Abstract
As an astrocytic protein specific to the central nervous system, S100b is a potentially useful marker in outcome prediction after traumatic brain injury (TBI). Some studies have questioned the validity of S100b, citing the extracerebral origins of the protein as reducing the specificity of the marker. This study evaluated S100b as a prognostic biomarker in adult subjects with severe TBI (sTBI) by comparing outcomes with S100b temporal profiles generated from both cerebrospinal fluid (CSF) (n=138 subjects) and serum (n=80 subjects) samples across a 6-day time course. Long-bone fracture, Injury Severity Score (ISS), and isolated head injury status were variables used to assess extracerebral sources of S100b in serum. After TBI, CSF and serum S100b levels were increased over healthy controls across the first 6 days post-TBI (p≤0.005 and p≤0.031). Though CSF and serum levels were highly correlated during early time points post-TBI, this association diminished over time. Bivariate analysis showed that subjects who had temporal CSF profiles with higher S100b concentrations had higher acute mortality (p<0.001) and worse Glasgow Outcome Scale (GOS; p=0.002) and Disability Rating Scale (DRS) scores (p=0.039) 6 months post-injury. Possibly as a result of extracerebral sources of S100b in serum, as represented by high ISS scores (p=0.032), temporal serum profiles were associated with acute mortality (p=0.015). High CSF S100b levels were observed in women (p=0.022) and older subjects (p=0.004). Multivariate logistic regression confirmed CSF S100b profiles in predicting GOS and DRS and showed mean and peak serum S100b as acute mortality predictors after sTBI.
Key words: cerebrospinal fluid, S100b, outcome, serum, traumatic brain injury
Introduction
Prognosis of patient outcome after traumatic brain injury (TBI) primarily incorporates clinical predictors, such as age, pupillary reactivity, motor score, and computerized tomography (CT) characteristics; these variables only provide moderate sensitivity and specificity for accurate prognosis.1,2 Additional studies suggest that predictive models, built using large populations with TBI, vary in their applicability to other populations. Predictive models are also subject to variation, based on covariate utilization3 and incorporation of baseline characteristics.1 The heterogeneity involved with TBI mechanism, injuries, treatments, and recovery patterns may contribute to this issue.4 The incorporation of biomarkers into predictive outcomes models may be one approach to address this heterogeneity.
Proteomic biomarkers are a part of mainstream clinical care used to quantitatively assess and define injury in almost every organ system, except the brain.5,6 With the absence of specific prognostic tools, there is increasing interest in identifying biomarkers that are both sensitive and specific to the central nervous system (CNS) to aid in diagnosis and prognosis for individuals sustaining TBI.6 Brain-specific markers measureable in serum have received the most attention for their translational potential in patient care and management, with more-limited studies of these same markers occurring in cerebrospinal fluid (CSF).7–9
S100b is a low-molecular-weight (9–13 kDa), Ca2+-binding protein primarily found in astrocytic glial cells of the CNS.10 S100b has significant calcium homeostatic functions and is secreted by astrocytes for neuroprotective and -trophic cellular functions in the CNS.11 As a neuroprotective factor, S100b prevents mitochondrial failure and cell death in the absence of glucose by increasing cellular [Ca2+] concentrations.12 Additionally, S100b functions as a neurotrophic factor by promoting neurite outgrowth and astrocytic proliferation that result in increased neuronal function.13
S100b is specifically found in the cytoplasm and nucleus, and less than 1% of this protein is secreted in a regulated fashion.14 Elevated CSF S100b levels after severe brain injury may reflect ongoing structural damage and cell death. Numerous studies have demonstrated increased S100b levels, significantly above control levels, after severe TBI (sTBI) in both CSF and serum.7,8,15 However, strategies employed for correlating S100b levels to outcome vary based on outcomes measures used, biofluid sampled, and time of sample collection relative to time of injury.9,16–23 Most of these studies have focused on serum measurements and have found the strongest positive predictive correlation with mortality. Further, these studies suggest predictive differences between serum S100b measures upon admission, compared to later time points (e.g., after 24 h postinjury), possibly resulting from the potential confounding factor of extracerebral sources of S100b contributing to values measured in serum, temporal changes in blood–brain barrier (BBB) integrity after injury, or evolving secondary injury cascades over time.
The promise of serum S100b as a viable diagnostic and/or prognostic biomarker is tempered by two major limitations. First, because the S100b protein is too large to pass through an intact BBB from the CNS to the blood, there have been limited attempts at establishing the correlation between serum and CSF S100b concentrations specific to injury.24 Watson and colleagues suggest that BBB permeability for S100b is increased after exercise in warm environments.25 Another early study demonstrates that CSF S100b levels are more predictive of outcome than serum S100b levels, citing that serum S100b may be derived more from an impaired BBB than intraparenchymal pathology.26,27 However, it is not yet fully understood whether serum S100b levels are primarily associated with extent of brain damage or BBB integrity. A second limitation of S100b is that the peripheral origins, or source contributions, of serum S100b are not fully elucidated, and serum levels may be attributed to extracerebral injuries, such as bone fracture, burns, and muscle injury.28,29 In a study evaluating patients with acute uncomplicated orthopedic fractures without head injury, serum S100b levels were higher than control in 29% of the patients within the first 24 h after injury.30 Also, some studies report acute increases in serum S100b after swimming and running,25,31 suggesting that exercise can cause extracerebral S100b release without neuronal damage. Others argue that extracerebral sources of S100b, such as in adipocytes,4 limit S100b as a viable brain-specific TBI biomarker.
Kleindienst and colleagues directly compared CSF and serum S100b concentrations in a sTBI population (n=71) by generating S100b CSF/serum ratios, analogous to the albuminCSF/albuminserum quotient (QA).32 Their data showed limited effectiveness of this S100b ratio as a clinical biomarker of BBB dysfunction. However, no studies have explored temporal relationships between CSF and serum S100b levels, particularly in relation to outcome. As such, our study quantitatively assessed both CSF and serum S100b concentrations in a large population with severe TBI and comparatively assessed the prognostic utility of single-point estimates, as well as longitudinal S100b biomarker profiles derived from CSF and from serum.
Based on the literature cited above, the primary aim of this study was to characterize serum and CSF time-course profiles during the first week after severe TBI. This study used a multivariate approach to comparatively assess the predictive value of longitudinal serum and CSF profiles versus single-point estimates for aggregated temporal S100b values for subjects with sTBI on global and functional outcome. Secondary aims were to evaluate sample-to-sample CSF to serum S100b correlations over time from each population and to demonstrate the degree to which extracerebral contributions of S100b in serum (e.g., long-bone fracture and anatomic injury scales) confound the utility of serum S100b to predict global outcome for those with sTBI over time.
Methods
Subjects
This prospective cohort study was approved by the University of Pittsburgh's Institutional Review Board (Pittsburgh, PA). Serum (n=80 subjects; n=224 samples) and CSF (n=138 subjects; n=499 samples) samples were collected from patients enrolled at our level 1 trauma center, where TBI was confirmed by CT scan. Enrollment criteria for this study included (1) age ≥16 years and (2) Glasgow Coma Scale (GCS) score ≤8 indicating sTBI. Subjects were included for analysis if there were at least two time points of CSF or serum sample collection during the first 6 days postinjury. Patients were excluded from enrollment if they had any of the following: cardiac or respiratory arrest before admission; documented prolonged hypoxia or hypotension before admission; evidence of brain death within the first 3 days after injury; an Abbreviated Injury Score (AIS) of 5 in any region other than the brain; or penetrating TBI. When possible, CSF was collected up to twice-daily for 6 days by an external ventricular drain (EVD) placed for clinical care. Serum was collected daily for 6 days. Clinical care issues, such as medical stability, minimal CSF output, clinical need for EVD, or removal from the intensive care unit (ICU), affected the collection of CSF and serum samples. Consecutive patients were recruited and consented by a next of kin. There was overlap between the CSF and serum cohorts (n=58 subjects; n=246 samples).
For comparison, healthy controls were recruited and CSF (n=15) and serum (n=6) S100b levels examined. CSF was collected by lumbar puncture, and serum was collected through a venous blood draw, specifically done for research. Control subjects had no previous record of TBI or neurological disease.
Brief description of clinical care
All subjects with TBI enrolled were admitted to the neurotrauma ICU and received treatment consistent with the Guidelines for the Management of Severe Traumatic Brain Injury.33 Temperature was monitored regularly, and a subset of subjects received moderate hypothermia if they were enrolled in a randomized, controlled clinical trial evaluating moderate hypothermia after sTBI. A small number of the subjects in the population (n=12 for the CSF cohort and n=11 for the serum cohort) received hypothermia treatment during time of sample collection. A small number of subjects (n=7 serum and n=14 CSF cohort) were also involved in the Citicoline Brain Injury Treatment (COBRIT) study, a randomized, double-blind, placebo-controlled, multi-center trial of the effects of 90 days of citicoline on functional outcome after TBI.34 For both hypothermia and COBRIT cohorts, S100b levels were not significantly different from the remainder of the cohort (data not shown).
Sample processing and protein measurement
Serum and CSF samples were centrifuged at 3000 rpm, for at least 5 min, and aliquoted and then stored at −80°C until batch analysis. CSF and serum samples matching up at specific time points postinjury were selected, where possible, for correlational analysis. CSF and serum S100b concentrations were measured by research staff unfamiliar with subject outcome data using Nexus Dx S100b enzyme-linked immunosorbent assay (ELISA) kits, in accord with the manufacturer's instructions (International Point of Care Inc., Toronto, Ontario, Canada). A four-parameter fit was used when generating the standard curve for calibration analysis and determination of protein concentration. For values with concentrations above the highest point in the calibration analysis, we reanalyzed samples using dilution factors, as appropriate. For values that had concentrations below the detectable range of the ELISA kit, the average of the respective lowest three detectable samples was used in place of out-of-range values. The detection limit of this assay was 0.02 ng/mL, although concentrations below this threshold were interpolated on the standard curve, allowing for analysis of some values below the detection limit.
Demographic and clinical injury variables
Demographic and clinical variables included the following: age; sex; body mass index (BMI); initial GCS score; Injury Severity Score (ISS); presence or absence of long-bone fracture; isolated head injury (IHI); mechanism of injury; and acute care mortality. Injury type, based on admission head CT, was also used in clinical analysis. Trained neurosurgical staff in the ICU made GCS assessments35 within 8 h of injury. This was done after intubation and initial resuscitation, with the temporary withdrawal of paralytics and active sedation. Trained trauma center registrars abstracted ISS and AIS information for each study participant with TBI. The ISS is a trauma scoring system that captures the overall severity of injury for a patient by quantifying injury over multiple anatomical regions using AIS, specifically for the three most injured regions. An ISS score of 75 represents a maximum score.36 Because of the potential confounding factor of extracerebral sources contributing to serum S100b concentrations, we assessed biomarker associations with the presence of a lower extremity femoral or tibial fractures in the serum cohort (n=6 subjects). Based on other large studies in the literature, IHI was defined as having sTBI with a head AIS value ≥3 and AIS values <3 for all other body regions.37–39 Of the 79 subjects in the serum cohort who had available AIS scores (necessary for IHI determination), 38 were designated as having an IHI. Acute care mortality data were collected for each patient.
Outcome variables
Primary outcomes measures used in this study were: mortality; Glasgow Outcome Scale (GOS) (1=dead; 5=good recovery) at 6 months40; and the Disability Rating Scale (DRS) (0=no disability; 30=dead) at 6 months.41 Research-trained neuropsychometrists, blinded to biomarker analyses, collected the data used to generate GOS and DRS scores for this cohort. For this analysis, we collapsed GOS scores into three groups (1, 2/3, and 4/5). For DRS, subjects were analyzed in three groups: none-to-partial disability (score, 0–3); moderate-to-severe disability (score, 4–14), and extremely severe disability to death (score, 15–30).41
Statistical analysis
Statistical analyses were performed using SAS (version 9.2; SAS Institute Inc., Cary, NC) and SPSS software (version 19.0; SPSS, Inc., Chicago, IL). Exploratory analysis was performed to describe each cohort. For continuous variables, summary statistics, including median, mean, and standard error of the mean (SEM), were computed. For categorical variables, frequencies and percentages were determined. Pearson's correlation analysis was used to determine the relationship between serum and CSF S100b levels in a subset of overlapping subjects with values taken from samples collected at the same time postinjury. Serum and CSF correlations were examined across 6 days after injury to assess interrelationships between levels in each compartment in the context of two independently varying biomarker source components.42
We used group-based trajectory analysis to explore biomarker levels over time. The PROC TRAJ Macro,43 available in SAS software (version 9.2 of the SAS system for windows) was used. In general, group-based trajectory modeling uses a probability function to identify clusters of individuals following a similar progression of some measure over time. Group-based trajectory modeling assumes that the population is composed of a finite number of unobserved groups, and leverages repeated subject sampling, to relate temporal patterns. We have used this approach in previous studies assessing the ability of temporal biomarker patterns to predict TBI outcome.44,45,46 S100b TRAJ groups were formulated with 80 patients having serum measurements and 138 subjects having CSF S100b measurements. Daily biomarker levels were not normally distributed because of a significant difference between sample variability and clustering of S100b values around the detection limits. As such, we applied the natural log transformation to biomarker data before deriving TRAJ groups for each sample. The number of TRAJ groups and their shape were determined by using the steps described in a companion study.
Daily S100b levels were graphed by TRAJ group membership in relation to levels observed in healthy controls. TRAJ group differences in daily biomarker values and other continuous data were compared using Wilcoxon's rank-sum and Kruskal-Wallis' tests. TRAJ group differences with regard to categorical data were assessed using chi-square analysis with Fisher's exact test, when appropriate.
Multivariate ordinal logistic regression models were used to evaluate how biomarker trajectory groups affected acute mortality, GOS, and DRS scores. Separate multivariate models were built in both CSF and serum profiles. Clinical and demographic variables having a p-value ≤0.2 in bivariate analyses, when compared to outcomes, were controlled for in multivariate ordinal logistic regression models. The use of TRAJ versus point estimates for S100b as predictors of outcome were comparatively assessed in both CSF and serum analysis. Using a stepwise selection method, variables were removed until all variables in models were significant at the 0.05 level, except S100b TRAJ group membership or levels that were forced in models, regardless of their significance. A stable multivariate logistic regression model for acute mortality in the CSF cohort was not possible because only 1 subject in the low TRAJ group died.
Results
Population description
Table 1 describes the cohorts with serum and with CSF S100b measurements. In the CSF cohort (n=138), 20.3% were women. The CSF report had a mean age of 35.6±1.3 years and a median GCS score of 6. Average ISS score in this cohort was 34.3±0.9, the median 6-month GOS score was 3, and 18.6% died during their acute care hospitalization. In the serum cohort (n=80), 26.2% were women. The serum cohort had a mean patient age of 37.1±1.8 years, and a median GCS score of 6. Average ISS score for this cohort was 34.8±1.2, the median GOS score was 3, and the acute mortality rate was 33.3%. Gender, age, median GCS, average ISS, and median GOS scores for the CSF and serum cohorts were not statistically different. The groups did differ, though, regarding DRS scores (p=0.040), with a median DRS for CSF and serum of 6 and 18, respectively.
Table 1.
S100b Demographic and Clinical Variables: Distributions by Trajectory Groups
| |
S100b CSF trajectory groups |
S100b serum trajectory groups |
|||||
|---|---|---|---|---|---|---|---|
| Variable | Low group (n=25) | Intermediate group (n=80) | High group (n=33) | p-value | Low group (n=45) | High group (n=35) | p-value |
| Gender, N (%) | |||||||
| Female | 2 (7.1) | 14 (50.0) | 12 (42.9) | 0.022 | 13 (61.9) | 8 (38.1) | 0.614 |
| Male | 23 (20.9) | 66 (60.0) | 21 (19.1) | 32 (54.2) | 27 (45.8) | ||
| Age, years mean±SEM | 36.3±3.0 | 32.3±1.6 | 42.8±2.9 | 0.004 | 35.2±2.2 | 39.5±2.9 | 0.226 |
| BMI mean±SEM | 27.0±0.9 | 26.5±0.6 | 28.1±1.1 | 0.394 | 25.2±0.6 | 28.3±1.2 | 0.054 |
| GCS, median | 7 | 6 | 6 | 0.234 | 6 | 6 | 0.296 |
| ISS mean±SEM | 37.6±2.0 | 33.4±1.1 | 33.9±1.8 | 0.261 | 32.5±1.6 | 37.6±1.6 | 0.032 |
| Hypothermia treatment, N (% yes) | 3 (18.8) | 9 (56.2) | 4 (25.0) | >0.999 | 8 (72.7) | 3 (27.3) | 0.332 |
| Long-bone fracture, N (% fractured) | 2 (18.2) | 6 (54.5) | 3 (27.3) | 0.913 | 4 (66.7) | 2 (33.3) | 0.691 |
| Isolated head injury, N (% with IHI) | 6 (10.0) | 40 (66.7) | 14 (23.3) | 0.081 | 22 (50.0) | 16 (45.7) | 0.821 |
| Mechanism of injury | |||||||
| Automobile/motorcycle | 22 (22.4) | 57 (58.2) | 19 (19.4) | 0.056 | 35 (61.4) | 22 (38.6) | 0.173 |
| Fall/jump | 2 (9.1) | 13 (59.1) | 7 (31.8) | 4 (33.3) | 8 (66.7) | ||
| Other | 0 (0.0) | 10 (58.8) | 7 (41.2) | 5 (50.0) | 5 (50.0) | ||
| Radiological injury type, N (% present) | |||||||
| Subdural hematoma | 14 (17.3) | 47 (58.0) | 20 (24.7) | 0.833 | 25 (51.0) | 24 (49.0) | 0.480 |
| Subarachnoid hemorrhage | 17 (17.4) | 55 (56.1) | 26 (26.5) | 0.421 | 34 (55.7) | 27 (44.3) | 0.781 |
| Diffuse axonal injury | 5 (11.6) | 31 (72.1) | 7 (16.3) | 0.110 | 13 (65.0) | 7 (35.0) | 0.308 |
| Epidural hematoma | 5 (29.4) | 8 (47.1) | 4 (23.5) | 0.295 | 7 (46.7) | 8 (53.3) | 0.570 |
| Contusion | 7 (12.1) | 33 (56.9) | 18 (31.0) | 0.270 | 19 (48.7) | 20 (51.3) | 0.363 |
| Intraventricular hemorrhage | 5 (11.6) | 26 (60.5) | 12 (27.9) | 0.610 | 13 (59.1) | 9 (40.9) | 0.800 |
| Intracerebral hemorrhage | 10 (21.7) | 22 (47.8) | 14 (30.4) | 0.134 | 14 (53.8) | 12 (46.2) | >0.999 |
| Acute care mortality | |||||||
| Dead | 1 (4.2) | 10 (41.7) | 13 (54.1) | <0.001 | 9 (34.6) | 17 (65.4) | 0.015 |
| Alive | 22 (20.9) | 66 (62.9) | 17 (16.2) | 34 (65.4) | 18 (34.6) | ||
SEM, standard error of the mean; BMI, body mass index; GCS, Glasgow Coma Scale; ISS, Injury Severity Scale; IHI, isolated head injury.
Bolded values represent statistically significant comparisons where alpha<0.05.
CSF and serum S100b levels
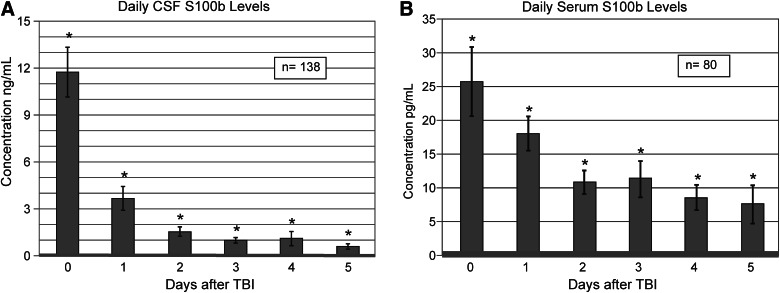
Figure 1A shows that daily mean CSF S100b levels were elevated above controls (control levels, 0.075±0.003 ng/mL) for all 6 days after injury (p≤0.005 all comparisons). Figure 1B shows that serum S100b levels were also elevated above controls (control levels, 0.328±0.101 pg/mL) for all 6 days postinjury (p≤0.031 all comparisons). Peak concentrations occurred on day 0 (d0) in both serum and CSF. Notably, there was a near 500-fold difference in concentration peaks for CSF and serum. Figure 1A,B shows that concentrations declined over the 6-day (d) period toward healthy control levels in both CSF and serum cohorts. In CSF, d0 values were significantly higher than d1 values (p<0.001), and d1 values were significantly higher than d2 values (p<0.001). Compared to CSF, the decline in serum values was not as marked, but d0 levels were significantly higher than d1 values (p=0.049) and also significantly higher than d2 values (p<0.001).
FIG. 1.
Daily biomarker levels over the first 6 days after severe traumatic brain injury, compared to healthy controls. For both cerebrospinal fluid (CSF) and serum, mean peak S100b levels occur on d0. (A) Daily mean CSF S100b levels are higher than controls across all days tested (p≤0.005 all comparisons). (B) Daily mean serum S100b levels are higher than controls across all days tested (p≤0.031 all comparisons). Healthy control CSF S100b values: 0.0754±0.0034 ng/mL; healthy control serum S100b values: 0.328±0.101 pg/mL.
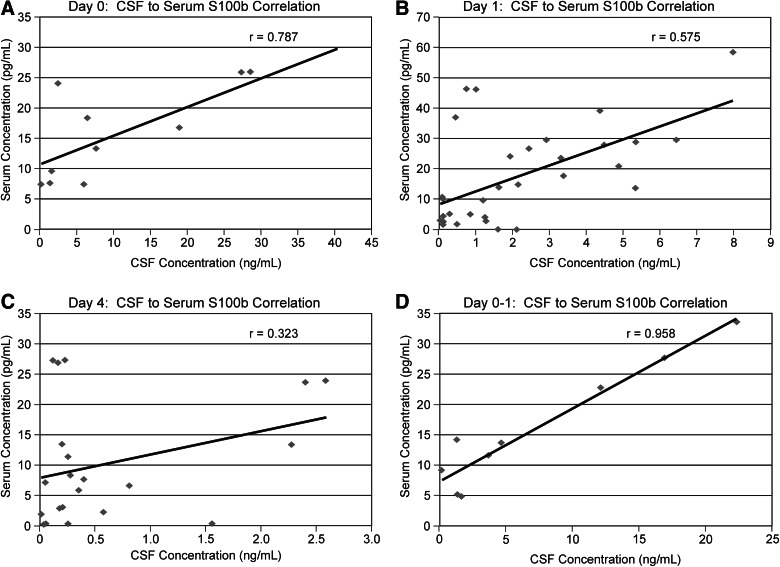
We examined the relationship between CSF and serum S100b levels for the overlapping subset using correlation analysis. When analyzed in 24-hour intervals, CSF/serum correlation was strongest on d0 (n=11; r=0.787; see Fig. 2A), followed by d1 (n=32; r=0.575; see Fig. 2B). Correlation coefficients between serum and CSF values collected at later time points were not significant (d4 data shown in Fig. 2C). When further restricting analysis to subjects with data points on both d0 and d1, and averaging their CSF values and corresponding serum values, the resulting correlation is shown in Figure 2D (n=9; r=0.958). Also, both mean and peak serum S100b levels were significantly correlated with ISS scores (r=0.260, p=0.021; r=0.266, p=0.018) for subjects in the serum cohort, but there were no associations between mean or peak S100b levels and long-bone fracture.
FIG. 2.
Serum S100b levels correlated to cerebrospinal fluid S100b levels in the overlapping sample between the two compartments showing decreasing correlation over time after severe traumatic brain injury. (A) Subjects who had CSF and serum S100b levels at d0 (n=11; r=0.787). (B) Subjects who had CSF and serum S100b levels at d1 (n=32; r=0.575). (C) Subjects who had CSF and serum S100b levels at d4 (n=21; r=0.323). (D) Subjects who had CSF and serum S100b levels at both d0 and d1 (n=9; r=0.958).
CSF and serum S100b: Bivariate associations with TRAJ group membership and outcome
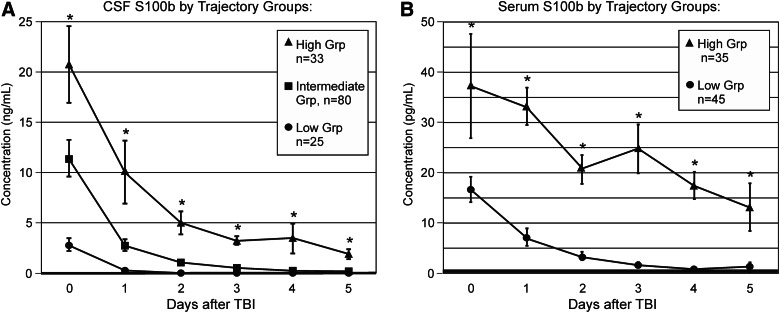
In our companion article, three statistically distinct TRAJ profiles were derived using samples from the CSF S100b cohort,46 and daily mean S100b concentrations over time were plotted for each TRAJ group (Fig. 3A). The “intermediate” group consisted of approximately 58% of the CSF cohort, whereas the “high” group comprised 24% of the cohort, and the “low” group comprised 18% of the cohort. At least one TRAJ group had daily S100b levels significantly different than the other TRAJ groups across all testing days (p<0.001 all comparisons). Notably, serial S100b values for all three groups trended downward toward controls by d5 after injury. However, only the high and intermediate groups sustained levels throughout the time course that were significantly above controls (high group [d5] CSF levels, 1.923±0.476 ng/mL; p<0.001 versus controls; intermediate group [d5] CSF levels, 0.203±0.029 ng/mL; p<0.001 versus controls). The low group returned to baseline by d2.
FIG. 3.
Trajectory groups for profiles (TRAJ) over the first 6 days after severe traumatic brain injury, compared to healthy controls. (A) Mean cerebrospinal fluid S100b levels are shown for three statistically distinct TRAJ groups. At least one TRAJ group was significantly different from the other two TRAJ groups across all time points tested (p<0.001 all comparisons). (B) Mean serum S100b levels are shown for two statistically distinct TRAJ groups. The two TRAJ groups were significantly different across all time points tested (p≤0.024 all comparisons). Healthy control CSF S100b values: 0.0754±0.0034 ng/mL; healthy control serum S100b values: 0.328±0.101 pg/mL.
Figure 3B shows that two statistically distinct TRAJ profiles were derived using samples from the serum S100b cohort. Mean S100b concentrations over time were plotted for each TRAJ group. In the serum cohort, the low group comprised approximately 56% of the cohort, whereas the high group comprised the other 44%. The two TRAJ groups were significantly different from each other across all testing days (p≤0.024 all comparisons). Unlike the high group, which had levels consistently above control levels (high group [d5] serum levels, 13.057±4.751 pg/mL; p=0.008 versus controls), the low group returned to control levels by d4.
CSF and serum TRAJ groups for S100b were compared to outcome, demographic, and clinical variables. In the CSF cohort, TRAJ groups were statistically different when compared by age (p=0.004) and gender (p=0.022; Table 1), with older and female subjects more likely to be in the high or intermediate TRAJ group. The CSF S100b TRAJ groups did not statistically differ by injury severity (GCS or ISS) or hypothermia treatment. However, acute mortality rates (p<0.001), GOS (p=0.002), and DRS (p=0.039) distributions were significantly different by CSF TRAJ group (see Table 2A). For this bivariate analysis, groups with higher S100b levels had higher mortality rates and worse outcomes. Mean and peak CSF S100b levels were associated with mortality (p=0.026) and GOS scores (p=0.047), but not DRS.
Table 2A.
S100b CSF Bivariate Associations between Outcomes, Demographic/Clinical Variables, S100b Average Levels, Peak Levels, and Trajectory Groups
| |
Acute care mortality |
Glasgow Outcome Score (GOS) |
Disability Rating Scale (DRS) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Alive | Dead | p-value | 1 | 2/3 | 4/5 | p-value | 0–3 | 4–14 | 15–30 | p-value |
| Gender, N (%) | |||||||||||
| Female | 17 (68.0) | 8 (32.0) | 0.083 | 9 (36.0) | 12 (48.0) | 4 (16.0) | 0.085 | 2 (7.7) | 10 (38.5) | 14 (53.8) | 0.017 |
| Male | 88 (84.6) | 16 (15.4) | 21 (22.6) | 36 (38.7) | 36 (38.7) | 33 (34.7) | 27 (28.4) | 35 (36.8) | |||
| Age, years, mean±SEM | 32.9±1.4 | 43.2±3.3 | 0.002 | 46.4±2.9 | 33.8±2.1 | 31.2±2.1 | <0.001 | 28.1±2.1 | 36.1±2.4 | 42.3±2.4 | <0.001 |
| BMI, mean±SEM | 26.4±0.5 | 27.9±1.3 | 0.253 | 27.9±1.0 | 25.8±0.8 | 27.6±0.8 | 0.072 | 26.5±0.9 | 27.4±0.9 | 27.9±0.9 | 0.473 |
| GCS, median | 7 | 5 | 0.013 | 6 | 6 | 7 | 0.017 | 7 | 7 | 6 | 0.003 |
| ISS, mean±SEM | 34.2±0.9 | 36.3±2.4 | 0.393 | 34.5±2.1 | 34.5±1.4 | 32.8±1.5 | 0.772 | 33.3±1.6 | 32.9±1.6 | 35.0±1.6 | 0.614 |
| Hypothermia treatment, N (% yes) | 15 (93.7) | 1 (6.3) | 0.303 | 1 (6.2) | 7 (43.8) | 8 (50.0) | 0.103 | 6 (42.9) | 6 (42.9) | 2 (14.3) | 0.080 |
| Long-bone fracture, N (% fractured) | 10 (90.9) | 1 (9.1) | 0.688 | 1 (11.1) | 6 (66.7) | 2 (22.2) | 0.305 | 2 (22.2) | 4 (44.5) | 3 (33.3) | 0.749 |
| Isolated head Injury, N (% with IHI) | 43 (74.1) | 15 (25.9) | 0.071 | 16 (30.8) | 19 (36.5) | 17 (32.7) | 0.487 | 17 (32.1) | 14 (26.4) | 22 (41.5) | 0.658 |
| Mechanism of injury | |||||||||||
| Automobile/motorcycle | 79 (85.9) | 13 (14.1) | 0.005 | 15 (18.3) | 34 (41.5) | 33 (40.2) | 0.013 | 30 (35.7) | 26 (31.0) | 28 (33.3) | 0.109 |
| Fall/jump | 10 (52.6) | 9 (47.4) | 11 (52.4) | 5 (23.8) | 5 (23.8) | 3 (13.6) | 6 (27.3) | 13 (59.1) | |||
| Other | 15 (88.2) | 2 (11.8) | 4 (28.6) | 8 (57.1) | 2 (14.3) | 2 (14.3) | 5 (35.7) | 7 (50.0) | |||
| Radiological injury type, N (% present) | |||||||||||
| Subdural hematoma | 58 (77.3) | 17 (22.7) | 0.256 | 22 (30.1) | 33 (45.2) | 18 (24.7) | 0.025 | 17 (22.7) | 23 (30.7) | 35 (46.6) | 0.040 |
| Subarachnoid hemorrhage | 71 (77.2) | 21 (22.8) | 0.122 | 26 (30.6) | 33 (38.8) | 26 (30.6) | 0.146 | 22 (25.6) | 25 (29.1) | 39 (45.3) | 0.063 |
| Diffuse axonal injury | 37 (90.2) | 4 (9.8) | 0.089 | 4 (12.1) | 12 (36.4) | 17 (51.5) | 0.022 | 18 (52.9) | 7 (20.6) | 9 (26.5) | 0.003 |
| Epidural hematoma | 13 (18.2) | 3 (18.8) | >0.999 | 3 (23.1) | 3 (23.1) | 7 (53.8) | 0.279 | 7 (50.0) | 3 (21.4) | 4 (28.6) | 0.229 |
| Contusion | 38 (69.1) | 17 (30.9) | 0.005 | 21 (38.9) | 18 (33.3) | 15 (27.8) | 0.014 | 12 (49.1) | 15 (28.3) | 26 (49.1) | 0.127 |
| Intraventricular hemorrhage | 33 (84.6) | 6 (15.4) | 0.625 | 8 (22.2) | 14 (38.9) | 14 (38.9) | 0.691 | 12 (30.8) | 11 (28.2) | 16 (41.0) | 0.939 |
| Intracerebral hemorrhage | 36 (87.8) | 5 (12.2) | 0.227 | 8 (22.2) | 17 (47.2) | 11 (30.6) | 0.585 | 8 (20.5) | 17 (43.6) | 14 (35.9) | 0.092 |
| S100b Levels (ng/mL), mean±SEM | |||||||||||
| S100b average levels | 2.60±0.44 | 3.89±0.79 | 0.006 | 4.34±0.97 | 3.29±0.75 | 2.04±0.44 | 0.009 | 2.05±0.43 | 3.49±0.97 | 3.64±0.65 | 0.080 |
| S100b peak levels | 6.34±1.01 | 9.01±2.14 | 0.026 | 9.07±1.99 | 7.15±1.60 | 6.51±1.62 | 0.047 | 6.79±1.81 | 7.89±2.14 | 7.77±1.36 | 0.110 |
| S100b trajectory groups, N (%) | |||||||||||
| Low group | 22 (95.7) | 1 (4.3) | <0.001 | 2 (10.5) | 8 (42.1) | 9 (47.4) | 0.002 | 7 (35.0) | 5 (25.0) | 8 (40.0) | 0.039 |
| Intermediate group | 66 (86.8) | 10 (13.2) | 11 (16.7) | 29 (43.9) | 26 (39.4) | 24 (35.3) | 23 (33.8) | 21 (30.9) | |||
| High group | 17 (56.7) | 13 (43.3) | 17 (51.5) | 11 (33.3) | 5 (15.2) | 4 (12.1) | 9 (27.3) | 20 (60.6) | |||
CSF, cerebrospinal fluid; SEM, standard error of the mean; BMI, body mass index; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; IHI, isolated head injury.
Bolded values represent statistically significant comparisons where alpha<0.05.
Serum TRAJ groups differed by ISS score (p=0.032), but not by GCS, gender, age, or hypothermia treatment (see Table 1). Subjects in the high serum TRAJ group had higher ISS scores than those in the low TRAJ group. Among serum TRAJ groups, the high TRAJ group had higher acute mortality rates (p=0.015), but they did not differ from the low group with GOS or DRS scores. Also, mean and peak S100b levels were associated with acute mortality (p<0.002 both comparisons; see Table 2B).
Table 2B.
S100b Serum Bivariate Associations between Outcomes, Demographic/Clinical Variables, S100b Average Levels, Peak Levels, and Trajectory Groups
| |
Acute care mortality |
Glasgow Outcome Score (GOS) |
Disability Rating Scale (DRS) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Alive | Dead | p-value | 1 | 2/3 | 4/5 | p-value | 0–3 | 4–14 | 15–30 | p-value |
| Gender, N (%) | |||||||||||
| Female | 9 (47.4) | 10 (52.6) | 0.052 | 11 (55.0) | 6 (30.0) | 3 (15.0) | 0.462 | 2 (10.0) | 5 (25.0) | 13 (65.0) | 0.360 |
| Male | 43 (72.9) | 16 (27.1) | 19 (37.3) | 20 (39.2) | 12 (23.5) | 13 (26.0) | 9 (18.0) | 28 (56.0) | |||
| Age, years, mean±SEM | 32.9±2.1 | 44.1±2.8 | 0.003 | 46.1±2.7 | 33.0±3.1 | 26.5±2.3 | <0.001 | 27.8±3.6 | 31.2±3.5 | 42.9±2.5 | 0.002 |
| BMI, mean±SEM | 25.9±0.8 | 27.1±1.1 | 0.193 | 27.1±1.0 | 26.4±1.3 | 25.9±1.8 | 0.356 | 25.4±1.9 | 28.0±2.0 | 26.6±0.8 | 0.280 |
| GCS, median | 7 | 5 | 0.030 | 6 | 6 | 7 | 0.034 | 7 | 7 | 6 | 0.001 |
| ISS, mean±SEM | 33.8±1.5 | 37.7±1.8 | 0.114 | 35.9±1.8 | 34.6±1.8 | 34.4±2.9 | 0.844 | 32.9±2.6 | 33.9±2.8 | 36.3±1.5 | 0.506 |
| Hypothermia treatment, N (% yes) | 9 (81.8) | 2 (18.2) | 0.319 | 2 (18.2) | 3 (27.3) | 6 (54.5) | 0.018 | 6 (60.0) | 1 (10.0) | 3 (30.0) | 0.010 |
| Long-bone fracture, N (% fractured) | 6 (100.0) | 0 (0.0) | 0.171 | 0 (0.0) | 4 (66.7) | 2 (33.3) | 0.048 | 2 (33.3) | 3 (50.0) | 1 (16.7) | 0.043 |
| Isolated head injury, N (% with IHI) | 23 (63.9) | 13 (36.1) | 0.810 | 16 (47.1) | 13 (38.2) | 5 (14.7) | 0.465 | 6 (18.2) | 6 (18.2) | 21 (63.6) | 0.756 |
| Mechanism of injury | |||||||||||
| Automobile/motorcycle | 40 (71.4) | 16 (28.6) | 0.084 | 17 (34.7) | 19 (38.8) | 13 (26.5) | 0.156 | 12 (25.0) | 11 (22.9) | 25 (52.1) | 0.665 |
| Fall/jump | 4 (36.4) | 7 (63.6) | 9 (75.0) | 2 (16.7) | 1 (8.3) | 2 (16.7) | 1 (8.3) | 9 (75.0) | |||
| Other | 7 (70.0) | 3 (30.0) | 4 (44.4) | 4 (44.4) | 1 (11.2) | 1 (11.1) | 2 (22.2) | 6 (66.7) | |||
| Radiological injury type, N (% present) | |||||||||||
| Subdural hhematoma | 30 (62.5) | 18 (37.5) | 0.615 | 21 (44.7) | 17 (36.2) | 9 (19.1) | 0.755 | 9 (19.6) | 10 (21.7) | 27 (58.7) | 0.774 |
| Subarachnoid hemorrhage | 36 (61.0) | 23 (39.0) | 0.153 | 27 (49.1) | 16 (29.1) | 12 (21.8) | 0.093 | 11 (20.4) | 8 (14.8) | 35 (64.8) | 0.025 |
| Diffuse axonal injury | 15 (75.0) | 5 (25.0) | 0.412 | 5 (29.4) | 6 (35.3) | 6 (35.3) | 0.259 | 6 (35.3) | 3 (17.6) | 8 (47.1) | 0.343 |
| Epidural hematoma | 10 (66.7) | 5 (33.3) | >0.999 | 5 (35.7) | 6 (21.4) | 6 (42.9) | 0.102 | 6 (42.9) | 2 (14.2) | 6 (42.9) | 0.127 |
| Contusion | 19 (50.0) | 19 (50.0) | 0.007 | 20 (54.1) | 11 (29.7) | 6 (16.2) | 0.157 | 7 (18.9) | 6 (16.2) | 24 (64.9) | 0.402 |
| Intraventricular hemorrhage | 15 (75.0) | 5 (25.0) | 0.412 | 8 (42.1) | 7 (36.8) | 4 (21.1) | >0.999 | 4 (21.1) | 2 (10.5) | 13 (68.4) | 0.435 |
| Intracerebral hemorrhage | 17 (68.0) | 8 (32.0) | 0.801 | 11 (50.0) | 8 (36.4) | 3 (13.6) | 0.565 | 2 (9.5) | 7 (33.3) | 12 (57.2) | 0.115 |
| S100b Levels (pg/mL), mean±SEM | |||||||||||
| S100b average levels | 9.61±1.37 | 21.15±3.98 | 0.001 | 19.39±3.64 | 10.26±1.80 | 10.68±3.08 | 0.112 | 10.49±3.03 | 10.06±2.63 | 17.12±2.82 | 0.236 |
| S100b peak levels | 16.12±2.11 | 33.43±5.89 | 0.002 | 30.42±5.41 | 17.58±2.73 | 18.44±4.78 | 0.181 | 18.21±4.62 | 16.56±3.96 | 27.39±4.19 | 0.267 |
| S100b trajectory groups, N (%) | |||||||||||
| Low group | 34 (79.1) | 9 (20.9) | 0.015 | 12 (31.6) | 16 (42.1) | 10 (26.3) | 0.150 | 9 (24.3) | 9 (24.3) | 19 (51.4) | 0.458 |
| High group | 18 (51.4) | 17 (48.6) | 18 (54.5) | 10 (30.3) | 5 (15.2) | 6 (18.2) | 5 (15.1) | 22 (66.7) | |||
SEM, standard error of the mean; BMI, body mass index; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; IHI, isolated head injury.
Bolded values represent statistically significant comparisons where alpha<0.05.
Multivariate outcome prediction models
Multivariate results for S100b in the CSF cohort comparing TRAJ groups to GOS are summarized in Table 3A. After accounting for demographic and clinical variables, S100b TRAJ groups were significantly associated with outcome. Specifically, the odds of having better GOS outcome for subjects with CSF S100b levels in the intermediate TRAJ group were 3.32 times higher (p=0.008), compared to the high TRAJ group. Those in the low TRAJ group were almost 6 times more likely to have better GOS outcome than patients in the high group (p=0.007). CSF S100b average and peak levels did not affect outcomes after adjusting for demographic and clinical variables. Similar results were observed when assessing predictors of DRS scores (see Table 3B). However, in the serum cohort, S100b TRAJ group membership did not significantly affect GOS, DRS, or acute mortality (models not shown). In contrast, average and peak S100b levels were predictive of acute mortality in the serum cohort, with higher levels associated with higher mortality rates (see Table 3C).
Table 3A.
Multivariate Logistic Regression Predicting GOS Outcome Using Clinical/Demographic Variables and CSF S100b Trajectory Groups
| Independent variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Using S100b trajectory groups | |||
| Age | 0.64 | (0.50, 0.83) | 0.001 |
| Injury severity (GCS) | 1.32 | (1.04, 1.70) | 0.022 |
| Subdural hematoma | 0.34 | (0.15, 0.74) | 0.007 |
| S100b low group | 5.92 | (1.64, 21.41) | 0.007 |
| S100b intermediate group | 3.32 | (1.36, 8.07) | 0.008 |
| Using mean S100b levels | |||
| Age | 0.62 | (0.47, 0.80) | <0.001 |
| Injury severity (GCS) | 1.39 | (1.10, 1.77) | 0.006 |
| Subdural hematoma | 0.42 | (0.19, 0.91) | 0.027 |
| Contusion | 0.48 | (0.23, 0.99) | 0.049 |
| S100b mean levels | 0.95 | (0.86, 1.04) | 0.229 |
| Using peak S100b levels | |||
| Age | 0.59 | (0.46, 0.77) | <0.001 |
| Injury severity (GCS) | 1.36 | (1.07, 1.72) | 0.013 |
| Subdural hematoma | 0.42 | (0.20, 0.91) | 0.027 |
| S100b peak levels | 0.83 | (0.64, 1.09) | 0.176 |
GOS, Glasgow Outcome Scale; CSF, cerebrospinal fluid; CI, confidence interval; GCS, Glasgow Coma Scale.
Bolded values represent statistically significant comparisons where alpha<0.05.
Table 3B.
Multivariate Logistic Regression Predicting DRS Outcome Using Clinical/Demographic Variables and CSF S100b Trajectory Groups
| Independent variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Using S100b trajectory groups | |||
| Age | 0.65 | (0.5, 0.84) | 0.001 |
| Injury severity (GCS) | 1.37 | (1.08, 1.74) | 0.010 |
| Subdural hematoma | 0.38 | (0.18, 0.83) | 0.015 |
| S100b low group | 2.84 | (0.84, 9.58) | 0.092 |
| S100b intermediate group | 2.73 | (1.11, 6.74) | 0.029 |
| Using mean S100b levels | |||
| Age | 0.65 | (0.50, 0.85) | 0.002 |
| Injury severity (GCS) | 1.42 | (1.12, 1.81) | 0.004 |
| Subdural hematoma | 0.36 | (0.16, 0.79) | 0.011 |
| Contusion | 0.38 | (0.15, 0.99) | 0.048 |
| S100b mean levels | 0.98 | (0.89, 1.07) | 0.639 |
| Using peak S100b levels | |||
| Age | 0.64 | (0.50, 0.83) | 0.001 |
| Injury severity (GCS) | 1.44 | (1.13, 1.83) | 0.003 |
| Subdural hematoma | 0.37 | (0.17, 0.83) | 0.011 |
| Gender | 0.37 | (0.14, 0.96) | 0.040 |
| S100b peak levels | 1.01 | (0.97, 1.04) | 0.755 |
DRS, Disability Rating Scale; CSF, cerebrospinal fluid; CI, confidence interval; GCS, Glasgow Coma Scale.
Bolded values represent statistically significant comparisons where alpha<0.05.
Table 3C.
Multivariate Logistic Regression Acute Mortality Outcome Using Clinical/Demographic Variables and Serum S100b Trajectory Groups
| Independent variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Using S100b trajectory groups | |||
| Age | 0.54 | (0.36, 0.82) | 0.003 |
| Injury severity (GCS) | 1.65 | (1.13, 2.40) | 0.010 |
| Contusion | 0.13 | (0.03, 0.48) | 0.002 |
| S100b low group | 2.95 | (0.90, 9.64) | 0.073 |
| Using mean S100b levels | |||
| Age | 0.53 | (0.35, 0.80) | 0.003 |
| Injury severity (GCS) | 1.52 | (1.04, 2.22) | 0.032 |
| Contusion | 0.14 | (0.04, 0.53) | 0.004 |
| S100b mean levels | 0.52 | (0.29, 0.92) | 0.026 |
| Using peak S100b levels | |||
| Age | 0.52 | (0.34, 0.80) | 0.003 |
| Injury severity (GCS) | 1.49 | (1.02, 2.19) | 0.041 |
| Contusion | 0.14 | (0.04, 0.53) | 0.004 |
| S100b peak levels | 0.53 | (0.30, 0.94) | 0.030 |
CI, confidence interval; GCS, Glasgow Coma Scale.
Bolded values represent statistically significant comparisons where alpha<0.05.
Discussion
As an astrocytic protein, highly specific to the CNS and released into CSF upon structural damage, S100b may be a good candidate for a biomarker of brain injury. Given the translational potential in patient care and management, S100b's presence in serum, as derived from CSF sources, has driven interest in this marker. Yet, the contributions to serum S100b levels are not fully understood.42,47 Additionally, the role of S100b as a marker of damage or neuroprotection in relation to TBI pathophysiology is not entirely clear. Because of these concerns, it is important to study S100b levels in both serum and CSF as a biomarker for sTBI. Although clinical studies have assessed the predictive value of admission and temporal serum S100b levels, to our knowledge, this is the first study to quantitatively examine and directly compare temporal profiles and point estimates from aggregated temporal data in both serum and CSF in an sTBI population while relating levels to outcome measures.
Using a TRAJ approach, we show that acute temporal profiles of elevated CSF S100b levels are significantly related to age and gender and, in bivariate analysis, are predictive of outcome. We also show that, at early time points postinjury, serum and CSF S100b levels closely correlate with each other, whereas at later time points, the correlation is much weaker. The reasons that S100b correlations wane over time, though likely multifactorial, may be linked with dynamic changes in BBB integrity as well as dynamic S100b contributions from extracerebral sources over time. As such, and consistent with other reports,20,48 the predictive value of serum S100b temporal profile membership is limited to acute mortality. Point estimates that captured overall mean and maximum serum S100b levels were also significant with regard to mortality prediction, findings that may reflect damage related to the early peak of CSF S100b levels and the overall elevations in CSF S100b, compared to controls.
Our temporal data show that CSF and serum S100b concentrations were significantly increased for 6 days above control levels in patients after sTBI, confirming previous reports.15,22,26,32,42,48 Peak concentrations were generally observed within the first 24 hours after injury in both CSF and serum. However, when utilizing TRAJ, statistically distinct longitudinal profiles with differing times to return to baseline over the 6-day time course were determined. CSF S100b TRAJ membership discriminated outcome across multiple variables, such that elevated S100b profiles in the high group predicted worse outcomes, compared to both the intermediate and the low groups.
CSF TRAJ groups were also correlated with age and gender and showed that women and older subjects were likely to be in the high CSF S100b TRAJ, which could be a reflection of more structural damage and cell death for older subjects and for women with TBI. Age and, to a lesser degree, gender, have been associated with TRAJ group membership for serum hormone profiles studied in sTBI.44,45 Older age has also been implicated in affecting other CSF biomarker levels after TBI, including BCL-2 and cytochrome C44 as well as F2-isoprostane.49 As with these other studies, older age was also independently associated with worse outcome, possibly resulting from increased medical complications and decreased repair mechanisms outside of the specific relationships with S100b and other markers.50,51
Although our results show CSF S100b levels increase with age, we found this to be untrue in the serum cohort. The examination of age effects on S100b levels has been limited to relatively few studies in pediatric TBI.5,6 Although injury levels of serum S100b in pediatric patients were comparable to those of adults, control levels in the pediatric population were higher than adults (16 vs. 0.326 pg/mL).52 The literature suggests that S100b levels vary with gestational age, early newborn development, and across the age span.53–55 Though our results are not directly applicable to the pediatric population, there are similarities with regard to the early elevation and then rapid decline in S100b levels after injury,52 as well as the ability to use temporal profiles to inform outcome.56 Future work should focus on age-adjusted evaluation of S100b injury response across the age spectrum.
Not surprisingly, increased TBI severity, as measured by lower GCS scores, was predictive of worse outcome. Yet, even when controlling for demographic and clinical variables, CSF S100b was still confirmed as an independent prognostic biomarker for severe TBI based on GOS and DRS. This finding shows that the degree of structural damage captured by S100b TRAJ groups provides unique prognostic information outside of that provided by standard clinical injury severity variables, such as GCS. This finding is consistent with the poor correlations between GCS and either serum or CSF S100b found by Kleindienst and colleagues.32,42 Given the independent relationships of both S100b and GCS to outcome, it is not surprising that S100b CSF TRAJ was not significantly related to GCS scores. The lack of relationship between biomarkers and GCS scores is not specific to S100b, but also has been shown in other studies,44,45 particularly when injury-induced breakdown products are not the biomarker of interest.57
Unlike CSF S100b TRAJ, point estimates such as mean and peak CSF S100b levels were not significant predictors of outcome, suggesting that persistently high levels, and the associated structural pathology and cell death that drives this phenomenon, is the key element when discriminating outcome. The representation of dynamic changes in CSF S100b are likely not defined by BBB permeability and the small amount of S100b leaked into serum after injury, which may be one reason why single-point estimates, such as mean and peak CSF S100b, are not as sensitive in discriminating outcome.
In contrast to CSF, BBB integrity does likely influence dynamic serum S100b levels over time. BBB compromise is known to persist for several days after a significant TBI.58 Consistent with this concept, serum TRAJ analysis S100b subpopulations showed that the low group returned to healthy control levels at around day 4. However, the high group did not return to baseline at any point during the time course. In addition to BBB dynamics, these data, demonstrating that concentrations declined more slowly toward control levels in serum compared to CSF, provide indirect evidence that peripheral sources of S100b contribute to serum levels, particularly because serum TRAJ group membership and the temporal S100b profiles they represent did not predict neurological outcome. In contrast to CSF TRAJ, serum TRAJ showed only discrimination toward predicting acute care mortality. However, serum point estimate values performed better than serum TRAJ for discriminating acute mortality. Reasons for serum S100b having less potential to discriminate outcomes may be multi-factorial. CSF/serum S100b correlations were best during the early points in the sampling period, supporting both the notion of a dynamic BBB and extracerebral sources as contributing to profiles. The fact that ISS scores are significantly different among the two serum S100b TRAJ groups, and that ISS scores are significantly correlated with both mean and peak S100b levels, provides further support for the latter point.19 However, the relatively smaller sample size for the serum cohort, compared to CSF, should also be considered when interpreting findings.
Other studies have shown potential extracerebral sources, including long-bone fracture, muscle and fat trauma, and burns. Kleindienst and associates suggest,32 based on a descriptive case analysis, that increases in S100b resulting from long-bone fracture could be transient and occur early after injury. In our serum cohort, less than 10% of our population sustained a long-bone fracture, thus limiting the power associated with this analysis particularly at early time points when the CNS contributes most to serum S100b levels. IHI was used as a proxy variable representing significant extracerebral injury. However, this variable did not significantly differentiate across serum S100b TRAJ, which may reflect the fact that some extracerebral injury is incorporated into the definition of IHI that we used.36–38 Notably peak serum levels were ∼500 times lower than peak CSF levels, suggesting that the CNS is necessarily the primary contributor to serum 100b profiles.
An intact BBB generally prevents normal diffusion of water-soluble proteins larger than approximately 500 Da, such as S100b (9–13 kDa).58 However, BBB disruption after TBI allows extravasation from CSF to serum and vice versa. Our correlation values between CSF and serum S100b levels are similar to the concept of CSF/serum albumin quotient (QA) correlations provided by Blyth and colleagues58 and support the idea that BBB extravasation is a significant contributor to serum S100b levels, particularly over the first 24–48 h. Accordingly, the most clinically useful information regarding serum S100b and prognosis in future studies would come from early sampling, when cerebral S100b contribution to serum S100b is highest. The idea that serum and CSF S100b each vary temporally, as a result of independently dynamic and different source contributions, may be one reason why serum/CSF S100b ratios assessed by Kleindienst and associates32,42 were inconsistent in their associations with outcome and when compared to the albumin ratio, an index generated on a protein with only one contribution source. Our study findings, evidence from others that peak serum S100b levels occur within the first 48 h after injury,21 and the fact that only approximately 1% of S100b is secreted in a regulatory fashion,14 suggest that the majority of S100b found in serum at peak levels is likely the result of cerebral S100b contributions from cell lysis and structural damage that pass along an impaired BBB. BBB damage itself is likely one contributor to serum S100b, from astrocytic foot processes that are damaged with BBB disruption after TBI.59,60
We believe our work helps clarify potential conflicts in the literature with regard to if and how serum S100b levels have prognostic value after TBI. In this sense, our peak serum S100b predictive associations with outcome support other studies where admission S100b levels are highly predictive of mortality and global outcome.21,23,48,61 Also, the limited predictive value of temporal profiles associated with serum S100b TRAJ are consistent with other time-course studies suggesting limited predictive value of this biomarker on outcome.32,42,62 Thus, future biomarker work can and should consider including S100b as a potentially informative biomarker, but only when used appropriately in the context of its limitations with regard to BBB dynamics and extracerebral source contributions.
There were certain limitations to our study. To fully assess CSF and serum correlations, a larger data set, specifically with more sample-to-sample overlap, would be desirable to demonstrate further a correlation between CSF and serum S100b levels. Multiple factors make sample collection after injury a challenge. These challenges were the result, in part, of the need for EVD, limited participant availability resulting from emergent procedures and hemodynamic stability, and variable CSF output with standard care. Further, the lack of mortality observed in the low TRAJ group in the CSF cohort prevented multivariate analysis from being performed on the mortality outcome measure. Nonetheless, the fact that those in the low CSF TRAJ group had almost no mortality is a striking feature of this TRAJ analysis and its discriminatory power with regard to outcome. Our study population included subjects who were also participating in clinical interventional trials. Although this inclusion increased the heterogeneity of the population studied, S100b was sufficiently discriminatory to predict global outcomes. This finding possibly indicates the potential generalizeability of S100b in discriminating outcomes in other populations.
Additional research will be useful in evaluating the additional predictive value of biomarkers, such as S100b, with established clinical variable prognostic models.63 Further research may be useful in linking S100b profiles and/or levels to other injury-relevant biomarkers for their additive prognostic utility and to determine to what extent BBB dysfunction and structural damage represented by S100b reflects the capacity of injury biomarker to prognosticate outcome. As recent research suggests, S100b is considered, by some, to be a marker of ongoing brain distress, including neurodegeneration, neuroinflammation, and psychiatric conditions.64 Future work might consider the utility of this marker in tracking long-term chronic conditions associated with TBI and as a measure sensitive to treatments.
Acknowledgments
This work was supported by the Centers for Disease Control (grant no.: R49/CCR323155) and the Department of Defense (grant no.: W81XWH-07-1-0701). The authors acknowledge the University of Pittsburgh Medical Center UPMC Trauma Registry team and the Brain Trauma Research Center for their role in data collection.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Steyerberg E.W. Mushkudiani N. Perel P. Butcher I. Lu J. McHugh G.S. Murray G.D. Marmarou A. Roberts I. Habbema J.D. Maas A.I. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:1251–1261. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MRC CRASH Trial Collaborators. Perel P. Arango M. Clayton T. Edwards P. Komolafe E. Poccock S. Roberts I. Shakur H. Steyerberg E. Yutthakasemsunt S. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336:425–429. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hukkelhoven C.W. Rampen A.J. Maas A.I. Farace E. Habbema J.D. Marmarou A. Marshall L.F. Murray G.D. Steyerberg E.W. Some prognostic models for traumatic brain injury were not valid. J. Clin. Epidemiol. 2006;59:132–143. doi: 10.1016/j.jclinepi.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Maas A.I. Marmarou A. Murray G.D. Teasdale S.G. Steyerberg E.W. Prognosis and clinical trial design in traumatic brain injury: the IMPACT study. J. Neurotrauma. 2007;24:232–238. doi: 10.1089/neu.2006.0024. [DOI] [PubMed] [Google Scholar]
- 5.Berger R.P. The use of serum biomarkers to predict outcome after traumatic brain injury in adults and children. J. Head Trauma Rehabil. 2006;21:315–333. doi: 10.1097/00001199-200607000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Kochanek P.M. Berger R.P. Bayir H. Wagner A.K. Jenkins L.W. Clark R.S. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr. Opin. Crit. Care. 2008;14:135–141. doi: 10.1097/MCC.0b013e3282f57564. [DOI] [PubMed] [Google Scholar]
- 7.Ingebrigtsen T. Romner B. Biochemical serum markers of traumatic brain injury. J. Trauma. 2002;52:798–808. doi: 10.1097/00005373-200204000-00038. [DOI] [PubMed] [Google Scholar]
- 8.Missler U. Wiesmann M. Wittmann G. Magerkurth O. Hagenström H. Measurement of glial firbrillary acidic protein in human blood: analytical method and preliminary clinical results. Clin. Chem. 1999;45:138–141. [PubMed] [Google Scholar]
- 9.Pelinka L.E. Kroepfl A. Leixnering M. Buchinger W. Raabe A. Redl H. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J. Neurotrauma. 2004;21:1553–1561. doi: 10.1089/neu.2004.21.1553. [DOI] [PubMed] [Google Scholar]
- 10.Donato R. Intracellular and extracellular roles of S100 proteins. Microsc. Res. Tech. 2003;60:540–551. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- 11.Kleindienst A. Bullock M.R. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J. Neurotrauma. 2006;23:1185–1200. doi: 10.1089/neu.2006.23.1185. [DOI] [PubMed] [Google Scholar]
- 12.Barger S.W. Van Eldik L.J. Mattson M.P. S100 beta protects hippocampal neurons from damage induced by glucose deprivation. Brain Res. 1995;677:167–170. doi: 10.1016/0006-8993(95)00160-r. [DOI] [PubMed] [Google Scholar]
- 13.Reeves R.H. Yao J. Crowley M.R. Buck S. Zhang X. Yarowsky P. Gearhart J.D. Hilt D.C. Astrocytosis and axonal proliferation in the hippocampus of S100b transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5359–5363. doi: 10.1073/pnas.91.12.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonçalves C.A. Leite M.C. Nardin P. Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin. Biochem. 2008;41:755–763. doi: 10.1016/j.clinbiochem.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Petzold A. Keir G. Lim D. Smith M. Thompson E.J. Cerebrospinal fluid (CSF) and serum S100B: release and wash-out pattern. Brain Res. Bull. 2003;61:281–285. doi: 10.1016/s0361-9230(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 16.Raabe A. Grolms C. Seifert V. Serum markers of brain damage and outcome prediction in patients after severe head injury. Br. J. Neurosurg. 1999;13:56–59. doi: 10.1080/02688699944195. [DOI] [PubMed] [Google Scholar]
- 17.Raabe A. Grolms C. Sorge O. Zimmermann M. Seifert V. Serum S-100B protein in severe head injury. Neurosurgery. 1999;45:477–483. doi: 10.1097/00006123-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Vos P.E. Lamers K.J. Hendricks J.C. van Haaren M. Beems T. Zimmerman C. van Geel W. de Reus H. Biert J. Verbeek M.M. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 2004;62:1303–1310. doi: 10.1212/01.wnl.0000120550.00643.dc. [DOI] [PubMed] [Google Scholar]
- 19.Vos P.E. Jacobs B. Andriessen T.M. Lamers K.J. Borm G.F. Beems T. Edwards M. Rosmalen C.F. Vissers J.L. GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology. 2010;75:1786–1793. doi: 10.1212/WNL.0b013e3181fd62d2. [DOI] [PubMed] [Google Scholar]
- 20.Pelinka L.E. Toegel E. Mauritz W. Redl H. Serum S 100 B: a marker of brain damage in traumatic brain injury with and without multiple trauma. Shock. 2003b;19:195–200. doi: 10.1097/00024382-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Elting J.W. de Jager A.E. Teelken A.W. Schaaf M.J. Maurits N.M. van der Naalt J. Sibinga C.T. Sulter G.A. De Keyser J. Comparison of serum S-100 protein levels following stroke and traumatic brain injury. J. Neurol. Sci. 2000;181:104–110. doi: 10.1016/s0022-510x(00)00442-1. [DOI] [PubMed] [Google Scholar]
- 22.Böhmer A.E. Oses J.P. Schmidt A.P. Perón C.S. Krebs C.L. Oppitz P.P. D'Avilla T.T. Souza D.O. Portela L.V. Stefani M.A. Neuron-specific enolase, S100B, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury. Neurosurgery. 2011;68:1624–1631. doi: 10.1227/NEU.0b013e318214a81f. [DOI] [PubMed] [Google Scholar]
- 23.Rainey T. Lesko M. Sacho R. Lecky F. Childs C. Predicting outcome after severe traumatic brain injury using the serum S100B biomarker: results using a single (24 h) time-point. Resuscitation. 2009;80:341–345. doi: 10.1016/j.resuscitation.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Dash P.K. Zhao J. Hergenroeder G. Moore A.N. Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurotherapeutics. 2010;7:100–114. doi: 10.1016/j.nurt.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson P. Shirreffs S.M. Maughan R.J. Blood-brain barrier integrity may be threatened by exercise in a warm environment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:1689–1694. doi: 10.1152/ajpregu.00676.2004. [DOI] [PubMed] [Google Scholar]
- 26.Ucar T. Baykal A. Akyuz M. Dosemeci L. Toptas B. Comparison of serum and cerebrospinal fluid protein S-100b levels after severe head injury and their prognostic importance. J. Trauma. 2004;57:95–98. doi: 10.1097/01.ta.0000071352.95491.75. [DOI] [PubMed] [Google Scholar]
- 27.Kapural M. Krizanac-Bengez L. Barnett G. Perl J. Masaryk T. Apollo D. Rasmussen P. Mayberg M.R. Janigro D. Serum S-100B as a possible marker of blood-brain barrier disruption. Brain Res. 2002;940:102–104. doi: 10.1016/s0006-8993(02)02586-6. [DOI] [PubMed] [Google Scholar]
- 28.Pelinka L.E. Szalay L. Jafarmadar M. Schmidhammer R. Redl H. Bahrami S. Circulating S100B is increased after bilateral femur fracture without brain injury in the rat. Br. J. Anaesth. 2003a;91:595–597. doi: 10.1093/bja/aeg225. [DOI] [PubMed] [Google Scholar]
- 29.Anderson A.E. Hansson L.O. Nilsson O. Dijlai-Merzoug R. Settergren G. High serum S100b levels for patients without head injuries. Neurosurgery. 2001;48:1255–1260. doi: 10.1097/00006123-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Undén J. Bellner J. Eneroth M. Alling C. Ingebrigtsen T. Romner B. Raised serum S100B levels after acute bone fractures without cerebral injury. J. Trauma. 2005;58:59–61. doi: 10.1097/01.ta.0000130613.35877.75. [DOI] [PubMed] [Google Scholar]
- 31.Hasselblatt M. Mooren F.C. von Ahsen N. Keyvani K. Fromme A. Schwarze-Eicker K. Senner V. Paulus W. Serum S100beta increases in marathon runners reflect extracranial release rather than glial damage. Neurology. 2004;62:1634–1636. doi: 10.1212/01.wnl.0000123092.97047.b1. [DOI] [PubMed] [Google Scholar]
- 32.Kleindienst A. Schmidt C. Parsch H. Emtmann I. Xu Y. Buchfelder M. The passage of S100b from brain to blood is not specifically related to the blood-brain barrier integrity. Cardiovasc. Psychiatry Neurol. 2010;2010:1–8. doi: 10.1155/2010/801295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bullock M.R. Povlishock J.T. Guidelines for the management of severe traumatic brain injury. J. Neurotrauma. 2007;24(Suppl 1):S1–S106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 34.Zafonte R. Friedewald W.T. Lee S.M. Levin B. Diaz-Arrastia R. Ansel B. Eisenberg H. Timmons S.D. Temkin N. Novack T. Ricker J. Merchant R. Jallo J. The citicoline brain injury treatment (COBRIT) trial: design and methods. J. Neurotrauma. 2009;26:2207–2216. doi: 10.1089/neu.2009.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teasdale G. Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 36.Baker S.P. O'Neill B. Haddon W., Jr. Long W.B. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J. Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 37.Berry C. Ley E.J. Tillou A. Cryer G. Margulies D.R. Salim A. The effect of gender on patients with moderate to severe head injuries. J. Trauma. 2009;67:950–953. doi: 10.1097/TA.0b013e3181ba3354. [DOI] [PubMed] [Google Scholar]
- 38.van Leeuwen N. Lingsma H.F. Perel P. Lecky F. Roozenbeek B. Lu J. Shakur H. Weir J. Steyerberg E.W. Maas A.I. International Mission on Prognosis and Clinical Trial Design in TBI Study Group, Corticosteroid Randomization After Significant Head Injury Trial Collaborators, and Trauma Audit and Research Network. Prognostic value of major extracranial injury in traumatic brain injury: an individual patient data meta-analysis in 39,274 patients. Neurosurgery. 2012;70:811–818. doi: 10.1227/NEU.0b013e318235d640. discussion, 818. [DOI] [PubMed] [Google Scholar]
- 39.Wafaisade A. Lefering R. Tjardes T. Wutzler S. Simanski C. Paffrath T. Fischer P. Bouillon B. Maegele M. Trauma Registry of DGU. Acute coagulopathy in isolated blunt traumatic brain injury. Neurocrit. Care. 2010;12:211–219. doi: 10.1007/s12028-009-9281-1. [DOI] [PubMed] [Google Scholar]
- 40.Jennett B. Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 41.Rappaport M. Hall K.M. Hopkins K. Belleza T. Cope D.N. Disability rating scale for severe head trauma: coma to community. Arch. Phys. Med. Rehabil. 1982;63:118–123. [PubMed] [Google Scholar]
- 42.Kleindienst A. Meissner S. Eyupoglu I.Y. Parsch H. Schmidt C. Buchfelder M. Dynamics of S100B release into serum and cerebrospinal fluid following acute brain injury. Acta Neurochir. Suppl. 2010;106:247–250. doi: 10.1007/978-3-211-98811-4_46. [DOI] [PubMed] [Google Scholar]
- 43.Jones B.L. Nagin D.S. Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol. Method. Res. 2001;29:374–393. [Google Scholar]
- 44.Wagner A.K. Amin K.B. Niyonkuru C. Postal B.A. McCullough E.H. Ozawa H. Dixon C.E. Bayir H. Clark R.S. Kochanek P.M. Fabio A. CSF Bcl-2 and cytochrome C temporal profiles in outcome prediction for adults with severe TBI. J. Cereb. Blood Flow Metab. 2011;31:1886–1896. doi: 10.1038/jcbfm.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner A.K. McCullough E.H. Niyonkuru C. Ozawa H. Loucks T.L. Dobos J.A. Brett C.A. Santarsieri M. Dixon C.E. Berga S.L. Fabio A. Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury. J. Neurotrauma. 2011;28:871–888. doi: 10.1089/neu.2010.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niyankuru C. Wagner A.K. Ozawa H. McCullough E.H. Goyal A. Amin K. Fabio A. Biomarker applications for group-based trajectory analysis and prognostic model development in severe TBI: A practical example. J. Neurotrauma. 2013 doi: 10.1089/neu.2012.2578. (in press). [DOI] [PubMed] [Google Scholar]
- 47.Korfias S. Stranjalis G. Papadimitriou A. Psachoulia C. Daskalakis G. Antsaklis A. Sakas D.E. Serum S-100B protein as a biochemical marker of brain injury: a review of current concepts. Curr. Med. Chem. 2006;13:3719–3731. doi: 10.2174/092986706779026129. [DOI] [PubMed] [Google Scholar]
- 48.Dimopoulou I. Korfias S. Dafni U. Anthi A. Psachoulia C. Jullien G. Sakas D.E. Roussos C. Protein S-100b serum levels in trauma-induced brain death. Neurology. 2003;60:947–951. doi: 10.1212/01.wnl.0000049931.77887.7f. [DOI] [PubMed] [Google Scholar]
- 49.Wagner A.K. Bayir H. Ren D. Puccio A. Zafonte R.D. Kochanek P.M. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: the impact of gender, age, and hypothermia. J. Neurotrauma. 2004;21:125–136. doi: 10.1089/089771504322778596. [DOI] [PubMed] [Google Scholar]
- 50.Crownover J. Galang G. Wagner AK. Neurobiological influences of aging and geriatric care concepts in TBI rehabilitation. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2012 In press. [Google Scholar]
- 51.Hukkelhoven C.W. Steyerberg E.W. Rampen A.J. Farace E. Habbema J.D. Marshall L.F. Murray G.D. Maas A.I. Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J. Neurosurg. 2003;99:666–673. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- 52.Berger R.P. Adelson P.D. Pierce M.C. Dulani T. Cassidy L.D. Kochanek P.M. Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J. Neurosurg. 2005;103(Suppl. 1):61–68. doi: 10.3171/ped.2005.103.1.0061. [DOI] [PubMed] [Google Scholar]
- 53.Gazzolo D. Bruschettini M. Lituania M. Serra G. Gandullia E. Michetti F. S100b protein concentrations in urine are correlated with gestational age in healthy preterm and term newborns. Clin. Chem. 2001;47:1132–1133. [PubMed] [Google Scholar]
- 54.Gazzolo D. Michetti F. Bruschettini M. Marchese N. Lituania M. Mangraviti S. Pedrazzi E. Bruschettini P. Pediatric concentrations of S100B protein in blood: age- and sex-related changes. Clin. Chem. 2003;49:967–970. doi: 10.1373/49.6.967. [DOI] [PubMed] [Google Scholar]
- 55.Michetti F. Gazzolo D. S100B protein in biological fluids: a tool for perinatal medicine. [Review]. Clin. Chem. 2002;48:2097–2104. [PubMed] [Google Scholar]
- 56.Berger R.P. Bazaco M.C. Wagner A.K. Kochanek P.M. Fabio A. Trajectory analysis of serum biomarker concentrations facilitates outcome prediction after pediatric traumatic and hypoxemic brain injury. Dev. Neurosci. 2010;32:396–405. doi: 10.1159/000316803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berger R.P. Hayes R.L. Richichi R. Beers S.R. Wang K.K. Serum concentrations of ubiquitin C-terminal hydrolase-L1 and αII-spectrin breakdown product 145 kDa correlate with outcome after pediatric TBI. J. Neurotrauma. 2012;29:162–167. doi: 10.1089/neu.2011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blyth B.J. Farhavar A. Gee C. Hawthorn B. He H. Nayak A. Stöcklein V. Bazarian J.J. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J. Neurotrauma. 2009;26:1497–1507. doi: 10.1089/neu.2008.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janzer R.C. Raff M.C. Astrocytes induce blood-brain-barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 60.Goldstein G.W. Betz A.L. The blood-brain-barrier. Sci. Am. 1986;255:70–83. doi: 10.1038/scientificamerican0986-74. [DOI] [PubMed] [Google Scholar]
- 61.Townend W. Ingebrigtsen T. Head injury outcome prediction: a role for protein S-100B? Injury. 2006;37:1098–1108. doi: 10.1016/j.injury.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 62.Jackson R.G. Samra G.S. Radcliffe J. Clark G.H. Price C.P. The early fall in levels of S-100 beta in traumatic brain injury. Clin. Chem. Lab. Med. 2000;38:1165–1167. doi: 10.1515/CCLM.2000.179. [DOI] [PubMed] [Google Scholar]
- 63.Mushkudiani N.A. Hukkelhoven C.W. Hernández A.V. Murray G.D. Choi S.C. Maas A.I. Steyerberg E.W. A systematic review finds methodological improvements necessary for prognostic models in determining traumatic brain injury outcomes. J. Clin. Epidemiol. 2008;61:331–343. doi: 10.1016/j.jclinepi.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 64.Michetti F. Corvino V. Geloso M.C. Lattanzi W. Bernardini C. Serpero L. Gazzolo D. The S100B protein in biological fluids: more than a lifelong biomarker of brain distress. J. Neurochem. 2012;120:644–659. doi: 10.1111/j.1471-4159.2011.07612.x. [DOI] [PubMed] [Google Scholar]