 110.Buttle DJ, Handley CJ, Ilic MZ, Saklatvala J, Murata M, Barrett AJ. Inhibition of cartilage proteoglycan release by a specific inactivator of cathepsin B and an inhibitor of matrix metalloproteinases. Evidence for two converging pathways of chondrocyte-mediated proteoglycan degradation. Arthritis and Rheumatism. 1993;36:1709–17. doi: 10.1002/art.1780361210. [DOI] [PubMed] [Google Scholar]
- 111.Murphy G, Ward R, Gavrilovic J, Atkinson S. Physiological mechanisms for metalloproteinase activation. Matrix Supplement. 1992;1:224–30. [PubMed] [Google Scholar]
- 112.Kostoulas G, Lang A, Nagase H, Baici A. Stimulation of angiogenesis through cathepsin B inactivation of the tissue inhibitors of matrix metalloproteinases. FEBS Letters. 1999;455:286–90. doi: 10.1016/s0014-5793(99)00897-2. [DOI] [PubMed] [Google Scholar]
- 113.Hara K, Kominami E, Katunuma N. Effect of proteinase inhibitors on intracellular processing of cathepsin B, H and L in rat macrophages. FEBS Letters. 1988;231:229–31. doi: 10.1016/0014-5793(88)80737-3. [DOI] [PubMed] [Google Scholar]
- 114.Cox SW, Eley BM, Kiili M, Asikainen A, Tervahartiala T, Sorsa T. Collagen degradation by interleukin-1beta-stimulated gingival fibroblasts is accompanied by release and activation of multiple matrix metalloproteinases and cysteine proteinases. Oral Diseases. 2006;12:34–40. doi: 10.1111/j.1601-0825.2005.01153.x. [DOI] [PubMed] [Google Scholar]
- 115.Winberg JO, Berg E, Kolset SO, Uhlin-Hansen L. Calcium-induced activation and truncation of promatrix metalloproteinase-9 linked to the core protein of chondroitin sulfate proteoglycans. European Journal of Biochemistry. 2003;270:3996–4007. doi: 10.1046/j.1432-1033.2003.03788.x. [DOI] [PubMed] [Google Scholar]
- 116.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biology. 2007;26:587–96. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Malla N, Berg E, Uhlin-Hansen L, Winberg JO. Interaction of pro-matrix metalloproteinase-9/proteoglycan heteromer with gelatin and collagen. Journal of Biological Chemistry. 2008;283:13652–65. doi: 10.1074/jbc.M709140200. [DOI] [PubMed] [Google Scholar]
- 118.Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. FEBS Journal. 2011;278:28–45. doi: 10.1111/j.1742-4658.2010.07920.x. [DOI] [PubMed] [Google Scholar]
- 119.Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, Carvalho RM, Tjäderhane L, Tay FR, Pashley DH. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. European Journal of Oral Sciences. 2006;114:160–6. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 120.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, Toledano M, Pashley EL, Tay FR. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27:4470–6. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 121.Carrilho MR, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, Breschi L, Foulger S, Pashley DH. Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. Journal of Biomedical Materials Research Part B, Applied Biomaterials. 2009;90:373–80. doi: 10.1002/jbm.b.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tezvergil-Mutluay A, Agee KA, Hoshika T, Carrilho M, Breschi L, Tjäderhane L, Nishitani Y, Carvalho RM, Looney S, Tay FR, Pashley DH. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dental Materials. 2010;26:1059–67. doi: 10.1016/j.dental.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rozman J, Stojan J, Kuhelj R, Turk V, Turk B. Autocatalytic processing of recombinant human procathepsin B is a bimolecular process. FEBS Letters. 1999;459:358–62. doi: 10.1016/s0014-5793(99)01302-2. [DOI] [PubMed] [Google Scholar]
- 124.Almeida PC, Nantes IL, Chagas JR, Rizzi CC, Faljoni-Alario A, Carmona E, Juliano L, Nader HB, Tersariol IL. Cathepsin B activity regulation. Heparin-like glycosaminogylcans protect human cathepsin B from alkaline pH-induced inactivation. Journal of Biological Chemistry. 2001;276:944–51. doi: 10.1074/jbc.M003820200. [DOI] [PubMed] [Google Scholar]
- 125.Ishidoh K, Kominami E. Multi-step processing of procathepsin L in vitro. FEBS Letters. 1994;352:281–4. doi: 10.1016/0014-5793(94)00924-4. [DOI] [PubMed] [Google Scholar]
- 126.Kopitar G, Dolinar M, Štrukelj B, Pungerčar J, Turk V. Folding and activation of human procathepsin S from inclusion bodies produced in Escherichia coli. European Journal of Biochemistry. 1996;236:558–62. doi: 10.1111/j.1432-1033.1996.00558.x. [DOI] [PubMed] [Google Scholar]
- 127.Li Z, Yasuda Y, Li W, Bogyo M, Katz N, Gordon RE, Fields GB, Brömme D. Regulation of collagenase activities of human cathepsins by glycosaminoglycans. Journal of Biological Chemistry. 2004;279:5470–9. doi: 10.1074/jbc.M310349200. [DOI] [PubMed] [Google Scholar]
- 128.Serveau C, Boulangé A, Lecaille F, Gauthier F, Authié E, Lalmanach G. Procongopain from Trypanosoma congolense is processed at basic pH: an unusual feature among cathepsin L-like cysteine proteases. Biological Chemistry. 2003;384:921–7. doi: 10.1515/BC.2003.103. [DOI] [PubMed] [Google Scholar]
- 129.Orsini G, Ruggeri A, Jr, Mazzoni A, Nato F, Mazzotti G, Putignano A, Tjäderhane L, Breschi L. A review on nature, role and functions of dentin non-collagenous proteins. Part I: proteoglycans and glycoproteins. Endodontic Topics. 2012;21:1–18. [Google Scholar]
- 130.Dung SZ, Gregory RL, Li Y, Stookey GK. Effect of lactic acid and proteolytic enzymes on the release of organic matrix components from human root dentin. Caries Research. 1995;29:483–9. doi: 10.1159/000262119. [DOI] [PubMed] [Google Scholar]
- 131.Nascimento FD, Rizzi CC, Nantes IL, Stefe I, Turk B, Carmona AK, Nader HB, Juliano L, Tersariol IL. Cathepsin X binds to cell surface heparan sulfate proteoglycans. Archives of Biochemistry and Biophysics. 2005;436:323–32. doi: 10.1016/j.abb.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 132.Tay FR, Pashley DH. Water treeing–a potential mechanism for degradation of dentin adhesives. American Journal of Dentistry. 2003;16:6–12. [PubMed] [Google Scholar]
- 133.Lehmann N, Debret R, Roméas A, Magloire H, Degrange M, Bleicher F, Sommer P, Seux D. Self-etching increases matrix metalloproteinase expression in the dentin-pulp complex. Journal of Dental Research. 2009;88:77–82. doi: 10.1177/0022034508327925. [DOI] [PubMed] [Google Scholar]
- 134.Pääkkönen V, Ohlmeier S, Bergmann U, Larmas M, Salo T, Tjäderhane L. Analysis of gene and protein expression in healthy and carious tooth pulp with cDNA microarray and two-dimensional gel electrophoresis. European Journal of Oral Sciences. 2005;113:369–79. doi: 10.1111/j.1600-0722.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- 135.Shin SJ, Lee JI, Baek SH, Lim SS. Tissue levels of matrix metalloproteinases in pulps and periapical lesions. Journal of Endodontics. 2002;28:313–5. doi: 10.1097/00004770-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 136.Gusman H, Santana RB, Zehnder M. Matrix metalloproteinase levels and gelatinolytic activity in clinically healthy and inflamed human dental pulps. European Journal of Oral Sciences. 2002;110:353–7. doi: 10.1034/j.1600-0722.2002.21347.x. [DOI] [PubMed] [Google Scholar]
- 137.Tsai CH, Chen YJ, Huang FM, Su YF, Chang YC. The upregulation of matrix metalloproteinase-9 in inflamed human dental pulps. Journal of Endodontics. 2005;31:860–2. doi: 10.1097/01.don.0000164851.55389.4e. [DOI] [PubMed] [Google Scholar]
- 138.Orsini G, Mazzoni A, Orciani M, Putignano A, Procaccini M, Falconi M, Pashley DH, Tay FR, Breschi L. Matrix metalloproteinase-2 expression induced by two different adhesive systems on human pulp fibroblasts. Journal of Endodontics. 2011;37:1663–7. doi: 10.1016/j.joen.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 139.Sano H, Yoshikawa T, Pereira PN, Kanemura N, Morigami M, Tagami J, Pashley DH. Long-term durability of dentin bonds made with a self-etching primer, in vivo. Journal of Dental Research. 1999;78:906–11. doi: 10.1177/00220345990780041101. [DOI] [PubMed] [Google Scholar]
- 140.Hashimoto M, Ohno H, Kaga M, Endo K, Sano H, Oguchi H. In vivo degradation of resin-dentin bonds in humans over 1 to 3 years. Journal of Dental Research. 2000;79:1385–91. doi: 10.1177/00220345000790060601. [DOI] [PubMed] [Google Scholar]
- 141.Takahashi A, Inoue S, Kawamoto C, Ominato R, Tanaka T, Sato Y, Pereira PN, Sano H. In vivo long-term durability of the bond to dentin using two adhesive systems. Journal of Adhesive Dentistry. 2002;4:151–9. [PubMed] [Google Scholar]
- 142.Hashimoto M, Tay FR, Ohno H, Sano H, Kaga M, Yiu C, Kumagai H, Kudou Y, Kubota M, Oguchi H. SEM and TEM analysis of water degradation of human dentinal collagen. Journal of Biomedical Materials Research Part B, Applied Biomaterials. 2003;66:287–98. doi: 10.1002/jbm.b.10560. [DOI] [PubMed] [Google Scholar]
- 143.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. Journal of Dental Research. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 144.Bornstein P, Sage H. Structurally distinct collagen types. Annual Review of Biochemistry. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- 145.Simsek B, Karacaer O, Karaca I. Urine products of bone breakdown as markers of bone resorption and clinical usefulness of urinary hydroxyproline: an overview. Chinese Medical Journal. 2004;117:291–5. [PubMed] [Google Scholar]
- 146.Veis A, Schlueter RJ. The macromolecular organization of dentine matrix collagen. I. Characterization of dentine collagen. Biochemistry. 1964;3:1650–7. doi: 10.1021/bi00899a009. [DOI] [PubMed] [Google Scholar]
- 147.Tezvergil-Mutluay A, Agee KA, Hoshika T, Tay FR, Pashley DH. The inhibitory effect of polyvinylphosphonic acid on functional matrix metalloproteinase activities in human demineralized dentin. Acta Biomaterialia. 2010;6(4136):42. doi: 10.1016/j.actbio.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, Breschi L, Nishitani Y, Tay FR, Pashley DH. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. Journal of Dental Research. 2011;90:535–40. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tezvergil-Mutluay A, Mutluay MM, Gu LS, Zhang K, Agee KA, Carvalho RM, Manso A, Carrilho M, Tay FR, Breschi L, Suh BI, Pashley DH. The anti-MMP activity of benzalkonium chloride. Journal of Dentistry. 2011;39:57–64. doi: 10.1016/j.jdent.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tezvergil-Mutluay A, Mutluay MM, Agee KA, Seseogullari-Dirihan R, Hoshika T, Cadenaro M, Breschi L, Vallittu P, Tay FR, Pashley DH. Carbodiimide cross-linking inactivates soluble and matrix-bound MMPs, in vitro. Journal of Dental Research. 2012;91:192–6. doi: 10.1177/0022034511427705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Charni-Ben Tabassi N, Desmarais S, Bay-Jensen AC, Delaissé JM, Percival MD, Garnero P. The type II collagen fragments Helix-II and CTX-II reveal different enzymatic pathways of human cartilage collagen degradation. Osteoarthritis and Cartilage. 2008;16:1183–91. doi: 10.1016/j.joca.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 152.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. Journal of Dental Research. 2005;84:741–6. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 153.Van Meerbeek B, Dhem A, Goret-Nicaise M, Braem M, Lambrechts P, VanHerle G. Comparative SEM and TEM examination of the ultrastructure of the resin-dentin interdiffusion zone. Journal of Dental Research. 1993;72:495–501. doi: 10.1177/00220345930720020501. [DOI] [PubMed] [Google Scholar]
- 154.Van Meerbeek B, Conn LJ, Jr, Duke ES, Eick JD, Robinson SJ, Guerrero D. Correlative transmission electron microscopy examination of nondemineralized and demineralized resin-dentin interfaces formed by two dentin adhesive systems. Journal of Dental Research. 1996;75:879–88. doi: 10.1177/00220345960750030401. [DOI] [PubMed] [Google Scholar]
- 155.Van Meerbeek B, Yoshida Y, Lambrechts P, Vanherle G, Duke ES, Eick JD, Robinson SJ. A TEM study of two water-based adhesive systems bonded to dry and wet dentin. Journal of Dental Research. 1998;77:50–9. doi: 10.1177/00220345980770010501. [DOI] [PubMed] [Google Scholar]
- 156.Tay FR, Gwinnett JA, Wei SH. Micromorphological spectrum from overdrying to overwetting acid-conditioned dentin in water-free acetone-based, single-bottle primer/adhesives. Dental Materials. 1996;12:236–44. doi: 10.1016/s0109-5641(96)80029-7. [DOI] [PubMed] [Google Scholar]
- 157.Tay FR, Gwinnett AJ, Wei SH. The overwet phenomenon: an optical, micromorphological study of surface moisture in the acid-conditioned, resin-dentin interface. American Journal of Dentistry. 1996;9:43–8. [PubMed] [Google Scholar]
- 158.Tay FR, Gwinnett AJ, Wei SH. The overwet phenomenon: a transmission electron microscopic study of surface moisture in the acid-conditioned, resin-dentin interface. American Journal of Dentistry. 1996;9:161–6. [PubMed] [Google Scholar]
- 159.Armstrong SR, Vargas MA, Chung I, Pashley DH, Campbell JA, Laffoon JE, Qian F. Resin-dentin interfacial ultrastructure and microtensile dentin bond strength after five-year water storage. Operative Dentistry. 2004;29:705–12. [PubMed] [Google Scholar]
- 160.Carrilho MR, Carvalho RM, de Goes MF, di Hipólito V, Geraldeli S, Tay FR, Pashley DH, Tjäderhane L. Chlorhexidine preserves dentin bond in vitro. Journal of Dental Research. 2007;86:90–4. doi: 10.1177/154405910708600115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Carrilho MR, Geraldeli S, Tay FR, de Goes MF, Carvalho RM, Tjäderhane L, Pashley DH. In vivo preservation of hybrid layer by chlorhexidine. Journal of Dental Research. 2007;86:529–3. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 162.Tay FR, Pashley DH, Loushine RJ, Weller RN, Monticelli F, Osorio R. Self-etching adhesives increase collagenolytic activity in radicular dentin. Journal of Endodontics. 2006;32:862–8. doi: 10.1016/j.joen.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 163.Mazzoni A, Carrilho M, Papa V, Tjäderhane L, Gobbi P, Nucci C, Di Lenarda R, Mazzotti G, Tay FR, Pashley DH, Breschi L. MMP-2 assay within the hybrid layer created by a two-step etch-and-rinse adhesive: biochemical and immunohistochemical analysis. Journal of Dentistry. 2011;39:470–7. doi: 10.1016/j.jdent.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 164.Min D, Lyons JG, Jia J, Lo L, McLennan SV. 2-Methoxy-2,4-diphenyl-3(2H)-furanone-labeled gelatin zymography and reverse zymography: a rapid real-time method for quantification of matrix metalloproteinases-2 and -9 and tissue inhibitors of metalloproteinases. Electrophoresis. 2006;27:357–64. doi: 10.1002/elps.200500484. [DOI] [PubMed] [Google Scholar]
- 165.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, Ruggeri A, Jr, Tay FR, Dorigo Ede S, Pashley DH. Chlorhexidine stabilizes the adhesive interface: a 2-year in vitro study. Dental Materials. 2010;26:320–5. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.De Munck J, Van den Steen PE, Mine A, Van Landuyt KL, Poitevin A, Opdenakker G, Van Meerbeek B. Inhibition of enzymatic degradation of adhesive-dentin interfaces. Journal of Dental Research. 2009;88:1101–6. doi: 10.1177/0022034509346952. [DOI] [PubMed] [Google Scholar]
- 167.De Munck J, Mine A, Van den Steen PE, Van Landuyt KL, Poitevin A, Opdenakker G, Van Meerbeek B. Enzymatic degradation of adhesive-dentin interfaces produced by mild self-etch adhesives. European Journal of Oral Sciences. 2010;118:494–501. doi: 10.1111/j.1600-0722.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 168.Kato MT, Leite AL, Hannas AR, Buzalaf MA. Gels containing MMP inhibitors prevent dental erosion in situ. Journal of Dental Research. 2010;89:468–72. doi: 10.1177/0022034510363248. [DOI] [PubMed] [Google Scholar]
- 169.Kato MT, Leite AL, Hannas AR, Oliveira RC, Pereira JC, Tjäderhane L, Buzalaf MA. Effect of iron on matrix metalloproteinase inhibition and on the prevention of dentine erosion. Caries Research. 2010;44:309–16. doi: 10.1159/000315932. [DOI] [PubMed] [Google Scholar]
- 170.Nakabayashi N, Pashley DH. Hybridization of Dental Hard Tissues. Chicago: Quintessence Publishing; 1998. [Google Scholar]