Abstract
Activation of the renin–angiotensin–aldosterone system (RAAS) plays a key role in the progression of chronic kidney disease (CKD). RAAS inhibitors, such as angiotensin converting enzyme inhibitors (ACEis) and angiotensin II receptor blockers (ARBs), decrease the rate of progression of diabetic and non-diabetic nephropathies and are first-line therapies for CKD. Although these agents are highly effective, current therapeutic strategies are unable to sufficiently suppress the RAAS and stop CKD progression. Aliskiren, the first in a new class of RAAS-inhibiting agents (direct renin inhibitors) has been approved to treat hypertension. Aliskiren exerts renoprotective, cardioprotective, and anti-atherosclerotic effects in animal models that appear to be independent of its blood pressure lowering activity. Early clinical studies using urinary protein excretion as a marker of renal involvement suggest a possibly novel role for aliskiren in treating CKD. This review discusses the antiproteinuric efficacy and safety of aliskiren and considers the evidence for its potential renoprotection.
Keywords: aliskiren, proteinuria, chronic kidney disease, renin-angiotensin-aldosterone system
Background
Chronic kidney disease (CKD) is a worldwide public health problem. The incidence and prevalence of kidney failure are increasing and are associated with poor outcomes and high cost [1]. Kidney failure requiring treatment with dialysis or transplantation is the most visible outcome of CKD, but cardiovascular disease is also frequently associated with CKD, and the risk of cardiovascular events correlates with kidney function [2]. The activity of the renin-angiotensin-aldosterone system (RAAS) plays a central role in the pathogenesis of CKD, hypertension, and cardiovascular complications; thus, targeting the RAAS is a logical therapeutic approach. Interruption of the RAAS using angiotensin-converting enzyme inhibitors (ACEis) and angiotensin II AT-1 receptor blockers (ARBs), alone or in combination, has become a leading therapeutic strategy to slow the progression of CKD [3]. Nevertheless, a considerable proportion of patients progress despite this therapy. New alternative RAAS blocking strategies are being tested to improve patient outcomes. The role of aliskiren, the recently available direct renin inhibitor (DRI), should be assessed. The purpose of this review is to critically evaluate the potential role of aliskiren in this regard.
RAAS and Kidney Damage
The RAAS plays an important biological homeostatic function in maintaining blood volume and salt-water balance, thus affecting the levels of blood pressure and tissue perfusion through a number of multiple complex actions, which have a global effect of vasoconstriction and sodium retention [4]. Despite the essential role of the RAAS in maintaining homeostasis, its long-lasting stimulation can lead to kidney lesions and the development and progression of CKD. These changes result from a range of hemodynamic and nonhemodynamic consequences of angiotensin II and aldosterone. Angiotensin II leads to increased intraglomerular pressure, which is recognized as an important factor in the progression of CKD. This increased pressure results mainly from a differential angiotensin II effect on afferent and efferent glomerular arterioles. The nonhemodynamic renal effects of angiotensin II and aldosterone include the stimulation of mitogenesis, local inflammation, and fibrosis [5].
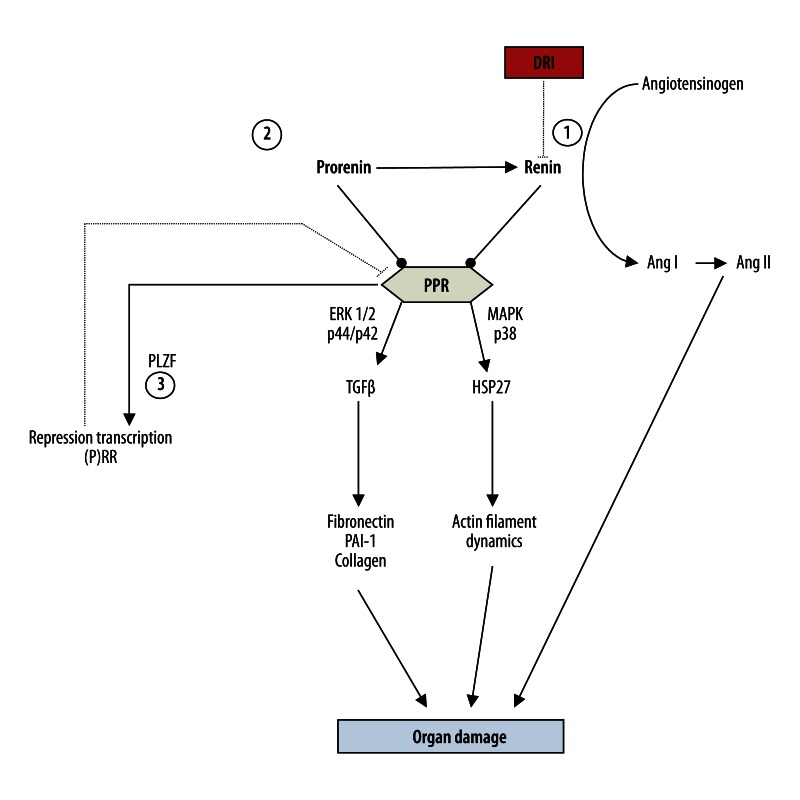
The discovery of the (pro)renin receptor (PRR) has provided a new role for renin – as a profibrotic agent. Both prorenin and renin have been found to stimulate transforming growth factor beta-1 (TGF-β1) production via MAPK p42/p44, which subsequently may result in an up-regulation of profibrotic and prothrombotic molecules, such as fibronectin, collagen-1, and PAI-1, and induce kidney fibrosis (Figure 1) [6–8]. Thus, both prorenin and renin are capable of mediating the angiotensin II-independent harmful signaling cascades, which cannot be halted by either an ACEi or ARB [9,10]. A high prorenin level is associated with more severe diabetic complications, microalbuminuria, and the development of nephropathy [11–14]. However, a high prorenin level is observed in pregnancy and is not associated with poor outcomes or organ damage [15]. Additionally, a high circulating prorenin level has been shown not to cause glomerulosclerosis in transgenic ren-2 rats [16]. Therefore, the majority of researchers believe that prorenin does not induce direct organ damage, but rather enhances the detrimental effects other risk factors, such as hyperglycemia or inflammation [8,17,18]. One should also note that PPR is downregulated only by prorenin production, which is mediated by the promyelocytic leukemia zinc finger pathway; perhaps only this mechanism prevents the harmful effects of PPR activation [19].
Figure 1.
Renin-angiotensin-aldosteron system: the role of prorenin/renin and target of direct rennin inhibitor (aliskiren). ERK 1/2 – extracellular regulated protein kinase 1/2; HSP27 – Heat shock protein 27; MAPK – mitogen activated protein kinase; PAI-1 – plasminogen-activator inhibitor 1; PLZF – promyelocytic leukaemia zinc finger; (P)RR – prorenin/renin receptor; TGF-β – transforming growth factor β. 1. DRI bind to the active site of renin and inhibit the binding of renin to angiotensinogen, which is the rate-determining step of the RAAS cascade, consequently prevent the formation of Ang I and Ang II. 2. DRI strongly increase renin and prorenin concentrations. 3. PPR is downregulated by prorenin production that is mediated by the PLZF pathway.
Pharmacological Blockade of RAAS in Renoprotection
RAAS plays an important role in the progression of CKD, and RAAS inhibition may reduce CKD progression. The renoprotective effects of the RAAS-inhibiting drugs have been shown to be in part independent of the reduction in systemic blood pressure, but to involve the normalization of glomerular hyperperfusion and hyperfiltration, restoration of glomerular barrier function, and a reduction of the nonhemodynamic effects of angiotensin II and aldosterone [3]. Several large, randomized controlled trials have shown the renoprotective potential of ACEis and ARBs in nephropathies of almost any etiology [20–23]. Patients with adult autosomal dominant polycystic kidney disease are an exception. Despite recent progress, there is still no optimal therapy that can stop the progression of this disease. Constant treatment with an ACEi or ARB has been shown to return angiotensin II and aldosterone to their pre-treatment levels [24]. One possible reason for this is suboptimal suppression of RAAS activity via an ACEi or ARB, causing a compensatory increase in renin, and angiotensin I and angiotensin II levels. Angiotensin II can also be formed using pathways that do not involve angiotensin-converting enzyme. Therefore, a therapeutic strategy that can enhance RAAS blockade and further improve renal outcomes is necessary. One possible alternative is a new class of drugs that inhibit this system – DRIs.
Aliskiren – the First Direct Renin Inhibitor
Aliskiren is a new orally active, nonpeptide, low-molecular-weight DRI that has a high affinity and specificity for human renin and inhibits the enzyme renin by binding to its catalytic site [25]. Aliskiren is poorly absorbed, with an absolute oral bioavailability of 2.5%, and maximum plasma aliskiren concentrations are reached within 1–3 hours of oral administration [26]. Steady-state plasma concentrations are reached 5–8 days after the initiation once-daily oral administration of aliskiren. Following a single oral 300 mg dose, aliskiren has an elimination half-life of 40 hours in healthy volunteers [27]. Excretion is almost completely via the fecal route (91.5%), with 77.5% of the dose excreted as unchanged drug. The pharmacokinetics of aliskiren in patients with hepatic impairment, mild to severe renal disease, or type 2 diabetes are no different from those of healthy volunteers. Thus, initial dose adjustments are not necessary in patients with renal or hepatic impairment. Aliskiren, in contrast to ACEis and ARBs, decreases plasma renin activity by approximately 50–80%. Aliskiren also reduces plasma angiotensin I and angiotensin II levels, but strongly increases renin and prorenin concentrations [28–30]. The increase in plasma renin during aliskiren treatment appears to be larger than the increase observed during treatment with either an ACEi or ARB [31,32]. Because the degree of the increase in renin is thought to reflect the degree of RAAS blockade [33], aliskiren may simply provide a more complete RAAS blockade. However, this effect may be an assay artifact, because DRIs allow prorenin to be recognized as renin in the renin immunoradiometric assays that are used to quantify renin.
One concern may be that high levels of renin and prorenin can activate the PPR receptor, which can possibly initiate ERK1/2 signaling, TGF-β activation, and other potentially serious complications [8,26]. Fortunately, some studies have shown that despite a significant increase in renin concentration, the concentrations of prorenin and TGF-β remain unchanged and aliskiren can reduce in vivo gene expression of the PPR receptor [30,34,35].
Aliskiren in Proteinuric CKD
Experimental studies
Some studies in animal models have suggested that aliskiren can protect against end-organ damage. These data are derived mainly from studies of double-transgenic rats (dTGR) that express human genes for renin and angiotensinogen. In dTGR, both low- and high-dose aliskiren and high-dose valsartan were capable of not only ameliorating but also reversing albuminuria and reducing mortality [36]. In hypertensive transgenic rats with diabetes, aliskiren has been shown to be as effective as perindopril, an ACEi, in reducing albuminuria, fibrosis, and glomerulosclerosis [37]. In the same animal model, Feldman et al. showed that aliskiren not only prevents the development of albuminuria but also suppresses production of TGF-β, a fibrosis-promoting cytokine [35,38]. In this study, aliskiren accumulated in renal tissue after 2 weeks of treatment and localized to the glomeruli and the arterial walls of the small cortical blood vessels. These findings suggest that suppression of the intrarenal RAAS may be a mechanism that contributes to the renoprotection afforded by aliskiren. Gross et al. demonstrated the antifibrotic effects of aliskiren in a normotensive mouse model [39]. Compared to placebo-treated controls, the accumulation of extracellular matrix, renal scarring, and the levels of TGF-β1 and connective tissue growth factor were decreased in the treated mice. Aliskiren has also been shown to reduce the renal expression of the PPR receptor in an animal model of diabetes [35]. Others have demonstrated that aliskiren may ameliorate oxidative stress and prevent atherosclerotic lesion formation [40,41]. These findings suggest that aliskiren may contribute to end-organ protection by reducing the deleterious actions of angiotensin II and aldosterone, as well as the angiotensin II-independent effects of PPR receptor activation.
Clinical studies
Diabetic nephropathy
Many groups have previously published studies on the use of aliskiren in patients with diabetic nephropathy (Table 1). Persson et al. investigated the effect of aliskiren in 15 patients with type 2 diabetes and a found significant reduction in albuminuria and proteinuria [42]. Importantly, the antiproteinuric effect was independent of blood pressure changes.
Table 1.
The clinical studies confirming antiproteinuric effect of direct renin inhibitor (aliskiren).
| Author/study | Population | Comparison | Results | Year [Ref.] |
|---|---|---|---|---|
| Abe M. et al | diabetic and non-diabetic nephropathy | added to standard treatment | ↓ 40% UACR | 2012 [57] |
| AVOID | diabetic nephropathy | placebo | ↓ 20% UACR | 2008 [43] |
| De Nicola L. Et al. | added to ACE-inhibitor and ARB therapy | ↓ 22% proteinuria | 2012 [47] | |
| Lizakowski S. et al. | non-diabetic nephropathy | placebo | ↓ 23% proteinuria (150 mg) ↓ 36% proteinuria (300 mg) |
2012 [53] |
| Moriyama T et al. | non-diabetic nephropathy | added to olmesartan | ↓ UACR 40% | 2012 [55] |
| Nakamura T. et al. | diabetic and non-diabetic nephropathy | olmesartan | no differences between study group | 2012 [58] |
| Persson F. et al | diabetic nephropathy | added to losartan | ↓ UACR 22% | 2011 [44] |
| Persson F. et al. | diabetic nephropathy | placebo | ↓ 48% UACR ↓ 71% with irbesartan 300 mg) |
2009 [45] |
| Persson F. et al. | diabetic nephropathy | placebo | ↓ UACR 36% (150 mg) ↓ 48% (300 mg) ↓ 52% (600 mg) |
2010 [46] |
| Tang S. et al | IgA nephropathy | added to losartan | ↓ UACR 26% | 2012 [56] |
AVOID – aliskiren in the evaluation of proteinuria in diabetes; UACR – urinary creatinine-to-albumin ratio.
In the AVOID trial (Aliskiren Combined with Losartan in Type 2 Diabetes and Nephropathy), a randomized, double-blind, placebo-controlled study that included hypertensive patients with type 2 diabetes and nephropathy, Parving et al. evaluated the renoprotective effects of dual blockade of the RAAS by adding aliskiren to losartan, an ARB, used at the maximum recommended dose of 100 mg [43]. In total, 599 patients were randomized to receive 6 months of treatment with aliskiren (dose titrated to 300 mg daily) or placebo, in addition to losartan. Treatment with aliskiren reduced the mean albuminuria by 20% (P=0.002), with a reduction of 50% or more in 24.7% of the patients who received aliskiren compared to 12.5% of those who received placebo (P<0.001). The total number of adverse and serious events was similar in both groups. The rate of decline in eGFR was comparable in both groups (2.4 ml/min/1.73 m2 in the aliskiren group and 3.8 ml/min/1.73 m2 in the placebo group, P=0.07). A detailed analysis of adverse events revealed that only the incidence of hyperkalemia was higher in the aliskiren group than in the placebo group (14/201 vs. 5/200 or 4.7 vs. 1.7%). No patient required renal replacement therapy for this reason [43]. A post hoc analysis published in 2011 showed that aliskiren added to losartan and recommended antihypertensive treatment reduced albuminuria independent of blood pressure changes and attenuated the decline in eGFR in the group with an insufficiently treated baseline blood pressure above 140/90 mmHg [44].
In another study, 26 patients with type 2 diabetes, hypertension, and albuminuria were randomly assigned to four 2-month treatment groups with placebo, 300 mg aliskiren, 300 mg irbesartan, an ARB, or a combination of aliskiren and irbesartan [45]. Aliskiren treatment reduced albuminuria by 48% compared to the placebo (P<0.001) but was not significantly different from the 58% reduction with irbesartan treatment (P<0.001). The combination therapy reduced albuminuria by 71%, which is significantly more than either monotherapy. Persson et al. analyzed the effects of increasing doses of aliskiren on albuminuria in patients with diabetic nephropathy and hypertension [46]. Aliskiren at 150, 300, and 600 mg daily significantly reduced albuminuria by 36%, 48%, and 52%, respectively, compared to the placebo (P<0.001). The albuminuria reduction after 600 mg aliskiren was not significantly different compared to 300 mg aliskiren.
Another study evaluated the effect of triple RAAS blockade, including aliskiren, on proteinuria. De Nicola et al. added aliskiren to therapy with an ACEi or ARB in proteinuric patients with diabetes [47]. After 6 months of treatment, the mean proteinuria decreased by 22% and remained unchanged at 12 months. Subgroup analysis showed a greater mean relative decrease in proteinuria in the younger patients (<65 vs. >65 years old) and those with a higher baseline sodium excretion (>160 vs. >160 mmol/24 h). The treatment was relatively well tolerated, with only 3 patients (out of 45) dropping out after 6 months (1 each for worsening eGFR, persistent hypotension, and steroid therapy). The safety analysis of more 12 000 patients in randomized, controlled clinical trials has demonstrated that aliskiren in combination with hydrochlorothiazide, valsartan, or losartan is well tolerated and has a good safety profile in patients with hypertension, including older individuals and those with additional risk factors such as diabetes [48].
The results of the ALTITUDE study (Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints) were highly anticipated [49,50]. This 4-year, multicenter, placebo-controlled trial evaluated the potential benefits of aliskiren 300 mg daily in addition to conventional therapy, including an ACEi or ARB, in reducing the risk of cardiovascular (CV) and renal events. In total, 8606 diabetic patients with renal impairment and/or CV disease were recruited into the study. The primary endpoint was the first occurrence of the one of the following events: CV death, resuscitated sudden death, non-fatal myocardial infarction, non-fatal stroke, unplanned hospitalization for heart failure, doubling of the serum creatinine concentration to above the upper limit of normal, and the onset of end-stage renal disease or renal disease-related death. The secondary CV endpoint was the first occurrence of the above-mentioned CV outcomes, while the secondary renal endpoint was the first occurrence of renal events. The ALTITUDE study was stopped early in December 2011 on the recommendation of its Data Monitoring Committee because the study patients were unlikely to benefit from aliskiren and there was an increased incidence of non-fatal stroke, renal complications (including acute renal failure), hyperkalemia, and hypotension in the patients randomized to the aliskiren group. There was an increased risk of primary outcome events associated with the aliskiren group, with a hazard ratio (HR) of 1.09 (95% CI 0.97–1.22). There was a higher incidence of adverse events, including fatal or non-fatal stroke (2.6% vs. 2.0%), serious renal adverse events (e.g., renal impairment), acute renal failure, chronic renal failure (4.7% vs. 3.3%), hyperkalemia (36.9% vs. 27.1%) and hypotension (18.4% vs. 14.6%), in the aliskiren group compared to the placebo group. Currently, in response to the FDA/EMEA recommendations, aliskiren in combination with an ACEi or ARB is contraindicated in all diabetic patients, and it is recommended that this combination be avoided in non-diabetic patients with impaired renal function (GFR <60 ml/min/1.73 m2) [50–52].
In summary, the majority of studies have demonstrated that aliskiren reduces albumin/protein excretion in patients with diabetic nephropathy and may be an additional therapeutic option for renoprotection. Until the results of other trials on aliskiren, the concomitant use of aliskiren and other RAAS blocking agents in diabetics is not indicated.
Non-diabetic nephropathy
There have been several small studies on the antiproteinuric effects of aliskiren in non-diabetic CKD patients (Table 1). In our recent study, we demonstrated that aliskiren significantly reduced proteinuria by 25% compared to the placebo [53]. In equivalent hypotensive doses, aliskiren 150 mg seems to decrease proteinuria at least as efficiently as perindopril (10 mg), an ACEi. Increasing the dose of aliskiren from 150 to 300 mg induced a further 13% decrease in protein excretion. Aliskiren also suppressed TGF-β1 production despite a large increase in renin and prorenin concentrations [30].
Abe et al. assessed the effect of aliskiren treatment on blood pressure, albuminuria, and kidney function in 67 patients with CKD [54]. The subjects were subdivided according to the presence of diabetes. Albuminuria in both groups decreased by approximately 40% over 24 weeks compared to baseline values. There were no significant differences in the percent reduction of albuminuria or changes in eGFR levels from baseline between the 2 groups.
Several recently published studies have demonstrated the beneficial effect of adding aliskiren for non-diabetic proteinuric patients being treated with an ACEi or ARB. In a prospective study, Moriyama et al. demonstrated that aliskiren 150 mg daily reduced urinary protein excretion by approximately 40% from baseline in patients treated with olmesartan, an ARB [55]. Additionally, the eGFR did not change throughout the study period. Likewise, aliskiren added to losartan in patients with immunoglobulin A nephropathy (IgAN) and significant residual proteinuria achieved a 26% reduction in proteinuria after 12 months of therapy [56]. The authors also showed that aliskiren significantly reduced circulating interleukin 6 (IL-6) and TGF-β levels. Thus, the suppression of inflammatory molecules and cytokines by aliskiren may contribute to renoprotection in the long term. In these studies, the antiproteinuric effect conferred by aliskiren was independent of changes in blood pressure.
Conclusions
Aliskiren is the first orally active DRI that provides an alternative to ACEis and ARBs for treating hypertension and organ protection in CKD. As monotherapy, aliskiren effectively reduces albuminuria and proteinuria and may be administered when therapy with an ACEi or ARB is ineffective or poorly tolerated. Aliskiren may also enhance RAAS blockade and be used in combination with an ACEi or ARB in patients with proteinuria in which monotherapy is not sufficiently effective. These concomitant therapies must be carefully monitored for hyperkalemia and worsening of kidney function, and should be avoided in patients with diabetes or renal insufficiency until the results from the other aliskiren trials become available.
Footnotes
Source of support: Self financing
References
- 1.Rutkowski B, Krol E. Epidemiology of chronic kidney disease in central and eastern europe. Blood Purif. 2008;26:381–85. doi: 10.1159/000137275. [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS. Cardiovascular disease and chronic renal disease: a new paradigm. Am J Kidney Dis. 2000;35:S117–31. doi: 10.1016/s0272-6386(00)70239-3. [DOI] [PubMed] [Google Scholar]
- 3.Tylicki L, Lizakowski S, Rutkowski B. Renin-angiotensin-aldosterone system blockade for nephroprotection: current evidence and future directions. J Nephrol. 2012;25(6):900–10. doi: 10.5301/jn.5000134. [DOI] [PubMed] [Google Scholar]
- 4.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system – an endocrine and paracrine system. Endocrinology. 2003;144:2179–83. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 5.Wolf G, Butzmann U, Wenzel U. The renin-angiotensin system and progression of renal disease: from hemodynamics to cell biology. Nephron Physiol. 2003;93:3–13. doi: 10.1159/000066656. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen G. Renin/prorenin receptors. Kidney Int. 2006;69:1503–6. doi: 10.1038/sj.ki.5000265. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Wongamorntham S, Kasting J, et al. Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 2006;69:105–13. doi: 10.1038/sj.ki.5000011. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen G, Muller DN. The biology of the (pro)renin receptor. J Am Soc Nephrol. 2010;21:18–23. doi: 10.1681/ASN.2009030300. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen G, Delarue F, Burckle C, et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–27. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Noble NA, Zhang J, et al. Renin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int. 2007;72:45–52. doi: 10.1038/sj.ki.5002243. [DOI] [PubMed] [Google Scholar]
- 11.Hollenberg NK. Direct renin inhibition and the kidney. Nat Rev Nephrol. 2010;6:49–55. doi: 10.1038/nrneph.2009.201. [DOI] [PubMed] [Google Scholar]
- 12.Deinum J, Ronn B, Mathiesen E, et al. Increase in serum prorenin precedes onset of microalbuminuria in patients with insulin-dependent diabetes mellitus. Diabetologia. 1999;42:1006–10. doi: 10.1007/s001250051260. [DOI] [PubMed] [Google Scholar]
- 13.Wilson DM, Luetscher JA. Plasma prorenin activity and complications in children with insulin-dependent diabetes mellitus. N Engl J Med. 1990;323:1101–6. doi: 10.1056/NEJM199010183231604. [DOI] [PubMed] [Google Scholar]
- 14.Sakoda M, Itoh H, Ichihara A. Podocytes as a target of prorenin in diabetes. Curr Diabetes Rev. 2011;7:17–21. doi: 10.2174/157339911794273955. [DOI] [PubMed] [Google Scholar]
- 15.Itskovitz J, Rubattu S, Levron J, Sealey JE. Highest concentrations of prorenin and human chorionic gonadotropin in gestational sacs during early human pregnancy. J Clin Endocrinol Metab. 1992;75:906–10. doi: 10.1210/jcem.75.3.1517384. [DOI] [PubMed] [Google Scholar]
- 16.Peters B, Grisk O, Becher B, et al. Dose-dependent titration of prorenin and blood pressure in Cyp1a1ren-2 transgenic rats: absence of prorenin-induced glomerulosclerosis. J Hypertens. 2008;26:102–9. doi: 10.1097/HJH.0b013e3282f0ab66. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Matavelli LC, Siragy HM. Renal (pro)renin receptor contributes to development of diabetic kidney disease through transforming growth factor-beta1-connective tissue growth factor signalling cascade. Clin Exp Pharmacol Physiol. 2011;38:215–21. doi: 10.1111/j.1440-1681.2011.05486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Siragy HM. Glucose promotes the production of interleukine-1beta and cyclooxygenase-2 in mesangial cells via enhanced (Pro)renin receptor expression. Endocrinology. 2009;150:5557–65. doi: 10.1210/en.2009-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schefe JH, Menk M, Reinemund J, et al. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res. 2006;99:1355–66. doi: 10.1161/01.RES.0000251700.00994.0d. [DOI] [PubMed] [Google Scholar]
- 20.Barnett AH, Bain SC, Bouter P, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–61. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 21.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 22.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 23.Ruggenenti P, Perna A, Gheradi G, et al. Renoprtective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354:359–64. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- 24.Lakkis J, Lu W, Weir M. RAAS escape: a real clinical entity that may be important in the progression of cardiovascular and renal disease. Curr Hypertens Rep. 2003;5:408–17. doi: 10.1007/s11906-003-0087-9. [DOI] [PubMed] [Google Scholar]
- 25.Riccioni G. Aliskiren in the treatment of hypertension and organ damage. Cardiovasc Ther. 2011;29:77–87. doi: 10.1111/j.1755-5922.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- 26.Trimarchi H. Role of aliskiren in blood pressure control and renoprotection. Int J Nephrol Renovasc Dis. 2011;4:41–48. doi: 10.2147/IJNRD.S6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cagnoni F, Njwe CA, Zaninelli A, et al. Blocking the RAAS at different levels: an update on the use of the direct renin inhibitors alone and in combination. Vasc Health Risk Manag. 2010;6:549–59. doi: 10.2147/vhrm.s11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luft FC, Weinberger MH. Antihypertensive therapy with aliskiren. Kidney Int. 2008;73:679–83. doi: 10.1038/sj.ki.5002732. [DOI] [PubMed] [Google Scholar]
- 29.Oparil S, Yarows SA, Patel S, et al. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet. 2007;370:221–29. doi: 10.1016/S0140-6736(07)61124-6. [DOI] [PubMed] [Google Scholar]
- 30.Lizakowski S, Tylicki L, Renke M, et al. Aliskiren and Perindopril Reduce the Levels of Transforming Growth Factor-beta in Patients With Non-Diabetic Kidney Disease. Am J Hypertens. 2012;25:636–39. doi: 10.1038/ajh.2012.14. [DOI] [PubMed] [Google Scholar]
- 31.Nussberger J, Wuerzner G, Jensen C, Brunner HR. Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril. Hypertension. 2002;39:E1–8. doi: 10.1161/hy0102.102293. [DOI] [PubMed] [Google Scholar]
- 32.Azizi M, Menard J, Bissery A, et al. Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II-renin feedback interruption. J Am Soc Nephrol. 2004;15:3126–33. doi: 10.1097/01.ASN.0000146686.35541.29. [DOI] [PubMed] [Google Scholar]
- 33.Azizi M, Bissery A, Lamarre-Cliche M, Ménard J. Integrating drug pharmacokinetics for phenotyping individual renin response to angiotensin II blockade in humans. Hypertension. 2004;43:785–90. doi: 10.1161/01.HYP.0000125698.00128.64. [DOI] [PubMed] [Google Scholar]
- 34.Gross O, Girgert R, Rubel D, et al. Renal Protective Effects of Aliskiren Beyond Its Antihypertensive Property in a Mouse Model of Progressive Fibrosis. Am J Hypertens. 2011;24(3):355–61. doi: 10.1038/ajh.2010.231. [DOI] [PubMed] [Google Scholar]
- 35.Feldman DL, Jin L, Xuan H, et al. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension. 2008;52:130–36. doi: 10.1161/HYPERTENSIONAHA.107.108845. [DOI] [PubMed] [Google Scholar]
- 36.Pilz B, Shagdarsuren E, Wellner M, et al. Aliskiren, a human renin inhibitor, ameliorates cardiac and renal damage in double-transgenic rats. Hypertension. 2005;46:569–76. doi: 10.1161/01.HYP.0000179573.91016.3f. [DOI] [PubMed] [Google Scholar]
- 37.Kelly DJ, Zhang Y, Moe G, et al. Aliskiren, a novel renin inhibitor, is renoprotective in a model of advanced diabetic nephropathy in rats. Diabetologia. 2007;50:2398–404. doi: 10.1007/s00125-007-0795-9. [DOI] [PubMed] [Google Scholar]
- 38.Feldman DL. New insights into the renoprotective actions of the renin inhibitor aliskiren in experimental renal disease. Hypertens Res. 2010;33:279–87. doi: 10.1038/hr.2010.19. [DOI] [PubMed] [Google Scholar]
- 39.Gross O, Girgert R, Rubel D, et al. Renal protective effects of aliskiren beyond its antihypertensive property in a mouse model of progressive fibrosis. Am J Hypertens. 2011;24:355–61. doi: 10.1038/ajh.2010.231. [DOI] [PubMed] [Google Scholar]
- 40.Ino J, Kojima C, Osaka M, et al. Dynamic observation of mechanically-injured mouse femoral artery reveals an antiinflammatory effect of renin inhibitor. Arterioscler Thromb Vasc Biol. 2009;29:1858–63. doi: 10.1161/ATVBAHA.108.182519. [DOI] [PubMed] [Google Scholar]
- 41.Poss J, Werner C, Lorenz D, et al. The renin inhibitor aliskiren upregulates pro-angiogenic cells and reduces atherogenesis in mice. Basic Res Cardiol. 2010;105:725–35. doi: 10.1007/s00395-010-0120-5. [DOI] [PubMed] [Google Scholar]
- 42.Persson F, Rossing P, Schjoedt KJ, et al. Time course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetes. Kidney Int. 2008;73:1419–25. doi: 10.1038/ki.2008.68. [DOI] [PubMed] [Google Scholar]
- 43.Parving HH, Persson F, Lewis JB, et al. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–46. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 44.Persson B, Lewis JB, Lewis EJ, et al. Aliskiren in combination with losartan reduces albuminuria independent of baseline blood pressure in patients with type 2 diabetes and nephropathy. Clin J Am Soc Nephrol. 2011;6:1025–31. doi: 10.2215/CJN.07590810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Persson F, Rossing P, Reinhard H, et al. Renal effects of aliskiren compared with and in combination with irbesartan in patients with type 2 diabetes, hypertension, and albuminuria. Diabetes Care. 2009;32:1873–79. doi: 10.2337/dc09-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Persson F, Rossing P, Reinhard H, et al. Optimal antiproteinuric dose of aliskiren in type 2 diabetes mellitus: a randomised crossover trial. Diabetologia. 2010;53:1576–80. doi: 10.1007/s00125-010-1789-6. [DOI] [PubMed] [Google Scholar]
- 47.De Nicola L, Zamboli P, Bellizzi V, et al. Antiproteinuric response to add-on aliskiren in proteinuric patients treated with dual blockade of the renin-angiotensin system: a 12-month prospective uncontrolled study. Am J Kidney Dis. 2011;57:961–63. doi: 10.1053/j.ajkd.2011.02.384. [DOI] [PubMed] [Google Scholar]
- 48.White WB, Bresalier R, Kaplan AP, et al. Safety and tolerability of the direct renin inhibitor aliskiren: a pooled analysis of clinical experience in more than 12,000 patients with hypertension. J Clin Hypertens (Greenwich) 2010;12(10):765–75. doi: 10.1111/j.1751-7176.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parving HH, Brenner BM, McMurray JJ, et al. Baseline characteristics in the Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE) J Renin Angiotensin Aldosterone Syst. 2012;13(3):387–93. doi: 10.1177/1470320311434818. [DOI] [PubMed] [Google Scholar]
- 50.Parving HH, Brenner BM, McMurray JJ, et al. Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE): rationale and study design. Nephrol Dial Transplant. 2009;24:1663–71. doi: 10.1093/ndt/gfn721. [DOI] [PubMed] [Google Scholar]
- 51.Novartis. Novartis announcestermination of ALTITUDE study with Rasilez/Tekturna in high-risk patients with diabetes and renal impairment. 2011 [Google Scholar]
- 52.de Leeuw PW. Dual renin-angiotensin system blockade. BMJ. 2012;344:e656. doi: 10.1136/bmj.e656. [DOI] [PubMed] [Google Scholar]
- 53.Lizakowski S, Tylicki L, Renke M, et al. Effect of aliskiren on proteinuria in non-diabetic chronic kidney disease: a double-blind, crossover, randomised, controlled trial. Int Urol Nephrol. 2012;44(6):1763–70. doi: 10.1007/s11255-011-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abe M, Suzuki H, Okada K, et al. Efficacy analysis of the renoprotective effects of aliskiren in hypertensive patients with chronic kidney disease. Heart Vessels. 2012 doi: 10.1007/s00380-012-0260-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Moriyama T, Tsuruta Y, Kojima C, et al. Beneficial effect of aliskiren combined with olmesartan in reducing urinary protein excretion in patients with chronic kidney disease. Int Urol Nephrol. 2012;44:841–45. doi: 10.1007/s11255-011-9991-0. [DOI] [PubMed] [Google Scholar]
- 56.Tang SC, Lin M, Tam S, et al. Aliskiren combined with losartan in immunoglobulin A nephropathy: an open-labeled pilot study. Nephrol Dial Transplant. 2012;27:613–18. doi: 10.1093/ndt/gfr349. [DOI] [PubMed] [Google Scholar]