Abstract

A convergent approach provides a convenient access to synthetically and biologically useful 3,4-disubstituted 5-hydroxy indoles. The one-pot procedure uses microwave heating to initiate an intramolecular [4+2]-cycloaddition of an alkynol segment onto a furan followed by a fragmentation, aromatization and N-Boc deprotection cascade. Yields range from 15-75%, with aromatic substituents providing better conversions. 4-Trimethylsilylated analogs undergo a 1,3-silatropic rearrangement to give the O-TMS ethers.
INTRODUCTION
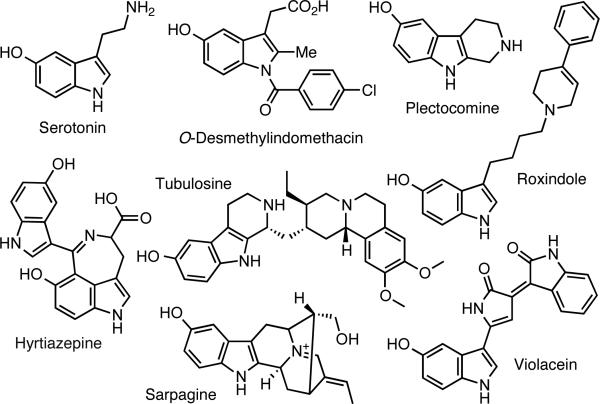
Second only to pyridines, indoles are among the most common aromatic scaffolds present in bioactive molecules.1 The 5-hydroxy indole moiety alone has currently >11,000 substructure hits from >100,000 literature references in SciFinder. In addition to their prominence in the neurotransmitter serotonin and its many analogs, 5-hydroxy indoles are found in a vast array of pharmacologically active agents and natural products (Figure 1). Furthermore, the hydroxy group can be readily converted to derivatives that allow scaffold extensions cross-coupling or nucleophilic addition reactions of the indole nucleus.2
Figure 1.
Representative biologically active 5-hydroxy indoles.
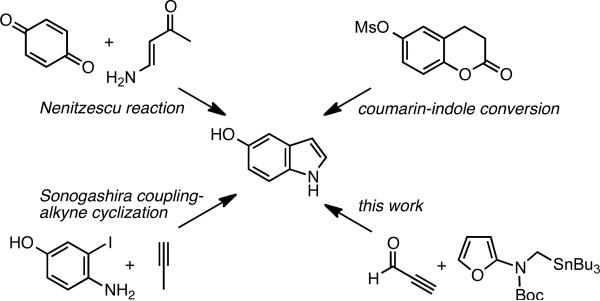
The Nenitzescu reaction is frequently used for the construction of 5-hydroxy indoles.3 Alternative protocols include the coumarin-indole transformation,4 and the Pd-catalyzed coupling of 2-iodoanilines with alkynes (Scheme 1).5 All of these protocols start with a functionalized 6-membered ring and add the pyrrole moiety in a linear sequence. We have recently reported an alternative strategy for a convergent indole synthesis that uses an intramolecular Diels-Alder furan (IMDAF) cycloaddition to assemble both benzene and pyrrole moieties simultaneously.6,7 We are now reporting an extension of this procedure to the direct preparation of 5-hydroxy indoles.
Scheme 1.
Methods for Formation of 5-Hydroxy Indoles
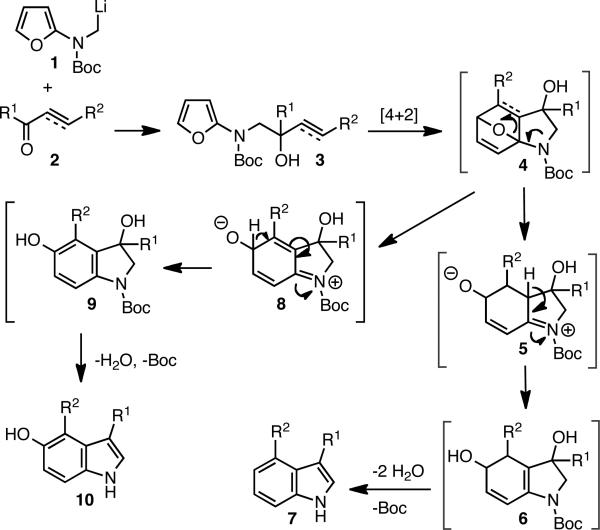
In our previous reaction sequence,6 the 1,2-addition of lithiated 1 to enone 2 provided the allylic alcohol 3 (Scheme 2). Microwave heating of 3 led to the tricyclic IMDAF product 4 and subsequently through sequential elimination of two equivalents of water via 5 and 6 led to indoles 7. We reasoned that the use of an alkynone in place of the enone would give us the opportunity to branch out from this reaction pathway, eliminate the bridging oxygen atom in 4 to give 8, aromatize the intermediate at an earlier stage to give phenol 9, and finally only eliminate a single molecule of water and, concomitantly, the N-protective group8 to yield 5-hydroxy indole 10.
Scheme 2.
Proposed Reaction Pathways for Indole and 5-Hydroxy Indole Formation by Intramolecular Diels-Alder Reaction with Furans
RESULTS AND DISCUSSION
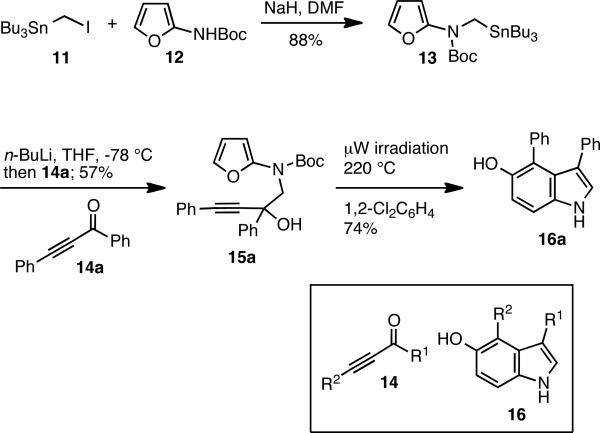
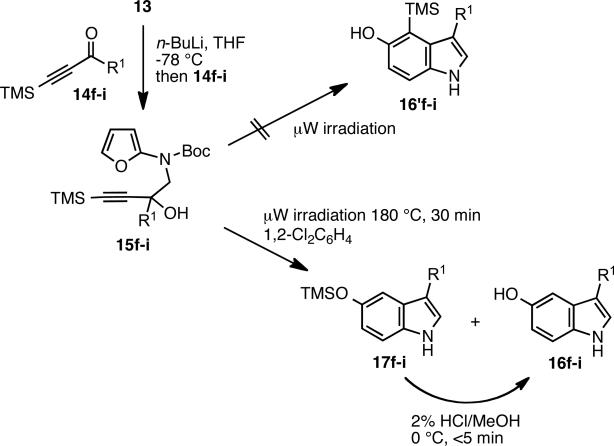
The hypothesis that an alkynyl intermediate 3 would lead to the formation of 5-hydroxy indole 10 was readily put to test. Furanyl stannane 13 was prepared from iodide 119 and N-Boc-2-aminofuran 1210 in the presence of NaH (Scheme 3). Stannane 13 underwent a rapid transmetalation (5 min) to 1 with n-Bu Li at -78 °C; and after the addition of 1 equivalent of 1,3-diphenylprop-2-yn-1-one 14a, the tertiary alcohol 15 was isolated in 57% yield. Microwave heating to 220 °C for 1 h effected the desired IMDAF process and aromatization to give 5-hydroxy indole 16 a in 74% yield after chromatographic purification of the reaction mixture on SiO2. The Boc group was cleaved off under the thermal conditions8. Interestingly, the ethoxycarbonyl protective group7c on nitrogen led to lower yields, possibly due to an N → O acyl shift in intermediate 3.
Scheme 3.
a Formation of Propargyl Alcohol and Thermal Cycloaddition Reaction
In order to further investigate the scope of this new process, we converted commercially available carbonyl compounds into ynones 14b-e according to literature protocols.11,12 The lithium reagent derived from stannane 13 was then added to these ynones to give the corresponding alkynols 15b-e in unoptimized 44-52% yield. As shown in Table 1, microwave irradiation of 15b-e in o-dichlorobenzene for 1 h produced the expected 5-hydroxy indoles 16b-e in moderate (36%) to good (63%) yields.
Table 1.
Synthesis of 5-Hydroxy Indoles 16b-e from Tertiary Alkynols 15b-e
| entry | 15 | [%]a | R1 | R2 | 16 | [%]b |
|---|---|---|---|---|---|---|
| 1 | b | 52 | 2-thiophenyl | Ph | b | 61 |
| 2 | c | 44 | n-hexyl | Ph | c | 58 |
| 3 | d | 47 | CH2CH2Ph | Ph | d | 63 |
| 4 | e | 49 | c-hexyl | n-butyl | e | 36 |
Yields of isolated alkynols 15.
Yields of isolated furans 16.
The reaction tolerated both aromatic and heteroaroma tic groups (i.e. phenyl, 16a, and thiophene, 16b) as well as branched and cyclic aliphatic substituents (i.e. n-hexyl, 16c, phenethyl, 16d, and c-hexyl, 16e) at the R1-position. We explored fewer variations of the R2 groups, but as the n-butyl derivative 16e demonstrated, aliphatic groups appear to be acceptable substituents at R2. The lowest yield, 36%, was obtained with R1 and R2 both being aliphatic residues, which could indicate that either indole stabilization through conjugative substituents at R1 and R2 or the presence of sterically bulky groups such as phenyl rings promotes product formation. Therefore, we tested silylated alkynes derived from silyl ketones as substrates that provide steric shielding in the absence of π-electron resonance effects.
When the four trimethylsilyl (TMS) alkynols 15f-i13 were subjected to the microwave mediated cycloaddition conditions at 180 °C, the cycloaddition occurred smoothly and provided indole products in good yields (Scheme 4 and Table 2). However, rather than the expected 4-trimethylsilyl derivatives 16'f-i, a mixture of 5-trimethylsilyloxy indoles 17f-i and the corresponding 5-hydroxy indoles 16f-i were isolated. Presumably, the TMS-ethers result from a 1,3-silatropic C to O rearrangement,14 induced by the thermal conditions and rendered quantitative by the strong O-Si bond. In order to generate a homogeneous product fraction, the crude reaction mixtures were treated with 2% HCl in MeOH to desilylate 17f-i to cleanly afford the 5-hydroxy indole products 16f-i.
Scheme 4.
a Formation of 3-Substituted 5-Hydroxy Indoles from Silylated Alkynones.
Table 2.
Synthesis of 5-Hydroxy Indoles 16f-i from Tertiary Alkynols 15f-i.
| entry | 15 | [%]a | R1 | 16 | [%]b |
|---|---|---|---|---|---|
| 1 | f | 58 | Ph | f | 63 |
| 2 | g | 60 | 2-naphthyl | g | 60 |
| 3 | h | 58 | 2-benzofuryl | h | 63 |
| 4 | i | 52 | c-propyl | i | 47 |
Yields of isolated alkynols 15.
Yields of isolated furans 16.
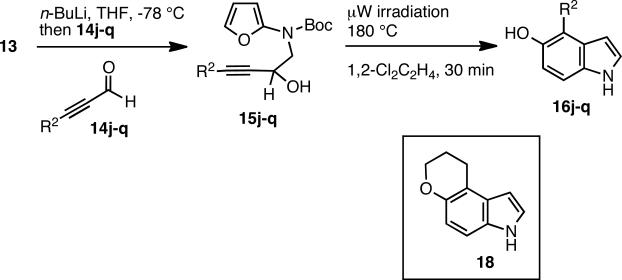
As a further test of this methodology, the reaction sequence was extended to aldehyde substrates, and the secondary alkynols 15j-q were prepared16 in unoptimized 20-57% yields by addition of lithiated 13 to ynals 14j-q (Scheme 5). As expected, the microwave conditions promoted cycloadditions to ultimately afford the 4-substituted-5-hydroxy indoles 16j-q. Similar to our previous observations, aromatic substrates gave better isolated yields compared to the alkyl substituted alkynols (Table 3).
Scheme 5.
a Formation of 4-Substituted 5-Hydroxy Indoles from Alkynals.
Table 3.
Synthesis of 5-Hydroxy Indoles 16j-q from Secondary Alkynols 15j-q.
| entry | 15 | [%]a | R2 | 16 | [%]b |
|---|---|---|---|---|---|
| 1 | j | 43 | Ph | j | 48 |
| 2 | k | 57 | 3-OMePh | k | 42 |
| 3 | l | 52 | 4-MePh | l | 47 |
| 4 | m | 26 | 4-CF3Ph | m | 64 |
| 5 | n | 20 | 4-F-Ph | n | 44 |
| 6 | o | 54 | (CH2)2CH(CH3)2 | o | 15 |
| 7 | p | 52 | (CH2)3Cl | p | 20c |
| 8 | q | 38 | 1-cyclohexene | q | 23 |
Yields of isolated alkynols 15.
Yields of isolated furans 16.
The pyran-containing indole product 1817 was also isolated in 7% (see Supporting Information).
Indoles with 4-aryl residues 16j-n were isolated in 42-64% yield, and no obvious trend for electron-withdrawing or –donating substituents was observed. The two alkyl-chain containing products 16o and 16p were formed in low (15-20%) yields, and the 3-chloropropyl analog 16p partially cyclized and produced an additional 7% of the corresponding pyran 18. The cyclized ether17 could also be obtained in 53% by treatment of isolated 16p with NaH in THF. Finally, the cyclohexene derivative 15q led to the introduction of a 4-alkene group in 16q, but the yield (23%) was similarly low as observed for aliphatic substrates. Nonetheless, this methodology allows for the rapid introduction of a diverse range of substituents at carbons 3 and 4 of the 5-hydroxy indole scaffold.
CONCLUSION
The use of alkynones and alkynals as starting materials extends our microwave-assisted IMDAF-aromatization cascade reaction to the direct formation of synthetically and biologically valuable 5-hydroxy indoles. The requisite substituted alkynes are readily available, and the methodology represents an unusual convergent formation of both the ben zene and the pyrrole subunits of the indole ring system. Yields range from 15-75%, and the efficiency of the conversion benefits from aromatic substituents that can stabilize the charged and/or reactive intermediates 4, 8 and 9 in the cascade indole format ion process. We also observed an interesting 1,3-silatropic rearrangement of 4-silylated intermediates 16’. Overall, due to the straightforward access to ynones and ynals, the convergent nature of the retrosynthetic disconnection, and the convenient thermal reaction conditions, this new reaction provides a significant alternative to other common methods for 5-hydroxy indole construction, especially for projects where a diverse range of 3-and 4-substitutions is desired.
EXPERIMENTAL SECTION
General Information
Microwave reactions were performed at 200-250 W using a Biotage Initiator. Ketones 14a and 14b are commercially available and were used without further purification. Ketones 14c,11,1814d,11,19, 14e,12,2014f,21,22 14g,21,2314h,2114i21,24 and aldehydes 14j,2514k,11,2614l,11,2714m,11,2814n,11,2914o,2514p,25,30 and 14q25,31 were prepared according to literature procedures.
1-(Benzofuran-2-yl)-3-(trimethylsilyl)prop-2-yn-1-one (14h)
According to a literature protocol,2114h was obtained as a yellow oil (2.50 g, 84%): IR (neat) 2963, 2158, 1630, 1548 cm-1; 1H NMR (400 MHz, acetone-d6) δ 7.94 (s, 1 H), 7.88 (d, J = 7.6 Hz, 1 H), 7.66 (d, J = 8.5 Hz, 1 H), 7.60 (ddd, J = 1.2, 7.0, 7.0 Hz, 1 H), 7.40 (ddd, J = 0.9, 8.0, 8.0 Hz, 1 H), 0.34 (s, 9 H); 13C NMR (100 MHz, acetone-d6) δ 165.9, 157.3, 154.0, 130.3, 127.9, 125.3, 124.9, 118.9, 113.2, 101.1, 99.7, -0.78; HRMS (TOF APCI+) m/z calcd for C14H15O2Si (M+H) 243.0841, found 243.0865.
General Protocol A. tert-Butyl furan-2-yl(2-hydroxy-2,4-diphenylbut-3-ynyl)carbamate (15a)
A flame dried 25-mL round bottom flask was charged with a solution of furanyl-stannane 13 (0.608 g, 1.25 mmol) in dry THF (4 mL). The solution was cooled to-78 °C and treated with BuLi (1.0 mL, 1.6 M in hexanes) using a syringe pump over 10 min. The reaction mixture was then treated with alkyne 14a (0.253 g, 1.23 mmol) in THF (4 mL) via syringe pump over 10 min. After 1 h, the orange solution was diluted with satd. NH4Cl, extracted with EtOAc, washed with brine, dried (Na2SO4), filtered and concentrated. The crude residue was purified by chromatography on SiO2 nt) (ISCO-Companion, 0-100% EtOAc/hexanes, 25 min gradie) to give alcohol 15a (0.29 g, 0.72 mmol, 57%) as a yellow oil: ; IR (neat) 3395, 2982, 1713, 1681 cm-1; 1H NMR (400 MHz, acetone-d6) δ 7.73 (d, J = 7.2 Hz, 2 H), 7.44-7.28 (m, 8 H), 7.24 (dd, J = 1.0, 2.2 Hz, 1 H), 6.32 (dd, J = 2.4, 3.2 Hz, 1 H), 6.02 (br s, 1 H), 5.39 (br s, 1 H), 4.16-4.04 (m,2H), 4.07 (d, J = 14.2 Hz, 1 H), 1.29 (s, 9 H); 13C NMR (75 MHz, acetone-d6) δ 150.0, 144.2, 138.9, 132.6, 129.4, 129.3, 128.8, 128.5, 127.1, 123.8, 111.7, 91.9, 86.4, 81.7, 73.8, 60.8, 28.2; HRMS (TOF ES+) m/z c alcd for C25H25NO4Na (M+Na) 426.1681, found 426.1698.
tert-Butyl furan-2-yl(2-hydroxy-4-phenyl-2-(thiophen-2-yl)but-3-ynyl)carbamate (15b)
According to General Protocol A, 15b was obtained as a yellow oil (0.350 g, 52%, SiO, EtOAc:hexanes, 1:6): IR (neat) 3384, 2982, 2918, 1713, 1681 cm-1; 1H NMR (400 MHz, acetone-d6) δ 7.46-7.37 (m, 6 H), 7.27-7.25 (m, 2 H), 7.01 (dd, J = 3.6, 4.4 Hz, 1 H), 6.34 (dd, J = 2.4, 3.2 Hz, 1 H), 6.08 (br s, 1 H), 5.78 (br s, 1 H), 4.20 (s, 2 H), 1.35 (s, 9 H); 13C NMR (100 MHz, acetone-d6) δ 149.8, 149.1, 138.9, 132.6, 132.4, 129.5, 129.3, 1 27.5, 126.1, 125.7, 123.5, 111.8, 91.2, 86.0, 81.8, 60.8, 28.2; HRMS (TOF ES+) m/z calcd for C23H23NO4SNa (M+Na) 432.1245, found 432.1281.
tert-Butyl furan-2-yl(2-hydroxy-2-(phenylethynyl)octyl)carbamate (15c)
According to General Protocol A, 15c was obtained as a yellow oil (0.225 g, 44%, SiO2, EtOAc:hexanes, 1:6): IR (neat) 3422, 2956, 2931, 2855, 1718, 1686, 1591 cm-1; 1H NMR (400 MHz, acetone-d6) δ 7.34-7.32 (m, 5 H), 7.27 (br s, 1 H), 6.35 (s, 1 H), 6.14 (s, 1 H), 4.53 (br s, 1 H), 3.91 (s, 2 H), 1.72-1.51 (m, 4 H), 1.39 (s, 9 H), 1.29 (br s, 6 H), 0.87 (t, J = 6.0 Hz, 3 H); 13C NMR (100 MHz, acetone-d6) δ 150.2, 139.0, 132.5, 129.2, 129.1, 124.1, 111.8, 102.9, 92.2, 85.4, 81.8, 72.1, 58.5, 40.8, 32.6, 28.3, 24.9, 23.3, 14.4; HRMS (TOF ES+) m/z calcd for C25H33NO4Na (M+Na) 434.2307, found 434.2281.
tert-Butyl furan-2-yl(2-hydroxy-2-phenethyl-4-phenylbut-3-ynyl)carbamate (15d)
According to General Protocol A, 15d was obtained as a yellow oil (0.310 g, 47%, SiO2, EtOAc:hexanes, 1:6): IR (neat) 3409, 3054, 2976, 2928, 1712, 1591 cm-1; 1H NMR (400 MHz, acetone-d6) δ 7.43-7.16 (m, 11 H), 6.37 (dd, J = 2.0, 3.2 Hz, 1 H), 6.17 (dd, J = 0.8, 3.2 Hz, 1 H), 4.78 (br s, 1 H), 4.01 (s, 2 H), 3.00-2.85 (m, 2 H), 2.06-1.99 (m, 2 H), 1.40 (s, 9 H); 13C NMR (100 MHz, acetone-d6) δ 150.2, 143.3, 139.1, 132.6, 129.3, 129.23, 129.20, 126.6, 124.0, 111.8, 103.0, 91.8, 85.8, 81.8, 71.8, 58.3, 42.9, 31.4, 28.3; HRMS (TOF ES+) m/z calcd for C27H29NO4Na (M+Na) 454.1994, found 454.2024.
tert-Butyl 2-cyclohexyl-2-hydroxyoct-3-ynyl(furan-2-yl)carbamate (15e)
According to General Protocol A, 15e was obtained as a yellow oil (0.438 g, 49%, SiO2, EtOAc:hexanes, 1:9): IR (neat) 3422, 3285, 2931, 2855, 1694, 1590 cm-1; 1H NMR (400 MHz, acetone-d6) δ 7.31 (dd, J = 0.8, 1.6 Hz, 1 H), 6.39 (dd, 1 H, J = 2.4, 3.2 Hz, 1 H), 6.13 (dd, J = 0.8, 3.2 Hz, 1 H), 4.15 (br s, 1 H), 3.89 (d, J = 14.4 Hz, 1 H), 3.79 (d, J = 14.4 Hz, 1 H), 2.16 (app t, J = 6.8 Hz, 2 H), 2.03-2.01 (m, 1 H), 1.75-1.63 (m, 4 H), 1.49-1.44 (m, 13 H), 1.22-1.13 (m, 6 H), 0.91 (t, J = 6.8 Hz, 3 H); 13C NMR (100 MHz, acetone-d6) δ 150.3, 139.0, 111.9, 111.7, 102.8, 86.3, 81.8, 81.7, 74.7, 57.1, 46.4, 31.7, 28.8, 28.4, 28.3, 27.5, 27.3, 27.1, 27.0, 22.6, 18.9, 13.9; HRMS (EI) m/z calcd for C23H35NO4Na (M+Na) 412.2464, found 412.2472.
tert-Butylfuran-2-yl(2-hydroxy-2-phenyl-4-(trimethylsilyl)but-3-ynyl)carbamate (15f)
According to General Protocol A, 15f was obtained as a yellow oil (0.335 g, 58%, SiO2, EtOAc:hexanes, 1:6): IR (neat) 3390, 2956, 2918, 1713, 1681 cm-1; 1H NMR (400 MHz, acetone-d6) δ 7.66 (d, J = 7.2 Hz, 2 H), 7.34 (dd, J = 6.8, 7.2 Hz, 2 H), 7.29-7.26 (m, 2 H), 6.34 (s, 1 H), 5.96 (br s, 1 H), 5.34 (br s, 1 H), 4.00 (s, 2 H), 1.30 (s, 9 H), 0.14 (s, 9 H); 13C NMR (100 MHz, acetone-d6) δ 149.8, 143.8, 138.7, 128.6, 128.4, 127.1, 111.7, 108.2, 102.7, 90.3, 81.7, 73.5, 60.8, 28.2, 0.09; HRMS (TOF ES+) m/z calcd for C22H29NO4SiNa (M+Na) 422.1764, found 422.1778.
tert-Butylfuran-2-yl(2-hydroxy-2-(naphthalen-2-yl)-4-(trimethylsilyl)but-3-ynyl)carbamate (15g)
According to General Protocol A, 15g was obtained as a yellow oil (0.413 g, 60%, SiO2, EtOAc:hexanes, 1:9): IR (neat) 3395, 2956, 2140, 1718, 1681 cm-1; 1H NMR (300 MHz, CDCl3) δ 8.18 (d, J = 1.5 Hz, 1 H), 7.90-7.83 (m, 3 H), 7.72 (dd, J = 1.8, 8.7 Hz, 1 H), 7.52-7.49 (m, 2 H), 7.13 (br s, 1 H), 6.26 (br s, 1 H), 5.78 (br s, 1 H), 4.09 (s, 2 H), 1.41 (s, 9 H), 0.22 (s, 9 H); 13C NMR (100 MHz, acetone-d6) δ 148.9, 140.4, 137.8, 133.1, 133.0, 128.2, 127.5, 127.4, 126.03, 125.99, 125.0, 124.6, 110.8, 107.3, 101.9, 89.8, 80.8, 72.7, 59.6, 27.2, -0.74; HRMS (TOF ES+) m/z calcd for C26H31NO4SiNa (M+Na) 472.1920, found 472.1915.
tert-Butyl-2-(benzofuran-2-yl)-2-hydroxy-4-(trimethylsilyl)but-3-ynyl(furan-2-yl)carbamate (15h)
According to General Protocol A, 15h was obtained as a yellow oil (0.151 g, 58%, SiO2, ISCO-Companion, 0-100% EtOAc/hexanes, 15 min gradient): IR (neat) 3377, 2969, 1718, 1681 cm-1; 1H NMR (400 MHz, acetone-d6) δ 7.59 (d, J = 7.6 Hz, 1 H), 7.44 (d, J = 8.0 Hz, 1 H), 7.28 (ddd, J = 1.2, 7.2, 7.2 Hz, 1 H), 7.26-7.21 (m, 1 H), 7.16 (dd, J = 0.8, 1.6 Hz, 1 H), 6.89 (d, J = 0.8 Hz, 1 H), 6.22 (br s, 1 H), 5.94 (br s, 1 H), 5.64 (br s, 1 H), 4.25 (s, 2 H), 1.27 (s, 9 H), 0.17 (s, 9 H); 13C NMR (75 MHz, acetone-d6) δ 158.0, 156.0, 149.3, 138.7, 129.0, 125.1, 123.6, 122.0, 112.0, 111.5, 105.3, 105.2, 103.0, 90.8, 81.7, 69.6, 57.4, 28.1, -0.06; HRMS (TOF ES+) m/z calcd for C24H29NO5SiNa (M+Na) 462.1713, found 462.1749.
tert-Butyl-2-cyclopropyl-2-hydroxy-4-(trimethylsilyl)but-3-ynyl(furan-2-yl)carbamate (15i)
According to General Protocol A, 15i was obtained as a yellow oil (0.139 g, 52%, SiO2, ISCO-Companion, 0-100% EtOAc/hexanes, 15 min gradient): IR (neat) 3427, 2974, 1718 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.17 (dd, J = 1.2, 2.0 Hz 1 H), 6.33 (dd, J = 2.4, 3.6 Hz, 1 H), 6.06 (br s, 1 H), 3.96 (br s, 2 H), 1.45 (s, 9 H), 1.15-1.08 (m, 1 H), 0.71-0.66 (m, 1 H), 0.49-0.45 (m, 3 H), 0.12 (s, 9 H); 13C NMR (75 MHz, CDCl3) δ 156.1, 148.9, 137.9, 110.9, 103.6, 101.7, 89.8, 82.2, 73.4, 59.0, 28.1, 18.2, 1.58, 1.35, -0.14; HRMS (TOF ES+) m/z calcd for C19H29NO4SiNa (M+Na) 386.1764, found 386.1757.
tert-Butyl furan-2-yl(2-hydroxy-4-phenylbut-3-ynyl)carbamate (15j)
According to General Protocol A, 15j was obtained as a yellow oil (0.210 g, 43%, SiO2, EtOAc:hexanes, 1:6): IR (neat) 3422, 2974, 2931, 1705, 1625 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.44-7.42 (m, 2 H), 7.32-7.26 (m, 3 H), 7.22 (s, 1 H), 6.37 (s, 1 H), 6.11 (br s, 1 H), 4.86-4.83 (m, 1 H), 4.00 (dd, J = 8.0, 14.4 Hz, 1 H), 3.88 (dd, J = 3.6, 14.4 Hz, 1 H), 1.47 (s, 9 H); 13C NMR (100 MHz, CDCl3) δ 148.2, 138.3, 134.8, 131.7, 128.4, 128.2, 122.3, 111.0, 101.8, 87.3, 82.1, 62.3, 54.8, 28.0; HRMS (EI) m/z calcd for C19H21NO4Na (M+Na) 350.13968, found 350.1361.
tert-Butyl-furan-2-yl(2-hydroxy-4-(3-methoxyphenyl)but-3-ynyl)carbamate (15k)
According to General Protocol A, 15k was obtained as a yellow oil (0.250 g, 57%, SiO2, EtOAc:hexanes, 1:6): IR (neat) 3459, 2982, 1712, 1599 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.22 (app t, J = 8.4 Hz, 2 H), 7.02 (d, J = 7.6 Hz, 1 H), 6.96 (s, 1 H), 6.89 (dd, J = 2.4, 8.4 Hz, 1 H), 6.37 (app t, J = 2.8 Hz, 1 H), 6.11 (br s, 1 H), 4.84 (dd, J = 4.0, 7.6 Hz, 1 H), 4.00 (dd, J = 8.0, 14.4 Hz, 1 H), 3.88 (dd, J = 4.0, 14.4 Hz, 1 H), 3.80 (s, 3 H), 1.47 (s, 9 H); 13C NMR (100 MHz, acetone-d6) δ 160.5, 149.8, 139.2, 130.4, 124.8, 117.3, 115.7, 111.8, 89.8, 85.4, 81.6, 61.5, 55.7, 55.0, 28.3; HRMS (TOF ES+) m/z calcd for C20H23NO5Na (M+Na) 380.1474, found 380.1477.
tert-Butyl furan-2-yl(2-hydroxy-4-(p-tolyl)but-3-yn-1-yl)carbamate (15l)
According to General Protocol A, 15l was obtained as a yellow oil (0.144 g, 52%, SiO2, EtOAc:hexanes, 1:15): 1H NMR (400 MHz, CDCl3) δ 7.30 (d, J = 8.0 Hz, 2 H), 7.19 (dd, J = 0.8, 2.0 Hz, 1 H), 7.10 (d, J = 7.6 Hz, 2 H), 6.34 (dd, J = 2.0, 3.2 Hz, 1 H), 6.08 (br s, 1 H), 4.83-4.78 (m, 1 H), 3.97 (dd, J = 8.0, 14.4 Hz, 1 H), 3.85 (dd, J = 4.0, 14.4 Hz, 1 H), 2.34 (s, 3 H), 1.61 (br s, 1 H), 1.45 (s, 9 H); 13C NMR (125 MHz, CDCl3) δ 155.0, 148.2, 138.4, 138.2, 131.6, 128.9, 119.3, 110.9, 101.8, 86.9, 85.8, 81.9, 62.0, 54.7, 28.0, 21.3; HRMS (TOF ES+) m/z calcd for C20H23NO4Na (M+Na) 364.1525, found 364.1494.
tert-Butyl furan-2-yl(2-hydroxy-4-(4-(trifluoromethyl)phenyl)but-3-yn-1-yl)carbamate (15m)
According to General Protocol A, 15m was obtained as a yellow oil (0.067 g, 26%, SiO2, EtOAc:hexanes, 1:15): 1H NMR (500 MHz, CDCl3) δ 7.54 (d, J = 8.5 Hz, 2 H), 7.49 (d, J = 8.5 Hz, 2 H), 7.18 (dd, J = 1.0, 2.0 Hz, 1 H), 6.34 (dd, J = 2.0, 3.0 Hz, 1 H), 6.07 (br s, 1 H), 4.84 (dd, J = 4.0, 8.0 Hz, 1 H), 3.98 (dd, J = 8.0, 14.5 Hz, 1 H), 3.89 (dd, J = 4.0, 14.5 Hz, 1 H), 1.44 (s, 9 H); 13C NMR (125 MHz, CDCl3) δ 148.2, 138.4, 132.0, 130.2 (q, J = 32.5 Hz), 126.2, 125.2 (q, J = 3.8 Hz), 123.8 (d, J = 270.5 Hz), 111.1, 101.8, 90.1, 84.4, 82.2, 62.2, 60.4, 54.6, 28.1; HRMS (TOF ES+) m/z calcd for C20H21F3NO4 (M+H) 396.1423, found 396.1404.
tert-Butyl (4-(4-fluorophenyl)-2-hydroxybut-3-yn-1-yl)(furan-2-yl)carbamate (15n)
According to General Protocol A, 15n was obtained as a yellow oil (0.042 g, 20%, SiO2, EtOAc:hexanes, 1:15): 1H NMR (400 MHz, CDCl3) δ 7.40-7.36 (m, 2 H), 7.19 (dd, J = 0.8, 2.0 Hz, 1 H), 7.02-6.96 (m, 2 H), 6.34 (dd, J = 2.0, 4.0 Hz, 1 H), 6.07 (br s, 1 H), 4.80 (dd, J = 4.0, 8.0 Hz, 1 H), 3.97 (dd, J = 8.0, 14.4 Hz, 1 H), 3.86 (dd, J = 4.0, 14.4 Hz, 1 H), 3.25-3.21 (m, 1 H), 1.45 (s, 9 H); 13C NMR (125 MHz, CDCl3) δ 162.6 (d, J = 248.8 Hz), 148.2, 138.4, 133.7 (d, J = 8.8 Hz), 118.4, 115.5 (d, J = 21.2 Hz), 111.0, 101.8, 87.1, 84.8, 82.2, 62.3, 54.8, 28.1; HRMS (TOF ES+) m/z calcd for C19H20FNO4Na (M+Na) 368.1274, found 368.1258.
tert-Butyl furan-2-yl(2-hydroxy-7-methyloct-3-ynyl)carbamate (15o)
According to General Protocol A, 15o was obtained as a yellow oil (0.073 g, 54%, SiO2, ISCO-Companion, 0-100% EtOAc/hexanes, 20 min gradient): IR (neat) 3429, 2933, 2881, 1714, cm-1; 1H NMR (400 MHz, acetone-d6) δ 7.29 (d, J = 1.2 Hz, 1 H), 6.38 (dd, J = 2.0, 3.2 Hz, 1 H), 6.10 (d, J = 2.8 Hz, 1 H), 4.50-4.41 (m, 2 H), 3.68 (d, J = 6.4 Hz, 2 H), 2.19 (ddd, J = 2.0, 7.2, 7.2 Hz, 2 H), 1.67 (sept, J = 6.8 Hz, 1 H), 1.42 (s, 9 H), 1.36 (d, J = 7.2 Hz, 1 H), 1.33 (d, J = 7.2 Hz, 1 H), 0.87 (d, J = 6.4 Hz, 6 H); 13C NMR (100 MHz, acetone-d6) δ 149.9, 139.1, 111.8, 102.6, 85.9, 81.4, 80.7, 61.2, 55.5, 38.4, 28.3, 27.8, 22.5, 17.2; HRMS (TOF ES+) m/z calcd for C18H27NO4Na (M+Na) 344.1838, found 344.1830.
tert-Butyl-7-chloro-2-hydroxyhept-3-ynyl(furan-2-yl)carbamate (15p)
According to General Protocol A, 15p was obtained as a yellow oil (0.089 g, 52%, SiO2, ISCO-Companion, 0-100% EtOAc/hexanes, 15 min gradient): IR (neat) 3440, 2969, 1705, 1617 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.19 (s, 1 H), 6.35 (app t, J = 2.4 Hz, 1 H), 6.05 (br s, 1 H), 4.58-4.55 (m, 1 H), 3.84 (dd, J = 8.4, 14.4 Hz, 1 H), 3.73 (dd, J = 4.4, 14.4 Hz, 1 H), 3.62 (t, J = 6.4 Hz, 2 H), 2.38 (ddd, J = 2.0, 6.8, 6.8 Hz, 2 H), 1.94 (ddd, J = 6.8, 6.8, 13.2 Hz, 2 H), 1.45 (s, 9 H); 13C NMR (100 MHz, acetone-d6) δ 149.8, 111.8, 84.2, 81.7, 81.5, 61.1, 55.3, 44.5, 32.3, 28.3, 16.6; HRMS (TOF ES+) m/z calcd for C16H22ClNO4Na (M+Na) 350.1135, found 350.1100.
tert-Butyl 4-cyclohexenyl-2-hydroxybut-3-ynyl(furan-2-yl)carbamate (15q)
According to General Protocol A, 15q was obtained as a yellow oil (0.313 g, 38%, SiO2, EtOAc:hexanes, 1:6): IR (neat) 3435, 2982, 1705, 1612 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.16 (dd, J = 0.8, 2.0 Hz, 1 H), 6.32 (dd, J = 2.4, 3.2 Hz, 1 H), 6.08-6.05 (m, 2 H), 4.69-4.65 (m, 1 H), 3.87 (dd, J = 8.0, 14.4 Hz, 1 H), 3.73 (dd, J = 8.0, 14.4 Hz, 1 H), 2.07-2.04 (m, 4 H), 1.62-1.52 (m, 4 H), 1.43 (s, 9 H); 13C NMR (100 MHz, CDCl3) δ 148.2, 138.3, 135.6, 119.9, 110.9, 101.7, 84.6, 81.9, 62.1, 54.8, 28.9, 28.0, 25.5, 22.1, 21.4; HRMS (TOF ES+) m/z calcd for C19H25NO4Na (M+Na) 354.1681, found 354.1674.
General Protocol B. 3,4-Diphenyl-1 H-indol-5-ol (16a)
A solution of alcohol 15a (0.087 g, 0.21 mmol) in o-DCB (2.1 mL) was subjected to microwave irradiation at 220 °C for 60 min. The crude solution was purified by chromatography on SiO2 (0-20%, EtOAc/hexanes) to give indole 16a (0.046 g, 74%) as a light brown solid: Mp 184-185 °C; IR (neat) 3504, 3403, 3054, 1599 cm-1; 1H NMR (400 MHz, acetone-d6) δ 10.32 (br s, 1 H), 7.37 (d, J = 8.8 H, 1 H), 7.28 (d, J = 2.8 Hz, 1 H), 7.12-7.10 (m, 2 H), 7.08 (s, 1 H), 7.03-6.99 (m, 3 H), 6.96-6.82 (m, 6 H); 13C NMR (100 MHz, acetone-d6) δ 148.0, 137.2, 132.9, 131.5, 129.5, 127.7, 127.4, 126.5, 125.6, 125.3, 124.9, 119.5, 119.0, 112.9, 112.0; HRMS (TOF APCI+) m/z calcd for C20H16NO 286.1232 (M+H), found 286.1226.
4-Phenyl-3-(thiophen-2-yl)-1H-indol-5-ol (16b)
According to General Protocol B, 16b was obtained as a brown solid (0.057 g, 61%, SiO2, EtOAc:hexanes, 1:6): Mp 166-167 °C; IR (neat) 3528, 3390, 3067, 1574 cm-1; 1H NMR (400 MHz, acetone-d6) δ 10.42 (br s, 1 H), 7.37 (d, J = 8.8 Hz, 1 H), 7.36 (s, 1 H), 7.19-7.16 (m, 2 H), 7.13-7.09 (m, 4 H), 7.01 (dd, J = 1.2, 5.2 Hz, 1 H), 6.95 (d, J = 8.8 Hz, 1 H), 6.55 (dd, J = 3.6, 5.2 Hz, 1 H), 6.04 (dd, J = 1.2, 3.6 Hz, 1 H); 13C NMR (100 MHz, acetone-d6) δ 148.5, 138.7, 137.3, 132.9, 131.5, 128.0, 127.2, 127.1, 126.9, 126.6, 125.8, 123.7, 120.0, 113.4, 112.4, 111.0; HRMS (TOF ES+) m/z calcd for C18H13NOSK (M+K) 330.0355, found 330.0370.
3-Hexyl-4-phenyl-1H-indol-5-ol (16c)
According to General Protocol B, 16c was obtained as a brown oil (0.054 g, 58%, SiO2, EtOAc:hexanes, 1:6): IR (neat) 3535, 3472, 3403, 1725, 1591 cm-1; 1H NMR (400 MHz, acetone-d6) δ 9.76 (br s, 1 H), 7.44-7.32 (m, 5 H), 7.21 (d, J = 8.4 Hz, 1 H), 6.99 (d, J = 2.4 Hz, 1 H), 6.80 (d, J = 8.0 Hz, 2 H), 2.09-2.03 (m, 2 H), 1.21-1.01 (m, 6 H), 0.97-0.89 (m, 2 H), 0.81 (t, J = 7.2 Hz, 3 H); 13C NMR (100 MHz, acetone-d6) δ 146.4, 137.4, 131.8, 130.7, 127.1, 126.2, 125.6, 123.0, 118.8, 115.8, 111.0, 110.6, 31.1, 30.8, 26.2, 22.0, 13.1; HRMS (TOF ES+) m/z calcd for C20H24NO 294.1858 (M+H), found 294.1841.
3-Phenethyl-4-phenyl-1H-indol-5-ol (16d)
According to General Protocol B, 16d was obtained as a brown solid (0.063 g, 63%, SiO2, EtOAc:hexanes, 1:6): Mp 102-103 °C; IR (neat) 3528, 3409, 3067, 3054, 1559 cm-1; 1H NMR (400 MHz, acetone-d6) δ 9.87 (br s, 1 H), 7.49-7.40 (m, 5 H), 7.27 (d, J = 8.4 Hz, 1 H), 7.18-7.14 (m, 2 H), 7.10-7.06 (m, 2 H), 6.92 (s, 1 H), 6.87 (d, J = 8.4 Hz, 1 H), 6.83-6.81 (m, 2 H), 2.49-2.37 (m, 4 H); 13C NMR (100 MHz, acetone-d6) δ 147.6, 143.1, 138.6, 132.9, 131.8, 128.8, 128.5, 128.4, 127.4, 126.4, 125.9, 124.7, 119.7, 116.0, 112.1, 111.7, 38.6; HRMS (EI) m/z calcd for C22H19NO 313.1467 (M+), found 313.1464.
4-Butyl-3-cyclohexyl-1H-indol-5-ol (16e)
According to General Protocol B, 16e was obtained as a brown oil (0.050 g, 36%, SiO2, 0-10%, EtOAc:hexanes): IR (neat) 3395, 1681 cm-1; 1H NMR (400 MHz, acetone-d6) δ 9.64 (br s, 1 H), 7.26 (s, 1 H), 7.02 (d, J = 2.4 Hz, 1 H), 7.00 (d, J = 8.4 Hz, 1 H), 6.69 (d, J = 8.4 Hz, 1 H), 3.00-2.87 (m, 4 H), 2.07-2.04 (m, 3 H), 1.87-1.76 (m, 3 H), 1.64-1.58 (m, 2 H), 1.54-1.43 (m, 7 H), 1.00 (t, J = 6.8 Hz, 3 H); 13C NMR δ 148.1, 132.8, 126.1, 123.1, 121.5, 119.7, 112.0, 109.5, 36.9, 36.7, 34.0, 29.0, 27.9, 27.0, 26.7, 23.7, 14.3; HRMS (EI) m/z calcd for C18H25NO (M+) 271.1936, found 271.1928.
General Protocol C. 3-Phenyl-1H-indol-5-ol (16f).15
A solution of alcohol 15f (0.057 g, 0.14 mmol) in o-DCB (1.0 mL) was subjected to microwave irradiation at 180 °C for 30 min. The reaction mixture was cooled to 0 °C and treated with dry methanolic HCl (2%, 0.2 mL). After 5 min, the solution was concentrated and the crude residue was purified by chromatography on SiO2 (ISCO-Rf, 0-100%, EtOAc/hexanes; 12 min gradient) to give indole 16f (0.019 g, 63%) as a light brown oil: IR (neat) 3385, 1687 cm-1; 1H NMR (300 MHz, acetone-d6) δ 10.26 (br s, 1 H), 7.76 (s, 1 H), 7.67 (dd, J = 1.2, 8.4 Hz, 2 H), 7.55 (d, J = 2.7 Hz, 1 H), 7.42 (app t, J = 7.8 Hz, 2 H), 7.38 (d, J = 2.4 Hz, 1 H), 7.32 (d, J = 8.7 Hz, 1 H), 7.25-7.19 (m, 1 H), 6.79 (dd, J = 2.4, 8.7 Hz, 1 H); 13C NMR (75 MHz, acetone-d6) δ 152.2, 137.2, 132.7, 129.2, 127.2, 126.9, 125.7, 123.9, 116.8, 112.8, 112.5, 103.9; HRMS (TOF ES+) m/z calcd for C14H12NO (M+H) 210.0919, found 210.0925.
3-(Naphthalen-2-yl)-1H-indol-5-ol (16g)
According to General Protocol C, 16g was obtained as a brown oil (0.028 g, 60%, SiO2, Flash-system, 0-100%, EtOAc/hexanes; 15 min gradient): IR (neat) 3390, 1699 cm-1; 1H NMR (300 MHz, acetone-d6) δ 10.27 (br s, 1 H), 8.12 (s, 1 H), 7.88 (d, J = 8.7 Hz, 2 H), 7.84-7.79 (m, 2 H), 7.72 (s, 1 H), 7.64 (d, J = 2.7 Hz, 1 H), 7.47-7.44 (m, 2 H), 7.41-7.35 (m, 2 H), 7.30 (d, J = 8.7 Hz, 1 H), 6.77 (dd, J = 2.4, 8.7 Hz, 1 H); 13C NMR (75 MHz, acetone-d6) δ 152.4, 134.9, 134.8, 132.8, 132.4, 128.7, 128.2, 127.1, 126.7, 126.6, 125.5, 124.7, 124.4, 116.6, 112.9, 112.6, 104.2; HRMS (TOF ES+) m/z calcd for C18H13NO (M+) 259.0997, found 259.0970.
3-(Benzofuran-2-yl)-1H-indol-5-ol (16h)
According to General Protocol C, 16h was obtained as a light orange solid (0.022 g, 63%, SiO2, ISCO-Rf, 0-100%, EtOAc/hexanes; 15 min gradient): Mp 169-172 °C; IR (neat) 3364, 3295, 1629 cm-1; 1H NMR (300 MHz, acetone-d6) δ 10.55 (br s, 1 H), 7.94 (s, 1 H), 7.88 (d, J = 3.0 Hz, 1 H), 7.62-7.53 (m, 1 H), 7.52-7.50 (m, 2 H), 7.38 (d, J = 8.7 Hz, 1 H), 7.25-7.21 (m, 2 H), 6.95 (d, J = 1.0 Hz, 1 H), 6.86 (dd, J = 2.4, 8.7 Hz, 1 H); 13C NMR (75 MHz, acetone-d6) δ 154.6, 154.3, 152.8, 132.3, 130.7, 126.0, 125.1, 123.4, 123.3, 120.5, 113.1, 110.8, 107.2, 104.7, 98.6; HRMS (TOF ES+) m/z calcd for C16H11NO2 (M+) 249.0790, found 249.0783.
3-Cyclopropyl-1H-indol-5-ol (16i)
According to General Protocol C, 16i was obtained as a light yellow oil (0.018 g, 47%, SiO2, Flash-system, 0-100%, EtOAc/hexanes; 15 min gradient): IR (neat) 3435, 3364, 1581 cm-1; 1H NMR (400 MHz, acetone-d6) δ 9.56 (br s, 1 H), 7.50 (s, 1 H), 7.10 (d, J = 8.4 Hz, 1 H), 7.00 (d, J = 2.4 Hz, 1 H), 6.89 (d, J = 1.6 Hz, 1 H), 6.63 (dd, J = 2.0, 8.4 Hz, 1 H), 1.83-1.76 (m, 1 H), 0.78-0.74 (m, 2 H), 0.52-0.50 (m, 2 H); 13C NMR (100 MHz, acetone-d6) δ 151.1, 132.3, 129.7, 122.2, 117.6, 112.2, 112.0, 103.4, 6.6, 6.0; HRMS (TOF ES+) m/z calcd for C11H12NO (M+H) 174.0919, found 174.0913.
General Protocol D. 4-Phenyl-1H-indol-5-ol (16j)
A solution of alcohol 15j (0.052 g, 0.16 mmol) in 1,2-dichloroethane (1.0 mL) was subjected to microwave irradiation at 180 °C for 30 min. The crude solution was concentrated and purified by chromatography on SiO2 (Flash-system, 0-100% EtOAc:hexanes, 15 min gradient) to give 16j as a light brown oil (0.016 g, 48%): IR (neat) 3535, 3409, 3045, 2918, 1580 cm-1; 1H NMR (400 MHz, acetone-d6) δ 9.95 (br s, 1 H), 7.48 (d, J = 7.6 Hz, 2 H), 7.31 (app t, J = 7.6 Hz, 2 H), 7.20-7.15 (m, 2 H), 7.13-7.11 (m, 2 H), 6.73 (d, J = 8.8 Hz, 1 H), 6.14 (br s, 1 H); 13C NMR (100 MHz, acetone- d6) δ 145.9, 135.6, 130.9, 129.9, 129.3, 127.8, 127.7, 125.1, 117.8, 111.9, 111.3, 101.6; HRMS (TOF APCI+) m/z calcd for C14H12NO (M+H) 210.0919, found 210.0895.
4-(3-Methoxyphenyl)-1H-indol-5-ol (16k)
According to General Protocol D, 16k was obtained as a light brown oil (0.015 g, 42%, SiO2, Flash-system, 0-100%, EtOAc/hexanes; 15 min gradient): IR (neat) 3528, 3403, 2924, 1712, 1580 cm-1; 1H NMR (400 MHz, acetone-d6) δ 10.11 (br s, 1 H), 7.37 (t, J = 8.4 Hz, 1 H), 7.30-7.26 (m, 3 H), 7.20-7.18 (m, 2 H), 6.90 (dd, J = 2.4, 8.0 Hz, 1 H), 6.87 (d, J = 8.8 Hz, 1 H), 6.30 (app t, J = 2.4 Hz, 1 H), 3.85 (s, 3 H); 13C NMR (100 MHz, acetone-d6) δ 160.5, 147.7, 140.1, 132.3, 129.8, 129.4, 126.3, 123.6, 118.8, 116.8, 113.2, 112.9, 112.0, 101.6, 55.5; HRMS (TOF ES+) m/z calcd for C15H13NO2 (M+) 239.0946, found 239.0940.
4-(p-Tolyl)-1H-indol-5-ol (16l)
According to General Protocol D, 16l was obtained as a light brown solid (0.011 g, 47%, SiO2, EtOAc:hexanes, 1:8): Mp 112-114 °C; IR (neat) 3523, 3409, 3021, 2917 cm-1; 1H NMR (500 MHz, CDCl3) δ 8.11 (br s, 1 H), 7.47 (d, J = 8.5 Hz, 2 H), 7.35 (d, J = 8.0 Hz, 2 H), 7.27 (dd, J = 0.5, 8.5 Hz, 1 H), 7.17 (app t, J = 3.0 Hz, 1 H), 6.94 (d, J = 9.0 Hz, 1 H), 6.32 (ddd, J = 1.0, 2.0, 3.0 Hz, 1 H), 5.00 (br s, 1 H), 2.45 (s, 3 H); 13C NMR (125 MHz, CDCl3) δ 146.0, 137.4, 132.5, 130.9, 130.0, 129.8, 127.9, 125.0, 117.8, 111.9, 111.1, 101.7, 21.3; HRMS (TOF ES+) m/z calcd for C15H14NO (M+H) 224.1075, found 224.1074.
4-(4-(Trifluoromethyl)phenyl)-1H-indol-5-ol (16m)
According to General Protocol D, 16m was obtained as a light brown solid (0.013 g, 64%, SiO2, EtOAc:hexanes, 1:8): Mp 140-142 °C; IR (neat) 3491, 2922 cm-1; 1H NMR (500 MHz, CDCl3) δ 8.17 (br s, 1 H), 7.79 (d, J = 8.0 Hz, 2 H), 7.73 (d, J = 8.0 Hz, 2 H), 7.32 (dd, J = 0.5, 8.5 Hz, 1 H), 7.21 (app t, J = 3.0 Hz, 1 H), 6.92 (d, J = 9.0 Hz, 1 H), 6.31 (ddd, J = 1.0, 2.5, 3.0 Hz, 1 H), 4.76 (br s, 1 H); 13C NMR (125 MHz, CDCl3) δ 145.9, 139.9, 131.0, 130.4, 129.5 (q, J = 32.2 Hz), 127.7, 126.0 (q, J = 3.6 Hz), 125.5, 124.2 (q, J = 270.1 Hz), 116.7, 112.3, 112.0, 101.4; HRMS (TOF ES+) m/z calcd for C15H11NOF3 (M+H) 278.0793, found 278.0794.
4-(4-Fluorophenyl)-1H-indol-5-ol (16n)
According to General Protocol D, 16n was obtained as a brown solid (0.006 g, 44%, SiO2, EtOAc:hexanes, 1:8): Mp 145-148 °C; IR (neat) 3491, 3409, 2919 cm-1; 1H NMR (500 MHz, CDCl3) δ 8.13 (br s, 1 H), 7.57-7.53 (m, 2 H), 7.29 (dd, J = 1.0, 9.0 Hz, 1 H), 7.25-7.21 (m, 2 H), 7.18 (app t, J = 3.0 Hz, 1 H), 6.93 (d, J = 8.5 Hz, 1 H), 6.28 (ddd, J = 1.0, 2.0, 3.0 Hz, 1 H), 4.80 (br s, 1 H); 13C NMR (125 MHz, CDCl3) δ 162.3 (d, J =245.0 Hz), 146.0, 131.8 (d, J = 7.5 Hz), 131.5 (d, J = 2.5 Hz), 130.9, 128.0, 125.2, 116.9, 116.2 (d, J = 24.2 Hz), 112.1, 111.4, 101.5; HRMS (TOF ES+) m/z calcd for C14H11NOF (M+H) 228.0825, found 228.0827.
4-Isopentyl-1H-indol-5-ol (16o)
According to General Protocol D, 16o was obtained as a brown oil (0.005 g, 15%, SiO2, ISCO-Rf, 0-100%, EtOAc/hexanes; 15 min gradient): IR (neat) 3405, 2974, 1686 cm-1; 1H NMR (400 MHz, acetone-d6) δ 9.92 (br s, 1 H), 7.31 (s, 1 H), 7.23 (app t, J = 2.0 Hz, 1 H), 7.07 (d, J = 8.4 Hz, 1 H), 6.73 (d, J = 8.4 Hz, 1 H), 6.41 (br s, 1 H), 2.90 (t, J = 8.0 Hz, 2 H), 1.77 (sept, J = 6.8 Hz, 1 H), 1.59-1.53 (m, 2 H), 0.99 (d, J = 6.4 Hz, 6 H); 13C NMR (75 MHz, acetone-d6) δ 148.0, 132.0, 129.7, 125.3, 118.9, 112.5, 109.5, 100.3, 39.7, 28.9, 25.5, 22.9; HRMS (TOF ES-) m/z calcd for C13H16NO (M-H) 202.1232, found 202.1229.
4-(3-Chloropropyl)-1H-indol-5-ol (16p)
According to General Protocol D, 16p was obtained as a brown oil (0.007 g, 20%, SiO2, Flash-system, 0-100%, EtOAc/hexanes; 15 min gradient): IR (neat) 3409, 2934, 1705 cm-1; H NMR (400 MHz, acetone-d6) δ 9.99 (br s, 1 H), 7.50 (s, 1 H), 7.26 (app t, J = 2.8 Hz, 1 H), 7.12 (d, J = 8.4 Hz, 1 H), 6.75 (d, J = 8.4 Hz, 1 H), 6.47 (app s, 1 H), 3.66 (t, J = 6.8 Hz, 2 H), 3.04 (t, J = 7.6 Hz, 2 H), 2.15 (pent, J = 7.2 Hz, 2 H); 13C NMR (75 MHz, acetone-d6) δ 148.3, 131.9, 129.9, 125.6, 116.9, 112.4, 110.1, 100.2, 45.7, 33.7, 24.9; HRMS (TOF ES+) m/z calcd for C11H13NOCl (M+H) 210.0686, found 210.0657.
4-Cyclohexenyl-1H-indol-5-ol (16q)
According to General Protocol B, 16q was obtained as a brown oil (0.021 g, 23%, SiO2, EtOAc:hexanes, 1:6): IR (neat) 3403, 2931, 1705, 1612 cm-1; 1H NMR (400 MHz, acetone-d6) δ 9.94 (br s, 1 H), 7.19 (t, J = 2.8 Hz, 1 H), 7.12 (dd, J = 0.8, 8.8 Hz, 1 H), 6.84 (s, 1 H), 6.70 (d, J = 8.8 Hz, 1 H), 6.30-6.29 (m, 1 H), 5.76-5.76 (m, 1 H), 2.41-2.37 (m, 2 H), 2.24-2.20 (m, 2 H), 1.82-1.78 (m, 4 H); 13C NMR (100 MHz, acetone-d6) δ NMR δ 147.1, 135.9, 132.1, 128.9, 127.6, 125.6, 121.3, 112.6, 110.9, 101.5, 26.3, 23.9, 23.2; HRMS (TOF ES+) m/z calcd for C14H15NO 213.1154, found 213.1171.
3,7,8,9-Tetrahydropyrano[3,2-e]indole (18)17
A solution of phenol 16p (0.023 g, 0.11 mmol) in THF (1.5 mL) was treated with NaH (0.008 g, 0.20 mmol, 60% dispersion) followed by TBAI (0.045 g, 0.19 mmol) at room temperature. After 30 min, the solution was diluted with brine and extracted with diethyl ether. The organic layers were dried (Na2SO4) filtered and concentrated. The crude residue was purified by chromatography on SiO2 (ISCO-Rf, 0-100% EtOAc/hexanes, 15 min gradient) to give pyran 18 (0.010 g, 53%) as a light yellow semi-solid: IR (neat) 3428, 2934, 1493 cm-1; H NMR (400 MHz, acetone-d6) δ 9.91 (br s, 1 H), 7.11 (app t, J = 2.8 Hz, 1 H), 6.99 (d, J = 8.8 Hz, 1 H), 6.43 (d, J = 8.8 Hz, 1 H), 6.21 (br s, 1 H), 3.99 (t, J = 5.2 Hz, 2 H), 2.76 (t, J = 6.8 Hz, 2 H), 2.72-2.68 (m, 2 H); 13C NMR (75 MHz, acetone-d6) δ 148.9, 131.4, 128.7, 125.3, 112.9, 112.2, 110.4, 99.7, 66.4, 23.1, 22.5; HRMS (TOF ES+) m/z calcd for C11H12NO (M+H) 174.0919, found 174.0895.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the NIH/NIGMS CMLD program (GM067082). We thank Dr. Nilesh Z aware (University of Pittsburgh) for preliminary synthetic studies on the synthesis of 16a.
Footnotes
Dedicated to the memory of Professor Robert E. Ireland.
Supporting Information. Copies of 1H NMR spectra for 14e, 14i, 14k, 14l, 14m, 14n, 14o, 14p, and 14q, and copies of 1H NMR and 13C NMR spectra for all other compounds, except the commercial compounds 14a and 14b. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.a Ertl P, Jelfs S, Mühlbacher J, Schuffenhauer A, Selzer P. J. Med. Chem. 2006;49:4568–4573. doi: 10.1021/jm060217p. [DOI] [PubMed] [Google Scholar]; b Taber DF, Tirunahari PK. Tetrahedron. 2011;67:7195–7210. doi: 10.1016/j.tet.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a Riendeau D, Aspiotis R, Ethier D, Gareau Y, Grimm EL, Guay J, Guiral S, Juteau H, Mancini JA, Méthot N, Rubin J, Friesen RW. Bioorg. Med. Chem. Lett. 2005;15:3352–3355. doi: 10.1016/j.bmcl.2005.05.027. [DOI] [PubMed] [Google Scholar]; b Im G-YJ, Bronner SM, Goetz AE, Paton RS, Cheong PH-Y, Houk KN, Garg NK. J. Am. Chem. Soc. 2010;132:17933–17944. doi: 10.1021/ja1086485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a Allen GR. Org. React. 1973;20:337–454. [Google Scholar]; b Humphrey GR, Kuethe JT. Chem. Rev. 2006;106:2875–2911. doi: 10.1021/cr0505270. [DOI] [PubMed] [Google Scholar]; c Velezheva VS, Sokolov AI, Kornienko AG, Lyssenko KA, Nelyubina YV, Godovikov IA, Peregudov AS, Mironov AF. Tetrahedron Lett. 2008;49:7106–7109. [Google Scholar]
- 4.Stoffman EJL, Clive DL. J. Org. Biomol. Chem. 2009;7:4862–4870. doi: 10.1039/b914580j. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 5.Ezquerra J, Pedregal C, Lamas C, Barluenga J, Pérez M, Garcia-Martin MA, González JM. J. Org. Chem. 1996;61:5804–5812. [Google Scholar]
- 6.a Petronijevic F, Timmons C, Cuzzupe A, Wipf P. Chem. Commun. 2009:104–106. doi: 10.1039/b816989f. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Petronijevic FR, Wipf P. J. Am. Chem. Soc. 2011;133:7704–7707. doi: 10.1021/ja2026882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For pioneering applications of IMDAF cycloadditions to indoline and natural product synthesis, see: Li G, Padwa A. Org. Lett. 2011;13:3767–3769. doi: 10.1021/ol201320v.Boonsompat J, Padwa A. J. Org. Chem. 2011;76:2753–2761. doi: 10.1021/jo200125c.Padwa A, Brodney MA, Liu B, Satake K, Wu T. J. Org. Chem. 1999;64:3595–3607. doi: 10.1021/jo982453g.
- 8.For thermal cleavage of Boc-groups, see: Wasserman HH, Berger GD, Cho KR. Tetrahedron Lett. 1982;23:465–468.Rawal VH, Cava MP. Tetrahedron Lett. 1985;26:6141–6142.Wipf P, Furegati M. Org. Lett. 2006;8:1901–1904. doi: 10.1021/ol060455e.
- 9.Ahman J, Somfai P. Synth. Commun. 1994;24:1117–1120. [Google Scholar]
- 10.Padwa A, Brodney MA, Lynch SM. Org. Synth. 2002;78:202–204. [Google Scholar]
- 11.Wang B, Bonin M, Micouin L. J. Org. Chem. 2005;70:6126–6128. doi: 10.1021/jo050760y. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen TE, Cubillo de Dios MA, Tanner D. J. Org. Chem. 2002;67:7309–7313. doi: 10.1021/jo0259008. [DOI] [PubMed] [Google Scholar]
- 13.For the preparation of TMS-substituted ynones, see: Morisaki Y, Luu T, Tykwinski RR. Org. Lett. 2006;8:689–692. doi: 10.1021/ol0528888.
- 14.a Eastham SA, Ingham SP, Hallett MR, Herbert J, Modi A, Morley T, Painter JE, Patel P, Quayle P, Ricketts DC, Raftery J. Tetrahedron. 2008;64:936–948. [Google Scholar]; b Wu H-I, Yen C-H, Chuang C-T. Tetrahedron Lett. 1996;37:7395–7398. [Google Scholar]
- 15.Satomura M. J. Org. Chem. 1993;58:3757–3760. [Google Scholar]
- 16.For synthesis of ynal substrates, see: Frimpong K, Wzorek J, Lawlor C, Spencer K, Mitzel T. J. Org. Chem. 2009;74:5861–5870. doi: 10.1021/jo900763u.
- 17.a Macor JE, Ryan K, Newman ME. Tetrahedron. 1992;48:1039–1052. [Google Scholar]; b Macor JE, Blank DH, Post RJ. Tetrahedron Lett. 1994;35:45–48. [Google Scholar]
- 18.Parker KA, Ledeboer MW. J. Org. Chem. 1996;61:3214–3217. doi: 10.1021/jo951712o. [DOI] [PubMed] [Google Scholar]
- 19.Jackson MM, Leverett C, Toczko JF, Roberts JC. J. Org. Chem. 2002;67:5032–5035. doi: 10.1021/jo025682i. [DOI] [PubMed] [Google Scholar]
- 20.Chen J-Y, Lin T-C, Chen S-C, Chen A-J, Mou C-Y, Tsai F-Y. Tetrahedron. 2009;65:10134–10141. [Google Scholar]
- 21.Morisaki Y, Luu T, Tykwinski RR. Org. Lett. 2006;8:689–692. doi: 10.1021/ol0528888. [DOI] [PubMed] [Google Scholar]
- 22.Friscourt F, Boons G-J. Org. Lett. 2010;12:4936–4939. doi: 10.1021/ol1022036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santra S, Ranjan P, Bera P, Ghosh P, Mandal SK. RSC Adv. 2012;2:7523–7533. [Google Scholar]
- 24.Wipf P, Weiner WS. J. Org. Chem. 1999;64:5321–5324. doi: 10.1021/jo990352s. [DOI] [PubMed] [Google Scholar]
- 25.Frimpong K, Wzorek J, Lawlor C, Spencer K, Mitzel T. J. Org. Chem. 2009;74:5861–5870. doi: 10.1021/jo900763u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SP, Ahn S-H, Lee K-J. Tetrahedron. 2010;66:3490–3498. [Google Scholar]
- 27.Wei W, Hamamoto Y, Ukaji Y, Inomata K. Tetrahedron Asymm. 2008;19:476–481. [Google Scholar]
- 28.Belot S, Vogt KA, Besnard C, Krause N, Alexakis A. Angew. Chem., Int. Ed. 2009;48:8923–8926. doi: 10.1002/anie.200903905. [DOI] [PubMed] [Google Scholar]
- 29.Wadsworth DH, Geer SM, Detty MR. J. Org. Chem. 1987;52:3662–3668. [Google Scholar]
- 30.M. C., Randell-Sly HE, Woodward RL, Currie GS. Org. Lett. 2005;7:2249–2251. doi: 10.1021/ol050638l. [DOI] [PubMed] [Google Scholar]
- 31.Hauptmann H, Mader M. Synthesis. 1978:307–309. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.