Abstract
The endophytic actinobacterial population in the roots of wheat grown in three different soils obtained from the southeast part of South Australia was investigated by terminal restriction fragment length polymorphism (T-RFLP) analysis of the amplified 16S rRNA genes. A new, validated approach was applied to the T-RFLP analysis in order to estimate, to the genus level, the actinobacterial population that was identified. Actinobacterium-biased primers were used together with three restriction enzymes to obtain terminal restriction fragments (TRFs). The TRFs were matched to bacterial genera by the T-RFLP Analysis Program, and the data were analyzed to validate and semiquantify the genera present within the plant roots. The highest diversity and level of endophytic colonization were found in the roots of wheat grown in a dark loam from Swedes Flat, and the lowest were found in water-repellent sand from Western Flat. This molecular approach detected a greater diversity of actinobacteria than did previous culture-dependent methods, with the predominant genera being Mycobacterium (21.02%) in Swedes Flat, Streptomyces (14.35%) in Red Loam, and Kitasatospora (15.02%) in Western Flat. This study indicates that the soil that supported a higher number of indigenous organisms resulted in wheat roots with higher actinobacterial diversity and levels of colonization within the plant tissue. Sequencing of 16S rRNA clones, obtained using the same actinobacterium-biased PCR primers that were used in the T-RFLP analysis, confirmed the presence of the actinobacterial diversity and identified a number of Mycobacterium and Streptomyces species.
The definition of an endophyte has evolved over time; the most precise definition is “fungi or bacteria, which for all or part of their life cycle, invade the tissues of living plants and cause unapparent and asymptomatic infections entirely within plant tissues, but cause no symptoms of disease” (43). Endophytic bacteria have been isolated from a variety of plants including tomato (Solanum lycopersicum L.) (17), potato (Solanum tuberosum L.) (38), wheat (Triticum aestivum L.) (9, 36), sweet corn (Zea mays L.) (29), cotton (Gossypium hirsutum L.) (29), oilseed rape (Brassica napus L.) (17), wild rice (Oryza officinalis L.) (16), and citrus plants (3, 4). The best-studied bacterial endophyte-plant interaction is the rhizobium-legume symbiosis. Rhizobia (Azorhizobium, Bradyrhizobium, Mesorhizobium, Rhizobium, and Sinorhizobium) are soil bacteria that can form a symbiosis with leguminous plants and produce nitrogen-fixing nodules. This symbiosis has been important agronomically as crop rotations with legumes can enhance the productivity of nonleguminous crops (20). The associations between bacterial endophytes and their plant hosts have been reviewed extensively (18, 39, 40), as well as the niches for bacterial endophytes (28).
Endophytic bacteria have been shown to have a number of beneficial effects on the host plant when reintroduced. Bacterial endophytes are reported to control fungal infections in cotton (6), peas (5), potatoes (38), and wheat (10). They can also increase nitrogen fixation in wild rice (15) and accelerate seed emergence, and endophytes isolated from oilseed rape and tomato have the ability to improve seed germination, seedling length, and plant growth (30).
A number of the biologically active endophytes isolated belong to the actinobacterial phylum and, specifically, the genus Streptomyces (9, 33, 35). The first actinobacterial endophyte isolated was Frankia, which is a nitrogen-fixing actinobacterium that forms actinorhizae with eight families of angiosperms (31). This symbiosis is closest to the rhizobium-legume symbiosis in terms of evolution, structure, and function (20, 31). In our laboratory we have focused on the filamentous actinobacteria due to their recognized ability to produce a range of useful bioactive molecules, including antibiotics and plant growth regulators. Recently, the isolation of endophytic actinobacteria from wheat plants by cultivation-based methods was reported, with the major genera obtained being Streptomyces, Microbispora, Micromonospora, and Nocardioides (9). The endophytic nature of these filamentous actinobacteria was further confirmed by tagging one of the isolates with green fluorescent protein and visualizing it within the tissues of wheat plants after reintroducing it to a germinating seed (8). A number of isolates were able to suppress fungal pathogens of wheat, including Rhizoctonia solani, Pythium spp., and Gaeumannomyces graminis var. tritici, both in vitro and in planta (10).
Endophytic populations have been isolated and characterized primarily by cultivation-based methods. However, due to the limitations of culture-based methods, molecular methods of community analysis need to be employed. The terminal restriction fragment (TRF) length polymorphism (T-RFLP) technique is gaining popularity as it is rapid and offers high resolution. It has previously been used to study the changes in the bacterial communities in soil (13, 19, 24) and marine sediments (34). From these studies it was concluded that the T-RFLP technique is a sensitive tool appropriate for analyzing endophytic microbial communities, with the added advantage that it allows the identification of the genera present by using the T-RFLP Analysis Program (TAP) software linked to the Ribosomal Database Project database (27).
The endophytic populations of a variety of plants are affected by a number of biological and environmental factors such as plant cultivar, plant age, tissue type, time of sampling, and soil environment (1, 3, 16, 36, 44). These have been investigated using culture-dependent studies of cotton, pea, canola, and wheat (5, 17, 36) and by denaturing gradient gel electrophoresis methods (16, 37), neither of which revealed the extent of the identifiable microbial population.
In this study our objectives were to investigate the effect of soil type on the endophytic actinobacterial populations in wheat roots by applying quantitative analysis to the culture-independent method, T-RFLP, so that changes could be revealed at the genus level. Results were confirmed by cloning and sequencing of 16S rRNA amplicons.
MATERIALS AND METHODS
Bacterial cultures.
The following pure actinobacterial cultures were used to validate the T-RFLP technique: Streptomyces galilaeus (EN 45), Streptomyces caviscabies/setonii (SE2), Streptomyces caviscabies/setonii (PM 23), Streptomyces bottropensis (AB6), Streptomyces scabies (ATCC 49173), and Streptomyces setonii (ATCC 25497). All bacteria, except ATCC type strains, were previously isolated from healthy wheat roots in our laboratory, and cultures were maintained on yeast malt extract agar, mannitol soy agar, or half-strength potato dextrose agar (9).
DNA extraction from actinobacterial cells.
Total genomic DNA was extracted using a modified cetryltrimethylammonium bromide (CTAB)-NaCl protocol (21). For each isolate, two loopfuls of mycelium and spores was scraped from colonies grown on agar media and resuspended in 500 μl of TE (10 mM Tris, 10 mM EDTA, pH 8.0) by vortexing. This suspension was lysed with 10 μl of lysozyme (10 mg · ml−1) for 1 h at 37°C. Subsequently, 10 μl of 1% (wt/vol) proteinase K (Sigma, St. Louis, Mo.) and 33.5 μl of 10% sodium dodecyl sulfate were added, followed by incubation for 1 h at 55°C. To this were added 100 μl of 5 M NaCl and 65 μl of CTAB-NaCl (700 mM NaCl, 275 mM CTAB), and the mixture was incubated for a further 10 min. The lysates were centrifuged (12,000 × g, 15 min) to precipitate the cell debris, before DNA was extracted with 500 μl of chloroform-isoamyl alcohol (24:1) at room temperature for 30 min with intermittent shaking. After centrifugation (12,000 × g, 15 min), DNA was precipitated from the supernatant with 3 volumes of absolute ethanol and 0.1 volume of 3 M sodium acetate. Precipitated DNA was washed with 70% ethanol, resuspended in 75 μl of sterile H2O, and stored at −20°C. The DNA was semiquantified on a 2% agarose gel in 0.5× Tris-borate-EDTA and visualized by staining with ethidium bromide.
Soil sampling sites and bacterial counts.
Three field soils were collected from the southeast of South Australia in December 2001. Two soil samples were collected from Swedes Flat, a sandy loam from a pasture (Red Loam) and a dark loam from a wheat field (Swedes Flat). Water-repellent sand from a virgin scrub was collected from Western Flat.
One gram of soil was diluted in 25 ml of 0.06 M potassium phosphate buffer, pH 7.6, and incubated at room temperature for 5 min with shaking at 125 rpm. Soil suspensions were sonicated for 1 min in a sonicator bath (SoniClean) to disperse the microorganisms, and serial dilutions were made in sterile saline. From each dilution 50 and 20 μl were spread on half-strength nutrient agar and starch casein agar, both containing 50 mg of Benomyl (DuPont) ml−1 to control fungi. Bacterial CFU per gram of soil−1 was calculated after the plates were incubated at 27°C for up to 7 days.
Cultivation of wheat plants.
Three wheat seeds (cultivar Krichauff) were sown in pots (100-mm height, 50-mm diameter) containing the field soil. Two to three replicate pots were prepared for each field soil. Plants were cultivated in a glasshouse for 6 weeks with watering with tap water as required.
Surface sterilization of wheat roots.
Plant root sections from the 6-week plants were washed thoroughly to remove all soil particles, suspended in NAP buffer (124 mM Na2HPO4 · H2O), and sonicated for 1 min in a sonicator bath (SoniClean). After being washed with sterile H2O, the root sections were surface sterilized, as described previously (9), in 99% ethanol (60 s), 3% sodium hypochlorite (6 min), and 99% ethanol (30 s). Plant material was rinsed in sterile H2O, dipped in absolute ethanol, and flamed. Surface sterilization of the plant material was checked by rolling the sterilized plant material onto nutrient agar and yeast malt extract agar plates, which were incubated for up to 7 days at 27°C.
Extraction of endophytic bacterial DNA.
One gram of surface-sterilized wheat roots was cut into 0.1- to 0.5-mm sections and transferred to sterile 1.5-ml screw-top tubes with 1 g of 0.1-mm-diameter glass beads (BioSpec Products) and 1 ml of TE (10 mM Tris, 10 mM EDTA, pH 8.0). Samples were homogenized in a mini-bead beater (Daintree Scientific) at 4,800 rpm for 3 min. Further treatment of the bacterial cell homogenate to extract DNA was performed as described above for actinobacterial cells, from the lysozyme treatment step onwards. Precipitated DNA was purified twice using a Bio-Rad DNA purification kit (Bio-Rad Laboratories). Any remaining inhibitors were removed by reprecipitating the DNA with ethanol and 3 M sodium acetate as stated previously before resuspending it in 30 μl of sterile H2O. The DNA was semiquantified as stated previously.
Analysis of 16S rRNA T-RFLP.
Partial 16S rRNA gene sequences were amplified using the actinobacterium-biased primers 243f (5′ GGA TGA GCC CGC CGC CTA 3′) (19) and 5′-TET (6-carboxy-2′,4,7,7′-tetrachlorofluorescein)-labeled 1492r (5′ TA CGG GTA CCT TGT TAC GAC TT 3′) (42), prepared by GeneWorks, Adelaide, Australia. Amplification was carried out in a 50-μl mixture containing 10 μl of 5× Taq buffer (5% 40 mM deoxynucleoside triphosphates, 40% 25 mM MgCl, 50% 10× PCR buffer, 5% water) (Biotech), 2 μl of each primer (20 ng · μl−1), 1 μl of Taq DNA polymerase (2 U · μl−1) (Biotech), 33 μl of water, and 2 μl of template DNA. Reaction mixtures were subjected to the following temperature cycling profile: 94°C for 5 min; followed by 40 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min; followed by 72°C for 10 min. The PCR was performed in duplicate for each sample. Amplification products (10 μl) were separated on a 2% agarose gel in 0.5× Tris-Borate-EDTA and visualized by ethidium bromide staining.
Single restriction digests of the 16S rRNA PCR products were performed using HinfI, HhaI, and MboI (Promega). Three to five units of restriction enzyme was used to digest 10 μl of the PCR mixture at 37°C for 16 to 18 h to achieve complete digestion and then stored at −20°C.
The sizes of the terminal 16S rRNA gene fragments present in the restriction digestions were determined on an automated Applied Biosystems 373 DNA sequencer (Stretch) with 1 μl of the restriction digest. Data were analyzed using the GeneScan Analysis program V.3.1.2 (Applied Biosystems). From the GeneScan data the TRF sizes present for each restriction enzyme were determined.
Statistical and data analysis.
For each soil treatment, replicate T-RFLP profiles were obtained. These were from two to three wheat root samples subjected to duplicate PCR. As the objective of the study was to quantify the actinobacterial genera present, the data were subjected to the following analytical steps. For each soil sample the TRFs obtained from each of the restriction enzymes for each of the replicates were aligned and the average length for each representative TRF was determined. Only TRFs above 35 bp and present in a minimum of two of the replicates were considered for further analysis. In some cases TRFs over 500 bp were visible on the electrophoresis gel but could not be detected by GeneScan. These sizes were recorded but without a corresponding peak area.
The Jaccard coefficient was used to determine the relative community relatedness. The presence and absence of peaks for each restriction enzyme were converted to binary data so that the proximity matrix could be determined; each restriction enzyme was considered separately. For the Jaccard coefficient a value of 1 means that the communities are identical and a value of 0 means that they are not related.
The relative abundance of each bacterial genus present in the sample was determined by first calculating the percentage of abundance (Ap) for each fragment and for each replicate separately with the formula Ap = (ni/N) × 100, where ni is the peak area of one distinct fragment and N is the sum of all peak areas in a given T-RFLP profile (24). The minimum and maximum Ap values for each TRF across the replicates were recorded.
The TRFs to be included for further analysis were then correlated with bacterial genera and species in the Ribosomal Database Project (25) with the TAP T-RFLP software available online (http://rdp.cme.msu.edu/html/TAP-trflp.html) (27). A bacterial genus or species was considered to be present in a sample only if all three corresponding TRFs (from the three separate restriction enzyme digests) within a 1- to 2-bp range were present in the sample. In those instances where the TRF for the third restriction enzyme digest (usually MboI) was not present in the TAP T-RFLP database, a decision to include the genus was made on a case-by-case basis after rechecking the T-RFLP electropherograms. A TRF was considered to be validated if it correlated with TRFs from the other two enzyme digests that matched a bacterial genus or species. These three TRFs were called a “triple TRF-genus match.”
A triple TRF-genus match often corresponded to more than one bacterial species or genus. When this was the case, the bacterial genera were listed under the same set of three TRFs. A table of the TRFs and Ap values for all three restriction enzymes that had a corresponding bacterial genus was prepared. The minimum and maximum Ap values for each of the validated TRFs were corrected for the number of times that the TRF was part of a combination in the prepared table. This was done for each of the TRFs to provide the theoretical minimum and maximum Ap values for the bacterial genus or species that was represented by the triple TRF-genus match.
As actinobacterial genera can have more than one set of triple TRF-genus matches due to the presence of more than one species, the minimum and maximum corrected Ap values for each genus were combined to give the final percentage for that genus.
Cloning actinobacterial 16S rRNA genes.
By using the extracted endophytic bacterial DNA as template, actinobacterial 16S rRNA genes were amplified using the 243f and (unlabeled) 1492r primers, as described above. The 16S rRNA PCR products were purified using the MoBio UltraClean PCR product purification kit. Purified 16S rRNA PCR products were ligated into the Bluescript pGEM-T vector (Promega) per the manufacturer's protocol. Competent Escherichia coli JM109 cells (Promega) were used for transformations per the manufacturer's protocol with 3 μl of the ligation product and transformants selected by standard protocols on Luria-Bertani agar with ampicillin, X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), and IPTG (isopropyl-β-d-thiogalactopyranoside) facilitating blue-white screening.
Plasmid preparation and selection of candidates for sequencing.
For each field soil group 100 transformants were selected and 2 ml of overnight cultures was prepared using Luria-Bertani medium with 100 μg of ampicillin · ml−1 and incubated overnight at 37°C with shaking at 120 rpm. Plasmid isolation was performed by the alkali lysis method (32).
For each field soil group 2 μl of each of the 100 plasmids was digested with 1 U of HhaI according to the manufacturer's instructions (Promega) to select 16S rRNA sequences with dissimilar RFLPs. Digestions were performed for 16 to 18 h at 37°C and separated by agarose gel electrophoresis. From the restriction patterns generated with HhaI, plasmids that showed different restriction fragment patterns were further digested with the restriction enzyme HinfI (Promega) to further identify plasmids with dissimilar 16S rRNA RFLPs.
Sequencing of actinobacterial 16S rRNA genes.
Sequencing was performed from the purified, screened pGEM-T vector with the SP6 forward primer and dynamic ET terminator sequencing chemistry (Amersham). Sequencing was performed on an automated Applied Biosystems 373 DNA sequencer (Stretch). Sequences were analyzed using the Sequencing Analysis V.3.4.1 program (Applied Biosystems) and compared to online databases by using the BLAST program located at the National Center for Biotechnology Information website (www.ncbi.nlm.nih.gov). The standard BLASTN (nucleotide-nucleotide) algorithm was used with the default settings (2).
RESULTS
Validation of the T-RFLP technique with pure cultures of actinobacteria.
The T-RFLP technique was first validated using pure actinobacterial cultures for which the 16S rRNA sequence was known. Table 1 shows expected terminal fragment sizes of the actinobacterial species according to the TAP T-RFLP database and the actual terminal fragment size detected. The actual size of the TRFs varied from 0.1 to 3.78 bp from the expected TRFs of the reference cultures. There were no entries in the database for some of the species, such as S. galilaeus and S. caviscabies/setonii, which were isolated previously from wheat roots. Nevertheless, they would have been identified as Streptomyces, as other Streptomyces species within the database contain the same triple TRF-genus match.
TABLE 1.
TRF sizes of the 16S rRNA genes from pure actinobacterial species digested with HinfI, HhaI, and MboI
| Name | Species | Enzyme | Expected fragment size (bp) | Actual fragment size (bp) |
|---|---|---|---|---|
| EN 45 | Streptomyces galilaeus | HinfI | NAa | 240.6 |
| HhaI | NA | 419.2 | ||
| MboI | NA | 158.1 | ||
| AB6 | Streptomyces bottropensis | HinfI | 236 | 238.6 |
| HhaI | 419 | 418.5 | ||
| MboI | 158 | 158.1 | ||
| PM 23 | Streptomyces caviscabies/setonii | HinfI | NA | 180.0 |
| HhaI | NA | |||
| MboI | NA | 158.1 | ||
| 162.4 | ||||
| S. scabies | Streptomyces scabies (ATCC 49173) | HinfI | 236 | 239.6 |
| HhaI | 418 | 415.7 | ||
| MboI | 157 | 157.3 | ||
| SE2 | Streptomyces caviscabies/setonii | HinfI | NA | 238.5 |
| HhaI | NA | 419.0 | ||
| MboI | NA | 158.0 | ||
| S. setonii | Streptomyces setonii (ATCC 25497) | HinfI | 236 | 239.9 |
| HhaI | 419 | |||
| MboI | 158 | 158.3 |
NA, no entry found in the RDP database by using the TAP T-RFLP software.
T-RFLP analysis of the endophytic actinobacterial population present in the roots of wheat grown in three different field soils.
Wheat was cultivated for 6 weeks in field soils obtained from two regions in the southeast of South Australia. The total bacterial load for each of the field soils was 5 × 105 CFU g of Western Flat sand−1, 6.25 × 105 CFU g of Red Loam soil−1, and 1 × 106 CFU g of Swedes Flat soil−1.
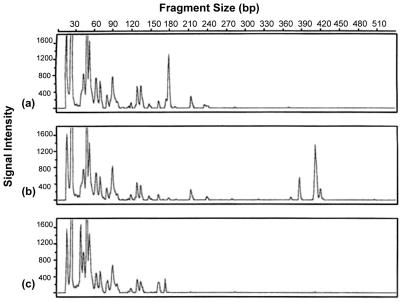
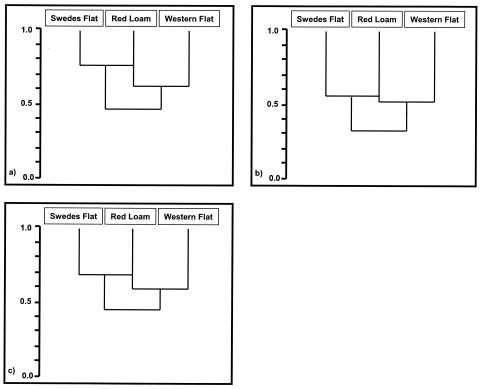
Figure 1 shows a representative GeneScan T-RFLP profile of the 16S rRNA terminal fragments amplified from endophytic DNA extracted from the roots of wheat which had been cultivated in the Swedes Flat soil and digested with the restriction enzymes HinfI, HhaI, and MboI. Each peak corresponded to a TET-labeled terminal fragment. The average TRFs for each plant sample and restriction enzyme were determined from GeneScan data as shown in Tables A1.1 to A1.3, A2.1 to A2.3, and A3.1 to A3.3 placed online at http://som.flinders.edu.au/FUSA/Biotech/Acrobatfiles/ButcherFranco/appendices.htm (hereafter referred to as the additional-data website) and summarized in Table 2. The Jaccard coefficient analyzed for each of the restriction enzymes (Fig. 2) showed that the endophytic actinobacterial populations for the roots of wheat grown in Swedes Flat and Red Loam soils were the most closely related, followed by Red Loam and Western Flat soils, with the bacterial population of plants grown in Western Flat sand being most different from that found with Swedes Flat soil.
FIG. 1.
T-RFLP profile of the 16S rRNA genes of the actinobacterial population amplified from the roots of 6-week-old wheat grown in the field soil obtained from Swedes Flat and digested with HinfI (a), HhaI (b), and MboI (c).
TABLE 2.
16S rRNA TRFs obtained with the enzymes HinfI, HhaI, and MboI for the roots of wheat grown for 6 weeks in field soils obtained from Red Loam, Swedes Flat, and Western Flata
| Fragment size (bp) | Red Loam | Swedes Flat | Western Flat |
|---|---|---|---|
| HinfI | |||
| 35 | + | + | + |
| 39 | − | + | + |
| 41 | + | + | − |
| 44 | + | + | + |
| 49 | + | + | − |
| 53 | + | + | + |
| 57 | + | − | + |
| 61 | − | − | + |
| 63 | + | + | + |
| 70 | + | + | + |
| 80 | + | + | + |
| 82 | + | + | + |
| 84 | + | + | + |
| 89 | + | + | + |
| 92 | − | − | + |
| 94 | + | + | + |
| 97 | + | − | + |
| 99 | + | − | + |
| 105 | + | − | + |
| 112 | − | − | + |
| 128 | + | + | + |
| 133 | + | + | + |
| 147 | + | + | + |
| 167 | + | − | + |
| 174 | + | + | + |
| 176 | + | + | + |
| 178 | + | + | + |
| 180 | − | + | − |
| 188 | − | − | + |
| 213 | + | + | + |
| 236 | + | + | + |
| 258 | − | − | + |
| 279 | − | − | + |
| 284 | − | − | + |
| 321 | − | − | + |
| 348 | − | − | + |
| 369 | − | − | + |
| 407 | − | − | + |
| 411 | − | − | + |
| HhaI | |||
| 36 | + | + | − |
| 39 | − | − | + |
| 41 | + | + | − |
| 44 | + | + | + |
| 49 | + | + | − |
| 53 | + | + | − |
| 58 | + | − | + |
| 61 | − | − | + |
| 63 | + | + | + |
| 70 | + | + | + |
| 81 | + | + | + |
| 85 | + | + | + |
| 89 | + | + | − |
| 91 | + | − | + |
| 94 | + | + | + |
| 97 | − | − | − |
| 99 | − | − | + |
| 106 | + | − | + |
| 113 | − | − | + |
| 120 | + | − | + |
| 127 | + | + | + |
| 133 | + | + | + |
| 147 | + | + | + |
| 175 | + | − | + |
| 178 | + | − | + |
| 190 | + | − | + |
| 213 | + | + | + |
| 227 | − | − | + |
| 239 | + | − | − |
| 258 | − | − | + |
| 279 | + | − | + |
| 321 | − | − | + |
| 348 | − | − | + |
| 368 | − | − | + |
| 372 | + | − | − |
| 385 | − | + | − |
| 387 | + | + | + |
| 410 | − | + | − |
| 412 | + | + | + |
| 414 | + | + | − |
| 416 | + | − | − |
| 418 | − | + | − |
| 420 | + | − | − |
| MboI | |||
| 34 | + | + | + |
| 39 | − | + | − |
| 43 | + | + | + |
| 49 | + | + | + |
| 53 | + | + | + |
| 57 | + | − | + |
| 63 | + | + | + |
| 70 | + | + | + |
| 72 | − | − | − |
| 80 | + | − | − |
| 82 | + | + | + |
| 89 | + | + | − |
| 91 | − | − | + |
| 95 | + | + | + |
| 97 | + | − | − |
| 105 | + | − | + |
| 113 | − | − | + |
| 118 | − | + | − |
| 128 | + | + | + |
| 133 | + | + | + |
| 147 | − | − | + |
| 156 | + | + | + |
| 158 | + | + | − |
| 162 | + | + | − |
| 173 | + | + | + |
| 175 | − | − | + |
| 178 | + | − | + |
| 214 | − | − | + |
| 258 | − | − | + |
+, present; −, absent.
FIG. 2.
Dendrograms showing the relative community relatedness of the endophytic actinobacterial population present in the roots of wheat grown in three different field soils according to the Jaccard coefficient. The three dendrograms show the Jaccard coefficient of the three populations with the T-RFLP profile obtained with HinfI (a), HhaI (b), and MboI (c).
From the average TRFs obtained for each restriction enzyme (Table 2), the endophytic actinobacterial population was determined using the TAP T-RFLP software (27) linked to the RDP database (25). The Ap values for each actinobacterial genus were calculated as described above and shown on the additional-data website in Tables A1.4 to A1.6 for Red Loam, A2.4 to A2.6 for Swedes Flat, and A3.4 to A3.6 for Western Flat. The detailed calculations showing how the Ap values were corrected for each triple TRF-genus match are shown in Tables A1.7, A2.7, and A3.7 on the additional-data website. In addition, the actinobacterial genera identified and the relative proportions present in the roots of wheat grown for 6 weeks in each field soil are shown on the additional-data website in Tables A.1.8 (Red Loam), A.2.8 (Swedes Flat), and A.3.8 (Western Flat). These results are summarized in Table 3, showing the maximum percentage of each actinobacterial genus identified in the wheat plants grown in each of the three soils. The maximum Ap values listed for each of the genera will be an overestimation because of the multiple genera that are correlated with some triple TRF-genus matches. For the same reason, the minimum Ap value for some genera may be 0, but a minimum value is presented to indicate the theoretical range for any bacterial genus that is present in the plant root.
TABLE 3.
Maximum Ap of each genus identified by T-RFLP in the roots of wheat grown for 6 weeks in field soils obtained from Red Loam, Swedes Flat, and Western Flat
| Genus | Max Ap
|
||
|---|---|---|---|
| Red Loam | Swedes Flat | Western Flat | |
| Mycobacterium | 1.30 | 21.02 | 2.45 |
| Bifidobacterium | 5.78 | 20.78 | 1.24 |
| Rhodococcus | 1.95 | 20.64 | 1.24 |
| Streptomyces | 14.35 | 18.53 | 0.00 |
| Nocardia | 6.22 | 16.75 | 1.18 |
| Geodermatophilus | 1.30 | 13.76 | 1.24 |
| Saccharomonospora | 1.30 | 9.45 | 0.62 |
| Arthrobacter | 11.49 | 7.52 | 0.62 |
| Microbacterium | 0.65 | 7.41 | 0.62 |
| Frankia | 1.30 | 6.51 | 0.62 |
| Gordonia | 0.65 | 6.88 | 1.26 |
| Saccharothrix | 0.00 | 6.44 | 0.59 |
| Brevibacterium | 4.50 | 6.05 | 1.24 |
| Thermonospora | 2.62 | 5.47 | 0.00 |
| Kitasatospora | 1.95 | 5.52 | 15.02 |
| Pimelobacter | 0.65 | 4.99 | 0.62 |
| Lentzea | 1.30 | 4.99 | 0.62 |
| Agromyces | 0.00 | 4.84 | 0.00 |
| Actinomyces | 0.00 | 4.72 | 0.00 |
| Williamsia | 0.65 | 4.46 | 0.00 |
| Thermocrispum | 2.62 | 4.46 | 0.00 |
| Sanguibacter | 0.65 | 4.46 | 0.00 |
| Rubrobacter | 4.93 | 4.46 | 0.00 |
| Promicromonospora | 0.65 | 4.46 | 0.00 |
| Corynebacterium | 1.30 | 1.06 | 1.24 |
| Micrococcus | 0.65 | 2.95 | 0.62 |
| Micromonospora | 0.00 | 2.51 | 0.59 |
| Leifsonia | 0.00 | 2.42 | 0.00 |
| Dietzia | 0.00 | 2.42 | 0.00 |
| Curtobacterium | 0.00 | 2.42 | 0.00 |
| Kineococcus-like bacterium | 11.49 | 1.54 | 0.62 |
| Actinosynnema | 2.46 | 1.98 | 1.18 |
| Actinoplanes | 4.92 | 2.00 | 0.59 |
| Catellatospora | 0.65 | 1.06 | 0.62 |
| Sarraceniospora | 0.65 | 1.06 | 0.00 |
| Nocardioides | 3.27 | 0.53 | 0.00 |
| Lechevalieria | 0.65 | 1.06 | 0.62 |
| Kribella | 0.00 | 1.06 | 0.00 |
| Streptoalloteichus | 2.46 | 1.01 | 0.00 |
| Spirilliplanes | 2.46 | 1.01 | 0.00 |
| Amycolatopsis | 7.73 | 0.00 | 0.00 |
The highest level of diversity and endophytic colonization was present in the roots of wheat grown in the Swedes Flat soil. Wheat grown in the Western Flat soil contained the lowest level of endophytic actinobacterial diversity and colonization.
A number of TRFs did not have a match in the database, or there was a match for one enzyme TRF but the corresponding TRFs from the other two enzyme digests were not present. Table 4 shows the percentage of peak areas for each restriction enzyme that matched bacterial species that had the other two corresponding matches.
TABLE 4.
Percentages of peak areas of TRF sizes obtained with restriction enzymes HinfI, HhaI, and MboI corresponding to a triple TRF-genus match in the TAP T-RFLP database and present in the roots of wheat grown in Red Loam, Swedes Flat, or Western Flat soil
| Restriction enzyme | % of peak area
|
||
|---|---|---|---|
| Red Loam | Swedes Flat | Western Flat | |
| HinfI | 52 | 65 | 31 |
| HhaI | 44 | 62 | 19 |
| MboI | 13 | 45 | 5 |
Partial 16S rRNA gene sequencing.
The partial 16S rRNA gene sequences for the cloned amplicons were compared to sequences available in the National Center for Biotechnology Information database, and the results of the three highest matches for each clone and the corresponding bit score and percentage of identity for each field soil are shown in Tables B.1, B.2, and B.3 on the additional-data website. These results are summarized in Table 5.
TABLE 5.
Number of clones identified on basis of highest sequence match of partial 16S rRNA actinobacterial sequences isolated from roots of wheat grown in field soils Red Loam, Western Flat, and Swedes Flat
| Highest sequence match | No. of clones from soil (n)
|
||
|---|---|---|---|
| Red Loam (17) | Swedes Flat (16) | Western Flat (12) | |
| Amycolatopsis sp. strain GY152 | 2 | ||
| Gordonia polyisoprenivorans | 1 | ||
| Micromonospora endolithica | 1 | ||
| Micromonospora peucetica | 1 | ||
| Mycobacterium aichiense | 1 | ||
| Mycobacterium bohemicum | 2 | ||
| Mycobacterium cookii | 1 | 1 | |
| Mycobacterium flavescens | 1 | ||
| Mycobacterium heidelbergense | 1 | ||
| Mycobacterium palustre | 1 | ||
| Mycobacterium sp. | 1 | ||
| Mycobacterium sp. strain 2333 | 1 | 2 | 2 |
| Mycobacterium sp. strain IMVS B76676 | 5 | 5 | 4 |
| Mycobacterium sp. strain MCRO 33 | 1 | ||
| Nocardia pseudobrasiliensis | 1 | ||
| Rhodococcus coprophilus | 1 | ||
| Streptomyces sp. strain EF-91 | 1 | ||
| Streptomyces sp. strain SE2 | 1 | 1 | |
| Streptomyces thermolineatus | 3 | ||
| Uncultured actinobacterium clone SMW4.128WL | 1 | ||
| Uncultured eubacterium WD294 | 1 | ||
| Uncultured maize root bacterium Zmrc174 | 1 | ||
DISCUSSION
This is the first study to investigate the endophytic actinobacterial population present in wheat roots grown in different field soils by the molecular biology-based T-RFLP technique. T-RFLP was used to assess the effect of different field soils on the endophytic actinobacterial population in the roots of wheat. As the same seed cultivar was sown, the results indicate how the soil microbial population influences the endophyte population. The field soils used in this study had different compositions: Red Loam was a sandy loam, Swedes Flat was a dark loam, and Western Flat was water-repellent sand. The endophytic populations were significantly different when grown in different field soils, as shown in the Jaccard coefficient analysis, as well as in the presence of the major genera associated with each soil type. The endophyte population was considered to be a subset of the rhizosphere population by Germida et al. (17), who investigated the diversity of bacteria in the rhizosphere and roots of canola and wheat. They found that, while the endophytic population was less diverse, the endophytes appeared to originate from the rhizosphere. Therefore, plants grown in soils that harbor a higher number of microorganisms are expected to contain a larger and more diverse number of endophytes. This has been confirmed in our study.
The dominant genera as determined by T-RFLP for the plants grown in Swedes Flat soil were Mycobacterium, Bifidobacterium, Rhodococcus, Streptomyces, Nocardia, and Geodermatophilus. In the Red Loam, which supported plants that had the next highest diversity, the major genera were identified as Streptomyces, Arthrobacter, Kineococcus-like bacterium, Amycolatopsis, and Nocardia. The major genus identified in wheat plants grown in Western Flat soil was Kitasatospora, followed by Mycobacterium.
The diversity of actinobacteria from wheat roots detected by T-RFLP was higher than that found by culture-dependent methods performed in our laboratory. Coombs and Franco (9) isolated four different genera (Microbispora, Micromonospora, Nocardioides, and Streptomyces) from wheat plants sampled from a range of field sites in South Australia. Nine actinobacterial genera with a maximum Ap value of over 1% (Actinosynnema, Bifidobacterium, Brevibacterium, Corynebacterium, Geodermatophilus, Kitasatospora, Mycobacterium, Nocardia, and Rhodococcus) were identified in all of the field soils, and a total of 41 different actinobacterial genera were identified as possible endophytes among the three different field soils. Actinobacterial genera that have been identified as endophytes include Arthrobacter, Kitasatospora, Micromonospora, Microbispora, Nocardia, Nocardioides, Streptomyces, and Tsukamurella (4, 9, 12, 16, 38).
The characterization of the endophytic actinobacterial community cannot be confirmed by T-RFLP alone; therefore, sequencing of 16S rRNA gene clones was performed to further identify the species present within the wheat roots. Isolation of these species from surface-sterilized roots needs to be performed to confirm their endophytic nature. With the limited number of clones sequenced, the eight different genera identified among the three soils were also identified by the T-RFLP analysis. The predominant actinobacterial genus identified by sequencing was Mycobacterium, with 64% of clones belonging to this genus. This is the first time that Mycobacterium spp. have been identified as endophytes of wheat, though they are known to be present in the rhizosphere. For example, Mycobacterium achiense JS618 was isolated from an industrial soil site, whereas a number of Mycobacterium species were isolated from groundwater, soil, and activated sludge that were capable of degrading vinyl chloride (7). As a number of Mycobacterium species are known to be human pathogens (41), it is significant that species similar to those reported as clinical isolates, including M. heidelbergense, M. palustre, Mycobacterium sp. strain IMVS B76676, and Mycobacterium sp. strain MCRO 33, have been identified. It is possible that some mycobacteria were introduced via the tap water used for watering the plants, as M. kansasii, M. fortuitum, M chelonae, M. avium, and M. xenopi have been identified in tap water (22). We are now pursuing the isolation of endophytic mycobacteria to gain more insight into the role of these species within healthy plant tissue.
The high percentage of the 16S rRNA clones related to Mycobacterium sequences was not reflected in the T-RFLP analysis, where the maximum abundance of Mycobacterium detected was 1.3% in Red Loam, 2.5% in Western Flat, and 21% in Swedes Flat samples. This high frequency of detection is possibly a result of the RFLP screening used to select clones for sequencing, with the mycobacterial clones possessing a degree of detectable intrageneric polymorphism higher than the degree of the intergeneric polymorphisms between the actinobacterial genera present. The RFLP patterns of the 16S rRNA gene sequences of the Mycobacterium spp. identified generated with HinfI and HhaI were significantly different from one another. Random sequencing of clones may have resulted in lower detection, though it would still not give information on abundance of genera.
The second most frequent genus identified by sequencing was Streptomyces. Endophytic Streptomyces bacteria appear to be ubiquitous and have been isolated from various plants including Ficus, Dieffenbachia, Allium porrum, Brassica oleracera, and Quercus sp. (33). Of the three Streptomyces species isolates identified by sequencing, Streptomyces strain SE2 was identified as a wheat endophyte in our laboratory (9), and Streptomyces sp. strain EF-91 is a known endophyte of potato (12). This the first report of Streptomyces thermolineatus detected as an endophyte.
Micromonospora yulongensis was cultured from healthy wheat roots previously (9), and in this study we have identified clones that are closely related to Micromonospora endolithica and Micromonospora peucetica. Nocardia endophytes have been isolated from citrus plants (4), and the clone identified in this study was closely related to the human pathogen Nocardia pseudobrasiliensis, the causative agent of nocardiosis, which is transmitted through soil (11). Also of interest is the presence of a close relative of Gordonia polyisoprenivorans, a rubber-degrading species (23). As this species and most of the others such as Amycolatopsis sp. strain GY152 and Rhodococcus coprophilus that have not been previously identified as endophytes are culturable, it should be possible to isolate them for further study, by using the appropriate conditions.
The study of heterogenous microbial populations by the T-RFLP technique has its limitations. The preferential extraction of genomic DNA and subsequent amplification bias during PCR are known limitations for all molecular techniques that rely on amplification of community DNA and PCR analysis (24, 26). To minimize problems at the PCR level, care was taken in primer design and the execution of the PCR, which was run in duplicate. In addition, the restriction enzymes were chosen carefully and the digests were performed for the maximum length of time to ensure complete digestion of amplicons. While the T-RFLP technique can be used to determine if microbial populations are significantly different from one another, the TAP T-RFLP software extends this technique to correlate these fragments with bacterial species. Further limitations arise at this point, and the most apparent is that the RDP database (25), while diverse, is not complete; hence, it is unlikely that a match with a genus and/or species will be obtained for all the TRFs in an environmental sample.
The use of three restriction enzymes to provide the triple TRF-genus match is expected to eliminate false positives and the effect of any pseudo-TRFs (14), which can occur when only one or two restriction enzymes are used. In our studies a maximum of 65% of the peak areas obtained from the GeneScan data matched bacterial species in the database. This resolution is relatively high for a genus-level identification, because it was expected that there were a significant number of bacterial species present in the samples for which there was no sequence entry in the database. Nevertheless, the ability to calculate Ap values for each genus allows for a semiquantitative comparison to be made between different microbial populations at the genus level. Therefore, this method of T-RFLP analysis combined with the semiquantitative calculation of validated TRF-genus matches provides a rapid and high-resolution molecular technique for the characterization of heterogenous microbial communities.
In conclusion, this study has determined that soil type significantly affects the endophytic actinobacterial population of wheat. Soils that support a higher indigenous microbial population appear to result in a higher and more diverse actinobacterial endophyte population. The endophytic actinobacterial population in the roots of wheat appears to be much more diverse than previously detected by culture-dependent techniques. This knowledge can now lead to selection of the appropriate medium for isolation of novel and potentially beneficial endophytes, as well as to the study of the effect of various factors on endophytic populations at the genus level.
Acknowledgments
This work was supported by the Australian Grains Research and Development Corporation (GRDC).
REFERENCES
- 1.Adams, P. D., and J. W. Kloepper. 2002. Effect of host genotype on indigenous bacterial endophytes of cotton (Gossypium hirsutum L.). Plant Soil 240:181-189. [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo, W. L., W. Maccheroni, C. I. Aguilar-Vidoso, and P. A. V. Barroso. 2001. Variability and interactions between endophytic bacteria and fungi isolated from leaf tissues of citrus rootstocks. Can. J. Microbiol. 47:229-236. [DOI] [PubMed] [Google Scholar]
- 4.Araujo, W. L., J. Marcon, W. Maccheroni, J. D. van Elsas, J. W. L. van Vuurde, and J. L. Azevedo. 2002. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl. Environ. Microbiol. 68:4906-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benhamou, N., J. W. Kloepper, A. Quadt-Hallmann, and S. Tuzun. 1996. Induction of defense-related ultrastructural modifications in pea root tissues inoculated with endophytic bacteria. Plant Physiol. 112:919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, C., E. M. Bauske, G. Musson, R. Rodriguez-Kaban, and J. W. Kloepper. 1995. Biological control of Fusarium wilt on cotton by use of endophytic bacteria. Biol. Control 5:83-91. [Google Scholar]
- 7.Coleman, N. V., T. E. Mattes, J. M. Gossett, and J. C. Spain. 2002. Phylogenetic and kinetic diversity of aerobic vinyl chloride-assimilating bacteria from contaminated sites. Appl. Environ. Microbiol. 68:6162-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coombs, J. T., and C. M. M. Franco. 2003. Visualization of an endophytic Streptomyces species in wheat seed. Appl. Environ. Microbiol. 69:4260-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coombs, J. T., and C. M. M. Franco. 2003. Isolation and identification of actinobacteria isolated from surface-sterilized wheat roots. Appl. Environ. Microbiol. 69:5303-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coombs, J. T., P. P. Michelsen, and C. M. M. Franco. Evaluation of endophytic actinobacteria as antagonists of Gaeumannomyces graminis var. tritici in wheat. Biol. Control 29:359-366.
- 11.Division of Bacterial and Mycotic Diseases, National Center for Infectious Diseases, Centers for Disease Control and Prevention. 2002. Disease information. Nocardiosis. [Online.] http://www.cdc.gov/ncidod/dbmd/diseaseinfo/nocardiosis_t.htm.
- 12.Doumbou, C. L., V. Akimov, M. Cote, P. M. Charest, and C. Beaulieu. 2001. Taxonomic studies on non-pathogenic streptomycetes isolated from common scab lesions on potato tubers. Syst. Appl. Microbiol. 24:451-456. [DOI] [PubMed] [Google Scholar]
- 13.Dunbar, J., L. O. Ticknor, and C. R. Kuske. 2000. Assessment of microbial diversity in four southwestern United States soils by 16S rRNA gene terminal restriction fragment analysis. Appl. Environ. Microbiol. 66:2943-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egert, M., and M. W. Friedrich. 2003. Formation of pseudo-terminal restriction fragments, a PCR-related bias affecting terminal restriction fragment length polymorphism analysis of microbial community structure. Appl. Environ. Microbiol. 69:2555-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elbeltagy, A., K. Nishioka, T. Sato, H. Suzuki, B. Ye, T. Hamada, T. Isawa, H. Mitsui, and K. Minamisawa. 2001. Endophytic colonization and in planta nitrogen fixation by a Herbaspirillum sp. isolated from wild rice species. Appl. Environ. Microbiol. 67:5285-5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garbeva, P., L. S. Overbeek, J. W. van Vuurde, and J. D. van Elsas. 2001. Analysis of endophytic bacterial communities of potato by plating and denaturing gradient gel electrophoresis (DGGE) of 16S rDNA-based PCR fragments. Microb. Ecol. 41:369-383. [DOI] [PubMed] [Google Scholar]
- 17.Germida, J. J., S. D. Siciliano, R. de Freitas, and A. M. Seib. 1998. Diversity of root-associated bacteria associated with field-grown canola (Brassica napus L.) and wheat (Triticum aestivum L.). FEMS Microbiol. Ecol. 26:43-50. [Google Scholar]
- 18.Hallmann, J., A. Quadt-Hallmann, W. F. Mahaffee, and J. W. Kloepper. 1997. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 43:895-914. [Google Scholar]
- 19.Heuer, H., M. Krsek, P. Baker, K. Smalla, and E. M. H. Wellington. 1997. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl. Environ. Microbiol. 63:3233-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch, A. M., M. R. Lum, and J. A. Downie. 2001. What makes the rhizobia-legume symbiosis so special? Plant Physiol. 127:1484-1492. [PMC free article] [PubMed] [Google Scholar]
- 21.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood (ed.). 2000. Practical Streptomyces genetics. John Innes Centre, Norwich, England.
- 22.Le Dantec, C., J.-P. Duguet, A. Montiel, N. Dumoutier, S. Dubrou, and V. Vincent. 2002. Occurrence of mycobacteria in water treatment lines and in water distribution systems. Appl. Environ. Microbiol. 68:5318-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linos, A., A. Steinbuchel, C. Sproer, and R. M. Kroppenstedt. 1999. Gordonia polyisoprenivorans sp. nov., a rubber-degrading actinomycete isolated from an automobile tyre. Int. J. Syst. Bacteriol. 49:1785-1791. [DOI] [PubMed] [Google Scholar]
- 24.Lukow, T., P. F. Dunfield, and W. Liesack. 2000. Use of the T-RFLP technique to assess spatial and temporal changes in the bacterial community structure within an agricultural soil planted with transgenic and non-transgenic potato plants. FEMS Microbiol. Ecol. 32:241-247. [DOI] [PubMed] [Google Scholar]
- 25.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, J. M. Stredwick, G. M. Garrity, B. Li, G. J. Olsen, S. Pramanik, T. M. Schmidt, and J. M. Tiedje. 1999. The RDP (Ribosomal Database Project) continues. Nucleic Acids Res. 28:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsh, T. L. 1999. Terminal restriction fragment length polymorphism (T-RFLP): an emerging method for characterizing diversity among homologous populations of amplification products. Curr. Opin. Microbiol. 2:323-327. [DOI] [PubMed] [Google Scholar]
- 27.Marsh, T. L., P. Saxman, J. Cole, and J. Tiedje. 2000. Terminal restriction fragment length polymorphism analysis program, a web-based research tool for microbial community analysis. Appl. Environ. Microbiol. 66:3616-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCully, M. E. 2001. Niches for bacterial endophytes in crop plants: a plant biologist's view. Aust. J. Plant. Physiol. 28:983-990. [Google Scholar]
- 29.McInroy, J. A., and J. W. Kloepper. 1995. Population dynamics of endophytic bacteria in field-grown sweet corn and cotton. Can. J. Microbiol. 41:895-901. [Google Scholar]
- 30.Nejad, P., and P. A. Johnson. 2000. Endophytic bacteria induce growth promotion and wilt disease suppression in oilseed rape and tomato. Biol. Control 18:208-215. [Google Scholar]
- 31.Provorov, N. A., A. Y. Borisov, and I. A. Tikhonovich. 2002. Developmental genetics and evolution of symbiotic structures in nitrogen-fixing nodules and arbuscular mycorrhiza. J. Theor. Biol. 214:215-232. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Sardi, P., M. Saracchi, S. Quaroni, B. Petrolini, G. E. Borgonovi, and S. Merli. 1992. Isolation of endophytic Streptomyces strains from surface-sterilized roots. Appl. Environ. Microbiol. 58:2691-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scala, D. J., and L. J. Kerkhof. 2000. Horizontal heterogeneity of denitrifying bacterial communities in marine sediments by terminal restriction fragment length polymorphism analysis. Appl. Environ. Microbiol. 66:1980-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sessitsch, A., B. Reiter, U. Pfeifer, and E. Wilhelm. 2001. Cultivation-independent population analysis of bacterial endophytes in three potato varieties based on eubacterial and actinomycetes-specific PCR of 16S rRNA genes. FEMS Microbiol. Ecol. 1305:1-10. [DOI] [PubMed] [Google Scholar]
- 36.Siciliano, S. D., C. M. Theoret, J. R. de Freitas, P. J. Huci, and J. J. Germida. 1998. Differences in the microbial communities associated with the roots of different cultivars of canola and wheat. Can. J. Microbiol. 44:844-851. [Google Scholar]
- 37.Smalla, K., G. Wieland, A. Buchner, A. Zock, J. Parzy, S. Kaiser, N. Roskot, H. Heuer, and G. Berg. 2001. Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl. Environ. Microbiol. 67:4742-4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sturz, A. V., B. R. Christie, B. G. Matheson, W. J. Arsenault, and N. A. Buchanan. 1999. Endophytic bacterial communities in the periderm of potato tubers and their potential to improve resistance to soil-borne plant pathogens. Plant Pathol. 48:360-369. [Google Scholar]
- 39.Sturz, A. V., and J. Nowak. 2000. Endophytic communities of rhizobacteria and the strategies required to create yield enhancing associations with crops. Appl. Soil Ecol. 15:183-190. [Google Scholar]
- 40.van Vuurde, J. W. L., and M. Elvira-Recuenco. 2000. Endophyte management as a tool to optimize plant quality. In Proceedings of the Fifth International PGPR Workshop. [Online.] Auburn University, Auburn, Ala. http://www.ag.auburn.edu/argentina/pdfmanuscripts/vanvuurde2.pdf.
- 41.Wayne, L. G., and G. P. Kubica. 1986. The mycobacteria, p. 1435-1457. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. Williams & Wilkins, Baltimore, Md.
- 42.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson, D. 1995. Endophyte—the evolution of a term, and clarification of its use and definition. Oikos 73:274-276. [Google Scholar]
- 44.Zinniel, D. K., P. Lambrecht, B. N. Harris, Z. Feng, D. Kuczmarski, P. Higley, C. A. Ishimaru, A. Arunakumari, R. G. Barletta, and A. K. Vidaver. 2002. Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Appl. Environ. Microbiol. 68:2198-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]